Abstract
Background:
The beneficial links between engagement in methadone maintenance therapy (MMT) and HIV treatment outcomes have been extensively described. Nevertheless, people who use drugs (PWUD) continue to experience suboptimal HIV treatment outcomes. In this systematic review, we sought to identify components of MMT service provision that are associated with improvements in HIV care outcomes across the HIV care cascade.
Methods:
We searched for peer-reviewed studies in online databases. To be eligible for inclusion in this review, studies must have involved a population or sub-population of PWUD engaged in MMT; report improved uptake of HIV testing, exposure to ART, or HIV-1 RNA plasma viral load suppression; provide details on MMT services; and be published in English between 1 January 2006 until 31 December 2018.
Results:
Out of the 5594 identified records, 22 studies were eligible for this systematic review. Components of MMT services associated with HIV care cascade outcomes described in the studies were classified in three categories of care models: 1) standard MMT care with adequate doses, 2) standard MMT care and alongside additional medical component(s), and 3) standard MMT care, additional medical component(s) as well as informational or instrumental social support.
Conclusion:
The few studies identified reflect a scarcity of evidence on the role of social support to increase the benefits of MMT for PWUD who are living with HIV. Further research is needed to assess the role of medical and social service components in MMT care delivery in advancing PWUD along the HIV care cascade.
Keywords: HIV, Methadone, Antiretroviral Therapy, Adherence, People Who Use Drugs
1. Introduction
The HIV continuum of care (or “care cascade”) denotes key steps in the progression of HIV treatment, including HIV testing and diagnosis, engagement and retention in clinical monitoring, prescription of antiretroviral therapy (ART), and achievement of optimal ART adherence and undetectable HIV-1 RNA plasma viral load (VL; Gardner et al., 2011; Centers for Disease and Control Prevention, 2014; Eaton et al., 2014; Lourenco et al., 2014). In 2014, the Joint United Nations Programme on HIV/AIDS (UNAIDS) announced three sequentially linked targets in relation to the HIV continuum of care to accelerate the end the AIDS epidemic by 2030. The goals, known as the 90-90-90 targets, aim to have 90% of all PLWH to know their HIV status, treat 90% of all people with diagnosed HIV infection with ART, and achieve virologic suppression in 90% of all people receiving the treatment (UNAIDS, 2014). Unfortunately, people who use illicit drugs (PWUD) living with HIV continue to experience suboptimal HIV care cascade engagement (Degenhardt et al., 2014; Nosyk et al., 2014; Risher et al., 2015).
There is a growing recognition of the importance of integrating health services, such as substance use disorder (SUD) treatment, with HIV care to form a comprehensive care program for PWUD who are living with HIV (Volkow and Montaner, 2011). Methadone maintenance treatment (MMT), a common treatment modality for individuals with opioid use disorder, is in many settings the first-line pharmacotherapy, and was added as one of the World Health Organizations (WHO) essential medicines in 2005 (Mattick et al., 2009). A 2017 systematic review has reported that MMT was more commonly used throughout the world, with MMT reported available in 81 countries while other opioid agonist therapies (OAT) such as buprenorphine in 56 countries (Larney et al., 2017). The benefits of MMT are not limited to reductions in the frequency of illicit opioid use and high-risk injection practices (Karki et al., 2016), but also extend to supporting the engagement of PWUD in HIV care (Spire et al., 2007; Uhlmann et al., 2010; Nosyk et al., 2015). This outcome may be improved through creating connections to institutional care networks, by decreasing the requirements of drug-seeking activity that may interfere with engagement in care, or incorporating case management within MMT services to coordinate care for vulnerable populations in accessing relevant services.
Across global health systems, MMT programs are delivered by a broad range of service providers. Treatment initiation and dose stabilization are generally conducted by addiction specialists, psychiatrists, or trained physicians in primary healthcare settings (Fiellin et al., 2001, Carrieri et al., 2014). In some settings, the dispensation of medication has extended to tertiary health facilities or pharmacies in the community (Fonseca et al., 2018). Given the numerous platforms in which MMT is delivered, guidelines and program components vary considerably, with some restricted to clinical care while others incorporate social services seeking to support optimal health outcomes. For example, MMT services in some settings have included the integrated management of other co-infections such as chronic hepatitis C treatment (Litwin et al., 2009) and tuberculosis treatment (Morozova et al., 2013), and differentiated MMT-HIV care in community-based organizations through outreach (Guise et a., 2019). Despite the well-known benefits of MMT for HIV care, HIV care outcomes among PWUD remain suboptimal, even in patients receiving MMT (Shrestha et al., 2019). Therefore, there is an urgent need to assess MMT service delivery components that help optimize HIV care outcomes and identify gaps in existing MMT services.
This systematic review aims to identify components of MMT service delivery that are associated with improved HIV-related outcomes among PWUD. These three main outcomes correspond to the 90-90-90 targets: 1) receipt of HIV testing, 2) exposure to ART and 3) HIV VL suppression (UNAIDS, 2014). In recent years, systematic reviews on HIV outcomes among PWUD have focused on quantifying the impact of opioid agonist therapy on adherence to ART and viral load suppression (Low et al., 2016), behavioral, psychosocial and medication-assisted interventions to promote ART adherence (Binford et al., 2012), and SUD treatment and HIV integrated models (Haldane et al., 2017). This systematic review shares the common goal of assessing the outcomes of integrated care for PWUD. However, it expands the scope of previous reviews to focus on the components of service delivery in the context of MMT across a wider set of HIV treatment outcomes. This review aims to provide a summary of the scientific evidence of best practices with regards to MMT service delivery and HIV care, and may assist decision-making among policy makers, and service providers on the provision of MMT among PWUD who are living with HIV.
2. Materials and Methods
This systematic review was registered in PROSPERO (Registration Number: CRD42018096727).
2.1. Definition of service delivery
Based on the WHO recommendations for multidisciplinary approaches to address PWUD’s needs for concurrent clinical and social services (WHO, 2016), a classification of MMT services that encompasses four general categories of care models was developed to inform this review: 1) standard MMT care, 2) standard MMT care and additional medical component(s) (e.g. HIV testing or treatment, treatment of other viral infections, mental health services) 3) standard MMT care and social support and 4) standard MMT care, additional medical component(s) and social support. The rationale for the four models of care classification is the emergence of global attention on integrated services for PWUD who are living with HIV given the complex social and structural barriers that impact effective HIV care outcomes (Volkow and Montaner, 2011; Kuchinad et al., 2016). We further delineated dimensions of social support delivered by service providers as instrumental or informational support (House, 1987; Heaney and Israel, 2008). Instrumental support was conceptualized as the various types of tangible aid and services such as provision of transportation, meals, money, housing or residence assistance (George et al., 2009). Informational support included providing individuals with advice, ideas, suggestions, guidance and information on topics including life skills training and educational programs not exclusive to health.
2.2. Inclusion/exclusion criteria
To be eligible for inclusion, studies must have assessed a population or sub-population of PWUD engaged in MMT care. Illicit drug use was defined as using substances including illicit opioids (e.g., heroin), cocaine/crack cocaine, or amphetamines through injection or non-injection use. While the focus on MMT service delivery would lend itself to a specific focus on people who use opioids, because polysubstance use is highly prevalent among PWUD (Degenhardt and Hall, 2012), studies that incorporated non-opioid substance use were also considered for inclusion.
Additionally, to be included, studies must have assessed the association of MMT services with at least one of the following outcomes: (1) HIV testing, including the proportion of participants who accepted HIV testing and received results, or the proportion of participants who were newly diagnosed with HIV, (2) Exposure to ART, including time to ART initiation, the proportion of participants who initiated or received ART, the proportion of participants who achieved optimal ART adherence, or increased ART adherence rates, and (3) HIV RNA-1 plasma VL suppression, such as the proportion of participants who achieved VL suppression. Only peer-reviewed studies that were randomized controlled trials, clinical trials, cross-sectional, case-control or cohort studies published between 1 January 2006 until 31 December 2018, to capture the time directly after methadone was added to the WHO list of essential medicine, and written in English were considered for inclusion.
2.3. Search strategy and data extraction
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009). An electronic search protocol to systematically identify potentially relevant studies was carried out on MEDLINE, EMBASE, CINAHL, EBM reviews, PsychInfo and Web of Science. Multiple search terms using both MeSH terms and keywords (See Appendix S1 for MEDLINE search strategy) were deployed to reflect the key concepts and themes of interest; 1) drug use (e.g., “heroin”, “cocaine”, “crack cocaine”, “amphetamines” or “substance abuse”, “intravenous”) and HIV (e.g., “HIV”, “HIV infections”, “AIDS serodiagnosis” or “HIV seropositivity”), 2) HIV testing (e.g., “HIV testing”, “HIV screening”, “rapid test”, “voluntary counselling and testing”), 3) exposure to ART (e.g., “antiretroviral therapy”, “highly active”, “initiation”, “uptake”, “receive”, “adherence” or “compliance”), 4) VL suppression (e.g., “viral load”, “virologic response”, “immunologic success”, “undetectable”, or “suppression”), 5) MMT (e.g., “methadone”, “opioid replacement” or “opioid substitution”), 6) provision of medical support (e.g., “comprehensive health care” or “delivery of health care” or “health services accessibility”) and (7) social support (e.g., “community-based”, “non-profit”, “non-government”, “educational program”, “housing assistance”, “life-skill training”, “peer education” or “peer support”).
Relevant articles were extracted from the above data sources using EndNote software as a citation management tool. Titles and abstracts of these articles were screened independently by two reviewers. Next, the full text of short-listed articles was reviewed based on the screening criteria. Disagreements about study eligibility were resolved through consultation with senior team members until consensus was reached. Additionally, article study authors were consulted when eligibility could not be determined or to confirm which service components were assessed. During the data extraction process, a standardized data collection form was used to extract the following information: first author’s name, publication year, country, study design, study setting and recruitment, types of drugs use, cascade stage(s) examined, outcomes assessed, and MMT service delivery components associated with the outcomes of interest.
2.4. Risk of bias assessment
Risk of bias was assessed using the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) for selected non-randomized studies (Sterne et al., 2016), and the Cochrane Collaboration’s tool for randomized controlled trials (RCT; Higgins and Green, 2011).
3. Results
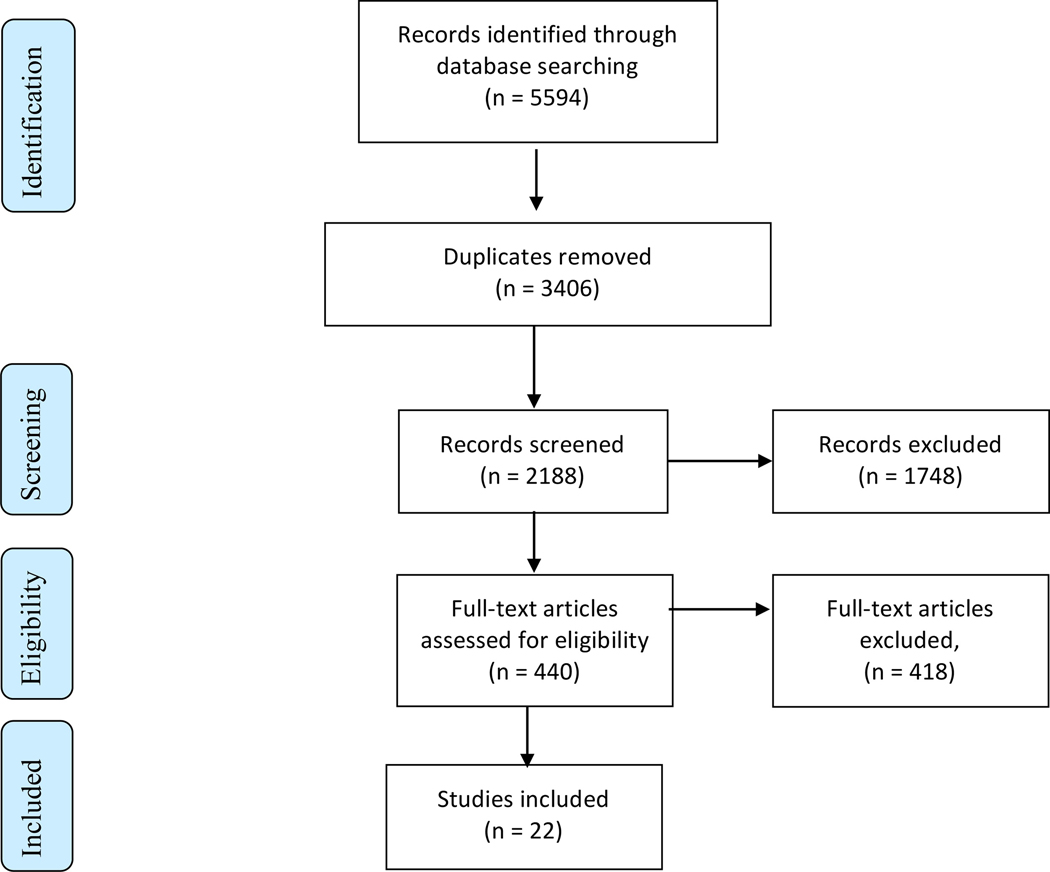
The initial database search yielded 5594 records (Figure 1) with 2188 records retained after duplicates were removed. Manuscripts were screened by title and abstract, removing 1748 records and yielding 440 records that were retrieved as full text. From the assessment of these texts, 418 records were excluded for reasons defined by the study criteria such as no inclusion or analysis of a drug-using population, no exposure to MMT services, failure to assess outcomes of defined interest in this systematic review, or insufficient information to determine the eligibility of those studies. Following full manuscript screening, 22 studies were included for the qualitative data synthesis.
Fig. 1.
PRISMA flow diagram for process of selecting included studies.
3.1. Study characteristics
Characteristics of the 22 selected studies are summarized in Table 1. Studies are organized according to the classification of MMT services operationalized earlier that encompassed the four general categories of care. Of these, twelve studies were conducted in North America (nine in United States and three in Canada), three studies were conducted in Europe (one study each from Spain, the Netherlands and Ukraine) and five in Asia (two studies each from Vietnam and China, and one from Indonesia). One multisite study incorporated data from three countries (Ukraine, Vietnam and Indonesia). In most studies, data on MMT services were drawn from clinical settings, such as specialized MMT clinics and hospitals. A number of studies drew data from participants recruited in community-based settings. There were eight cohort studies, seven cross-sectional studies, five RCT and one study with a pre-post design. Study-specific risk of bias scores are presented in Appendices S2 and S3. Overall, the risk of bias in non-randomized studies was assessed as moderate whereas the risk of bias in the randomized controlled studies was assessed as low.
Table 1:
Characteristics of 22 studies on MMT service delivery and improvements in HIV care cascade outcomes
| Study (Year) | Country | Design (Period) | Treatment Setting | Sample Population (%) | Report on types of drugs or drug use route (%) | Non-male (%) | Ethnicity | Exposure to MMT services (%) |
|---|---|---|---|---|---|---|---|---|
| Lappalainen et al., 2015 | Canada | Longitudinal cohort (2005 – 2013) | Following PWUD with access to HIV and addiction care | PWUD, 297 (100.0) | Crack smoking, 297 (100.0); <daily cocaine injection 263 (88.6); | 116 (39.1) | Caucasian, 175 (58.9) | 297 (100.0) |
| Pang et al., 2007 | China | Repeated cross-sectional surveys (2004 – 2005) | 8 MMT clinics | PWUD: 3,546 (100.0) | Heroin use, 3,546 (100.0) | 134 (22.9) a | Han, 515 (88.0) a | 585 (100.0) a |
| Tran et al., 2016 | Vietnam | Cross-sectional (2013) | 4 health centers incorporating MMT | PWUD: 1,016 (100.0) | Injection drug use 746 (73.4) | Not reported | Not reported | 1016 (100.0) |
| Xia et al., 2013 | China | Cross-sectional (2008) | 45 MMT clinics | PWUD: 13,270 (100.0); | Heroin (99.6) | 807 (6.1) | Not reported | 13,270 (100.0) |
| Seewald et al., 2013 | USA | Cross-sectional (2007 – 2009); two-year period | Hospital-based MMT clinic | PWUD: Phase 1; 7,875 (100.0), Phase 2; 7,870 (100.0). | Opioid-dependence | 4,418 (28.1) | Black, 4,496 (28.6) Hispanic, 7,732 (49.1) White, 3,992 (2.4) | 15,745 (100.0) |
| Hung et al., 2016 | Viet Nam | Pre-post study design (2013 – 2014) | Community-based centers with HIV care and MMT | PWUD: PreIntegration: 3,618 (100.0), PostIntegration: 3,903 (100.0) d | Heroin Amphetamine Morphine Benzodiazine | Not reported | Not reported | 3,903 (100.0) b |
| Safren et al., 2012 | USA | RCT (2005 – 2008) | MMT and HIV clinics | PWUD, 89 (100.0) with depression | Cocaine, 20 (22.5); Opiate, 11(12.7) | 35 (39.3) | African-American, 26 (33.0) | 89 (100.0) |
| Lambers et al., 2011 | The Netherlands | Longitudinal cohort | Following PWUD with access to HIV and addiction care | PWUD, 102 (100.0), with 733 number of visits | Heroin, cocaine or a mix of both (92.2); | 31 (30.4) | Not reported | 91 (89.2) |
| Sanchez et al., 2012 | Spain | Cohort (2005 – 2009) | Outpatient SUD treatment facility | PWUD: 71 (100.0) | Heroin (87.3); Cocaine (12.7); | 30 (42.3) | White, 69 (97.2) | 71 (100.0) |
| Fingerhood et al., 2006 | USA | Retrospective cohort study | Primary care setting in a hospital | PWUD: 175 (100.0); Employed(17.0); Homeless (15.0) | Heroin (94.0); Cocaine (91.0) | 85 (48.6) | African-American, 131 (74.9); White/Other, 44 (25.1) | 63 (36.0) |
| Berg et al., 2011 | USA | 24-week RCT (2004 – 2007) | 12 MMT clinics | PWUD: 77 (100%) | Opioid, 24 (31.0), Cocaine, 42 (55.0) | 36 (46.7) | Hispanic, 35 (45.0) | 77 (100.0) |
| Lucas et al., 2006 | USA | 18-month observational study (2006) | 3 MMT clinics | PWUD: 401 (45.0) Non-PWUD: 490 (55.0) | Injection drug use, 401 (45.0) | 310 (34.8) | African-American, 702 (78.8) | 157 (17.6) |
| Achmad et al. 2009 | Indonesia | Cohort study (2006 –2009) | Hospital-based MMT clinic | PWUD: 223 (100.0) | Opioid-dependence | 14 (6.3) | Not reported | 223 (100.0) |
| Rothman et al., 2007 | USA | Cohort (1990 – 2002) | 12 substance use treatment facilities (7 MMT facilities) | PWID 2,271 (23.0) c | Injection drug use | 3,571 (36.2) c | Black, 3263 (33.0); Hispanic, 4072 (41.2) c | >527 (55.7) c |
| Bachireddy et al., 2014 | Ukraine | Cross-sectional (2010) | Integrated and fully, co-located (ICL); non-co-located (NCL); and harm reduction and outreach (HRO) sites | PWUD: 296 (100.0); Unemployed (46.0) | Opioids | 292 (34.0) | Not reported | 201 (67.9) |
| Cooperman et al., 2012 | USA | RCT | 12 MMT clinics with on-site HIV care | PWUD: 60 (100.0); Unemployed (97.0); | Opioid-dependence | 30 (50.0) | White, 7 (12.0) | 60 (100.0) |
| Parashar et al., 2011 | Canada | Cross sectional study (2007 – 2010) | Community health clinic | PLWH 644 (100.0), Unstably housed 212 (32.9) | Current illicit drug use 162 (76.4) d | 68 (32.1) d | Aboriginal, 87 (41.0) d | 42 (19.8) d |
| Barker et al., 2018 | Canada | Longitudinal cohort study | Community health clinic | PWUD: 354 (100.0) | Injection drug use in the past six months; 256 (72.3) | 175 (49.4) | Indigenous, 354 (100.0) | 35 (9.9) e |
| Sorensen et al., 2007 | USA | RCT (2001 – 2004) | 2 MMT clinics | PWUD, 66 (100.0); Employed (9.0); | Opiates, 25 (38.0); Cocaine, 34 (52.0) | 27 (41.0) | Caucasian, 24 (36.4); | 66 (100.0) |
| Simeone et al., 2017 | USA | Cross-sectional | 3 different clinics; MMT, HIV and community clinic | PWUD: 65 (100.0) | Opioid-dependence | 24 (36.9) | African-American, 27 (41.5); Caucasian, 26 (40.0) | 57 (87.7) |
| Ti et al., 2017 | Canada | Longitudinal cohort study | HIV/AIDS non governmental, service organization | PWUD: 746 (100.0); Homeless, 222 (29.8) | Heroin, 109 (14.6); Cocaine, 61 (8.2); Crack, 258 (34.6) | 246 (33.0) | Indigenous, 302 (40.5) | 269 (36.1) |
| Miller et al., 2018 | Ukraine, Vietnam and Indonesia | Multi-site RCT | Community-based center (Ukraine), health centers (Vietnam) and hospital (Indonesia) | PWID: 502 (100.0) | Active injection drug use (100.0) | 75 (15.0) | Not reported | 502 (100.0) |
Study characteristic were drawn at baseline (the first wave of survey, n = 585), year 2004
Reporting study sample from facilities providing 3-services (HIV testing and counselling, MMT and HIV outpatient care) only, do not account for the whole study participants
Proportions of people who inject drugs (PWID) was reported in this sample of people with SUD. Study characteristics were drawn during the year 2002 and do not account for the whole study participants between study period 1990 – 2002.
Among those who were unstably housed
Number of participants reported enrolled in a medically-assisted therapy (MAT) program, inclusive of a MMT component, at baseline
3.2. Study outcomes
Each study documented service components in conjunction with MMT provision and their association with one or more HIV care cascade outcomes. Among all 22 selected studies, four studies reported more than one HIV care cascade outcome. Seven studies reported HIV testing outcomes; thirteen studies reported exposure to HIV treatment, including five other studies that evaluated initiation or receipt of ART, and eight studies that examined adherence to ART. Eight studies reported on HIV VL suppression. ART adherence levels in the included studies were predominantly assessed using pill count and/or medication event monitoring systems (MEMS), an electronic adherence monitoring system placed on medication bottles that records a presumptive dose each time the cap is opened (Arnsten et al., 2001). One study adopted measurement of adherence self-reports from participants in addition to MEMS caps and pill count but found an overestimation of adherence levels in self-reports compared to other methods.
3.3. MMT service delivery components associated with HIV cascade of care outcomes
Models of care that were described in these studies were: 1) standard MMT care, 2) standard MMT care and additional medical components, 3) standard MMT care, an additional medical component(s) and social support. None of the studies reported on services that consisted of MMT care and social support with no additional medical component. All service components were delivered in the context of MMT in facility-based settings, either in an HIV facility where HIV and SUD treatment services were combined, or in SUD treatment centers where HIV care was incorporated. A summary of results assessing components of MMT and attendant services and their relationship with cascade outcomes are summarized in Table 2. The following sub-sections describe findings in relation the HIV care cascade outcomes of HIV testing, exposure to ART and/or HIV RNA-1 plasma VL suppression, and are organized according to MMT models of care.
Table 2:
Components of MMT service delivery associated with HIV testing, exposure to ART and VL suppression in 22 studies
| Study (Year) | MMT service delivery | HIV care cascade outcomes | Other relevant analyses | ||
|---|---|---|---|---|---|
| Number of participants tested for HIV (%) | Number of participants who initiate, receive, or achieve ART adherence, (%), and/or adherence rates (%) | Number of participants with VL suppression (%) | |||
| 1) Standard MMT care | |||||
| Lappalainen et al., 2015 | Optimal methadone dose > 100mg/day | More than 50% participants receiving methadone dose ≥150mg/day achieved optimal ≥ 95% ART adherence | Dose of ≥100mg/day positively associated with optimal ART adherence (AOR =1.38; 95% CI=1.08–1.77) | ||
| 2) Standard MMT care and additional medical component(s) | |||||
| Pang et al., 2007 | Provision of voluntary HIV Counselling and Testing (VCT) one month after entry into the MMT program | 3069 (86.5) | |||
| Tran et al., 2016 | Integration of voluntary, VCT service in MMT clinics | 957 (94.2) | MMT service model (with VCT vs without VCT) associated with improved HIV testing (r = 0.6, p-value <0.05 95% CI: 0.1 – 1.1) | ||
| Xia et al., 2013 | Implementation of voluntary HIV and Hepatitis C (HCV) testing | 10,046 (75.7) | A significant correlation between HIV test uptake and HCV test uptake (correlation coefficient = 0.64, p-value < 0.001) | ||
| Seewald et al., 2013 | A routine, HIV rapid testing was offered on admission to MMT during medical care or at the mandatory annual physical examinations, as opposed to targeted testing in which patients identified by substance abuse counselors as being high risk were offered HIV pre- and post-test counselling and testing, as well as incentives | 7870 (34.3) | More patients were tested for HIV in routine testing compared with targeted testing (p-value < 0.0001, OR: 3.2: 95% CI: 2.9–3.4) | ||
| Hung et al., 2016 | Services counselling for all three VCT, ART and MMT by the same counsellor who underwent crosstraining, under the same administrative structure | PreIntegration: 3,614 (99.9), PostIntegration: 3,896 (99.8) | 266 (68.9%) initiated ART after service integration, as compared 311 (44.4%) before service integration, p-value < 0.05. | ||
| Safren et al., 2012 | Cognitive Behavioral Therapy (CBT) for treatment of depression, medication adherence counselling and adherence tools (n= 44), as opposed to comparison group with medication adherence counseling and adherence tools, but no CBT | Increase in adherence rate, measured using MEMS-caps was significantly greater over time among those who received CBT (γ- slope = 0.8873, p-value= 0.02) | |||
| Lambers et al., 2011 | Complete use of harm reduction (HR), defined as injecting drugs, high MMT dosage (≥80 mg/day) and full NSEP; or not injecting drugs and high MMT dosage (number of visits: 452, 61.7%), as opposed to participants with no/incomplete dependence on HR (number of visits: 81, 11.1%) | 51 (7.5%) number of visits reporting non-adherence among participants with complete use of HR, as compared to 6 (11.4%) with no/incomplete dependence | |||
| Sanchez et al., 2012 | Infectious disease specialist monitored ART, TB and HCV, with monthly provision of ART; nurses dispensed MMT and identify drug interaction and adherence problems; provide education on adherence; perform directly administered treatment to homeless participants; psychiatrists evaluated mental health and prescribed psychotropic medications such as antidepressants. | 62 (87.3%) achieved VL suppression (50 copies/ml), in comparison to a comparator arm consisted of a control group of sexually transmitted HIV-infected participants in which 42 (87.5%) achieved VL suppression | |||
| Fingerhood et al., 2006 | Multidisciplinary team consisting of general internists, a nurse practitioner and a nurse case manager/educator. Provision of short-term counseling and treatment of depression and a weekly HIV patient support group. | 80 (45.7%) received ART | 52 (61.0) who received ART achieved undetectable VL, after 5 years from initial visit | ||
| Berg et al., 2011 | Directly administered antiretroviral therapy (DAART) with methadone doses and counselling (n = 39), as opposed to comparison group, treatment as usual (TAU) with self-administered medication | Adherence rate, measured using pill count or MEMS-caps, was 86% in the DAART group, as compared to 56% in the TAU, p-value < 0.0001 | 54 (70%) in DAART achieved undetectable VL (<75 copies/ml), as compared to 32 (42%) in TAU groups | At week 24, the odds of having undetectable VL were 3-fold greater for DAART than TAU participants (p-value < 0.02, 95% CI: 1.1–5.4) | |
| Lucas et al., 2006 | DAART with methadone doses (n = 82); as opposed to self-administered ART in comparison groups: 1) PWID-MMT (n = 75), 2) PWID-Non-MMT (n = 244) and 3) Non-PIUD (n = 490). | 46 (56%) DAART participants achieved VL suppression (<400 copies/ml), as compared to 24 (32%) PWID-MMT, 81 (33%) PWID- Non-MMT and 216 (44%) non-PWID groups | In adjusted analyses, DAART participants were significantly more likely to achieve VL suppression than participants in each of the 3 cohort comparison groups | ||
| Achmad et al., 2009 | VCT offered consistently throughout enrollment in MMT, in addition to on-site HIV care, CD4 cell measurement; weekly provision of ART | 95 (42.6) | 16 (72.8%) out of 22 HIV-positive individuals with indication for ART (CD4 count less than 200 cell/mm3) initiated ART | 34 (97.1%) patients out of 35 HIV positive individuals who received ART at the clinic achieved VL suppression (< 400 copies/mL) | |
| 3) Standard MMT care, an additional medical component(s) and social support | |||||
| Rothman et al., 2007 | Co-location of HIV prevention services in substance use treatment program. Service components include VCT, risk reduction education, group and individual supportive counseling, and linkage with hospitals for subspecialty services, community-based organizations for social services and local government agencies responsible for health insurance and welfare programs | Among individuals with SUD, 52,562 (31.2%) tests were conducted on PWIDs out of 168,340 HIV tests, between 1990 – 2002 | In 2002, 527/946 (55.7%) HIV-positive PWUD were from MMT treatment settings. | ||
| Bachireddy et al., 2014 | In integrated and co-located (ICL) services, on-site daily observed opioid agonist therapy (OAT), free screening and treatment services for HIV and TB, psychosocial counseling were provided, (n = 97), as opposed to comparison groups: 1) non-co-located (NCL) services with only OAT and psychosocial counseling (n = 104), and 2) harm reduction only (HRO) with needle- syringe exchange (NSEP but no OAT), case management, referral to ancillary services (including HIV and TB), and psychosocial counseling, (n=95) | 48 (49.5%) participants in the ICL group received ART, as compared to 20 (19.2%) participants in the NCL group and 25 (26.3%) participants in the HRO group, p-value <0.001 | |||
| Cooperman et al., 2012 | DAART with 15-month period adherence counselling to identify unaddressed mental health, substance abuse, financial, vocational, and housing issues (n =22), as opposed to comparison group, DAART with no counseling (n=38) | Adherence rate, measured using MEMS- caps and pill count, was 61% in participants with adherence counselling, as compared to 78% in participants with no counselling, p-value < 0.05 | Among participants who received adherence counseling, each additional hour of counseling was associated with a 20% increase in adherence rate | ||
| Parashar et al., 2011 | Enrolment in a maximally assisted therapy (MAT) program which included provision of MMT, HIV care and other social support, including daily meals, arrangement of transportation to specialist visits, assistance in securing stable housing, medication delivery by outreach workers | Among unstably housed participants (n = 212), 42 (19.8%) enrolled in MAT and of these, 32 (76.2%) participants enrolled in MAT achieved optimal ≥ 95% ART adherence | Unstably housed participants enrolled in MAT were significantly associated with optimal ≥ 95% ART adherence than those who did not (AOR = 4.76, 95% CI 1.7213.13) | ||
| Barker et al., 2018 | Enrolment in a maximally assisted therapy (MAT) program which included provision of MMT and ART, adherence support by pharmacist, as well as social support, including meals, arrangement of transportation to specialist visits, assistance in securing stable housing, medication delivery by outreach workers. | In adjusted analysis, engagement in MAT was positively associated with increased odds of achieving 95% adherence to ART, after adjusting for potential confounders (AOR = 4.92, 95% CI: 3.18 – 7.62, p-value <0.001) | |||
| Sorensen et al., 2007 | Adherence coaching and voucher reinforcement for opening electronic medication (MEMS) caps on time, as opposed to comparison group with adherence coaching only. Participants could earn up to $1172.40 in vouchers if all medication doses are taken as scheduled through the 12-week period. | Adherence rate, measured using MEMS- caps, was 77.6% among participants who received vouchers and 55.5% in the comparison group, p-value < 0.0001 | Adherence rate, measured by pill count, in voucher and comparison groups was 85.9% vs. 75.4%. Adherence rate, measured by self-reports, in voucher and comparison groups was 87.3% vs. 68.7% | ||
| Simeone et al., 2017 | On-site opt-out HIV screening, DAART, integrated HIV primary care and psychiatric services, social HIV case management at a MMT clinic (n = 15, 23.1%), as opposed to comparison groups, 1) HIV specialty clinic (n = 42, 64.6%) and 2) community clinic (n = 8, 12.3%) | 14 (93.3%) participants from MMT clinic achieved VL suppression (<40 copies/ml), as compared to 33 (78.6%) in HIV specialty clinic and 5 (62.5%) in community clinic, p-value: 0.164 | |||
| Ti et al., 2017 | Enrolment in a HIV/AIDS non-government service organization which provides support including nursing care residence, enhanced supported housing program, therapeutic and harm reduction service, counseling and food. | At baseline, 83 (51.6%) participants who were enrolled in a HIV/AIDS service organization achieved VL suppression (<50 copies/mL), as compared to 206 (35.2%) who were not enrolled | The odds of VL suppression in those who were enrolled in a HIV/AIDS service organization was higher compared to those who did not (AOR = 1.51, 95% CI:1.13–2.02, p-value <0.001) | ||
| Miller et al., 2018 | Participants in the intervention group received the standard harm- reduction package and the following interventions: a) systems navigation to facilitate engagement in HIV care and MAT, and costs of any required laboratory testing and transportation; b) psychosocial counselling to facilitate initiation of ART and MAT, counselling to address ART adherence and improve adherence communication skills with health providers; depression; social support; encourage the presence of a support person (ie. family members) to counselling sessions (n = 126). Participants in the comparison group received a standard of care, harm reduction package, including referrals to HIV and MAT (use of MMT), HIV VCT, diagnosis of other infectious diseases (n = 376) | At week 52, 80/111 (72%) in the intervention group received ART, as compared to 140/329 (43%) in the standard of care group (PR = 1.7, 95% CI: 1.4 – 1.9) | At week 52, 45/111 (41%) achieved VL suppression (<50 copies/mL), as compared to 80/328 (24%) in the standard of care group (PR = 1.7, 95% CI: 1.3 – 2.2) | ||
AOR: Adjusted Odds Ratio; PR: Probability Ratio; 95% CI: 95% Confidence Interval
Abbreviations
ART: antiretroviral therapy, CBT: cognitive behavioral therapy, DAART: directly administered antiretroviral therapy, HCV: Hepatitis C, MEMS-cap: medication event monitoring system, MAT: maximally assisted therapy, MMT: methadone maintenance therapy, TB: tuberculosis, OAT: opioid agonist therapy, VCT: voluntary HIV counselling and testing, VL: viral load
3.3.1. Standard MMT care
Standard MMT care was reported in one study included in the review, documenting the potential role of MMT care with adequate dosing in improving HIV care cascade outcome among participants who were recruited from the community (Lappalainen et al., 2015). In British Columbia, Canada, Lappalainen et al. found that more than 50% of participants receiving daily methadone doses of more than 150 mg achieved optimal ART adherence in a longitudinal study. They also observed a positive association between provision of higher daily methadone doses (i.e., more than 100mg) with achieving ≥95% adherence, with a dose-dependent relationship between higher methadone doses and likelihood of optimal ART adherence (Lappalainen et al., 2015).
3.3.2. Standard MMT care and additional medical components
Among the twelve studies documenting standard MMT care and additional medical care components, HIV testing outcomes were reported in six studies, HIV treatment exposure was reported in six studies and HIV VL suppression was reported in five studies with three studies reporting more than one HIV care cascade outcome.
Four studies documented MMT provision with routine HIV testing, including the implementation of voluntary HIV counselling and testing (VCT) as standard upon MMT initiation and throughout enrollment period (Pang et al., 2007; Seewald et al., 2013; Xia et al., 2013; Tran et al. 2016). Pang et al. found that in response to the high rate of HIV transmission among PWUD in China, the establishment of MMT clinics in southwest China incorporated HIV testing in which VCT was offered after one month enrollment in the MMT program (Pang et al., 2007). This led to 3069 (86.5%) PWUD being tested for HIV, with a resulting seroprevalence rate of 11.4% (Pang et al., 2007). Similarly, Tran et al. found that MMT care models integrating VCT services in Vietnam were associated with improved HIV testing uptake compared to care models that did not integrate the same service, with 957 (94.2%) PWUD undergoing HIV testing (Tran et al., 2016). In a different province in China, Xia et al. reported an MMT program offering free, voluntary, routine HIV testing bundled with hepatitis C (HCV) screening in which 10,046 (75.7) participants were tested for HIV, with a significant correlation between HIV and HCV testing uptake (Xia et al., 2013). Furthermore, one study in MMT clinics in New York by Seewald et al. found a 20% increase in the proportion of participants tested for HIV in routine universal testing upon admission in an MMT program or at the mandatory annual physical examinations for MMT patients, compared to a previous approach of targeted HIV rapid testing whereby counsellors identified individuals at a high risk for HIV transmission (Seewald et al., 2013).
In one study, additional medical components in MMT care were associated with ART initiation (Hung et al., 2016). In Vietnam, Hung et al. evaluated the effect of integrated services in a community-based center which incorporated counselling for MMT and VCT and HIV by staff who were cross-trained, in comparison to a period when such services were provided within separate administrative structures (Hung et al., 2016). It was found that there was a 24.5% increase in the proportion of ART-eligible participants who initiated ART after service integration was implemented.
Furthermore, two studies found better adherence to ART linked to additional medical components in conjunction with MMT. Safren et al. evaluated the use of cognitive behavioral therapy to assist with depression and self-care in addition to a single-session intervention on ART adherence among participants with depression (Safren et al., 2012). They found that participants who received cognitive behavioral therapy had significantly higher ART adherence rates, compared to participants receiving only the single session intervention. A study by Lambers et al. explored the potential impacts of exposure to a comprehensive harm reduction strategy in Amsterdam to improve medical conditions associated with problematic drug use by promoting MMT uptake as well as the use of a needle and syringe exchange program (NSEP; Lambers et al., 2011). This longitudinal cohort study has highlighted the potential role of comprehensive harm reduction utilization, consisting of participation in NSEP and MMT with daily dosage of more than 80mg on self-reported ART adherence among a group of PWUD with recent injection and non-injection use (Lambers et al., 2011). Participants who received comprehensive harm reduction care (i.e., both NSEP and MMT with doses more than 80mg) had lower ART non-adherence rates, compared to those who did not use comprehensive harm reduction care.
Additional medical components associated with VL suppression was reported in two studies (Fingerhood et al., 2006; Sanchez et al., 2012). In Spain, Sanchez et al. demonstrated multidisciplinary care in a SUD treatment facility, including the monitoring of co-morbidities (HIV, HCV, tuberculosis and mental health care), prescription of psychotropic medications, monthly on-site ART provision as well as directly administered treatment to homeless participants (Sanchez et al., 2012). Study results showed that VL suppression (i.e., <50 copies/mL plasma) was achieved in 62 (87.3%) PWUD attending this facility, in comparison to a control group of sexually transmitted HIV-infected participants in which 42 (87.5%) achieved VL suppression (p-value = 0.1779). In Baltimore, USA, Fingerhood et al. reported the outcomes of a five-year study among participants receiving HIV care in a primary care setting (Fingerhood et al., 2006). The study included a multidisciplinary team that provided clinical services such as short-term counselling and the treatment of depression follow-up (Fingerhood et al., 2006). In this study, undetectable VL was achieved in 52 (61%) of the participants who received ART (Fingerhood et al., 2006).
Notably, additional medical components were associated with more than one care cascade outcomes in three studies (Lucas et al., 2006; Achmad et al., 2009; Berg et al., 2011). For example, the implementation of a directly administered ART (DAART) approach in conjunction with MMT services improved ART adherence and VL suppression in several studies (Lucas et al., 2006; Berg et al., 2011). In an RCT, Berg et al. reported an adherence rate of 86% among participants who received DAART along with their daily methadone doses compared to a 56% rate among participants who self-administered ART by week 24 of the intervention (Berg et al., 2011). Additionally, in this study, the percentage of participants who achieved VL suppression was approximately 28% higher in the DAART group than in the comparison group, with the odds to achieve undetectable levels (<75 copies/mL) three times greater among those who received DAART (Berg et al., 2011). In an observational study, Lucas et al. have observed a higher proportion of DAART participants who achieved low VL (i.e., <400 copies/ml), compared to participants who self-administered ART in three different subgroups; PWUD enrolled in MMT, PWUD not enrolled in MMT and non-PWUD (Lucas et al., 2006).
Only one study described the potential role of additional medical components that are not exclusive to MMT across all three cascade outcomes (Achmad et al., 2009). In Indonesia, Achmad et al. reported 95 (42.6%) participants were tested for HIV at a hospital-based MMT clinic, where VCT with repeated counselling was provided throughout participants’ enrolment in the MMT program. Among them, 72 (75.8%) were identified as HIV-positive (Achmad et al., 2009). In addition to VCT, on-site HIV care, including regular CD4 cell measurement was carried out to examine ART eligibility for those who were diagnosed positive for HIV, and ART medications were provided where indicated (Achmad et al., 2009). Following this, among 22 individuals who presented low CD4 cell counts (i.e., <200 cell/mm3), 16 (72.8%) initiated ART, with 34 (97.1%) patients out of 35 HIV-positive individuals who received ART at the MMT clinic achieved low VL (i.e., < 400 copies/mL; Achmad et al., 2009).
3.3.3. Standard MMT care, an additional medical component(s) and social support
Among the nine studies documenting standard MMT care, an additional medical component(s) and social support, one study reported HIV testing outcomes; six studies reported exposure to HIV treatment and three studies reported HIV VL suppression, with only one of these studies reporting more than one HIV care cascade outcomes.
The integration of social support as an additional component of care to supplement to MMT and other medical services barriers has been observed. In a 12-year study by Rothman et al., the co-location of HIV care in substance use treatment centers, including seven MMT facilities, was linked to improvements in HIV testing uptake among PWUD in New York (Rothman et al., 2007). Medical services described in the study consisted of the provision of VCT, education to reduce risk for HIV acquisition, and on-site primary HIV care with the delivery of ART medication. Social support components included referrals to community-based organizations that offer social support as well as government agencies that offer welfare assistance (Rothman et al., 2007). Between 1990 and 2002, 168,340 HIV tests were conducted, with 52,562 (31.2%) tests conducted among participants identified as engaging in higher risk injection drug use (Rothman et al., 2007).
The provision of standard MMT care, additional medical components and informational social services was associated with ART initiation in one study. In Ukraine, Bachireddy et al. demonstrated that higher proportions of participants received ART in a single location where multiple services were delivered concurrently—including on-site daily observed opioid agonist therapy (i.e., methadone and buprenorphine), screening and treatment services for HIV and tuberculosis, and psychosocial counselling—compared to two other locations providing addiction or needle-syringe exchange programs but no on-site HIV care (Bachireddy et al., 2014).
The provision of all three services (i.e., standard MMT care, additional medical components, and support services) was linked to higher ART adherence levels in four studies (Sorensen et al., 2007; Parashar, et al., 2011; Cooperman et al., 2012; Barker et al., 2018). The use of counselling to improve adherence levels among PWUD who receive MMT was also investigated by extending Berg et al.’s study on a DAART approach (Berg et al., 2011). Cooperman et al. reported the potential role of adherence counsellors through counselling sessions that identified unaddressed adherence barriers including mental health, SUD, financial, vocational and housing issues, for DAART participants who were enrolled in an MMT program (Cooperman et al., 2012). They found that there was a 20% increase in the ART adherence rate for each additional hour of counselling among those who received this intervention. In British Columbia, Canada, Parashar et al. reported that enrolment in a maximally-assisted therapy (MAT) program in a community health clinic was associated with higher ART adherence levels among participants with unstable housing compared to participants with stable housing (Parashar et al., 2011). This MAT program consisted of medication support, including provision of MMT and HIV medications, adherence support, as well as assistance for transportation needs to access medical care, securing stable housing and referrals to mental health and addiction counselling. Similarly, in the same setting, Barker et al. reported that among HIV-positive Indigenous populations, enrolment in the same MAT program which included the social support identified in the study by Parashar et al. (Parashar et al., 2011), was associated with increased odds of achieving ≥ 95% adherence (Barker et al., 2018). Further, a study by Sorensen et al. in the United States reported on the effectiveness of financial incentives, as a form of tangible social support, to promote ART adherence in a setting where MMT patients received ART on-site (Sorensen et al., 2007). In this RCT, the mean adherence rate was 78% among participants who received a voucher that was exchangeable for goods and services each time a medication cap was opened. This rate was 22% higher than those who did not receive these incentives.
Two studies demonstrated associations between MMT provision models that included additional medical and social support, and VL suppression (Simeone et al., 2017; Ti et al., 2017). Simeon and colleagues reported that 93% of participants achieved VL suppression (i.e., <50 copies/mL) in an urban MMT clinic in San Francisco, United States, that offers on-site opt-out HIV screening, integrated HIV primary care including a DAART approach and psychiatric services, as well as informational support through social case management. This compares to the 79% of participants who achieved VL suppression at an HIV specialty clinic with on-site MMT and ART but no provision of case management, and 62 % of participants who achieved VL suppression at a community clinic, (p-value = 0.164; Simeone et al., 2017). In one study conducted in British Columbia, Ti et al. found that participants enrolled in a non-governmental AIDS service organization had greater odds of achieving VL suppression compared to those who were not enrolled (Ti et al., 2017). The authors noted that these positive results could potentially be attributed to the provision of multidisciplinary services covering a wide range of social and clinical needs to PWUD accessing the organization, including nursing care, enhanced supportive housing, counselling and food.
In a multi-site RCT study in three different countries, Miller et al. reported that the provision of standard MMT care, additional medical components and social support were associated with two care cascade outcomes (Miller et al., 2018). In addition to the standard of care which included a harm reduction package which included referrals to HIV care and MAT (ie. MMT use), VCT and screening for other infectious diseases, participants in the intervention group received two additional components. These included (1) the availability of a system navigator that facilitated engagement, retention and adherence in HIV care and MAT, as well as covering costs of testing for infectious disease and transportation; and (2) psychosocial counselling sessions to address ART adherence, depression and social support, as well as to help improve communication skills with health providers pertaining to adherence to treatment. At week 52, a higher proportion of participants in the intervention group received ART and achieved VL suppression (i.e., <40 copies/mL), compared to participants in the standard of care group.
4. Discussion
In this systematic review, we identified 22 studies assessing the impacts of components of MMT service components on HIV cascade of care-related outcomes among PWUD engaged in MMT care. Taken together, these studies provide evidence that health and social service delivery components were associated with better likelihood of optimal HIV treatment-related outcomes. In each of these studies, at least one of the three key HIV care outcomes of interest linked to the UNAIDS 90-90-90 goals were reported: uptake in HIV testing; exposure to ART, including treatment initiation, receipt or adherence; and HIV-1 RNA VL suppression. From the findings, MMT services demonstrated in the selected studies encompassed three models of care. The first model was composed of standard MMT care with adequate doses. The second model included standard MMT care and an additional medical component(s), such as: on-site voluntary HIV testing and HIV care; clinical management of other concurrent disorders, including viral hepatitis, mental health and tuberculosis; medication dispensing, including DAART; and delivery of ART medication into the community. The third model encompassed standard MMT care, an additional medical component(s) and social support, including ART adherence counselling to address social barriers to effective engagement in HIV care; linkage and referrals to relevant support services; food provision, housing support or transportation assistance.
Improved HIV testing outcomes are potentially explained by the implementation of voluntary VCT within MMT programs that follow the guidelines set by health agencies (Pang et al., 2007; Seewald et al., 2013). In line with WHO recommendations for more widespread screening, the expansion of HIV testing venues to include settings where high-risk populations receive treatment for SUD is a strategic approach to identify PWUD living with HIV. In the included studies, voluntary VCT was offered upon admission to and throughout enrolment in MMT treatment as a standard procedure with a view to increase rates of PWUD being tested for HIV. Additionally, the routine offering of VCT rather than a targeted, mandatory or coercive approach as practiced in many drug treatment settings of studies included in this analysis is an important feature of service delivery as it may represent a strategy to reduce stigma and discrimination associated with HIV and illicit drug use in primary care settings (Bazazi et al., 2017).
The studies in this review suggest that on-site HIV care alongside MMT is linked to better HIV care outcomes among PWUD (Fingerhood et al., 2006; Lucas, et al., 2006, Achmad et al., 2009; Berg et al., 2011; Lambers et al., 2011; Safren et al., 2012; Sanchez et al., 2012; Bachireddy et al., 2014). In countries where there are challenges in following WHO guidelines to initiate ART regardless of CD4 cell count, provision of CD4 cell count monitoring in MMT settings is a promising step to ensure HIV-positive PWUD receive timely treatment, particularly in resource-limited settings (Achmad et al., 2009). Further, on-site medication dispensing through approaches such as DAART represents a key support for ART adherence where ART medication is dispensed concurrently with daily methadone doses. Although studies in this review have demonstrated the effectiveness of DAART alongside MMT in improving adherence rates and reducing VL among participants who received DAART (Lucas et al., 2006; Berg et al., 2011), it is worth noting that the same effects were not sustained after DAART was discontinued (Berg et al., 2011). While this would support calls for the long-term implementation of DAART within MMT settings, it is also important to consider the potential role of a broader range of more sustainable supplementary services to support PWUD in achieving long term medication adherence, such as the delivery of ART in accessible community settings and the incorporation of social support.
Notably, this review describes evidence for the integrated management of co-morbidities in settings where ART is offered alongside MMT to enhance clinical HIV outcomes (Cooperman et al., 2012; Simeone et al., 2017). The presence of HIV-associated comorbidities, such as viral hepatitis, tuberculosis and mental illness among PWUD has an impact on health outcomes, including a heightened risk of HIV disease progression (Dieterich et al., 2016). On-site management of these illnesses helps to reduce barriers to optimal HIV treatment through multidisciplinary teams that support PWUD with co-occurring disorders to successfully move along the continuum of care (Kamarulzaman and Altice, 2015; Simeone et al., 2017). Service users have previously reported that co-located SUD treatment and HIV services have improved accessibility by offering multiple services at a single location and reducing travel time (Egan et al., 2011), whereas for service providers, there is an opportunity to improve monitoring of medication interaction within the same clinical team (Sylla et al., 2007). While primary care settings may be more structured to provide an exhaustive list of medical services compared to settings that focus primarily on MMT provision, studies in the review have shown that the incorporation of multiple services to manage comorbidities in MMT settings is practical (Sanchez et al., 2012; Simeone et al., 2017). Nevertheless, given the lack of understanding on the conclusive impact of multidisciplinary health services within SUD treatment settings on HIV care cascade outcome among PWUD, future research should explore the feasibility and sustainability of such services, especially in MMT platforms.
A key contribution of this review is the consideration of the potential role of social services in supporting clinical substance use and HIV treatment outcomes. These services may reduce the impact of socio-economic marginalization on HIV treatment engagement and retention, especially in contexts where inadequate social services may contribute to health inequities (Rumptz et al., 2007; Wang et al., 2016; Kennedy et al., 2017). For instance, previous studies have shown that unmet subsistence needs, such as housing, transportation and income were linked to lower levels of engagement in HIV care among vulnerable sub-groups of PLWH including PWUD (Rumptz et al., 2007). While MMT programs may be well positioned to enable access to such support, there is a dearth of recent evidence that provides understanding of the provision or use of these services in conjunction with MMT and subsequent HIV care cascade outcomes. For example, in the absence of MMT services, PWUD receiving a family-focused intervention to strengthen medication adherence and facilitate relapse prevention did not show improved ART adherence (Feaster et al., 2010). Although none of the studies in this current systematic review described a model of care that encompassed MMT care and social support without additional medical services, this review did identify a limited number of studies that report services that integrate all three: MMT care, additional medical services and social support (Rothman et al., 2007; Cooperman et al., 2012; Bachireddy et al., 2014; Simeone et al., 2017; Ti et al., 2017).
A small number of these studies outline the potential role of informational support in facilitating PWUD along the HIV care cascade through counselling services to address adherence barriers such as housing or financial issues (Cooperman et al., 2012) and case management (Simeone et al., 2017). The use of psychosocial counselling has been recognized as a useful informational social support not only to address mental and emotional well-being (Dugosh et al., 2016), but also in providing information on resources for housing, legal aid and meal programs for PWUD in non-MMT platforms (Hesse and Pedersen, 2008; George et al., 2009). In our assessment of the current literature, informational support was not commonly described in MMT platforms. However, provision of such services may potentially improve access to instrumental support, as such it links PWUD to specific agencies such as social assistance departments through referral services (Rothman et al., 2007). Thus, informational support is crucial for PWUD to be able to access relevant instrumental social support, such as income assistance, supported or subsidized housing or vocational training to support employment uptake and retention opportunities (Wolitski et al., 2010; Conyers and Boomer, 2014; Nachega etal., 2014).
Additionally, studies in this review have pointed out the relevance of instrumental social support in MMT platforms (Parashar et al., 2011; Ti et al., 2017; Barker et al., 2018; Miller et al., 2018). Specifically, monetary incentives, meal provision, housing assistance or residential services as well as supports that reduce care access barriers, including transportation assistance, were key components of the broader instrumental social supports (Parashar et al., 2011; Ti et al., 2017; Barker et al., 2018; Miller et al., 2018). Such interventions have been successful in providing support services specifically to vulnerable populations, including Indigenous groups and PWUD who experience homelessness (Parashar, Palmer et al. 2011, Barker, Adams et al. 2018). Importantly, this review suggests that social support may potentially address concerns around social and structural-level barriers to engagement in HIV care among PWUD. Given the paucity of data in this area, further research is needed to confirm the role of integrated services in MMT platforms and explore how different forms of support could reinforce each other in delivering comprehensive support services for this population.
Despite the potential of community-based organizations to improve program acceptability among PWUD, the evaluation of HIV care cascade outcomes in community-based facilities integrating all three elements of support assessed in the current review is limited. In this review, only one study evaluated associations between HIV clinical outcomes and enrolment in an HIV/AIDS non-government service organization supporting health and basic life needs including on-site ART and MMT dispensation, provision of meals, and housing assistance (Ti et al., 2017). Further, no gender-specific services were assessed in the review despite consistent reports on gender-related inequities in service access among PWUD, particularly within SUD and HIV treatment environments (Ayon et al,. 2018; Des Jarlais et al., 2018). The lack of strategies to address needs specific to women, such as reproductive health, childcare or domestic violence support may represent barriers to adequately engaged in SUD and HIV care (El-Bassel and Strathdee, 2015). Similarly, none of the studies reported enhanced MMT services for incarcerated PWUD despite the role of MMT in incarceration settings to improve HIV clinical outcomes (Springer et al., 2012). Given previously documented interruptions to ART, MMT and social stability among recently incarcerated PWUD (Freudenberg et al., 2008; Stone et al., 2018), the role of support services that facilitate MMT and ART continuity in the community upon release such as housing assistance may be of particular importance.
This review has several limitations to note. First, since the aim was to systematically describe evidence on health and non-health service delivery that enhance HIV care cascade outcomes in the context of MMT service delivery, we have not reviewed qualitative studies and gray literature, such as policy reports, which may contain descriptions of services delivered in MMT platforms as part of standard operating procedures. Although these studies offer additional understandings of how specific service delivery models facilitate efforts to improve treatment outcomes, for the purpose of this study, qualitative studies and gray literature may be less informative in terms of documenting progress towards the 90-90-90 targets. Second, our operationalization of ART adherence did not require studies to report the optimal 95% adherence level for the achievement of VL suppression. A strong majority of studies demonstrated adherence levels that were below this level (Sorensen et al., 2007; Berg et al., 2011; Cooperman et al., 2012). Using adherence measures below the international standard for optimal adherence may obfuscate conclusions about the potential role of associated services in improving optimal adherence to ART among PWUD. Third, despite using multiple database platforms to search for eligible studies, most studies were from higher income countries. There are very limited studies from non-Western settings that have a substantial number of PWUD, such as in East and South Asia. Therefore, this review may over-represent findings from resourceful countries. Fourth, limited availability of opioid agonist therapy in multiple settings has contributed to the low number of studies identified in the review. For example, the prohibition of opioid agonist therapy in Russia is not only a barrier to SUD care but also disrupts the effective delivery of HIV care for PWUD (Sarang et al., 2013). Further, a recent review has shown that even in regions where opioid agonist therapy is included within national treatment guidelines, the treatment continues to be underutilized, partly due to a complex array of policy-level barriers (Degenhardt et al., 2017). Finally, many studies explored provision of health and social support in general terms, for instance, by referring to “counselling” or “case management” to address social needs that would impede treatment success. Similarly, health services often do not differentiate between different types of health services, such as mental health services. It was not possible to differentiate between the services potentially included under these general terms, therefore, a more standardized reporting of well-defined and separately measured health and social services would better advance scientific understandings in this area.
5. Conclusion
Evidence in this review has identified the potential role of multi-modal strategies to manage health and social comorbidities in supporting optimized HIV clinical outcomes among PWUD across the HIV care cascade. However, a paucity of data evaluating different complementary MMT service models may hinder the implementation of person-centered approaches which seek to address the complex needs of members of this key population. While such approaches are key to ending the HIV pandemic, evidence-based practices that promote models of care that optimize treatment for opioid use are currently underdeveloped. Nonetheless, studies included in this review indicate that there is a growing empirical basis for integrated models to improve HIV care cascade outcomes among PWUD. This systematic review underscores a clear need for further research that assesses the effectiveness of integrated services.
Supplementary Material
Highlights.
We reviewed methadone-related services across HIV care cascade outcomes.
Services include standard care, additional medical components and social support.
Integrated services that optimise methadone and HIV care are scarce.
In particular, social support alongside methadone services remains underexplored.
Acknowledgements (optional).
NA.MS’s postgraduate studies at UBC are sponsored by the University of Malaya and the Malaysian Ministry of Higher Education. Pauline Voon is supported by doctoral scholarships from the Pierre Elliott Trudeau Foundation and the CIHR Vanier Graduate Scholarship. Mohammad Karamouzian is supported by Vanier Canada Graduate Scholarship and Pierre Elliott Trudeau Foundation Doctoral Scholarships. L.R and M-J.M are supported by MSFHR Scholar awards and Canadian Institutes of Health Research (CIHR) New Investigator awards (MSH 217672). L.R is additionally supported by a CIHR Foundation Award (FDN-154320). M-JM is additionally supported by the National Institutes of Health (U01-DA0251525). His institution has received an unstructured gift from NG Biomed Ltd., a private firm seeking a government license to produce medical cannabis, to support him. M-JM is the Canopy Growth professor of cannabis science at the University of British Columbia, a position established by arms’ length gifts from Canopy Growth, a licensed producer of cannabis, and the Government of British Columbia’s Ministry of Mental Health and Addictions. The funders had no role in study design, preparation of the manuscript or decision to publish.
Role of Funding Source
Nothing declared
Footnotes
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Achmad YM, Istiqomah AN, Iskandar S, Wisaksana R, van Crevel R. and Hidayat T, 2009. Integration of methadone maintenance treatment and HIV care for injecting drug users: a cohort study in Bandung, Indonesia. Acta Med. Indones. 41 Suppl 1: 23–27. [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang C-J, Buono D, Eckholdt H, Howard AA and Schoenbaum EE, 2001. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin. Infect. Dis 33(8): 1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayon S, Ndimbii J, Jeneby F, Abdulrahman T, Mlewa O, Wang B, Ragi A. and Mburu G, 2018. Barriers and facilitators of access to HIV, harm reduction and sexual and reproductive health services by women who inject drugs: role of community-based outreach and drop-in centers. AIDS Care. 30(4): 480–487. [DOI] [PubMed] [Google Scholar]
- Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K. and Altice FL, 2014. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend. 134: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K. and Altice FL, 2014. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend. 134: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker B, Adams E, Wood E, Kerr T, DeBeck K, Dong H, Shoveller J, Montaner J. and Milloy M-J, 2018. Engagement in Maximally-Assisted Therapy and Adherence to Antiretroviral Therapy Among a Cohort of Indigenous People Who Use Illicit Drugs. AIDS Behav. 23(5):1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazazi AR, Vijay A, Crawford FW, Heimer R, Kamarulzaman A. and Altice FL, 2017. HIV Testing and awareness of HIV status among people who inject drugs in greater Kuala Lumpur, Malaysia. AIDS Care. 30(1):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Litwin A, Li X, Heo M. and Arnsten JH, 2011. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug Alcohol Depend. 113(2): 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Litwin AH, Li X, Heo M. and Arnsten JH, 2011. Lack of sustained improvement in adherence or viral load following a directly observed antiretroviral therapy intervention. Clin. Infect. Dis 53(9): 936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binford MC, Kahana SY and Altice FL, 2012. A systematic review of antiretroviral adherence interventions for HIV-infected people who use drugs. Curr. HIV/AIDS Rep. 9(4): 287–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri PM, Michel L, Lions C, Cohen J, Vray M, Mora M, Marcellin F, Spire B, Morel A. and Roux P, 2014. Methadone induction in primary care for opioid dependence: a pragmatic randomized trial (ANRS Methaville). PloS One. 9(11): e112328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2014. Understanding the HIV care continuum. Retrieved 10 December 2018 from http://www.cdc.gov/hiv/pdf/dhap_continuum.pdf.
- Conyers L. and Boomer K, 2014. Examining the role of vocational rehabilitation on access to care and public health outcomes for people living with HIV/AIDS. Disabil Rehabil. 36(14): 1203–1210. [DOI] [PubMed] [Google Scholar]
- Cooperman NA, Heo M, Berg KM, Li X, Litwin AH, Nahvi S. and Arnsten JH, 2012. Impact of adherence counseling dose on antiretroviral adherence and HIV viral load among HIV-infected methadone maintained drug users. AIDS Care. 24(7): 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L. and Hall W, 2012. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 379(9810): 55–70. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Mathers BM, Wirtz AL, Wolfe D, Kamarulzaman A, Carrieri MP, Strathdee SA, Malinowska-Sempruch K, Kazatchkine M. and Beyrer C, 2014. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int. J. Drug Policy. 25(1): 53–60. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, McKnight C, Feelemyer J, Arasteh K, Tross S, Campbell AN, Cooper HL and Perlman DC, 2018. Heterosexual male and female disparities in HIV infection at the end of an epidemic: HIV infection among persons who inject drugs in New York City, 2001–2005 and 2011–2015. Drug Alcohol Depend. 185:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich D, Fusco J, Henegar C, D’Amico R, Schulman K, Zelt S. and Lackey P, 2016. HIV/hepatitis C co-infected patients are significantly more complex to manage than HIV mono-infected patients in a large cohort of treatment-naive, HIV-positive individuals. Presented at 2016 International Congress on Drug Therapy in HIV Infection (HIV Glasgow) Retrieved 10 December 2018 from https://www.epividian.com/wp-content/uploads/2017/06/09_HIVGlasgow2016-OPERA-HIV-HCV-Coinfected-Patients-are-Significantly-More-Complex-to-Manage-than-HIV-Mono-infected-Patients.pdf [Google Scholar]
- Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M. and Festinger D, 2016. A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. J Addict. Med 10(2): 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton EF, Saag MS and Mugavero M, 2014. Engagement in human immunodeficiency virus care: linkage, retention, and antiretroviral therapy adherence. Infect. Dis. Clin. North Am 28(3): 355–369. [DOI] [PubMed] [Google Scholar]
- Egan JE, Netherland J, Gass J, Finkelstein R, Weiss L. and Collaborative B, 2011. Patient perspectives on buprenorphine/naloxone treatment in the context of HIV care. J. Acquir. Immune Defic. Syndr 56: S46–S53. [DOI] [PubMed] [Google Scholar]
- El-Bassel N. and Strathdee SA, 2015. Women who use or inject drugs: an action agenda for women-specific, multilevel and combination HIV prevention and research. J. Acquir. Immune Defic. Syndr 69(Suppl 2): S182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaster DJ, Mitrani VB, Burns MJ, et al. , 2010. A randomized controlled trial of Structural Ecosystems Therapy for HIV medication adherence and substance abuse relapse prevention. Drug Alcohol Depend. 111(3):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, O’connor PG, Chawarski M, Pakes JP, Pantalon MV and Schottenfeld RS, 2001. Methadone maintenance in primary care: a randomized controlled trial. JAMA. 286(14): 1724–1731. [DOI] [PubMed] [Google Scholar]
- Fingerhood M, Rastegar DA and Jasinski D, 2006. Five year outcomes of a cohort of HIV-infected injection drug users in a primary care practice. J Addict. Dis 25(2): 33–38. [DOI] [PubMed] [Google Scholar]
- Fonseca J, Chang A. and Chang F, 2018. Perceived Barriers and Facilitators to Providing Methadone Maintenance Treatment Among Rural Community Pharmacists in Southwestern Ontario. J. Rural Health. 34(1): 23–30. [DOI] [PubMed] [Google Scholar]
- Freudenberg N, Daniels J, Crum M, Perkins T. and Richie BE, 2008. Coming home from jail: the social and health consequences of community reentry for women, male adolescents, and their families and communities. Am. J. Public Health. 98(Supplement_1): S191–S202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, Del Rio C. and Burman WJ, 2011. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin. Infect. Dis 52(6): 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Garth B, Wohl AR, Galvan FH, Garland W. and Myers HF, 2009. Sources and types of social support that influence engagement in HIV care among Latinos and African Americans.” J. Health Care Poor Underserved. 20(4): 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise A, Seguin M, Mburu G, McLean S, Grenfell P, Islam Z, Filippovych S, Assan H, Low A, Vickerman P. and Rhodes T, 2017. Integrated opioid substitution therapy and HIV care: a qualitative systematic review and synthesis of client and provider experiences. AIDS Care. 29(9), pp.1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane V, Cervero‐ Liceras F, Chuah FL, Ong SE, Murphy G, Sigfrid L, Watt N, Balabanova D, Hogarth S. and Maimaris W, 2017. Integrating HIV and substance use services: a systematic review. J. Int. AIDS Soc 20(1):21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney CA and Israel BA, 2008. Social networks and social support. Health behavior and health education: Theory, research, and practice. 4: 189–210. [Google Scholar]
- Hesse M. and Pedersen MU, 2008. Easy-access services in low-threshold opiate agonist maintenance. Int. J. Ment. Health Ad 6(3): 316–324. [Google Scholar]
- Higgins JP, & Green S, 2011. Cochrane handbook for systematic reviews of interventions (Vol.4). John Wiley & Sons. [Google Scholar]
- House JS, 1987. Social support and social structure Sociological forum, Springer. [Google Scholar]
- Hung V, Nguyen ST, Tieu VTT, Nguyen TTT, Duong TH, Lyss S. and Oeltmann JE, 2016. Evaluation of the integrated clinic model for HIV/AIDS services in Ho Chi Minh City, Viet Nam, 2013–2014. Public Health Action. 6(4): 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarulzaman A. and Altice FL, 2015. The challenges in managing HIV in people who use drugs. Curr. Opin. Infect. Dis 28(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Shrestha R, Huedo-Medina TB and Copenhaver M, 2016. The impact of methadone maintenance treatment on HIV risk behaviors among high-risk injection drug users: a systematic review. Evid. Based Med Public Health. 2016;2. pii: e1229 [PMC free article] [PubMed] [Google Scholar]
- Kennedy MC, Kerr T, McNeil R, Parashar S, Montaner J, Wood E. and Milloy M-J, 2017. Residential eviction and risk of detectable plasma HIV-1 RNA viral load among HIV-positive people who use drugs. AIDS Behav. 21(3): 678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinad KE, Hutton HE, Monroe AK, Anderson G, Moore RD and Chander G, 2016. A qualitative study of barriers to and facilitators of optimal engagement in care among PLWH and substance use/misuse. BMC Res. Notes. 9(1): 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers FAE, Stolte IG, van den Berg CHSB, Coutinho RA and Prins M, 2011. Harm reduction intensity—Its role in HAART adherence amongst drug users in Amsterdam. Int. J. Drug Policy. 22(3): 210–218. [DOI] [PubMed] [Google Scholar]
- Lappalainen L, Nolan S, Dobrer S, Puscas C, Montaner J, Ahamad K, Dong HR, Kerr T, Wood E. and Milloy MJ, 2015. Dose-response relationship between methadone dose and adherence to antiretroviral therapy among HIV-positive people who use illicit opioids. Addiction. 110(8): 1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larney S, Peacock A, Leung J, Colledge S, Hickman M, Vickerman P, et al. , 2017. Global, regional, and country‐ level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health. 5: e1208–e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin AH, Harris KA Jr, Nahvi S, Zamor PJ, Soloway IJ, Tenore PL, Kaswan D, Gourevitch MN and Arnsten JH, 2009. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 37(1), pp.32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low AJ, Mburu G, Welton NJ, May MT, Davies CF, French C, Turner KM, Looker KJ, Christensen H. and McLean S, 2016. Impact of opioid substitution therapy on antiretroviral therapy outcomes: a systematic review and meta-analysis. Clin. Infect. Dis 63(8): 1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Mullen AB, Weidle PJ, Hader S, McCaul ME and Moore RD, 2006. Directly Administered Antiretroviral Therapy in Methadone Clinics Is Associated with Improved HIV Treatment Outcomes, Compared with Outcomes among Concurrent Comparison Groups. Clin. Infect. Dis 42(11): 1628–1635. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J. and Davoli M, 2009. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst. Rev 2003;(2):CD002209 [DOI] [PubMed] [Google Scholar]
- Miller WC, Hoffman IF, Hanscom BS, Ha TV, Dumchev K, Djoerban Z, Rose SM, Latkin CA, Metzger DS and Lancaster KE, 2018. A scalable, integrated intervention to engage people who inject drugs in HIV care and medication-assisted treatment (HPTN 074): a randomised, controlled phase 3 feasibility and efficacy study. Lancet. 392(10149): 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J. and Altman DG, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med 151(4): 264–269. [DOI] [PubMed] [Google Scholar]
- Morozova O, Dvoryak S. and Altice FL, 2013. Methadone treatment improves tuberculosis treatment among hospitalized opioid dependent patients in Ukraine. Int J Drug Pol. 24(6), pp.e91–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachega JB, Uthman OA, Peltzer K, Richardson LA, Mills EJ, Amekudzi K. and Ouédraogo A, 2014. Association between antiretroviral therapy adherence and employment status: systematic review and meta-analysis. Bull World Health Organ. 93: 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, Min JE, Evans E, Li L, Liu L, Lima VD, Wood E. and Montaner JS, 2015. The effects of opioid substitution treatment and highly active antiretroviral therapy on the cause-specific risk of mortality among HIV-positive people who inject drugs. Clin. Infect. Dis 61(7): 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, Montaner JS, Colley G, Lima VD, Chan K, Heath K, Yip B, Samji H, Gilbert M. and Barrios R, 2014. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect. Dis 14(1): 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L, Hao Y, Mi G, Wang C, Luo W, Rou K, Li J. and Wu Z, 2007. Effectiveness of first eight methadone maintenance treatment clinics in China. AIDS. 21: S103–S107. [DOI] [PubMed] [Google Scholar]
- Parashar S, Palmer AK, O’Brien N, Chan K, Shen A, Coulter S, Montaner JSG and Hogg RS, 2011. Sticking to It: The Effect of Maximally Assisted Therapy on Antiretroviral Treatment Adherence Among Individuals Living with HIV Who are Unstably Housed. AIDS Behav. 15(8): 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher K, Mayer KH and Beyrer C, 2015. HIV treatment cascade in MSM, people who inject drugs, and sex workers. Curr. Opin. HIV AIDS. 10(6): 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J, Rudnick D, Slifer M, Agins B, Heiner K. and Birkhead G, 2007. Co-located substance use treatment and HIV prevention and primary care services, New York State, 1990–2002: a model for effective service delivery to a high-risk population. J. Urban Health. 84(2): 226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumptz MH, Tobias C, Rajabiun S, Bradford J, Cabral H, Young R. and Cunningham WE, 2007. Factors associated with engaging socially marginalized HIV-positive persons in primary care. AIDS Patient Care STDS. 21(S1): S-30-S-39. [DOI] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh CM, Bullis JR, Otto MW, Stein MD and Pollack MH, 2012. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: a randomized controlled trial. J. Consult. Clin. Psychol 80(3): 404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez GV, Llibre JM, Torrens M, Sanvisens A, Mateu G, Knobel H, Langohr K, Santos JR and Muga R, 2012. Effectiveness of antiretroviral therapy in HIV-1-infected active drug users attended in a drug abuse outpatient treatment facility providing a multidisciplinary care strategy. Curr. HIV Res 10(4): 356–363. [DOI] [PubMed] [Google Scholar]
- Seewald R, Bruce RD, Elam R, Tio R, Lorenz S, Friedmann P, Rabin D, Garger YB, Bonilla V Jr. and Perlman DC, 2013. Effectiveness and feasibility study of routine HIV rapid testing in an urban methadone maintenance treatment program. Am. J. Drug Alcohol Abuse. 39(4): 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha R, Altice FL and Copenhaver MM, 2019. HIV-Related Stigma, Motivation to Adhere to Antiretroviral Therapy, and Medication Adherence Among HIV-Positive Methadone-Maintained Patients. J. Acquir. Immune Defic. Syndr 80(2): 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone C, Shapiro B. and Lum PJ, 2017. Integrated HIV care is associated with improved engagement in treatment in an urban methadone clinic. Addict. Sci. Clin. Pract 12(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen JL, Haug NA, Delucchi KL, Gruber V, Kletter E, Batki SL, Tulsky JP, Barnett P. and Hall S, 2007. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial.” Drug Alcohol Depend. 88(1): 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spire B, Lucas GM and Carrieri MP, 2007. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST). Int. J. Drug Policy. 18(4): 262–270. [DOI] [PubMed] [Google Scholar]
- Springer SA, Qiu J, Saber-Tehrani AS and Altice FL, 2012. Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PloS One. 7(5): e38335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT and Boutron I, 2016. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Fraser H, Lim AG, Walker JG, Ward Z, MacGregor L, Trickey A, Abbott S, Strathdee SA and Abramovitz D, 2018. Incarceration history and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. Lancet Infect. Dis 18(12): 1397–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylla L, Bruce RD, Kamarulzaman A. and Altice FL, 2007. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy. 18(4): 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ti L, Dong H, Kerr T, Turje R, Parashar S, Min J, Montaner J, Wood E. and Milloy MJ, 2017. The effect of engagement in an HIV/AIDS integrated health programme on plasma HIV‐ 1 RNA suppression among HIV‐ positive people who use illicit drugs: a marginal structural modelling analysis. HIV Med. 18(8):580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran BX, Nguyen LH, Nguyen LP, Nguyen CT, Phan HTT and Latkin CA, 2016. Methadone maintenance treatment promotes referral and uptake of HIV testing and counselling services amongst drug users and their partners. PloS One. 11(4): e0152804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS, The Joint United Nations Programme on HIV/AIDS, 2014. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; Retrieved 10 December 2018 from https://www.unaids.org/en/resources/909090. [Google Scholar]
- Uhlmann S, Milloy MJ, Kerr T, Zhang R, Guillemi S, Marsh D, Hogg RS, Montaner JS and Wood E, 2010. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 105(5): 907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND and Montaner J, 2011. The urgency of providing comprehensive and integrated treatment for substance abusers with HIV. Health Aff. 30(8): 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Panagiotoglou D, Min JE, DeBeck K, Milloy M, Kerr T, Hayashi K. and Nosyk B, 2016. Inability to access health and social services associated with mental health among people who inject drugs in a Canadian setting. Drug Alcohol Depend. 168: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolitski RJ, Kidder DP, Pals SL, Royal S, Aidala A, Stall R, Holtgrave DR, Harre D, Courtenay-Quirk C. and Housing and Health Study Team, 2010. Randomized Trial of the Effects of Housing Assistance on the Health and Risk Behaviors of Homeless and Unstably Housed People Living with HIV. AIDS Behav. 14(3): 493–503. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2016). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Retrieved from: https://www.who.int/hiv/pub/arv/arv-2016/en/ [PubMed]
- Xia YH, Chen W, Tucker JD, Wang C. and Ling L, 2013. HIV and hepatitis C virus test uptake at methadone clinics in Southern China: opportunities for expanding detection of bloodborne infections. BMC Public Health. 13: 899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.