Abstract
Introduction
Cystic lymphangioma is a benign tumor originating from the lymph vessels. It commonly occurs in childhood, in the head or neck regions. However, abdominal Cystic lymphangioma is extremely rare in adult patients and often asymptomatic. Considering abdominal space, it may attain huge sizes whilst causing minimal symptoms. Due to this insidious presentation, these tumors become massive and can be diagnosed late at the complication stage.
Presentation of case
This case report describes a rare and exceptional case of giant cystic lymphangioma of the stomach presented with a perforation in the abdominal cavity. The diagnosis was suspected following an abdominal CT scan, but could not confirm that the lesion was derived from the stomach. Therefore, an exploratory laparotomy found a multi-cystic mass occupying most of the abdominal space, adherent to the small gastric curvature and without delimitation line. This mass presents a small perforation responsible for an ascites of medium abundance. Then, the patient underwent a subtotal gastrectomy removing the entire cystic mass. Pathological analysis of the surgical specimen confirmed the diagnosis of cystic lymphangioma of the stomach.
The postoperative recovery was uneventful, and the patient was discharged after 6 days. At the 3-month follow-up, the patient was in good health.
Discussion
The cystic lymphangioma of the stomach is rare and exceptionally described in the literature. However, if this tumor is benign, it has the potential to grow, invade vital structures, and develop life-threatening complications.
Conclusion
We stress the importance of complete surgical excision to prevent cyst complications and to reduce the recurrence risk.
Keywords: Cystic Lymphangioma, Stomach Lymphangioma, Subtotal gastrectomy, Case report
Highlights
-
•
Cystic lymphangiomas of the stomach are rare benign tumors originating from the lymph.
-
•
Its clinical presentation is usually variable and often asymptomatic. However, it can cause mass effect and result in serious complications.
-
•
The radical resection remains a sufficiently safe and effective treatment to avoid cyst complications and recurrence risk.
-
•
Long-term follow-up is required after incomplete resection.
1. Introduction
Lymphangiomas are rare benign neoplasms, described for the first time by Koch in 1913 [1]. It is mostly encountered in the pediatric population in the neck and axillary regions. They are rare and slow-growing.
The majorities of abdominal lymphangiomas, which account for 1% of all lymphangioma cases, are common of cystic type and occur in the mesentery, followed by the omentum, mesocolon, and retroperitoneum. They are usually asymptomatic and found accidently. Therefore, the clinical presentations of abdominal cystic lymphangiomas are variable, nonspecific, and generally don't help establish the diagnosis.
Abdominal ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) are necessary for describing the cyst and giving important information about its location, size, and adjacent organ involvement. However, the diagnosis is confirmed after the pathological examination.
This case study describes an extremely rare case of giant cystic lymphangioma of the stomach in an adult male with a cyst perforation in the abdominal cavity. This case report aims to sensitize medical team about the management of this exceptional case. it was demonstrated that the diagnosis of stomach lymphangioma should always be considered at any age.
This work has been reported in line with the SCARE 2018 criteria [2].
1.1. Presentation of case
A 21-year-old man patient single, with BMI 24 kg/m2, was referred by a family physician to our emergency department of a university hospital center for management of a diffuse acute abdominal pain that has evolved from a week without nausea, vomiting, fever, or any symptom of gastrointestinal obstruction.
His medical and surgical past was unremarkable. He had no history of trauma or any drug histories and allergies. His family history is unremarkable, and there are no proven genetic abnormalities. He is normally independent and is a non-smoker.
On physical examination, his abdomen was markedly distended, tender. Moreover, a soft mass occupying all quadrants of the abdomen was palpated. The mass was freely mobile, non-compressible, and non-pulsatile. Rectal examination was normal.
The Laboratory studies at admission indicated leukocytosis (13760/mm3) with neutrophilia, and the inflammatory marker, C-reactive protein, was elevated (PCR 211 mg/dl). Moreover, hemoglobin value and platelet count were normal. The tumor markers (ACE, and CA19-9) were within normal limits, and other blood laboratory tests were normal.
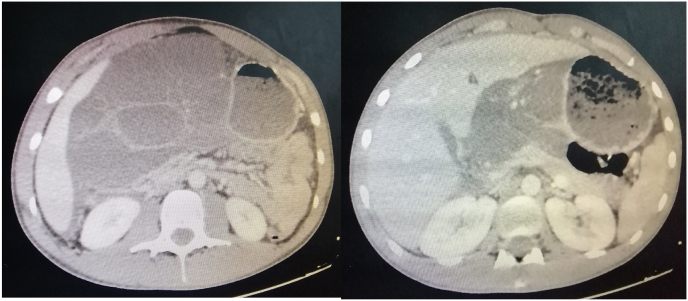
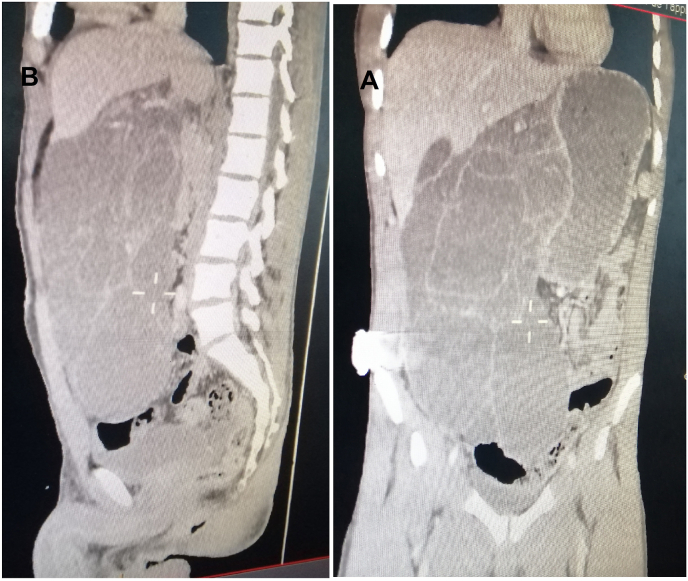
An abdominal CT-scan was performed (Fig. 1, Fig. 2) to evaluate the abdominal mass detected by physical examination, which showed a giant multi-lobulated cystic process, measuring 26 × 15 × 5 cm in size, without enhancement, crossed by septa, and occupied most of the abdominal cavity. However, the origin of this mass was not determined by CT. Additionally, there is a parietal defect of this cystic mass communicating with an intra-peritoneal effusion confirming its perforation. The main feeder artery for the tumor seemed to be the left gastric artery. Therefore the preoperative findings indicated that the lesion was cystic lymphangioma with perforation but could not confirm that the lesion was derived from the stomach. Correlating the clinical and abdominal CT scan, the primary diagnosis was an abdominal cystic lymphangioma perforated in the abdominal cavity that indicated the need for urgent laparotomy.
Fig. 1.
Findings of the computed tomography analysis; A multilobulated cystic mass was observed.
Fig. 2.
Abdominal CT scan: the cystic mass occupied most of the abdominal cavity. A: Coronal image; B: Sagittal image.
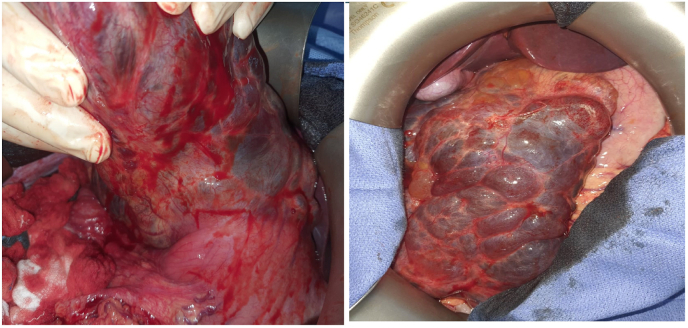
He underwent a midline exploratory laparotomy under general anesthesia. Intraoperative findings showed a multi-cystic mass originating from the lesser curvature of the stomach (Fig. 3). Moreover, the cyst occupied most of the abdominal cavity, and the caudal side of this tumor had no adhesions with pelvic organs. It has intimate contact with the stomach and without delimitation line, suggesting that the tumor had likely arisen from the stomach. Ascites have been observed in the peritoneal cavity suggested a cyst perforation in the abdomen. We decided to perform subtotal gastrectomy with mass excision for curative resection.
Fig. 3.
Intraoperative image showing a multi-cystic mass in the lesser curvature of the stomach.
We gradually dissected along with the capsule and moved the tumor outside the body. The large size of this tumor created considerable challenges for the surgical team.
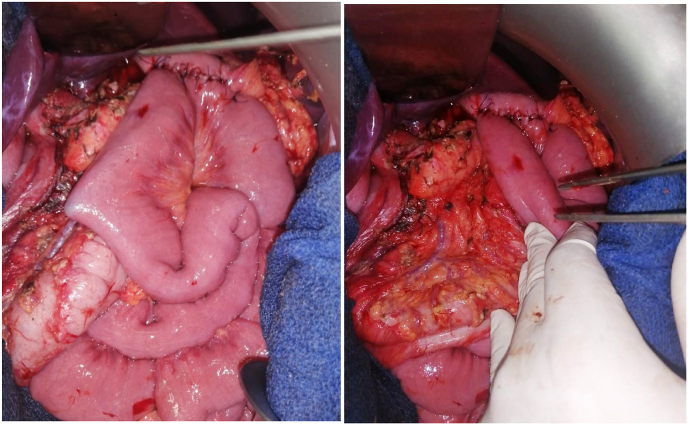
Firstly, the greater omentum was dissected from the transverse colon, and the right gastroepiploic vessels were resected at the greater curvature. Secondly, the right gastric vessels were resected, and the first portion of the duodenum was resected about 1cm distal to the pylorus, and the left gastric vessels were subsequently resected. Thirdly, Short Gastric Vessels were divided cautiously with an energy device, and the proximal portion of the stomach was resected. Then, the mass with the stomach was extracted. The resection margins are set at the proximal third of the stomach (Fig. 4). Thereafter, reconstruction by gastrojejunostomy anastomosis was performed by hand-sewn end-to-side anastomosis of stomach to proximal jejunum (Polya's intervention) (Fig. 5), and an abdominal drain was placed next to the anastomosis. Finally, peritoneal cavity irrigation with saline was performed, and the resection margin in the stomach was identified. The operation is performed by a professor of surgery with 20 years of experience.
Fig. 4.
Status after radical resection with subtotal gastrectomy and Gastrojejunostomy.
Fig. 5.
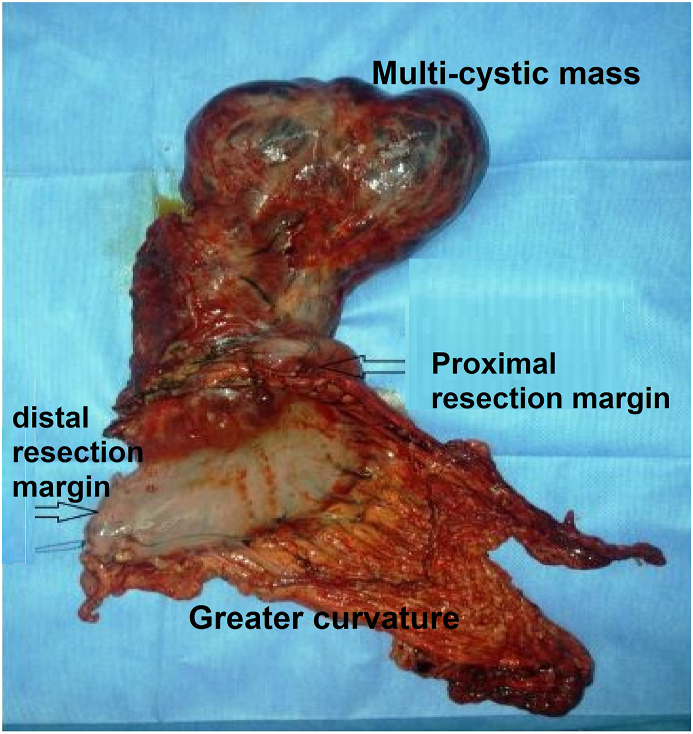
Photo of the surgical specimen showing the multilobulated cystic tumor arising from the lesser curvature of the Stomach.
Postoperatively, prophylaxis for thromboembolism, analgesia, and ceftriaxone (2g IV per day) with metronidazole 500mg IV (500 mg every 8 hours) antibiotics were administered. The oral diet was started cautiously on postoperative day 4. Patient post-operative recovery was uneventful. The drain was removed on the fifth day postoperative day and he was discharged on the sixth postoperative day.
He was instructed to eat multiple and separate meals a day. Thus, he is followed up at the outpatient clinic.
Macroscopic examination of the resected specimen revealed a 25 × 12 × 5-cm cystic multilocular mass and localized in the small gastric curvature.
The histological examination found a gastric wall with large confluent cavities, bordered by septae lined with flattened endothelial cells and filled with a slightly eosinophilic product. These cavities contain collagen fibers with some smooth muscle fibers and lymphocyte islands. This proliferation involved the submucosa, the muscular tissue, and comes into contact with the serous.
The definitive pathology reported is cystic lymphangioma of the stomach, without a sign of malignancy. The patient was followed up at one month and three months after surgery. On this visit, a clinical examination was done, which showed no evidence of recurrence. At three months follow-up, the patient was in good health.
The procedure and follow-up were done according to what is generally accepted with the general guidelines for abdominal cystic lymphangioma.
Scare 2018 paper was used for the construction of this paper [2].
2. Discussion
Lymphangioma is an uncommon benign tumor of the lymphatic vessels that rarely occurs in adults. The etiology is probably a congenital abnormality of the lymphatic system, causing sequestrations of lymphatic tissue that does not communicate with the lymphatic flow [3]. This theory would explain why lymphangiomas occur mainly in children. However, the adult manifestations may be related to inflammatory conditions or physical trauma such as those created by surgical or radiation therapies [4]. Our patient had no precise medical history and did not report any notion of trauma. This data leads us to think of an insidious evolution since childhood.
A total of 90% of lymphangiomas occur in children under two years of age. They are preferentially located in the head and neck (75%) and axilla (20%). They are rarely observed in adult patients and exceptionally occur in the abdomen [5] with less than 1% [6].
They are classified into three types: the simple capillary type, the cavernous, and the cystic type. The latter is the most common is characterized by cystic spaces and dilated endothelium, containing abundant lymphoid tissue and smooth muscle in the cyst wall with islands of lymphocytes in the lumen.
The most common location of these intraperitoneal tumors is in the mesentery and accounts for 70% of all such tumors [7].
Four varieties of abdominal lymphangiomas are known [8]:
I – pedicled, which attract attention by torsion;
II – sessile, frequently caught between leaves of mesentery;
III – retroperitoneal; and
IV – multicentric, which can involve at the same time, intra- and retroperitoneal organs.
The gastric origin of these tumors is exceptional. To our knowledge to date, only a small number of studies have described cystic lymphangiomas occurring intra-abdominally in adolescent patients. A PubMed search with the words “lymphangiomatosis” found a total of 625 articles and 78 articles for intra-abdominal cystic lymphangioma. In particular, only four cystic lymphangiomas originating from the Stomach have been documented [[9], [10], [11], [12]]. But the current case is the first with a cyst perforation in the abdominal cavity.
In these reported cases, it has a delimitation line between the gastric wall and the cyst in two cases, which leads to resection of the cyst without removing the stomach. But in two other cases, the cyst resection was associated with partial gastrectomy or subtotal gastrectomy.
In our case, there is no delimitation line, which led us to perform a subtotal gastrectomy to remove the entire cyst and to prevent the risk of recurrence.
The clinical presentations of abdominal cyst lymphangioma are usually variable, nonspecific and a large part of the patients are asymptomatic. However, it may discover accidently during investigation or laparotomy for unrelated pathology.
In the symptomatic cases, the clinical presentation include; nausea, vomiting, weight loss, abdominal distension, and acute abdominal pain [13]. The mass effect explains the transition to the asymptomatic stage. In our case, the patient is admitted for abdominal pain that has been evolving for a week. It is explained by the increase of the cyst size and its perforation in the peritoneal cavity.
In some cases, the patient is admitted at stages of complication such as rupture, hemorrhage, secondary infection, or intestinal obstruction [14].
Ultrasonography, Computed tomography, and Magnetic resonance imaging studies are useful for diagnosis and surgical planning of abdominal cyst lymphangioma by determining its location and its relation to surrounding structures [15].
The ultrasonographic presentation is a cystic mass with multiple thin septa. On abdominal CT scan and nuclear magnetic resonance imaging, it appears as multi-cystic fluid-filled masses.
In the current case, the tumor occupied most of the abdominal cavity, and it was vascularized by a branch of the left gastric artery. But we were unable to confirm the origin of the tumor. Thus preoperative imaging and knowledge of distorted vascular anatomy were pivotal to operative planning. Therefore the large size of this tumor poses significant challenges for radical treatment.
In most instances, diagnosis is confirmed only at the histological examination of the surgical specimen. Macroscopically, they appear as a soft, multiloculated cystic mass that contains serous, serosanguinous, or lymphatic fluid. Histological diagnosis is based on dilated lymphatic channels of varying sizes and delimited by thin septae, supported by connective tissue stromata of variable thickness that contain lymphoid tissue, round cells with smooth muscle. These spaces are lined with flattened or cuboidal endothelial cells.
The differential diagnosis of these lesions is broad to include benign and malignant cyst-like lesions as pancreatic pseudo-cyst, cystic teratoma, ovarian cyst, duplication cyst, cystic mesothelioma, malignant mesenchyma, undifferentiated sarcoma, and adnexal torsion.
Cystic lymphangiomas are generally benign tumors, but they can grow to an enormous size, invade adjacent vital structures, and develop fatal complications such as rupture, infection, compression, or hemorrhage [16]. Additionally, they may cause significant morbidity or mortality. On the other hand, these tumors can recur after incomplete resection [17]. Therefore, it should be excised completely to avoid cyst complications and reduce the risk of recurrence. However, sometimes radical resection might be technically impossible. Moreover, the other therapeutic methods have been described, such as surgical enucleation, sclerotherapy, radiation therapy, chemotherapy, and percutaneous drainage [18]. However, all these methods, unfortunately, carry a risk of recurrence.
Drainage and sclerotherapy are employed in select cases. Generally, they have indicated in cases where complete excision is not possible because of its enormous size and deep location with an invasion of vascular structures. But the infection and recurrence rates are high [19].
Often, the prognosis following complete resection is excellent [20]. But it will invariably recur if treated by aspiration or partial resection. The recurrence rate ranges from 0% to 13.6%, and most recurrences occur in patients who had only a partial excision.
Therefore, follow-up is not necessary after complete resection of the tumor because of the low risk of recurrence. However, long-term follow-up is necessary for other situations because of the risk of recurrence.
3. Conclusion
This case report describes an exceptionally adult case of cystic lymphangioma of the stomach. The surgical exploration has found a multi-cystic mass attached to the small gastric curvature without a delimitation line. The standard surgical treatment by radical resection, including a subtotal gastrectomy removing the cyst, remains a sufficiently safe and effective treatment option for this type of benign tumor to avoid long-term recurrence.
Diagnosis of this tumor is usually difficult due to its variable clinical presentations (asymptomatic or insidious) and its rarity. Although Ultrasonography, Computed tomography, and Magnetic resonance imaging can only suspect the disease; however, the definitive diagnosis is made only after histological examination of the surgical specimen.
The prognosis following complete resection is generally excellent, but it is poor after incomplete resection because of the recurrence. Therefore, long-term follow-up is required.
Patient perspective
The procedure of surgery was explained to the patient with all advantages and possible complications. He agreed on the procedure and informed consent was taken from her.
Conflicts of interest
The authors declared no potential conflicts of interests with respect to research, authorship and/or publication of the article.
Ethics approval
No ethical approval necessary.
Consent of the patient
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
The author(s) received no financial support for the research, authorship and/or publication of this article.
Author's contribution
Hassane Ait Ali; Writing, review and editing of the manuscript.
Brahim Zeriouh, Leila Bouziane: contributed for diagnose and treatment of the patient.
Rachid Jabi, Mohamed Bouziane: Review, Supervision and surgeons of the patient.
Registration of research studies: Our paper is a case report; no registration was done for it.
Guarantor: Hassane Ait Ali.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.amsu.2020.12.010.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Zhou Q., Zheng J.W., Mai H.M., Luo Q.F., Fan X.D., Su L.X., Wang Y.A. Treatment guidelines of lymphatic malformations of the head and neck. Oral Oncol. 2011;47:1105–1109. doi: 10.1016/j.oraloncology.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Agha Riaz A., Borrelli Mimi R., Farwana Reem, Koshy Kiron, Fowler Alexander J., Orgill Dennis P. For the SCARE Group. The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Schneider G., Seidel R., Altmeyer K., Remberger K., Pistorius G., Kramann B. Lymphangioma of the pancreas and the duodenal wall: MR imaging findings. Eur. Radiol. 2001;11:2232–2235. doi: 10.1007/s003300100846. [DOI] [PubMed] [Google Scholar]
- 4.Kang B.H., Hur H., Joung Y.S., Kim do K., Kim Y.B., Ahn C.W. Giant mesenteric cystic lymphangioma originating from the lesser omentum in the abdominal cavity. J Gastric Cancer. 2011;11:243–247. doi: 10.5230/jgc.2011.11.4.243. [PMID: 22324018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang Y.F., Qiu L.F., Du Y., Jiang Z.N., Gao M. Small intestinal hemolymphangioma with bleeding: a case report. World J. Gastroenterol. 2012;18:2145–2146. doi: 10.3748/wjg.v18.i17.2145. [PMID: 22563205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu H., Wu Z.Y., Lin X.Z., Shi B., Upadhyaya M., Chen K. Gastrointestinal tract lymphangiomas: findings at CT andendoscopic imaging with histopathologic correlation, Abdom. Imaging. 2008;33(6):662–668. doi: 10.1007/s00261-007-9354-6. [DOI] [PubMed] [Google Scholar]
- 7.Losanoff J.E., Richman B.W., El-Sherif A., Rider K.D., Jones J.W. Mesenteric cystic lymphangioma. J. Am. Coll. Surg. 2003;196:598–603. doi: 10.1016/S1072-7515(02)01755-6. [DOI] [PubMed] [Google Scholar]
- 8.Gayen R., Mahata M., Dasgupta S., Dasgupta J. Giant retroperitoneal cystic lymphangioma – a case report with review of literature. IOSR J. Dent. Med. Sci. 2015;14:69–71. [Google Scholar]
- 9.Hyuma A.L., James T.L., Justin H.T., Lorene E.R., Vishal B. Cystic lymphangioma of the lesser curvature of the stomach - case report. J. Radiol. Case Rep. 2011;5(5):31–37. doi: 10.3941/jrcr.v5i5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thijs Ralf V., Simon W.N., Thomas Bernard J.D., Michael Derek P.L., Ignace Hubertus J.T. Giant cystic lymphangioma originating from the lesser curvature of the stomach. World J. Gastrointest. Surg. 2013 Oct 27;5(10):264–267. doi: 10.4240/wjgs.v5.i10.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sathasivam S.K., Sri Aurobindo P.D., Vikram K. Cystic lymhangioma of the lesser sac in adult presenting with features of gastric outlet obstruction - a case report. J. Clin. Diagn. Res. 2015 Nov;9(11) doi: 10.7860/JCDR/2015/14789.6830. PD15–PD16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng C., Mingqing L., Tayyab H.M., Shouying L., Ying T., Hong X. Giant cystic lymphangioma originating from the cardia of the stomach: a case report. Exp Ther Med. 2016 May;11(5):1943–1946. doi: 10.3892/etm.2016.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safdar A., Bakhsh M., Ahmed I., Kibria R. An unusual cause of haemoperitoneum in a child. J. Pakistan Med. Assoc. 2008;58:458–460. [PMID: 18822648] [PubMed] [Google Scholar]
- 14.Elukoti H.N., Alcasoas S., Vernekar J., Hegde P., Pereira S. Mesenteric lymphangioma presenting as ileal volvulus. J. Clin. Diagn. Res. 2015;9:TJ05–TJ06. doi: 10.7860/JCDR/2015/16631.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marom E.M., Moran C.A., Munden R.F. Generalized lymphangiomatosis. AJR Am. J. Roentgenol. 2004;182:1068. doi: 10.2214/ajr.182.4.1821068. [PMID: 15039189. [DOI] [PubMed] [Google Scholar]
- 16.Yoon H.K., Han B.K. Chronic midgut volvulus with mesenteric lymphangioma: a case report. Pediatr. Radiol. 1998;28:611. doi: 10.1007/s002470050429. [DOI] [PubMed] [Google Scholar]
- 17.Rieker R.J., Quentmeier A., Weiss C., Kretzschmar U., Amann K., Mechtersheimer G. Cystic lymphangioma of the small-bowel mesentery: case report and a review of the literature. Pathol. Oncol. Res. 2000;6:146–148. doi: 10.1007/BF03032366. [PMID: 10936792. [DOI] [PubMed] [Google Scholar]
- 18.Wani I. Mesenteric lymphangioma in adult: a case series with a review of the literature. Dig. Dis. Sci. 2009;54:2758–2762. doi: 10.1007/s10620-008-0674-3. [DOI] [PubMed] [Google Scholar]
- 19.Martín-Pérez E., Tejedor D., Brime R. Cystic lymphangioma of the lesser omentum in an adult. Am. J. Surg. 2010;199:e20–e22. doi: 10.1016/j.amjsurg.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Durgakeri P., Penington B. Cystic mesenteric lymphangioma: a case report. ANZ J. Surg. 2018;88:E861–E862. doi: 10.1111/ans.13950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.