Abstract
Background:
There are significant knowledge gaps of the vulnerabilities faced by youth from families with histories of alcohol or substance misuse. This study aimed to provide a comprehensive assessment of problems experienced by substance-naive children with positive family histories of substance misuse (FHP).
Methods:
Baseline data from up to 11,873 children (52.1% male), aged 9.0–10.9 years (M=9.9±0.6), enrolled in the US-based Adolescent Brain Cognitive Development Study® were utilized. Mixed models tested cross-sectional associations between family history of substance misuse, assessed categorically and continuously, with neurobiological, cognitive, behavioral, and psychological outcomes, when controlling for confounding factors, including family history of psychopathology, and correcting for multiple comparisons.
Results:
One in four (26.3%) youth were categorized as FHP (defined as ≥one parent or ≥two grandparents with misuse history). Controlling for confounding, FHP youth exhibited thinner whole cortices and greater surface area in frontal and occipital regions than youth with no such history (ps<.001, |ds|≥0.04). FHP youth experienced greater psychopathology and sleep disturbance (ps<.001, |ds| ≥ 0.36) and were more likely to be diagnosed with multiple mental disorders (odds ratios≥1.22, ps<.001), with severity of effects dependent on family history density of substance misuse. Differences in cognition, impulsivity, and motivation were non-significant. Psychopathology, mental disorders, and sleep disturbance were negatively correlated with various neural indices (|rs|=0.01–0.05, ps<.05).
Conclusions:
At age 9–10 years, FHP youth can experience numerous problems, with psychopathology and mental disorders being some of the most significant. Therefore, prevention efforts should target psychopathology vulnerabilities in FHP children.
Keywords: family history, substance use disorder, alcohol use disorder, addiction, mental disorder, brain structure
1. Introduction
Alcohol and other substance use disorders (SUDs) are widespread disorders that are increasing in occurrence, with a recent 12-month prevalence rate of 7.8% to 12.7% among US adults (Grant et al., 2017, SAMHSA, 2019). SUDs are associated with negative outcomes such as work absence, homelessness, mental disorders, suicide, accidents, and violence (Teesson et al., 2010, Schifano et al., 2020). Additionally, SUDs are related to a number of cognitive impairments and changes in brain structure and connectivity (Ramey and Regier, 2018, Lees et al., 2020b). Several factors lead to the development of SUDs, including genetics as demonstrated through twin and adoption studies, as well as environmental and psychological influences (Enoch and Goldman, 2001, Enoch, 2006). Heritability estimates of SUDs range from 30% to 60% (Verhulst et al., 2015, Knopik et al., 2004), and a shared environment with an individual with SUD explains an additional 10% of the risk (Verhulst et al., 2015). Approximately one in four children in the US grow up with an individual with SUD in their immediate family (Grant, 2000). Individuals with a first-degree relative who has a history of SUD (i.e., family history positive [FHP]) are approximately four to nine times more likely to develop a SUD in their lifetimes, compared to individuals with no such history (FHN) (Merikangas et al., 1998). In addition, the likelihood of developing a SUD is correlated with the number of affected first- and second-degree relatives (i.e., family history density [FHD]) (Dawson et al., 1992). For these reasons, FHP substance-naïve youth with affected first- and second-degree relatives have been identified as ‘at-risk’ individuals for onset of SUD within the research community (Cservenka, 2016).
A large body of literature has examined the neurobiological mechanisms by which heritable risk for SUD may be partly transmitted. Functional neuroimaging studies suggest that FHP youth exhibit altered neural activation during executive functioning, reward responsiveness, and emotional processing tasks (Cservenka, 2016, Lees et al., 2020a, Tervo-Clemmens et al., 2020). Likewise, cognitive studies have identified poorer behavioral performance on measures of inhibition, working memory, and impulsivity for FHP compared to FHN youth (for review, see Squeglia and Cservenka, 2017). Relatively few studies, however, have focused on structural brain differences in FHP youth. A 2019 review identified that FHP youth have altered brain volume in several frontal and subcortical regions compared to FHN, with little to no information available on cortical thickness and area differences (Comstock et al., 2019). When compared to FHN peers, FHP youth had lower frontal (Benegal et al., 2007) and amygdala volumes (Benegal et al., 2007; Dager et al., 2015; Hill et al., 2001, 2013) and greater cerebellar volumes (Benegal et al., 2007; Hill et al., 2007, 2011, 2016). Some studies reported no group differences in hippocampal (Dager et al., 2015; Hill et al., 2001) or nucleus accumbens volume (Squeglia et al., 2014), while others had reported greater hippocampal volume among FHP males (Hanson et al., 2010) and greater nucleus accumbens volumes among FHP females (Cservenka et al., 2015). Several studies identified have utilized small samples that are underpowered to detect small to moderate effects and have often used a selective regions-of-interest approach. Furthermore, a number of studies have examined offspring who use alcohol or other substances themselves, which likely confounds family history results (Comstock et al., 2019). A comprehensive evaluation of multiple morphometric indicators (i.e., volume, cortical thickness, surface area) examining widespread brain regions in a large sample of substance-naïve youth may resolve previous conflicting findings and provide more reliable biomarkers in ‘at-risk’ youth of later substance use problems.
In addition to neurobiological and cognitive risk indicators in FHP youth, heightened psychopathology may also increase vulnerability to onset of SUDs. Multiple studies show that FHP children have an increased risk for developing externalizing disorders, including conduct disorder, oppositional defiant disorder, and attention deficit hyperactivity disorder (ADHD) (Vidal et al., 2012, Hill et al., 2011). Increased odds of anxiety and affective disorders have also been observed in some studies (Hill and Muka, 1996, Hill et al., 2008). Limitations of these studies exist. First, the large majority of studies have not adjusted for factors other than family history of SUD that may influence the risk of psychopathology in FHP youth, such as comorbid psychopathology in family members, parenting style, or prenatal exposures (Lees et al., 2020c). Second, only a small number of studies have assessed elevations in risk with the number of affected first- and second-degree FHP relatives (i.e., FHD). Third, potential mechanisms underlying, or contributing to, these disorders are understudied in this population. For example, psychopathology may be a function of altered neurobiological mechanisms in FHP youth (Benegal et al., 2007), as well as environmental stressors and familial relationships (Ryan et al., 2016, Barnow et al., 2002). In summary, there are significant gaps in knowledge of the specific vulnerabilities that may antedate the development of SUDs in this ‘at-risk’ population. A better understanding of the problems FHP youth experience is essential in order to improve targeted prevention initiatives.
The aim of the current study was to provide a comprehensive assessment of neurobiological, cognitive, behavioral, and psychological factors related to FHP and FHD. Data from youth aged 9 to 10 years enrolled in the Adolescent Brain Cognitive Development (ABCD) Study® were utilized. Based on the current evidence base, it was hypothesized that FHP youth, with at least one parent or two grandparents with a history of substance use problems, would exhibit altered brain morphometry, greater impulsivity, and poorer executive functioning compared to FHN youth, with no first- or second-degree relatives with a history of SUD. It was also hypothesized that FHP youth would experience greater psychopathology and would be more likely to endorse low-level alcohol use experimentation (e.g., sipping) than FHN peers. Finally, it was predicted that greater FHD would be associated with poorer outcomes in youth.
2. Methods
2.1. Participants
This study examined baseline cross-sectional data from 11,873 children (52.1% male), aged 9.0–10.9 years (M=9.9±0.6), included in the ABCD Study® annual release 2.0.1. A detailed account of the recruitment strategy has been previously published (Garavan et al., 2018). A probability sample was recruited through schools, selected based on sex, race/ethnicity, socioeconomic status, and urbanicity. All parents/caregivers provided written informed consent and all children provided assent to the research protocol approved by the institutional review board at each of the 21 data collection sites.
2.2. Explanatory Measures
The presence of lifetime symptoms associated with SUDs in biological parents and grandparents were assessed in the modified, parent-reported Family History Assessment Module Screener (Rice et al., 1995). Other second-degree relatives (i.e., aunts, uncles, half siblings) were not considered in the primary analyses due to >20% missing data and because parents’ account of more distal relatives are likely to be less accurate (Andreasen et al., 1977, 1986; Thompson et al., 1982). Based on previous definitions (Cservenka, 2016), a three-level categorical variable was derived where youth were categorized as: 1) ‘FHP’ if they had ≥one biological parent or ≥two biological grandparents with histories of alcohol and/or other substance use problems, 2) ‘FHN’ if they had no parent or grandparent with histories of problems, or 3) ‘N/A’ if they had just one grandparent with histories of problems (data available for n=11,873). To evaluate the extent to which the presence of substance-related problems may contribute to childhood outcomes, FHD scores were calculated based on the sum of positive reports of problems from biological parents (+0.5) and biological grandparents (+0.25). The FHD scores could range from 0 to 4, with a score of 0 indicating absence of problems (data available for n=11,298). Participants categorized as ‘N/A’ were excluded from FHP categorical analyses but were included in the FHD analyses. See the Supplement for relevant questionnaire items and further details on derived variables.
2.3. Outcome Measures
2.3.1. Structural brain indices (youth)
Indices of cortical thickness, surface area, and volume were examined from 34 cortical parcellations and 8 subcortical (volume only) segmentations per hemisphere based on the Desikan-Killiany brain registration atlas (Desikan et al., 2006). Volumetric and area, but not thickness, measures were corrected for intracranial volume (Schmansky, 2020). Details on MRI data acquisition are described in detail elsewhere (Casey et al., 2018) and briefly in the Supplement.
2.3.2. Cognition (youth performance)
The cognitive assessment included seven NIH Toolbox® tasks and composite scores for total cognition (based on all tasks), fluid cognition (Pattern Comparison, Working Memory, Sequential Memory, Flanker, Card Sort), and crystallized cognition (Vocabulary, Reading Recognition) (Luciana et al., 2018). All scores were age-corrected standard scores, where higher scores indicate greater cognitive performance. Both composite scores and individual task scores were examined as outcomes of interest.
2.3.3. Impulsivity and motivation (youth report)
Impulsivity was assessed using the Urgency, Premeditation, Perseverance, Sensation Seeking, Positive Urgency, Impulsive Behavior Scale for Children-Short Form (UPPS-P)(Whiteside et al., 2005). Motivation was examined using the four subscales of the Behavioral Avoidance/Inhibition Scales (BIS/BAS)(Pagliaccio et al., 2016).
2.3.4. Psychopathology and lifetime mental disorder diagnoses (parent report)
Psychopathology was examined in children using the eight empirically based syndrome scales and higher order factors of the Child Behavior Checklist (CBCL)(Achenbach, 2009). The internalizing factor was calculated from three scales (anxious depressed, withdrawal, somatic complaints), the externalizing factor from two scales (rule breaking, aggressive), and the total problems factor from all syndrome scales (previous scales in addition to social problems, thought problems, attention deficits). Here, higher scores indicate greater psychopathology. Both syndrome scales and higher order factors were examined as outcomes of interest. Lifetime mental disorder diagnoses (i.e., past and/or present) were determined from the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS), based on DSM-V criteria (Kobak et al., 2013). The low prevalence of panic disorder (n=27), hallucinations (n=45), and agoraphobia (n=55), precluded the examination of these disorders in the current study.
2.3.5. Sleep disturbance (parent report)
The Sleep Disturbance Scale for Children total score was utilized, where higher scores reflect greater sleep disturbance (Bruni et al., 1996).
2.3.6. Youth alcohol experimentation (youth report)
Lifetime endorsement of non-religious low-level alcohol use (i.e., sip of alcohol) was assessed via the youth-reported iSay Sip Inventory (Jackson et al., 2015).
2.4. Covariates
The following twelve fixed covariates were included in all statistical models and were dummy coded (see Supplement for details): 1) sex, 2) race/ethnicity, 3) parent education, 4) household income, 5) marital status, 6) prenatal alcohol exposure, 7) prenatal substance use exposure, and family history of psychopathology in first and/or second degree relatives related to 8) psychosis, 9) depression, 10) anxiety, 11) antisocial behavior, and 12) mania (Table 1). Youth age was included as a continuous fixed effect. During sensitivity analysis, youth-reported parental monitoring and parental warmth/acceptance and parent-reported family conflict were included as three additional continuous fixed effects.
Table 1:
Sociodemographic characteristics of youth with a negative (FHN) or positive family history of substance use problems (FHP). (n=10,0811).
| FHN | FHP | p | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Total Participants | 6,955 | 69.0 | 3,126 | 31.0 | |
| Male | 3,631 | 52.2 | 1,597 | 51.1 | .292 |
| Age (m [SD]) | 9.9 | 0.6 | 9.9 | 0.6 | .943 |
| Race/Ethnicity a | <.001 | ||||
| White | 3,632 | 52.2 | 1,534 | 49.1 | |
| Black | 1,064 | 15.3 | 479 | 15.3 | |
| Hispanic | 1,371 | 19.7 | 694 | 22.2 | |
| Asian | 219 | 3.1 | 12 | 0.4 | |
| Other | 659 | 9.5 | 402 | 12.9 | |
| Household Income a | <.001 | ||||
| 25–50K | 1,669 | 24.0 | 1,192 | 38.1 | |
| 50–100K | 1,688 | 24.3 | 869 | 27.8 | |
| >100K | 2,972 | 42.7 | 818 | 26.2 | |
| Don’t know/refuse to answer | 626 | 9.0 | 247 | 7.9 | |
| Parent Education a | <.001 | ||||
| <High school | 468 | 6.7 | 233 | 7.5 | |
| High school/GED equivalent | 705 | 10.1 | 402 | 12.9 | |
| College | 1,726 | 24.8 | 1,250 | 40.0 | |
| Bachelor’s degree | 2,080 | 29.9 | 711 | 22.7 | |
| Post graduate degree | 1,963 | 28.2 | 527 | 16.9 | |
| Married | 5,466 | 78.6 | 1,822 | 58.3 | <.001 |
| In utero alcohol exposure | 1,401 | 20.1 | 1,004 | 32.1 | <.001 |
| In utero drug exposure | 248 | 3.6 | 506 | 16.2 | <.001 |
| Family history of depression b | 2,986 | 42.9 | 2,151 | 68.8 | <.001 |
| Family history of anxiety | 1,368 | 19.7 | 1,197 | 38.3 | <.001 |
| Family history of anti-social behavior | 1,199 | 17.2 | 1,756 | 56.2 | <.001 |
| Family history of mania | 619 | 8.9 | 702 | 22.5 | <.001 |
| Family history of psychosis | 464 | 6.7 | 548 | 17.5 | <.001 |
Of 11,873 participants with available data on family history of substance use-related problems, 1,792 participants had just one grandparent (and no parent) with a history of problems and therefore did not meet criteria for FHP or FHN.
There was missing data for the following variables: Race/ethnicity (FHN=10, FHP=5), Parent education (FHN=13, FHP=3).
Family history of psychopathology variables includes any first or second degree relative with past or present problems.
2.5. Statistical Analysis
All continuous outcome variables were winsorized to ±3 SD to minimize the influence of extreme values. A series of linear mixed models were performed using R version 4.0.0 (‘glmmTMB’ package (Wood, 2017)). Model parameters were estimated by the Restricted Maximum Likelihood. Random intercept parameters accounted for family membership (i.e., siblings, twins, triplets) and research site or MRI scanner (i.e., for analyses with brain indices only), where family membership was nested within 22 research sites or 29 scanner sites (as some research sites have multiple scanners). Participants with missing data were excluded from analyses.
First, associations between the categorical family history variable (FHP/FHN) and outcomes of interest were examined when accounting for fixed effects (see section 2.4). Youth categorized as ‘N/A’ (i.e., with just one grandparent with a history of alcohol or substance-use related problems) were excluded from this analysis (n=1,792). Second, additive effects of family problems were explored by entering FHD continuous scores as predictors alongside the fixed covariates. Spline regression models were estimated for continuous outcome variables to model smooth functions, including linear and non-linear associations (‘mgcv’ package). Third, in order to explore whether aberrant structural brain indices were related to other problems observed in FHP youth, follow-up bivariate Pearson correlations were conducted between all outcome variables significantly associated with FHP. Finally, two sensitivity analyses were conducted where: 1) significant behavioral analyses were re-run after entering parental monitoring, parental warmth/acceptance, and family conflict as additional fixed covariates to adjust for possible confounding effects of parenting, and 2) categorical family history analyses were re-run after excluding other relatives (i.e., aunts, uncles, siblings) from the FHN group with known parent-reported histories of alcohol or other substance use problems. The false discovery rate (FDR) was utilized to correct for multiple comparisons in primary and sensitivity analyses, run across all behavioral models (38 FDR-corrected comparisons) and run separately across each series of morphometric indices (34 FDR-corrected comparisons for cortical thickness and area parcellations, 42 FDR-corrected comparisons for cortical volume parcellations and subcortical volume segmentations), where pFDR<.05 was equivalent to p<.001 (Benjamini and Hochberg, 1995). A post-hoc analysis confirmed there were no matrilineal- or patrilineal-specific associations with child outcomes.
3. Results
3.1. FHP child outcomes
Of the 11,873 youth (52.1% male, 9.9±0.6 years) with data available on family history of SUD, 4,918 (41.4%) had at least one biological parent or grandparent with a history of alcohol or substance use related problems. For the categorical analysis, 3,126 (26.3%) were classified as FHP (i.e., ≥one parent or ≥two grandparents with a history of problems), 6,955 (58.6%) were classified as FHN (i.e., no first-degree family history of problems), and 1,792 (15.1%) were excluded because they had just one grandparent with a history of problems. A summary of all covariate associations is provided in the Supplement.
3.1.1. Brain structure, cognition, impulsivity, and motivation
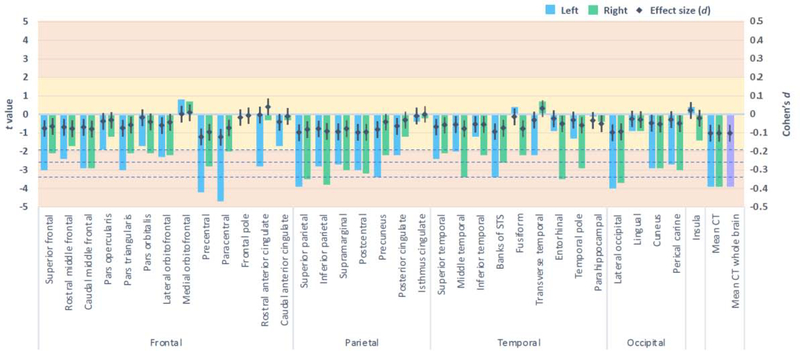
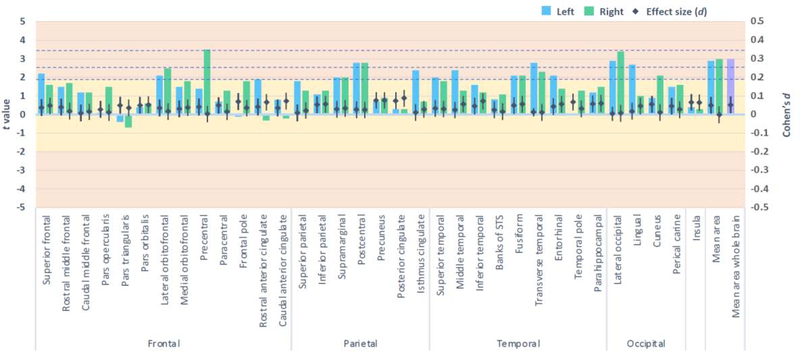
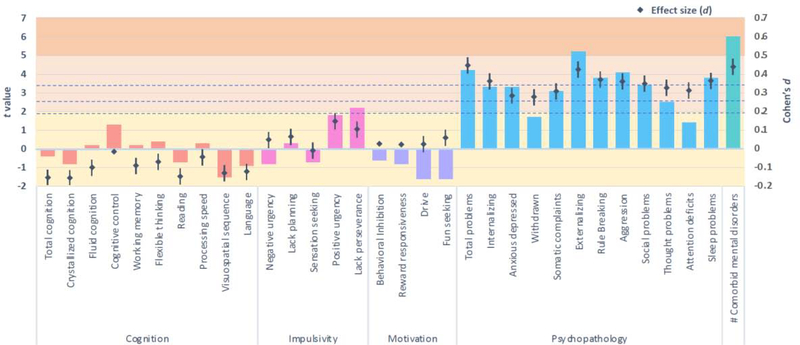
FHP youth exhibited lower whole brain cortical thickness than FHN youth (pFDR<.05, d = −0.1), when adjusting for covariates, including age, sex, race/ethnicity, parent education, household income, marital status, prenatal alcohol exposure, prenatal substance use exposure, and family histories of five types of psychopathology (Figure 1). In adjusted models, FHP youth also exhibited thinner cortices in nine specific regions, including the left precentral and paracentral lobules, bilateral superior and right inferior parietal lobules, left precuneus, right middle temporal gyrus, left banks of the superior temporal sulcus, right entorhinal cortex, and bilateral lateral occipital sulcus (pFDRs<.05, ds ≤ −0.05)(Figure 1). FHP youth also exhibited greater cortical area in the right precentral lobule and right lateral occipital sulcus than FHN peers (pFDRs<.05, ds = 0.04)(Figure 2). No group differences for brain volume passed FDR correction (Supplement Figure 1). Furthermore, no group differences for cognitive performance or for measures of impulsivity or motivation passed FDR correction (Figure 3).
Figure 1:
Cortical thickness among family history positive compared to negative youth, when adjusting for fixed and random effects.
Columns represent the t values derived from mixed models (left axis) and the dot plot illustrates the accompanying effect sizes (Cohen’s d with 95% confidence intervals; right axis). Dotted horizontal lines reflect p values .05, .01, and .001 (pFDR=.05). Yellow shading indicates the very small effect size range and orange indicates the small range.
Figure 2:
Cortical area among family history positive compared to negative youth, when adjusting for fixed and random effects.
Columns represent the t values derived from mixed models (left axis) and the dot plot illustrates the accompanying effect sizes (Cohen’s d with 95% confidence intervals; right axis). Dotted horizontal lines reflect p values .05, .01, and .001 (pFDR=.05). Yellow shading indicates the very small effect size range and orange indicates the small range.
Figure 3 :
Associations between family history positive youth and behavioural outcomes, when adjusting for fixed and random effects.
Columns represent the t values derived from mixed models (left axis) and the dot plot illustrates the accompanying effect sizes (Cohen’s d with 95% confidence intervals; right axis). Dotted horizontal lines reflect p values .05, .01, and .001 (pFDR=.05). Yellow shading indicates the very small effect size range, light orange indicates small, and dark orange indicates the medium range.
3.1.2. Psychopathology and lifetime mental disorders
Compared to FHN peers, FHP youth exhibited significantly greater total psychopathological problems, driven by externalizing syndrome scales, in addition to greater sleep disturbance, when adjusting for covariates (pFDRs<.05, ds ≥ 0.36)(Figure 3). In adjusted models, FHP youth were significantly more likely to have a lifetime mental disorder diagnosis than FHN youth and were at increased odds of presenting with multiple mental disorders, when examined both categorically (Table 2) and continuously (Figure 3)(pFDRs<.001). Compared to FHN, FHP youth were more likely to have a lifetime diagnosis of separation anxiety, post-traumatic stress disorder, and specific phobias in adjusted models (all ORs>1.22, pFDRs<.001)(Table 2). Prevalence rates of all mental disorders examined are available in Table 2 and severity of psychopathology for all CBCL measures are available in Supplement Figure 2.
Table 2:
Summary of the odds of youth from families with substance use problems (FHP) experiencing mental disorders compared to youth from families with no problems (FHN).
| Lifetime mental disorder diagnoses | FHN (%) | FHP (%) | aORa | 95% CI | t | p(FDR) |
|---|---|---|---|---|---|---|
| Number of disorders | ||||||
| 1+ Disorder(s) | 45.1 | 60.0 | 1.22 | 1.09–1.36 | 3.39 | <.001 |
| 2+ Disorders | 20.3 | 36.5 | 1.37 | 1.20–1.58 | 4.49 | <.001 |
| 3+ Disorders | 9.9 | 22.4 | 1.55 | 1.30–1.84 | 5.00 | <.001 |
| 4+ Disorders | 4.4 | 13.5 | 1.83 | 1.47–2.28 | 5.44 | <.001 |
| Specific diagnoses | ||||||
| Depression | 1.7 | 4.7 | 1.46 | 1.07–1.99 | 2.38 | .578 |
| Generalized Anxiety | 3.1 | 7.3 | 1.46 | 0.49–4.29 | 0.68 | .496 |
| Separation Anxiety | 6.5 | 14.1 | 1.54 | 1.29–1.84 | 4.70 | <.001 |
| Social Anxiety | 3.8 | 6.4 | 1.15 | 0.90–1.46 | 1.12 | .261 |
| Delusions | 1.4 | 2.9 | 1.29 | 0.90–1.086 | 1.37 | .171 |
| ADHD | 17.1 | 28.5 | 1.18 | 1.03–1.34 | 2.38 | .578 |
| Oppositional Defiant | 11.3 | 20.0 | 1.25 | 1.07–1.46 | 2.85 | .136 |
| Conduct Disorder | 2.1 | 5.7 | 1.43 | 1.06–1.92 | 2.34 | .646 |
| OCD | 7.9 | 12.9 | 1.17 | 0.98–1.40 | 1.72 | .085 |
| Bipolar/Related Disorder | 3.2 | 4.9 | 1.09 | 0.83–1.43 | 0.65 | .517 |
| PTSD | 0.9 | 4.9 | 2.68 | 1.82–3.96 | 4.96 | <.001 |
| Specific Phobia | 23.6 | 32.7 | 1.22 | 1.08–1.38 | 3.25 | .034 |
aOR = adjusted odds ratio, ADHD = attention deficit hyperactivity disorder, CI = confidence interval, OCD = obsessive compulsive disorder, PTSD = post-traumatic stress disorder.
Reference group = FHN youth.
3.1.3. Youth alcohol experimentation
No significant group difference was observed for youth lifetime endorsement of sipping alcohol by ages 9 to 10 years, when adjusting for covariates (OR = 0.98, 95% CI 0.85–1.14, p = .80).
3.2. FHD child outcomes
Of the 11,298 youth with available FHD data, 4,478 (39.6%) had at least one parent or grandparent with a history of alcohol or other substance use-related problems. Among those with a parent and/or grandparent with SUD-related problems, the mean density score was 0.8±0.8 and the maximum was 4 (see Supplement Figure 3 for histogram of FHD scores).
Negative associations were observed between FHD and regional cortical thickness in the left paracentral and right inferior parietal lobules and in the left banks of superior temporal sulcus (pFDRs<.05; Supplement Table 1). All behavioral associations observed for FHP youth were replicated in FHD analyses, excluding phobia disorders. Additionally, greater FHD was also associated with greater internalizing psychopathology, social and thought problems, as well as greater odds of a lifetime diagnosis of depression, oppositional defiant disorder, and conduct disorder (pFDRs<.05; Supplement Table 2). Spline models demonstrated linear associations between FHD and all significant outcomes.
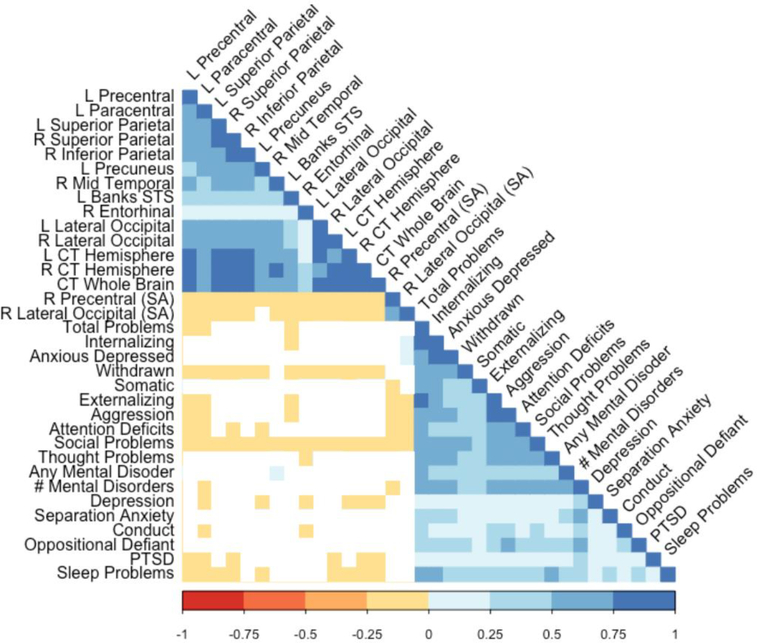
Based on the finding that there were significant group and FHD differences for neural indices, additional analyses were conducted to determine whether structural differences were related to variability in psychopathology and mental disorders. Figure 4 illustrates follow-up bivariate correlational analyses which indicated that various cortical thickness and surface area measures were significantly and negatively related to psychopathology symptoms, sleep disturbance, as well as lifetime post-traumatic stress disorder diagnoses (rs=−0.01 to −0.05, p<.05). Correlations by FHP and FHN groups are reported in Supplement Figures 4–5.
Figure 4:
Significant correlations (colored squares) are illustrated for childhood outcomes found to be associated with family history of substance use problems (n=10,299). White squares indicate non-significant correlations.
3.3. Sensitivity analyses
When parental monitoring, parental warmth/acceptance, and family conflict were included as additional fixed covariates, the majority of significant findings remained consistent with the exceptions of greater total and internalizing psychopathology and greater odds of meeting criteria for oppositional defiant disorder and conduct disorder (Supplement Table 3–4). Findings were consistent when excluding youth with known positive histories of SUD-related problems among aunts, uncles, or siblings (Supplement Table 5).
4. Discussion
The present study provided a comprehensive assessment of the neurobiological, cognitive, behavioral, and psychological problems experienced by substance-naïve youth aged 9–10 years with a family history of alcohol or other substance use problems. Consistent with hypotheses, the findings indicate that, even after controlling for sociodemographic factors, prenatal substance exposure, and family history of psychopathology, FHP youth exhibit altered brain structure (very small effect), greater psychopathology (small effect), and sleep disturbance (small effect), with the severity of most problems dependent upon FHD. Compared to FHN, FHP youth were 22% (95% CI 9–36%) more likely to have at least one mental disorder, with greater FHD linked to increased odds of both externalizing (conduct, oppositional defiant) and internalizing (depression, separation anxiety, PTSD) diagnoses. FHP youth also experienced clinical comorbidity. FHP youth, compared to FHN, were 37% (20–58%) more likely to have ≥two mental disorders, 55% (30–84%) more likely to have ≥three disorders, and 83% (47–128%) more likely to have ≥four mental disorders. In contrast to hypotheses, FHP youth did not perform significantly worse on cognitive tasks, were not more impulsive, and did not endorse alcohol sips more than FHN youth. These findings were in a largely substance-naïve cohort of youth who had not consumed a full drink of alcohol or tried other substances (99.999%), negating potential confounding effects of offspring substance use.
4.1. Comparison of identified FHP vulnerabilities with previous studies
The current neurobehavioral findings replicate a study by Henderson et al.(2018) who reported lower cortical thickness in FHP compared to FHN substance-naïve adolescents and a study by Ryan et al.(2016) who reported null findings for cognition and impulsivity in a similarly aged substance-naïve cohort (M=10.97±0.84). However, in contrast to the present results, previous studies have also reported volumetric differences between FHP and FHN youth, and related cognitive and impulsivity deficits (for review, see Comstock et al., 2019, Cservenka, 2016, Squeglia and Cservenka, 2017, McPhee et al., 2018). While conflicting findings have been reported for hippocampal and nucleus accumbens volume (as summarized in the introduction), previous studies have consistently reported greater cerebellar and lower amygdala volume among FHP individuals (Comstock et al., 2019). Disparities in results are partly due to rigorous multiple comparisons correction in the current study (p<.001/pFDR<.05), where nominally significant group differences were observed for several morphometric indices (including cerebellar volume; see Supplement Figure 1) and a measure of impulsivity (p<.05). Additionally, post-hoc analysis of the psychometric properties of the impulsivity (UPPS-P) and motivation (BIS/BAS) scales indicated poor internal consistency for ~50% of the subscales which may explain the null findings in the current study (see Supplement Table 6). Conflicting findings with prior studies may also relate to the earlier neurodevelopment stage under investigation (i.e., preadolescence), possible sampling biases in previously underpowered studies, and disparities in covariate adjustments, such as prenatal alcohol and substance use exposures, family history of psychopathology, race/ethnicity, and socioeconomic factors, which accounted for much of the variance in brain volume and area, cognition, and impulsivity in the current study. Furthermore, much of the previous work on FHP and FHD have recruited older samples who already engage in substance use which is known to affect neurodevelopment (Lees et al., 2019). It has been suggested that reciprocal relations exist between impulsiveness, reward responsiveness, and substance use (Ryan et al., 2016), where past substance use is linked to increased sensation seeking and greater subsequent use, which in turn may negatively affect cognition (e.g., MacPherson et al., 2010). Considering the current cohort are largely substance-naïve, it will be important to examine growth trajectories of neurodevelopment, cognition, and impulsiveness as youth enter adolescence and some begin to experiment further with substance use.
Unique regional variations between cortical thickness and area measures were observed among FHP youth. While cortical thickness and surface area are both highly heritable, these measurements are found to be genetically and phenotypically independent, with unique regional variations particularly among youth undergoing critical neurodevelopmental changes (Panizzon et al., 2009). Additionally, small negative correlations between some cortical thickness and area measures were found, perhaps as a result of ‘cortical stretching’ (lower thickness with greater surface area); a normal neurodevelopmental process occurring throughout childhood involving early growth of white matter which stretches the adjacent gray matter tissue (Hogstrom et al., 2013). FHP youth may exhibit an exaggerated stretching pattern, although longitudinal examination of gray and white matter trajectories is required.
FHP youth were more likely to experience mental illness than FHN peers and an additive effect of FHD was found for the prevalence of mental disorders and severity of psychopathology. These findings are consistent with other studies that have compared psychopathology symptoms and disorders between FHP and FHN youth (e.g., Ryan et al., 2016, Vidal et al., 2012, Hill and Muka, 1996, El-Sheikh and Buckhalt, 2003). Of note, the severity of psychopathology symptoms did not reach clinically significant levels in the current study (CBCL T-Scores ≤ 60, pFDR<.05), which is also consistent with previous work in FHP preadolescents (Ryan et al., 2016). Importantly, this study adds to the existing literature by demonstrating that the effect of FHP and FHD on psychopathology and mental disorders were above and beyond that of socio-demographic characteristics, prenatal exposures, family history of psychopathology, and parenting factors.
Finally, despite FHP youth experiencing greater externalizing symptoms, these youth did not report significantly higher levels of impulsivity. Post-hoc correlation analyses indicated these variables were only weakly correlated (Supplement Figure 6), which could reflect the poor internal consistency of impulsivity subscales in the ABCD Study.
4.2. Potential mechanisms underlying psychopathology and pathways to future SUDs
The follow-up correlational analyses demonstrated that psychopathology and mental disorders were related to thinner cortices and greater regional cortical area, which adds to a growing body of literature proposing that neurobiological mechanisms may underlie or contribute to psychopathology in childhood and adolescence (Benegal et al., 2007, Parker et al., 2020, Lees et al., 2020f). Additionally, psychopathology was related to family functioning (see ‘Covariate Associations’ in Supplement File), albeit, independently of family history of substance use problems. Nevertheless, indications of adverse childhood experiences were observed in FHP youth, including youth experiencing the greatest odds of PTSD (OR=3.3, 95% CI=2.3–4.8) compared to any other mental disorder, high sleep disturbance, and reports of high family conflict. This is in accord with previous research which suggests FHP youth experience greater family disharmony and childhood psychopathology which increases vulnerability to later SUD (Barnow et al., 2002, El-Sheikh and Buckhalt, 2003). Future longitudinal analysis is required to delineate causal pathways between neurobiology, adverse childhood experiences, and psychopathology.
Unexpectedly, no group differences were observed for alcohol experimentation in the current cohort despite previous research demonstrating that FHP offspring are at much higher odds of having early-onset SUD themselves (Merikangas et al., 1998). This may suggest that a family history of SUD does not directly lead to curiosity about substance use, or substance use initiation and escalation in offspring. Instead, it may be the case that a number of mediating and moderating factors in FHP youth lead to later substance use problems. Previous studies have found that childhood externalizing disorders including conduct disorder and oppositional defiant disorder often precede SUDs in FHP young adults (Hill et al., 2011) and general samples (Nock et al., 2006, Nock et al., 2007). In contrast, the presence of anxiety disorders alone in FHP children do not appear to predict later development of SUDs (Hill et al., 2011), although, internalizing symptoms coupled with poor executive functioning may do so (Lees et al., 2020e). FHP youth in the current study with greater FHD were at greater odds of meeting criteria for these disorders and this may increase their risk for future SUDs as they enter adolescence. Furthermore, our recent study showed that youth were more likely to endorse sipping alcohol by ages 9–10 if they had been exposed to alcohol while in utero (Lees et al., 2020d). There is relatively high overlap among FHP and prenatally exposed individuals (here, 32%), thus, in utero exposures may account for some of the variance in substance use initiation previously not accounted for in FHP studies.
Based on the current evidence, prevention efforts designed to interrupt the intergenerational transmission of SUDs should consider targeting psychopathology and mental disorders in FHP substance-naïve children before they enter adolescence and begin experimenting with alcohol and other substances. Intervention initiatives should continue to treat and aim to reduce the prevalence of SUDs in parents.
4.3. Strengths and limitations
A key strength of this study was the utilization of a mixed model analytic approach which allowed for appropriate adjustment of the complexity of factors that may influence youth neurobiology, cognition, psychopathology, and behavior. Using this approach showed for the first time in a large-scale study that FHP youth experience greater psychopathology and mental disorders which are related to aberrant brain structure. This study also has several limitations. First, this is a cross-sectional assessment, which enabled establishment of associations but does not address causation. The longitudinal component of ABCD will be essential to begin to delineate causal pathways. Second, FHP classification in the current study was based on histories of any alcohol or other substance use problems (e.g., marital separation, DUI, alcohol treatment program), rather than a positive SUD diagnosis. A threshold of just one problem was required, as sensitivity of symptoms was prioritized over specificity. It is likely that a SUD threshold for FHP classification would have resulted in stronger rather than weaker associations. Third, unmeasured confounding factors may be contributing to the observed associations. Fourth, data were not available on the percentage of family members with past versus current SUD-related symptoms, precluding examination of the impact of current problems on child outcomes. Fifth, cortical surface reconstruction and subcortical segmentation were performed using Freesurfer v5.3. Versions 6.0 and 7.0 have since been released and appear to have improved volumetric segmentation (as determined by dice scores) and perhaps the utilization of v5.3 contributed to the null findings of volumetric measures. Lastly, genetic influences of FHP were not examined and are likely to have an important underlying role in the observed associations.
4.4. Conclusions
Overall, FHP youth exhibit aberrant brain structure, greater psychopathology symptoms and increased odds of mental disorders, with severity of problems related to FHD of substance misuse. At ages 9–10, FHP youth do not appear to present with problems related to impulsiveness, reward responsiveness, or cognitive deficits. Associations preceded offspring alcohol or other substance use and were robust to the inclusion of potential confounding factors, such as socio-demographic characteristics, prenatal exposures, family history of psychopathology, and parenting factors. The results indicate that psychopathology and mental disorders could be partially underpinned by neurobiological differences, although longitudinal analysis is required to determine causal mechanisms. Based on the current findings and wider evidence base, psychopathology in FHP children may increase risk of chronic problems with mental and substance use disorders, resulting in reduced quality of life, and should therefore be targeted in prevention efforts.
Supplementary Material
Highlights.
Child outcomes related to family history of substance misuse were explored.
Among a US cohort of >11,000 children, one in four had a family history of misuse.
These youth exhibited thinner whole cortices and greater regional surface area.
Aberrant brain structure was linked to psychopathology and mental disorders.
Severity of effects were dependent on family history density of substance misuse.
Acknowledgments
Funding: This work was supported by the National Health and Medical Research Council (GNT1169377 to BL; GNT1132853 to LAS; GNT1041756 and GNT1078407 to MT); the National Institutes of Health (U01 DA041093, K23 AA025399, and R01 AA027399 to LMS; R21 DA047953 and U01 DA041089 to JJ); and the California Tobacco-Related Disease Research Grants Program Office of the University of California (Grant 580264 to JJ).
Role of funding source:
The funding sources had no role in the writing of the manuscript or the decision to submit for publication. Content is solely the responsibility of the authors and does not necessarily represent the official views of the Australian National Health and Medical Research Council, the National Institutes of Health, or the California Tobacco-Related Disease Research Grants Program Office of the University of California.
Disclaimer:
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners “under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147”. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from the curated annual release 2.0.1.
Footnotes
Conflict of interest:
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM 2009. The Achenbach system of empirically based assessment (ASEBA): Development, findings, theory, and applications, University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL & Winokur G 1977. The family history method using diagnostic criteria: Reliability and validity. Archives of General Psychiatry, 34(10), 1229–1235. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Rice J, Endicott J, Reich T & Coryell W 1986. The family history approach to diagnosis: How useful is it? Archives of General Psychiatry, 43(5), 421–429. [DOI] [PubMed] [Google Scholar]
- Barnow S, Schuckit MA, Lucht M, John U & Freyberger HJ 2002. The importance of a positive family history of alcoholism, parental rejection and emotional warmth, behavioral problems and peer substance use for alcohol problems in teenagers: a path analysis. J Stud Alcohol, 63, 305–15. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G & Jayakumar PN 2007. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict. Biol, 12, 122–132. [DOI] [PubMed] [Google Scholar]
- Benjamini Y & Hochberg Y 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57, 289–300. [Google Scholar]
- Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F & Giannotti F 1996. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res, 5, 251–61. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, Orr CA, Wager TD, Banich MT, Speer NK, Sutherland MT, Riedel MC, Dick AS, Bjork JM, Thomas KM, Chaarani B, Mejia MH, Hagler DJ Jr., Daniela Cornejo M, Sicat CS, Harms MP, Dosenbach NUF, Rosenberg M, Earl E, Bartsch H, Watts R, Polimeni JR, Kuperman JM, Fair DA, Dale AM & Workgroup AIA 2018. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci, 32, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock SM, Vaidya JG & Niciu MJ 2019. Neurophysiological correlates and differential drug response in subjects with a family history of an alcohol use disorder. Chronic Stress (Thousand Oaks), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A 2016. Neurobiological phenotypes associated with a family history of alcoholism. Drug and alcohol dependence, 158, 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Gillespie AJ, Michael PG, & Nagel BJ 2015. Family history density of alcoholism relates to left nucleus accumbens volume in adolescent girls. Journal of Studies on Alcohol and Drugs, 76(1), 47–56. [PMC free article] [PubMed] [Google Scholar]
- Dager AD, McKay DR, Kent JW Jr, Curran JE, Knowles E, Sprooten E, Göring HH, Dyer TD, Pearlson GD, Olvera RL, Fox PT, Lovallo WR, Duggirala R, Almasy L, Blangero J, & Glahn DC 2015. Shared genetic factors influence amygdala volumes and risk for alcoholism. Neuropsychopharmacology, 40(2), 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Harford TC & Grant BF 1992. Family history as a predictor of alcohol dependence. Alcohol Clin Exp Res, 16, 572–5. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP & Hyman BT 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31, 968–980. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M & Buckhalt JA 2003. Parental problem drinking and children’s adjustment: attachment and family functioning as moderators and mediators of risk. J Fam Psychol, 17, 510–20. [DOI] [PubMed] [Google Scholar]
- Enoch MA 2006. Genetic and environmental influences on the development of alcoholism: resilience vs. risk. Ann N Y Acad Sci, 1094, 193–201. [DOI] [PubMed] [Google Scholar]
- Enoch MA & Goldman D 2001. The genetics of alcoholism and alcohol abuse. Curr Psychiatry Rep, 3, 144–51. [DOI] [PubMed] [Google Scholar]
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, Jernigan T, Potter A, Thompson W & Zahs D 2018. Recruiting the ABCD sample: Design considerations and procedures. Dev Cogn Neurosci, 32, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF 2000. Estimates of US children exposed to alcohol abuse and dependence in the family. American journal of public health, 90, 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A & Hasin DS 2017. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: Results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry, 74, 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, & Tapert SF 2010. Hippocampal volumes in adolescents with and without a family history of alcoholism. The American Journal of Drug and Alcohol Abuse, 36(3), 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson KE, Vaidya JG, Kramer JR, Kuperman S, Langbehn DR & O’leary DS 2018. Cortical thickness in adolescents with a family history of alcohol use disorder. Alcohol Clin Exp Res, 42, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, & Pitts T 2001. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological Psychiatry, 49(11), 894–905. [DOI] [PubMed] [Google Scholar]
- Hill SY, Lichenstein SD, Wang S, & O’Brien J 2016. Volumetric differences in cerebellar lobes in individuals from multiplex alcohol dependence families and controls: their relationship to externalizing and internalizing disorders and working memory. Cerebellum, 15(6), 744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, & Keshavan M 2007. Cerebellar volume in offspring from multiplex alcohol dependence families. Biological Psychiatry, 61(1), 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY & Muka D 1996. Childhood psychopathology in children from families of alcoholic female probands. J Am Acad Child Adolesc Psychiatry, 35, 725–33. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke-Wellman J, Matthews AG & Mcdermott M 2008. Psychopathology in offspring from multiplex alcohol dependence families with and without parental alcohol dependence: a prospective study during childhood and adolescence. Psychiatry research, 160, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Tessner KD & Mcdermott MD 2011. Psychopathology in offspring from families of alcohol dependent female probands: a prospective study. J Psychiatr Res, 45, 285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, McDermott MD, Zezza N, & Stiffler S 2013. Amygdala Volume in Offspring from Multiplex for Alcohol Dependence Families: The Moderating Influence of Childhood Environment and 5-HTTLPR Variation. Journal of Alcoholism and Drug Dependence, Suppl 1, 001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogstrom LJ, Westlye LT, Walhovd KB & Fjell AM 2013. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cerebral Cortex, 23(11), 2521–2530. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Barnett NP, Colby SM & Rogers ML 2015. The prospective association between sipping alcohol by the sixth grade and later substance use. Journal of Studies on Alcohol and Drugs, 76, 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, Statham D, Whitfield JB & Martin NG 2004. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychol Med, 34, 1519–30. [DOI] [PubMed] [Google Scholar]
- Kobak KA, Kratochvil C, Stanger C & Kaufman J 2013. Computerized screening of comorbidity in adolescents with substance or psychiatric disorders. Anxiety Disorders and Depression.(La Jolaa, CA). [Google Scholar]
- Lees B, Aguinaldo L, Squeglia LM, Infante MA, Wade NE, Hernandez Mejia M & Jacobus J 2020a. Parental Family History of Alcohol Use Disorder and Neural Correlates of Response Inhibition in Children From the Adolescent Brain Cognitive Development (ABCD) Study. Alcoholism: Clinical and Experimental Research, 44, 1234–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees B, Meredith LR, Kirkland AE, Bryant BE & Squeglia LM 2020b. Effect of alcohol use on the adolescent brain and behavior. Pharmacol Biochem Behav, 192, 172906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees B, Mewton L, Jacobus J, Valadez EA, Stapinski LA, Teesson M, Tapert SF & Squeglia LM 2020c. Association of prenatal alcohol exposure with psychological, behavioral, and neurodevelopmental outcomes in children from the Adolescent Brain Cognitive Development Study. Am J Psychiatry, doi: 10.1176/appi.ajp.2020.20010086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees B, Mewton L, Stapinski LA, Squeglia LM, Rae CD & Teesson M 2019. Neurobiological and cognitive profile of young binge drinkers: A systematic review and meta-analysis. Neuropsychology Review, 29, 357–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees B, Mewton L, Stapinski LA, Teesson M & Squeglia LM 2020d. Association of prenatal alcohol exposure with preadolescent alcohol sipping in the ABCD Study. Drug & Alcohol Dependence, 214, 108187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees B, Stapinski LA, Prior K, Sunderland M, Newton N, Baillie A, Teesson M & Mewton L 2020e. Exploring the complex inter-relations between internalising symptoms, executive functioning and alcohol use in young adults. Addictive Behaviors, 106, 106351. [DOI] [PubMed] [Google Scholar]
- Lees B, Squeglia LM, Mcteague LM, Forbes MK, Krueger RF, Sunderland M, Baillie AJ, Koch F, Teesson M & Mewton L 2020f. Altered neurocognitive functional connectivity and activation patterns underlie psychopathology in preadolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, doi: 10.1016/j.bpsc.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ & Banich MT 2018. Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Developmental Cognitive Neuroscience, 32, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson L, Magidson Jessica F, Reynolds Elizabeth K, Kahler Christopher W & Lejuez CW 2010. Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcoholism: Clinical and Experimental Research, 34, 1400–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcphee MD, Claus ED, Boileau I, Lee ACH, Graff-Guerrero A & Hendershot CS 2018. Does family history of alcohol use disorder relate to differences in regional brain volumes? A descriptive review with new data. Alcohol Clin Exp Res, 42, 2369–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’malley SS & Rounsaville BJ 1998. Familial transmission of substance use disorders. Arch Gen Psychiatry, 55, 973–9. [DOI] [PubMed] [Google Scholar]
- Moos RH 1994. Family environment scale manual: Development, applications, research, Consulting Psychologists Press. [Google Scholar]
- Nock MK, Kazdin AE, Hiripi E & Kessler RC 2006. Prevalence, subtypes, and correlates of DSM-IV conduct disorder in the National Comorbidity Survey Replication. Psychological medicine, 36, 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Kazdin AE, Hiripi E & Kessler RC 2007. Lifetime prevalence, correlates, and persistence of oppositional defiant disorder: results from the National Comorbidity Survey Replication. J Child Psychol Psychiatry, 48, 703–13. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, Luking KR, Anokhin AP, Gotlib IH, Hayden EP, Olino TM, Peng CZ, Hajcak G & Barch DM 2016. Revising the BIS/BAS Scale to study development: Measurement invariance and normative effects of age and sex from childhood through adulthood. Psychol Assess, 28, 429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A & Kremen WS 2009. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 19(11), 2728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker N, Patel Y, Jackowski AP, Pan PM, Salum GA, Pausova Z, Paus T, For The Saguenay Youth, S. & The, I. C. 2020. Assessment of neurobiological mechanisms of cortical thinning during childhood and adolescence and their implications for psychiatric disorders. JAMA Psychiatry, doi: 10.1001/jamapsychiatry.2020.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey T & Regier PS 2018. Cognitive impairment in substance use disorders. CNS Spectr, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI Jr., Schuckit MA & Begleiter H 1995. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res, 19, 1018–23. [DOI] [PubMed] [Google Scholar]
- Ryan SR, Acheson A, Charles NE, Lake SL, Hernandez DL, Mathias CW & Dougherty DM 2016. Clinical and social/environmental characteristics in a community sample of children with and without family histories of substance use disorder in the san antonio area: A descriptive study. J Child Adolesc Subst Abuse, 25, 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifano F, Zangani C, Chiappini S, Guirguis A, Bonaccorso S & Corkery JM 2020. Substance-Use Disorders and Violence. In: CARPINIELLO B, VITA A & C. M (eds.) Violence and Mental Disorders. Comprehensive Approach to Psychiatry. Springer, Cham. [Google Scholar]
- Schmansky NF 2020. eTIV - estimated Total Intracranial Volume, aka ICV. Available: https://surfer.nmr.mgh.harvard.edu/fswiki/eTIV [Accessed: 27 May 2020].
- Squeglia LM & Cservenka A 2017. Adolescence and drug use vulnerability: Findings from neuroimaging. Curr Opin Behav Sci, 13, 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Brumback T, Meloy MJ, & Tapert SF 2014. White matter integrity in alcohol-naive youth with a family history of alcohol use disorders. Psychological Medicine, 44(13), 2775–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse And Mental Health Services Administration 2019. Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health In: (HHS PUBLICATION NO. PEP19–5068, N. S. H.−. (ed.). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Teesson M, Hall W, Slade T, Mills K, Grove R, Mewton L, Baillie A & Haber P 2010. Prevalence and correlates of DSM-IV alcohol abuse and dependence in Australia: Findings of the 2007 National Survey of Mental Health and Wellbeing. Addiction, 105, 2085–94. [DOI] [PubMed] [Google Scholar]
- Tervo-Clemmens B, Quach A, Calabro FJ, Foran W & Luna B 2020. Meta-analysis and review of functional neuroimaging differences underlying adolescent vulnerability to substance use. Neuroimage, 209, 116476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WD, Orvaschel H, Prusoff BA & Kidd KK 1982. An evaluation of the family history method for ascertaining psychiatric disorders. Archives of General Psychiatry, 39(1), 53–58. [DOI] [PubMed] [Google Scholar]
- Verhulst B, Neale MC & Kendler KS 2015. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med, 45, 1061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal SI, Vandeleur C, Rothen S, Gholam-Rezaee M, Castelao E, Halfon O, Aubry JM, Ferrero F & Preisig M 2012. Risk of mental disorders in children of parents with alcohol or heroin dependence: A controlled high-risk study. European Addiction Research, 18, 253–264. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR, Miller JD & Reynolds SK 2005. Validation of the UPPS impulsive behaviour scale: A four factor model of impulsivity. European Journal of Personality: Published for the European Association of Personality Psychology, 19, 559–574. [Google Scholar]
- Wood SN 2017. Generalized Additive Models: an Introduction with R, 2nd ed., Chapman Hall/CRC, New York. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.