Abstract
Cannabis edibles are becoming more common in an increasingly diverse population of users, and the impact of first pass metabolism on cannabis’s pharmacological profile across age and sex is not well understood. The present study examined the impact of age, sex and rodent species on the effects of intraperitoneal (i.p.) delta-9-tetrahydrocannabinol (THC) and its primary psychoactive metabolite, 11-OH-THC, in rodent models of psychoactivity and molecular assays of cannabinoid receptor type-1 (CB1) pharmacology. Like oral THC, i.p. THC also undergoes first pass metabolism. In both species and sexes, 11-OH-THC exhibited marginally higher affinity (~1.5 fold) than THC and both served as partial agonists in [35S]GTPγS binding with equivalent potency; 11-OH-THC exhibited slightly greater efficacy in rat brain tissue. In ICR mice, 11-OH-THC exhibited greater potency than THC in assays of catalepsy (7- to 15-fold) and hypothermia (7- to 31-fold). Further, 11-OH-THC was more potent in THC drug discrimination (7- to 9-fold) in C57Bl/6J mice, with THC-like discriminative stimulus effects being CB1-, but not CB2-, mediated. THC’s discriminative stimulus also was stable across age in mice, as its potency did not change over the course of the experiment (~17 months). While sex differences in THC’s effects were not revealed in mice, THC was significantly more potent in females Sprague-Dawley rats than in males trained to discriminate THC from vehicle. This study demonstrates a cross-species in the psychoactive effects of i.p. THC across sex that may be related to differential metabolism of THC into its psychoactive metabolite 11-OH-THC, suggesting that species is a crucial design consideration in the preclinical study of phytocannabinoids.
Keywords: cannabinoid, delta-9-tetrahydrocannabinol, 11-OH-THC, sex differences, age differences, drug discrimination, metabolite
1.0. Introduction
Recent policy shifts in the U.S. and internationally have loosened legal restrictions on access to cannabis and cannabis-based products, resulting in corresponding increases in use in some demographic groups (Smart and Pacula, 2019). While recreational use in young men (aged ≤ 24) still outpaces use in women and older adults (Carliner et al., 2017), the population of cannabis users is diverse and use is increasing in middle-aged adults (aged 50–64) and in seniors (> 65) of both sexes (Han and Palamar, 2018; Han and Palamar, 2020). Further, methods of cannabis delivery have diversified. Although the most common route of cannabis administration for non-medical use continues to be inhalation through smoking or vaping, ingestion of cannabis edibles and multi-modal use has become more prevalent (Borodovsky et al., 2017; Schauer et al., 2020), with an estimated 25% of adults in states where cannabis is legal for recreational use reporting consumption of edibles (Schauer et al., 2020).
Unlike inhalation, oral consumption is accompanied by first pass metabolism, which raises the issue of the potential role of metabolites in determination of the effects of edibles containing Δ9-tetrahydrocanabinol (THC), the major psychoactive constituent of Cannabis sativa. Metabolism of orally administered THC occurs primarily in the liver and is extensive, with almost 100 metabolites having been identified (Grotenhermen, 2003). The two most referenced metabolites are 11-OH-Δ9-tetrahydrocannabinol (11-OH-THC) and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (11-COOH-Δ9-THC or THC-COOH). 11-OH-THC, formed by the cytochrome-P450-(CYP)-mediated hydroxylation of the C-11 site, is the initial step of THC biotransformation in most species, including humans (Harvey and Brown, 1991; Yamamoto et al., 1984). A secondary metabolite, THC-COOH, is formed through subsequent oxidation of 11-OH-THC (Watanabe et al., 1980). Whereas THC-COOH lacks cannabimimetic effects (Huestis, 2007; Mazur et al., 2009), 11-OH-THC readily crosses the blood-brain barrier, perhaps with even greater brain penetrance than THC (Huestis, 2007; Schou et al., 1977).
Given the increasing use of THC-infused edibles by both women and men in the U.S. (Schauer et al., 2020), identifying biological or pharmacological mechanisms through which sex influences THC’s behavioral effects is crucial for development of appropriate cannabis therapeutics or treatments for cannabis dependence. Design of such mechanistic studies in humans is complicated by self-selection of users and lack of control over their prior history; hence, animal models are often employed. To date, the most commonly used research model in this effort has been rats (Cooper and Craft, 2018; Craft et al., 2013b). For example, prior research has revealed that female rats are more sensitive to analgesic and discriminative stimulus effects of THC (Craft et al., 2013a; Wiley et al., 2017). In contrast, mice have been used in relatively few studies investigating sex differences in cannabinoid effects, albeit their use is increasing concurrent with the U.S. federal mandate to consider sex as a biological variable in NIH-funded research (Clayton and Collins, 2014).
The goal of the present study was two-fold: (1) to evaluate the effects of sex, age, and species in an animal model of the psychoactive effects of THC and (2) to provide data on the in vitro effects of THC and 11-OH-THC, the primary psychoactive metabolite of THC, in male and female rodents. Because one of our interests was in the behavioral effects of a THC metabolite, we chose a route of administration (intraperitoneal, i.p.) wherein THC undergoes first-pass metabolism (Lukas et al., 1971). Although oral gavage may have been more translationally relevant, it has been shown to be stressful for rodents (Walker et al., 2012) and results in more variable absorption than i.p. (Daublain et al., 2017). Subjective effects were assessed using drug discrimination, in which mice and rats were trained to discriminate between the effects of THC and vehicle. Subsequently, we evaluated sex differences in THC’s discriminative stimulus effects in both species. Because cannabinoid research on sex differences in mice is sparse, we also examined additional pharmacological effects of cannabinoids in mice, including antagonist tests and tests with 11-OH-THC in drug discrimination and evaluation of mice in a classical cannabinoid tetrad of in vivo assays for which centrally acting cannabinoids typically produce a characteristic profile of effects: reduction in body temperature, reduction in locomotor activity, antinociception, and catalepsy (Martin et al., 1991). We also assessed the binding affinity of 11-OH-THC and THC for the CB1 cannabinoid receptor and their activation of this G-protein coupled receptor (GPCR) in both sexes in mice and rats.
2.0. Materials and methods
2.1. Subjects
Adult drug naïve male and female ICR mice (31–34g; Envigo, Frederick, MD) and C57/Bl6J mice (20–25g; Jackson Laboratories, Bar Harbor, ME) were used in the tetrad battery and mouse drug discrimination experiments, respectively. Adult drug naïve male and female Sprague-Dawley rats (200–274 g; Envigo, Frederick, MD) were used in the rat drug discrimination study. While mice in the tetrad battery experiment received free access to food in their home cage, mice and rats in the drug discrimination experiment were maintained at 85–90% of free-feeding body weights by restricting daily ration of standard rodent chow. All rodents were singly housed in temperature-controlled conditions (20–24 °C) with a 12 h standard light-dark cycle (lights on at 0700) and had ad libitum access to water in their home cages. Studies were carried out in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and in accordance with our Institutional Animal Care and Use Committee (IACUC), and other federal and state regulations.
2.2. Apparatus
For the in vivo test battery, measurement of spontaneous activity occurred in Plexiglas locomotor activity chambers (47 cm × 25.5 cm × 22 cm) with beam breaks (4 × 8 beam array) recorded by San Diego Instruments Photobeam Activity System software (San Diego, CA) on a computer located in the experimental room. A standard tail flick device for rodents (Stoelting, Dale, IL) was used to assess antinociception. A digital thermometer (Physitemp Instruments, Clifton, NJ) was used to measure rectal temperature. The ring immobility device consisted of an elevated metal ring (diameter, 5.5 cm; height, 28 cm) attached to a metal stand.
Rodents in the drug discrimination procedure were trained and tested in mouse or rat operant chambers (Coulbourn Instruments, Whitehall, PA) housed within light- and sound-attenuating cubicles. Each chamber was outfitted with a house light, white noise generator, and two nose-poke apertures (mice) or response levers (rats) with stimulus lights over each aperture/lever. A pellet feeder delivered 20 mg (mice) or 45 mg (rats) food pellets (Bioserv Inc., Frenchtown, NJ) into an illuminated pellet trough centered between the two apertures/levers. Chamber operations (i.e., illumination of lights, generation of white noise, delivery of food pellets, and recording of responses) were controlled by a computer system (Graphic State Software, v 3.03; Coulbourn Instruments).
2.3. Chemicals
THC (National Institute on Drug Abuse, NIDA, Rockville, MD), 11-OH-THC (NIDA), rimonabant [5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide; NIDA], and SR144528 [5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)methyl]-N-[(1S,2S,4R)-1,3,3-trimethylbicyclo[2.2.1]hept-2-yl]-1H-pyrazole-3-carboxamide; NIDA] were suspended in a 7.8% polysorbate 80 (Fisher Scientific, Pittsburgh, PA) and saline (Patterson Veterinary Supply, Devens, MA) mixture and were injected intraperitoneally (i.p.) at a volume of 10 ml/kg for mice and 1 ml/kg for rats.
For in vitro studies, THC, CP55,940 (NIDA), and [3H]CP55,940 (81.1 Ci/mmol; NIDA) were dissolved in 100% ethanol and stored at −80°C in silanized amber vials. Non-radiolabeled drugs were stored as 10 mM stocks and diluted to final concentration of 0.1% solvent in the assay. GDP (Sigma Aldrich, St. Louis, MO), unlabeled GTPγS (Sigma Aldrich, St. Louis, MO), and [35S]GTPγS (1261–1269 Ci/mmol; Perkin Elmer Life Sciences, Boston, MA) were dissolved in distilled water, aliquotted for single used and stored at −80°C. Adenosine deaminase (Sigma Aldrich, St. Louis, MO) was diluted in distilled water and stored at 4°C. High-performance liquid chromatography grade acetonitrile and water were purchased from Fisher Scientific (Fairlawn, NJ). Reference standards and metabolite reference standards for all compounds were obtained from Cayman Chemical (Ann Arbor, MI).
2.4. Procedures
2.4.1. Tissue collection and processing
The cerebellum was selected for in vitro study because of its high density of CB1 receptors and its distinct morphology which ensured a homogenous pool of receptors. Male and female ICR mice and Sprague-Dawley rats were euthanized by CO2 and brains were rapidly removed. Cerebella from were dissected on ice, snap frozen in liquid nitrogen, and stored at −80°C until the day of the experiment. On the day of the experiment, cerebella were thawed on ice and homogenized by Brinkmann Polytron 3000 with Kinematica 95/ PT-DA 3012/2 S generator probe in membrane buffer (50 mM Tris, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, pH 7.4) on ice, centrifuged for 10 min at 40,000 × g at 4°C. The supernatant was discarded, and the pellet was suspended in membrane buffer, homogenized, and centrifuged again for 10 min at 40,000 × g. The pellet was resuspended in membrane buffer and the protein amount was quantified by the Bradford method.
2.4.2. Radioligand Binding and Agonist-Stimulated [35S]GTPγS Binding
For [3H]CP55,940 displacement binding, reactions were carried out in assay buffer (membrane buffer containing 1 mg/ml bovine serum albumin; BSA) and membranes (10 μg protein) were incubated for 60 min at 30°C in with ~1 nM [3H]CP55,940. Non-specific binding was determined by including excess unlabeled ligand (10 μM). Total bound of [3H]CP55,940 was approximately 5% of total added (minimal ligand depletion).
For receptor signaling, membranes (10 μg protein) were preincubated in assay buffer for 10 min with 3 units/ml adenosine deaminase to remove endogenous adenosine and lower the basal [35S]GTPγS binding. The amount of solvent (0.1% ethanol) was fixed across all reaction tubes (including basal) to control for potential effects on observed function. CP55,940 (0.01 nM – 100 nM), THC and 11-OH-THC (0.1 nM – 10 μM) were then incubated for 60 min at 30°C with 30 μM GDP, ~0.12 nM [35S]GTPγS, and non-specific binding was determined by including 30 μM unlabeled GTPγS. After 1 h of incubation at 30°C, binding was terminated by vacuum filtration through GF/C glass fiber filter plates (Perkin Elmer) in a 96-well sampling manifold. Reaction vessels were washed three times with ~2 mL of 4°C rinse buffer at (50 mM Tris-HCl, 1 mg/mL BSA). The filter plates were air-dried and sealed on the bottom. Thirty-five μL of Microscint-20 (Perkin Elmer) was then added to the wells and the top was sealed. After at least 30 min of incubation, the radioactivity was quantified by liquid scintillation spectrophotometry using a Packard NXT Topcount.
2.4.3. Cannabinoid Tetrad in Mice
Male and female ICR mice (n=6 of each sex per dose) were administered vehicle, THC or 11-OH-THC and tested in a battery of four tests in which cannabinoid agonists produce a characteristic profile of in vivo effects: suppression of locomotor activity, antinociception, decreased rectal temperature, and catalepsy. Baseline rectal temperature and tail flick latency were measured, then vehicle or drug was administered. Following the 30 min pretreatment interval, mice were placed into individual locomotor activity chambers for 10 min. Locomotor activity was measured as the total number of beam breaks during the session. Rectal temperature and tail-flick latency were measured again immediately after removal from the locomotor chambers. At 50 min post-injection, the mice were placed on the elevated ring apparatus and amount of time spent immobile was recorded during a 5 min period.
2.4.4. Drug discrimination
Male and female C57BL/6J mice (n=20 and 15, respectively) and Sprague-Dawley rats (n=8/sex) were trained to respond on one of the two nose-poke apertures (mice) or levers (rats) in operant chambers following intraperitoneal (i.p.) administration of the THC training dose and to respond on the other aperture/lever following i.p. vehicle administration according to a fixed ratio 10 (FR10) schedule of food reinforcement, under which 10 consecutive responses on the correct (injection-appropriate) aperture/lever resulted in delivery of a food pellet. The THC training dose for mice was 5.6 mg/kg whereas the training dose for rats was 1.7 mg/kg. Training doses were chosen based upon our previous research showing that THC potency for producing discriminative stimulus effects is less in mice than in rats (Vann et al., 2009; Wiley et al., 2017) and were administered i.p. 30 min prior to the start of the training session for each species. Responses on the incorrect aperture/lever reset the ratio requirement on the correct aperture/lever. Prior to each daily training session, animals received a single injection of THC (5.6 mg/kg for mice; 1.7 mg/kg for rats) or vehicle in a double alternation schedule (e.g., two sessions with THC pre-injection followed by two sessions with vehicle pre-injection). These single daily 15 min training sessions were held on weekdays until the rodents consistently met three criteria: (1) the first completed FR10 was on the correct aperture/lever, (2) ≥ 80% of the total responding occurred on the correct aperture/lever, and (3) response rate was ≥ 0.1 responses/s. When these criteria had been met for the most recent THC training dose and vehicle sessions and 8 of the 10 most recent sessions, reliable discrimination had been established and stimulus substitution testing began.
During 15 min stimulus substitution tests, 10 consecutive responses on either aperture/lever delivered reinforcement. If a rodent responded on the other aperture/lever prior to completing 10 responses on an aperture/lever, the ratio requirement on the original aperture/lever was reset. To be eligible for a stimulus substitution test, a rodent needed to have completed a training session the previous day in which the criteria for reliable discrimination (i.e., ≥ 0.1 responses/s with the first completed FR10 registered on the injection-appropriate aperture/lever and ≥ 80% of responses on this aperture/lever) had been met. In addition, the rodent must have met these same criteria during the most recent training session with the alternate training compound (i.e., THC training dose or vehicle). Baseline discrimination training sessions continued other weekdays. After passing stimulus substitution tests for the training drug and vehicle (i.e., responses consistent with reliable discrimination criteria), an initial substitution dose-response curve was determined for THC in each sex.
Mice were subsequently tested with other cannabinoids over the course of an average of 15 (females) to 19 (males) months, as reported elsewhere (Gamage et al., 2018; Gamage et al., 2017; Wiley et al., 2019). At the end of these studies, a dose-effect curve for THC was re-determined. In addition, the effects of the CB1 and CB2 receptor antagonists, SR141716A (rimonabant) and SR144528, respectively, on THC substitution were assessed in a sub-group of the mice (n=8/sex) followed by a substitution dose-response curve for 11-OH-THC and evaluation of 1 mg/kg 11-OH-THC in combination with rimonabant and SR144528. For substitution tests, THC and 11-OH-THC were administered 30 min pre-session; for antagonist tests, 1 mg/kg rimonabant or 3 mg/kg SR144528 was administered 10 min prior to THC or 11-OH-THC. Choice of rimonabant and SR144538 doses and dosing regimen was based our prior work with these two compounds (Wiley et al., 2019; Wiley et al., 2017). All compounds were administered intraperitoneally (i.p.) to both species.
2.5. Data analysis
2.5.1. Analysis of tetrad data
Locomotor activity was measured as total number of photocell beam breaks during the 10-min session. For the purpose of potency calculation, locomotor activity was expressed as percentage of inhibition of activity of the vehicle group. Rectal temperature values were expressed as the difference between baseline (pre-injection) temperature and temperature following drug administration (Δ°C). For catalepsy, the total amount of time (in s) that the mouse spent immobile on the ring apparatus (excluding breathing and whisker movement) was used as an indication of catalepsy-like behavior. This value was divided by 300 s and multiplied by 100 to obtain a percent immobility. For compounds that produced one or more cannabinoid effects, ED50 was calculated separately using least-squares linear regression on the linear part of the dose-effect curve for each measure in the mouse tetrad, plotted against log10 transformation of the dose. ED50 was defined as the dose at which half-maximal effect occurred. Based on data obtained from numerous previous studies with cannabinoids, maximal cannabinoid effect in each procedure was estimated as follows: 100% inhibition of spontaneous activity, −6°C change in rectal temperature, and 100% ring immobility. Separate between-subjects ANOVAs were also used to analyze the four measures for each compound. Significant differences from control (vehicle) were further analyzed with Tukey post hoc tests (α = 0.05), as necessary. Factorial ANOVAs (rimonabant dose × compound dose) were used to analyze results of antagonist tests. Significant main effects and interactions were further analyzed with Tukey post hoc tests (α = 0.05), as necessary.
2.5.2. Analysis of drug discrimination data
For each drug discrimination session, responses on the drug aperture/lever as a percentage of all responses during the session and response rate (all responses/s) were calculated. As appropriate, ED50 values were calculated using linear least squares regression analysis. Data from sessions in which a rodent did not earn food reinforcement (i.e., fewer than 10 consecutive responses were registered on either aperture/lever) were excluded from analysis of percentage of drug response selection, but response rate data were included. Response rate data were analyzed with separate mixed (sex X dose or sex X dose X test) ANOVAs, followed by Tukey post hoc tests (α=0.05) to determine differences between means. Sex differences in acquisition were analyzed via t-test. Separate mixed model (sex X rimonabant dose) ANOVAs were used to analyze rimonabant antagonism of the discriminative stimulus and response rate effects of 1 mg/kg 11-OH-THC. Body weights were analyzed by a mixed model (sex X time) ANOVA, followed by Tukey post hoc tests (α=0.05) to determine differences between means.
2.5.3. Analysis of in vitro data
Data for [3H]CP55,940 displacement and [35S]GTPγS-binding experiments are reported as mean (± SEM) of at least three biological replicates. Net-stimulated [35S]GTPγS binding was defined as agonist-stimulated minus basal [35S]GTPγS binding, and percent stimulation was defined as (net-stimulated/basal [35S]GTPγS binding) × 100%. Data were plotted and analyzed with GraphPad Prism 6.0. [3H]CP55,940 displacement data were fit using a “One site - Fit Ki” binding model for fitting of Ki values for THC and 11-OH-THC. Functional data were fit using the “log(agonist) vs. response (three parameters)” model. Mean averages of best fit values were analyzed by two-way ANOVA within each species with drug (THC vs. 11-OH-THC) and sex (male vs. female) as factors. Significance was defined as P ≤ 0.05.
3.1. Results
3.1.1. CB1 receptor binding and activation
As shown in Table 1, both THC and its psychoactive metabolite 11-OH-THC bind to CB1 receptors in the cerebella of mice of both sexes with moderate affinity (Ki = 27.3–41.3 nM). Both compounds exhibited similar affinities across sex, with 11-OH-THC showing slightly (1.3–1.5-fold) greater affinity than THC in both sexes. Functionally, THC and 11-OH-THC were partial agonists, as measured in the [35S]GTPγS assay, and activated the CB1 receptor at similar potencies across sex (Table 1).
Table 1.
CB1 receptor affinity and efficacy in rodent cerebellum.
| Ligand | CB1 Binding Affinity (Ki in nM) | Potency (EC50 ± SEM) nM | Efficacy (Emax ± SEM) % | |||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| Mice | ||||||
| THC | 37.5 (0.06) |
41.3 (0.09) |
68.2 (15.0) |
64.6 (12.4) |
69 (4.65) |
56 (3.76) |
| 11-OH-THC | 28.5 (0.03) |
27.3 (0.04) |
81.4 (27.2) |
85.3 (20.1) |
60 (139) |
52 (5.94) |
| CP55,940 | 0.48 (0.07) |
0.56 (0.06) |
nd | nd | nd | nd |
| Rats | ||||||
| THC | 36.2 (4.61) |
44.3 (10.27) |
47.9 (12.58) |
43.3 (7.33) |
43.5 (6.69) |
40.5 (2.84) |
| 11-OH-THC | 26.5 (140) |
28 (3.79) |
108.6 (13.06) |
69.8 (9.97) |
51.4 (5.74) |
51.7 (5.42) |
| CP55,940 | 0.62 (0.10) |
0.53 (0.06) |
nd | nd | nd | nd |
N = 4 / value
Similarly, THC and 11-OH-THC also bind to CB1 receptors in the cerebella of rats of both sexes with moderate affinity (Ki = 26.5–44.3 nM; Table 1). Affinity differences between the two compounds were approximately the same as for mice, with 11-OH-THC showing slightly (1.4–1.6-fold) greater affinity than THC in both sexes. Functionally, THC and 11-OH-THC were partial agonists as measured in the [35S]GTPγS assay, but 11-OH-THC had slightly greater efficacy than THC and activated cerebellar CB1 receptors at potencies that were not significantly different across sex (Table 1).
3.1.2. Mouse tetrad
Figure 1 (panel A) shows the effects of THC and 11-OH-THC on locomotor activity in female and male mice. Whereas females exhibited greater activity than males across all THC doses [sex X dose interaction: F(1,50)=9.29, p<0.05], the effects of 11-OH-THC on locomotion were not significantly different across sex. Neither drug significantly decreased locomotor activity, although the lack of significance may be related to a large degree of variability (especially in females). In contrast, THC and 11-OH-THC produced clear and significant dose-dependent increases in ring immobility [THC dose main effect: F(4,50)=42.50, p<0.05; 11-OH-THC: sex X dose interaction: F(4,50)=41.40, p<0.05] and decreases in body temperature [THC: sex X dose interaction: F(4,50)=2.88, p<0.05; 11-OH-THC: sex X dose interaction: F(4,50)=5.83, p<0.05] in mice of both sexes (Figure 1, panels B and C, respectively), with 11-OH-THC showing 7- to 31-fold greater potency than THC across the two assays (Table 2). Because pre-session tail flick latencies were abnormally variable in these mice (for undetermined reasons), data for drug-induced antinociception were also highly variable and are not shown.
Figure 1.
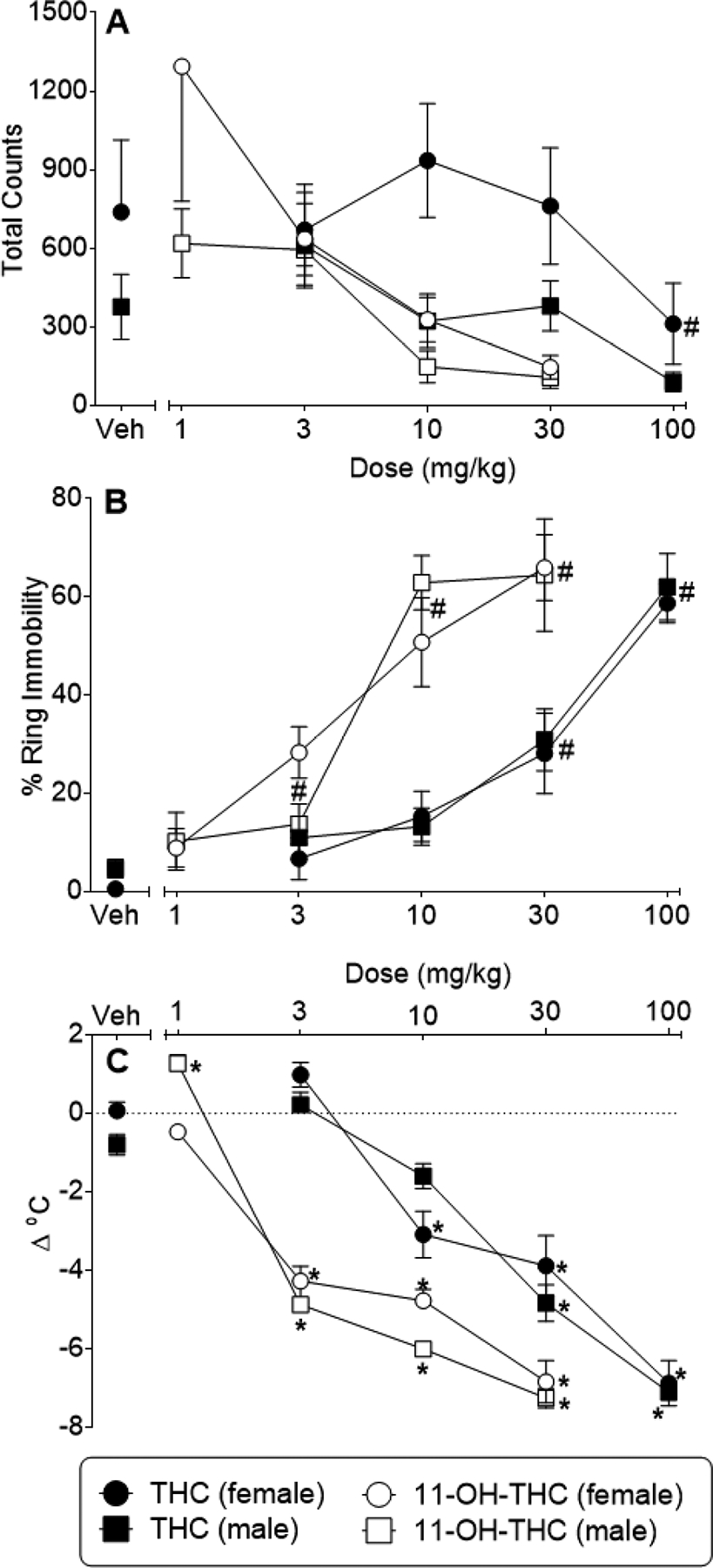
Effects of THC (filled symbols) and 11-OH-THC (open symbols) on locomotor activity (panel A), ring immobility (panel B), and rectal temperature (panel C) in female (circles) and male (squares) adult ICR mice. Values represent the mean (± SEM) of 6 mice. Asterisks (*) indicate a significant sex X dose interaction, with a significant post hoc difference for the indicated sex and dose (p<0.05) compared to the respective vehicle (at left side of each panel). Pound signs (#) indicate a significant main effect for dose across both sexes (p<0.05), as compared to vehicle (at left side of each panel).
Table 2.
Potency to produce hypothermia and ring immobility in female and male ICR mice.
| Sex | Body Temperature ED50 (± SEM)* | Ring Immobility ED50 (± SEM)* | ||
|---|---|---|---|---|
| THC | 11-OH-THC | THC | 11-OH-THC | |
| Female | 5.95 (1.81–19.53) |
0.19 (0.006–6.1) |
2.41 (0.53–10.92) |
0.16 (out of range) |
| Male | 5.21 (1.51–17.93) |
0.70 (0.22–2.26) |
3.85 (1.98–7.49) |
0.57 (0.07–4.57) |
n=6 per sex
3.1.3. THC drug discrimination in mice
Average sessions to successful acquisition of the THC discrimination did not differ between the sexes, with male (n=20) and female (n=15) mice requiring 40.2 (± 3.85) and 35.2 (± 2.61) sessions, respectively (p>0.05). Figure 2 shows effects of two dose-effect curves for THC, one at the beginning of the study and one at the end. In both curves, THC dose-dependently substituted for the 5.6 mg/kg training dose and was equipotent across sexes and across the time span of 15–19 months between the curves (Figure 2, top panel). ED50 values ranged from 1.76 to 2.14 mg/kg, with overlapping 95% confidence limits (Table 3). Analysis of response rate data revealed that THC significantly increased overall responding across both sexes at doses of 3 and 5.6 mg/kg during the first test and over a dose range of 3–10 mg/kg during the second test [test X dose interaction: F(5,165)=2.42, p<0.05; Figure 2, bottom panel]; however, significant response rate decreases compared to vehicle were not observed. Males showed significantly enhanced responding compared to females during the first test [sex X test interaction: F(1,33)=56.17, p<0.05]. Over time, however, response rates changed in opposite directions in both sexes. Whereas males exhibited a significant decrease in responding during the second test compared to the first, females showed significantly increased responding during the second test [sex X test interaction: F(1,33)=56.17, p<0.05]. Hence, during the second test, response rates were similar across sex.
Figure 2.
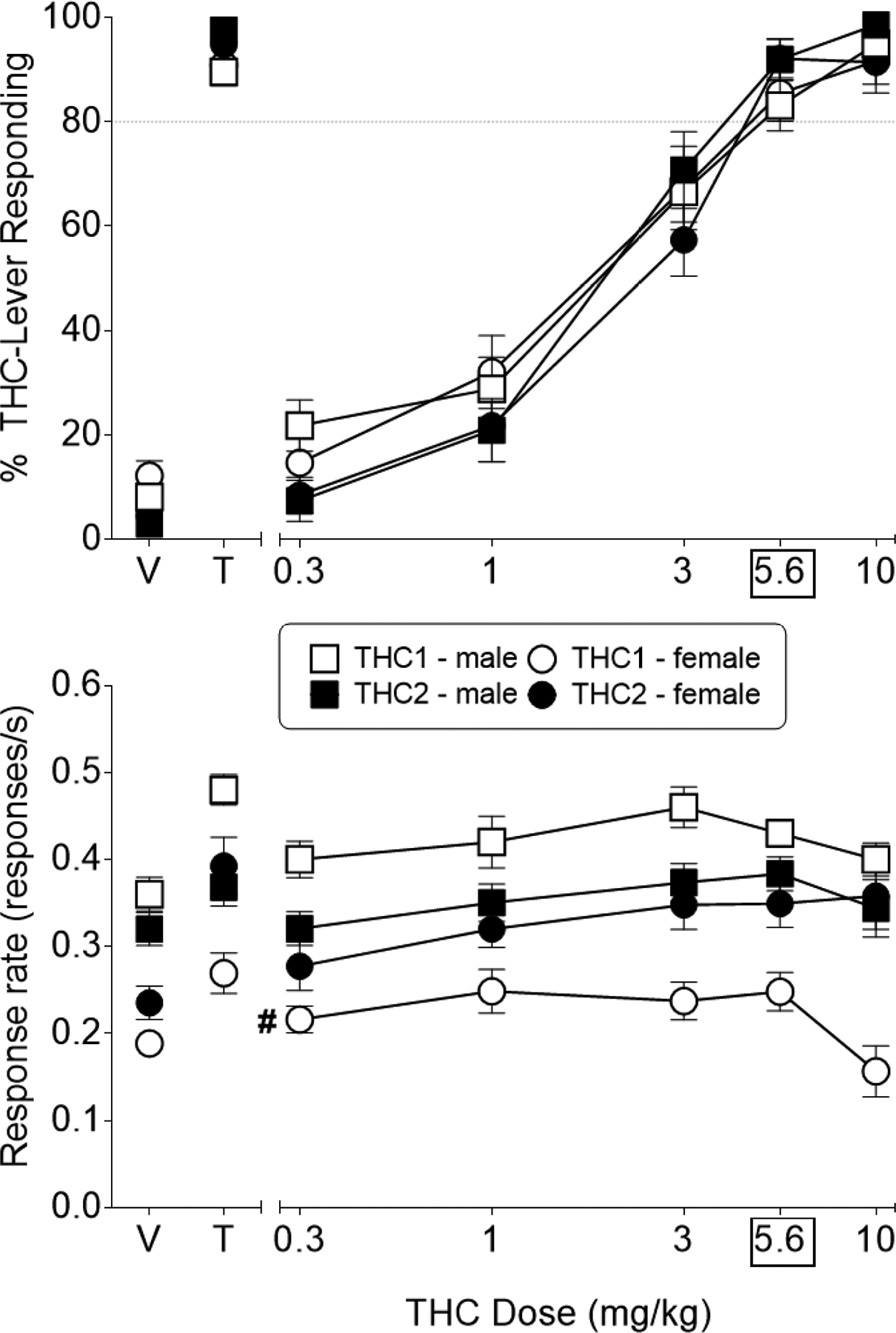
Dose response effects of THC at the beginning of the study (unfilled symbols) and at the end of the study (filled symbols) in female (circles) and male (squares) adult C57Bl/6J mice trained to discriminate 5.6 mg/kg THC from vehicle. Top panel shows THC effects on percentage of nose-pokes that occurred on the THC-associated aperture and bottom panel shows overall response rate. Control tests with vehicle (V) and the 5.6 mg/kg THC training dose (T) are shown at the left side of the panels. Each point represents the mean (± SEM) of data for 15 female and 20 male C57Bl/6J mice, except for n=13 for %THC aperture responding for 10 mg/kg THC in the second dose-effect curve for females (i.e., data for 2 female mice were excluded due to fewer than 10 overall responses). Dollar sign ($) indicates a significant main effect for sex across all doses (p<0.05) during the first THC dose-effect curve.
Table 3.
Potency in THC drug discrimination in female and male C57BL/6 mice.
| Sex | ED50 (± SEM) | ED50 (± SEM)c | ||||
|---|---|---|---|---|---|---|
| THC-1 | THC-2 | THC | THC + 1 mg/kg Rimonabant | THC + 3 mg/kg SR144528 | 11-OH-THC | |
| Female | ||||||
| (1.40–2.22) | (0.54–8.55) | (0.61–6.26) | (7.79–20.60) | (1.93–4.05) | (0.05–1.53) | |
| Male | ||||||
| (1.05–3.44) | (1.02–3.76) | (1.05–4.42) | (4.25–50.22) | (1.64–3.82) | (0.04–1.49) | |
n=15;
n=20;
n=8
Body weights were measured before every training and test session throughout the study. During the period of THC testing at the beginning and end of the study, average body weights for male mice were 23 g (± 0.20) and 27 g (± 0.21), respectively, and for female mice, 20 g (± 0.25) and 23 g (± 0.23), respectively. As expected, male mice weighed significantly more than female mice during both THC testing periods [sex main effect: F(1,33)=206, p<0.05] and both sexes gained weight over the course of testing [time main effect: F(1,33)=336, p<0.05].
Figure 3 shows results of evaluation of substitution and antagonism of THC’s discriminative stimulus effects in a subset of mice (n=8/sex). THC dose-dependently substituted for the 5.6 mg/kg THC training dose in both male (Figure 3, panel A) and female (Figure 3, panel B) mice. Whereas 1 mg/kg rimonabant shifted the THC dose-effect curves ~7-fold rightward for both sexes, 3 mg/kg SR144524 did not alter THC substitution in either sex (Figure 3, panels A and B; Table 3). Response rate was significantly decreased by 30 mg/kg THC [sex X dose interaction: F(6,84)=6.94, p<0.05] in males (compared to vehicle), but not in females (Figure 3, panels C and D, respectively); however, overall response rate across all doses was significantly suppressed in females compared to males [main effect of sex for THC: F(6,84)=18.25, p<0.05]. Hence, lack of significant cannabinoid-induced decreases in responding by females may have resulted from their decreased baseline responding across all doses, including vehicle. Response rates for males treated with 1 mg/kg rimonabant alone also were significantly suppressed compared to vehicle alone treatment [Figure 3, left side of panel C; sex X dose interaction: F(6,84)=4.15, p<0.05]. Females showed overall decreased responding compared to males but were not affected by rimonabant treatment compared to vehicle (Figure 3, panel D).
Figure 3.

Effects of THC on percentage of responses that occurred on the THC-associated aperture in male (filled squares; panel A) and female (filled circles; panel B) mice trained to discriminate 5.6 mg/kg THC from vehicle in a nose poke procedure. Response rates are also shown for both sexes (panels C and D for male and female mice, respectively). Rimonabant (1 mg/kg) produced rightward shifts in the THC dose-effect curves for %THC-aperture responding in male and mice (panels A and B, respectively), with changes in response rates shown in panel C and D. In contrast, SR144528 (3 mg/kg) did not alter %THC-aperture responding in male (unfilled triangles; panel A) or female (inverted unfilled triangles; panel B) mice, with changes in response rates shown in panel C and D, respectively. Control tests with vehicle (V), 5.6 mg/kg THC (T), and rimonabant (1 mg/kg) and SR144528 (3 mg/kg) alone (SR) were conducted prior to each dose-effect curve, with results shown at the left side of the panels. Each point represents the mean (± SEM) of data for 8 mice, except for %THC aperture responding for 30 mg/kg THC (males, n=1; females, n=4), 10 mg/kg THC (females, n=7), and 30 mg/kg THC + rimonabant (males, n=7). In each case, the n was reduced because data for %THC aperture responding were excluded due to fewer than 10 overall responses. Asterisks (*) indicate a significant sex X dose interaction, with a significant post hoc difference for the indicated sex and dose (p<0.05) compared to the respective vehicle (at left side of each panel). Dollar sign ($) indicates a significant main effect for sex across all doses (p<0.05) for the specific dose-effect curve.
11-OH-THC, also tested in this subset of mice, dose-dependently substituted for THC (Figure 4, top panel). While maximum levels of substitution for 11-OH-THC were similar to those obtained with THC (see Figure 3, panels A and C for males and females, respectively), 11-OH-THC was 7-fold and 9-fold more potent than THC in females and males, respectively (Table 3). Probe tests with CB1 and CB2 antagonists showed that 1 mg/kg rimonabant blocked maximal substitution produced by 1 mg/kg 11-OH-THC [main effect of rimonabant dose: F(1,14)=297.76, p<0.05] whereas 3 mg/kg SR144524 did not significantly affect the degree of substitution (Figure 4, top panel). Response rate was significantly decreased by 3 mg/kg 11-OH-THC [sex X dose interaction: F(4,56)=5.84, p<0.05] in males (compared to vehicle), but not in females (Figure 4, bottom panel); however, as with THC, overall response rate across all doses was significantly suppressed in females compared to males [main effect of sex for 11-OH-THC: F(4,56)=14.52, p<0.05]. Rimonabant (1 mg/kg) also decreased responding in 11-OH-THC-treated male, but not female, mice [Figure 4, bottom panel; sex X dose interaction: F(1,14)=5.30, p<0.05].
Figure 4.
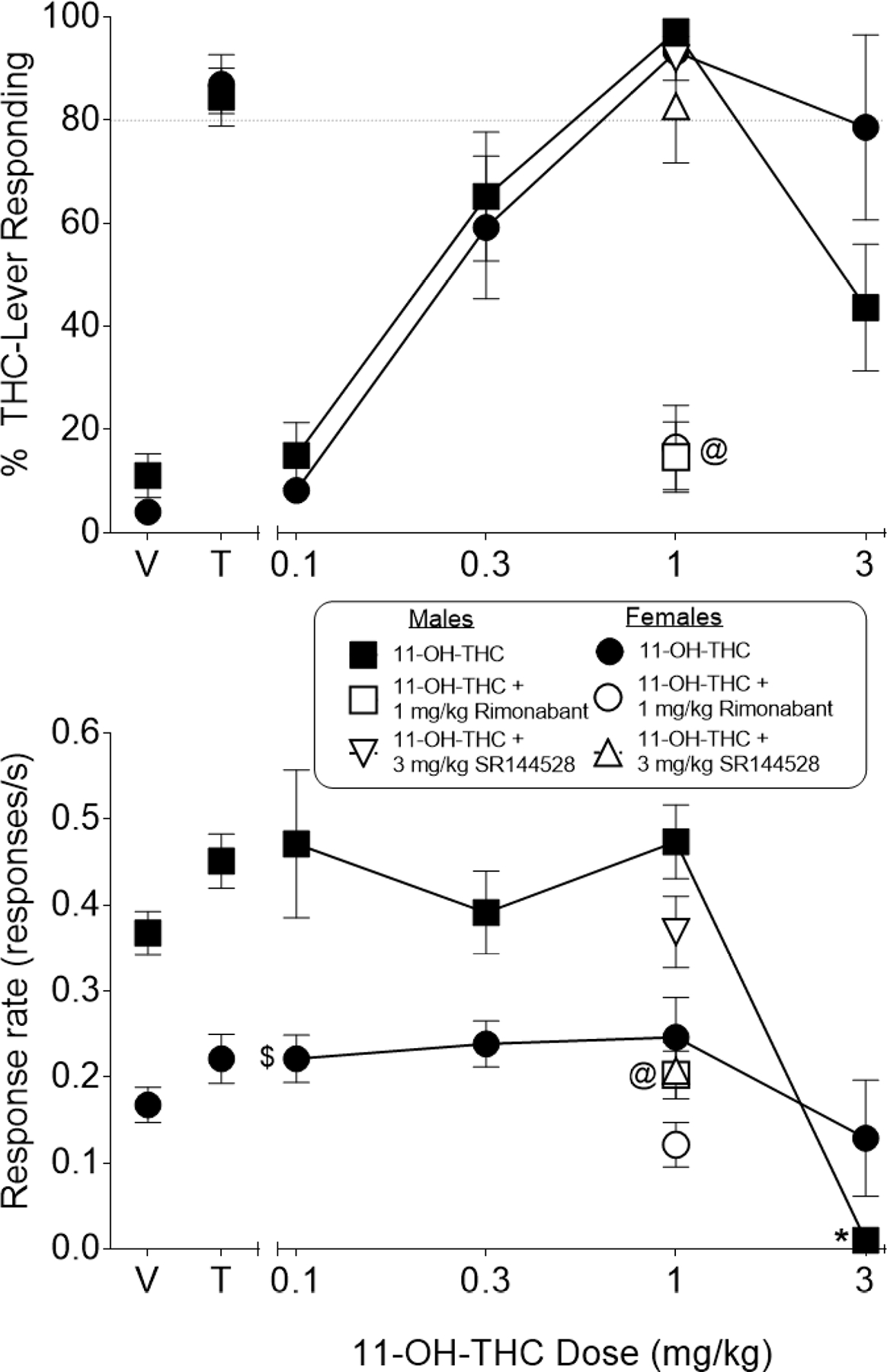
Effects of 11-OH-THC on percentage of responses that occurred on the THC-associated aperture (top panel) and response rate (bottom panel) in female (filled circles) and male (filled squares) mice trained to discriminate 5.6 mg/kg THC from vehicle in a nose poke procedure. Rimonabant (1 mg/kg) reversed the THC-like effects of 1 mg/kg 11-OH-THC in female (unfilled circles) and male (unfilled squares) mice (top panel), with comparable decreases in response rates shown in the bottom panel. In contrast, SR144528 (3 mg/kg) did not alter %THC-aperture responding for 1 mg/kg 11-OH-THC in female (inverted unfilled triangles) or male (unfilled triangles) mice (top panel), with changes in response rates shown in the bottom panel. Control tests with vehicle (V) and 5.6 mg/kg THC (T) are shown at the left side of the panels. Each point represents the mean (± SEM) of data for 8 mice, except for %THC aperture responding for 3 mg/kg 11-OH-THC (females, n=4) and 1 mg/kg 11-OH-THC + rimonabant (females, n=7). In each case, the n was reduced because data for %THC aperture responding were excluded due to fewer than 10 overall responses. Dollar sign ($) indicates a significant main effect for sex across all doses (p<0.05) for the specific dose-effect curve. At sign (@) indicates a significant main effect of the antagonist across both sexes (p<0.05) compared to the same dose without the antagonist.
3.1.4. THC drug discrimination in rats
Average sessions to successful acquisition of the THC discrimination did not differ between the sexes, with male (n=8) and female (n=8) rats requiring 26.1 (± 3.75) and 17.9 (± 2.78) sessions, respectively (p>0.05). Further, THC dose-dependently substituted for the 1.7 mg/kg training dose in both sexes (Figure 5, top panel). ED50s were 0.85 mg/kg (95% CI: 0.63–1.14) for males and 0.30 mg/kg (95% CI: 0.13–0.72) for females. Non-overlapping 95% confidence intervals suggest that THC was more potent (2.8-fold) in female than in male rats. Analysis of response rate data revealed that 3 mg/kg THC significantly decreased overall responding across both sexes, as compared with vehicle [dose main effect: F(5,70)=22.86, p<0.05; Figure 5, bottom panel].
Figure 5.
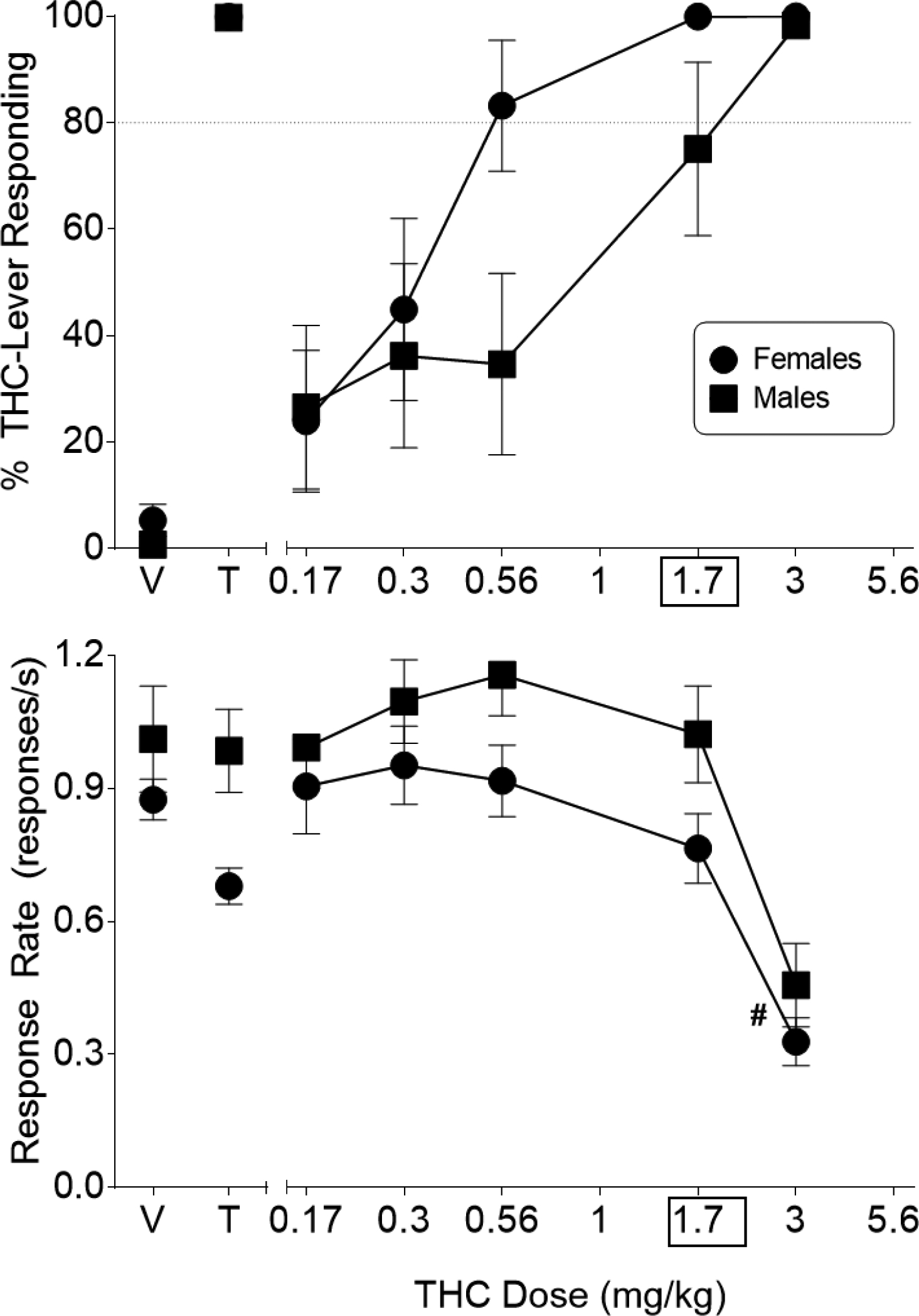
Effects of THC on percentage of responses that occurred on the THC-associated lever (top panel) and response rate (bottom panel) in female (filled circles) and male (filled squares) Sprague-Dawley rats trained to discriminate 1.7 mg/kg THC from vehicle in a lever procedure. Control tests with vehicle (V) and 1.7 mg/kg THC (T) are shown at the left side of the panels. Each point represents the mean (± SEM) of data for 8 rats, except for %THC aperture responding for 3 mg/kg THC (males, n=7). Data for %THC aperture responding at 3 mg/kg THC for 1 male rat were excluded due to fewer than 10 overall responses. Pound sign (#) indicates a significant main effect for dose across both sexes (p<0.05) compared to vehicle (at left side of each panel).
4.0. Discussion
Given the increasingly widespread use of cannabis edibles therapeutically and their continued abuse by both females and males in the U.S., identifying how sex influences cannabinoid pharmacology is imperative. One of the first steps in achieving this goal is to choose the most appropriate animal model. To date, most studies that have examined sex differences in the behavioral effects of cannabinoids have been conducted in rats (Cooper and Craft, 2018; Craft et al., 2013b), with findings suggesting that female rats are more sensitive to several cannabinoid effects, including antinociceptive (Craft et al., 2013a; Tseng and Craft, 2001), discriminative stimulus (Wiley et al., 2017), and reinforcing effects (Fattore et al., 2007). Notably, female rats required a lower THC training dose than males for optimal acquisition and maintenance of stimulus control in THC discrimination procedures (Wiley et al., 2017; Winsauer et al., 2012). The present results replicate these previous reports showing that THC is also more potent at producing discriminative stimulus effects in female Sprague-Dawley rats than in their male conspecifics when rats of each sex were trained to discriminate an identical THC dose (1.7 mg/kg). In contrast, THC potency to produce discriminative stimulus effects was similar in C57BL/6J mice of both sexes trained to discriminate 5.6 mg/kg THC from vehicle (present study) and in mice trained to discriminate a higher (30 mg/kg) dose of THC (Wiley et al., 2011). Together, these results suggest a species X sex interaction in potency for THC-like discriminative stimulus effects with i.p. administration.
Because this study represents one of the few to examine sex differences in THC-like discriminative stimulus effects in mice, our first goal was to provide further characterization of these effects in female mice compared to male mice, as has already been done in rats (Wiley et al., 2017). In mice of both sexes, the dose-effect curve for THC’s discriminative stimulus effects was shifted to the right by the CB1 receptor antagonist rimonabant, but not by the CB2 receptor antagonist SR144528, suggesting CB1 receptor mediation of these effects. Previous studies have also demonstrated CB1 receptor mediation of THC’s discriminative stimulus effects in male rats (Wiley et al., 1995) and nonhuman primates (Hruba et al., 2012; Wiley et al., 1995). Further, 11-OH-THC, THC’s primary psychoactive metabolite, dose-dependently substituted for THC in mice of both sexes, as has also been shown in male mice (Watanabe et al., 1990), rats (Browne and Weissman, 1981), pigeons (Jarbe and McMillan, 1980), and in men (Lemberger et al., 1973; Perez-Reyes et al., 1972). Sex differences in potencies in 11-OH-THC substitution for THC were not apparent in the present study. In addition, neither THC or 11-OH-THC showed differential potency across sex for producing hypothermia or catalepsy in ICR mice (present study). In THC discrimination in mice, the THC-like effects of 1 mg/kg 11-OH-THC were reversed by pre-injection with rimonabant, but not with SR144528, suggesting that these effects were also CB1 receptor mediated. These results complement previous studies in male rodents showing that psychoactive cannabinoids based upon several chemical templates (e.g., tetrahydrocannabinols, bicyclic, indole-derived, aminoalkylindoles, and anandamide analogs) produce THC-like discriminative stimulus effects (reviewed in Wiley et al., 2018). Interestingly, several synthetic cannabinoids have been reported to be more potent at producing THC-like discriminative stimulus effects in male rats (Wiley et al., 2017) and mice (Wiley et al., 2019) than in females, albeit interpretation of these results in rats was complicated because of different THC training doses.
In the present study, mice were maintained in the THC discrimination procedure for 15–19 months, a significant part of their natural lifespan. Over this time, they showed remarkable consistency in maintaining stimulus control for THC. Dose-effect curves for THC substitution for the 5.6 mg/kg THC training dose completed shortly after acquisition and at the end of the study showed substantial overlap for both male and female mice. These results suggest stability of THC’s discriminative stimulus effects over time, as has also been reported for ethanol in nonhuman primates (Helms and Grant, 2011); however, whether mice became tolerant to THC’s discriminative stimulus effects is not clear. Because training and concomitant THC administration were ongoing processes over the entire time span, mice could have been developing tolerance, but also learning to discriminate a functionally lower dose of THC (e.g., see Wiley et al., 1993).
Although the potency of THC’s discriminative stimulus effects in mice did not differ across sex or at different ages, the overall response rate for female mice was significantly lower than for males during the initial THC dose-effect curve. However, further examination of the data revealed that this difference most likely resulted from a difference in baseline rates of responding, as female mice exhibited lower response rates when they were injected with vehicle or with THC or 11-OH-THC. Over time, response rates became more similar across sex, as rates increased for females and decreased for males (see Figure 2). Further research will be necessary to parse out sex differences in baseline behavior from those related to THC’s pharmacological effects, as investigation of mechanisms underlying these sex differences in baseline responding was beyond the scope of this study.
To initiate the process of determination of possible mechanisms underlying the observed species X sex interaction in THC’s discriminative stimulus effects, we conducted a preliminary examination of sex differences in binding and activation of CB1 cannabinoid receptors across species. Results failed to show any sex or species differences in CB1 receptor affinities for THC or 11-OH-THC in the cerebella of drug naïve rats and mice. In both species and sexes, 11-OHTHC showed slightly greater affinity for the receptor than THC. Similarly, potency and efficacy of CB1 receptor activation did not significantly differ between sex in either species; however, there was a trend for less 11-OH-THC potency for activation in female rats compared to their male counterparts, an effect that, if replicated, would be opposite of what would be expected if greater metabolism to 11-OH-THC in female rats contributed to enhanced THC potency in vivo (see Narimatsu et al., 1991). The current results are consistent with previous work that has shown a lack of sex differences in CB1 receptor densities and G-protein coupling in the cerebella of adult Long-Evans and Sprague-Dawley rats (Burston et al., 2010; Farquhar et al., 2018). Investigation of cross-species sex differences in brain regions more relevant to cannabinoid abuse related effects (e.g., mesolimbic areas) may have yielded different results. While several investigations of regional CB1 receptor binding and function in adult rat brain have failed to find widespread sex differences (Burston et al., 2010; Castelli et al., 2014; Farquhar et al., 2018), others have reported differences in isolated areas such as hippocampus or prefrontal cortex as well as fluctuation across the estrus cycle in females (Castelli et al., 2014; Rodriguez de Fonseca et al., 1994; Silva et al., 2015). To date, however, research in this area remains sparse, with many procedural differences across studies complicating generalization of data from rats and lack of comparable data from mice. Hence, it is not possible to rule out regional species or sex differences in CB1 receptor density or function as contributory factors in the observed species X sex interaction effect in THC discrimination.
Another potential contribution to sex differences in THC’s effects in rats that has been identified previously is differential metabolism to 11-OH-THC. Whereas THC biotransformation in male rats includes formation of 11-OH-THC and a variety of largely inactive hydroxylated metabolites, females preferentially metabolize THC to 11-OH-THC (Narimatsu et al., 1991), resulting in elevated brain concentrations of 11-OH-THC in females compared to males despite comparable brain THC concentrations (Wiley and Burston, 2014). Because 11-OH-THC produces THC-like psychoactivity, females (vs males) may experience prolonged and more intense cannabimimetic effects from comparable doses of THC. Consistent with this idea, Tseng and Craft (2001) showed that 11-OH-THC was about twice as potent in female than male rats and a later study showed that inhibition of CYP-mediated THC metabolism attenuated THC’s behavioral effects in females (Tseng et al., 2004), which may contribute to observed sex differences in rats. While early studies showed that 11-OH-THC is also a major metabolite of THC in mice, these studies included only male mice (Borys and Karler, 1979; Harvey and Brown, 1991); hence, it is as yet unknown whether mice lack the sex difference in 11-OH-THC production that has been observed in rats. A further limitation is that the observed results may only apply to routes of administration that involve first-pass metabolism (oral, i.p.). Additional investigation of sex differences with other routes of administration (e.g., inhalation) is still needed.
In summary, the most notable finding of the present study is demonstration of a species X sex interaction in THC’s discriminative stimulus effects. Whereas THC shows greater potency for producing discriminative stimulus effects in female rats than males trained at the same dose, mice do not exhibit a sex difference in this behavior, even though other aspects of THC’s discriminative stimulus effects in mice resemble those reported previously in rats. For example, male and female mice readily acquire THC discrimination, with dose-effect curves indicating equipotency across sex and rightward shifts when the CB1 antagonist rimonabant, but not the CB2 antagonist SR144528, is pre-administered. Remarkable consistency across age was also seen. Preliminary investigation did not reveal cross-sex receptor differences in receptor densities or function that could account for the observed species X sex interaction, although only one brain region (i.e., cerebellum) was assayed here and other cross-species research in abuse relevant brain areas remains scant. While previous research showed that preferential metabolism of THC to its psychoactive metabolite 11-OH-THC occurred in female rats as compared to males, comparable research has not yet been completed in mice. Hence, a species X sex interaction in THC metabolism remains a viable mechanism that may underlie the major finding reported here. The present study also demonstrated that 11-OH-THC is THC-like in mice of both sexes and is more potent than THC. Increasing emphasis on sex differences in substance abuse has been spurred by the U.S. federal mandate to consider sex as a biological variable in NIH-funded research. In this milieu, characterization of sex differences across species is also important and will provide an empirical basis for choice of models with which to investigate cannabis abuse and medicinal use, with the ultimate goals of discovery of its underlying mechanisms and development of targeted treatment approaches.
Highlights.
Choice of animal model is important in study of THC-containing edibles.
We compared the effects of THC and 11-OH-THC in mice and rats of both sexes.
11-OH-THC had marginally higher affinity than THC for cerebellar CB1 receptors.
Sex differences in THC-like in vivo effects occurred in rats, but not in mice.
Results support consideration of sex and species in choice of animal model.
Acknowledgements
Research was supported by U.S. National Institutes of Health / National Institute on Drug Abuse [grants DA-045003, DA-003672]. NIDA had no further role in the writing of the manuscript or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None of the authors have any conflicts of interest with respect to the research described in this manuscript. The funding sources provided funding for the research, but did not otherwise influence the conduct of the experiments, writing on the manuscript, or decision to submit.
Ethical Statement
This manuscript and the data it contains are original, are not under current consideration for publication in any other journal, and have not previously been published. The studies reported in this manuscript were carried out in accordance with National Institutes of Health guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available.
References
- Borodovsky JT, Lee DC, Crosier BS, Gabrielli JL, Sargent JD, Budney AJ, 2017. U.S. cannabis legalization and use of vaping and edible products among youth. Drug Alcohol Depend 177, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borys HK, Karler R, 1979. Cannabidiol and delta-9-tetrahydrocannabinol metabolism: In vitro comparison of mouse and rat liver crude microsome preparations. Biochem Pharmacol 28, 1553–1559. [DOI] [PubMed] [Google Scholar]
- Browne RG, Weissman A, 1981. Discriminative stimulus properties of Δ9-THC : mechanistic studies. J Clin Pharmacol 21, 227s–234s. [DOI] [PubMed] [Google Scholar]
- Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ, 2010. Regional enhancement of CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure. Br J Pharmacol 161, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carliner H, Mauro PM, Brown QL, Shmulewitz D, Rahim-Juwel R, Sarvet AL, Wall MM, Martins SS, Carliner G, Hasin DS, 2017. The widening gender gap in marijuana use prevalence in the U.S. during a period of economic change, 2002–2014. Drug Alcohol Depend 170, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli MP, Fadda P, Casu A, Spano MS, Casti A, Fratta W, Fattore L, 2014. Male and female rats differ in brain cannabinoid CB1 receptor density and function and in behavioural traits predisposing to drug addiction: effect of ovarian hormones. Curr Pharm Des 20, 2100–2113. [DOI] [PubMed] [Google Scholar]
- Clayton JA, Collins FS, 2014. Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Craft RM, 2018. Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology 43, 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Kandasamy R, Davis SM, 2013a. Sex differences in anti-allodynic, anti-hyperalgesic and anti-edema effects of Delta(9)-tetrahydrocannabinol in the rat. Pain 154, 1709–1717. [DOI] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, Wiley JL, 2013b. Sex differences in cannabinoid pharmacology: A reflection of differences in the endocannabinoid system? Life Sci 92, 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daublain P, Feng KI, Altman MD, Martin I, Mukherjee S, Nofsinger R, Northrup AB, Tschirret-Guth R, Cartwright M, McGregor C, 2017. Analyzing the potential root causes of variability of pharmacokinetics in preclinical species. Mol Pharm 14, 1634–1645. [DOI] [PubMed] [Google Scholar]
- Farquhar CE, Breivogel CS, Gamage TF, Gay EA, Thomas BF, Craft RM, Wiley JL, 2018. Sex, THC, and hormones: Effects on density and sensitivity of CB1 cannabinoid receptors in rats. Drug Alcohol Depend 194, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W, 2007. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol 152, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage TF, Farquhar CE, Lefever TW, Marusich JA, Kevin RC, McGregor IS, Wiley JL, Thomas BF, 2018. Molecular and behavioral pharmacological characterization of abused synthetic cannabinoids MMB- and MDMB-FUBINACA, MN-18, NNEI, CUMYL-PICA, and 5-Fluoro-CUMYL-PICA. J Pharmacol Exp Ther 365, 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage TF, Farquhar CE, Lefever TW, Thomas BF, Nguyen T, Zhang Y, Wiley JL, 2017. The great divide: Separation between in vitro and in vivo effects of PSNCBAM-based CB1 receptor allosteric modulators. Neuropharmacology 125, 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F, 2003. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42, 327–360. [DOI] [PubMed] [Google Scholar]
- Han BH, Palamar JJ, 2018. Marijuana use by middle-aged and older adults in the United States, 2015–2016. Drug Alcohol Depend 191, 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BH, Palamar JJ, 2020. Trends in Cannabis Use Among Older Adults in the United States, 2015–2018. JAMA Intern Med 180, 609–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DJ, Brown NK, 1991. Comparative in vitro metabolism of the cannabinoids. Pharmacol Biochem Behav 40, 533–540. [DOI] [PubMed] [Google Scholar]
- Helms CM, Grant KA, 2011. The effect of age on the discriminative stimulus effects of ethanol and its GABA(A) receptor mediation in cynomolgus monkeys. Psychopharmacology (Berl) 216, 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruba L, Ginsburg B, McMahon LR, 2012. Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by {Delta}9-tetrahydrocannabinol treatment in rhesus monkeys. J Pharmacol Exp Ther 342, 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, 2007. Human cannabinoid pharmacokinetics. Chem Biodivers 4, 1770–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, McMillan DE, 1980. delta 9-THC as a discriminative stimulus in rats and pigeons: generalization to THC metabolites and SP-111. Psychopharmacology (Berl) 71, 281–289. [DOI] [PubMed] [Google Scholar]
- Lemberger L, Martz R, Rodda B, Forney R, Rowe H, 1973. Comparative pharmacology of Delta9-tetrahydrocannabinol and its metabolite, 11-OH-Delta9-tetrahydrocannabinol. J Clin Invest 52, 2411–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas G, Brindle SD, Greengard P, 1971. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther 178, 562–564. [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ, 1991. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav 40, 471–478. [DOI] [PubMed] [Google Scholar]
- Mazur A, Lichti CF, Prather PL, Zielinska AK, Bratton SM, Gallus-Zawada A, Finel M, Miller GP, Radominska-Pandya A, Moran JH, 2009. Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab Dispos 37, 1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H, 1991. Sex differences in the oxidative metabolism of delta-9-tetrahydrocannabinol in the rat. Biochem Pharmacol 41, 1187–1194. [DOI] [PubMed] [Google Scholar]
- National Research Council, 2011. Guide for the Care and Use of Laboratory Animals. National Academies Press (US), Washington, D.C. [Google Scholar]
- Perez-Reyes M, Timmons MC, Lipton MA, Davis KH, Wall ME, 1972. Intravenous injection in man of 9 -tetrahydrocannabinol and 11-OH- 9 -tetrahydrocannabinol. Science 177, 633–635. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ, 1994. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci 54, 159–170. [DOI] [PubMed] [Google Scholar]
- Schauer GL, Njai R, Grant-Lenzy AM, 2020. Modes of marijuana use - smoking, vaping, eating, and dabbing: Results from the 2016 BRFSS in 12 States. Drug Alcohol Depend 209, 107900. [DOI] [PubMed] [Google Scholar]
- Schou J, Prockop LD, Dahlstrom G, Rohde C, 1977. Penetration of Δ9-tetrahydrocannabinol and 11-OH-Δ9-tetrahydrocannabinol through the blood-brain barrier. Acta Pharmacol Toxicol 41, 33–38. [DOI] [PubMed] [Google Scholar]
- Silva L, Harte-Hargrove L, Izenwasser S, Frank A, Wade D, Dow-Edwards D, 2015. Sex-specific alterations in hippocampal cannabinoid 1 receptor expression following adolescent delta-9-tetrahydrocannabinol treatment in the rat. Neurosci Lett 602, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart R, Pacula RL, 2019. Early evidence of the impact of cannabis legalization on cannabis use, cannabis use disorder, and the use of other substances: Findings from state policy evaluations. Am J Drug Alcohol Abuse 45, 644–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AH, Craft RM, 2001. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol 430, 41–47. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Harding JW, Craft RM, 2004. Pharmacokinetic factors in sex differences in Delta 9-tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res 154, 77–83. [DOI] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL, 2009. Discriminative stimulus properties of Delta9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol 615, 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MK, Boberg JR, Walsh MT, Wolf V, Trujillo A, Duke MS, Palme R, Felton LA, 2012. A less stressful alternative to oral gavage for pharmacological and toxicological studies in mice. Toxicol Appl Pharmacol 260, 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Kijima T, Narimatsu S, Nishikami J, Yamamoto I, Yoshimura H, 1990. Comparison of pharmacological effects of tetrahydrocannabinols and their 11-hydroxy metabolites in mice. Chem Pharm Bull (Tokyo) 38, 2317–2319. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Yamamoto I, Oguri K, Yoshimura H, 1980. Comparison in mice of pharmacological effects of delta 8-tetrahydrocannabinol and its metabolites oxidized at 11-position. Eur J Pharmacol 63, 1–6. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Balster RL, Martin BR, 1993. Tolerance to the discriminative stimulus effects of Δ9-tetrahydrocannabinol. Behav Pharmacol 4, 581–585. [PubMed] [Google Scholar]
- Wiley JL, Burston JJ, 2014. Sex differences in Delta(9)-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci Lett 576, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Glass M, Thomas BF, 2019. Do you feel it now? Route of administration and Delta(9)-tetrahydrocannabinol-like discriminative stimulus effects of synthetic cannabinoids in mice. Neurotoxicology 73, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Marusich JA, Craft RM, 2017. Comparison of the discriminative stimulus and response rate effects of Delta9-tetrahydrocannabinol and synthetic cannabinoids in female and male rats. Drug Alcohol Depend 172, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR, 1995. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther 275, 1–6. [PubMed] [Google Scholar]
- Wiley JL, Owens RA, Lichtman AH, 2018. Discriminative stimulus properties of phytocannabinoids, endocannabinoids, and synthetic cannabinoids. Curr Top Behav Neurosci 39, 153–173. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Walentiny DM, Vann RE, Baskfield CY, 2011. Dissimilar cannabinoid substitution patterns in mice trained to discriminate Delta(9)-tetrahydrocannabinol or methanandamide from vehicle. Behav Pharmacol 22, 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Filipeanu CM, Bailey EM, Hulst JL, Sutton JL, 2012. Ovarian hormones and chronic administration during adolescence modify the discriminative stimulus effects of delta-9-tetrahydrocannabinol (Delta(9)-THC) in adult female rats. Pharmacol Biochem Behav 102, 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto I, Narimatsu S, Shimonishi T, Watanabe K, Yoshimura H, 1984. Difference in epoxides formation and their further metabolism between delta 9- and delta 8-tetrahydrocannabinols by human liver microsomes. J Pharmacobiodyn 7, 254–262. [DOI] [PubMed] [Google Scholar]


