Abstract
Purpose:
To investigate the impact of intraocular pressure (IOP) control on rates of change of spectral-domain optical coherence tomography (SD OCT) retinal nerve fiber layer (RNFL) thickness in a real-world large clinical population.
Design:
Retrospective cohort study.
Subjects:
85,835 IOP measurements and 60,223 SD OCT tests from 14,790 eyes of 7,844 patients.
Methods:
Data were extracted from the Duke Glaucoma Registry, a large database of electronic medical records of patients with glaucoma and suspected of the disease followed over time at the Duke Eye Center and satellite clinics. All records from patients with a minimum of 6 months of follow-up and at least 2 good quality SD OCT scans and 2 clinical visits with Goldmann applanation tonometry were included. Eyes were categorized according to the frequency of visits with IOP below cutoffs of 21 mmHg, 18mmHg, and 15 mmHg over time. Rates of change for global RNFL thickness were obtained using linear mixed models and classified as slow, if change was slower than −1.0 μm/year; moderate, if between −1.0 and −2.0 μm/year; and fast, if faster than −2.0 μm/year. Multivariable models were adjusted for age, gender, race, diagnosis, central corneal thickness, follow-up time and baseline disease severity.
Main Outcome Measures:
Rates of change in SD OCT RNFL thickness according to levels of IOP control.
Results:
Eyes had a mean follow-up of 3.5±1.9 years. Average rate of change in RNFL thickness was −0.68±0.59μm/year. Each 1mmHg higher mean IOP was associated with 0.05μm/year faster RNFL loss (P<0.001), after adjustment for potentially confounding variables. For eyes that had fast progression, 41% of them had IOP<21mmHg in all visits during follow-up, whereas 20% of them had all visits with IOP<18mmHg, but only 9% of them had all visits with IOP<15mmHg.
Conclusion:
IOP was significantly associated with rates of progressive RNFL loss in a real-world large clinical population. Eyes with stricter IOP control over follow-up visits had much smaller chance of exhibiting fast deterioration. Our findings may assist clinicians in establishing target pressures in clinical practice.
PRÉCIS
In a longitudinal study involving a large clinical population, stricter intraocular pressure control reduced the chance of fast structural loss from glaucoma. These findings may guide clinicians in establishing target pressures in clinical practice.
Glaucoma is a progressive optic neuropathy estimated to affect approximately 80 million people worldwide and remains the leading cause of irreversible blindness.1 Elevated intraocular pressure (IOP) remains the main and only modifiable risk factor in glaucoma, and understanding the impact of levels of IOP on risk of disease progression helps clinicians establish target pressures and individualized therapeutic approaches.2–4
Several clinical trials have attempted to investigate the relationship between IOP and risk of glaucoma progression.5–11 The Early Manifest Glaucoma Trial estimated a 10% less risk of progression for each 1-mmHg reduction in IOP.8 In a post-hoc analysis, the Advanced Glaucoma Intervention Study (AGIS) demonstrated that visual field loss was significantly more frequent in eyes with mean IOP above 17.5 mmHg compared to those with mean IOP below 14 mmHg; those eyes with IOP less than 18 mmHg in 100% of visits during follow-up demonstrated no average change on visual fields, as measured by standard automated perimetry (SAP).5 However, such estimations of the impact of IOP lowering on glaucoma progression have mostly relied on studies with restrictive criteria and rigid treatment schemes, which do not necessarily represent how disease progresses under routine clinical care.12, 13
Recently, the widespread incorporation of electronic health records (EHR) in clinical practice has allowed a rapid growth in the availability of clinical data. Utilization of EHR data may better reflect how the disease progresses in a real-world setting, reducing issues of random selection and bias that can occur in patients enrolled in clinical research studies.14–16 In addition, although preservation of visual function is the primary goal of glaucoma management, assessment of how IOP impacts neural loss in glaucoma using SAP may be confounded by the subjective nature of perimetry, as well as by nonlinearities in translating retinal ganglion cell (RGC) loss to visual sensitivity thresholds.17–19 In that regard, analysis of imaging data from a large EHR database may have advantages when investigating the impact of IOP on glaucoma progression. By providing an objective and quantitative assessment of neural loss, spectral-domain optical coherence tomography (SD OCT) may be better able to capture the direct relationship between IOP and RGC losses in glaucoma.
In the present work, we used a large database of glaucoma patients and glaucoma suspects under routine clinical care to evaluate the impact of IOP control on rates of structural change over time, as measured by SD OCT retinal nerve fiber layer (RNFL) thickness.
METHODS
This was a retrospective cohort study of patients from the Duke Glaucoma Registry (DGR),20 an EHR database developed by the Vision, Imaging and Performance (VIP) Laboratory. The database consisted of adults 18 years or older with glaucoma or glaucoma suspect diagnoses who were evaluated at the Duke Eye Center or its satellite clinics between January 2009 and September 2019. The Duke University Institutional Review Board approved this study with a waiver of informed consent due to the retrospective nature of this work. All methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects and were conducted in accordance with regulations of the Health Insurance Portability and Accountability Act.
The database used for this study contained clinical information from baseline and follow-up visits, including patient diagnostic and procedure codes, medical history, best-corrected visual acuity, slit-lamp biomicroscopy, IOP measurement using the Goldmann applanation tonometry (GAT; Haag-Streit, Konig, Switzerland), central corneal thickness (CCT), gonioscopy, ophthalmoscopy examination, stereoscopic optic disc photographs, and the results of all Spectralis SD OCT (Heidelberg Engineering, GmbH, Dossenheim, Germany) scans during the study period.
Participant selection
Patients were included in the study if they had glaucoma or suspicion of glaucoma based on International Classification of Diseases (ICD) codes (Supplemental Table 1, available at http://www.aaojournal.org) at baseline. Subjects were also required to have at least 2 good-quality SD OCT scans and 2 IOP measures with GAT in different days over a minimum follow-up period of 6 months. Tests performed after any diagnosis of retinal detachment, retinal or malignant choroidal tumors, non-glaucomatous disorders of the optical nerve and visual pathways, uveitis and venous or arterial retinal occlusion according to ICD codes were excluded. In addition, tests performed after treatment with panretinal photocoagulation, according to Current Procedural Terminology (CPT) codes, were also excluded. ICD and CPT codes used for inclusion and exclusion in the study are further described in Supplemental Table 1 (available at http://www.aaojournal.org). Tests were further censored after any filtration procedure (i.e. trabeculectomy or tube shunt surgery) happening during follow-up. The baseline characteristics and demographics were drawn from the date when the first valid SD OCT test for each eye was performed.
SD OCT testing
RNFL thickness measurements were obtained from a 12-degree (for single circle scans) or a 3.45mm-diameter peripapillary circle scan (for scans from the Glaucoma Mode Premium Edition) acquired using the Spectralis SD OCT, as described in detail previously.21 Tests were acquired using the latest available software version at the time of the scan and exported using the latest available version at the time of the analysis (Software version 6.8). For each scan, the global average RNFL thickness was calculated as the average of thicknesses of all points from the 360 degrees around the optic nerve head. This parameter was used to assess rates of change in RNFL thickness over time.
The device’s eye-tracking capability was used during image acquisition to adjust for eye movements. All scans that had a quality score lower than 15 were excluded from this analysis. Furthermore, since manual review of all tests was impractical, scans that had average global RNFL thickness measurements with implausible values were excluded (i.e., lower than 20 and greater than 150 μm). Those cutoffs represent measurements above the higher range of reported RNFL thickness for normal controls and below the lower range for glaucoma subjects22–24 and may indicate the presence of acquisition or segmentation errors in the presence of otherwise good quality scores.25 From the total of 136,322 eligible circle scans from the database (i.e. after exclusions for ICD and CPT codes), 6,337 (4.6%) tests were excluded due to low quality score and 1,678 (1.2%) were further excluded due to implausible average RNFL thickness values. When more than one good-quality test was available for the same date, the mean global RNFL thickness of all tests from that date was used in the analyses. Remaining tests were excluded when eyes had less than 6 months of follow-up, or due to the unavailability of complete IOP or CCT data.
Data Analyses
Linear mixed models (LMM) were used to evaluate the effect of IOP parameters on the rates of change in SD OCT global peripapillary RNFL thickness over time for each group while adjusting for potential correlations between both eyes from the same individual. This standard technique has been described in detail elsewhere.26 In brief, mixed models take into account the natural correlation of such data over time, as well as the fact that each patient may contribute with two eyes for the analyses. Differences in rates of change between eyes and subjects are taken into account by introducing random slopes and random intercepts. Multivariable LMMs were also adjusted for baseline age, gender, race, glaucoma diagnosis, CCT, follow-up time and baseline disease severity (as defined by the baseline RNFL thickness). Best linear unbiased prediction was used to estimate individual slopes of change for each eye.27–29
Individual slopes were then classified into groups according to pre-established cutoffs for rates of global RNFL thickness loss: slow, if change was slower than −1.0 μm/year; moderate, if between −1.0 and −2.0 μm/year; and fast, if faster than −2.0 μm/year.20 For each RNFL slope from the LMM, a mean IOP value was calculated as the average measurement for a given eye during the corresponding follow-up. Peak IOP was determined as the highest value during follow-up. Eyes were also categorized in 4 groups according to the frequency of IOP measurements below 18mmHg at each visit over time, previously proposed by the AGIS investigators:5 A: 100% of visits; B: 75% to less than 100% of visits, C: 50% to less than 75% of visits, D: less than 50% of visits. We also evaluated eyes classified according to looser and stricter levels of IOP control of 21mmHg and 15mmHg, respectively.
All statistical analyses were completed in Stata (version 16, StataCorp LP, College Station, TX) within the Protected Analytics Computing Environment (PACE), a highly protected virtual network space developed by Duke University for analysis of identifiable protected health information.
RESULTS
In this study, we included a total of 60,223 SD OCT tests acquired over 56,153 SD OCT visits from 14,790 eyes of 7,844 patients. Of these patients, 4,502 (57.4%) were female and 2,240 (28.6%) were self-identified as Black or African-American. 4,509 (30.5%) eyes had a diagnosis of POAG, 7,007 (47.4%) glaucoma suspect, and 3,274 (22.1%) of “other glaucoma”, according to ICD codes from the baseline visit. Table 2 summarizes the demographic and clinical characteristics of the eyes included in the study according to their baseline diagnosis. There was a large variation in baseline characteristics (Supplemental Figure 1, available at http://www.aaojournal.org) comprising the whole spectrum of the disease. Of note, mean ± standard deviation (SD) age of subjects at baseline was 65.3 ± 13.1 years and eyes had a mean ± SD follow-up time of 3.5 ± 1.9 (range: 0.5 to 9.5) years, with a mean ± SD number of 3.8 ± 1.7 SD OCT visits, ranging from 2 to 14. The dataset had a total of 85,835 valid visits where IOP was measured with GAT, with an average of 5.8 ± 3.6 visits per eye.
Table 2.
Demographics and Clinical Characteristics of Subjects Included in the Study.
| Characteristic | Overall | Diagnosis at baseline | ||
|---|---|---|---|---|
| GS | POAG | Other | ||
| Subject-specific | ||||
| Number of patients, n (%) | 7,844(100.0) | 3,691 (47.0) | 2,468 (31.4) | 1,821 (23.2) |
| Age (years), Mean ± SD | 65.3 ±13.1 | 62.9 ±12.6 | 68.8 ±12.2 | 65.6 ±13.8 |
| Sex, female (%) | 4,502 (57.4) | 2,214 (60.0) | 1,319 (53.4) | 1,040 (57.1) |
| Race, (%) | ||||
| White or Caucasian | 4,822 (61.5) | 2,265 (61.4) | 1,505 (61.0) | 1,124 (61.7) |
| Black or African American | 2,240 (28.6) | 1,037(28.1) | 771 (31.2) | 482 (26.5) |
| Other | 782 (9.9) | 389 (10.5) | 192 (7.8) | 215 (11.8) |
| Eye-specific | ||||
| Number of eyes, n (%) | 14,790 (100.0) | 7,007 (47.4) | 4,509 (30.5) | 3,274 (22.1) |
| Follow-up, years Mean ± SD | 3.5 ±1.9 | 3.6 ±1.9 | 3.3 + 2.0 | 3.5 ±1.8 |
| CCT,μm Mean ± SD | 549.5 ±41.8 | 554.7 ±41.2 | 544.0 ±40.8 | 546.0 ±43.0 |
| SD OCT | ||||
| Number of visits, n (%) | 56,153 (100.0) | 25,971 (46.2) | 17,147 (30.6) | 13,035 (23.2) |
| Number of visits per eye, Mean ± SD [range] | 3.8 ±1.7 [2–14] | 3.7 ±1.6 [2–12] | 3.8 ±1.9 [2–12] | 4.0 ±1.9 [2–14] |
| Baseline mean RNFL thickness, μm | ||||
| Mean + SD | 82.4 ±17.0 | 89.2 ±13.6 | 74.7 ±17.0 | 78.5 ±17.9 |
| Median (IQR) | 84.0 (72.0; 94.0) | 90.0 (81.0; 98.0) | 75.0 (63.0; 87.0) | 79.8 (66.0; 91.0) |
| Baseline mean SD OCT quality, | ||||
| Mean ± SD | 24.6 ± 4.3 | 24.7 ±4.3 | 24.5 ±4.3 | 24.4 ±4.2 |
| Median (IQR) | 25.0 (22.0; 28.0) | 25.0 (22.0; 28.0) | 25.0 (21.0; 28.0) | 25.0 (21.0; 27.0) |
| IOP | ||||
| Number of visits, n (%) | 85,835 (100.0) | 33,661 (39.2) | 29,647 (34.6) | 22,527 (26.2) |
| Number of visits per eye, n (%) Mean + SD [range] | 5.8 ± 3.6 [2–34] | 4.8 ± 2.9 [2–24] | 6.6 ± 3.9 [2–28] | 6.9 ± 3.9 [2–34] |
| Average IOP during follow-up, mmHg Mean + SD | 16.1 ±3.5 | 16.6 ±3.4 | 15.5 ±3.6 | 15.9 ±3.5 |
| Peak IOP during follow-up, mmHg Mean + SD | 19.4 ±5.5 | 19.2 ±4.8 | 19.2 ±6.0 | 19.9 ±6.1 |
| Visits with IOP<21mmHg during follow-up, n (%) | ||||
| Visits with IOP<18mmHg during follow-up, n (%) | ||||
| Visits with IOP<15mmHg during follow-up, n (%) | ||||
CCT = central corneal thickness; GS = glaucoma suspect; IOP = intraocular pressure; IQR = interquartile range; POAG = primary open-angle glaucoma; SD = standard deviation; SD OCT = Spectral-Domain Optical Coherence Tomography; RNFL = Retinal Nerve Fiber Layer.
The mean ± SD rate of change for global RNFL thickness in the overall population was −0.68 ± 0.59 μm/year (median −0.64, IQR: −0.88 to −0.42). Table 3 details the distribution of the rates of RNFL change according to each diagnostic group. POAG patients had faster rates of change than glaucoma suspects, but slower than other glaucoma types, on average. Results of univariable and multivariable analysis for the effect of each variable of interest on the rates of RNFL thickness loss over time are detailed in Table 4. Each 1 mmHg higher mean IOP was associated with 0.051 μm/year faster loss of RNFL, and each 1 mmHg higher of peak IOP was associated with 0.038 μm/year faster RNFL loss, after adjustment for potentially confounding variables.
Table 3.
Rates of Change for Spectral-Domain Optical Coherence Tomography Retinal Nerve Fiber Layer (RNFL) Thickness According to Glaucoma Diagnosis at Baseline for All Subjects Included in the Study.
| Diagnosis | Rates of global RNFL change (pm/year) 14,790 eyes of 7,844 subjects |
||||||
|---|---|---|---|---|---|---|---|
| mean | SD | median | IQR | p5 | p15 | ||
| GS | −0.60 | 0.55 | −0.57 | −0.78 | −0.36 | −1.42 | −0.94 |
| POAG | −0.68 | 0.53 | −0.67 | −0.88 | −0.48 | −1.46 | −1.03 |
| Other | −0.84 | 0.70 | −0.79 | −1.07 | −0.52 | −1.85 | −1.30 |
| Overall | −0.68 | 0.59 | −0.64 | −0.88 | −0.42 | −1.56 | −1.06 |
GS = glaucoma suspect; IQR = interquartile range; POAG = primary open-angle glaucoma; SD = standard deviation; RNFL = retinal nerve fiber layer.
Table 4.
Univariable and Multivariable Linear Mixed Models of the Effect of Each Clinical Characteristic on the Rate of Change of Spectral-Domain Optical Coherence Tomography Retinal Nerve Fiber Layer (RNFL) Thickness Over Time.
| Characteristic | Univariable Model | Multivariable Model 1 | Multivariable Model 2 | |||
|---|---|---|---|---|---|---|
| Coefficient | P‒value | Coefficient | P‒value | Coefficient | P‒value | |
| Diagnosis, | ||||||
| Age at baseline, per 10 years older | −0.028 | 0.006 | −0.063 | <0.001 | −0.055 | <0.001 |
| Sex, female | 0.041 | 0.116 | 0.053 | 0.089 | 0.046 | 0.132 |
| Race, Black or African American | −0.055 | 0.056 | −0.028 | 0.451 | −0.019 | 0.594 |
| Follow-up, years | −0.011 | 0.092 | −0.038 | <0.001 | −0.034 | <0.001 |
| Baseline mean global RNFL thickness, per 10μm thicker | −0.045 | 0.001 | −0.050 | <0.001 | −0.063 | <0.001 |
| CCT, per40μm thinner | 0.007 | 0.551 | −0.019 | 0.262 | −0.012 | 0.431 |
| Average IOP during follow-up, per 1 mmHg higher | −0.054 | <0.001 | −0.051 | 0.001 | --- | --- |
| Peak IOP during follow-up, per 1 mmHg higher | −0.049 | <0.001 | --- | --- | −0.038 | <0.001 |
Boldface indicates statistical significance (P<0.05).
CCT = central corneal thickness; IOP = intraocular pressure; GS = glaucoma suspect; POAG = primary open-angle glaucoma; SD = standard deviation; RNFL = Retinal Nerve Fiber Layer.
Figure 2 shows average rates of global RNFL loss for groups of eyes according to the percentage of visits with IOP<18mmHg. The proportions of eyes in each of the 4 categories of IOP control (IOP<18mmHg at each visit for A: 100% of visits; B: 75%−100% of visits, C: 50%−75% of visits, D: less than 50% of visits) were 40%, 14%, 19% and 27%, and they had mean ± SD IOP values of 13.1±2.0, 15.2±1.4, 17.1±1.3, and 20.3±2.4mmHg, respectively. Corresponding mean ± SD rates of RNFL thickness change for each group were −0.61±0.53, −0.68±0.60, −0.71±0.63, and −0.75±0.62 μm/year, respectively. Figure 2 also shows a similar analysis for eyes divided by the cutoffs of 15mmHg and 21mmHg.
Figure 2.
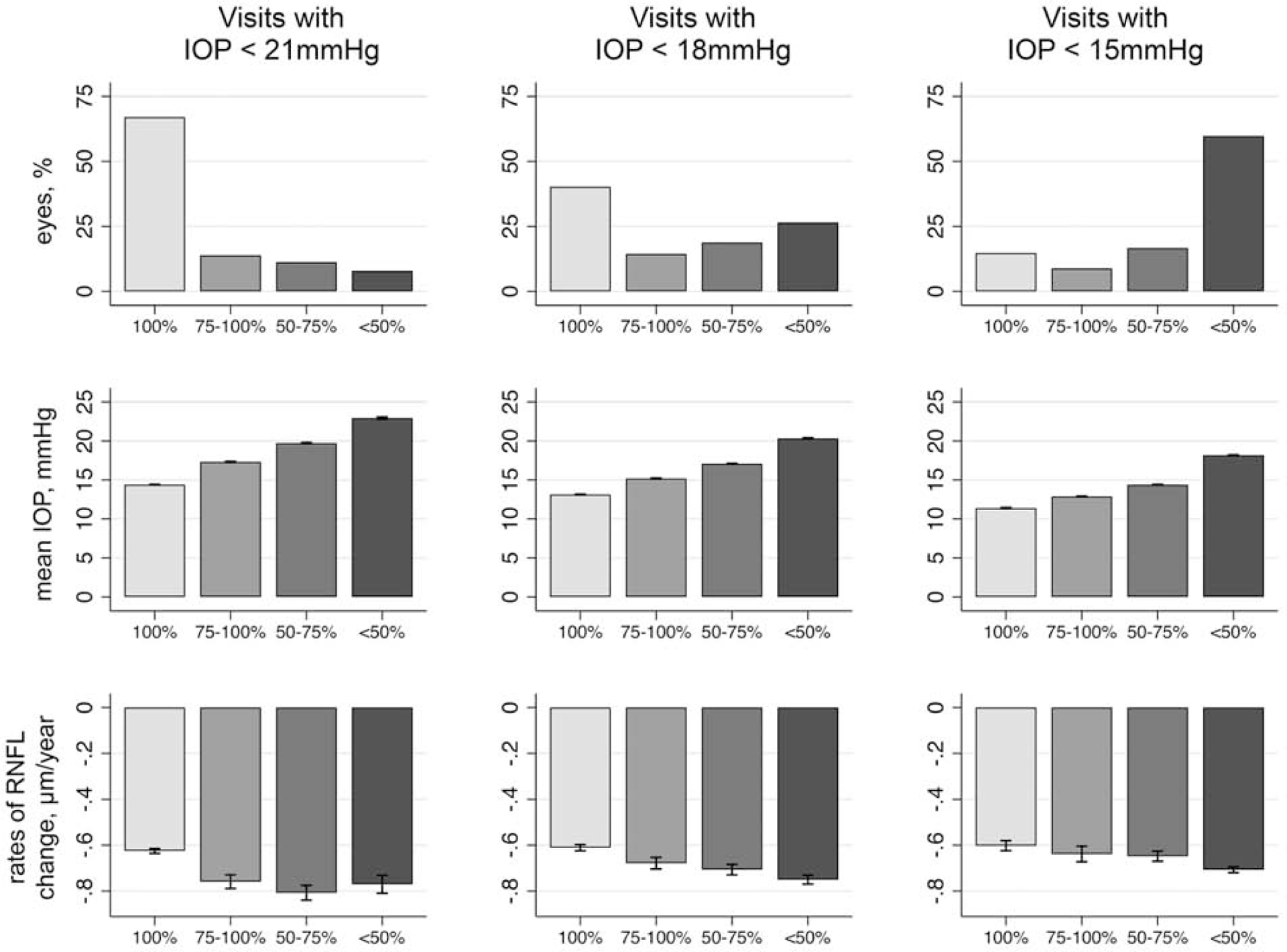
Proportion of Eyes, Mean Intraocular Pressure (IOP), and Mean Rates of Change of Spectral-Domain Optical Coherence Tomography (SD OCT) Retinal Nerve Fiber Layer (RNFL) Thickness According to the Percentage of Visits with IOP Below 21mmHg, 18mmHg, and 15mmHg During Follow-up. Capped Spikes Represent 95% Confidence Intervals.
Figure 3 shows the proportion of eyes classified according to the rates of RNFL loss as slow (slower than −1μm/year), moderate (between −1 and −2 μm/year) or fast progressors (faster than −2 μm/year) for groups of eyes according to the percentage of visits with IOP<21mmHg (Figure 3A), <18mmHg (Figure 3B), and <15mmHg (Figure 3C). Supplemental Figure 4 (available at http://www.aaojournal.org) displays additional plots according to glaucoma diagnosis at baseline. Overall, eyes progressing at fast rates had a relatively lower frequency of visits with “satisfactory” IOP measures. For example, for eyes that had fast progression, 20% of them had IOP<18mmHg in all visits, while in 40% of them, the IOP was above that cutoff in more than half of the visits. For a stricter cutoff of 15mmHg, only 9% of the eyes with fast progression had all visits with IOP below that cutoff. As expected, for a looser cutoff of 21mmHg, 41% of fast progressors had IOP below this level in all visits.
Figure 3.

Proportion of Eyes Classified as Slow, Moderate, and Fast Progressors According to the Rates of Change of Spectral-Domain Optical Coherence Tomography (SD OCT) Retinal Nerve Fiber Layer (RNFL) Thickness. Eyes Are Grouped According to the Percentage of Visits with Intraocular Pressure (IOP) Below (A) 21mmHg, (B) 18mmHg and (C) 15mmHg During Follow-up.
Rates of SD OCT global RNFL thickness change: slow, if slower −1.0 μm/year; moderate, if between −1.0 and −2.0 μm/year; fast, if faster than −2.0 μm/year.
We also investigated the relationship between levels of mean IOP during follow-up and rates of RNFL loss. Rather than looking at the proportion of visits with IOP below a certain cut-off, this additional analysis investigates the proportion of eyes with slow, moderate and fast progression according to pre-defined levels of mean IOP during follow-up, as proposed by the AGIS study.5 27% of the eyes had mean IOP below 14 mmHg, 42% had mean IOP between 14 and 17.5 mmHg and 31% had mean IOP above 17.5 mmHg during follow-up. The results are illustrated on Figure 5. Of note, of eyes with fast RNFL progression, 18% had mean IOP below 14 mmHg, 35% had mean IOP between 14 and 17.5 mmHg and 47% had mean IOP above 17.5 mmHg.
Figure 5.
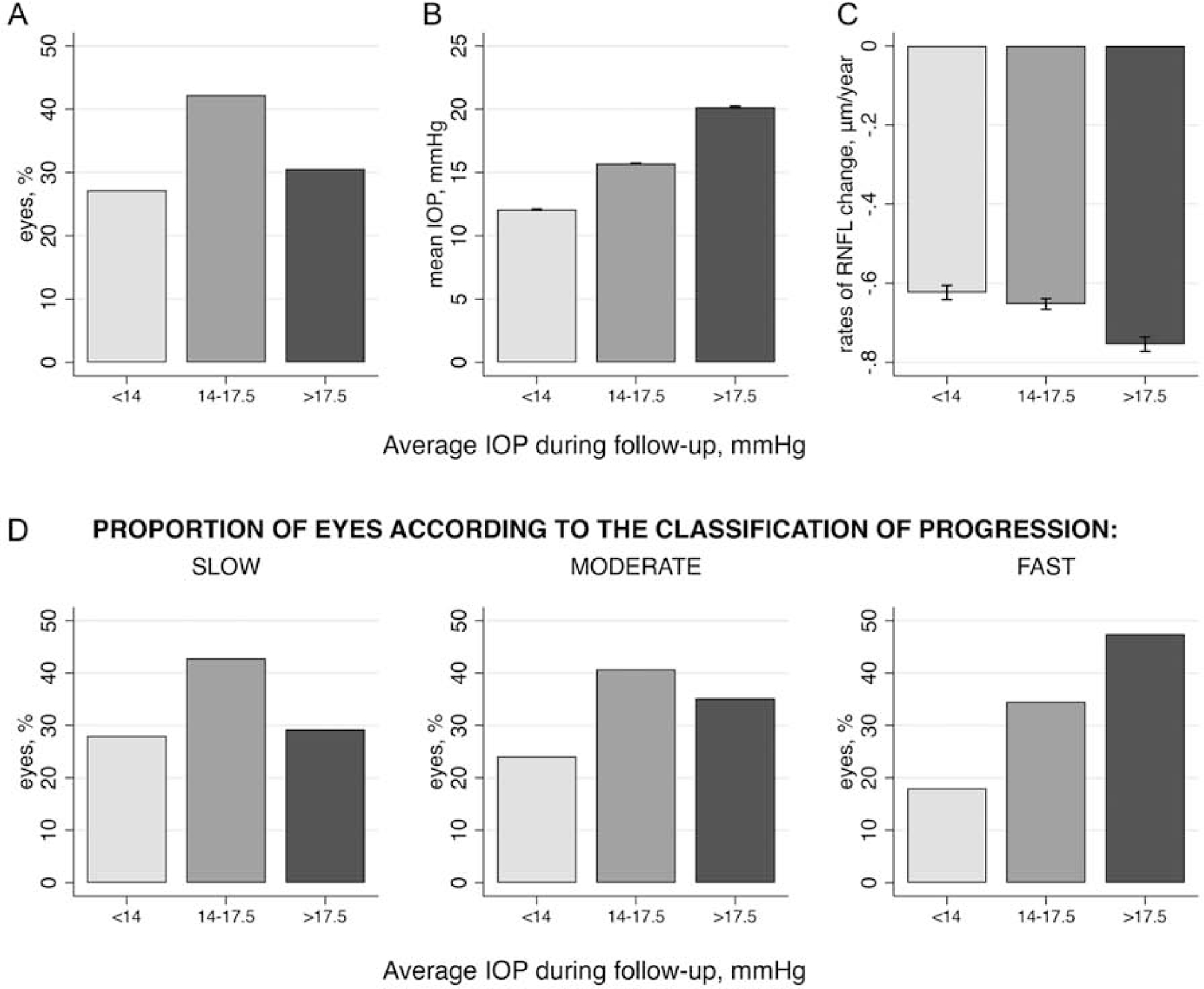
(A) Proportion of Eyes, (B) Mean Intraocular Pressure (IOP) and (C) Mean Rates of Change for Each Category of Average IOP During Follow-up, and (D) the Proportion of Eyes Classified as Slow, Moderate, and Fast Progressors Within Each Category According to the Rates of Change of Spectral-Domain Optical Coherence Tomography (SD OCT) Retinal Nerve Fiber Layer (RNFL) Thickness. Capped Spikes Represent 95% Confidence Intervals.
Rates of SD OCT global RNFL thickness change: slow, if slower than −1.0 μm/year; moderate, if between −1.0 and −2.0 μm/year; fast, if faster than −2.0 μm/year.
DISCUSSION
In this large integrated study of EHR and structural imaging data, we assessed the effect of IOP control on rates of RNFL thickness change from a population of patients followed at a tertiary care center. We present the largest analysis of longitudinal SD OCT results to date, with 14,790 eligible eyes undergoing routine clinical care over a follow-up period that extended up to 9.5 years. Although most patients under routine clinical care had relatively slow rates of progression and satisfactory IOP control, eyes with higher IOP over follow-up had significantly faster rates of change. Most importantly, by investigating the relationship between the frequency of visits with IOP below certain cutoff levels and the risk for fast progression, our results may assist clinicians in establishing target IOP levels in clinical practice.
In our study, for each 1 mmHg increase in mean IOP during follow-up, global RNFL thickness declined at a 0.05 μm/year faster rate. This effect was of similar magnitude even after adjusting for other relevant covariates such as age, sex, race, CCT, baseline disease severity and follow-up time, and is similar to that found in previous studies from smaller populations.30–32 Such estimate, however, offers little guidance on how clinicians should think about target IOP levels to prevent RNFL loss over time. We therefore conducted analyses investigating the relationship between rates of progression and the frequency of visits where IOP levels would be below a certain cutoff. This more closely replicates how clinicians set target IOP levels in clinical practice. We investigated a cutoff of 18mmHg based on the AGIS study,5 but also stricter and looser cutoffs of 15mmHg and 21mmHg, respectively. In the AGIS, eyes with IOP consistently lower than 18 mmHg in all visits did not show apparent visual field progression as measured by the AGIS score, a summary metric of visual field damage that behaves very similarly to mean deviation. In our study, we found that although having a higher frequency of visits with IOP<18mmHg translated into slower RNFL change over time, it was not sufficient to prevent moderate or fast progression. In eyes with moderate progression, still 33% had all their visits with IOP<18mmHg. In eyes with fast progression, 20% had all their visits below such cutoff. These findings may indicate a relative lack of sensitivity of the AGIS criteria to assess visual field progression, as found by other authors.33–35 It may also reflect the fact that structural change may often be seen in the absence of detectable visual field change.17–19 In fact, rates of RNFL change have been shown to be predictive of future visual field loss and decline in quality of life in glaucoma.36–38 In addition, studies such as the AGIS rely on very restrictive criteria and rigid treatment schemes, which do not necessarily represent how glaucoma progresses under routine clinical care.14, 16, 39
The contrast between results using cutoffs of 21mmHg and 15mmHg as shown on Figure 3 gives further insight into the relationship between IOP control over time and progression. Only 9% of the eyes with fast progression had IOP<15mmHg in all visits, whereas 41% of eyes with fast progression had IOP<21mmHg in all visits. These results suggest that a target IOP of 15mmHg would make it much less likely to have fast progression, while a target of 21mmHg would still be insufficient for a large number of eyes in preventing fast deterioration. On average, eyes with fast progression had an IOP of 17.5 ± 3.8 mmHg during follow-up, with 82% of them with mean IOP of 14 mmHg and above (Figure 5). However, it should be noted that the association between levels of IOP control over time and rates of RNFL thickness change was not perfect and many eyes continued to progress despite relatively low IOP levels. Similarly, many eyes had slow progression despite having at least some of their visits with IOP>21mmHg, as Figure 3 indicates. Ultimately, the required target pressure for each eye will depend on full consideration of risks and benefits of more aggressive therapy, including risk of disability, side effects of therapeutic options, disease severity at baseline, life expectancy, and the patient’s perceptions about treatment.40, 41 In addition, pre-treatment levels of IOP may also need to be considered when assessing target IOP levels. As many of the patients included in our study were already under treatment at baseline and most did not undergo washout, it was not possible to determine the impact of percent reductions in IOP in risk of progression over time. This, however, replicates common scenarios seen in practice.
The assessment of the true relationship between IOP and progression can be affected by the variability in IOP and RNFL measurements over time as well as by possible effects of treatment. In our study, patients were treated at the discretion of the attending ophthalmologists, and each attending may have had a different way of setting target IOP values. Overall, it is likely that patients with more severe stages of the disease underwent more aggressive treatment, which led to lower IOP values, whereas mild cases were allowed higher IOP values. Ultimately, the effect of treatment is expected to translate into changes in IOP over time, and would therefore be captured by our analyses. However, it is possible that the limited office measurements acquired over time may not have fully captured the true IOP behavior in many eyes.42 In fact, large variations of IOP outside office hours have been reported in glaucoma patients and in-office measurements may not fully reflect a patient’s medication-taking behavior or adherence to treatment at home.12, 43 Nevertheless, office IOP measurements remain the most commonly applied metric for both deciding on appropriate therapy and defining the success of a given intervention.41 Therefore, our results are relevant to common clinical practice.
Of note, our inclusion criteria required a minimum of two SD OCT tests over a follow-up period of at least six months. It could be argued that eyes with only two or a few SD OCT tests would not have a sufficient number of datapoints to reliably estimate a rate of change. However, in a large cohort study such as ours, estimates of rates of change in eyes with few observations can be improved by making use of data from the whole population sample.29 For example, it is reasonable to assume that the best estimator of the rate of change in a patient in whom we have no follow-up measurements collected over time is the average rate of change in the subgroup of the population that shares similar baseline characteristics to those of the patient. As more measurements are acquired for this patient, however, his/her rate of change will most likely deviate from the population average. For patients with few measurements, the precision of the estimates can be increased by “borrowing strength” from the population, whereas for patients with large number of measurements, precise estimates can be obtained relying almost only on the individual data and the need to borrow strength from the population decreases. These estimates correspond to empirical Bayes estimators (i.e., the BLUPs) from linear mixed models used in our study. We and others have previously shown that BLUPs improve the precision of slopes of change in glaucoma and result in improved prediction of future observations.27, 30, 44 Importantly, this approach also reduces the potential for selection bias that may occur from throwing away data from eyes with few observations. For example, eyes with very fast progression occurring over a relatively short period of time could be inadvertently excluded if one were to keep only eyes with a large number of tests during follow-up. Of note, the inclusion of eyes with few IOP measurements could lead to difficulties in properly categorizing these eyes according to the percentage of visits with IOP below cutoff points, a main outcome measure in our study. We therefore repeated the analysis using only eyes with at least 4 measurements, but the results remained essentially unchanged (Supplemental Figure 6, available at http://www.aaojournal.org).
While the size of our study is a major strength, working with EHR data also imposes limitations. Retrospective data may have missing values or be inaccurately coded. Coding for glaucoma diagnosis was done by the attending physicians, without following prespecified guidelines other than general billing coding guidelines, and thus may differ from physician to physician. As such, many eyes classified as suspects may indeed have had glaucomatous damage. Conversely, many eyes with glaucoma may have received such diagnosis based on previous history or other factors, without necessarily having confirmed optic nerve damage. In fact, although one would expect the effect of increased IOP to be different in glaucoma suspect eyes versus those with confirmed damage, we found similar effects of IOP on rates of RNFL loss when we performed analyses separately according to diagnostic groups (Supplemental Figure 4, available at http://www.aaojournal.org). As another limitation of our study, we assumed that the effect of treatment on the rate of RNFL loss was mediated by the IOP-lowering effect and not by any other potential mechanism of action that may be particular to a specific class of drugs. However, this assumption is not unreasonable since no class of medication has been clearly demonstrated to exert effects on glaucoma progression by mechanisms other than IOP. Given the large number of therapeutic options and the frequent changes in treatment scheme during a patient follow-up, it would be very difficult to obtain reliable estimates of individual drug effects on rates of RNFL change. Finally, adjustment for confounders in our study was obviously limited by the availability of EHR data. For example, refractive error, which has been shown to potentially impact SD OCT measurements, was only available for a subset of the patients (13,393 eyes, 90.5% of the sample). For those eyes, spherical equivalent had no statistically significant effect on rates of RNFL change over time (ß=0.006 μm/year for each diopter lower, P=0.346) in multivariable analysis. Future studies assembling even larger samples of data might be able to address issues of related to the effect of different classes of medications or other potential confounding factors.
In conclusion, our results indicate that IOP was significantly associated with rates of progressive RNFL loss in a real-world large clinical population. Most importantly, we were able to quantify the impact of different levels of IOP control on rates of RNFL change over time, showing that eyes with stricter IOP control over follow-up visits had much smaller chance of exhibiting fast deterioration. Our findings may assist clinicians in establishing individualized target pressures in clinical practice.
Supplementary Material
Supplemental Figure 1. Distribution of the Eyes Included in the Study According to Baseline (A) Age, (B) Spectral-Domain Optical Coherence Tomography (SD OCT) Global Retinal Nerve Fiber Layer (RNFL) Thickness and (C) Mean Intraocular Pressure (IOP) During Follow-up, (D) Follow-up Time, and (E) Number of SD OCT and (F) IOP Visits Per Eye.
Supplemental Figure 6. Proportion of Eyes Classified as Slow, Moderate, and Fast Progressors According to the Rates of Change of Spectral-Domain Optical Coherence Tomography (SD OCT) Retinal Nerve Fiber Layer (RNFL) Thickness Among Eyes with at Least Four Intraocular Pressure (IOP) Measurements During Follow-up. Eyes Are Grouped According to the Percentage of Visits with IOP Below (A) 21mmHg, (B) 18mmHg and (C) 15mmHg During Follow-up.
Rates of SD OCT global RNFL thickness change: slow, if slower than −1.0 μm/year; moderate, if between −1.0 and −2.0 μm/year; fast, if faster than −2.0 μm/year.
Supplemental Figure 4. Proportion of Eyes Classified as Slow, Moderate, and Fast Progressors According to Rates of Change of Spectral-Domain Optical Coherence Tomography (SD OCT) Retinal Nerve Fiber Layer (RNFL) Thickness for Glaucoma Suspects (GS), Primary Open-Angle Glaucoma (POAG) and Other Glaucoma Diagnoses. Eyes Are Grouped According to the Percentage of Visits with Intraocular Pressure (IOP) Below 18mmHg During Follow-up.
Rates of SD OCT global RNFL thickness change: slow, if slower than −1.0 μm/year; moderate, if between −1.0 and −2.0 μm/year; fast, if faster than −2.0 μm/year.
Financial Support:
Supported in part by National Institutes of Health/National Eye Institute grant EY029885 (FAM). The funding organization had no role in the design or conduct of this research.
Abbreviations:
- AGIS
Advanced Glaucoma Intervention Study
- BLUP
best linear unbiased predictions
- CCT
central corneal thickness
- CPT
Current Procedural Terminology
- EHR
electronic health records
- GAT
Goldmann applanation tonometry
- ICD
international classification of diseases
- IOP
intraocular pressure
- GS
glaucoma suspects
- LMM
linear mixed models
- POAG
primary open-angle glaucoma
- SAP
standard automated perimetry
- SD OCT
spectral-domain optical coherence tomography
- RNFL
retinal nerve fiber layer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: A.A.J.: none. A.C.T.: none. E.B.M.: none. T.E.: none. L.S.S.: none. S.I.B.: none. S.S.S.: none. F.A.M.: Aeri Pharmaceuticals (C); Allergan (C, F), Annexon (C); Biogen (C); Carl Zeiss Meditec (C, F), Galimedix (C); Google Inc. (F); Heidelberg Engineering (F), IDx (C); nGoggle Inc. (P), Novartis (F); Stealth Biotherapeutics (C); Reichert (C, F)
REFERENCES
- 1.Mariotti SP. Global Data on Vision Impairments 2010 Bull World Health Organ. Switzerland: World Health Organization, 2012. [Google Scholar]
- 2.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007;114(11):1965–72. [DOI] [PubMed] [Google Scholar]
- 3.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120(6):714–20; discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 4.European Glaucoma Prevention Study G, Miglior S, Pfeiffer N, et al. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology 2007;114(1):3–9. [DOI] [PubMed] [Google Scholar]
- 5.The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am J Ophthalmol 2000;130(4):429–40. [DOI] [PubMed] [Google Scholar]
- 6.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet 2015;385(9975):1295–304. [DOI] [PubMed] [Google Scholar]
- 7.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003;121(1):48–56. [DOI] [PubMed] [Google Scholar]
- 8.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002;120(10):1268–79. [DOI] [PubMed] [Google Scholar]
- 9.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120(6):701–13; discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 10.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108(11):1943–53. [DOI] [PubMed] [Google Scholar]
- 11.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 1998;126(4):487–97. [DOI] [PubMed] [Google Scholar]
- 12.Newman-Casey PA, Niziol LM, Gillespie BW, et al. The Association between Medication Adherence and Visual Field Progression in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology 2020;127(4):477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Moraes CG, Juthani VJ, Liebmann JM, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol 2011;129(5):562–8. [DOI] [PubMed] [Google Scholar]
- 14.Krumholz HM. Big data and new knowledge in medicine: the thinking, training, and tools needed for a learning health system. Health Aff (Millwood) 2014;33(7):1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Research Council. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington, DC: The National Academies Press, 2011. [PubMed] [Google Scholar]
- 16.Patel S, Sternberg P Jr. Real-World Data in Ophthalmology. Am J Ophthalmol 2020. [DOI] [PubMed] [Google Scholar]
- 17.Medeiros FA, Lisboa R, Weinreb RN, et al. A combined index of structure and function for staging glaucomatous damage. Arch Ophthalmol 2012;130(9):1107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garway–Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the Hill of Vision: The Physiological Relationship between Light Sensitivity and Ganglion Cell Numbers. Investigative Ophthalmology & Visual Science 2000;41(7):1774–82. [PubMed] [Google Scholar]
- 19.Abe RY, Diniz-Filho A, Zangwill LM, et al. The Relative Odds of Progressing by Structural and Functional Tests in Glaucoma. Invest Ophthalmol Vis Sci 2016;57(9):OCT421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jammal AA, Thompson AC, Mariottoni EB, et al. Rates of Glaucomatous Structural and Functional Change from a Large Clinical Population: The Duke Glaucoma Registry Study. Am J Ophthalmol 2020;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leite MT, Rao HL, Zangwill LM, et al. Comparison of the diagnostic accuracies of the Spectralis, Cirrus, and RTVue optical coherence tomography devices in glaucoma. Ophthalmology 2011;118(7):1334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varma R, Bazzaz S, Lai M. Optical tomography-measured retinal nerve fiber layer thickness in normal latinos. Invest Ophthalmol Vis Sci 2003;44(8):3369–73. [DOI] [PubMed] [Google Scholar]
- 23.Patel NB, Lim M, Gajjar A, et al. Age-associated changes in the retinal nerve fiber layer and optic nerve head. Invest Ophthalmol Vis Sci 2014;55(8):5134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowd C, Zangwill LM, Weinreb RN, et al. Estimating Optical Coherence Tomography Structural Measurement Floors to Improve Detection of Progression in Advanced Glaucoma. Am J Ophthalmol 2017;175:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asrani S, Essaid L, Alder BD, Santiago-Turla C. Artifacts in spectral-domain optical coherence tomography measurements in glaucoma. JAMA Ophthalmol 2014;132(4):396–402. [DOI] [PubMed] [Google Scholar]
- 26.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38(4):963–74. [PubMed] [Google Scholar]
- 27.Medeiros FA, Zangwill LM, Weinreb RN. Improved prediction of rates of visual field loss in glaucoma using empirical Bayes estimates of slopes of change. J Glaucoma 2012;21(3):147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medeiros FA, Zangwill LM, Mansouri K, et al. Incorporating risk factors to improve the assessment of rates of glaucomatous progression. Invest Ophthalmol Vis Sci 2012;53(4):2199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson GK. That BLUP is a Good Thing: The Estimation of Random Effects. Statistical Science 1991;6(1):15–32. [Google Scholar]
- 30.Diniz-Filho A, Abe RY, Zangwill LM, et al. Association between Intraocular Pressure and Rates of Retinal Nerve Fiber Layer Loss Measured by Optical Coherence Tomography. Ophthalmology 2016;123(10):2058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu T, Tatham AJ, Gracitelli CP, et al. Rates of Retinal Nerve Fiber Layer Loss in Contralateral Eyes of Glaucoma Patients with Unilateral Progression by Conventional Methods. Ophthalmology 2015;122(11):2243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C, Tatham AJ, Abe RY, et al. Corneal Hysteresis and Progressive Retinal Nerve Fiber Layer Loss in Glaucoma. Am J Ophthalmol 2016;166:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nouri-Mahdavi K, Hoffman D, Ralli M, Caprioli J. Comparison of methods to predict visual field progression in glaucoma. Arch Ophthalmol 2007;125(9):1176–81. [DOI] [PubMed] [Google Scholar]
- 34.Rabiolo A, Morales E, Mohamed L, et al. Comparison of Methods to Detect and Measure Glaucomatous Visual Field Progression. Transl Vis Sci Technol 2019;8(5):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayama C, Araie M, Suzuki Y, et al. Statistical evaluation of the diagnostic accuracy of methods used to determine the progression of visual field defects in glaucoma. Ophthalmology 2004;111(11):2117–25. [DOI] [PubMed] [Google Scholar]
- 36.Gracitelli CP, Abe RY, Tatham AJ, et al. Association between progressive retinal nerve fiber layer loss and longitudinal change in quality of life in glaucoma. JAMA Ophthalmol 2015;133(4):384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuang TM, Zhang C, Zangwill LM, et al. Estimating Lead Time Gained by Optical Coherence Tomography in Detecting Glaucoma before Development of Visual Field Defects. Ophthalmology 2015;122(10):2002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medeiros FA, Alencar LM, Zangwill LM, et al. Prediction of functional loss in glaucoma from progressive optic disc damage. Arch Ophthalmol 2009;127(10):1250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chauhan BC, Malik R, Shuba LM, et al. Rates of glaucomatous visual field change in a large clinical population. Invest Ophthalmol Vis Sci 2014;55(7):4135–43. [DOI] [PubMed] [Google Scholar]
- 40.European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Chapter 3: Treatment principles and options Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 3 Treatment principles and options. Br J Ophthalmol 2017;101(6):130–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prum BE Jr., Rosenberg LF, Gedde SJ, et al. Primary Open-Angle Glaucoma Preferred Practice Pattern((R)) Guidelines. Ophthalmology 2016;123(1):P41–P111. [DOI] [PubMed] [Google Scholar]
- 42.Jasien JV, Turner DC, Girkin CA, Downs JC. Cyclic Pattern of Intraocular Pressure (IOP) and Transient IOP Fluctuations in Nonhuman Primates Measured with Continuous Wireless Telemetry. Curr Eye Res 2019;44(11):1244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barkana Y, Anis S, Liebmann J, et al. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol 2006;124(6):793–7. [DOI] [PubMed] [Google Scholar]
- 44.Vianna JR, Danthurebandara VM, Sharpe GP, et al. Importance of Normal Aging in Estimating the Rate of Glaucomatous Neuroretinal Rim and Retinal Nerve Fiber Layer Loss. Ophthalmology 2015;122(12):2392–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Distribution of the Eyes Included in the Study According to Baseline (A) Age, (B) Spectral-Domain Optical Coherence Tomography (SD OCT) Global Retinal Nerve Fiber Layer (RNFL) Thickness and (C) Mean Intraocular Pressure (IOP) During Follow-up, (D) Follow-up Time, and (E) Number of SD OCT and (F) IOP Visits Per Eye.
Supplemental Figure 6. Proportion of Eyes Classified as Slow, Moderate, and Fast Progressors According to the Rates of Change of Spectral-Domain Optical Coherence Tomography (SD OCT) Retinal Nerve Fiber Layer (RNFL) Thickness Among Eyes with at Least Four Intraocular Pressure (IOP) Measurements During Follow-up. Eyes Are Grouped According to the Percentage of Visits with IOP Below (A) 21mmHg, (B) 18mmHg and (C) 15mmHg During Follow-up.
Rates of SD OCT global RNFL thickness change: slow, if slower than −1.0 μm/year; moderate, if between −1.0 and −2.0 μm/year; fast, if faster than −2.0 μm/year.
Supplemental Figure 4. Proportion of Eyes Classified as Slow, Moderate, and Fast Progressors According to Rates of Change of Spectral-Domain Optical Coherence Tomography (SD OCT) Retinal Nerve Fiber Layer (RNFL) Thickness for Glaucoma Suspects (GS), Primary Open-Angle Glaucoma (POAG) and Other Glaucoma Diagnoses. Eyes Are Grouped According to the Percentage of Visits with Intraocular Pressure (IOP) Below 18mmHg During Follow-up.
Rates of SD OCT global RNFL thickness change: slow, if slower than −1.0 μm/year; moderate, if between −1.0 and −2.0 μm/year; fast, if faster than −2.0 μm/year.


