Abstract
Hendra virus (HeV) continues to pose a serious public health concern as spillover events occur sporadically. Terminally ill horses can exhibit a range of clinical signs including frothy nasal discharge, ataxia or forebrain signs. Early signs, if detected, can include depression, inappetence, colic or mild respiratory signs. All unvaccinated ill horses in areas where flying foxes exist, may potentially be infected with HeV, posing a significant risk to the veterinary community. Equivac® HeV vaccine has been fully registered in Australia since 2015 (and under an Australian Pesticides and Veterinary Medicines Authority special permit since 2012) for immunization of horses against HeV and is the most effective and direct solution to prevent disease transmission to horses and protect humans. No HeV vaccinated horse has tested positive for HeV infection. There is no registered vaccine to prevent, or therapeutics to treat, HeV infection in humans. Previous equine HeV outbreaks tended to cluster in winter overlapping with the foaling season (August to December), when veterinarians and horse owners have frequent close contact with horses and their bodily fluids, increasing the chance of zoonotic disease transmission. The most southerly case was detected in 2019 in the Upper Hunter region in New South Wales, which is Australia's Thoroughbred horse breeding capital. Future spillover events are predicted to move further south and inland in Queensland and New South Wales, aligning with the moving distribution of the main reservoir hosts. Here we (1) review HeV epidemiology and climate change predicted infection dynamics, (2) present a biosecurity protocol for veterinary clinics and hospitals to adopt, and (3) describe diagnostic tests currently available and those under development. Major knowledge and research gaps have been identified, including evaluation of vaccine efficacy in foals to assess current vaccination protocol recommendations.
Keywords: One health, Vaccine, Zoonosis, Climate change, Infectious disease, Biosecurity
Abbreviations: OIE, World Organization for Animal Health; NiV, Nipah virus; HeV, Hendra virus; RNA, Ribonucleic acid; QLD, Queensland; NSW, New South Wales; sG, Soluble G; PPE, Personal protective equipment; Se, Sensitivity; Sp, Specificity; PC, Physical containment; qRT-PCR, Real-time reverse transcription polymerase chain reaction; iELISA, Indirect enzyme-linked immunosorbent assay; SNT, Serum neutralization test; MFI, Median fluorescent intensity; LAMP, Loop-mediated isothermal amplification
Highlights
-
•
Hendra virus (HeV) continues to pose a serious public health threat to the equine and veterinary industries.
-
•
HeV cases are likely to expand further south and inland due to climate change.
-
•
Strict HeV specific biosecurity protocols should be implemented to protect veterinary staff.
-
•
Research into HeV vaccination protocols in foals is required for evidence-based recommendations.
-
•
Point-of-care and other diagnostic tests for HeV are currently under development.
1. Introduction
An emerging zoonosis is defined by the World Health Organization, Food and Agriculture Organization of the United Nations and the World Organization for Animal Health (OIE) as “a zoonosis that is newly recognized or newly evolved, or that has occurred previously but shows an increase in incidence or expansion in geographical, host or vector range” [1]. Zoonotic pathogens account for 60% of emerging infectious diseases, of which 72% originate from wildlife [2]. Bats (order: Chiroptera) are important reservoir hosts for emerging zoonotic viruses such as Nipah virus (NiV) in Malaysia, and Hendra virus (HeV) in Australia, and are predicted to be a reservoir hosts of Ebola virus in Africa [3,4]. The most recent zoonosis is severe acute respiratory syndrome coronavirus 2 (abbreviated as SARS-CoV-2; disease known as COVID-19) first detected in Wuhan, China in December 2019 [5,6], however, the origin of this virus is yet to be determined.
Hendra virus is an important emerging zoonotic disease in Australia, characterized by high mortality rates [7]. It is classified as a biosafety level 4 organism (which is the highest level of biocontainment, and the same classification as Ebola virus). HeV, together with NiV and Cedar virus, belongs to the family Paramyxoviridae, under the genus Henipavirus [[8], [9], [10], [11]]. HeV is a pleomorphic (varying from spherical to filamentous), enveloped virus containing a single stranded, negative-sense, unsegmented ribonucleic acid (RNA) genome [11,12]. To date, there have been 62 spillover events, involving 105 horses and seven humans of which four died and three recovered with varying sequalae. The aim of this paper is to present a biosecurity protocol suitable for use in veterinary hospitals in areas where flying foxes exit. Where flying foxes are endemic horses (and therefore humans) are at risk for HeV infection. It can be adapted across a range of situations, and will be invaluable to the equine veterinary practitioner. To put the protocol in context, we start with a review of the epidemiology of HeV infection and dynamics in relation to climate change, and we discuss the current and developing diagnostic tests.
2. Epidemiology
Hendra virus was first described in September 1994 in Hendra, a suburb of Brisbane, Australia following an investigation of an outbreak of severe acute respiratory disease and high fever in 14 of the 20 horses on a single property [7]. Two people with a history of close contact with the affected horses were infected; one died within a week of infection, and the other recovered [8]. A similar event occurred in Mackay, Queensland, Australia involving two horses and a human the month prior (August 1994) [7]. This event was only recognized in 1995 after the previously infected person died from relapsing encephalitis [13,14]. Overall, the current approximate case fatality rate in horses and humans is 80% and 60% respectively [15]. The precise equine mortality rate is not possible to determine as all horses with a positive diagnosis of HeV are euthanized once the diagnosis is confirmed [15].
2.1. Clinical features
Horses infected with HeV generally have an acute non-specific illness, showing variable clinical signs with no pathognomonic signs. Clinical signs can be divided into three categories (1) respiratory: tachypnoea, with or without a frothy nasal discharge; (2) neurological: ataxia, head tilt, circling, seizures, urinary incontinence, altered mentation, recumbency; (3) other: colic, depression, fever, tachycardia, inappetence and restlessness [8,[16], [17], [18]]. Due to the high mortality rate and public health concerns, HeV infection, although rare, is always a high priority differential for any sick horse residing in an identified at-risk region. Therefore, a HeV exclusion test should be performed before proceeding with other diagnostics and/or treatment in any horse presenting with acute non-specific illness. Workplace health and safety fines (maximum penalty AUD $100,000) have been imposed on veterinarians who failed to comply with the Work Health and Safety Act 2011 when managing potentially HeV infected horses [19,20].
The reported incubation period of HeV in horses is 3 to 16 days [8,14,16,17]. Similarly, the incubation period of HeV in humans has ranged from a few days to two weeks [[21], [22], [23]]. In humans, mild clinical signs include fever, headache, drowsiness and influenza-like symptoms [21,22]. Severe infections are often fatal with respiratory and/or neurological signs (e.g. confusion, motor deficits and seizures) [21,22]. Relapsing encephalitis is possible after recovery from an acute infection and appears to be due to recrudescence of viral replication in the central nervous system [22,24]. To date, there have only been seven human infections, making the characterization of clinical features less well defined. Currently, there is no approved therapeutics or vaccines to treat or prevent HeV infection in humans. However, a human monoclonal antibody m102.4 is currently being administered in a clinical trial involving humans and is showing promising results [25].
2.2. Host species
Pteropid bats (also known as flying foxes or fruit bats) have been identified as the reservoir host. The virus circulates between asymptomatic flying foxes, and is maintained in these species, providing a common source of infection to horses. Serological surveys of over 5000 sera samples from 46 animal species detected neutralizing HeV antibodies in all four main species of flying foxes in eastern Australia: Pteropus conspicillatus (spectacled fruit bat), P. alecto (black fruit bat), P. scapulatus (little red flying fox), and P. poliocephalus (grey-headed flying fox) [26,27]. Viral RNA has been detected in a range of tissues in both naturally and experimentally infected P. alecto and P. poliocephalus [[28], [29], [30]]. Spatial analysis and molecular studies showed that P. alecto and P. conspicillatus are the two main natural reservoir hosts and are likely to be responsible for spillover events [[31], [32], [33], [34]].
Horses act as an amplifying host and are the only known mammalian species that has been infected directly from bats. To date, two asymptomatic dogs have been reported to have been infected due to a natural infection, arising from exposure to infected horses [35,36]. Experimental infections have been successful in dogs [36], pigs [37], hamsters [38], guinea pigs [30,39,40], ferrets [41], African green monkeys [42], cats [40,43] and horses [17]. Notwithstanding the very high viral doses used in experimental inoculations, dogs, cats, guinea pigs, ferrets and pigs housed outdoors are plausible susceptible hosts of HeV infection and may conceivably transmit the virus to humans. Horse-to-horse and horse-to-human transmissions are likely via contact with infected bodily fluids, especially nasal or oral secretions, from an infected horse during all stages of disease from preclinical to post-mortem [17]. The veterinary profession is at a particularly high risk with four infected people belonging to this profession and an additional one being the husband of a veterinarian who assisted with a necropsy on a HeV infected horse. Therefore, extra precautions should be taken especially when performing diagnostic, treatment or necropsy procedures involving the upper/lower airway and oral cavity of horses.
2.3. Risk factors related to horse management
After decades of research, the exact mode of transmission remains inferred from field data. Viral isolation from liver and lung of aborted flying fox fetuses and uterine fluid from aborting female flying fox suggests possible transmission to horses via direct contact with a recently aborted fetus or associated fetal fluid during parturition [29]. Another hypothesis is oro-nasal mucosal contact with infected flying fox urine, or less likely with other fluids such as blood, feces, nasal discharge and saliva, via contaminated pasture or feed, especially when horses are feeding under trees where flying foxes are foraging or roosting [32,33]. Considering HeV survival is relatively short (likely to be less than 96 h) and very sensitive to environmental conditions [[44], [45], [46]], direct contact of the mucosal membranes (such as conjunctiva) with fresh infectious flying fox urine is more likely to allow HeV transmission to occur. Interestingly, studies demonstrated higher prevalence of HeV genetic material in P. alecto and P. conspicillatus as compared to the two other species [[32], [33], [34],47] suggesting they are more important in maintaining and transmitting infection both within-species and cross-species. Spatial analysis studies showed that previous spillover events were associated with close proximity and denser populations of P. alecto and P. conspicillatus roosts [31,[48], [49], [50]]. A recent study suggested that immunologically naïve P. alecto sub-adults may be important in maintaining HeV infection within-species at a population level [47]. The larger population size and geographic distribution of P. alecto makes P. alecto potentially the most important species in transmission and spillover of HeV, followed by P. conspicillatus, P. poliocephalus and P. scapulatus. While densities of dominant flying fox species in a region can be affected by urbanization [51,52], P. alecto territories are expanding and could potentially be replacing or dominating current P. conspicillatus territory in northern and eastern Australia [53], increasing the risk of spillover events to additional areas in northern Queensland (QLD). Likewise, P. alecto is expanding its territories further south, with implications for southern New South Wales (NSW), Victoria and South Australia.
Hendra virus infection dynamics vary spatio-temporally. HeV excretion from flying fox urine fluctuates within-year and peaks in autumn and winter especially along the eastern coast (southern QLD and northern NSW) and central NSW [34,47,54]. This coincides with many mid-year spillover events in these locations. This is especially concerning to veterinarians as foaling season commences in August (winter) and horse owners and veterinarians have increased close contact with broodmares and foals and their bodily fluids, increasing the chance of zoonotic disease transmission. While infection prevalence in pteropid bats may be a main driver for horse infection [34], spillover events are rare and sporadic. In addition, HeV antibodies were first detected in archived samples from flying foxes collected at least 10 years before the first recorded equine HeV case [27]. It is unknown if unidentified equine cases occurred throughout this time, but HeV appears to be a true emerging infectious disease of horses and humans. Around 20% of cases occurred in spring and summer months, therefore, there are still some drivers within the complex multifactorial HeV transmission cycle that remain unidentified.
2.4. Impact of climatic variables on HeV epidemiology
Flying fox distributions are highly dependent on food sources, a combination of nectar, pollen and fruit especially those produced by Eucalyptus trees in woodlands and open forests [[55], [56], [57]]. Flying foxes have an important role in maintaining a balanced ecosystem through pollination and seed dispersion [55]. Seasonal climatic triggers (e.g. normal changes in temperature) are one of the many factors that influences eucalypts flowering patterns [58,59]. Extreme dry conditions are associated with poor flowering [59]. Therefore, a lack of food resources due to tree clearance and climate change may disperse flying foxes outside of typical territories with resultant increased levels of stress. Studies using urine cortisol concentrations to measure physiological stress in pteropid bats have demonstrated lower winter temperatures increase cortisol concentrations which is associated with HeV excretion in flying foxes [60,61]. Body condition score is a proxy of nutritional status. Poor body condition is associated with increased seroconversion and HeV infection risk in flying foxes. This increases urinary excretion of viable virus, with increased risk of transmission to horses [47,62].
Specific weather patterns have been associated with spillover events. A number of studies have consistently demonstrated associations between dry conditions and spillover events [49,50,57,63]. El Niño cycles (warmer and drier conditions) in spring/summer correlates with spillover events in autumn/winter [49,57]. Reduced flowering of eucalypts in winter following El Niño events causes flying foxes to disperse over wider areas for resources which coincides with the observed peaks of HeV prevalence in bats and winter HeV spillover events [34,50,57]. Although most HeV outbreaks tend to cluster in cooler months, individual equine cases present all year round. Studies showed that flying foxes excrete HeV throughout the year and it is possible for spillover events to peak in other seasons [50,54]. El Niño and drought events are predicted to occur more frequently in the future due to climate change [64,65]. Therefore, these extreme weather conditions coupled with the cascading effects to flying foxes may increase the risk and frequency of HeV spillover events/outbreaks and expansion into novel areas.
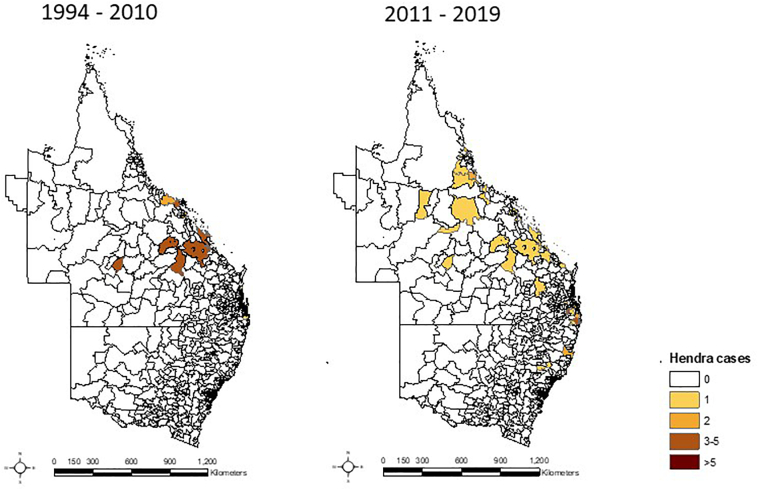
The territory of P. alecto, the main reservoir host is expanding southward, and it was predicted equine HeV infections would occur within the high horse density Hunter Valley, the Thoroughbred racehorse breeding capital of Australia [53]. A year after this prediction, the first and southernmost case of HeV was recorded in June 2019 in the Upper Hunter Valley. HeV spillover events have been expanding southward along the eastern coast and inland (Fig. 1).
Fig. 1.
Hendra virus spillover events categorized by postcode in Queensland and New South Wales from 1994 to 2010 (left); and from 2011 to 2019 (right).
3. Vaccine
Equivac® HeV vaccine is a subunit-based vaccine, manufactured by Zoetis Australia, containing recombinant HeV soluble G (sG) glycoprotein and thiomersal (adjuvant) [66,67]. The primary immunization protocol requires two doses administered as an intramuscular injection three to six weeks apart, then another dose 6 months later. An annual booster is required thereafter to maintain immunity to HeV. The vaccine contains a subunit of the virus, making viral replication impossible and therefore safe for horses. The reported adverse reaction rate is low (estimated 0.001% in 100,000 horses), with the majority being local injection site reactions [68,69]. The vaccine does not affect horse racing performance [70]. To date, all HeV infected horses were unvaccinated. In addition, studies demonstrated all vaccinated horses, as well as other animal models, were protected when challenged with HeV or NiV [41,66,[71], [72], [73], [74]].
Hendra virus vaccine uptake has been low [75], due to a wide range of reasons, including owner's perceived knowledge of HeV and vaccine safety and efficacy concerns [69,76,77]. This is possibly due to the effects of amplified reports of anecdotal adverse events from social media. However, a Queensland state government parliamentary enquiry concluded that the vaccine was the best way to prevent HeV infections in horses and humans [78,79]. Veterinarians should build rapport and educate horse owners with scientific evidence about HeV and the importance of vaccination as a public health initiative [69,80,81]. HeV vaccination remains to be the most effective, reliable and direct approach to prevent infection in horses and therefore transmission to humans. Other management strategies, such as targeted tree clearance, are possible to implement but can be overwhelmingly complex, operationally challenging and would disturb existing ecosystems.
4. Veterinary hospital biosecurity
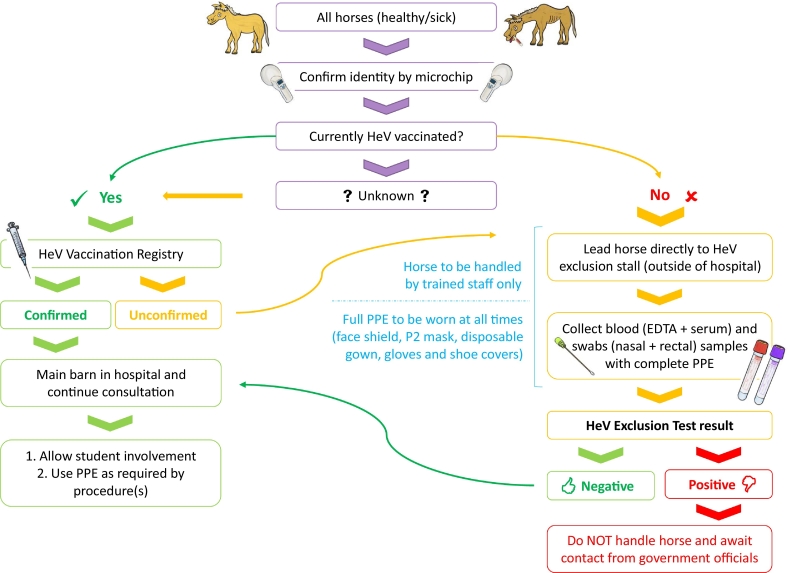
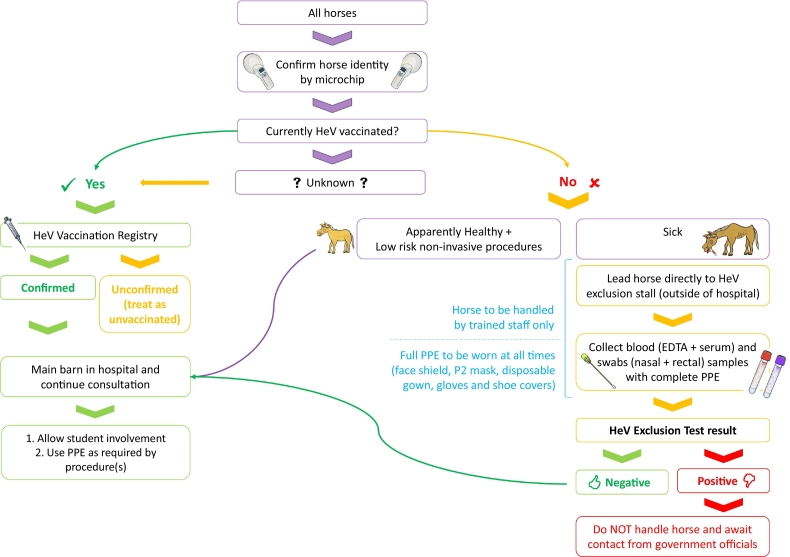
As the risk of HeV spillover events are likely to expand to novel areas, it is important for veterinarians in areas where flying foxes are endemic to establish a biosecurity plan to protect horses, horse owners, veterinarians and veterinary nurses/technicians. The authors propose the following strict biosecurity protocol1 for individual veterinary clinics and hospitals to adopt to minimize risk (Fig. 2). A less rigorous protocol that allows routine procedures to be performed on unvaccinated or unexcluded horses with use of personal protective equipment (PPE) at the discretion of the veterinarians (Fig. 3) is also presented. These models may also be suitable to adapt for other infectious diseases.
Fig. 2.
Flowchart of a strict Hendra virus biosecurity protocol. HeV, Hendra virus; EDTA, ethylenediaminetetraacetic acid; PPE, personal protective equipment.
Fig. 3.
Flowchart of a less rigorous Hendra virus biosecurity protocol. HeV, Hendra virus; EDTA, ethylenediaminetetraacetic acid; PPE, personal protective equipment.
4.1. Vaccinated horses
For a horse to be considered adequately HeV vaccinated its vaccination status must be recorded on an online Hendra vaccination registry (https://www.health4horses.com.au/) by the vaccinating veterinarian after confirming the horse identity by scanning an implanted microchip. Although HeV vaccines can be directly purchased by horse owners, only vaccines administered by veterinarians have the added assurance that the vaccine was appropriately stored and the horse correctly identified prior to vaccination. Prior to examining any horse, HeV vaccination status should be checked via the Hendra vaccination registry or request a current HeV vaccination certificate (obtained from the registry) to ensure it is adequately vaccinated. Appropriate vaccination is indicated by a “green tick” on the online registry. In an experimental study, horses that had a HeV titer ≥1:32 did not develop clinical signs and survived while unvaccinated horses succumbed to infection [17,66]. However, low risk still exists in vaccinated horse as viral genome was recovered from nasal secretions in immunized horses when challenged with HeV oro-nasally [66]. Therefore, as a precaution, PPE should still be worn. Gloves should be worn if clinical signs suggest HeV infection. As no vaccine is 100% effective, additional precautions include use of gloves and a face shield when performing upper respiratory diagnostic testing and surgery with high risk of exposure to aerosols, such as endoscopy and dental procedures.
4.2. Unvaccinated horses
Horses >6 months of age that have not been appropriately vaccinated and foals born from unvaccinated mares should be considered as unvaccinated. To minimize risk to veterinary hospital personnel, in the strict policy (Fig. 2), only horses that have had a negative HeV exclusion test within the preceding 3 days and have been kept stabled are admitted directly to the hospital. Sick unvaccinated/unexcluded horses are never admitted, and no care can be provided until a negative exclusion test is obtained. For all healthy unvaccinated/unexcluded horses a HeV exclusion test should be performed utilizing full PPE including: face shield, P2 mask, disposable gown, gloves and shoe covers. PPE should also be provided to the horse handler. A blood sample should be collected, via the jugular vein, using an ethylenediaminetetraacetic acid (EDTA; purple top) tube and serum (red top) tube. The horse should be kept in a dedicated stall separate from all other horses to minimize risk of transmission whilst exclusion results are pending. No further diagnostic procedures or treatment should be performed and the horse should only be monitored at a distance until a negative result of the HeV exclusion test is returned. All PPE should only be used once and disposed of appropriately in a clinical waste bag. Once a negative HeV exclusion test result is returned, the horse can then be moved to a routine hospital stall for further assessment and treatment.
Many veterinarians may elect to follow the less rigorous policy (Fig. 3) where exclusion testing is only performed on clinically ill horses and routine procedures on healthy unvaccinated horses are performed with PPE as appropriate. However, this does put veterinary personnel and horse owners at risk as horses can excrete HeV virus for 5 days prior to the onset of clinical signs [17]. Post mortem examination of unvaccinated horses, or those showing clinical signs consistent with HeV infection should be delayed until a negative HeV exclusion result is obtained. In cases where HeV infection remains as a differential diagnosis, in addition to blood collection, a nasal and rectal swab should be collected and placed in viral transport media to be sent immediately for HeV exclusion testing prior to performing any other diagnostic procedure, including blood sample analysis, to minimize risk to laboratory personnel. The horse should be euthanized or isolated from all animals and humans until the results are available. For welfare reasons, only administration of analgesics should be performed by the veterinarian in full PPE.
4.3. Foals
Although HeV has not been studied in foals, a foal born from a vaccinated mare is assumed to be protected by passive transfer of immunity before 6 months of age if colostrum intake was adequate. Currently it is recommended that foals from unvaccinated mares commence HeV vaccination at 4-month-of-age, and vaccination be delayed until 6-month-of-age for foals born to vaccinated mares. However, these recommendations are based on studies from other diseases and there are no published studies on the persistence of HeV-specific maternal antibody and HeV vaccination responses in foals. While all documented HeV cases only involve adult horses, considering the serious consequences of HeV infection, foals should be considered as susceptible hosts. Therefore, a cross-sectional longitudinal study of HeV-specific antibody concentrations in foals is warranted.
4.4. Veterinary students
Veterinary institutions and some privately owned veterinary clinics have workplace health and safety responsibilities to equine and veterinary students rotating through the equine hospitals for practical experiences. This makes a strict biosecurity protocol (Fig. 2) even more crucial to protect all stakeholders. It is advisable that students should not be in-contact with any unvaccinated horse before a negative exclusion test result is obtained. The assigned HeV exclusion test stalls should be physically away from the hospital vicinity and only accessible by trained staff members.
4.5. Paddock/yards
While fruit trees and eucalypts should be removed from equine paddocks to discourage flying foxes foraging in the area, solid overhead covering should be installed to provide shade for horses to fulfil fundamental animal welfare responsibilities. Water troughs and feed should be placed away from trees in open areas or under a solid covering to prevent contamination from flying fox excreta.
5. Diagnostics tests
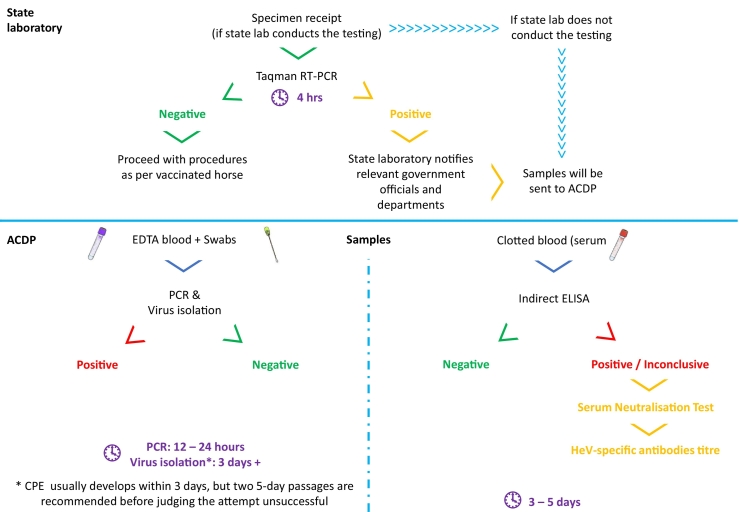
A range of diagnostics tests are available through government veterinary laboratories, ranging from viral isolation, serology and molecular testing (Table 1, Fig. 4). Diagnostic sensitivity (Se) and specificity (Sp) have been included where available. Veterinarians can submit swabs and blood samples to the state veterinary laboratories for molecular TaqMan real-time reverse transcription polymerase chain reaction (qRT-PCR) assay specific for the matrix (M) and nucleoprotein (N) gene of HeV [[82], [83], [84]]. This highly sensitive molecular test detects HeV-specific ribonucleic acid, and can provide results within four hours [83]. A negative result should be interpreted within the clinical and epidemiological context of that case. Positive results indicate presence of viral genome.
Table 1.
Summary of currently available diagnostic tests for Hendra virus in clinically unwell horses.
| Category | Diagnostic test | Preferred sample(s) [84] | Purpose(s) |
|---|---|---|---|
| Detect presence of virus (antigen) | qRT-PCR | EDTA blood | First step of exclusion test. Quick to perform, results within 4 h. Only detects virus genetic material. |
| Viral isolation | Swabs (nasal or oro-naso-pharyngeal), clotted blood | Influenced by multiple factors. Must be performed in PC4 laboratory. Takes days to perform test. | |
| Loop-mediated isothermal amplification (LAMP) | N/A | Point-of-care diagnostics. Allow quick detection of potential HeV infection. Should not replace PCR. | |
| Detect immune response (antibodies) | iELISAa | Clotted blood | Detect HeV specific antibodies. Allows for high throughput in 96 well plate format. |
| Viral/Serum neutralization test | Clotted blood | Reference standard for the detection of neutralizing antibodies to HeV and provide antibody titer. Must be performed in PC4 laboratory. Takes 3 days to perform test. | |
| Bead-based microsphere immuno-assay (Luminex®)a | Clotted blood | Detect HeV antibodies and a surrogate of HeV neutralization test. Can be performed in PC2 laboratories. |
N/A, not available; qRT-PCR, real-time reverse transcription polymerase chain reaction; iELISA, indirect enzyme linked immunosorbent assay; HeV, Hendra virus; PC, physical containment; EDTA, ethylenediaminetetraacetic acid.
Sensitivity/Specificity of (1) iELISA: 84.2%/97.1% [88, 2) Luminex® blocking assay: 95.24%/100%, binding assay: 95.24%/99.64% [86].
Fig. 4.
Flowchart of diagnostics procedures for HeV exclusion and confirmatory tests. qRT-PCR, real-time reverse transcription polymerase chain reaction; Indirect ELISA, indirect enzyme-linked immunosorbent assay; EDTA, ethylenediaminetetraacetic acid; CPE, cytopathic effect.
As HeV is a notifiable disease in Australia [85], when a positive exclusion test is detected, relevant government officials/department are notified by the state laboratory and a series of biosecurity responses will be triggered. Submitted samples will be transported to Australian Centre for Disease Preparedness (ACDP) in Victoria for further testing, namely PCR and viral isolation [86], for a definitive diagnosis. As HeV is a biosafety level 4 agent, any laboratory activities involving live HeV, such as virus isolation and serum neutralization tests are required to be performed under strict regulations in a physical containment (PC) 4 laboratory. Virus isolation takes several days to weeks. A negative result does not rule out the presence of viable and infectious virus as success of virus isolation can be influenced by multiple factors [87].
Indirect enzyme-linked immunosorbent assay (iELISA) and serum neutralization tests (SNT, also known as virus neutralization tests) are used to detect antibodies. A new iELISA assay was recently developed as a screening test (Se = 84.2%, Sp = 97.1%), using a recombinant-expressed HeVsG glycoprotein [67], which has improved specificity compared to the previous HeV iELISA which uses inactivated virus, and both tests can be performed in a PC2/3 laboratory [84,88,89]. A positive result (S/P ratio > 0.4) indicates presence of HeV-specific antibodies [88]. All inconclusive and positive results from HeVsG iELISA are subjected to SNT as a confirmatory test, which is highly specific, and allows for the determination of HeV-specific antibody titer [84,90]. Furthermore, due to the biological nature of the SNT which uses live virus, repeatability is an issue, making test results difficult to compare across runs.
Bead-based fluorescent microsphere immuno-assays (Luminex®) allow detection and differentiation of HeV and NiV specific antibodies in one test via a total antibody-binding format, and pseudo-viral neutralization using recombinant proteins via a restricted-receptor blocking format [86,90]. Both assays utilize recombinant sG proteins of HeV or NiV and require the use of Bio-Plex® Protein Array System and software for data acquisition and analysis. The result of the binding assay is recorded as median fluorescent intensity (MFI) of 100 beads which can then be transformed to percent positive relative to the MFI from positive control, while the blocking assay is reported as percent inhibition [84,86,90]. Luminex® is highly sensitive and specific (blocking assay Se = 95.24%, Sp = 100%; binding assay Se = 95.24%, Sp = 99.64%) [86]. As no live HeV is used, a PC2 laboratory is adequate. Although the blocking assay is designed as a surrogate of viral neutralization, it does not provide an antibody titer value. For this reason, further investigation is required to determine if there is a correlation between the test results of SNT and Luminex®.
Equivac® HeV vaccine and all existing serological tests use an expressed recombinant truncated HeVsG protein. Therefore, presence of antibodies does not differentiate whether immune response originates from either seroconversion due to previous natural infection, or vaccination. This complicates international trade and travel of horses, as certain countries require a certificate of “proof of freedom” of Hendra virus infection. This has potentially contributed to the reduced uptake of the HeV vaccine. To overcome this, an approach differentiating infected and vaccinated animals (also known as DIVA) using ELISA is currently being developed specific for the N protein of HeV [86].
Loop-mediated isothermal amplification (LAMP) assay first described by Notomi et al. [91], is an emerging rapid point-of-care diagnostic tool for a number of animal diseases, such as footrot caused by Dichelobacter nodosus [92], foot and mouth disease [93], bovine viral diarrhea virus [94] and other OIE notifiable diseases [95]. Currently, all diagnostic tests for HeV require at least several hours to days and require specialized equipment. Some assays also require a PC4 laboratory. LAMP for HeV detection is currently under development. Once validated and commercially available, veterinarians may be able to carry a portable device to perform preliminary HeV testing in the field. However, LAMP is not likely to become a reference standard for confirmatory diagnosis. Taqman qRT-PCR assay performed in a laboratory should remain the gold standard for HeV exclusion testing. Nevertheless, LAMP technology can be used for early detection of HeV thus guiding implementation of biosecurity protocols to protect human and animal lives.
6. Gaps and conclusion
HeV continues to pose a serious public health threat, animal welfare concerns and wildlife conservation issues. While it is important to understand the ecology and transmission of HeV, these studies are time consuming and further research is required to reduce human and horse infections. HeV vaccination of horses remains the most effective and direct one health approach to solve this issue. Vaccination breaks the chain of infection. However, major knowledge gaps have been identified. Immunity in foals to HeV from transfer of passive immunity and vaccination remains unknown, and current vaccination recommendations may need revision. Correlation of diagnostic test results of Luminex® and SNT would result in reduced turnaround time for reporting, address workplace health and safety concerns, and technical difficulties due to the need for a PC4 laboratory for SNT. SNT titer cut-off at which annual booster could be safely delayed (due to previous vaccination reactions) requires further study. Development of point-of-care diagnostics will improve biosecurity response, thus animal and human health. Lastly, while this review focused on the specific characteristics of HeV epidemiology, especially in regards to diagnostics and biosecurity, human behaviors and attitudes towards HeV vaccination and associated side effects and adverse events are likely to play a major role in HeV prevention.
Funding information
This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
Acknowledgement
The authors would like to thank Mr. Zakary Mychal Jaques and Ms. Shani Tripcony, veterinary science students from The University of Queensland, for their assistance in graphic designs for Fig. 2, Fig. 3, Fig. 4.
Footnotes
Adapted from HeV biosecurity protocol (internal document) from the School of Veterinary Science, The University of Queensland, Australia.
References
- 1.World Health Organization, Food and Agriculture Organization of the United Nations & World Organization for Animal Health . World Health Organization; Geneva: 2004. Report of the WHO/FAO/OIE joint consultation on emerging zoonotic diseases / in collaboration with the Health Council of the Netherlands. [Google Scholar]
- 2.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–994. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006;19(3):531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith I., Wang L.F. Bats and their virome: An important source of emerging viruses capable of infecting humans. Curr. Opin. Virol. 2013;3(1):84–91. doi: 10.1016/j.coviro.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorbalenya A., Baker S., Baric R., Drosten C., Gulyaeva A., Haagmans B. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Coronavirus disease (COVID-19) pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 3 June 2020)
- 7.Business Queensland, Summary of Hendra virus incidents in horses. https://www.business.qld.gov.au/industries/service-industries-professionals/service-industries/veterinary-surgeons/guidelines-hendra/incident-summary (accessed 7 Feburary 2020).
- 8.Murray K., Selleck P., Hooper P., Hyatt A., Gould A., Gleeson L. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268(5207):94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- 9.Mayo M.A. A summary of taxonomic changes recently approved by ICTV. Arch. Virol. 2002;147(8):1655–1656. doi: 10.1007/s007050200039. [DOI] [PubMed] [Google Scholar]
- 10.Marsh G.A., de Jong C., Barr J.A., Tachedjian M., Smith C., Middleton D. Cedar virus: A novel henipavirus isolated from Australian bats. PLoS Pathog. 2012;8(8) doi: 10.1371/journal.ppat.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L.F., Mackenzie J.S., Broder C.C. Henipaviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 6th ed. Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 1070–1085. [Google Scholar]
- 12.Hyatt A.D., Zaki S.R., Goldsmith C.S., Wise T.G., Hengstberger S.G. Ultrastructure of Hendra virus and Nipah virus within cultured cells and host animals. Microbes Infect. 2001;3(4):297–306. doi: 10.1016/S1286-4579(01)01383-1. [DOI] [PubMed] [Google Scholar]
- 13.O’Sullivan J.D., Allworth A., Paterson D.L., Snow T.M., Boots R., Gleeson L. Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet. 1997;349(9045):93–95. doi: 10.1016/S0140-6736(96)06162-4. [DOI] [PubMed] [Google Scholar]
- 14.Rogers R.J., Douglas I.C., Baldock F.C., Glaville R.J., Seppanen K.T., Gleeson L.J. Investigation of a second focus of equine morbillivirus infection in coastal Queensland. Aust. Vet. J. 1996;74(3):243–244. doi: 10.1111/j.1751-0813.1996.tb15413.x. [DOI] [PubMed] [Google Scholar]
- 15.Queensland Government Hendra virus. 2018. https://www.business.qld.gov.au/industries/farms-fishing-forestry/agriculture/livestock/animal-welfare/pests-diseases-disorders/hendra-virus (accessed 18 May 2020)
- 16.Field H., Schaaf K., Kung N., Simon C., Waltisbuhl D., Hobert H. Hendra virus outbreak with novel clinical features, Australia. Emerg. Infect. Dis. 2010;16(2):338–340. doi: 10.3201/eid1602.090780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh G.A., Haining J., Hancock T.J., Robinson R., Foord A.J., Barr J.A. Experimental infection of horses with Hendra virus/Australia/Horse/2008/Redlands. Emerg. Infect. Dis. 12, 2011;17:2232–2238. doi: 10.3201/eid1712.111162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Field H.E., Breed A.C., Shield J., Hedlefs R.M., Pittard K., Pott B. Epidemiological perspectives on Hendra virus infection in horses and flying foxes. Aust. Vet. J. 2007;85(7):268–270. doi: 10.1111/j.1751-0813.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- 19.Buchanan K. Queensland vet found guilty of breaching workplace laws while treating a horse with Hendra virus: ABC News. 2016. https://www.abc.net.au/news/rural/2016-09-30/vet-found-guilty-over-hendra-case/7892316 (accessed 21 July 2020)
- 20.WorkCover Queensland Hendra virus: Queensland Government. 2019. https://www.worksafe.qld.gov.au/injury-prevention-safety/hazardous-exposures/biological-hazards/diseases-from-animals/hendra-virus (accessed 21 July 2020).
- 21.Hanna J.N., McBride W.J., Brookes D.L., Shield J., Taylor C.T., Smith I.L. Hendra virus infection in a veterinarian. Med. J. Aust. 10, 2006;185:562–564. doi: 10.5694/j.1326-5377.2006.tb00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Playford E.G., McCall B., Smith G., Slinko V., Allen G., Smith I. Human Hendra virus encephalitis associated with equine outbreak, Australia, 2008. Emerg. Infect. Dis. 2010;16(2):219–223. doi: 10.3201/eid1602.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvey L.A., Wells R.M., McCormack J.G., Ansford A.J., Murray K., Rogers R.J. Infection of humans and horses by a newly described morbillivirus. Med. J. Aust. 1995;162(12):642–645. doi: 10.5694/j.1326-5377.1995.tb126050.x. [DOI] [PubMed] [Google Scholar]
- 24.Wong K.T., Robertson T., Ong B.B., Chong J.W., Yaiw K.C., Wang L.F. Human Hendra virus infection causes acute and relapsing encephalitis. Neuropathol. Appl. Neurobiol. 2009;35(3):296–305. doi: 10.1111/j.1365-2990.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- 25.Playford E.G., Munro T., Mahler S.M., Elliott S., Gerometta M., Hoger K.L. Safety, tolerability, pharmacokinetics, and immunogenicity of a human monoclonal antibody targeting the G glycoprotein of henipaviruses in healthy adults: a first-in-human, randomised, controlled, phase 1 study. Lancet Infect. Dis. 2020;20(4):445–454. doi: 10.1016/S1473-3099(19)30634-6. [DOI] [PubMed] [Google Scholar]
- 26.Young P.L., Halpin K., Selleck P.W., Field H.E., Gravel J.L., Kelly M.A. Serologic evidence for the presence in Pteropus bats of a paramyxovirus related to equine morbillivirus. Emerg. Infect. Dis. 1996;2(3):239–240. doi: 10.3201/eid0203.960315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hume E.F. The University of Queensland; Brisbane, Australia: 2004. The ecology of Hendra virus and Australian bat lyssavirus. Ph.D. thesis. [Google Scholar]
- 28.Halpin K., Hyatt A.D., Fogarty R., Middleton D., Bingham J., Epstein J.H. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: A comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg. 2011;85(5):946–951. doi: 10.4269/ajtmh.2011.10-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halpin K., Young P.L., Field H.E., Mackenzie J.S. Isolation of Hendra virus from pteropid bats: A natural reservoir of Hendra virus. J. Gen. Virol. 2000;81(8):1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- 30.Williamson M.M., Hooper P.T., Selleck P.W., Westbury H.A., Slocombe R.F. Experimental Hendra virus infectionin pregnant guinea-pigs and fruit bats (Pteropus poliocephalus) J. Comp. Pathol. 2000;122(2−3):201–207. doi: 10.1053/jcpa.1999.0364. [DOI] [PubMed] [Google Scholar]
- 31.Smith C., Skelly C., Kung N., Roberts B., Field H. Flying-fox species density - A spatial risk factor for Hendra virus infection in horses in eastern Australia. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldspink L.K., Edson D.W., Vidgen M.E., Bingham J., Field H.E., Smith C.S. Natural Hendra virus infection in flying-foxes - Tissue tropism and risk factors. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0128835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edson D., Field H., McMichael L., Vidgen M., Goldspink L., Broos A. Routes of Hendra virus excretion in naturally-infected flying-foxes: Implications for viral transmission and spillover risk. PLoS One. 10, 2015;10 doi: 10.1371/journal.pone.0140670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Field H., Jordan D., Edson D., Morris S., Melville D., Parry-Jones K. Spatiotemporal aspects of Hendra virus infection in Pteropid bats (flying-foxes) in eastern Australia. PLoS One. 12, 2015;10 doi: 10.1371/journal.pone.0144055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirkland P.D., Gabor M., Poe I., Neale K., Chaffey K., Finlaison D.S. Hendra virus infection in dog, Australia, 2013. Emerg. Infect. Dis. 12, 2015;21:2182–2185. doi: 10.3201/eid2112.151324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Middleton D., Riddell S., Klein R., Arkinstall R., Haining J., Frazer L. Experimental Hendra virus infection of dogs: Virus replication, shedding and potential for transmission. Aust. Vet. J. 2017;95(1–2):10–18. doi: 10.1111/avj.12552. [DOI] [PubMed] [Google Scholar]
- 37.Li M., Embury-Hyatt C., Weingartl H.M. Experimental inoculation study indicates swine as a potential host for Hendra virus. Vet. Res. 2010;41(3):33. doi: 10.1051/vetres/2010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guillaume V., Wong K.T., Looi R.Y., Georges-Courbot M.C., Barrot L., Buckland R. Acute Hendra virus infection: Analysis of the pathogenesis and passive antibody protection in the hamster model. Virology. 2009;387(2):459–465. doi: 10.1016/j.virol.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Williamson M.M., Hooper P.T., Selleck P.W., Westbury H.A., Slocombe R.F.S. A guinea-pig model of Hendra virus encephalitis. J. Comp. Pathol. 2001;124(4):273–279. doi: 10.1053/jcpa.2001.0464. [DOI] [PubMed] [Google Scholar]
- 40.Westbury H.A., Hooper P.T., Selleck P.W., Murray P.K. Equine morbillivirus pneumonia: Susceptibility of laboratory animals to the virus. Aust. Vet. J. 1995;72(7):278–279. doi: 10.1111/j.1751-0813.1995.tb03549.x. [DOI] [PubMed] [Google Scholar]
- 41.Pallister J., Middleton D., Wang L.-F., Klein R., Haining J., Robinson R. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine. 34, 2011;29:5623–5630. doi: 10.1016/j.vaccine.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rockx B., Bossart K.N., Feldmann F., Geisbert J.B., Hickey A.C., Brining D. A novel model of lethal Hendra virus infection in African Green monkeys and the effectiveness of ribavirin treatment. J. Virol. 2010;84(19):9831–9839. doi: 10.1128/JVI.01163-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williamson M.M., Hooper P.T., Selleck P.W., Gleeson L.J., Daniels P.W., Westbury H.A. Transmission studies of Hendra virus (equine morbillivirus) in fruit bats, horses and cats. Aust. Vet. J. 1998;76(12):813–818. doi: 10.1111/j.1751-0813.1998.tb12335.x. [DOI] [PubMed] [Google Scholar]
- 44.Martin G., Plowright R., Chen C., Kault D., Selleck P., Skerratt L.F. Hendra virus survival does not explain spillover patterns and implicates relatively direct transmission routes from flying foxes to horses. J. Gen. Virol. 2015;96(6):1229–1237. doi: 10.1099/vir.0.000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scanlan J., Kung N., Selleck P., Field H. Survival of Hendra virus in the environment: Modelling the effect of temperature. EcoHealth. 2015;12(1):121–130. doi: 10.1007/s10393-014-0920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fogarty R., Halpin K., Hyatt A.D., Daszak P., Mungall B.A. Henipavirus susceptibility to environmental variables. Virus Res. 2008;132(1–2):140–144. doi: 10.1016/j.virusres.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edson D., Peel A.J., Huth L., Mayer D.G., Vidgen M.E., McMichael L. Time of year, age class and body condition predict Hendra virus infection in Australian black flying foxes (Pteropus alecto) Epidemiol. Infect. 2019;147 doi: 10.1017/S0950268819001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin G.A., Yanez-Arenas C., Roberts B.J., Chen C., Plowright R.K., Webb R.J. Climatic suitability influences species specific abundance patterns of Australian flying foxes and risk of Hendra virus spillover. One Health. 2016;2:115–121. doi: 10.1016/j.onehlt.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McFarlane R., Becker N., Field H. Investigation of the climatic and environmental context of Hendra virus spillover events 1994–2010. PLoS One. 12, 2011;6 doi: 10.1371/journal.pone.0028374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Páez D.J., Giles J., McCallum H., Field H., Jordan D., Peel A.J. Conditions affecting the timing and magnitude of Hendra virus shedding across pteropodid bat populations in Australia. Vol. 145. 15, 2017. et al; pp. 3143–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plowright R.K., Foley P., Field H.E., Dobson A.P., Foley J.E., Eby P. Urban habituation, ecological connectivity and epidemic dampening: The emergence of Hendra virus from flying foxes (Pteropus spp.) Proc. R. Soc. B. 2011;278(1725):3703–3712. doi: 10.1098/rspb.2011.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tait J., Perotto-Baldivieso H.L., McKeown A., Westcott D.A. Are flying-foxes coming to town? Urbanisation of the spectacled flying-fox (Pteropus conspicillatus) in Australia. PLoS One. 10, 2014;9 doi: 10.1371/journal.pone.0109810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin G., Yanez-Arenas C., Chen C., Plowright R., Webb R., Skerratt L. Climate change could increase the geographic extent of Hendra virus spillover risk. EcoHealth. 2018;15(3):509–525. doi: 10.1007/s10393-018-1322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Field H., de Jong C., Melville D., Smith C., Smith I., Broos A. Hendra virus infection dynamics in Australian fruit bats. PLoS One. 12, 2011;6 doi: 10.1371/journal.pone.0028678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palmer C., Price O., Bach C. Foraging ecology of the black flying fox (Pteropus alecto) in the seasonal tropics of the Northern Territory, Australia. Wildl. Res. 2000;27(2):169–178. doi: 10.1071/WR97126. [DOI] [Google Scholar]
- 56.Courts S. Dietary strategies of Old World fruit bats (Megachiroptera, Pteropodidae): How do they obtain sufficient protein? Mammal Rev. 1998;28(4):185–193. [Google Scholar]
- 57.Giles J.R., Eby P., Parry H., Peel A.J., Plowright R.K., Westcott D.A. Environmental drivers of spatiotemporal foraging intensity in fruit bats and implications for Hendra virus ecology. Sci. Rep. 2018;8:9555. doi: 10.1038/s41598-018-27859-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hudson I.L., Keatley M.R. In: Phenological research: Methods for environmental and climate change analysis. Hudson I.L., Keatley M.R., editors. Springer Netherlands; Dordrecht: 2010. pp. 209–228. Eds. [Google Scholar]
- 59.Law B., Mackowski C., Schoer L., Tweedie T. Flowering phenology of myrtaceous trees and their relation to climatic, environmental and disturbance variables in northern New South Wales. Aust. Ecol. 2000;25(2):160–178. doi: 10.1046/j.1442-9993.2000.01009.x. [DOI] [Google Scholar]
- 60.Edson D., Field H., McMichael L., Jordan D., Kung N., Mayer D. Flying-fox roost disturbance and Hendra virus spillover risk. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0125881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McMichael L., Edson D., Smith C., Mayer D., Smith I., Kopp S. Physiological stress and Hendra virus in flying-foxes (Pteropus spp.), Australia. PLoS One. 2017;12(8):e0182171. doi: 10.1371/journal.pone.0182171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plowright R.K., Field H.E., Smith C., Divljan A., Palmer C., Tabor G. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus) Proc. R. Soc. B. 2008;275(1636):861–869. doi: 10.1098/rspb.2007.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin G., Yanez-Arenas C., Plowright R., Chen C., Roberts B., Skerratt L. Hendra virus spillover is a bimodal system driven by climatic factors. EcoHealth. 2018;15(3):526–542. doi: 10.1007/s10393-017-1309-y. [DOI] [PubMed] [Google Scholar]
- 64.Timmermann A., Oberhuber J., Bacher A., Esch M., Latif M., Roeckner E. Increased El Niño frequency in a climate model forced by future greenhouse warming. Nature. 1999;398(6729):694–697. doi: 10.1038/19505. [DOI] [Google Scholar]
- 65.Mpelasoka F., Hennessy K., Jones R., Bates B. Comparison of suitable drought indices for climate change impacts assessment over Australia towards resource management. Int. J. Climatol. 10, 2008;28:1283–1292. doi: 10.1002/joc.1649. [DOI] [Google Scholar]
- 66.Middleton D., Pallister J., Klein R., Feng Y.-R., Haining J., Arkinstall R. Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg. Infect. Dis. 2014;20(3):372–379. doi: 10.3201/eid2003.131159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bossart K., Crameri G., Dimitrov A., Mungall B.A., Feng Y., Patch Jr. Receptor binding, fusion inhibition, and induction of cross-reactive neutralizing antibodies by a soluble G glycoprotein of Hendra virus. J. Virol. 2005;79(11):6690–6702. doi: 10.1128/jvi.79.11.6690-6702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Australian Pesticides and Veterinary Medicine Authority Hendra virus vaccine. 2020. https://apvma.gov.au/node/10946 (accessed 24 May 2020)
- 69.Manyweathers J., Field H., Longnecker N., Agho K., Smith C., Taylor M. “Why won’t they just vaccinate?” Horse owner risk perception and uptake of the Hendra virus vaccine. BMC Vet. Res. 2017;13:103. doi: 10.1186/s12917-017-1006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schemann K., Annand E., Reid P., Lenz M., Thomson P., Dhand N. Investigation of the effect of Equivac® HeV Hendra virus vaccination on thoroughbred racing performance. Aust. Vet. J. 2018;96(4):132–141. doi: 10.1111/avj.12679. [DOI] [PubMed] [Google Scholar]
- 71.Mungall B.A., Middleton D., Crameri G., Bingham J., Halpin K., Russell G. Feline model of acute Nipah virus infection and protection with a soluble glycoprotein-based subunit vaccine. J. Virol. 2006;80(24):12293–12302. doi: 10.1128/JVI.01619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McEachern J.A., Bingham J., Crameri G., Green D.J., Hancock T.J., Middleton D. A recombinant subunit vaccine formulation protects against lethal Nipah virus challenge in cats. Vaccine. 2008;26(31):3842–3852. doi: 10.1016/j.vaccine.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bossart K.N., Rockx B., Feldmann F., Brining D., Scott D.P., LaCasse R. A Hendra virus G glycoprotein subunit vaccine protects African Green Monkeys from Nipah virus challenge. Sci. Transl. Med. 2012;4(146):146ra107. doi: 10.1126/scitranslmed.3004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mire C.E., Geisbert J.B., Agans K.N., Feng Y.-R., Fenton K.A., Bossart K.N. A recombinant Hendra virus G glycoprotein subunit vaccine protects non-human primates against Hendra virus challenge. J. Virol. 2014;88(9):4624–4631. doi: 10.1128/JVI.00005-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor M., Dhand N., Toribio J.-A., Wiethoelter A., Schembri N., Sawford K. HHALTER: Horse owners and Hendra Virus: A longitudinal study to evaluate risk. Rural Industries Research and Development Corporation; 2016. Longitudinal cohort study of horse owners. [Google Scholar]
- 76.Goyen K.A., Wright J.D., Cunneen A., Henning J. Playing with fire - What is influencing horse owners’ decisions to not vaccinate their horses against deadly Hendra virus infection? PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0180062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kung N., McLaughlin A., Taylor M., Moloney B., Wright T., Field H. Hendra virus and horse owners - Risk perception and management. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Department of Agriculture, Fisheries and Forestry . Queensland Government; Brisbane, Queensland: 2013. Guidelines for veterinarians handling potential Hendra virus infection in horses. [Google Scholar]
- 79.Agriculture and Environment Committee . Parliament House; Queensland: 2016. Report no. 24. Hendra virus EquiVac® vaccine and its use by veterinary surgeons in Queensland. [Google Scholar]
- 80.Wiethoelter A.K., Schembri N., Dhand N.K., Sawford K., Taylor M.R., Moloney B. Australian horse owners and their biosecurity practices in the context of Hendra virus. Prev. Vet. Med. 2017;148:28–36. doi: 10.1016/j.prevetmed.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 81.Manyweathers J., Field H., Jordan D., Longnecker N., Agho K., Smith C. Risk mitigation of emerging zoonoses: Hendra virus and non-vaccinating horse owners. Transbound. Emerg. Dis. 2017;64(6):1898–1911. doi: 10.1111/tbed.12588. [DOI] [PubMed] [Google Scholar]
- 82.Feldman K.S., Foord A., Heine H.G., Smith I.L., Boyd V., Marsh G.A. Design and evaluation of consensus PCR assays for henipaviruses. J. Virol. Methods. 2009;161:52–57. doi: 10.1016/j.jviromet.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 83.Smith I.L., Halpin K., Warrilow D., Smith G.A. Development of a fluorogenic RT-PCR assay (TaqMan) for the detection of Hendra virus. J. Virol. Methods. 2001;98:33–40. doi: 10.1016/S0166-0934(01)00354-8. [DOI] [PubMed] [Google Scholar]
- 84.World Organisation for Animal Health (OIE) 2018. Nipah and Hendra virus disease. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; pp. 526–543. [Google Scholar]
- 85.Department of Agriculture, Water and the Environment National list of notifiable animal diseases. 2020. https://www.agriculture.gov.au/pests-diseases-weeds/animal/notifiable#national-list-of-notifiable-diseases-of-terrestrial-animals-at-april-2019 (accessed 2 Jun 2020)
- 86.McNabb L., Barr J., Crameri G., Juzva S., Riddell S., Colling A. Henipavirus microsphere immuno-assays for detection of antibodies against Hendra virus. J. Virol. Methods. 2014;200:22–28. doi: 10.1016/j.jviromet.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leland D.S., Ginocchio C.C. Role of cell culture for virus detection in the age of technology. Clin. Microbiol. Rev. 2007;20(1):49–78. doi: 10.1128/CMR.00002-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colling A., Lunt R., Bergfeld J., McNabb L., Halpin K., Juzva S. A network approach for provisional assay recognition of a Hendra virus antibody ELISA: Test validation with low sample numbers from infected horses. J. Vet. Diagn. Investig. 2018;30(3):362–369. doi: 10.1177/1040638718760102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daniels P., Ksiazek T., Eaton B.T. Laboratory diagnosis of Nipah and Hendra virus infections. Microbes Infect. 2001;3(4):289–295. doi: 10.1016/S1286-4579(01)01382-X. [DOI] [PubMed] [Google Scholar]
- 90.Bossart K.N., McEachern J.A., Hickey A.C., Choudhry V., Dimitrov D.S., Eaton B.T. Neutralization assays for differential henipavirus serology using bio-Plex protein array systems. J. Virol. Methods. 2007;142:29–40. doi: 10.1016/j.jviromet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 91.Notomi T., Okayama H., Masubuchi H., Yonekawa T., Watanabe K., Amino N. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12) doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Best N., Rodoni B., Rawlin G., Beddoe T. The development and deployment of a fieldbased loop mediated isothermal amplification assay for virulent Dichelobacter nodosus detection on Australian sheep. PLoS One. 2018;13(9) doi: 10.1371/journal.pone.0204310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bath C., Scott M., Sharma P.M., Gurung R.B., Phuentshok Y., Pefanis S. Further development of a reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the detection of foot-and-mouth disease virus and validation in the field with use of an internal positive control. Transbound. Emerg. Dis. 2020 doi: 10.1111/tbed.13589. [DOI] [PubMed] [Google Scholar]
- 94.Fan Q., Xie Z., Xie L., Liu J., Pang Y., Deng X. A reverse transcription loop-mediated isothermal amplification method for rapid detection of bovine viral diarrhea virus. J. Virol. Methods. 2012;186(1−2):43–48. doi: 10.1016/j.jviromet.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mansour S.M.G., Ali H., Chase C.C.L., Cepica A. Loop-mediated isothermal amplification for diagnosis of 18 World Organization for Animal Health (OIE) notifiable viral diseases of ruminants, swine and poultry. Anim. Health Res. Rev. 2015;16(2):89–106. doi: 10.1017/S1466252315000018. [DOI] [PubMed] [Google Scholar]