Abstract
Background
The mitochondrial cofactors α-lipoic acid (ALA), coenzyme Q10 (CoQ10) and carnitine (CARN) play distinct and complementary roles in mitochondrial functioning, along with strong antioxidant actions. Also termed mitochondrial nutrients (MNs), these cofactors have demonstrated specific protective actions in a number of chronic disorders, as assessed in a well-established body of literature.
Methods
Using PubMed, the authors searched for articles containing information on the utilization of MNs in inflammatory disorders as assessed from in vitro and animal studies, and in clinical trials, in terms of exerting anti-inflammatory actions.
Results
The retrieved literature provided evidence relating acute pathologic conditions, such as sepsis and pneumonia, with a number of redox endpoints of biological and clinical relevance. Among these findings, both ALA and CARN were effective in counteracting inflammation-associated redox biomarkers, while CoQ10 showed decreased levels in proinflammatory conditions. MN-associated antioxidant actions were applied in a number of acute disorders, mostly using one MN. The body of literature assessing the safety and the complementary roles of MNs taken together suggests an adjuvant role of MN combinations in counteracting oxidative stress in sepsis and other acute disorders, including COVID-19-associated pneumonia.
Conclusions
The present state of art in the use of individual MNs in acute disorders suggests planning adjuvant therapy trials utilizing MN combinations aimed at counteracting proinflammatory conditions, as in the case of pneumonia and the COVID-19 pandemic.
Keywords: Sepsis, Pneumonia, α-Lipoic acid, Coenzyme Q10, Carnitine, COVID-19
Introduction
Acute pathological conditions display well-established links with oxidative stress (OS), through a number of different or complementary mechanistic features, as early studies have reported [1–4]. Fighting acute diseases has long been a target in medicine and over time has come to encompass a range of pharmacological and immunological tools, including the use of adjuvant means for mitigating the inflammatory conditions in relevant therapeutical strategies [5–8].
A major contemporary case of inflammatory pneumonia is presented by the global COVID-19 (SARS-CoV2) outbreak. Clinical presentations in COVID-19 include, but are not limited to, cough, fever, and acute respiratory distress sydrome, which can lead to serious complications for those with underlying cardiovascular disease, diabetes mellitus, chronic pulmonary disorders, renal disease and other co-morbidities [9, 10]. COVID-19 causes neutrophilia, lymphopenia, leukopenia, thrombopenia, and anemia as well as increased expression of systemic inflammatory proteins IL-6, C-reactive protein (CRP), innate chemokines (CXCL10, CCL2, CCL3) and the proinflammatory cytokine TNF-α [11–13]. A relevant involvement of mitochondrial dysfunction (MDF) in COVID-19 pathogenesis was recently reported by [14–16].
On a molecular level, the virus has several binding centers, including a transmembrane receptor for angiotensin-converting enzyme 2 (ACE-2) that facilitates viral entry into cells. The expression level of ACE-2 is increased with age [17, 18] and ACE-2 accumulates on alveolar, ciliated and goblet cells in the airways, the intestinal epithelium, cardiac cells and vascular endothelia [19, 20]. COVID-19 also exhibits genomic regions encoding the viral spike protein [21] which may attach to immunoglobulin CD147 on the surface of erythrocytes and some lymphocytes to attack the 1b-chain of hemoglobin, causing inhibition of heme metabolism [22–24]. This results in a strong OS and uncontrolled release of proinflammatory cytokines which has been termed “cytokine storm” [25].
Sepsis, on the other hand, is a pathogenesis caused by bacterial, viral, fungal, or protozoan infection, and also results in an inflammatory response and poor delivery of oxygen to tissues [26]. The most common consequences are impaired vascular permeability, cardiac malfunction, and MDF leading to impaired respiration [27]. As in COVID-19, the course of sepsis is often accompanied by a cytokine storm, leading to OS [14]. An important target of altered inflammation in the COVID-19 pathology has been shown to be also the endothelium with recent evidences indicating that the clinical condition produced by COVID-19 infection is not primarily a respiratory pathology, but rather a coagulative disorder [23, 28]. The endothelium plays a major role in the regulation of coagulative processes; thus, OS may disturb endothelial function, promoting the inactivation of beneficial endothelial-derived nitric oxide.
The relationship between OS and the risk of death in patients infected with COVID-19 suggests the need for alternative approaches to counteract this infection [28]. In addition, a recent report on the possible participation of COVID-19 in weakening mitochondrial functions suggests the need to consider these organelles as an object for adjuvant therapeutic effects targetting [16, 17, 29, 30].
In view of contributing to the mitigation of prooxidant state in COVID-19, the use of several antioxidants has been proposed, as in the case of melatonin [31], vitamin C [32], vitamin D [33], vitamin B12 and nicotinamide [34], resveratrol [35], and herbal preparations [36–38]. The rationale of these adjuvant strategies has been recenty reviewed by Quiles et al. [39].
This concurs with antioxidant therapy against sepsis that also suggests focuses on improving mitochondrial functions [40]. A range of studies has assessed the adjuvant role of three mitochondrial cofactors in mitigating a prooxidant state, with background data deriving from experimental and clinical studies.
MDF and energy deficiency during sepsis and COVID-19
Many researchers have postulated that systemic inflammation, accompanied by elevated levels of TNF-α, IL-1 and PDGF, was the main determinant of the pathogenesis of sepsis and septic shock [41]. The relationship between increased production of nitric oxide, antioxidant depletion and a decrease in the activity of complex I of the respiratory chain in patients with sepsis has been well demonstrated [42, 43]. Persistent inflammation during sepsis can be caused by overproduction of mitochondrial ROS (mtROS) with consequent mitochondrial damage and MDF. Since the main role of mitochondria is to supply cells with energy, the above consequences should lead to a decrease in the synthesis of ATP. Indeed, decreased levels of ATP in the liver [44], kidney [45] and blood [46] were associated with the severity of sepsis [46, 47]. Although data on ATP levels and mitochondrial function are still emerging in COVID-19 patients, there are many reasons for drawing parallels with sepsis. In particular, the most recent work by Gibellini et al. [48] shows a decrease in ATP and MDF levels in patients infected with SARS-CoV-2. This means that mitochondria may be dysfunctional and unable to cope with the hypermetabolic demands associated with COVID-19 sepsis. Excessive ROS levels have also been seen in critically ill patients with COVID-19, indicating MDF’s involvement in the disease [49, 50]. In general, approaches targeting mtROS should be incorporated into preventive and therapeutic strategies against sepsis [7] and COVID-19-associated sepsis [51]. One such approach may be metabolic resuscitation with MNs, which can prevent uncontrolled production of mtROS and help maintain tissue homeostasis during these diseases.
Mitochondrial nutrients: action mechanisms and antioxidant properties
Over the past several decades, a body of literature has established distinct, yet complementary, roles of MNs in mitochondrial functions [52, 53]. Comprehensive recent reviews have been focused on the roles and on the prospective potential clinical utilization application of α-lipoic acid (ALA) [54–56], coenzyme Q10 (CoQ10) [8, 57–60] and carnitine (CARN) [61, 62]. We have reported previously on the combined features of MDF, prooxidant state and prospective use of MNs in an extensive number of chronic, age-related or genetic disorders [6, 63–67]. Unlike chronic disorders, a relatively lesser body of literature has been focused on acute disorders, in spite of their—quite obvious—association with a prooxidant state as in, for example, sepsis.
The relative roles of each MN in counteracting acute prooxidant conditions are reported in the following tables, with data deriving from in vitro and animal studies and from clinical trials.
As shown in Table 1, in vitro studies have shown the relevance of each MN in a number of prooxidant-related conditions. Murine, rat and human cell lines, characterized by prooxidant state endpoints, were tested for antioxidant effects of ALA, which was found to inhibit signal-regulated kinase-1 (ERK1), prooxidant interleukins and other OS biomarkers [68–73]. An analogous antioxidant action was found by Schmelzer et al. [74] by testing CoQ10 in murine cells, which exerted anti-inflammatory properties via NFκB1-dependent gene expression. Further studies on models of inflammation included human endothelial cells at different levels of replicative senescence which were challenged with LPS. In this context, the reduced form of CoQ10 was particularly effective in preventing the modulation of inflammatory markers that characterize the senescence-associated inflammatory phenotype [75]. Further, CARN, when tested in rat renal cells or cardiomyocytes, was found to enhance SOD2 expression and to counteract OS and inflammation [76, 77]. Taken together, these studies of the in vitro MN-associated antioxidant effects provide a body of evidence suggesting a protective antioxidant action of MNs at the organismal level.
Table 1.
Reports on in vitro effects of mitochondrial nutrients [MN: ALA, CoQ10 and (acyl-)CARN] focused on anti-inflammatory end points
| MN | Test model | Effects | References |
|---|---|---|---|
| ALA | C2C12 myotubes | Regulating IL-6R and gp130 expression | [53] |
| SK-N-BE neuroblastoma cells | Repression of IL-1b and IL-6 dependent on DNA methylation | [54] | |
| Murine RAW 264.7 cells | Inhibited ERK, p38 and NFκB | [55] | |
| Murine RAW 264.7 cells | Inhibited signal-regulated kinase-1 (ERK1) and peroxisome proliferator-activated receptor gamma (PPARγ) | [56] | |
| Rat embryonic fibroblasts | Decreased β-galactosidase, oxidative stress biomarkers, and number of apoptotic cells via the caspase-dependent pathway | [57] | |
| Human glioblastoma cells | Decreased apoptotic, inflammatory and oxidant effects of TRPA1 activation | [58] | |
| CoQ10 | Murine RAW 264.7 cells | Anti-inflammatory properties via NFκB1-dependent gene expression | [59] |
| Human dermal fibroblasts human umbilical vein endothelial cells (HUVECs) | CoQ10-induced improvement of oxidative status via miR-146a modulation | [60] | |
| CARN | Rat renal cells (NRK-52E) | Leptin-induced oxidative stress and inflammation were reversed by CARN | [61] |
| H9c2 rat cardiomyocytes | Promotes STAT3 activation and increases the expression of SOD2 | [62] |
Testing of the effects of MNs in animal models of acute inflammation conditions is summarized in Table 2. A number of studies in the recent decade have tested the ALA-associated anti-inflammatory effects on rats [78–86] and mice [87, 88]. The model disorders included multiple-organ sepsis [78, 81, 82, 86], endotoxemia [79], metal or organic poisoning [81, 84], and radiation-induced damage [88]. Altogether, ALA administration was found to decrease inflammatory response, H2O2, MDA levels, myeloperoxidase activity, and cytokine levels. Thus, the body of evidence for ALA-associated anti-inflammatory actions provides strong suggestions toward the adjuvant use of this MN in counteracting inflammatory conditions.
Table 2.
Reports on the effects of mitochondrial nutrients (MN) on anti-inflammatory endpoints tested in animal studies
| MN | Species (strain) | Effects | References |
|---|---|---|---|
| ALA | Rats | Decreased kidney injury in a model of sepsis | [63] |
| Wistar rats | Attenuated inflammatory response and improved multiple organ dysfunction syndrome caused by endotoxemia | [64] | |
| Rats | Decreased H2O2, MDA levels, and myeloperoxidase activity in ulcerative colitis | [65] | |
| Wistar rats | Reduced inflammation and oxidative stress in liver and kidney after sepsis | [66] | |
| Wistar–Kyoto rats | Counteracting counteracting gold nanoparticle-induced oxidative stress | [67] | |
| Sprague–Dawley rats | Decreased renal and gut injury, levels of IL-1β, TNF-α, and NO synthase | [68] | |
| Wistar rats | Decreased oxidative stress and the level of C-reactive protein and increased antioxidant potential in Cd-induced oxidative stress | [69] | |
| Ovariectomized rats | Prevented GSH and total non-enzymatic antioxidants depletion, and restored GPx and GR activities, TNF-α, and IL-6 in ovariectomized rats | [70] | |
| Rats | Decreased cytokine levels in acute respiratory distress syndrome | [71] | |
| Mice | Mitigated infiltration of most inflammatory cells, inflammation and vascular damage in radiation-induced pneumonitis | [72] | |
| C57BL/6 Mice | Decreased lipopolysaccharide-induced acute inflammatory response | [73] | |
| CoQ10 | Lewis rats | Decreased TBARS and IL-1 in methotrexate-induced rheumatoid arthritis | [74] |
| Wistar rats | Protective effects on multiple organ damage and histopathologically following cecal ligation and puncture-induced sepsis | [75] | |
| C57BL/6J mice | Decreased NFκB phosphorylation; abrogated MDA and 8-OHDG, and restored cellular glutathione in experimental cerebral malaria | [76] | |
| CARN | STAM mice | Prevented progression of non-alcoholic steatohepatitis by upregulating the mitochondrial β-oxidation and redox system | [77] |
| CARN | Sprague–Dawley rats | Peritonitis positively affected by CARN following puncture sepsis | [78] |
| Albino Wistar rats | Proinflammatory cytokines following inflammation-induced osteoporosis | [79] | |
| Mice | Ameliorated liver inflammation and serum proinflammatory markers in cancer cachexia through regulating CPT I-dependent PPARγ signaling | [80] | |
| Acetyl-CARN | Swiss Albino mice | Protective and therapeutic effect in neuroinflammation | [81] |
| Wistar rats | Decreased inflammation by the overexpression of NFκB and IL-1 and IL-6 following as-induced oxidative damage | [82] |
CoQ10 was also tested in rat and mouse models (Table 2), for its ability to counteract inflammatory conditions as drug-induced [89], or in puncture-induced sepsis [90], or experimental cerebral malaria [91].
Overall, CoQ10 was found to decrease MDA, TBARS and 8-OH-dG. Though through a more limited body of evidence compared to ALA, also CoQ10-associated anti-inflammatory properties may suggest the grounds for the design of adjuvant clinical treatments in acute disorders.
The animal studies of CARN- or acetyl-CARN-induced protection against proinflammatory conditions were focused on the same set of test-induced noxae (steatohepatitis, peritonitis, neuroinflammation) [92–95], as shown in Table 2. The results showed that (acetyl-)CARN decreased the levels of several proinflammatory endpoints, including proinflammatory markers, NF-ĸB and IL-1 and IL-6, and ameliorated organ inflammation [96, 97].
Thus, from the evidence provided in animal studies, each MN provides multiple means of protection against a number of proinflammatory conditions.
The reports from clinical trials on MNs in acute disorders are relatively few compared to the wealth of literature assessing the positive effects of MNs in several chronic diseases, such as type 2 diabetes and aging-related or cardiovascular disorders. An example of this growing body of literature on clinical trials in a number of chronic disorders may be found in our review [6], which cites a total of 262 reports on clinical trials testing MN-associated protective effects in patients affected by an extensive number of chronic disorders. As shown in Table 3, ALA was administered to patients admitted for hemodialysis [98], or undergoing cardiopulmonary surgery [99], or affected by ischemia—reperfusion injury [100]. Following ALA administration, patients underwent decrease in inflammatory markers, C-reactive protein (CRP), and IL-6 and IL-8 levels.
Table 3.
Reports on the effects of mitochondrial nutrients (MN) on anti-inflammatory end points tested in clinical trials on patients with acute disorders
| MN | Disease/condition | No. Of patients | Duration | Effects | References |
|---|---|---|---|---|---|
| ALA | Hemodialysis | 63 | 8 weeks | Decreased C-reactive protein (CRP) | [83] |
| Cardiopulmonary surgery | 30 | 24 ± 9.4 months | Significantly decreased IL-6 and IL-8 levels | [84] | |
| Ischemia–reperfusion injury | 26 | > 14 days | Decreased inflammatory markers, and early kidney dysfunction and pancreatitis | [85] | |
| CoQ10 | Septic shock | 14 | 72 h | Significantly lower CoQ10 plasma levels in septic shock patients than in healthy controls. CoQ10 negatively associated with inflammatory molecules | [86] |
| Acute influenza | 50 | 3 influenza seasons | Significantly lower CoQ10 plasma levels in patients with acute influenza infection | [87] | |
| Papillomavirus skin warts | 156 | 90 days | Decreased viral load and increased antiviral cytokine levels | [88] | |
| CARN | Hemodialysis or chronic peritoneal dialysis | 113 | 6 months | Suppressed inflammation, CRP | [89] |
| Hemodialysis | 42 | 6 months | Decreased CRP | [90] | |
| Hemodialysis | 36 | 12 weeks | Decreased CRP | [91] | |
| Septic shock | 31 | 28 days | Decreased mortality | [92] | |
| Coronary artery disease | 47 | 12 weeks | Decreased inflammation markers(CRP, IL-6 and TNF-α) | [93] | |
| Perioperative atrial fibrillation | 134 | 48 h post-operation | Decreased CRP | [94] |
CoQ10 levels were significantly lower in patients with acute influenza infection [101, 102]. CoQ10-supplemented patients showed decreased levels of inflammatory markers such as IL‐2 and TNF‐α, although no correlation with IL‐6 and IL‐10 was found [102]. Patients affected by papillomavirus skin warts and administered with CoQ10 underwent decreased viral load and increased antiviral cytokine levels [103] (Table 3).
CoQ10 was shown to improve clinical parameters as well as MDF in septic patients who received 100 mg CoQ10 twice a day for 7 days. In a randomized trial (n = 40), decreased levels of TNF-α and malondialdehyde were obtained in the early phase of septic shock patients [104].
Concurrent reports on CARN administration to patients undergoing hemodialysis [105–107], or septic shock [108] or affected by coronary artery disease [109], or perioperative atrial fibrillation [110] found CARN-induced significant decrease in CRP or decreased mortality, as shown in Table 3. The relevance of CRP in inflammation and OS had been established in early studies [111], thus the adjuvant role of CARN in mitigating a number of proinflammatory conditions should be ascertained.
Mitochondrial nutrients: safety, and their combined administration in counteracting proinflammatory conditions
Safety
α-Lipoic acid
α-Lipoic acid is a physiological compound produced in the mitochondria as a part of their basic metabolism (Krebs cycle). Degradation of ALA is similar in humans and in rats [112], and the safety of ALA has been demonstrated in multiple clinical studies [113, 114]. Only one report of acute ALA-induced toxicity [115] was related to a suicidal attempt that was, however, reversed after a 3-d supportive treatment. Overall, a body of literature has assessed the protective action of ALA against a number of xenobiotics in in vivo and in vitro investigations [reviewed by 116].
Coenzyme Q10
Coenzyme Q identifies a family of lipohilic cofactors with ubiquitous presence in many organisms [117]. The most abundant form in humans is CoQ10, being characterized by a side chain consisting of ten isoprenoid units. As the other MNs considered, it is an endogenous molecule also introduced through the diet. Coenzyme Q10 is a natural—and indispensable—compound present in mitochondria (electron transport chain). The use of CoQ10 as a dietary supplement offers very low toxicity and does not induce serious adverse effects in humans [118]. CoQ10 was well tolerated at up to 900 mg/day according to Ikematsu et al. [119]. In addition, administration of exogenous CoQ10 does not inhibit the physiological production of CoQ10 [120, 121]. A recent study by Sadeghiyan Galeshkalami et al. [122] reported on the benefits of ALA and coQ10 combination on experimental diabetic neuropathy by modulating OS and apoptosis.
Carnitine
The amino acid derivative CARN and its active stereoisomer acetyl-CARN (ALC) have been used in a number of human studies alone or as part of a combination therapy since the early 1980s [123]. ALC is synthesized in many tissues and has low toxicity [124]. Administration of CARN in clinical studies including an extensive number of disorders (Alzheimer’s disease, depression, aging, diabetes, ischemia and other neurological diseases) did not report major toxic effects [6, 124]. Song et al. [125] performed a meta-analysis of randomized controlled trials and reported that CARN had good tolerance in patients with chronic heart failure, improving clinical symptoms and cardiac functions.
Toward combined MN administration
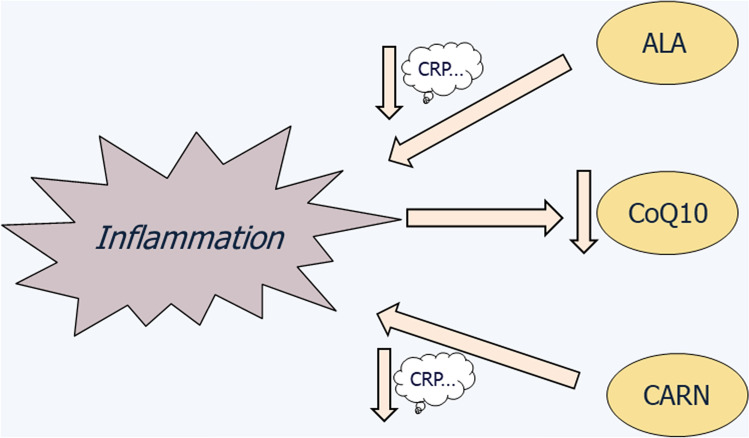
Based on the evidence from experimental studies and from clinical trials, it may be concluded that separate administration of ALA, coQ10, or CARN is safe in human and in animal health. Thus, as conceptually depicted in Fig. 1, both ALA and CARN were found to lower the levels of several inflammation biomarkers, such as CRP, both in animal models [71] and in humans [100, 101, 104, 105]. Another direct link of proinflammatory conditions with MNs was provided by Donnino et al. [101] and by Chase et al. [102], who reported decreased CoQ10 plasma levels in patients affected by septic shock or by acute influenza.
Fig. 1.
Outline of the major relationships between proinflammatory conditions and MNs, displaying decreased CoQ10 levels in plasma of patients with acute disorders, while ALA and CARN exert decreased levels of CRP and other inflammation biomarkers
A question may be raised about using individual MN administration in acute disorders, without any known attempt to test two or three combined MNs. Only a few clinical trials [122, 126, 127] investigated the effects of two combined MNs in chronic disorders, while no report is available—to the best of our knowledge—in testing three MNs concurrently.
Although there are no reports of the combined use of the three MNs in humans, any combined administration should not present potential problems when administered in patients suffering from acute disorders. According to the major and distinct interactions of MNs displayed in inflammatory conditions, as summarized in Fig. 1, it may be expected that: (a) CoQ10 administration should counteract the reported CoQ10 deficiency associated with inflammation and (b) both ALA and CARN administration should contribute to decreasing a set of inflammation biomarkers including, but not confined to, CRP. The present state-of-art is confined to clinical trials in one MN. This might be seen as a self-mutilation in the frame of adjuvant strategies targeted to mitigation of inflammatory conditions, such as sepsis, influenza, pneumonia, or other acute disorders. Taken together, the available knowledge about safety and anti-inflammatory effectiveness of each MN should prompt the combined use of these autochthonous cofactors in adjuvant therapeutic design originally designed for mitochondrial diseases [128]. The same rationale may be designed in view of mitigating acute disorders such as pneumonia infections. So far, clinical management of COVID-19 has been suggested by means of blocking cytokine storm through corticosteroids [129] or cytokine inhibitors [130, 131], controlling systemic inflammation via intravenous immunoglobulins injection [132], or inhibition of Janus kinases [133], and intervention with antimalarial drugs to inhibit tissue infection and viral replication [134]. It is worth noting that tocilizumab, as tested in COVID-19 [132, 133], is a well-established IL-6-blocking drug used in rheumatoid arthritis, both decreasing OS and MDF [135, 136].
Working hypothesis: comparing redox potential of MNs and of other antioxidant agents
Counteracting the course of disorders characterized by a prooxidant state has been a goal of an extensive body of experimental and clinical literature (as summarized in Tables 2, 3). Apart from the attempts to utilize MNs for this purpose, a long list of natural or synthetic antioxidants, vitamins and herbal preparations has been reported in the literature focused on mitigating COVID-19 progression [31–38]. Without regarding MNs as alternative means in counteracting inflammation, one might suggest combining these agents with well-established antioxidants, such as melatonin and/or resveratrol [31, 35].
However, with regard to MNs and the broader field, a major question arises about the quantification of the redox properties of any unspecified “antioxidant”, such as redox potential. To date, attempts to accomplish this task are frustrated by the multiplicity of parameters to be considered to obtain an endpoint that may be considered valid for this purpose. These parameters, mostly obtained in physico-chemical studies, encompass a number of variables, such as temperature, pH, concentration, dimerization, and multiple free radical formation [137–139]; thus, an effort to compare the antioxidant actions of several chemicals is presently unavailable. This therefore may suggest the timeliness of a quantitative comparison of antioxidant actions, under defined—physiological—conditions such as ionic strength, pH, and temperature, which may reflect the parameters detected in basal vs. pathological conditions. To provide an experimental and clinical choice among the multitude of antioxidants, this investigation, as yet unaccomplished, is much warranted.
Conclusions
The present paper reviews the experimental and clinical literature regarding the use of MNs in acute disease conditions, rather than presenting the more extensive literature about chronic disorders. The available literature provides definite evidence for the protective roles of ALA, CoQ10 and CARN in counteracting inflammation in acute disorders, such as sepsis and viral infections.
A rationale is presented for the clinical design of “triad” combinations of MNs [128] in countering the progression of acute disorders, by means of adjuvant protocols that may contribute to counteracting disease-related inflammation.
A working hypothesis is raised to achieve a comparative evaluation toward the antioxidant properties of several candidate antioxidant agents.
Acknowledgement
Our thanks are due to Agostino Esposito for his conceptual advice on paper finalization.
Author contributions
Conceptualization: GP, CM. Methodology: AL. Writing—original draft preparation: G|P, FP. Writing—review and editing: LT, AL. Supervision: MT.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ryrfeldt A, Bannenberg G, Moldéus P. Free radicals and lung disease. Br Med Bull. 1993;49:588–603. doi: 10.1093/oxfordjournals.bmb.a072633. [DOI] [PubMed] [Google Scholar]
- 2.Gutteridge JM, Mitchell J. Redox imbalance in the critically ill. Br Med Bull. 1999;55:49–75. doi: 10.1258/0007142991902295. [DOI] [PubMed] [Google Scholar]
- 3.MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. 2001;429:195–207. doi: 10.1016/s0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- 4.de Vega JMA, Díaz J, Serrano E, Carbonell LF. Oxidative stress in critically ill patients with systemic inflammatory response syndrome. Crit Care Med. 2002;30:1782–1786. doi: 10.1097/00003246-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Luft R. The development of mitochondrial medicine. Proc Natl Acad Sci USA. 1994;91:8731–8738. doi: 10.1073/pnas.91.19.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagano G, Talamanca AA, Castello G, Cordero MD, d’Ischia M, Gadaleta MN, Pallardó FV, Petrović S, Tiano L, Zatterale A. Current experience in testing mitochondrial nutrients in disorders featuring oxidative stress and mitochondrial dysfunction: rational design of chemoprevention trials. Int J Mol Sci. 2014;15:20169–20208. doi: 10.3390/ijms151120169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prauchner CA. Oxidative stress in sepsis: pathophysiological implications justifying antioxidant co-therapy. Burns. 2017;43:471–485. doi: 10.1016/j.burns.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Hargreaves IP, Mantle D. Supplementation with selenium and coenzyme Q10 in critically ill patients. Br J Hosp Med (Lond). 2019;80:589–593. doi: 10.12968/hmed.2019.80.10.589. [DOI] [PubMed] [Google Scholar]
- 9.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, Liu L, Shan H, Lei C, Hui DSC, Du B, Li L, Zeng G, Yuen KY, Chen R, Tang C, Wang T, Chen P, Xiang J, Li S, Wang J, Liang Z, Peng Y, Wei L, Liu Y, Hu Y, Peng P, Wang J, Liu J, Chen Z, Li G, Zheng Z, Qiu S, Luo J, Ye C, Zhu S, Zhong N, China Medical Treatment Expert Group for COVID-19 Clinical characteristics of coronavirus disease 2019 in China. New Eng J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, Wang X, Hu C, Ping R, Hu P, Li T, Cao F, Chang C, Hu Q, Jin Y, Xu G. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saleh J, Peyssonnaux C, Singh KK, Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1–7. doi: 10.1016/j.mito.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh KK, Chaubey G, Chen JY, Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in pathogenesis of COVID-19. Am J Physiol Cell Physiol. 2020;19:C258–267. doi: 10.1152/ajpcell.00224.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu KE, Fazal FM, Parker KR, Zou J, Chang HY. RNA-GPS predicts SARS-CoV-2 RNA residency to host mitochondria and nucleolus. Cell Syst. 2020;11:102–108. doi: 10.1016/j.cels.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan AS, Xiang Q, Wojdyla D, Khatana SM, Dayoub EJ, Wadhera RK, Bhatt DL, Kolansky DM, Kirtane AJ, Rao SV, Yeh RW, Groeneveld PW, Wang TY, Giri J. Performance of hospitals when assessing disease-based mortality compared with procedural mortality for patients with acute myocardial infarction. JAMA Cardiol. 2020;5:765–772. doi: 10.1001/jamacardio.2020.0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xudong X, Junzhu C, Xingxiang W, Furong Z, Yanrong L. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL, HCA Lung Biological Network SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Mao Y, Chen G. Risk factors for 2019 novel coronavirus disease (COVID-19) patients progressing to critical illness: a systematic review and meta-analysis. Aging (Albany NY). 2020;12:12410–12421. doi: 10.18632/aging.103383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lansiaux E, Pébaÿ PP, Picard J-L, Son-Forget J. COVID-19: beta-thalassemia subjects immunised. Med Hypotheses. 2020;142:109827. doi: 10.1016/j.mehy.2020.109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bian H, Zheng ZH, Wei D, Zhang Z, Kang WZ, Hao CQ, Dong K, Kang W, Xia JL, Miao JL, Xie RH, Wang B, Sun XX, Yang XM, Lin P, Geng JJ, Wang K, Cui HY, Zhang K, Chen XC, Tang H, Du H, Yao N, Liu SS, Liu LN, Zhang Z, Gao ZW, Nan G, Wang QY, Lian JQ, Chen ZN, Zhu P. Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. medRxiv. 2020; 10.1101/2020.03.21.20040691.
- 23.Wang K, Chen W, Zhou Y-S, Lian J-Q, Zhang Z, Du P, Gong L, Zhang Y, Cui H-Y, Geng J-J, Wang B, Sun X-X, Wang C-F, Yang X, Lin P, Deng Y-Q, Wei D, Yang X-M, Zhu Y-M, Zhang K, Zheng Z-H, J Miao J-L, Guo T, Shi Y, Zhang J, Fu L, Wang Q-Y, Bian H, Zhu P, Chen Z-N. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020; 10.1101/2020.03.14.988345
- 24.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tasaka S, Amaya F, Hashimoto S, Ishizaka A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid Redox Signal. 2008;10:739–753. doi: 10.1089/ars.2007.1940. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Costa Lisboa T, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Taylor Thompson B, Townsend SR, Van der Poll T, Vincent J-L, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016, Conference reports and expert panel. Intens Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 27.Mantzarlis K, Tsolaki V, Zakynthinos E. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid Med Cell Longev. 2017;2017:5985209. doi: 10.1155/2017/5985209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delgado-Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Priya Gatti P, Ilamathi HS, Todka K, Germain M. Mitochondria targeted viral replication and survival strategies—prospective on SARS-CoV-2. Front Pharmacol. 2020;11:578599. doi: 10.3389/fphar.2020.578599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, Liu C, Reiter RJ. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carr AC. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care. 2020;24:133. doi: 10.1186/s13054-020-02851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:E988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kandeel M, Al-Nazawi M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020;251:117627. doi: 10.1016/j.lfs.2020.117627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin SC, Ho CT, Chuo WH, Li S, Wang TT, Lin CC. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17:144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ang L, Lee HW, Choi JY, Zhang J, Soo LM. Herbal medicine and pattern identification for treating COVID-19: a rapid review of guidelines. Integr Med Res. 2020;9:100407. doi: 10.1016/j.imr.2020.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, Chufang L, Jin Z, Zhenhua J, Haiming J, Kui Z, Shuxiang H, Jun D, Xiaobo L, Xiaotao H, Lin W, Nanshan Z, Zifeng Y. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang YX, Ma JR, Wang SQ, Zeng YQ, Zhou CY, Ru YH, Zhang L, Lu ZG, Wu MH, Li H. Utilizing integrating network pharmacological approaches to investigate the potential mechanism of Ma Xing Shi Gan Decoction in treating COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:3360–3384. doi: 10.26355/eurrev_202003_20704. [DOI] [PubMed] [Google Scholar]
- 39.Quiles JL, Rivas-García L, Varela-López A, Llopis J, Battino M, Sánchez-González C. Do nutrients and other bioactive molecules from foods have anything to say in the treatment against COVID-19? Environ Res. 2020;191:110053. doi: 10.1016/j.envres.2020.110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocha M, Herance R, Rovira S, Hernandez-Mijares A, Victor VM. Mitochondrial dysfunction and antioxidant therapy in sepsis. Infect Disord Drug Targets. 2012;12:161–178. doi: 10.2174/187152612800100189. [DOI] [PubMed] [Google Scholar]
- 41.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Mervyn Singer M, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 43.Brealey D, Singer M. Mitochondrial dysfunction in sepsis. Curr Infect Dis Rep. 2003;5:365–371. doi: 10.1007/s11908-003-0015-9. [DOI] [PubMed] [Google Scholar]
- 44.Satoi S, Kamiyama Y, Kitade H, Kwon AH, Takahashi K, Wei T, Inoue T, Takahashi H. Nitric oxide production and hepatic dysfunction in patients with postoperative sepsis. Clin Exp Pharmacol Physiol. 2000;27:197–201. doi: 10.1046/j.1440-1681.2000.03228.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen GD, Zhang JL, Chen YT, Zhang JX, Wang T, Zeng QY. Insulin alleviates mitochondrial oxidative stress involving upregulation of superoxide dismutase 2 and uncoupling protein 2 in septic acute kidney injury. Exp Ther Med. 2018;15:3967–3975. doi: 10.3892/etm.2018.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jang DH, Orloski CJ, Owiredu S, Shofer FS, Greenwood JC, Eckmann DM. Alterations in mitochondrial function in blood cells obtained from patients with sepsis presenting to an emergency department. Shock. 2019;51:580–584. doi: 10.1097/SHK.0000000000001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arulkumaran N, Deutschman CS, Pinsky MR, Zuckerbraun B, Schumacker PT, Gomez H, Gomez A, Murray P, Kellum JA, ADQI XIV Workgroup Mitochondrial function in sepsis. Shock. 2016;45:271–281. doi: 10.1097/SHK.0000000000000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gibellini L, De Biasi S, Paolini A, Borella R, Boraldi F, Mattioli M, Lo Tartaro D, Fidanza L, Caro-Maldonado A, Meschiari M, Iadisernia V, Bacca E, Riva G, Cicchetti L, Quaglino D, Guaraldi G, Busani S, Girardis M, Mussini C, Cossarizza A. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol Med. 2020;19:e13001. doi: 10.15252/emmm.202013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laforge M, Elbim C, Frère C, Hémadi M, Massaad C, Nuss P, Benoliel JJ, Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Las HN, Martín Giménez VM, Ferder L, Manucha W, Lahera V. Implications of oxidative stress and potential role of mitochondrial dysfunction in COVID-19: therapeutic effects of Vitamin D. Antioxidants (Basel) 2020;9:897. doi: 10.3390/antiox9090897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shenoy S. Share coronavirus (COVID-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm Res. 2020;69:1077–1085. doi: 10.1007/s00011-020-01389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palade GE. The organization of living matter. Proc Natl Acad Sci USA. 1964;52:613–634. doi: 10.1073/pnas.52.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haas RH. Mitochondrial dysfunction in aging and diseases of aging. Biology (Basel) 2019;8:E48. doi: 10.3390/biology8020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tibullo D, Li Volti G, Giallongo C, Grasso S, Tomassoni D, Anfuso CD, Lupo G, Amenta F, Avola R, Bramanti V. Biochemical and clinical relevance of alpha lipoic acid: antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm Res. 2017;66:947–959. doi: 10.1007/s00011-017-1079-6. [DOI] [PubMed] [Google Scholar]
- 55.Saboori S, Falahi E, Eslampour E, Zeinali Khosroshahi M, Yousefi RE. Effects of alpha-lipoic acid supplementation on C-reactive protein level: a systematic review and meta-analysis of randomized controlled clinical trials. Nutr Metab Cardiovasc Dis. 2018;28:779–786. doi: 10.1007/10.1016/j.numecd.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Rahimlou M, Asadi M, Banaei Jahromi N, Mansoori A. Alpha-lipoic acid (ALA) supplementation effect on glycemic and inflammatory biomarkers: a systematic review and meta-analysis. Clin Nutr ESPEN. 2019;32:16–28. doi: 10.1016/j.clnesp.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 57.Fan L, Feng Y, Chen GC, Qin LQ, Fu CL, Chen LH. Effects of coenzyme Q10 supplementation on inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2017;119:128–136. doi: 10.1016/j.phrs.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 58.Zhai J, Bo Y, Lu Y, Liu C, Zhang L. Effects of coenzyme Q10 on markers of inflammation: a systematic review and meta-analysis. PLoS ONE. 2017;12:e0170172. doi: 10.1371/journal.pone.0170172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Littarru GP, Tiano L, Belardinelli R, Watts GF. Coenzyme Q(10), endothelial function, and cardiovascular disease. BioFactors. 2011;37:366–373. doi: 10.1002/biof.154. [DOI] [PubMed] [Google Scholar]
- 60.Sabbatinelli J, Orlando P, Galeazzi R, Silvestri S, Cirilli I, Marcheggiani F, Dludla PV, Giuliani A, Bonfigli AR, Mazzanti L, Olivieri F, Antonicelli R, Tiano L. Ubiquinol ameliorates endothelial dysfunction in subjects with mild-to-moderate dyslipidemia: a randomized clinical trial. Nutrients. 2020;12:1098. doi: 10.3390/nu12041098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCoin CS, Knotts TA, Adams SH. Acylcarnitines—old actors auditioning for new roles in metabolic physiology. Nat Rev Endocrinol. 2015;11:617–625. doi: 10.1038/nrendo.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohammadi M, Hajhossein Talasaz A, Alidoosti M. Preventive effect of l-carnitine and its derivatives on endothelial dysfunction and platelet aggregation. Clin Nutr ESPEN. 2016;15:1–10. doi: 10.1016/j.clnesp.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Pallardó FV, Lloret A, Lebel M, d'Ischia M, Cogger VC, Le Couteur DG, Gadaleta MN, Castello G, Pagano G. Mitochondrial dysfunction in some oxidative stress-related genetic diseases: Ataxia-Telangiectasia, Down Syndrome Fanconi Anaemia and Werner Syndrome. Biogerontol. 2010;11:401–419. doi: 10.1007/s10522-010-9269-4. [DOI] [PubMed] [Google Scholar]
- 64.Pagano G, Castello G. Oxidative stress and mitochondrial dysfunction in Down syndrome. Adv Exp Med Biol. 2012;724:291–299. doi: 10.1007/978-1-4614-0653-2_22. [DOI] [PubMed] [Google Scholar]
- 65.Pagano G, Castello G, Pallardó FV. Sjøgren’s syndrome-associated oxidative stress and mitochondrial dysfunction: prospects for chemoprevention trials. Free Radic Res. 2013;47:71–73. doi: 10.3109/10715762.2012.748904. [DOI] [PubMed] [Google Scholar]
- 66.Pagano G, Talamanca AA, Castello G, Cordero MD, d’Ischia M, Gadaleta MN, Pallardó FV, Petrović S, Tiano L, Zatterale A. Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: toward a rational design of chemoprevention strategies by means of mitochondrial nutrients. Oxid Med Cell Longev. 2014;2014:541230. doi: 10.1155/2014/541230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pagano G, Pallardó FV, Porto B, Fittipaldi MR, Trifuoggi M. Evaluating in vivo mitochondrial dysfunction in type 2 diabetes and in Fanconi anemia patients: toward mitoprotective clinical strategies. Antioxidants. 2020;9:82. doi: 10.3390/antiox9010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee CT, Chang LC, Wu PF. Lipoic acid exerts antioxidant and anti-inflammatory effects in response to heat shock in C2C12 myotubes. Inflammation. 2016;39:1160–1168. doi: 10.1007/s10753-016-0350-2. [DOI] [PubMed] [Google Scholar]
- 69.Dinicola S, Proietti S, Cucina A, Bizzarri M, Fuso A. Alpha-lipoic acid downregulates IL-1β and IL-6 by DNA hypermethylation in SK-N-BE neuroblastoma cells. Antioxidants (Basel) 2017;6:E74. doi: 10.3390/antiox6040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang P, Liu J, Yu Y, Cui SY, Guo ZH, Chen GM, Huang Q, Liu ZG. Alpha-lipoic acid suppresses extracellular histone-induced release of the inflammatory mediator tumor necrosis factor-α by macrophages. Cell Physiol Biochem. 2017;42:2559–2568. doi: 10.1159/000480217. [DOI] [PubMed] [Google Scholar]
- 71.Lee KJ, Ko YJ, Kang SK, Kim WS, Cho CS, Choi YJ. Additive anti-inflammation by a combination of conjugated linoleic acid and α-lipoic acid through molecular interaction between both compounds. Food Sci Biotechnol. 2019;29:419–429. doi: 10.1007/s10068-019-00677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baeeri M, Bahadar H, Rahimifard M, Navaei-Nigjeh M, Khorasani R, Rezvanfar MA, Gholami M, Abdollahi M. α-Lipoic acid prevents senescence, cell cycle arrest, and inflammatory cues in fibroblasts by inhibiting oxidative stress. Pharmacol Res. 2019;141:214–223. doi: 10.1016/j.phrs.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Deveci HA, Akyuva Y, Nur G, Nazıroğlu M. Alpha lipoic acid attenuates hypoxia-induced apoptosis, inflammation and mitochondrial oxidative stress via inhibition of TRPA1 channel in human glioblastoma cell line. Biomed Pharmacother. 2019;111:292–304. doi: 10.1016/j.biopha.2018.12.077. [DOI] [PubMed] [Google Scholar]
- 74.Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, Döring F. Functions of coenzyme Q10 in inflammation and gene expression. BioFactors. 2008;32:179–183. doi: 10.1002/biof.5520320121. [DOI] [PubMed] [Google Scholar]
- 75.Olivieri F, Lazzarini R, Babini L, Prattichizzo F, Rippo MR, Tiano L, Di Nuzzo S, Graciotti L, Festa R, Brugè F, Orlando P, Silvestri S, Capri M, Palma L, Magnani M, Franceschi C, Littarru GP, Procopio AD. Anti-inflammatory effect of ubiquinol-10 on young and senescent endothelial cells via miR-146a modulation. Free Radic Biol Med. 2013;63:410–420. doi: 10.1016/j.freeradbiomed.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 76.Blanca AJ, Ruiz-Armenta MV, Zambrano S, Salsoso R, Miguel-Carrasco JL, Fortuño A, Revilla E, Mate A, Vázquez CM. Leptin induces oxidative stress through activation of NADPH oxidase in renal tubular cells: antioxidant effect of l-carnitine. J Cell Biochem. 2016;117:2281–2288. doi: 10.1002/jcb.25526. [DOI] [PubMed] [Google Scholar]
- 77.Vacante F, Senesi P, Montesano A, Frigerio A, Luzi L, Terruzzi I. l-carnitine: an antioxidant remedy the survival of cardiomyocytes under hyperglycemic condition. J Diabetes Res. 2018;2018:4028297. doi: 10.1155/2018/4028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li G, Gao L, Jia J, Gong X, Zang B, Chen W. α-Lipoic acid prolongs survival and attenuates acute kidney injury in a rat model of sepsis. Clin Exp Pharmacol Physiol. 2014;41:459–468. doi: 10.1111/1440-1681.12244. [DOI] [PubMed] [Google Scholar]
- 79.Shen HH, Lam KK, Cheng PY, Kung CW, Chen SY, Lin PC, Chung MT, Lee YM. Alpha-lipoic acid prevents endotoxic shock and multiple organ dysfunction syndrome induced by endotoxemia in rats. Shock. 2015;43:405–411. doi: 10.1097/SHK.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 80.Petronilho F, Florentino D, Danielski LG, Vieira LC, Martins MM, Vieira A, Bonfante S, Goldim MP, Vuolo F. Alpha-lipoic acid attenuates oxidative damage in organs after sepsis. Inflammation. 2016;39:357–365. doi: 10.1007/s10753-015-0256-4. [DOI] [PubMed] [Google Scholar]
- 81.Della Giustina A, Goldim MP, Danielski LG, Florentino D, Mathias K, Garbossa L, Oliveira Junior AN, Fileti ME, Zarbato GF, da Rosa N, Martins Laurentino AO, Fortunato JJ, Mina F, Bellettini-Santos T, Budni J, Barichello T, Dal-Pizzol F, Petronilho F. Alpha-lipoic acid attenuates acute neuroinflammation and long-term cognitive impairment after polymicrobial sepsis. Neurochem Int. 2017;108:436–447. doi: 10.1016/j.neuint.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 82.Abdelhalim MAK, Moussa SAA, Qaid HA, Al-Ayed MS. Potential effects of different natural antioxidants on inflammatory damage and oxidative-mediated hepatotoxicity induced by gold nanoparticles. Int J Nanomed. 2018;13:7931–7938. doi: 10.2147/IJN.S171931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jia J, Gong X, Zhao Y, Yang Z, Ji K, Luan T, Zang B, Li G. Autophagy enhancing contributes to the organ protective effect of alpha-lipoic acid in septic rats. Front Immunol. 2019;10:1491. doi: 10.3389/fimmu.2019.01491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Markiewicz-Górka I, Pawlas K, Jaremków A, Januszewska L, Pawłowski P, Pawlas N. Alleviating effect of α-lipoic acid and magnesium on cadmium-induced inflammatory processes, oxidative stress and bone metabolism disorders in Wistar rats. Int J Environ Res Public Health. 2019;16:E4483. doi: 10.3390/ijerph16224483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delgobo M, Agnes JP, Gonçalves RM, Dos Santos VW, Parisotto EB, Zamoner A, Zanotto-Filho A. N-acetylcysteine and alpha-lipoic acid improve antioxidant defenses and decrease oxidative stress, inflammation and serum lipid levels in ovariectomized rats via estrogen-independent mechanisms. J Nutr Biochem. 2019;67:190–200. doi: 10.1016/j.jnutbio.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 86.Erol N, Saglam L, Saglam YS, Erol HS, Altun S, Aktas MS, Halici MB. The protection potential of antioxidant vitamins against acute respiratory distress syndrome: a rat trial. Inflammation. 2019;42:1585–1594. doi: 10.1007/s10753-019-01020-2. [DOI] [PubMed] [Google Scholar]
- 87.Guo J, Gao S, Liu Z, Zhao R, Yang X. Alpha-lipoic acid alleviates acute inflammation and promotes lipid mobilization during the inflammatory response in white adipose tissue of mice. Lipids. 2016;51:1145–1152. doi: 10.1007/s11745-016-4185-2. [DOI] [PubMed] [Google Scholar]
- 88.Azmoonfar R, Amini P, Yahyapour R, Rezaeyan A, Tavassoli A, Motevaseli E, Khodamoradi E, Shabeeb D, Musa AE, Najafi M. Mitigation of radiation-induced pneumonitis and lung fibrosis using alpha-lipoic acid and resveratrol. Antiinflamm Antiallergy Agent Med Chem. 2020;19:149–157. doi: 10.2174/1871523018666190319144020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goktas MT, Kilinc I, Pehlivan S, Bariskaner H, Ugurluoglu C, Iskit AB. Coenzyme Q10 improves the survival, mesenteric perfusion, organs and vessel functions in septic rats. Biomed Pharmacother. 2017;91:912–919. doi: 10.1016/j.biopha.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 90.Pala R, Beyaz F, Tuzcu M, Er B, Sahin N, Cinar V, Sahin K. The effects of coenzyme Q10 on oxidative stress and heat shock proteins in rats subjected to acute and chronic exercise. J Exerc Nutrition Biochem. 2018;22:14–20. doi: 10.20463/jenb.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nyariki JN, Ochola LA, Jillani NE, Nyamweya NO, Amwayi PE, Yole DS, Azonvide L, Isaac AO. Oral administration of Coenzyme Q10 protects mice against oxidative stress and neuro-inflammation during experimental cerebral malaria. Parasitol Int. 2019;71:106–120. doi: 10.1016/j.parint.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 92.Ishikawa H, Takaki A, Tsuzaki R, Yasunaka T, Koike K, Shimomura Y, Seki H, Matsushita H, Miyake Y, Ikeda F, Shiraha H, Nouso K, Yamamoto K. l-carnitine prevents progression of non-alcoholic steatohepatitis in a mouse model with upregulation of mitochondrial pathway. PLoS ONE. 2014;9:e100627. doi: 10.1371/journal.pone.0100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ercan U, Kiraz A, Çikman Ö, Türkön H, Kilinç N, Otkun MT, Özkan ÖF, Kiraz HA, Karaayvaz M. The effect of systemic carnitine administration on colon anastomosis healing in an experimental sepsis model. J Invest Surg. 2015;28:334–340. doi: 10.3109/08941939.2015.1029652. [DOI] [PubMed] [Google Scholar]
- 94.Aydin A, Halici Z, Albayrak A, Polat B, Karakus E, Yildirim OS, Bayir Y, Cadirci E, Ayan AK, Aksakal AM. Treatment with carnitine enhances bone fracture healing under osteoporotic and/or inflammatory conditions. Basic Clin Pharmacol Toxicol. 2015;117:173–179. doi: 10.1111/bcpt.12384. [DOI] [PubMed] [Google Scholar]
- 95.Jiang F, Zhang Z, Zhang Y, Wu J, Yu L, Liu S. l-carnitine ameliorates the liver inflammatory response by regulating carnitine palmitoyltransferase I-dependent PPARγ signaling. Mol Med Rep. 2016;13:1320–1328. doi: 10.3892/mmr.2015.4639. [DOI] [PubMed] [Google Scholar]
- 96.Kazak F, Yarim GF. Neuroprotective effects of acetyl- l-carnitine on lipopolysaccharide-induced neuroinflammation in mice: Involvement of brain-derived neurotrophic factor. Neurosci Lett. 2017;658:32–36. doi: 10.1016/j.neulet.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 97.Bodaghi-Namileh V, Sepand MR, Omidi A, Aghsami M, Seyednejad SA, Kasirzadeh S, Sabzevari O. Acetyl- l-carnitine attenuates arsenic-induced liver injury by abrogation of mitochondrial dysfunction, inflammation, and apoptosis in rats. Environ Toxicol Pharmacol. 2018;58:11–20. doi: 10.1016/j.etap.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 98.Khabbazi T, Mahdavi R, Safa J, Pour-Abdollahi P. Effects of alpha-lipoic acid supplementation on inflammation, oxidative stress, and serum lipid profile levels in patients with end-stage renal disease on hemodialysis. J Ren Nutr. 2012;22:244–250. doi: 10.1053/j.jrn.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 99.Uyar IS, Onal S, Akpinar MB, Gonen I, Sahin V, Uguz AC, Burma O. Alpha lipoic acid attenuates inflammatory response during extracorporeal circulation. Cardiovasc J Afr. 2013;24:322–326. doi: 10.5830/CVJA-2013-067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ambrosi N, Arrosagaray V, Guerrieri D, Uva PD, Petroni J, Herrera MB, Iovanna JL, León L, Incardona C, Chuluyan HE, Casadei DH. α-Lipoic acid protects against ischemia-reperfusion injury in simultaneous kidney—pancreas transplantation. Transplantation. 2016;100:908–915. doi: 10.1097/TP.0000000000000981. [DOI] [PubMed] [Google Scholar]
- 101.Donnino MW, Cocchi MN, Salciccioli JD, Kim D, Naini AB, Buettner C, Akuthota P. Coenzyme Q10 levels are low and may be associated with the inflammatory cascade in septic shock. Crit Care. 2011;15:R189. doi: 10.1186/cc10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chase M, Cocchi MN, Liu X, Andersen LW, Holmberg MJ, Donnino MW. Coenzyme Q10 in acute influenza. Influenza Other Respir Viruses. 2019;13:64–70. doi: 10.1111/irv.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Luca C, Kharaeva Z, Raskovic D, Pastore P, Luci A, Korkina L. Coenzyme Q(10), vitamin E, selenium, and methionine in the treatment of chronic recurrent viral mucocutaneous infections. Nutrition. 2012;28:509–514. doi: 10.1016/j.nut.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 104.Soltani R, Alikiaie B, Shafiee F, Amiri H, Mousavi S. Coenzyme Q10 improves the survival and reduces inflammatory markers in septic patients. Bratisl Lek Listy. 2020;121:154–158. doi: 10.4149/BLL_2020_022. [DOI] [PubMed] [Google Scholar]
- 105.Savica V, Santoro D, Mazzaglia G, Ciolino F, Monardo P, Calvani M, Bellinghieri G, Kopple JD. l-carnitine infusions may suppress serum C-reactive protein and improve nutritional status in maintenance hemodialysis patients. J Ren Nutr. 2005;15:225–230. doi: 10.1053/j.jrn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 106.Duranay M, Akay H, Yilmaz FM, Senes M, Tekeli N, Yücel D. Effects of l-carnitine infusions on inflammatory and nutritional markers in haemodialysis patients. Nephrol Dial Transplant. 2006;21:3211–3214. doi: 10.1093/ndt/gfl356. [DOI] [PubMed] [Google Scholar]
- 107.Hakeshzadeh F, Tabibi H, Ahmadinejad M, Malakoutian T, Hedayati M. Effects of l-carnitine supplement on plasma coagulation and anticoagulation factors in hemodialysis patients. Ren Fail. 2010;32:1109–1114. doi: 10.3109/0886022X.2010.510617. [DOI] [PubMed] [Google Scholar]
- 108.Puskarich MA, Kline JA, Krabill V, Claremont H, Jones AE. Preliminary safety and efficacy of l-carnitine infusion for the treatment of vasopressor-dependent septic shock: a randomized control trial. JPEN J Parenter Enteral Nutr. 2014;38:736–743. doi: 10.1177/0148607113495414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee BJ, Lin JS, Lin YC, Lin PT. Antiinflammatory effects of l-carnitine supplementation (1000 mg/day) in coronary artery disease patients. Nutrition. 2015;31:475–479. doi: 10.1016/j.nut.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 110.Dastan F, Talasaz AH, Mojtahedzadeh M, Karimi A, Salehiomran A, Bina P, Jalali A. Randomized trial of carnitine for the prevention of perioperative atrial fibrillation. Semin Thorac Cardiovasc Surg. 2018;30:7–13. doi: 10.1053/j.semtcvs.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 111.Wanner C, Metzger T. C-reactive protein a marker for all-cause and cardiovascular mortality in haemodialysis patients. Nephrol Dial Transplant. 2002;17(Suppl 8):29–32. doi: 10.1093/ndt/17.suppl_8.29. [DOI] [PubMed] [Google Scholar]
- 112.Schupke H, Hempel R, Peter G, Hermann R, Wessel K, Engel J, Kronbach T. New metabolic pathways of alpha-lipoic acid. Drug Metab Dispos. 2001;29:855–862. [PubMed] [Google Scholar]
- 113.Ziegler D, Hanefeld M, Ruhnau KJ, Hasche H, Lobisch M, Schütte K, Kerum G, Malessa R. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7 month multicenter randomized controlled trial (ALADIN III Study) ALADIN III Study Group. Diabetes Care. 1999;22:1296–1301. doi: 10.2337/diacare.22.8.1296. [DOI] [PubMed] [Google Scholar]
- 114.Heinisch BB, Francesconi M, Mittermayer F, Schaller G, Gouya G, Wolzt M, Pleiner J. Alpha-lipoic acid improves vascular endothelial function in patients with type 2 diabetes: a placebo-controlled randomized trial. Eur J Clin Invest. 2010;40:148–154. doi: 10.1111/j.1365-2362.2009.02236.x. [DOI] [PubMed] [Google Scholar]
- 115.Emir DF, Ozturan IU, Yilmaz S. Alpha lipoic acid intoxicatıon: an adult. Am J Emerg Med. 2018;36:1125.e3–1125.e5. doi: 10.1016/j.ajem.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 116.Cremer DR, Rabeler R, Roberts A, Lynch B. Safety evaluation of alpha-lipoic acid ALA. Regul Toxicol Pharmacol. 2006;46:29–41. doi: 10.1016/j.yrtph.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 117.Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2000;40:445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 118.Hidaka T, Fujii K, Funahashi I, Fukutomi N, Hosoe K. Safety assessment of coenzyme Q10 (CoQ10) BioFactors. 2008;32:199–208. doi: 10.1002/biof.5520320124. [DOI] [PubMed] [Google Scholar]
- 119.Ikematsu H, Nakamura K, Harashima S, Fujii K, Fukutomi N. Safety assessment of coenzyme Q10 Kaneka Q10 in healthy subjects: a double-blind, randomized, placebo-controlled trial. Regul Toxicol Pharmacol. 2006;44:212–218. doi: 10.1016/j.yrtph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 120.Alehagen U, Aaseth J, Alexander J, Johansson P. Still reduced cardiovascular mortality 12 years after supplementation with selenium and coenzyme Q10 for four years: a validation of previous 10-year follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly. PLoS ONE. 2018;13:e0193120. doi: 10.1371/journal.pone.0193120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suksomboon N, Poolsup N, Juanak N. Effects of coenzyme Q10 supplementation on metabolic profile in diabetes: a systematic review and meta-analysis. J Clin Pharm Ther. 2015;40:413–418. doi: 10.1111/jcpt.12280. [DOI] [PubMed] [Google Scholar]
- 122.Sadeghiyan Galeshkalami N, Abdollahi M, Najafi R, Baeeri M, Jamshidzade A, Falak R, Davoodzadeh Gholami M, Hassanzadeh G, Mokhtari T, Hassani S, Rahimifard M, Hosseini A. Alpha-lipoic acid and coenzyme Q10 combination ameliorates experimental diabetic neuropathy by modulating oxidative stress and apoptosis. Life Sci. 2019;216:101–110. doi: 10.1016/j.lfs.2018.10.055. [DOI] [PubMed] [Google Scholar]
- 123.De Leonardis V, Neri B, Bacalli S, Cinelli P. Reduction of toxicity of anthracyclines by l-carnitine: preliminary overview of clinical data. Int J Clin Pharmacol Res. 1985;5:137–142. [PubMed] [Google Scholar]
- 124.Wainwright MS, Mannix MK, Brown J, Stumpf DA. Carnitine reduces brain injury after hypoxic-ischemia in newborn rats. Pediat Res. 2003;54:688–695. doi: 10.1203/01.PDR.0000085036.07561.9C. [DOI] [PubMed] [Google Scholar]
- 125.Song X, Qu H, Yang Z, Rong J, Cai W, Zhou H. Efficacy and safety of l-carnitine treatment for chronic heart failure: a meta-analysis of randomized controlled trials. Biomed Res Int. 2017;2017:6274854. doi: 10.1155/2017/6274854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McMackin CJ, Widlansky ME, Hamburg NM, Huang AL, Weller S, Holbrook M, Gokce N, Hagen TM, Keaney JF, Jr, Vita JA. Effect of combined treatment with alpha-lipoic acid and acetyl-l-carnitine on vascular function and blood pressure in patients with coronary artery disease. J Clin Hypertens. 2007;9:249–255. doi: 10.1111/j.1524-6175.2007.06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Palacka P, Kucharska J, Murin J, Dostalova K, Okkelova A, Cizova M, Waczulikova I, Moricova S, Gvozdjakova A. Complementary therapy in diabetic patients with chronic complications: a pilot study. Bratisl Lek Listy. 2010;111:205–211. [PubMed] [Google Scholar]
- 128.Tarnopolsky MA. The mitochondrial cocktail: rationale for combined nutraceutical therapy in mitochondrial cytopathies. Adv Drug Deliv Rev. 2008;60:1561–1567. doi: 10.1016/j.addr.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 129.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ascierto PA, Fox BA, Urba WJ, Anderson AC, Atkins MB, Borden EC, Brahmer JR, Butterfield LH, Cesano A, Chen DC, de Gruijl TD, Dillman RO, Drake CG, Emens LA, Gajewski TF, Gulley JL, Stephen Hodi FJ, Hwu P, Kaufman D, Kaufman HL, Lotze MT, McNeel DG, Margolin KM, Marincola FM, Mastrangelo MJ, Maus MV, Parkinson DR, Romero PJ, Sondel PM, Spranger S, Sznol M, Weiner GJ, Wigginton JM, Weber JS. Insights from immuno-oncology: the society for immunotherapy of cancer statement on access to IL-6-targeting therapies for COVID-19. J Immunother Cancer. 2020;8:e000878. doi: 10.1136/jitc-2020-000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Michot JM, Albiges L, Chaput N, Saada V, Pommeret F, Griscelli F, Balleyguier C, Besse B, Marabelle A, Netzer F, Merad M, Robert C, Barlesi F, Gachot B, Stoclin A. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol. 2020;31:961–964. doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, Tang H, Nishiura K, Peng J, Tan Z, Wu T, Cheung KW, Chan KH, Alvarez X, Qin C, Lackner A, Perlman S, Yuen KY, Chen Z. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2020;4:e123158. doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cron RQ, Winn CW. The rheumatologist’s role in COVID-19. J Rheumatol. 2020;47:639–642. doi: 10.3899/jrheum.200334. [DOI] [PubMed] [Google Scholar]
- 134.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, Zeng X, Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hirao M, Yamasaki N, Oze H, Ebina K, Nampei A, Kawato Y, Shi K, Yoshikawa H, Nishimoto N, Hashimoto J. Serum level of oxidative stress marker is dramatically low in patients with rheumatoid arthritis treated with tocilizumab. Rheumatol Int. 2012;2012(32):4041–4045. doi: 10.1007/s00296-011-2135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Omoyinmi M, Hamaoui R, Bryant A, Chao Jiang M, Athigapanich T, Eleftheriou D, Hubank M, Brogan P, Woo P. Mitochondrial and oxidative stress genes are differentially expressed in neutrophils of sJIA patients treated with tocilizumab: a pilot microarray study. Pediatr Rheumatol Online J. 2016;14:7. doi: 10.1186/s12969-016-0067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Krishnan CV, Garnett M. Electrochemical behavior of the super antioxidant, α-lipoic acid. Int J Electrochem Sci. 2011;6:3607–3630. [Google Scholar]
- 138.Li D, Deng W, Xu H, Sun Y, Wang Y, Chen S, Ding X. Electrochemical investigation of coenzyme Q10 on silver electrode in ethanol aqueous solution and its determination using differential pulse voltammetry. J Lab Automat. 2016;21:579–589. doi: 10.1177/2211068216644442. [DOI] [PubMed] [Google Scholar]
- 139.Kusznierewicz B, Baranowska M, Suliborska K, Chrzanowski W, Namieśnik J, Bartoszek A. A three-step approach to estimation of reduction potentials of natural mixtures of antioxidants based on DPPH test. Illustration for catechins and cocoa Proceedings. 2019;11:4. doi: 10.3390/proceedings2019011004. [DOI] [Google Scholar]