Abstract
Aim:
To determine the effects of gonadotropin-releasing hormone analog treatment on final height and body mass index in girls with central precocious puberty.
Material and Methods:
All cases with diagnosis age <8 years constituted group 1 (n=19) and those with ≥8 years constituted group 2 (n=35).
Results:
There was no significant difference in height standard deviation score, body mass index standard deviation score, bone age/chronologic age, predicted final height at the time of diagnosis, and follow-up between group 1 and group 2. There was no significant difference in final height (standard deviation score) between the groups. The number of obese and overweight cases at diagnosis and final height was similar. The target height (standard deviation score), predicted final height (standard deviation score), and final height (standard deviation score) were similar in both Group 1 and Group 2.
Conclusion:
We found that between the ages of 6–9.8 years, girls with central precocious puberty who received gonadotropin-releasing hormone analog treatment reached a final height within their target height range. It is concluded that gonadotropin-releasing hormone analog treatment increases body mass index during treatment and when patients reach the final height, they return to their pretreatment body mass index. Younger age and greater height at the time of diagnosis are the positive factors on final height.
Keywords: Central precocious puberty, early puberty, final height, gonadotropin-releasing hormone analog treatment, rapidly progressive puberty
Abstract
Amaç:
Santral erken puberteli olgularda Gonadotropin Releasing Hormon agonist tedavisinin final boy ve vücut kitle indeksi üzerine etkisinin değerlendirilmesini amaçladık.
Gereç ve Yöntemler:
Çalışmaya alınan 54 kız hasta tanı yaşlarına göre 2 gruba ayrıldı. Tanı yaşı 8 yaş altı olan olgular Grup 1’i, 8 yaş ve üzeri olan olgular Grup 2’yi oluşturdu. Grup 1’de 19 hasta, Grup 2’de 35 hasta vardı.
Bulgular:
Tanıda ve izlem yıllarında boy standart deviasyon skoru, vücut kitle indeksi standart deviasyon skoru, kemik yaşı/takvim yaşı, öngörülen son boy açısından Grup 1 ile Grup 2 arasında istatistiksel olarak anlamlı fark yoktu. Gruplar arasında final boy, final boy standart deviasyon skoru açısından fark yoktu. Tanıda ve finalde obez ve kilolu olgu sayısı benzerdi. Hem Grup 1 hem Grup 2’de hedef boy, öngörülen son boy ve final boylar arasında ve bunların standart deviasyon skorları arasında istatistiksel olarak anlamlı fark saptanmadı.
Çıkarımlar:
Bu çalışmada Gonadotropin Releasing Hormon agonist tedavisi verilen 6–9.8 yaş aralığındaki santral erken puberteli kızların hedef boyları ile uyumlu final boya ulaştıkları belirlendi. Gonadotropin Releasing Hormon agonist tedavisinin tedavi sırasında vücut kitle indeksini arttırdığı, tedavi kesiminden sonra final boya ulaşıldığında hastaların tanı vücut kitle indekslerine geri döndükleri sonucuna varıldı. Tanı yaşının küçük olması ve tanı boyunun uzun olması final boy üzerine pozitif etkili etmenler olduğu bulundu.
Keywords: Erken puberte, final boy, gonadotropin releasing hormon analog tedavisi, santral puberte prekoks
Introduction
Precocious puberty is defined for the girls as onset of puberty before 8 years of age. Central precocious puberty develops due to premature activation of the hypothalamic-pituitary-ovarian axis. In these cases, early estradiol secretion shortens the growth period by increasing the growth velocity and bone maturation, resulting in short final stature (1, 2). Treatment with gonadotropin-releasing hormone analog (GnRHa) suppresses the hypothalamic-pituitary-ovarian axis in patients with central precocious puberty and reduces estradiol secretion. As a result, it decelerates rapid bone maturation, preserving growth potential. In general, puberty is delayed until 10–11 years of age by GnRHa treatment (2, 3).
There are conflicting results regarding the effects of GnRHa treatment on final adult height. The group with the largest gain in height is consists of patients with pubertal findings before 6 years of age (4). The girls with central precocious puberty aged 6–8 years at onset of puberty and patients with early puberty aged 8–10 years at onset of puberty are very heterogeneous groups. In these groups, factors affecting final height are not definitively determined (5, 6). The effect of GnRHa therapy on body mass index (BMI) during and after treatment is not clear. Studies have been published indicating that BMI possibly increase with treatment (7, 8).
In this study, it was aimed to establish factors influencing final height by determining demographic characteristics and evaluating effects of GnRHa treatment on final height, body weight (BW) and BMI in girls aged 6–10 years with central precocious puberty who underwent GnRHa treatment and reached their final height.
Material and Methods
This study included 54 girls who were diagnosed as having precocious puberty in the pediatric endocrinology outpatient clinic between April 2008 and December 2011, who underwent GnRHa treatment and reached their final height. The study was approved by the local Ethics Committee (approval number: 73799008). The study was conducted in accordance with the principles of the Declaration of Helsinki. We retrospectively reviewed patient files. The patients were divided into two groups based on age at diagnosis as follows: group 1 (n=19) including patients aged <8 years at diagnosis, and group 2 (n=35) including patients aged ≥8 years at diagnosis.
During the clinical assessment, BW, and height measurements were performed using a SECA 767 scale and Harpenden stadiometer, respectively. In all cases, BMI was calculated using the following formula: BMI=weight (kg) x height2 (m2). The height, weight, and BMI values were compared according to standard curves for Turkish children and standard deviation scores (SDS) for height, BW, and BMI were calculated according to calendar age. The patients with BMI-SDS >2 were considered as obese, and those with BMI-SDS of 1–2 were considered as overweight (9). Pubertal development was assessed using the Tanner and Marshall criteria (10). At diagnosis, left wrist radiograph was obtained from all cases and bone age (BA) was determined according to the Greulich and Pyle method (11). The predicted final height (PFH) according to bone age was calculated using the Bayley-Pinneau (12) method. To calculate PFH, accelerated standards were used when bone age was advanced by >1 year than chronologic age (CA). The predicted final height -SDS was calculated for each patient. Final height was defined as height achieved when growth rate within the preceding year was <1 cm or following closure of the epiphyses (13). Maternal and paternal heights were measured during outpatient clinic visits. The target height (TH) and TH-SDS were also estimated (14).
In all patients, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) were measured in blood samples obtained at morning hours. Luteinizing hormone and FSH were measured using an ADVIA Centaur analyzer system (Bayer Diagnostics, Tarrytown, NY, USA) using immunochemiluminescence (ICMA) methodology. The ADVIA Centaur LH assay has an analytical sensitivity of 0.1 mIU/mL. The ADVIA Centaur FSH assay has an analytical sensitivity of 0.3 mIU/mL (IU/L). A basal serum LH level >0.83 mIU/mL (being compatible with findings as a requirement) was accepted as activation of the hypothalamic-pituitary-gonadal (HPG) axis (15). In patients with basal LH <0.83 mIU/mL, a GnRH stimulation test was performed through intravenous injection of gonadorelin acetate (100 μg) between 8.00 and 8.30 a.m. for assessment of early puberty. The blood samples for serum LH and FSH level measurements were taken at 0, 15, 30, 45, 60, 90 min. In the test, peak LH ≥5 mIU/mL was accepted as the diagnostic criterion for activation of the HPG axis (16). A GnRH stimulation test was performed in all patients in group 1 and 10 of 35 patients in group 2. GnRHa treatment was started when baseline LH or peak LH met the criteria given for patients aged 6–8 years. In patients aged between 8 and 10 years, GnRHa treatment was started in the presence of pubertal laboratory findings with rapid progression of pubertal findings. A rapid progression of pubertal findings was considered to be a one-stage progression of breast stage within 3–6 months and emergence of uterine volume over 35 mm in pelvic USG. All patients received 3.75 mg depot leuprolide acetate every 28 days.
Cranial magnetic resonance imaging (MRI) was normal in all patients. Patients with problems that could affect growth and puberty such as growth hormone deficiency, thyroid pathology, adrenal and gonadal pathology, dysmorphic syndrome, skeletal dysplasia, chronic illness, cerebral palsy, hydrocephalus and those with a history of chronic drug use were excluded.
The criteria for adequate HPG axis suppression were defined as discontinuation or regression in breast development and achieving GnRHa-stimulated LH levels <4 mIU/mL (17). The HPG axis was suppressed by treatment in both groups.
Bone age was evaluated annually during treatment. The bone maturation ratio was calculated as the BA/CA ratio using the annual change in the years following treatment compared with baseline. The predicted final height and PFH SDS at baseline and during follow-up were used to evaluate the short-term effectiveness of the treatment regarding height.
Gonadotropin-releasing hormone analog treatment was withdrawn when the bone age reached 12–12.5 years, provided that the chronologic age reached at least 10 years (5). The patients were followed up until the achievement of final height after the cessation of therapy.
Statistical Analysis
Statistical analysis were performed using the Statistical Package for the Social Sciences software package for Windows version 22.0 statistics program. Continuous variables are presented as means±standard deviation or median (interquartile range). Percentages and frequencies were calculated for categorical variables. Mean values were compared using the t-test when data distribution was normal, and the Mann-Whitney U-test when data distribution was skewed. Friedman analysis of variance (ANOVA) was used for the comparisons of BMI SDS at the time of diagnosis, follow-up, and final BMI SDS of both groups because parametric the test hypothesis was not provided in repeated measures. The Wilcoxon test was used for binary comparisons. Stepwise multiple regression analysis was used to determine the best predictors of final height. A p-value <0.05 was considered significant for all tests.
Results
Demographic characteristics and clinical findings are shown for both groups and the entire sample in Table 1. The mean treatment duration was 44.7 (39–48.5) months in group 1 and 24.4 (18.9–28.5) months in group 2. The treatment duration was significantly longer in group 1 than in group 2 (p<0.001). The mean age at menarche age was 12±0.57 years for the entire group and there was no significant difference between the groups. The anthropometric data of group 1, group 2, and the entire sample at the time of diagnosis and in the follow-up years are shown in Table 2. The mean age at final height was 14.5±0.48 years in group 1 and 14.4±0.52 years in group 2.
Table 1.
Demographic characteristics, clinical findings and target, diagnosis and final height of Group 1, Group 2 and of total patients
| Group 1 n=19 Mean±SD | Group 2 n=35 Mean±SD | Total n=54 Mean±SD | |
|---|---|---|---|
| Age (years) | 7.26±0.53 | 8.97±0.51 | 8.37±0.97 |
| Thelarche stage, n (%) | Stage 2 =10 (52.6) | Stage 2 =10 (28.5) | Stage 2 =20 (37) |
| Stage 3 =9 (47.4) | Stage 3 =20 (57.2) | Stage 3 =29 (53.7) | |
| Stage 4 = (0) | Stage 4 =5 (14.3) | Stage 4 =5 (9.3) | |
| Pubarche stage, n (%) | Stage 1 =16 (84.2) | Stage 1 =9 (25.7) | Stage 1 =25 (46.3) |
| Stage 2 =2 (10.5) | Stage 2 =11 (31.4) | Stage 2 =13 (24.1) | |
| Stage 3 =1 (5.3) | Stage 3 =12 (34.3) | Stage 3 =13 (24.1) | |
| Stage 4 = (0) | Stage 4 =3 (8.6) | Stage 4 =3 (5.5) | |
| Duration of symptoms, (month) | 4.4±4.35 | 9.11±7.06 | 7.67±6.68 |
| Term/preterm, n (%) | 17/2 | 29/4 | 46/6 |
| Birth weight, (g) | 3157±616 | 2891±693 | 2988±673 |
| Maternal menarche age, (years) | 12.6±0.54 | 12.5±1.29 | 12.5±1.16 |
| Mother’s height, (cm) | 156.8±6.97 | 158.2±5.68 | 157.7±6.14 |
| Father’s height, (cm) | 172.1±5.64 | 171.7±6.09 | 171.9±5.88 |
| Target height (cm) | 157.9±5.14 | 158.4±4.45 | 158.2±4.67 |
| Target height, SDS | -0.4±0.86 | -0.3±0.74 | -0.33±0.78 |
| Height SDS (diagnosis) | 0.87±1.05 | 0.87±1 | 0.87±1.01 |
| BW SDS (diagnosis) | 0.63±0.86 | 0.9±0.75 | 0.8±0.79 |
| BA/CA (diagnosis) | 1.22±0.14 | 1.19±0.11 | 1.2±0.12 |
| Duration of treatment (month) | 44.7±6.4 | 24.4±7.7 | 31.6±12.1 |
| Final height (cm) | 159.2±5.9 | 157.8±5.59 | 158.3±5.7 |
| Final height SDS | 0.03±1.05 | -0.4±0.93 | -0.24±0.9 |
*: Continuous variables were presented as means±standard deviation. SDS: Standart deviation score; BW: Body weight; BA/CA: Ratio of bone age to chronological age. The percent and frequencies were calculated for categorical variables
Table 2.
Anthropometric data of Group 1, Group 2 and total pateints at the time of diagnosis and during the follow-up years
| Group 1 n=19 Mean±SD | Group 2 n=35 Mean±SD | Total n=54 Mean±SD | Group 1 vs. Group 2 p | |
|---|---|---|---|---|
| Height SDS (at diagnosis) | 0.87±1.05 | 0.87±1 | 0.87±1.01 | 0.995* |
| Height SDS (at the first year) | 0.85±1.08 | 0.77±1.06 | 0.8±1.06 | 0.276* |
| Height SDS (at the econd year) | 0.72±1.33 | 0.6±0.98 | 0.66±1.15 | 0.191* |
| Final height SDS | 0.03±1.05 | 0.47±0.93 | -0.24±0.9 | 0.409* |
| BW SDS (at diagnosis) | 0.63±0.86 | 0.9±0.75 | 0.8±0.79 | 0.636* |
| BW SDS (at the first year) | 0.86±0.79 | 0.94±0.86 | 0.91±0.83 | 0.893* |
| BW SDS (at the second year) | 0.78±0.83 | 0.88±0.92 | 0.83±0.87 | 0.590* |
| Final BW SDS | 0.34±1.31 | 0.4±1.14 | 0.38±1.1 | 0.25* |
| BMI SDS (at diagnosis) | 0.38±0.83 | 0.72±0.76 | 0.6±0.8 | 0.266* |
| BMI SDS (at the first year) | 0.68±0.87 | 0.82±0.68 | 0.77±0.75 | 0.669* |
| BMI SDS (at the second year) | 0.79±0.72 | 0.84±0.8 | 0.82±0.75 | 0.799* |
| Final BMI SDS | 0.44±1.16 | 0.57±1.04 | 0.53±1.07 | 0.32* |
| PFH (cm) (at diagnosis) | 157.3±7.68 | 157.7±6.45 | 157.6±6.84 | 0.556* |
| PFH (cm) (at the first year) | 156.6±8.53 | 158.4±6.2 | 157.7±7.1 | 0.705δ |
| PFH (cm) (at the second year) | 158.5±7.13 | 159.5±7.08 | 159±7 | 0.878δ |
| Final height (cm) | 159.2±5.9 | 157.8±5.59 | 158.3±5.7 | 0.409* |
| PFH SDS (at diagnosis) | -0.48±1.29 | -0.36±1.08 | -0.4±1.15 | 0.835* |
| PFH SDS (at the first year) | -0.45±1.34 | -0.3±1.04 | -0.36±1.15 | 0.971δ |
| PFH SDS (at the second year) | -0.29±1.20 | -0.11±1.18 | -0.2±1.18 | 0.879δ |
| Final height SDS | 0.03±1.05 | 0.47±0.93 | -0.24±0.9 | 0.409* |
: Mean values were compared using t-test when data distribution was normal;
: Mean values were compared using Mann-Whitney U-test when the data distribution was skewed; SDS: Standard deviation score; BW: Body weight; BMI: Body mass index; PFH: Predicted final height
There was no significant difference in the mean age at final height between group 1 and group 2. Final height and final height-SDS were similar between the groups. Bone age/chronologic age and growth velocity SDS of group 1, group 2 and the entire sample at the time of diagnosis and in the follow-up years are shown in Table 3.
Table 3.
Bone age/chronological age and growth velocity SDS of Group 1, Group 2 and of total patients at the time of diagnosis and during the follow-up years
| Group 1 n=19 Mean±SD | Group 2 n=35 Mean±SD | Total n=54 Mean±SD | Group 1 vs. Group 2 p | |
|---|---|---|---|---|
| BA/CA (at diagnosis) | 1.22±0.14 | 1.19±0.11 | 1.2±0.12 | 0.32* |
| BA/CA (at the first year) | 1.17±0.14 | 1.16±0.82 | 1.17±0.1 | 0.436* |
| BA/CA (at the second year) | 1.15±0.12 | 1.13±0.08 | 1.14±0.1 | 0.125* |
| ΔBA/ΔCA (at the first year) | 0.88±0.58 | 0.97±0.84 | 0.94±0.75 | 0.401* |
| ΔBA/ΔCA (at the second year) | 0.93±0.8 | 0.7±0.69 | 0.81±0.74 | 0.404* |
| Growth velocity SDS (at the first year) | 0.51±1.19 | 0.11±1.36 | 0.25±1.3 | 0.074* |
| Growth velocity SDS (at the second year) | 0.17±1.43 | -1.25±1.16 | -0.55±1.47 | 0.005* |
: Mean values were compared using Mann-Whitney U-test; BA/CA: Ratio of bone age to chronological age; SDS: Standard deviation score
Height gain at the first year of follow-up in group 1 was 5.93±1.11 cm and in group 2 it was 5.74±1.23 cm, there was no significant difference between group 1 and group 2. Height gain in the second year of follow-up in group 1 was 5.6±1.31 cm and in group 2 it was 4.8±1.1 cm. Height gain from the time of the first-year follow-up to the second-year follow-up was significantly greater in group 1 (p=0.005). Height gain from the time of diagnosis to final height was significantly greater in group 1 (32.6±1.63 cm/7.24 year) when compared with group 2 (21.1±0.35 cm/5.43 year) (p<0.001). In the entire group, delta height SDS from the time of diagnosis to final was -1.12±0.76. Between group 1 and group 2, there was no significant difference in target height (SDS), PFH at the time of diagnosis (SDS), PFH at the first-year follow-up (SDS), PFH at the second-year follow-up (SDS), final height (SDS) (Fig. 1, 2). The ΔBA/ΔCA of group 1, group 2, and the entire group were similar in the follow-up years. A linear regression analysis showed that the age and height at the time of diagnosis could explain 74% of the variation in final height. The regression equation established for the final height was as follows: FH=71.424+(1xHdiagnosis-5.829xCAdiagnosis). It is concluded that final height was affected positively by height at the time of diagnosis and negatively by age at the time of diagnosis in the multiple regression model including chronologic age, height, body weight, target height, and BA/CA at the time of diagnosis (Table 4).
Figure 1.
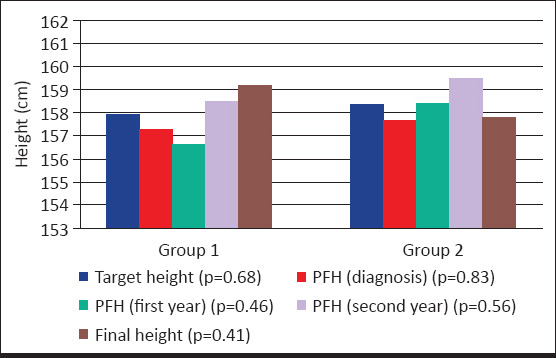
Comparisons of target height, PFH and final height within the Group 1 and Group 2
Figure 2.
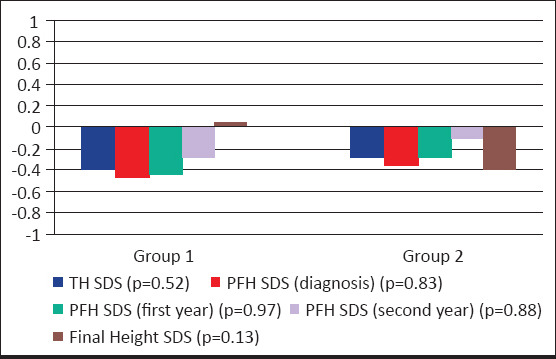
Comparisons of TH SDS, PFH SDS and Final height SDS within the Group 1 and Group 2
Table 4.
Multiple regression analysis of final height
| Predictive factor | ß | Standard error | p |
|---|---|---|---|
| Height (diagnosis) | 1.000 | 0.132 | 0.000 |
| BW (diagnosis) | -0.106 | 0.108 | 0.333 |
| CA (diagnosis) | -5.829 | 0.726 | 0.000 |
| TH | 0.082 | 0.106 | 0.443 |
| BA/CA (diagnosis) | -5.788 | 4.157 | 0.170 |
n=54; r2=0.741; p<0.001. Stepwise multiple regression analysis of the following independent variables: Chronological age, height, body weight, target height and BA/CA at the time of diagnosis. BW: Body weight; CA: Chronological age; TH: Target height; BA/CA: Ratio of bone age to chronological age
At the time of diagnosis 3.7% of all patients were obese and 22.2% were overweight. Ten of the 14 obese and overweight cases (71.4%) were in group 2. At the time of the final height assessment, 7.4% of all patients were obese and 25.9% of all cases were overweight. The number of obese and overweight patients at diagnosis and the final assessment were similar. BW SDS at diagnosis and follow-up in group 1 and group 2 are given in Figure 3. In group 1, BMI SDS was significantly increased over time at the first, second, and third year of follow-up compared with the BMI SDS at baseline. The final BMI SDS was similar to the BMI SDS at the time of diagnosis. In group 2, BMI SDS at the time of diagnosis, BMI SDS in first and second follow-up years, and the final BMI SDS were similar.
Figure 3.
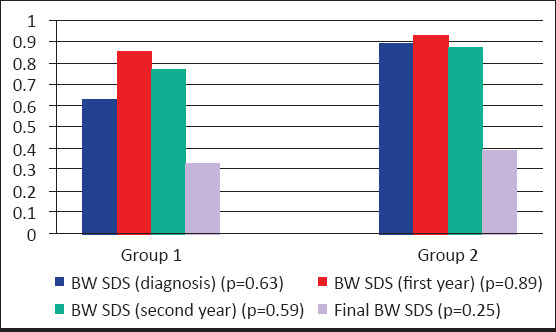
Comparisons of BW SDS at diagnosis and follow up in Group 1 andGroup 2
Discussion
It has been shown that, in patients with precocious puberty, treatment with GnRH analogs significantly improves the predicted final height during treatment (16, 18, 19). In recent studies, different results were reported on final height in treated and untreated girls with early puberty (20–24).
In a study on 71 Korean girls with central precocious puberty (three groups: <8 years, 8–9 years and 9–10 years), Baek et al. (25) investigated the near-final height, pretreatment PFH, and PFH after treatment with GnRHa. The authors reported that PFH after treatment, which was accepted as near-adult height, was significantly higher than pretreatment PFH, and that there was no significant difference in mean height SDS for CA and BA between the time of diagnosis and end of treatment. Lazar et al. (5) divided 115 girls with idiopathic central precocious puberty into three groups according to age at the time of diagnosis (<6 years, 6–8 years, 8–9 years) and investigated their final height after GnRHa treatment. The authors showed that only patients with age at puberty onset <6 years achieved the PFH at that point, but the final height was significantly lower than PFH in patients with age at puberty onset between 6–8 years and those >8 years, concluding that pretreatment intrinsic changes in growth plate negatively affected the final height of girls diagnosed after the age of 6 years.
In their study, Paterson et al. (26) assessed 46 girls with idiopathic central precocious puberty (8–10.5 yr) who had completed goserelin treatment, reporting that 11 of 46 patients had reached their final height. The mean target height was 160.9 cm (-0.48 SDS) and the mean final height was 159.7 cm (-0.63 SDS), indicating no significant difference. The authors suggested that these children did not gain in height, they only reached their target height. However, the whole point of treatment with GnRHa treatment was to allow the child to make it to their target height, to prevent loss of height potential (which is determined by target height) due to advancement of skeletal maturation. In another study, researchers reported that GnRHa administration did not contribute to final height in patients aged 7–8.5 years with early puberty, only provided to reach final height within their target height, and final height in this age group was positively influenced by target height and height at the time of diagnosis (27).
In some studies, it was reported that children with higher height at baseline would have a higher final height (28–31). It was suggested that growth after the withdrawal of treatment had a significant inverse correlation with chronologic age and bone age at the end of treatment, indicating that the timing of initiating treatment and withdrawal could modify the final result (28). Baek et al. (25) showed that factors affecting PFH, thus near-adult height indirectly, included BA/CA at baseline and target height. In our study, it was found that height at diagnosis was positively correlated while chronologic age and was negatively correlated with final height in the multiple regression model including chronologic age, height, BW, target height, and BA/CA at the time of diagnosis. It was found that the age and height at the time of diagnosis could explain 74% of the variation in final height.
In April 2017, Bertelloni et al. (32) published a meta-analysis reviewing studies published between 1980 and 2016 that addressed the effects of GnRHa on final height in girls aged 7–10 years with early puberty. In the meta-analysis, 300 untreated girls with early puberty from seven studies that reported on adult height were defined as group 1, and 183 girls who underwent GnRHa treatment from six studies were defined as group 2. In untreated patients, the target height SDS was -0.557 and PFH SDS was -0.559 at baseline with a final height SDS of -0.663. The Authors found that final height SDS was comparable to target height SDS and PFH SDS at baseline in untreated cases. In patients who underwent GnRHa treatment, target height SDS was -0.678 and PFH SDS was -0.939 at baseline with a final height SDS of -0.604. It was reported that PFH SDS at baseline was significantly lower than final height SDS in this group, but final height SDS was similar to target height SDS.
There are many studies and one meta-analysis claiming that GnRHa therapy does not affect height gain, especially after 6 years of age (5, 27, 32). These data were also supported in our study.
There is an ongoing debate about the effect of GnRHa treatment on BMI during and after treatment. Some studies reported GnRHa treatment increased BMI (26, 31, 32). By contrast, in other studies, it was reported that GnRHa treatment had no effect on BMI (7, 29, 33) or decreased BMI (8, 34). Seung Jae Lee et al. (35) investigated 38 girls with early puberty with a mean age of 7.93±0.84 years. The authors reported that BMI SDS was significantly increased at month 12 (0.79±0.84) and month 18 (0.96±0.83) when compared with BMI SDS at baseline (0.58±1.18). Paterson et al. (26) evaluated 46 girls with precocious puberty who completed goserelin treatment. The mean age at the beginning of treatment was 8.3 years and the duration of treatment was 2.9 years. The authors reported that mean BMI SDS was significantly increased from 0.93 to 1.2 during treatment. Forty-one percent of the patients were overweight, whereas 28% were obese before treatment. At the end of the treatment, these rates increased to 59% and 39%. However, 46 patients returned to their pretreatment BMI. Of these, 11 reached the final height. In our study, GnRH analog treatment increased BMI during treatment and when they reached the final height, they returned to their pretreatment BMI.
In this study, we found that girls aged 6–10 years with central precocious puberty and early puberty who underwent GnRH analog treatment reached the final height within their target height range. It was concluded that GnRH analog treatment increased BMI during treatment, which returned to pretreatment values after the cessation of treatment when they reached the final height. It was found that PFH at the time of diagnosis and the follow-up years can accurately estimate the final height, and the age and height at the time of diagnosis could explain 74% of the variation in final height. Younger age and higher height at the time of diagnosis are positive factors on final height. However, our study has some limitations. One of the limitations is the absence of a control group. Another limitation is that the number of patients for such an analysis, also considering the number of the factors analyzed in the regression model, is not sufficient.
Acknowledgement:
Special thanks to Nermin Kıbrıslıoğlu Uysal for her contribution to statistical analysis.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from Dr. Sami Ulus Maternity and Children’s Health and Diseases Training and Research Hospital Education Planning Board (2015, No: 73799008).
Informed Consent: Patient consent was not obtained in the study due to the retrospective design.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Ş.S.E., Z.A., S.Ç.; Design - Ş.S.E., P.Ş.O.; Supervision - Z.A., Ş.S.E., S.Ç.; Funding - Z.A., Ş.S.E., S.Ç.; Materials - P.Ş.O., Ş.S.E.; Data Collection and/or Processing - P.Ş.O., Ş.S.E.; Analysis and/or Interpretation - P.Ş.O., Ş.S.E.; Literature Review - P.Ş.O., Ş.S.E.; Writing - P.Ş.O., Ş.S.E.; Critical Review - Z.A., S.Ç., Ş.S.E.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
Teşekkür: İstatistiksel analizlere katkısından dolayı Nermin Kıbrıslıoğlu Uysal’a teşekkürler.
Etik Kurul Onayı: Bu çalışma için etik kurul onayı Dr. Sami Ulus Kadın Doğum, Çocuk Sağlığı ve Hastalıkları Eğitim ve Araştırma Hastanesi Eğitim Planlama Kurulu’ndan alınmıştır (2015, No: 73799008).
Hasta Onamı: Çalışmada geriye dönük tasarımdan dolayı hasta onamı alınmamıştır.
Hakem Değerlendirmesi: Dış bağımsız.
Yazar Katkıları: Fikir - Ş.S.E., Z.A., S.Ç.; Tasarım - Ş.S.E., P.Ş.O.; Denetleme - Z.A., Ş.S.E., S.Ç.; Kaynaklar - Z.A., Ş.S.E., S.Ç.; Malzemeler - P.Ş.O., Ş.S.E.; Veri Toplanması ve/veya İşlemesi - P.Ş.O., Ş.S.E.; Analiz ve/veya Yorum - P.Ş.O., Ş.S.E.; Literatür Taraması - P.Ş.O., Ş.S.E.; Yazıyı Yazan - P.Ş.O., Ş.S.E.; Eleştirel İnceleme - Z.A., S.Ç., Ş.S.E.
Çıkar Çatışması: Yazarlar çıkar çatışması bildirmemişlerdir.
Mali Destek: Yazarlar bu çalışma için mali destek almadıklarını beyan etmişlerdir.
References
- 1.Lee PA, Houk CP. Puberty and Its Disorders. In: Liftshitz F, editor. Pediatric Endocrinology. New York, USA: Informa Healthcare Inc; 2007. pp. 274–90. [Google Scholar]
- 2.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity:variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 3.Jung MK, Song KC, Kwon AR, Chae HW, Kim DH, Kim HS. Adult height in girls with central precocious puberty treated with gonadotropin-releasing hormone agonist with or without growth hormone. Ann Pediatr Endocrinol Metab. 2014;19:214–9. doi: 10.6065/apem.2014.19.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein KO, Barnes KM, Jones JV, Feuillan PP, Cutler GB., Jr Increased final height in precocious puberty after long-term treatment with LHRH agonists:the National Institutes of Health experience. J Clin Endocrinol Metab. 2001;86:4711–6. doi: 10.1210/jcem.86.10.7915. [DOI] [PubMed] [Google Scholar]
- 5.Lazar L, Padoa A, Phillip M. Growth pattern and final height after cessation of gonadotropin-suppressive therapy in girls with central sexual precocity. J Clin Endocrinol Metab. 2007;92:3483–9. doi: 10.1210/jc.2007-0321. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka T, Niimi H, Matsuo N, et al. Results of long-term follow-up after treatment of central precocious puberty with leuprorelin acetate:evaluation of effectiveness of treatment and recovery of gonadal function. The TAP-144-SR Japanese Study Group on Central Precocious Puberty. J Clin Endocrinol Metab. 2005;90:1371–6. doi: 10.1210/jc.2004-1863. [DOI] [PubMed] [Google Scholar]
- 7.Palmert MR, Mansfield MJ, Crowley WF, Jr, Crigler JF, Jr, Crawford JD, Boepple PA. Is obesity an outcome of gonadotropin-releasing hormone agonist administration?Analysis of growth and body composition in 110 patients with central precocious puberty. J Clin Endocrinol Metab. 1999;84:4480–8. doi: 10.1210/jcem.84.12.6204. [DOI] [PubMed] [Google Scholar]
- 8.Heger S, Partsch CJ, Sippell WG. Long-term outcome after depot gonadotropin-releasing hormone agonist treatment of central precocious puberty:final height, body proportions, body composition, bone mineral density, and reproductive function. J Clin Endocrinol Metab. 1999;84:4583–90. doi: 10.1210/jcem.84.12.6203. [DOI] [PubMed] [Google Scholar]
- 9.Neyzi O, Bundak R, Gökçay G, et al. Reference Values for Weight, Height, Head Circumference, and Body Mass Index in Turkish Children. J Clin Res Pediatr Endocrinol. 2015;7:280–93. doi: 10.4274/jcrpe.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 1st edition. Stanford University Press; 1999. [Google Scholar]
- 12.Bayley N, Pinneau Sr. Tables for predicting adult height from skeletal age:revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952;40:423–41. doi: 10.1016/s0022-3476(52)80205-7. [DOI] [PubMed] [Google Scholar]
- 13.Mauras N, Attie KM, Reiter EO, Saenger P, Baptista J. High dose recombinant human growth hormone (GH) treatment of GH-deficient patients in puberty increases near-final height:a randomized, multicenter trial. Genentech, Inc., Cooperative Study Group. J Clin Endocrinol Metab. 2000;85:3653–60. doi: 10.1210/jcem.85.10.6906. [DOI] [PubMed] [Google Scholar]
- 14.Tanner JM, Goldstein H, Whitehouse RH. Standards for children's height at ages 2-9 years allowing for heights of parents. Arch Dis Child. 1970;45:755–62. doi: 10.1136/adc.45.244.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houk CP, Kunselman AR, Lee PA. Adequacy of a single unstimulated luteinizing hormone level to diagnose central precocious puberty in girls. Pediatrics. 2009;123:e1059–63. doi: 10.1542/peds.2008-1180. [DOI] [PubMed] [Google Scholar]
- 16.Neely EK, Hintz RL, Wilson DM, et al. Normal ranges for immunochemiluminometric gonadotropin assays. J Pediatr. 1995;127:40–6. doi: 10.1016/s0022-3476(95)70254-7. [DOI] [PubMed] [Google Scholar]
- 17.Badaru A, Wilson DM, Bachrach LK, et al. Sequential comparisons of one-month and three-month depot leuprolide regimens in central precocious puberty. J Clin Endocrinol Metab. 2006;91:1862–7. doi: 10.1210/jc.2005-1500. [DOI] [PubMed] [Google Scholar]
- 18.Hümmelink R, Oostdijk W, Partsch CJ, Odink RJ, Drop SL, Sippell WG. Growth, bone maturation and height prediction after three years of therapy with the slow release GnRH-agonist Decapeptyl-Depot in children with central precocious puberty. Horm Metab Res. 1992;24:122–6. doi: 10.1055/s-2007-1003273. [DOI] [PubMed] [Google Scholar]
- 19.Brauner R, Malandry F, Rappaport R. Predictive factors for the effect of gonadotrophin releasing hormone analogue therapy on the height of girls with idiopathic central precocious puberty. Eur J Pediatr. 1992;151:728–30. doi: 10.1007/BF01959077. [DOI] [PubMed] [Google Scholar]
- 20.Boepple PA, Mansfield MJ, Crawford JD, Crigler JF, Jr, Blizzard RM, Crowley WF., Jr Gonadotrophin-releasing hormone agonist treatment of central precocious puberty:an analysis of growth data in a developmental context. Acta Paediatr Scand Suppl. 1990;367:38–43. doi: 10.1111/j.1651-2227.1990.tb11630.x. [DOI] [PubMed] [Google Scholar]
- 21.Oostdijk W, Rikken B, Schreuder S, et al. Final height in central precocious puberty after long term treatment with a slow release GnRH agonist. Arch Dis Child. 1996;75:292–7. doi: 10.1136/adc.75.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauli R, Galatzer A, Kornreich L, Lazar L, Pertzelan A, Laron Z. Final height of girls with central precocious puberty, untreated versus treated with cyproterone acetate or GnRH analogue. A comparative study with re-evaluation of predictions by the Bayley-Pinneau method. Horm Res. 1997;47:54–61. doi: 10.1159/000185432. [DOI] [PubMed] [Google Scholar]
- 23.Oerter KE, Manasco PK, Barnes KM, Jones J, Hill S, Cutler GB., Jr Effects of luteinizing hormone-releasing hormone agonists on final height in luteinizing hormone-releasing hormone-dependent precocious puberty. Acta Paediatr Suppl. 1993;388:62–8. doi: 10.1111/j.1651-2227.1993.tb12846.x. [DOI] [PubMed] [Google Scholar]
- 24.Cacciari E, Cassio A, Balsamo A, et al. Long-term follow-up and final height in girls with central precocious puberty treated with luteinizing hormone-releasing hormone analogue nasal spray. Arch Pediatr Adolesc Med. 1994;148:1194–9. doi: 10.1001/archpedi.1994.02170110080014. [DOI] [PubMed] [Google Scholar]
- 25.Baek JW, Nam HK, Jin D, Oh YJ, Rhie YJ, Lee KH. Age of menarche and near adult height after long-term gonadotropin-releasing hormone agonist treatment in girls with central precocious puberty. Ann Pediatr Endocrinol Metab. 2014;19:27–31. doi: 10.6065/apem.2014.19.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paterson WF, McNeill E, Young D, Donaldson MD. Auxological outcome and time to menarche following long-acting goserelin therapy in girls with central precocious or early puberty. Clin Endocrinol (Oxf) 2004;61:626–34. doi: 10.1111/j.1365-2265.2004.02146.x. [DOI] [PubMed] [Google Scholar]
- 27.Savaş-Erdeve Ş, Şıklar Z, Hacıhamdioğlu B, et al. Gonadotropin-Releasing Hormone Analogue Treatment in Females with Moderately Early Puberty:No Effect on Final Height. J Clin Res Pediatr Endocrinol. 2016;8:211–7. doi: 10.4274/jcrpe.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Fátima Borges M, de Melo Franciscon P, Contursi Cambraia T, et al. Evaluation of central precocious puberty treatment with GnRH analogue at the Triangulo Mineiro Federal University (UFTM) Arch Endocrinol Metab. 2015;59:515–22. doi: 10.1590/2359-3997000000101. [DOI] [PubMed] [Google Scholar]
- 29.Brito VN, Latronico AC, Cukier P, et al. Factors determining normal adult height in girls with gonadotropin-dependent precocious puberty treated with depot gonadotropin-releasing hormone analogs. J Clin Endocrinol Metab. 2008;93:2662–9. doi: 10.1210/jc.2007-2183. [DOI] [PubMed] [Google Scholar]
- 30.Benetti-Pinto CL, Camargo LB, Magna LA, Garmes HM, Petta CA. Predictive factors for height gain in idiopathic central precocious puberty treated with GnRH analogues. Rev Bras Ginecol Obstet. 2008;30:609–13. doi: 10.1590/s0100-72032008001200004. [DOI] [PubMed] [Google Scholar]
- 31.Carel JC, Roger M, Ispas S, et al. Final height after long-term treatment with triptorelin slow release for central precocious puberty:importance of statural growth after interruption of treatment. French study group of Decapeptyl in Precocious Puberty. J Clin Endocrinol Metab. 1999;84:1973–8. doi: 10.1210/jcem.84.6.5647. [DOI] [PubMed] [Google Scholar]
- 32.Bertelloni S, Massart F, Miccoli M, Baroncelli GI. Adult height after spontaneous pubertal growth or GnRH analog treatment in girls with early puberty:a meta-analysis. Eur J Pediatr. 2017;176:697–704. doi: 10.1007/s00431-017-2898-8. [DOI] [PubMed] [Google Scholar]
- 33.Traggiai C, Perucchin PP, Zerbini K, Gastaldi R, De Biasio P, Lorini R. Outcome after depot gonadotrophin-releasing hormone agonist treatment for central precocious puberty:effects on body mass index and final height. Eur J Endocrinol. 2005;153:463–4. doi: 10.1530/eje.1.01975. [DOI] [PubMed] [Google Scholar]
- 34.Antoniazzi F, Zamboni G, Bertoldo F, et al. Bone mass at final height in precocious puberty after gonadotropin-releasing hormone agonist with and without calcium supplementation. J Clin Endocrinol Metab. 2003;88:1096–101. doi: 10.1210/jc.2002-021154. [DOI] [PubMed] [Google Scholar]
- 35.Lee SJ, Yang EM, Seo JY, Kim CJ. Effects of gonadotropin-releasing hormone agonist therapy on body mass index and height in girls with central precocious puberty. Chonnam Med J. 2012;48:27–31. doi: 10.4068/cmj.2012.48.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]


