Abstract
Aim:
To evaluate the birth prevalence of specifically selected major congenital anomalies and to determine the correlated neonatal and maternal characteristics.
Material and Methods:
Data were collected retrospectively from hospital-based records of infants who were born at 22 completed weeks of gestation with a birth weight of more than 500 g in Zekai Tahir Burak Gynecology Training and Research Hospital between 2013 and 2018. Abortions, stillbirths, and terminated pregnancies due to fetal anomalies were excluded. Average annual prevalences were calculated for each selected major congenital anomaly.
Results:
The total prevalence of congenital anomalies was 9.97 per 1000 in 102 379 live birth cohorts. The prevalence of severe congenital heart anomalies (SI-SII) was found as 21.1 per 10 000 live births. Down syndrome and meningomyelocele were the second and third most common anomalies, after congenital heart defects (13.87 and 9.97 per 10 000 live births, respectively). The prevalence of anomalies requiring specific surgery was found as 4.3 per 1000 live births. Congenital heart disease was present in 31.7% of patients who had Down syndrome. Atrioventricular septal defect accounted for 53.3% of congenital heart anomalies detected in Down syndrome. The prevalence of Down syndrome in babies of mothers aged 35 years and older was found as 46.67 per 10 000, which was significantly higher than in the group aged under 35 years (8.24 per 10 000). On the other hand, the prevalence of gastroschisis in babies of mothers aged 19 years and under was found as 5.81 per 10 000, which was higher than in the group aged 20 years and over (0.84 per 10 000).
Conclusion:
The actual magnitude of the number of births affected by congenital anomalies in Turkey is unknown. In our study, congenital heart diseases, Down syndrome, and meningomyelocele were found to be the most common congenital anomalies, respectively.
Keywords: Congenital anomalies, newborn, prevalence
Abstract
Amaç:
çalışmanın amacı, özel olarak seçilmiş majör konjenital anomalilerin doğum yaygınlığını değerlendirmek ve bu anomalilerle ilişkili yenidoğan ve anneye ait özellikleri belirlemekti.
Gereç ve Yöntemler:
Veriler, Ankara Zekai Tahir Burak Kadın Hastalıkları Eğitim ve Araştırma hastanesinde 2013 ile 2018 yılları arasında, gestasyonel 22 haftasını tamamlamış ya da 500 g üstü canlı doğan bebeklerin hastane tabanlı kayıtlarından geriye dönük olarak toplandı. Düşükler, ölü doğumlar ve fetal anomali nedeniyle gebelik terminasyonu yapılan bebekler çalışmaya alınmadı. Seçilmiş majör konjenital anomali türünün her biri için ortalama yıllık prevalanslar hesaplandı.
Bulgular:
Majör konjenital anomalilerin genel prevalansı 102 379 canlı doğum kohortunda 1 000’de 9,97 hesaplandı. Ağır konjenital kalp anomalilerinin (SI-SII) total prevalansı 10 000 canlı doğumda 21,1 bulundu. Konjenital kalp hastalıklarından sonra, Down sendromu ve meningomiyelosel doğumda en sık gözlenen anomalilerdi (sırasıyla 10 000 canlı doğumda 13,87 ve 9,97). Özgün cerrahi ameliyat gerektiren anomaliler 1 000 canlı doğumda 4,3 bulundu. Down sendromlu olguların %31,7’inde konjenital kalp hastalığı vardı. Down sendromunda atriyoventriküler septal defekt, bu sendromda saptanan konjenital kalp anomali olgularının %53,3’ünü oluşturdu. Down sendromu prevalansı 35 yaş ve üstü anne bebeklerinde 10 000’de 46,67 (buna karşın 35 yaş altı grupta 8,24) sıklıkta belirgin düzeyde daha yüksek bulunurken, bunun aksine gastroşizis prevalansı 19 yaş ve altı anne bebeklerinde 10 000’de 5,81 (buna karşın 20 yaş ve üstü grupta 0,84) oranı ile daha yüksek bulundu.
Çıkarımlar:
Türkiye’de konjenital anomalilerden etkilenen doğum sayısının gerçek büyüklüğü bilinmemektedir. Majör konjenital anomalilerinin doğum sıklığının belirlenmesi yönünde yapılan çalışmamızda; konjenital kalp hastalıkları, Down sendromu ve meningomiyelosel sırasıyla en sık görülen konjenital anomaliler olarak saptandı.
Introduction
Congenital anomalies are defined as anomalies of body composition or body function that occur in the prenatal period and are present at birth (1). Congenital anomalies affect about 3% of all live births (2). Again, congenital anomalies had a share of 20.8% in all infant deaths in 2015 in the United States of America (USA) and they have become the main cause of death in infants as a result of the reduction in the frequency of other common causes of death such as diseases related to preterm birth and low birth weight, bacterial sepsis, and neonatal respiratory distress (3). In our country, congenital malformations and chromosomal anomalies were responsible for 1.23% of all causes of death in 2018 according to the Turkish Statistical Institute, taking second place to diseases related to preterm birth and low birth weight among causes of infant deaths (4).
Congenital anomalies are present at birth even if they are diagnosed months or years later. The calculation of the total prevalence of congenital anomalies is complex because of differences in surveillance methods and the periods selected when obtaining records, and because of differences in the criteria for including and excluding anomalies, which makes it difficult to make comparisons between records. Therefore, congenital anomaly epidemiologists are generally reluctant to give total prevalence predictions for congenital anomalies in a defined population (5–8).
Many studies have evaluated the prevalence and predisposition for selected major anomalies involving a single anomaly or anomaly category that is observed most commonly (6, 7, 9, 10). The anomalies selected in this study were preferred because they were relatively easy to recognize at birth, had an important public health impact, and there was primary protection potential for some of them. This approach makes it easy to collect and analyze data, increases data quality, and reduces the complexity to a great extent. The list to be selected may vary depending on the capacity and resources of the healthcare system and surveillance program (11).
There is little information about the prevalences of congenital anomalies in Turkey and the actual magnitude of the number of births affected by congenital anomalies is unknown because there is no national congenital anomaly surveillance system. Knowing the prevalence and predispositions for congenital anomalies is important in terms of specifying causative or preventive factors.
The aim of our study was to calculate the prevalence of selected congenital anomalies that have a great disease load in the community and are significantly involved in neonatal deaths, and to emphasize that our country needed a community-based surveillance program.
Material and Methods
The surveillance involved 102 379 consecutive newborn live babies who were born between January 2013 and December 2018 at Ankara Zekai Tahir Burak Women’s Health Education and Research Hospital. Individually defined data for the selected major congenital anomalies were obtained from hospital records, retrospectively. Infants born live with a gestational age of 22 weeks or with a birth weight of more than 500 g who were diagnosed as having major congenital anomaly at birth or in the first 1 week of life were included in the study. Cases of fetal death, terminated pregnancy, and minor anomalies were excluded. In the prevalence calculation, the denominator data were obtained from hospital birth records.
Fifteen major congenital anomalies including the central nervous system (meningomyelocele), cardiovascular system (truncus arteriosus, transposition of the great arteries, Fallot tetralogy, atrioventricular septal defect, hypoplastic left heart syndrome, coarctation of the aorta), gastrointestinal system (esophageal atresia, intestinal atresias, rectal and bowel atresia), musculoskeletal system (diaphragmatic hernia, gastroschisis, omphalocele) and orofacial system (cleft lip and/or palate) were selected for the study. Down syndrome (DS) was added to the list outside these organ system categories. This example list was chosen because these anomalies were relatively easy to recognize at birth, manifested themselves in the first week of life, had important public health impact, and some of these had primary prevention potential.
Cardiovascular anomalies were chosen among severe congenital heart diseases, which were classified as SI and SII according to the European Concerted Action on Congenital Anomalies and Twins (EUROCAT) system. In this classification, congenital heart anomalies are categorized into three groups according to the perinatal mortality rate (SI, SII and SIII). According to this classification, SI involves single ventricle, hypoplastic left heart, hypoplastic right heart, Ebstein anomaly and tricuspid atresia; SII involves pulmonary valve atresia, truncus arteriosus, atrioventricular septal defect, aortic valve atresia/stenosis, transposition of the great arteries, Fallot tetralogy, total anomalous pulmonary venous return and coarctation of the aorta; SIII involves isolated ventricular septal defect, atrial septal defect and pulmonary valve stenosis in the absence of any congenital heart anomaly included in SI and SII (12).
Anomalies that required surgery were categorized according to the EUROCAT system’s classification system, and a separate prevalence rate was calculated. This classification system involved anomalies requiring specific surgery such as selected gastrointestinal malformations, gastroschisis, omphalocele, severe congenital heart anomalies, and orofacial clefts (13).
In addition, atrioventricular septal defect was categorized as syndromic (if chromosomal or syndromic conditions were present) and nonsyndromic. Cleft lip and/or palate was also classified into two separate groups as syndromic and isolated (14).
As maternal age is strongly associated with DS and gastroschisis, the number of cases for these anomalies was collected under six categories by maternal age at the time of birth: below the age of 20 years, 20–24 years, 25–29 years, 30–34 years, 35–39 years, and 40 years and over. In the evaluation of maternal age in cases of DS, the age groups were collected under two main age groups, <35 years and ≥35 years (15).
Ethics approval for our study was obtained from our hospital’s ethics committee (Date: 15.11.2018, No.: 66/2018). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Statistical Analysis
The total and 3-year-interval birth prevalence rate for each selected major congenital anomaly was specified and calculated as the number of babies born live with a congenital anomaly per 10 000 live births.
The prevalence rates for major congenital anomalies and 95% confidence intervals (CI) were calculated separately over the years and with 3-year intervals. The difference between the male and female sexes in terms of the frequency of congenital anomalies and maternal age significance levels in cases of DS and gastroschisis were calculated using the Chi-square and Fisher’s exact tests over a p-value of 0.05. All analyses were performed using the SPSS 21.0 program.
Results
In our study, congenital anomalies were found in 1011 babies among 102 379 live births; the total prevalence was calculated as 0.99% (95% CI: 0.87–1.12) (Table 1). No difference was found between the male and female sexes in terms of congenital anomaly frequency (p=0.427).
Table 1.
Distribution of major congenital anomalies by years and sex and prevalences by years
| Year | Number of live births | Number of major congenital anomaly | Prevalence by years (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | p | Total | Male | Female | p | ||
| 2013 | 18 034 | 9171 | 8863 | 167 | 85 | 82 | 0.93 | ||
| 2014 | 18 551 | 9505 | 9046 | 161 | 72 | 89 | 0.87 | ||
| 2015 | 17 795 | 9138 | 8657 | 158 | 76 | 82 | 0.89 | ||
| 2016 | 16 771 | 8550 | 8221 | 176 | 105 | 71 | 1.05 | ||
| 2017 | 16 111 | 8150 | 7961 | 164 | 81 | 83 | 1.02 | ||
| 2018 | 15 117 | 7681 | 7436 | 185 | 109 | 76 | 1.22 | ||
| Total | 102 379 | 52 195 | 50 184 | 0.737a | 1 011 | 528 | 483 | 0.427b | 0.99 |
: Statistical comparison of the number of male and female cases of live births by years. Chi-square test was used; (p>0.05).
: Statistical comparison of the total number of male and female subjects with a congenital anomaly. Chi-square test was used; (p>0.05)
The major congenital anomalies included in the study were selected among a total of 1011 babies with major congenital anomalies found in our hospital’s records. The prevalences per 10 000 live births for these specifically selected major congenital anomalies were calculated in total and in 3-year periods (Table 2). The total prevalence for severe congenital heart anomalies (SI-SII) was found as 21.1 per 10 000 births, and severe congenital heart anomalies were the most common type of anomaly, followed by DS and meningomyelocele (13.87 and 9.97 per 10 000 live births, respectively).
Table 2.
Prevalences of selected major congenital anomalies between 2013 and 2018 (per 10 000 live births)
| Birth year periods (numbers of live births) | ||||||
|---|---|---|---|---|---|---|
| Congenital anomaly category | Total (102 379) | 2013–2015 (54 380) | 2016–2018 (47 999) | |||
| n | Prevalence(95% CI) | n | Prevalence(95% CI) | n | Prevalence(95% CI) | |
| Cardiovascular anomalies | ||||||
| Truncus arteriosus | 8 | 0.78 (0.1–1.47) | 5 | 0.93 (0–1.99) | 3 | 0.64 (0–1.39) |
| Transposition of the great arteries | 30 | 2.93 (1.67–4.18) | 17 | 3.14 (1.83–4.46) | 13 | 2.71 (0.46–4.97) |
| Fallot tetralogy | 35 | 3.38 (2.0–4.75) | 18 | 3.29 (2.02–4.57) | 17 | 3.46 (0.81–6.11) |
| Atrioventricular septal defect | 52 | 5.07 (3.46–6.68) | 27 | 4.95 (4.03–5.87) | 25 | 5.19 (1.77–8.60) |
| Hypoplastic left heart syndrome | 24 | 2.37 (1.58–3.15) | 13 | 2.40 (2.06–2.73) | 11 | 2.34 (0.63–4.04) |
| Coarctation of the aorta | 25 | 2.47 (1.75–3.2) | 11 | 2.04 (0.94–3.14) | 14 | 2.91 (2.65–3.17) |
| Nervous system anomalies | ||||||
| Meningomyelocele | 103 | 9.97 (8.80–11.43) | 56 | 10.30 (9.02–11.76) | 47 | 9.79 (7.29–12.37) |
| Orofacial clefts | ||||||
| Cleft lip and/or palate | 83 | 8.11 (5.49–10.94) | 37 | 6.80 (5.63–7.96) | 46 | 9.58 (4.34–14.93) |
| Gastrointestinal anomalies | ||||||
| Esophageal atresia | 38 | 3.67 (2.26–5.08) | 21 | 3.85 (1.16–6.55) | 17 | 3.49 (1.90–5.09) |
| Anorectal anomalies | 41 | 4.16 (2.21–6.10) | 12 | 2.22 (1.10–3.34) | 29 | 6.09 (4.26–7.92) |
| Intestinal atresias | 23 | 2.23 (1.69–2.76) | 14 | 2.58 (1.60–3.55) | 9 | 1.88 (1.77–1.99) |
| Musculoskeletal anomalies | ||||||
| Diaphragmatic hernia | 63 | 6.10 (4.34–7.86) | 41 | 7.56 (5.48–9.64) | 22 | 4.63 (2.92–6.35) |
| Gastroschisis | 12 | 1.18 (0.86–1.50) | 5 | 0.92 (0.56–1.28) | 7 | 1.45 (1.12–1.79) |
| Omphalocele | 24 | 2.40 (1.12–3.69) | 8 | 1.48 (0.72–2.23) | 16 | 3.33 (1.23–5.42) |
CI: Confidence interval
The prevalence of DS found as 13.87 per 10 000 live births (95% CI: 10.43–17.31). The prevalence of DS among mothers aged ≥35 years was found as 46.67 per 10 000 live births, which was statistically significantly higher than in the group aged <35 years (8.24 per 10 000 live births) (p<0.001) (Table 3, Fig. 1).
Table 3.
Distribution of the cases of Down syndrome by maternal age range and prevalences
| Maternal age range | Number of live births (n) | Number of Down syndrome (n) | Prevalence (per 10 000 live births) | pa | |
|---|---|---|---|---|---|
| 19 years and below | 6919 | 6 | 8.67 | <0.001 | |
| 20–24 years | 27 470 | 16 | 5.82 | 8.24 | |
| 25–29 years | 30 524 | 16 | 5.24 | ||
| 30–34 years | 22 472 | 34 | 15.13 | ||
| 35–39 years | 11 841 | 36 | 30.40 | 46.67 | |
| 40 years and above | 3153 | 34 | 107.83 | ||
| Total | 102 379 | 142 | 13.87 | ||
: Statistical comparison of the mothers aged below and above 35 years (p< 0.01)
Figure 1.
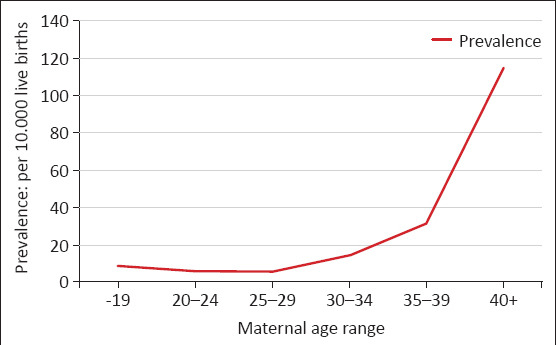
Prevalence rates by maternal age in babies with Down syndrome
Forty-five (31.7%) of 142 patients with DS had congenital heart disease. Atrioventricular septal defect (AVSD) constituted 53.3% of cases of congenital heart anomalies found in this syndrome (n=24). In our patients with DS, 16 isolated ventricular septal defects, four fallot tetralogies, and one total anomalous pulmonary venous return anomaly were found besides AVSD.
Thirty-five (67.3%) of 52 patients with atrioventricular septal defect (AVSD) had an additional syndrome or congenital anomaly (syndromic form) and 24 of these syndromic forms were DS. On the other hand, 49% of all patients with AVSD had DS.
Orofacial clefts were classified according to anatomic forms and the presence of additional anomaly/syndrome. A syndromic form was found in 51.8% of the cases of orofacial cleft (Table 4).
Table 4.
Classification and prevalence rates of orofacial clefts
| Cleft type/Anatomic form | Male (n) | Female (n) | Total (n) | Syndromic form (SF) (n) | Isolated form (I) (n) | Ratio SF/I |
|---|---|---|---|---|---|---|
| Cleft lip | 3 | 1 | 4 | 1 | 3 | 0.33 |
| Cleft palate | 20 | 21 | 41 | 24 | 17 | 1.41 |
| Cleft lip-palate | 21 | 17 | 38 | 18 | 20 | 0.90 |
| Total/Ratio (%) | 44 (53%) | 39 (47%) | 83 | 43 (51.8%) | 40 (49.2%) | 1.08 |
Among the mothers aged 19 years and below, the prevalence of gastroschisis was found as 5.81 per 10 000 live births, which was statistically significantly higher than in mothers aged ≥20 years (0.84 per 10 000 live births) (p=0.007) (Table 5, Fig. 2).
Table 5.
Distribution of the babies with gastroschisis by maternal age range and prevalences
| Maternal age range | Number of live births (n) | Number of gastroschises (n) | Prevalence (per 10 000 live births) | pa |
|---|---|---|---|---|
| 19 years and below | 6919 | 4 | 5.81 | 0.007 |
| 20 years and above | 95 460 | 8 | 0.84 | |
| Total | 102 379 | 12 | 1.18 |
: Statistical comparison of the mothers aged below 19 years and above 20 years (p<0.01)
Figure 2.
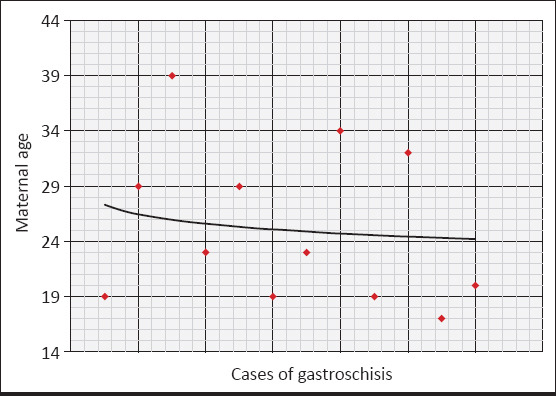
Distribution of babies with gastroschisis by maternal age
The prevalence of anomalies requiring specific surgery was found as 4.3 per 1000 live births (95% CI: 3.89–4.71).
Discussion
The total birth prevalence of major congenital anomalies was found as 0.99%. In the USA, the Centers for Disease Control and Prevention (CDC) reported that approximately 3% of all live births were complicated by congenital anomalies (9, 16). The mean prevalence of congenital anomalies in live births was reported as 1.87% in European countries, 1–1.5% in Brazil, 1.74% in Japan, 1.13% in Iran, and 1.25% in India (17–21). In a study conducted by Tunçbilek et al. (22), which was the only multicenter, prospective study involving 22 university hospitals from different regions in our country, the birth prevalence of congenital anomalies was found as 3.65%. In another study, the general incidence of congenital anomalies in newborns in the Turkish population was reported as 1.11% (23). The heterogeneity in the prevalence rates in our study and the literature arises from the differences in the surveillance methods selected when obtaining records. Because our study was a retrospective record study, many isolated major malformations were entered deficiently in the records, and this was thought to be associated with the low total prevalence of congenital anomalies found in our study.
The total prevalence of severe congenital heart anomalies (SI-SII), which were found with the highest frequency, was found as 21.1 per 10 000 live births. In European countries, the total prevalence of severe congenital heart anomalies was reported as 17.20 per 10 000 live births, which was close to the prevalence found in our study (12, 24).
In our cases of DS, AVSD constituted 53.3% of the congenital heart anomalies found in this syndrome. In a large-scale study conducted in European countries, the most common cardiac anomalies among patients with DS with congenital heart defects included AVSD (30%), atrial septal defect (25%), ventricular septal defect (22%), patent ductus arteriosus (5%), coarctation of the aorta (5%), and Fallot tetralogy (3%) (25). In our study, the rate of AVSD was observed to be high because asymptomatic atrial septal defect and patent ductus arteriosus in the neonatal period were not included in the anomaly group.
The prevalence of AVSD was found as 5.07 per 10 000 live births (95% CI: 3.46–6.68). In the data obtained from EUROCAT, which is a European network of population-based registries collecting data about congenital anomalies in Europe, the prevalence of AVSD was reported as 5.3 per 10 000 live births. This prevalence was similar to the prevalence found in our study (26).
The prevalence rate for DS in live births was found as 13.87 per 10 000 live births, which was higher compared with European countries (9.79 per 10 000 live births) (Table 6) (27). The prevalence of meningomyelocele was found as 9.97 per 10 000 live births, which was higher compared with the USA and European countries (3.5 and 1.63 per 10 000 live births, respectively) (Table 6). In these countries, very high rates of pregnancy termination due to spina bifida and DS caused marked reductions in the prevalence rates (9, 14, 27).
Table 6.
Comparison of the prevalence rates of congenital anomalies in our study with the surveillance studies in the literature
| Prevalence: per 10 000 live births | Our study 2013–2018 Ankara Hospital-based 102 379 |
Le MT et al. (14) 1999–2015 Texas Population-based 4 655 400 |
Parker SE et al. (9) 2004–2006 USA Population-based 12 515 956 |
EUROCAT (27) 2010–2017 Europ Population-based 5 555 027 |
Ko JK et al. (10) 2008–2014 Korea Population-based 3 208 617 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Prev. | n | Prev. | n | Prev. | n | Prev. | n | Prev. | |
| Truncus arteriosus | 8 | 0.78 | 551 | 0.84 | 301 | 0.72 | 242 | 0.44 | 75 | 0.23 |
| Transposition of the great arteries | 30 | 2.93 | 3354 | 5.13 | 1252 | 3.00 | 1683 | 2.92 | 574 | 1.8 |
| Fallot tetralogy | 35 | 3.38 | 2466 | 3.77 | 1657 | 3.97 | 1577 | 2.84 | 1365 | 4.3 |
| Atrioventricular septal defect | 52 | 5.07 | 2794 | 4.27 | 1966 | 4.71 | 1740 | 3.13 | ||
| Hypoplastic left heart syndrome | 24 | 2.25 | 1440 | 2.20 | 960 | 2.30 | 721 | 1.30 | 130 | 0.41 |
| Coartation of the aorta | 25 | 2.47 | 3340 | 5.10 | 2006 | 3.61 | 979 | 3.1 | ||
| Meningomyelocele | 103 | 9.97 | 2437 | 3.72 | 1460 | 3.50 | 903 | 1.63 | 2759 | 8.6 |
| Cleft palate and/or lip | 83 | 8.11 | 10 897 | 16.66 | 7088 | 16.98 | 7958 | 12.67 | 5309 | 16.5 |
| Esophageal atresia | 38 | 3.67 | 1399 | 2.14 | 905 | 2.17 | 1298 | 2.34 | 484 | 1.5 |
| Intestinal atresias | 23 | 2.23 | 2149 | 3.28 | 1160 | 2.09 | 1956 | 6.1 | ||
| Anorectal anomalies | 41 | 4.16 | 3441 | 5.26 | 1952 | 4.68 | 1393 | 2.51 | 1456 | 4.5 |
| Diaphragmatic hernia | 63 | 6.10 | 1795 | 2.74 | 1871 | 4.49 | 1123 | 2.02 | 408 | 1.3 |
| Omphalocele | 24 | 2.40 | 1377 | 2.10 | 775 | 1.86 | 691 | 1.24 | 1345 | 4.2 |
| Gastroschisis | 12 | 1.18 | 3432 | 5.24 | 1871 | 4.49 | 1217 | 2.19 | 77 | 0.24 |
| Down syndrome | 142 | 1.87 | 8809 | 13.46 | 6037 | 14.47 | 5437 | 9.79 | 1301 | 4.1 |
The prevalence of gastroschisis was found as 1.18 per 10 000 live births, which was markedly lower compared with the prevalence rates in the USA and European countries (9, 15, 27). In addition, gastroschisis was found to be associated with young maternal age (younger than 20 years). Both in Europe and other regions of the world, high prevalence rates and increments were reported for gastroschisis, which was partially associated with young pregnancy rates (28).
The prevalence of diaphragmatic hernia was found as 6.1 per 10 000 live births, which was markedly higher compared with the prevalence rates reported in the literature (10, 14, 27). The reason for this high prevalence was thought to be associated with the referral of patients who were diagnosed prenatally in external centers to our hospital in the prenatal period.
The prevalence of cleft lip and/or palate was found as 8.11 per 10 000 live births. This was markedly lower prevalence compared with the literature, suggesting that these cases were entered deficiently in the records in our study (Table 6).
The prevalence of anomalies requiring specific surgery was found as 4.3 per 10 000 live births. In European countries, the birth prevalence of anomalies requiring specific surgery was reported as 3–5 per 10 000 live births in most records, similar to our study. According to the data in the registry obtained between 2005 and 2007 (Odense, Denmark), 1.2% of all live births underwent surgery in early childhood because of congenital anomalies (13).
Our retrospective study involves the list of entrances in a defined hospital database. The limitations of our study include poor quality data provided by hospital-based surveillance and misleading results in prevalence rates because of clustering of cases in certain hospitals (29).
In conclusion, a national study group should be constituted for coordination and planning based on international plans, and a high-capacity, population-based congenital anomaly surveillance system should be established to prevent and reduce congenital anomalies in our country (30). The program may be initiated with a limited number of selected major congenital anomalies similar to our study, and later be extended such that additional anomalies are included when experience is gained and resources are increased.
Footnotes
Ethics Committee Approval: Ethics committee approval for our study was obtained from our hospital’s committee (date: 15.11.2018, number: 66/2018).
Informed Consent: Patient consent was not obtained due to the retrospective design of the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - D.T.; Design - D.T., F.E.C., T.D.; Supervision - F.E.C., N.A., Y.Y.; Data Collection and/or Processing - D.T., T.D., N.A., Y.Y.; Analysis and/or Interpretation - D.T., T.D.; Literature Review - D.T., T.D.; Writing - D.T.; Critical Review - F.E.C., N.A., Y.Y.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
Etik Kurul Onayı: Çalışmamız için etik kurul onayı hastanemiz kurulundan 15.11.2018 tarih ve 66/2018 numarası ile alındı.
Hasta Onamı: Çalışmanın geriye dönük tasarımından dolayı hasta onamı alınmamıştır.
Hakem Değerlendirmesi: Dış bağımsız.
Yazar Katkıları: Fikir - D.T.; Tasarım - D.T., F.E.C., T.D.; Denetleme - F.E.C., N.A., Y.Y.; Veri Toplanması ve/veya İşlemesi - D.T., T.D., N.A., Y.Y.; Analiz ve/veya Yorum - D.T., T.D.; Literatür Taraması - D.T., T.D.; Yazıyı Yazan - D.T.; Eleştirel İnceleme - F.E.C., N.A., Y.Y.
Çıkar Çatışması: Yazarlar çıkar çatışması bildirmemişlerdir.
Mali Destek: Yazarlar bu çalışma için mali destek almadıklarını beyan etmişlerdir.
References
- 1.World Health Organization. Congenital anomalies. Fact sheet No 370. 2012 Oct [Google Scholar]
- 2.CDC Update on overall prevalence of major birth defects-Atlanta, Georgia, 1978–2005. MMWR Morb Mortal Wkly Rep. 2008;57:1–5. [PubMed] [Google Scholar]
- 3.Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths:Final Data for 2015. Natl Vitl Stat Rep. 2017;66:1–75. [PubMed] [Google Scholar]
- 4.Türkiye İstatistik Kurumu (TÜİK) Ölüm Nedeni İstatistikleri. 2013-2018 [Google Scholar]
- 5.Mason CA, Kirby RS, Sever LE, Langlois PH. Prevalence is the preferred measure of frequency of birth defects. Birth Defects Res A Clin Mol Teratol. 2005;73:690–2. doi: 10.1002/bdra.20211. [DOI] [PubMed] [Google Scholar]
- 6.Kirby RS. The prevalence of selected major birth defects in the United States. Semin Perinatol. 2017;41:338–44. doi: 10.1053/j.semperi.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 7.St Louis AM, Kim K, Browne ML, et al. Prevalence trends of selected major birth defects:A multi-state population-based retrospective study, United States, 1999 to 2007. Birth Defects Res. 2017;109:1442–50. doi: 10.1002/bdr2.1113. [DOI] [PubMed] [Google Scholar]
- 8.Egbe AC. Birth defects in the newborn population:race and ethnicity. Pediatr Neonatol. 2015;56:183–8. doi: 10.1016/j.pedneo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Parker SE, Mai CT, Canfield MA, et al. National Birth Defects Prevention Networks. Updated national birth prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–16. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 10.Ko JK, Lamichhane DK, Kim HC, Leem JH. Trends in the prevalences of selected birth defects in Korea (2008–2014) Int J Environ Res Public Health. 2018;15:E923. doi: 10.3390/ijerph15050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Birth defects surveillance a manual for programme managers. Available from:URL:https://apps.who.int/iris/bitstream/handle/10665/110223 /978924154∔_eng.pdf .
- 12.Dolk H, Loane M, Garne E European Surveillance of Congenital Anomalies (EUROCAT) Working Group. Congenital heart defects in Europe:prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123:841–9. doi: 10.1161/CIRCULATIONAHA.110.958405. [DOI] [PubMed] [Google Scholar]
- 13.Khoshnood B, Greenlees R, Loane M, Helen Dolk H EUROCAT Project Management Committee;EUROCAT Working Group. EUROCAT Public health ındicators for congenital anomalies in Europe. Birth defects research (Part A):Clinical and Molecular Teratology. 2011;91:16–22. doi: 10.1002/bdra.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le MT, Shumate CJ, Hoyt AT, Wilkinson AV, Canfield MA. The prevalence of birth defects among non-Hispanic Asian/Pacific Islanders and American Indians/Alaska Natives in Texas, 1999–2015. Birth Defects Res. 2019;111:1380–8. doi: 10.1002/bdr2.1543. [DOI] [PubMed] [Google Scholar]
- 15.Mai CT, Isenburg J, Langlois PH, et al. Population-based birth defects data in the United States, 2008 to 2012:Presentation of state-specific data and descriptive brief on variability of prevalence. Birth Defects Res A Clin Mol Teratol. 2015;103:972–93. doi: 10.1002/bdra.23461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Update on overall prevalence of major birth defects-Atlanta, Georgia, 1978–2005. MMWR Morb Mortal Wkly Rep. 2008;57:1–5. [PubMed] [Google Scholar]
- 17.Feldkamp ML, Carey JC, Byrne JLB, Krikov S, Botto LD. Etiology and clinical presentation of birth defects:population based study. BMJ. 2017;357:j2249. doi: 10.1136/bmj.j2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luz GDS, Karam SM, Dumith SC. Congenital anomalies in Rio Grande do Sul State:a time series analysis. Rev Bras Epidemiol. 2019;22:e190040. doi: 10.1590/1980-549720190040. [DOI] [PubMed] [Google Scholar]
- 19.Hanaoka T, Tamura N, Ito K, et al. Prevalence and risk of birth defects observed in a prospective cohort study:The Hokkaido Study on Environment and Children's Health. J Epidemiol. 2018;28:125–32. doi: 10.2188/jea.JE20160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mashhadi Abdolahi H, Kargar Maher MH, Afsharnia F, Dastgiri S. Prevalence of congenital anomalies:a community-based study in the Northwest of Iran. ISRN Pediatr. 2014;2014:920940. doi: 10.1155/2014/920940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherian AG, Jamkhandi D, George K, et al. Prevalence of congenital anomalies in a secondary care hospital in South India:A Cross-Sectional Study. J Trop Pediatr. 2016;62:361–7. doi: 10.1093/tropej/fmw019. [DOI] [PubMed] [Google Scholar]
- 22.Tuncbilek E, Boduroglu K, Ali Kasifoglu M. Results of the Turkish congenital malformation survey. Turk J Pediatr. 1999;41:287–97. [PubMed] [Google Scholar]
- 23.Himmetoglu O, Tiras MB, Gursoy R, et al. The incidence of congenital malformations in a Turkish population. Int J Gynecol Obstet. 1996;55:117–21. doi: 10.1016/s0020-7292(96)02743-9. [DOI] [PubMed] [Google Scholar]
- 24.Morris JK, Springett AL, Greenlees R, et al. Trends in congenital anomalies in Europe from 1980 to 2012. PLoSOne. 2018;13:e0194986. doi: 10.1371/journal.pone.0194986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoll C, Dott B, Alembik Y, Roth MP. Associated congenital anomalies among cases with Down syndrome. Eur J Med Genet. 2015;58:674–80. doi: 10.1016/j.ejmg.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Christensen N, Andersen H, Garne E, et al. Atrioventricular septal defects among infants in Europe:a population-based study of prevalence, associated anomalies, and survival. Cardiol Young. 2013;23:560–7. doi: 10.1017/S1047951112001400. [DOI] [PubMed] [Google Scholar]
- 27.European Surveillance of Congenital Anomalies (EUROCAT) Prevalence Tables 2010–2017. Available from:URL:https://eu-rd-platform.jrc.ec.europa.eu /eurocat / eurocat-data /prevalence .
- 28.Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Adv Exp Med Biol. 2010;686:349–64. doi: 10.1007/978-90-481-9485-8_20. [DOI] [PubMed] [Google Scholar]
- 29.Bhide P, Kar A. A national estimate of the birth prevalence of congenital anomalies in India:systematic review and meta-analysis. BMC Pediatr. 2018;18:175. doi: 10.1186/s12887-018-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO. World Birth Defects Day:Raising Awareness of Preventable Birth Defects. Geneva: WHO; 2016. [Google Scholar]


