Abstract
Peroxiredoxin-2 (PRX-2) is known to be released from erythrocytes and induce brain damage after intracerebral hemorrhage (ICH); lipocalin-2 (LCN-2) is involved in neuroinflammation following ICH. This study examined the role of LCN-2 in PRX-2 induced brain injury and involved three parts. In the first part, adult male C57BL/6 wild-type (WT), LCN-2 heterozygous (LCN-2 HET), and LCN-2 knockout (LCN-2 KO) mice received either an intracaudate injection of recombinant PRX-2 or saline. In the second part, adult male C57BL/6 WT and male LCN-2 KO mice received recombinant PRX-2 with either recombinant mouse LCN-2 protein or control. In the third part, adult female C57BL/6 WT, LCN-2HET, and LCN-2 KO mice received recombinant PRX-2. Behavioral tests, and T2- and T2*- weighted magnetic resonance imaging was obtained for all mice. Mice were then euthanized, and their brains used for Western blotting, histology and immunohistochemistry.
Intracerebral PRX-2 injections resulted in increased expression of LCN-2 protein. PRX-2-induced brain swelling, neutrophil infiltration, microglia/macrophage activation, neuronal cell death, and neurological deficits were reduced in male LCN-2 HET and LCN-2 KO mice (P<0.01) compared to WT and were exacerbated by exogenous LCN-2 co-injection. Additionally, intracerebral PRX-2 injections caused brain injury and neurological deficits in female WT mice; effects reduced in female LCN-2 KO mice.
In conclusion, intracerebral injection of PRX-2 upregulates LCN-2, and LCN-2 is crucial in the effects of PRX-2 on neutrophil infiltration and microglia/macrophage activation, and ultimately brain damage.
Keywords: Intracerebral hemorrhage, Peroxiredoxin-2, Lipocalin-2, Mice, brain swelling, microglia, neutrophils
Introduction
Hemolysis in the hematoma occurs as early as the first day after intracerebral hemorrhage (ICH) 1. Lysis of red blood cells (RBCs) is associated with neuronal death, brain edema, and neurological deficits2. Peroxiredoxin-2 (PRX-2) is the third most common protein found in RBCs and it plays a key role as an initiator of inflammation3, 4. Large amounts of PRX-2 are released from RBC lysis after ICH, leading to brain damage. This brain damage was reduced by conoidin A, a specific inhibitor of PRX-25.
Lipocalin 2 (LCN-2) is a protein secreted by brain astrocytes under inflammatory conditions 6. Its expression as an acute phase reactant is increased in response to various nervous system injuries, including ICH- and SAH-induced white matter injury 7–9. Studies show it is a key factor that caused brain injury after stroke 10–14. Our prior studies showed that the expression of LCN-2 and PRX-2 are increased after ICH, however whether there is a link between LCN-2 and PRX-2 and their effects is unclear.
This study examined the role of LCN-2 protein in the neuroinflammation and brain injury induced by intracaudate injection extracellular PRX-2 using LCN-2 KO, LCN-2 heterozygous and wild-type (WT) mice.
Materials and Methods
Animal Preparation and Intracerebral Infusion
The University of Michigan Institutional Animal Care and Use Committee approved the protocols of all animals. The reporting of the in vivo experiments with this study complies with ARRIVE guidelines. Animals were randomized to either an odd or even number. A total of 133 mice ages 2 to 3 months old were obtained for the experiment. A single male wild-type (WT) mouse died at day 7 after intracerebral injection extracellular PRX-2 and was therefore excluded from this study. LCN-2 KO and LCN-2 HET mice came from the U of M Breeding Core, while C57BL/6 WT mice came from Charles River Laboratories (Roanoke, IL).
Mice were anesthetized using ketamine (90 mg/kg)/xylazine (5 mg/kg, i.p.) and their body temperature was maintained at 37.5 °C as described previously 9. The mice were fixed to a stereotaxic frame (Model 500; Kopf Instruments, Tujunga, CA). A drill was used to create a cranial burr hole (1 mm) close to the right coronal suture, 2.5 mm lateral to the midline. A microinfusion pump was used to inject recombinant rat PRX-2 (10 μl, 1 μg/μl, Novus biological, NBP2–52150) with or without recombinant mouse LCN-2 protein (1μg, R&D System) into the right basal ganglia at a speed of 1.5 μl/min using the following coordinates: 0.2 mm anterior, 3.5 mm ventral and 2.5 mm lateral to the bregma. The animals used for the control were injected with 10-μL saline. Co-injections of recombinant mouse LCN-2 (1 μg) in male LCN-2 KO mice (e.g. LCN-2 replacement) were used to further examine the potential role of LCN-2 in PRX-2 induced brain injury. In our previous study, intracaudate injection of 1μg LCN2 into LCN2 knockout mice did not cause marked brain injury but enhanced thrombin-induced brain damage9. The needle was kept in place for 10 minutes to restrict backflow and then withdrawn. Bone wax was used to fill the hole and sutures were used to close the skin incision.
Experimental Groups
This study included three parts. Animals were randomized to either an odd or even number. In the first part, male WT mice (n=12) received an intracerebral injection of 10μl saline, while male LCN-2 KO mice (n=18), LCN-2 HET mice (n=18), and WT mice (n=18) received an intracerebral injection of 10 μl PRX-2. Following MRI and behavioral testing on day 1, 12 saline control mice and 10 mice in each of the LCN-2 KO, LCN-2 HET, and WT PRX-2 groups were euthanized for brain histology and Western blotting. The remaining 8 mice in the LCN-2 KO, LCN-2 HET, and WT PRX-2 groups underwent behavioral testing on days 3, 7, and 14 and had a MRI on the day 14 before being euthanized for brain histology.
In the second part, male LCN-2 KO mice (n=8 each group) received an intracerebral injection of 10 μl PRX-2 with 1 μg recombinant mouse LCN-2 protein or vehicle. Male WT mice (n=8) had only 10 μl PRX-2 with vehicle. Behavioral tests and MRI were performed at day 1 before the mice were euthanized for brain histology.
In the third part, female LCN-2 KO mice (n=14), LCN-2 HET mice (n=14), and WT mice (n=14) received an intracerebral injection of 10 μl PRX-2. Following MRI and behavioral testing on day 1, 6 mice in each of the LCN-2 KO, LCN-2 HET, and WT groups were euthanized for brain histology. The remaining 8 mice in each of the groups received behavioral tests on days 1, 3, 7, and 14. MRI was obtained on day 14. The mice were then euthanized for histology.
MRI and Brain Swelling/Atrophy Measurement
T2- weighted MRIs were performed using a 9.4-T Varian MR scanner (183-mm horizontal bore; Varian, Palo Alto, CA) on days 1 and 14, as described preciously9,15. A blinded investigator measured all MRI images using NIH ImageJ. Brain swelling (ventricular compression) calculations at day 1 were based on every other third section around the needle tract. The value was calculated as follows: [(contralateral ventricular volume−ipsilateral ventricular volume)/contralateral ventricular volume] × 100%. Brain atrophy was measured at day 14 by two methods. 1) Enlargement of the ipsilateral ventricle: [(ipsilateral ventricular volume-contralateral ventricular volume)/contralateral ventricular volume] × 100%; and 2) Hemisphere brain tissue loss: [(contralateral hemisphere – ipsilateral hemisphere)/contralateral hemisphere] x 100%.
Immunohistochemistry and Immunofluorescence Double Staining
Immunostaining was performed as previously described 15, 16. Mice brains were sliced into 18μm sections on a cryostat. For immunohistochemistry, the primary antibodies (Ab) were goat anti-lipocalin 2 (1:200 Dilution, B&D Systems), goat anti-Iba-1(1:200 dilution, abcam), rabbit anti myeloperoxidase IgG (1:200 dilution, Thermo Fisher). Negative controls omitted the primary Ab. We selected three different areas in each region from every other section in high-power images (×40 magnification) for counting (expressed as cells/mm2). All measurements were repeated 3 times using ImageJ by a blinded investigator 1, 17.
For immunofluorescence double staining, the primary Abs were goat anti-lipocalin 2 (1:200 dilution, B&D Systems), polyclonal rabbit anti-glial fibrillary acidic protein (GFAP; astrocyte marker; 1:400 dilution, Millipore, Billerica), rabbit anti myeloperoxidase (MPO; neutrophil marker; 1:200 dilution, Thermo Fisher), rabbit anti-Iba-1 IgG (microglia /macrophage marker; 1:200 dilution, Proteintech), rabbit anti-von Willebrand factor (vWF; endothelial marker; 1:200 dilution, Sigma), polyclonal rabbit anti-neuronal specific nuclear protein (NeuN; 1:500 dilution, Abcam). Secondary Abs were Alexa Fluro 488-conjugated donkey anti-goat mAb (1:500 dilution, Invitrogen), Alexa Fluro 594-conjugated donkey anti-rabbit mAb (1:500 dilution, Invitrogen). Negative controls excluded the primary Ab.
Fluoro-Jade C Staining
Fluoro-Jade C Staining was used to assess neuronal death. Brain sections were stained with Fluoro-Jade C (Millipore, AG325) as previously described 18. Briefly, a digital camera was used to take three high-power images (×40 magnification) around the ipsilateral basal ganglia injection site. All measurements were repeated 3 times using ImageJ by a blinded investigator.
Western Blot Analysis
Western blot analysis was performed as described previously 19. Briefly, mice were perfused with pH 7.4 phosphate-buffered saline (PBS, 0.1mmol/L) after euthanasia. Then samples of the contralateral and ipsilateral basal ganglia tissues were obtained. The concentrations of protein were calculated using a Bio-Rad protein assay kit (Hercules, CA) and 30 μg protein of each sample was used for the Western blot analysis. The primary Abs were polyclonal goat anti-LCN2 (R&D System, 1:200 dilution), and rabbit anti-β-actin (1:20000, dilution, Cell Signaling). The secondary Abs were rabbit anti-goat IgG (Bio-Rad, 1:2000 dilution) and goat anti-rabbit IgG (Bio-Rad, 1:5000 dilution). Western blots were analyzed using the enhanced chemiluminescence system (Amersham) followed by exposure to Kodak X-OMAT film. Analysis of the relative densities of bands was obtained using NIH Image J. LCN-2 protein levels were normalized with beta-actin.
Cell Counting
Cell counting of brain coronal sections was obtained by a blinded investigator, as previously described5. Three brain locations in the ipsilateral and contralateral caudate were observed using high power images (× 40 magnification). Three slides from each brain were obtained; each slide contained three fields of interest were digitized. Mean values were obtained from three repeated measurements.
Behavioral Tests
Forelimb use asymmetry and corner turn tests were performed for behavioral assessment as our previous study elaborated 20. For the forelimb use asymmetry test, we observed the forelimb use of mice in a standing transparent cylinder. We recorded the numbers of times the ipsilateral forelimb (A), the contralateral forelimb (B), and both forelimbs (C) were used by the mice to explore the sides of the cylinder. Forelimb use asymmetry scores were calculated as follows: forelimb use asymmetry score=[A/(A+B+C)]-[B/(A+B+C)]. For the corner turn test, mice was allowed to move forward into a corner whose angle was 30°. The mice could turn either left or right. The percentage of right turns was calculated after repeating the test 10 times. All animals received behavioral tests before surgery and at days 1, 3, 7, and 14 after surgery. Both tests were performed by an investigator blinded to the study.
Statistical Analysis
Results are expressed as mean ± SD. The data was analyzed using Student’s t test, or one-way ANOVA tests using a Tukey post hoc test. Significant levels were set at P < 0.05.
Results
Intracaudate injection of PRX-2 upregulates brain LCN-2 in male WT mice
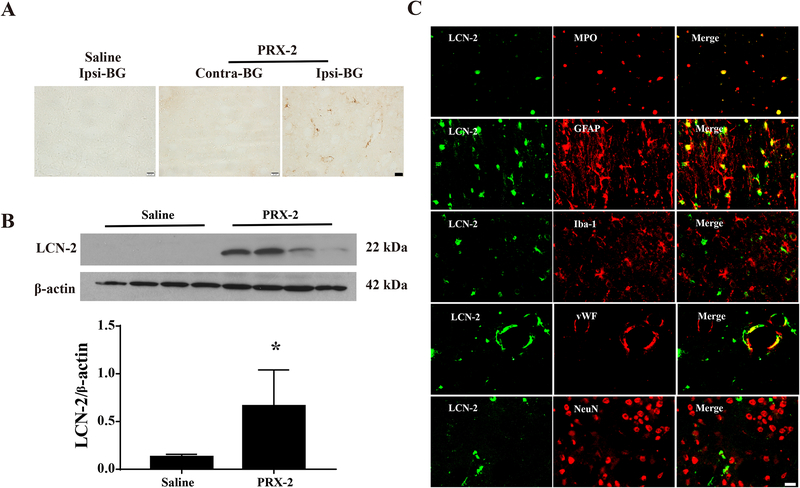
In the current study, we used immunohistochemical staining to show where and which cells expressing LCN-2. We then used Western blots to quantify LCN-2 levels. A large number of LCN-2 positive cells were detected in the ipsilateral basal ganglia at day 1 after PRX-2 injection in male WT mice. In contrast, there were minimal LCN-2 positive cells in the ipsilateral basal ganglia after saline injection (Fig. 1A). LCN-2 protein levels in the ipsilateral basal ganglia were significantly higher after PRX-2 injection than saline injection (ratio of LCN-2/beta-actin: 0.66 ± 0.38 vs. 0.13 ± 0.03, P<0.01; a 5-fold increase, Fig. 1B). LCN-2 positive cells mainly colocalized with MPO- (neutrophil marker), GFAP- (astrocyte marker), and VWF- (endothelial cell marker) positive cells. The main LCN-2-positive cells were neutrophils in the lesion center and astrocytes in the basal ganglia around the injection center. Only a few LCN-2 positive cells were Iba-1 (microglia/macrophage marker) or NeuN (neuronal marker) positive (Fig. 1C).
Figure 1: Intracaudate injection of PRX-2 upregulates brain LCN-2 in male WT mice.
A) LCN-2 immunoreactivity at day 1 in the ipsilateral or contralateral basal ganglia (BG) after injection of PRX-2 or saline. Scale bar=20 μm; B) LCN-2 protein levels in the ipsilateral BG after injection of PRX-2 or saline (Western blot). Values (ratio to β-actin) are means ± SD; n=4 per group, *P<0.05, PRX-2 vs. saline, by the Student t test. C) Double labeling of LCN-2 with either MPO (neutrophil marker), GFAP (astrocyte marker), Iba-1 (microglia/macrophage marker), VWF (endothelial cell marker) or NeuN (neuronal marker) after PRX-2 injection into the ipsilateral BG at day 1. Scale bar=20 μm.
LCN-2 deficiency reduces PRX-2 induced brain swelling and neuronal death in male mice
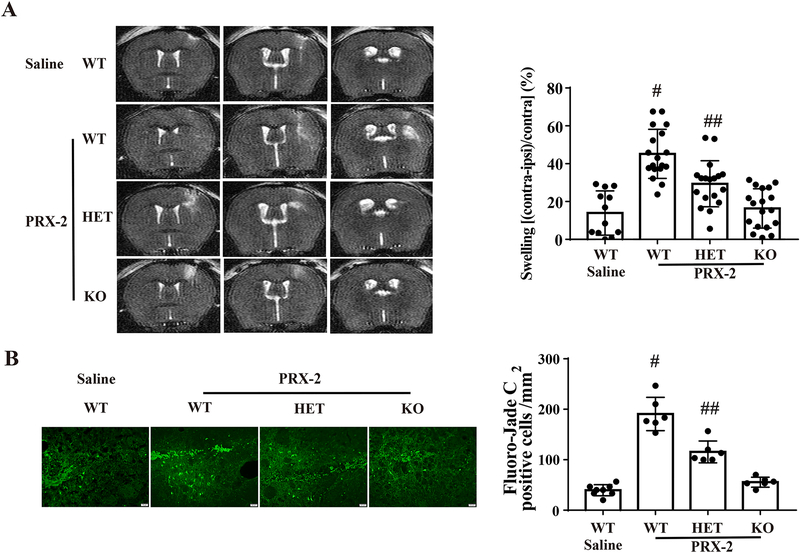
Brain swelling was measured by lateral ventricle compression in T2-weighted MRI coronal sections at day 1 after PRX-2 injection. PRX-2 caused significant ventricular compression in male WT mice compared to the saline control (45.2 ± 12.9 vs. 13.9 ± 11.7% in saline controls, P<0.01; Fig. 2A). However, the ventricular compression induced by PRX-2 was significantly less in LCN-2 HET (29.4 ± 12.2% vs. 45.2 ± 12.9% in WT group, P<0.01; Fig. 2A) and the LCN-2 KO male mice (16.3 ± 10.4% vs. 29.4 ± 12.2% in HET group, P<0.01; Fig. 2A).
Figure 2: Intracaudate injection of PRX-2 results in less brain swelling and neuronal death in male LCN-2 HET and LCN-2 KO mice.
A) Representative T2 MRI at day 1 after an intracerebral injection of PRX-2 or saline in four groups. Brain swelling was calculated as the following: ([contralateral ventricular volume−ipsilateral ventricular volume]/contralateral ventricular volume) × 100%]. Values are recorded as mean ± SD, n=12 in saline treated group and n=18 in PRX-2 treated groups; #P<0.01, vs. the Saline-WT, PRX-2-HET and PRX-2-KO group; ##P<0.01 vs. the Saline-WT group; by one-way ANOVA with Tukey’s post-hoc test.
B) Fluoro-Jade C staining shows degenerative neurons in the ipsilateral BG at day 1 after the injection of PRX-2. Results are recorded as mean ± SD. n=8 in the saline treated group and n=6 in PRX-2 treated groups. #P<0.01, vs. the Saline-WT, PRX-2-HET and PRX-2-KO group; ##P<0.01 vs. the Saline-WT group; by one-way ANOVA with Tukey’s post-hoc test. Scale bar=20 μm.
PRX-2 injection induced more Fluoro-Jade C positive cells in the WT group than in saline controls at day 1 (191 ± 33 vs. 40 ± 13 cells/mm2 in saline controls, P<0.01; Fig. 2B). Again, Fluoro-Jade C positive cells induced by PRX-2 was reduced in LCN-2 HET male mice (116± 22 vs. 191 ± 33 cells/mm2 in WT group, P<0.01; Fig. 2B) and even more so in LCN-2 KO male mice (56 ± 10 vs. 116 ± 22 cells/mm2 in HET group, P<0.01; Fig. 2B).
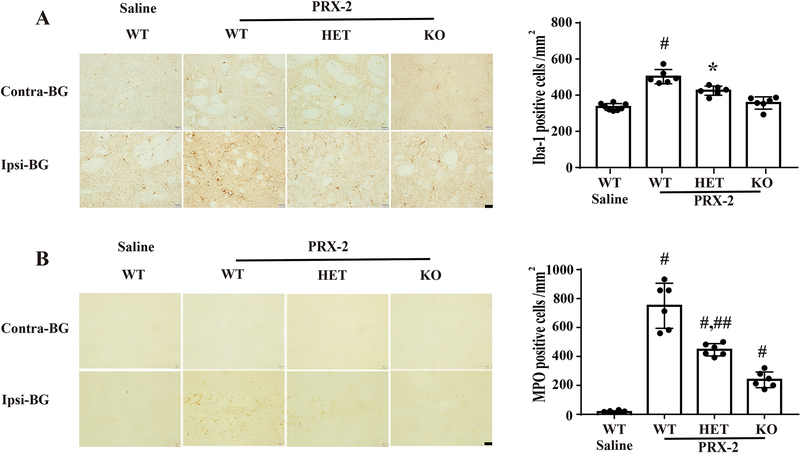
LCN-2 deficiency reduces PRX-2 induced microglia/macrophages activation and neutrophil infiltration in male mice
Microglia/macrophages were identified using Iba-1. Intracaudate injection of PRX-2 significant increased the number of Iba-1 positive cells in the ipsilateral basal ganglia at day 1 (502 ± 40 vs. 335 ± 19 cells/mm2 in saline group, P < 0.01; Fig 3A). This increase was reduced both in LCN-2 HET (425 ± 26 cells/mm2; Fig. 3A) and LCN-2 KO male mice groups (357 ± 34 cells/mm2; Fig. 3A).
Figure 3: Intracaudate injection of PRX-2 causes less microglia/macrophage activation and neutrophil infiltration in male LCN-2 HET and LCN-2 KO mice.
A) Iba-1 immunoreactivity in the basal ganglia (BG) at day 1 after an intracerebral injection of PRX-2 or saline. Results in the ipsilateral basal ganglia are means ± SD, n=8 in saline treated WT group, n=6 in PRX-2 treated groups; #P<0.01 vs. saline-WT and PRX-2-KO group, *P<0.05 vs. saline-WT and PRX-2-WT group by one-way ANOVA with Tukey’s post-hoc test. Scale bar=20 μm. B) MPO staining showing neutrophil infiltration in the BG at day 1 after an intracerebral injection of PRX-2 or saline. Values are means ± SD, n=8 in saline treated group; n=6 in the PRX-2 treated groups, #P<0.01 vs. saline-WT group, ##P<0.01 vs. the PRX-2-WT and PRX-2-KO group by one-way ANOVA with Tukey’s post-hoc test. Scale bar=20 μm.
Neutrophils were monitored using myeloperoxidase (MPO). Injection of PRX-2 resulted in a significantly increased number of MPO positive cells in the ipsilateral basal ganglia in WT mice at day 1 (751 ± 156 vs. 15 ± 9 cells/mm2 in saline group; P < 0.01; Fig. 3B). Again, there were less MPO positive cells after PRX-2 injection in the LCN-2 HET (446 ± 42 cells/mm2; Fig. 3B) and LCN-2 KO male mice (239 ± 54 cells/mm2; Fig. 3B).
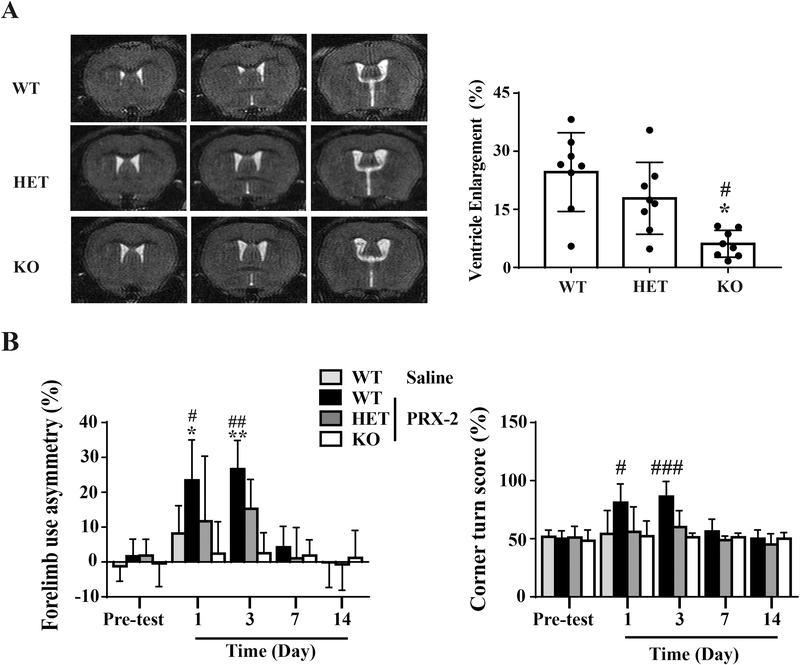
Intracaudate injection of PRX-2 causes less long-term brain atrophy and neurological deficits in male LCN-2 HET and LCN-2 KO mice
Brain atrophy was determined by measuring ipsilateral ventricular enlargement and hemispheric brain tissue loss on T2 MRI at day 14. Injection of PRX-2 resulted in a significant brain tissue loss in WT mice (ipsilateral ventricular enlargement: 24.6 ± 10.2%). This was markedly reduced in LCN-2 KO mice (6.1 ± 3.5%, P<0.01 vs. WT; Fig. 4A). Hemispheric brain tissue loss was also reduced in LCN-2 KO and LCN-2 HET mice (3.2 ±1.9%, 8.7 ± 4.1 % vs. 11.2 ± 3.1% in the WT mice, P<0.05).
Figure 4: Intracaudate injection of PRX-2 caused less long-term brain atrophy and neurological deficits in male LCN-2 HET and LCN-2 KO mice.
(A) T2-weighted images at 14 days after PRX-2 injection. Brain atrophy was measured at day 14 by enlargement of the ipsilateral ventricle: [(ipsilateral ventricular volume-contralateral ventricular volume)/contralateral ventricular volume] × 100%. #P<0.01 vs. WT, *P<0.05 vs. HET, n=8) by one-way ANOVA with Tukey’s post-hoc test. (B) Forelimb use asymmetry and corner turn test at pre-test (n=18), and day 1 (n=18), day 3 (n=8), day 7 (n=8), day 14 (n=8) after injection of PRX-2 in male WT, LCN-2 HET, and LCN-2 KO mice. Results are recorded as mean ± SD, #P < 0.01, *P<0.05 vs. the Saline-WT, PRX-2-HET and PRX-2-KO group at day 1; **P<0.05 vs. PRX-2-HET and ##P< 0.01 vs. PRX-2-KO group at day 3; ###P<0.01 vs. PRX-2-HET and PRX-2-KO group at day 3 by one-way ANOVA with Tukey’s post-hoc test.
Corner turn and forelimb use asymmetry tests were used to measure functional deficits. LCN-2 deficiency (KO or HET) ameliorated the behavioral deficits induced by PRX-2. WT mice injected with PRX-2 had more behavioral deficits compared with mice in the following groups: saline control group (forelimb use asymmetry, day 1, P<0.05, Fig. 4B; corner turn, day 1, P<0.01, Fig. 4B), LCN-2 HET mice group (forelimb use asymmetry, day 1, P < 0.05 and day 3, P<0.05; corner turn, day 1, P<0.01 and day 3, P<0.01, Fig. 4B) and the LCN KO mice group (forelimb use asymmetry, day 1, P<0.01 and day 3, P<0.01; corner turn, day 1, P<0.01, and day 3, P<0.01, Fig. 4B).
Effects of exogenous LCN-2 on PRX-2 induced brain damage in male LCN-2 KO mice
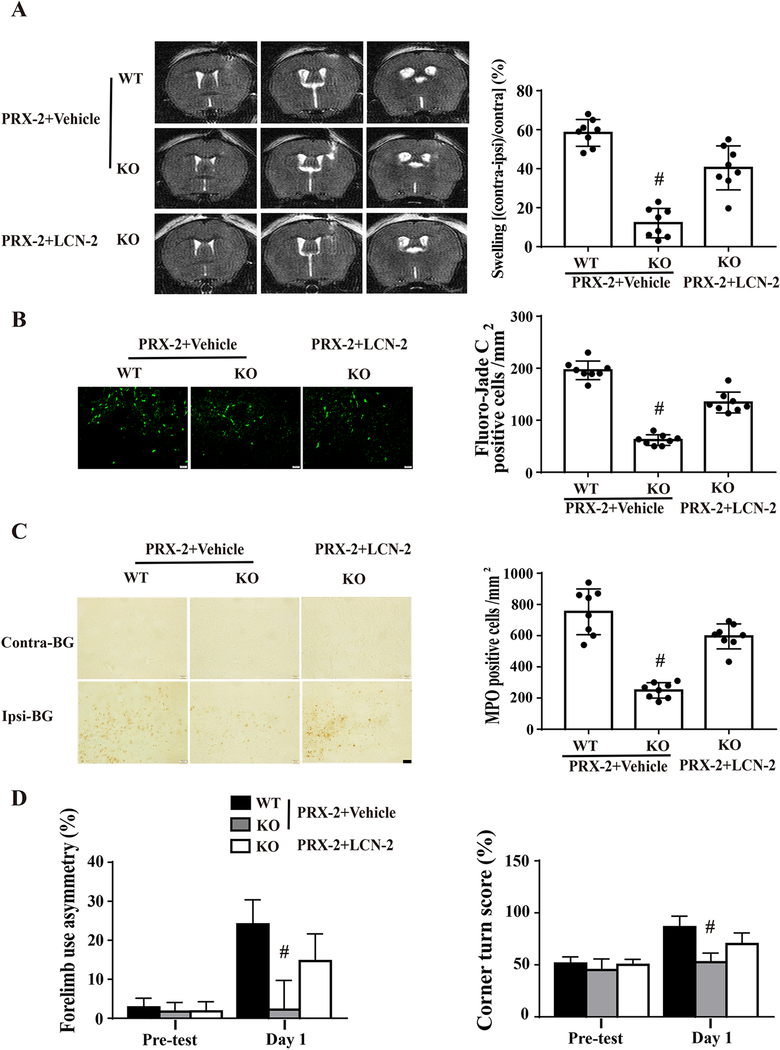
Co-injections of recombinant mouse LCN-2 (1 μg) in male LCN-2 KO mice (e.g. LCN-2 replacement) were used to further examine the potential role of LCN-2 in PRX-2 induced brain injury. Co-injection of LCN-2 with PRX-2 resulted in more severe brain swelling, neuron death, neutrophil infiltration, and behavioral deficits compared with PRX-2 plus vehicle in male LCN-2 KO mice at day 1. The brain injury was still less than PRX-2 with vehicle in male WT mice (Fig. 5).
Figure 5: Exogenous LCN-2 enhances PRX-2 induced brain damage in male LCN-2 KO mice.
Male LCN-2 KO mice received an intracerebral co-injection of PRX-2 with recombinant mouse LCN-2 protein or vehicle. Another control group of male WT mice received PRX-2 with vehicle. A) Representative T2 MRI at day 1 showing brain swelling by PRX-2. Brain swelling was quantified by ventricular compression. B) Fluoro-Jade C staining of degenerative neurons in the ipsilateral basal ganglia (BG) at day 1. C) Neutrophil infiltration in the ipsilateral BG at day 1. D) Forelimb use asymmetry and corner turn test. Values are mean ± SD, n=8, #P < 0.01 vs. the PRX-2+Vehicle-WT and PRX-2-KO group by one-way ANOVA with Tukey’s post-hoc test.
Intracaudate injection of PRX-2 induces less brain damage and neurological deficits in female LCN-2 KO mice
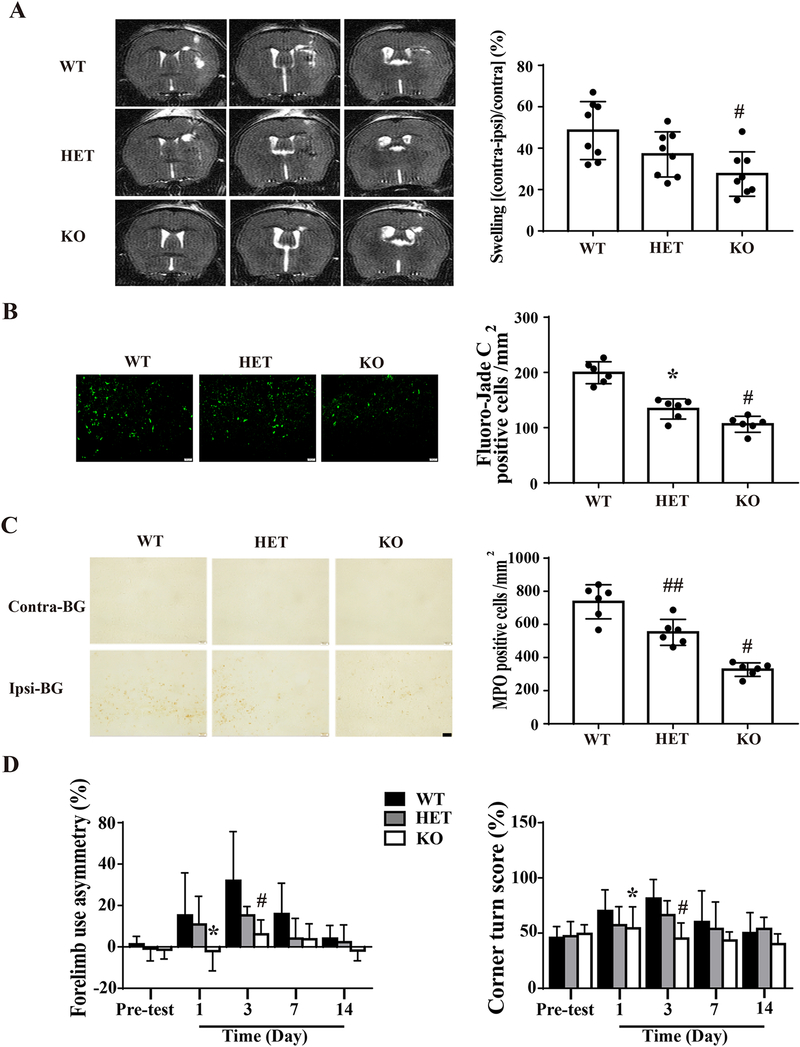
PRX-2 caused more brain swelling in female WT mice than LCN-2 KO mice at day 1 (ventricle compression: 48.5±13.9% vs. 27.5±10.7% in LCN-2 KO group; P<0.01; Figure.6A). However, they’re no significant statistical significance between LCN-2 HET and WT female mice.
Figure 6: Intracaudate injection of PRX-2 cause less brain damage and neurological deficits in female LCN-2 deficient mice.
Female WT, LCN-2 HET and LCN-2 KO mice had an intracerebral injection of PRX-2. A) Representative T2 MRIs at day 1 after an intracerebral injection of PRX-2. Brain swelling was assessed by ventricular compression at day 1. Values are mean ± SD. n=8, #P < 0.01 vs. WT by one-way ANOVA. B) Fluoro-Jade C staining showing degenerative neurons in the ipsilateral BG at day 1. Results are mean ± SD. n=6, #P < 0.01 vs. the WT group; *P<0.05 vs. the KO group by one-way ANOVA with Tukey’s post-hoc test. Scale bar =20 μm. C) MPO staining in the ipsilateral BG at day 1. Values are mean ± SD. n=6, #P < 0.01 vs. WT and HET groups; ##P<0.01 vs. WT group by one-way ANOVA with Tukey’s post-hoc test. Scale bar = 20 μm. D) Forelimb use asymmetry and corner turn tests at pre-test (n=14), day 1 (n=14), day 3 (n=8), day 7 (n=8), and day 14 (n=8). Results are mean ± SD, *P<0.05 vs. the WT group at day 1, #P < 0.01 vs. the WT group at day 3 by one-way ANOVA with Tukey’s post-hoc test.
PRX-2 induced less Fluoro-Jade C positive cells in female LCN-2 HET than WT mice at day 1 (134 ± 18 vs. 199 ± 20 cells/mm2 in WT group, P<0.01; Fig. 6B). There were even fewer positive cells in female LCN-2 KO mice (106 ±15 cell/mm2; Fig. 6B). Additionally, there were fewer MPO positive cells in the ipsilateral basal ganglia in female LCN-2 HET mice than WT mice at day 1 (552 ± 78 vs. 737 ± 103 cells/mm2 in WT group, P<0.01; Fig. 6C). There were even less MPO positive cells female LCN-2 KO mice (327 ± 41 cells/mm2; Fig. 6C).
PRX-2 injected female WT mice had more behavioral deficits compared to LCN-2 KO mice (forelimb use asymmetry, day 1, P < 0.05 and day 3, P<0.01; corner turn, day 1, P<0.05 and day 3, P<0.01, Fig. 6D). However, there were no significant statistical significances between female LCN-2 HET mice and WT mice on days 1 and 3. PRX-2 also caused less brain atrophy in female LCN-2 KO mice than in WT mice (4.0 ± 3.8% vs. 24.6 ± 13.0% in WT group, n=8, P<0.01).
Discussion
The key discoveries of this study were: (1) injection of exogenous PRX-2 upregulates brain LCN-2 levels in male WT mice; (2) PRX-2 induced brain injury and neuroinflammation were reduced in LCN2 KO and heterozygous male mice; (3) repleting LCN2 with exogenous recombinant protein exacerbated PRX-2 induced brain injury in LCN-2 KO mice; (4) LCN-2 deficiency also resulted in less PRX-2 induced injury in female mice.
PRX-2, which acts as a danger-associated molecular pattern (DAMP), is largely expressed in both neurons and RBCs. Excess PRX-2 from RBC lysis results in brain injury. Our prior studies found that intracaudate injections of PRX-2 can induce brain swelling, neuronal degeneration, neutrophil infiltration, blood-brain barrier leakage, and neurological deficits. Those injuries were attenuated by co-injection of conoidin A, a PRX-2 inhibitor, or PRX-2 heat inactivation 5. Intraventricular PRX-2 also results in hydrocephalus21. The mechanism by PRX-2 causes damage is unclear. Lu Y et al 22 found that exogenous PRX-2 can activate microglial toll-like 4 receptors (TLR4). Upon activation, the microglia activate many factors which promote inflammation, such as IL-1β and TNF-α, which may ultimately lead to neuronal cell death. Activation of TLR4 signaling can be attenuated by blocking PRX-2 release, which reduced inflammatory mediator production and ameliorated neurological deficits after cerebral ischemia 23.
We focused on PRX-2 in this study because PRX-2 levels in erythrocytes are very high (5.6 mg/ml)4. Thirty μl of blood could release 71 μg PRX-2 if the hematocrit is 42%. It should be noted that neurons could also release PRX-2 after injury. Therefore, 10 μg PRX-2 were used in this study. However, damaged brain cells could also release other DAMPs following ICH. For example, other PRXs and S100 proteins as DAMPs have been reported in brain ischemia24.
A previous study also suggested that LCN-2 acts as an element in brain injury after ICH 9. LCN-2 is released by activated astrocytes after a stroke 6. In this study, male LCN-2 KO and LCN-2 HET mice were used to examine if LCN-2 participates in PRX-2 mediated brain injury. Expression of LCN-2 was significantly enhanced after injection of PRX-2 indicating it might be involved in PRX-2 mediated brain injury. That hypothesis was confirmed by the finding that PRX-2 induced brain swelling, neutrophil infiltration, microglia/macrophage activation, neuronal cell death, and neurological deficits were all reduced in male LCN2 HET and LCN-2 KO mice. This study only found significant differences in neurological deficits among the WT, LCN-2 HET, and KO groups at days 1 and 3 after PRX-2 injection. This may be related to PRX-2 being cleared within a few days after being released outside the cells. We did, however, observing longer-term effects on brain atrophy, where there was a statistically significant difference at day 14 between WT and KO groups.
To further confirm the role of LCN-2 in PRX-2 induced brain injury, recombinant mouse LCN-2 was co-injected with PRX-2 in male LCN-2 KO mice. The co-injection of LCN-2 blocked the attenuation of PRX-2 induced brain damage found in male LCN-2 KO mice. We also found that brain injury in male WT mice (PRX-2 + vehicle injected group) is more severe than LCN-2 KO mice (PRX-2 + LCN-2 injected group); this may be because WT mice can express larger amounts of LCN-2 after injection of PRX-2.
Sex differences are a critical element affecting the prognosis of a cerebrovascular accident 25, 26. This study examined the role of PRX-2 in female WT, HET, KO mice. Brain swelling, neutrophil infiltration, and neurological deficits were compared among these groups. We found that significant differences between WT and KO groups on day 1, but there were no significant differences comparing brain swelling and neurological deficits between WT and HET groups. There were significant differences among the three groups in neutrophil infiltration.
There are several limitations to this study. It is a proof of principle study examining the effects of PRX-2 in LCN-2 deficient mice. PRX-2 is one blood component that appears to contribute to brain injury after ICH. Other components, e.g. hemoglobin/iron and thrombin are also involved in both injury and neuroinflammation. How those pathways interact with the PRX-2/LCN-2 axis is uncertain and merits examination. PRX-2 release into brain from a hematoma will be gradual as red blood cell lyse, a time course not reflected by the single injection employed here. The current study did not explore age, which is a major modifier of brain injury after ICH.
In summary, exogenous PRX-2 upregulates LCN-2, which in turn induces brain swelling, inflammation, neuronal cell death, and neurological deficits with these injuries being attenuated in LCN-2 deficient male and female mice. These and prior results indicate that the PRX-2/LCN-2 axis is involved in brain injury after hemorrhagic stroke and can potentially act as a novel therapeutic target.
Highlights.
PRX-2 upregulates the expression of LCN-2 and causes brain damage after intracerebral hemorrhage in male mice
The deficiency of LCN-2 can alleviate PRX-2 induced brain injury in male and female mice
Co-injection of recombinant LCN-2 protein can block the attenuation of PRX-2 induced brain damage in male mice
Acknowledgments
Funding: YH, RFK and GX were supported by grants NS-091545, NS-090925, NS-096917, NS106746 and NS116786 from the National Institutes of Health (NIH).
Footnotes
Conflict of Interests: Jingwei Zhang, Nemanja Novakovic, Ya Hua, Richard F. Keep, and Guohua Xi declare no conflict of interests.
Ethical approval: All institutional and national guidelines for the care and use of laboratory animals were followed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dang G, Yang Y, Wu G, Hua Y, Keep RF, Xi G. Early erythrolysis in the hematoma after experimental intracerebral hemorrhage. Transl Stroke Res. 2017;8:174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63 [DOI] [PubMed] [Google Scholar]
- 3.Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nature medicine. 2012;18:911–917 [DOI] [PubMed] [Google Scholar]
- 4.Low FM, Hampton MB, Winterbourn CC. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid Redox Signal. 2008;10:1621–1630 [DOI] [PubMed] [Google Scholar]
- 5.Bian L, Zhang J, Wang M, Keep RF, Xi G, Hua Y. Intracerebral hemorrhage-induced brain injury in rats: The role of extracellular peroxiredoxin 2. Transl Stroke Res. 2020;11:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S, Jha MK, Suk K. Lipocalin-2 in the inflammatory activation of brain astrocytes. Crit Rev Immunol. 2015;35:77–84 [DOI] [PubMed] [Google Scholar]
- 7.Egashira Y, Hua Y, Keep RF, Iwama T, Xi G. Lipocalin 2 and blood-brain barrier disruption in white matter after experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 2016;121:131–134 [DOI] [PubMed] [Google Scholar]
- 8.Egashira Y, Hua Y, Keep RF, Xi G. Acute white matter injury after experimental subarachnoid hemorrhage: Potential role of lipocalin 2. Stroke. 2014;45:2141–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao S, Xi G, Keep RF, Hua Y. Role of lipocalin-2 in thrombin-induced brain injury. Stroke. 2016;47:1078–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berard JL, Zarruk JG, Arbour N, Prat A, Yong VW, Jacques FH, et al. Lipocalin 2 is a novel immune mediator of experimental autoimmune encephalomyelitis pathogenesis and is modulated in multiple sclerosis. Glia. 2012;60:1145–1159 [DOI] [PubMed] [Google Scholar]
- 11.Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–1305 [DOI] [PubMed] [Google Scholar]
- 12.Jha MK, Lee S, Park DH, Kook H, Park KG, Lee IK, et al. Diverse functional roles of lipocalin-2 in the central nervous system. Neurosci Biobehav Rev. 2015;49:135–156 [DOI] [PubMed] [Google Scholar]
- 13.Jin M, Kim JH, Jang E, Lee YM, Soo Han H, Woo DK, et al. Lipocalin-2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2014;34:1306–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rathore KI, Berard JL, Redensek A, Chierzi S, Lopez-Vales R, Santos M, et al. Lipocalin 2 plays an immunomodulatory role and has detrimental effects after spinal cord injury. J Neurosci. 2011;31:13412–13419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Hua Y, Keep RF, Xi G. Brain ceruloplasmin expression after experimental intracerebral hemorrhage and protection against iron-induced brain injury. Transl Stroke Res. 2019;10:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni W, Mao S, Xi G, Keep RF, Hua Y. Role of erythrocyte cd47 in intracerebral hematoma clearance. Stroke. 2016;47:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, Hua Y, Keep RF, Wan S, Novakovic N, Xi G. Complement inhibition attenuates early erythrolysis in the hematoma and brain injury in aged rats. Stroke. 2019;50:1859–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo F, Hua Y, Wang J, Keep RF, Xi G. Inhibition of carbonic anhydrase reduces brain injury after intracerebral hemorrhage. Transl Stroke Res. 2012;3:130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin H, Xi G, Keep RF, Wu J, Hua Y. Darpp-32 to quantify intracerebral hemorrhage-induced neuronal death in basal ganglia. Transl Stroke Res. 2013;4:130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484 [DOI] [PubMed] [Google Scholar]
- 21.Tan X, Chen J, Keep RF, Xi G, Hua Y. Prx2 (peroxiredoxin 2) as a cause of hydrocephalus after intraventricular hemorrhage. Stroke. 2020;51:1578–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y, Zhang XS, Zhou XM, Gao YY, Chen CL, Liu JP, et al. Peroxiredoxin 1/2 protects brain against h2o2-induced apoptosis after subarachnoid hemorrhage. FASEB J. 2019;33:3051–3062 [DOI] [PubMed] [Google Scholar]
- 23.Mao XN, Zhou HJ, Yang XJ, Zhao LX, Kuang X, Chen C, et al. Neuroprotective effect of a novel gastrodin derivative against ischemic brain injury: Involvement of peroxiredoxin and tlr4 signaling inhibition. Oncotarget. 2017;8:90979–90995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shichita T, Ito M, Morita R, Komai K, Noguchi Y, Ooboshi H, et al. Mafb prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through msr1. Nature medicine. 2017;23:723–732 [DOI] [PubMed] [Google Scholar]
- 25.Davis CM, Fairbanks SL, Alkayed NJ. Mechanism of the sex difference in endothelial dysfunction after stroke. Transl Stroke Res. 2013;4:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorai Z, Hynan LS, Kopitnik TA, Samson D. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52:763–769; discussion 769–771 [DOI] [PubMed] [Google Scholar]