Summary
Previous studies have shown that live cyanobacteria can produce photocurrent in bio-photoelectrochemical cells (BPECs) that can be exploited for clean renewable energy production. Electron transfer from cyanobacteria to the electrochemical cell was proposed to be facilitated by small molecule(s) mediator(s) whose identity (or identities) remain unknown. Here, we elucidate the mechanism of electron transfer in the BPEC by identifying the major electron mediator as NADPH in three cyanobacterial species. We show that an increase in the concentration of NADPH secreted into the external cell medium (ECM) is obtained by both illumination and activation of the BPEC. Elimination of NADPH in the ECM abrogates the photocurrent while addition of exogenous NADP+ significantly increases and prolongs the photocurrent production. NADP+ is thus the first non-toxic, water soluble electron mediator that can functionally link photosynthetic cells to an energy conversion system and may serve to improve the performance of future BPECs.
Subject areas: Bio-Electrochemistry, Microbiology, Biotechnology, Energy Engineering
Graphical abstract
Highlights
-
•
NADPH is the electron mediator in cyanobacterial bio-photoelectrochemical cells
-
•
Operation of the electrochemical cell induces NADPH release from cyanobacteria
-
•
Addition of exogenous NADP+ to cyanobacteria enhances photocurrent production
-
•
NADPH is released by different fresh or sea water cyanobacterial species
Bio-Electrochemistry; Microbiology; Biotechnology; Energy Engineering
Introduction
World energy consumption continues to increase each year. To meet the increasing demand, use of fossil fuels continues at ever growing amounts, and as a result, more pollution is released into the environment. Estimation of the energy consumption between the years 2008 and 2035 has predicted an increase of >50 % that will reach an annual average of ∼26 TW (U.S. IEA, 2011). The increase in pollution has recently become a major concern because it might cause severe climate change which will be globally detrimental. Therefore, there is an urgent need for replacement of polluting energy production technologies with non-polluting, renewable, green energy technologies.
One such technology, which is believed to be a good base for production of green energy, is microbial fuel cells (MFCs). The main principle of MFCs is to use bacteria in electrochemical cells as electron donors (Fang et al., 2020; Park et al., 1999) at the anode or acceptors (Bergel et al., 2005; Fang et al., 2020; Gregory et al., 2004) at the cathode. The electron transfer between the inner membrane of live bacteria and the anode of the electrochemical cell was first demonstrated by Potter in 1910 (Ieropoulos et al., 2005). Since the membranes of most bacterial species have low conductivity, MFCs utilize small molecules which can mediate electrons between the inner membrane and the electrodes. Over the years, the use of various bacterial species has been reported (Bond et al., 2002; Bond and Lovley, 2003; Chaudhuri and Lovley, 2003; Choi et al., 2003; Gregory et al., 2004; Kim et al., 1999; Lee et al., 2002; Liu et al., 2006; Menicucci et al., 2006; Min et al., 2005; Park and Zeikus, 2002; 2000; 1999; Park et al., 1999; Pham et al., 2003; Rabaey et al., 2005; 2004; Rhoads et al., 2005; Ringeisen et al., 2006; Tanaka et al., 1983; Thurston et al., 1985; U.S. IEA, 2011; Wei et al., 2016). Some of these species were able to produce endogenous electron mediators (Bond et al., 2002; Bond and Lovley, 2003; Chaudhuri and Lovley, 2003; Liu et al., 2006; Min et al., 2005; Pham et al., 2003). Addition of different exogenous electron mediators was reported to significantly increase the produced current including cystine, neutral red, thionin, sulphides, ferric chelated complexes, quinones, phenazines, and humic acids (Ieropoulos et al., 2005; Lovley et al, 1996, 2004; Rabaey et al, 2004, 2005; Simoska et al., 2019). As most of these compounds are considered to be toxic, their use must be tightly regulated. Current production can also be performed by direct electron transfer (DET) mechanisms. Such a mechanism was reported for Geobacter sulfurreducens biofilms which use conductive pili and c-type cytochromes to reduce the anode (Heidary et al., 2020; Lovley, 2012; Nevin et al., 2008; Yi et al., 2009). Shewanella oneidensis MR-1 biofilms were reported to conduct direct electron transfer through Mtr complexes (Hartshorne et al., 2007; Shi et al., 2012) and an indirect electron transfer which is mediated by flavin molecules.
Recent studies have shown that oxygenic and anoxygenic photosynthetic microorganisms can be used as the source of electrons that can be collected in bio-photoelectrochemical cells (BPEC) for solar energy conversion to electrical current and/or clean fuels (such as hydrogen) (Grattieri, 2020; Grattieri et al., 2019a, 2019b; Tschörtner et al., 2019). Therefore, it has been suggested that their use could serve as the main photo-active component in the development of innovative renewable energy applications (Deng and Coleman, 1999; Efrati et al., 2016; Gizzie et al., 2015; Grattieri, 2020; Kaiser et al., 2013; Larom et al., 2015; McCormick et al, 2011, 2015; Pinhassi et al., 2016; Sawa et al., 2017; Schmetterer et al., 1994; Sekar et al, 2014, 2016; Zhang et al., 2013; Zhao et al., 2015). In some systems, light energy was converted utilizing reaction center/light harvesting proteins (Mershin et al., 2012) or isolated photosystems conductively attached to electrodes in BPECs (Bombelli et al., 2015; Efrati et al, 2013, Efrati et al., 2016; Hartmann et al., 2020; Li et al., 2016; Yehezkeli et al., 2012; Zhao et al., 2014). Some of these systems require the addition of an exogenous mediator and the photosystems must be attached to the anode which can be done by utilizing an immobilized electron mediator such as redox polymer (Zhao et al., 2014). Use of Photosystem I (PSI) or reaction centers from non-oxygenic photosynthetic organisms requires addition of an electron donor (Efrati et al, 2013, Efrati et al., 2016; Hartmann et al., 2020; Zhao et al., 2015), while in systems based on Photosystem II (PSII), water serves as the initial electron donor (Bombelli et al., 2015). Use of isolated complexes, especially when PSII is utilized, is hampered by their relative instability, leading to loss of activity. Utilization of thylakoid membranes, whose isolation process is much easier and cheaper than isolating complexes (Hasan et al., 2017; Pinhassi et al., 2016), entails only cell disruption and centrifugation, without expensive detergent treatment and chromatography. While the complexes are still unstable and cannot be repaired, replacement with fresh aliquots of membranes is inexpensive and non-polluting. However, their ability to perform DET is significantly lower than that of the isolated complexes, leading to use of mediating molecules. A potentially more stable system can be achieved by utilizing living photosynthetic microorganisms, which have internal repair mechanisms for replacing photodamaged components. Use of intact cells in BPECs leads to a new problem however: how to enable external electrodes to acquire electrons from internal membrane components that are sequestered behind multiple layers of membranes that make up the external barrier of the cells.
This problem can be solved by performing mediated electron transfer (MET) with mediators that are able to cross these barriers and efficiently interact with either inorganic or metallic electrodes and photosynthetic biological components. Previous studies have used exogenously added mediators such as ferricyanide (Huang et al., 2015; Lan et al., 2013; Laohavisit et al., 2015; McCormick, 2011, 2013; Ochiai et al., 1983), 2,6-dichloro-1,4 benzoquinone (Calkins et al., 2013; Larom et al., 2010; Pinhassi et al, 2015, 2016; Sekar et al., 2014; Tanaka et al., 1985), 2-hydroxy-1,4-naphthoquinone (Tanaka et al., 1988; Torimura et al., 2001; Yagishita et al., 1993; Zou et al, 2009, 2010), or 1,4-benzoquinone (Longatte et al., 2018; Sekar et al., 2014). However, all bacteria, and especially cyanobacteria, have outer membranes that limit the ability of such molecules to enter and exit the cells. Some of the mediators are toxic and most have a low solubility in water-based media (Cereda, 2014). The use of exogenous mediators might be avoided using a semi-dry cyanobacterial monolayer which can produce current by a DET mechanism (Sekar et al., 2016; Thirumurthy et al., 2020; Wei et al., 2016); however, such systems might damage the survival of the cyanobacteria and limit its ability to multiply which might be very important for construction of useful systems. One of the possible mechanisms for DET with cyanobacteria is through type IV pili. However, in a recent study, no significant difference was found between the photocurrent production of wt Synechocystis and the pili-deficient mutant ΔpilD (Thirumurthy et al., 2020). A different option for cyanobacteria is MET that could be conducted by an endogenous mediator which can be secreted by the cells in similar with other small inorganic molecules that can pass the outer membrane through porin channels (Kowata et al., 2017). It has recently been shown that live cyanobacteria can also produce photocurrent without the addition of any exogenous mediator (Saper et al., 2018). It was suggested that the electron source originates from contributions of both the photosynthetic and respiratory pathways which can combine use reduced plastoquinone molecules which then continue to transfer electrons between a sequence of molecules until they reach the final step of the photosynthetic pathway, which is the reduction of NADP+ to NADPH (Saper et al., 2018). It was proposed that these reducing equivalents could then reduce metabolites that can be either passively or actively transported out of the cells and serve to mediate the biological processes with an electrochemical cell. Efforts were made to identify the endogenous mediators using cyclic voltammetry and metabolomic measurements of the cyanobacterial external cell medium (ECM). However, the identity of the mediator(s) remained elusive (Saper et al., 2018).
In the present work, we use two-dimensional fluorescence map (2D-FM) fingerprinting to identify NADPH as a major component that is exported from cyanobacteria and accumulates in the BPEC medium upon the application of light and electric potential. NADH can also mediate between the cells and the BPEC, but it is not accumulated in the external medium upon illumination. Therefore, NADPH serves as the endogenous electron mediator of cyanobacteria, and since its addition to the system increased the produced electrical current, this finding would assist the generation of bio-photoelectrochemical devices that use living photosynthetic organisms to produce electricity and hydrogen.
Results and discussion
Light-dependent generation of electricity by live Synechocystis in a mini-BPEC system
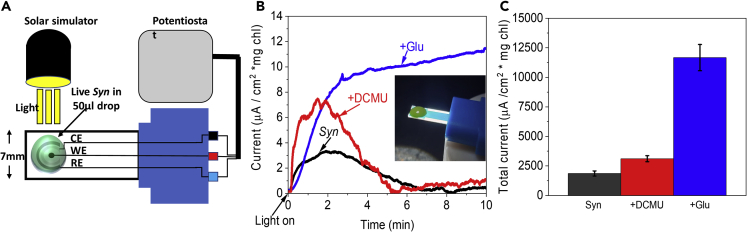
We have previously shown that photocurrent could be produced by live Synechocystis sp. PCC 6803 (Syn) cells in a BPEC (Saper et al., 2018). This BPEC was composed of 3 bulk electrodes: a graphite disk anode (the working electrode [WE]), a platinum rod cathode (the counter electrode [CE]), and an Ag/AgCl/3M NaCl reference electrode (RE), with direct contact between the cells and the anode in 50 mL of buffered solution. The large ECM volume made chemical identification of the internal mediator, released from the cyanobacteria, difficult (Saper et al., 2018). In order to chemically analyze the BPEC solution and identify the mediator, while measuring current, we utilized commercial screen-printed electrode (SPE) systems that formed the basis of a mini-BPEC, where a minimal ECM volume would be sufficient. The new mini-BPEC consisted of SPEs with carbon WE anode (0.79 mm2), platinum CE cathode, and Ag coated with AgCl RE enabling recording a current of about 3 μA cm−2 using 50 μL live Syn cells (containing 5 μg of chlorophyll, about 1.4x108 cells) (Figure 1A). In agreement with the previous results that were obtained with the larger BPEC (Saper et al., 2018), live Syn cells produced appreciable light-dependent electrical current without the addition of an exogenous electron mediator. In addition, as previously reported for the large BPEC, the current remained constant for a longer period of time when the cells were pre-incubated with glucose (Glu, Figures 1B and 1C) (Larom et al., 2015; Saper et al., 2018). Addition of Glu enhances the activity of the respiratory pathway, generating a multistep reduction process that ends in reduction of plastoquinone molecules (PQs). PQs are also an intermediate electron carrier in the photosynthetic pathway. Therefore, they link between the two pathways and continue to transfer electrons downstream in a multistep reduction process that ends in reduction of NADP+ to NADPH. Addition of the herbicide 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), which inhibits the electron flow from PSII downstream the photosynthetic pathway to the PQ, was shown to significantly enhance the photocurrent (Saper et al., 2018). It was concluded that the electron source from the cyanobacteria is the respiratory pathway from which the electrons are transferred to PSI and from this light-driven reaction to an internal mediator(s) that is/are secreted from the cell and reduce(s) the graphite anode (Saper et al., 2018). In addition, it was reported that PSII is not the major source of electrons in this pathway since the enhancing effect of DCMU happens also with Syn mutant lacking PSII (Saper et al., 2018). The reason why addition of DCMU increases the photocurrent in cyanobacterial BPECs is still unclear. In our previous work, we suggested that DCMU affects the production of the photocurrent at secondary site(s) by either inhibiting forward electron flow within the respiratory system or by enhancing the release of the external electron mediator (Saper et al., 2018). It has been shown that unlike plants or green algae, DCMU effects additional cyanobacterial functions. Addition of DCMU has been shown to affect Mn2+ uptake and release (Keren et al., 2002), Fe3+ reduction (Kranzler et al., 2014), and increase in the flexibility of thylakoid membranes in light (Stingaciu et al., 2019). When the ECM was collected following operation of the mini-BPEC and filtered through a 3 kDa filter, the filtrate was able to reduce oxidized cytochrome c (Figure S1). When the Syn cells were not illuminated in the mini-BPEC, no current was obtained (Figure S2).
Figure 1.
Light-dependent generation of electricity by live Syn
Syn cells (5μg chlorophyll) were placed in a 50-μL drop on a minielectrode-based BPEC, and the light-dependent chronoamperometry (CA) measurement was performed applying a voltage bias of 0.5 V on the anode.
(A) Schematic drawing of the mini-BPEC setup.
(B) CA of the light-dependent produced current of live Syn (black), with the addition of 100 μM of the herbicide DCMU (+DCMU, red) and after a pre-incubation in dark for 1 hr with 5 mM glucose (+Glu, blue). A picture of the minielectrode BPEC is shown in the inset.
(C) Calculation of the total current that was accumulated during 10 min in CA measurements of Syn (black), +DCMU (red), and +glucose (blue). The error bars represent the standard deviation over 3 independent measurements.
In our previous work using the 50 mL BPEC, we have found that mechanical treating the live Syn by a low-pressure microfluidizer or by osmotic shock significantly enhanced the amount of current produced in the BPEC (Saper et al., 2018). To simplify our system, in the following experiments, the cells were not pre-treated, other than centrifugation from their growth medium (see Transparent Methods for additional details). All other experimental parameters are similar to those previously described (Saper et al., 2018), and we assume that the same reactions occur on the CE, including reduction of protons to molecular hydrogen, as well as reduction of other molecules in the ECM.
Identification of NADPH and/or NADH in the ECM.
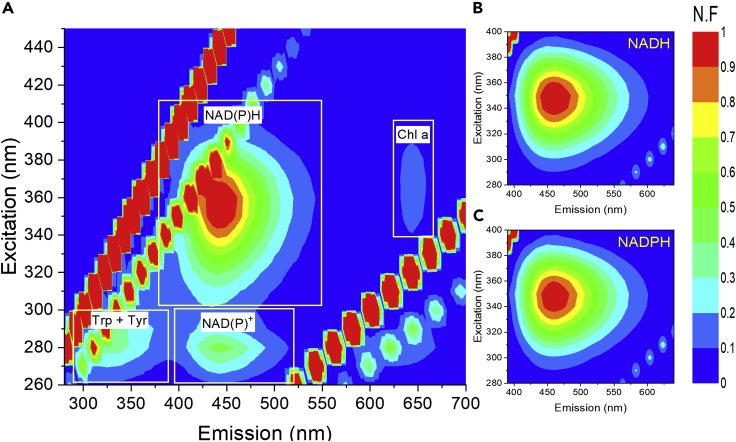
Production of photocurrent from live Syn without addition of an exogenous electron mediator has been suggested to occur by the secretion of endogenous electron mediator(s) to the ECM. We previously performed metabolomic assays on whole cells used in the BPEC (Saper et al., 2018); however, no significant change in any of the known metabolites could be observed. The stability of redox molecules in the ECM may be low. Therefore, we wished to utilize less destructive spectroscopic methods to screen the ECM for known redox components. Absorption spectra, FTIR, and NMR measurements did not result in the identification of a known redox-active molecule, especially due to the challenge of the presence of hundreds of molecules in the ECM mixture. Another method that can identify molecule(s) in the ECM is 2D-FMs, as many redox-active molecules contain moieties that absorb and fluoresce energy at specific wavelengths. When the ECMs, taken following the current production experiment in the BPEC, were analyzed by this method, a strong signal that matched the fluorescence fingerprint of NADPH and/or NADH (NAD(P)H) was observed. The signal in the ECM was compared to commercial samples of NAD(P)H used as standards and to previously published measurements of these molecules (Dartnell et al., 2010) (Figure 2). It should be noted that the 2D-FM analysis measures the sum of NADP(H) that is present in the solution at the moment of removal of the cells from the BPEC followed by cell removal by filtration. The total concentration of both of these species is of course greater than the immediate concentration of NADPH that is transferring electrons to the anode at any given moment, as only a subset of molecules are attractively associated with the anode.
Figure 2.
Identification of NAD(P)H in the ECM
Normalized 2D-FM spectra of the ECM of Syn, NADH, and NADPH.
(A) Normalized 2D-FMs of the ECM of Syn spectral fingerprints of the NAD(P)H, NAD(P)+, the amino acids Trp and Tyr, and chl a are marked above each peak. The lines of diagonal spots that appear in all of the maps presented here and in the following figures result from light scattering of the xenon lamp and Raman scattering of the water (Lawaetz and Stedmon, 2009).
(B) Normalized 2D-FM spectra of 5 mM NADH.
(C) Normalized 2D-FM spectra of 5 mM NADPH. In all panels, normalization was done by division of the intensity values of the whole map by the value of NAD(P)H signal obtained at (λ(ex) = 350, λ(em) = 450 nm). The fluorescence intensities in the color bar are displayed as normalized fluorescence (N.F).
Accumulation of NAD(P)H in the ECM is dependent on light and the connection between the cells and the electrochemical system
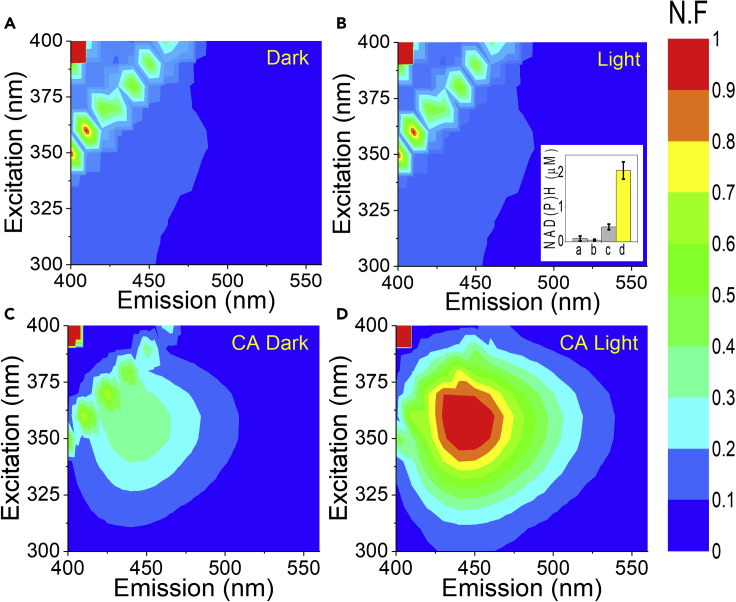
We suggest that the presence of NAD(P)H in the ECM is the result of the secretion of NADH, NADPH, or both to the ECM by the live Syn. Therefore, we next ask if this secretion is light dependent and whether or not it depends on physical linkage between the cells and the electrochemical system in the mini-BPEC. To this end, we performed 2D-FM spectra on the ECM from Syn cells, which were kept in the dark or exposed to light in either small transparent test tubes or in the mini-BPEC on the electrodes during 100 s of chronoamperometry (CA) measurement. No significant difference was observed between the fluorescence of the ECMs which were illuminated or not illuminated without any connection to the electrochemical system (in tubes). The NAD(P)H fluorescence intensity of Syn ECMs after CA measurement was significantly higher and further enhanced about upon illumination (Figure 3). NADPH is involved in a variety of enzymatic processes in the periplasmic space, and export of NADPH from the cyanobacterial cells to the ECM is prevented by the outer cell membrane. Under certain condition, cells invest energy to import NAD+ (released from lysed cells in the culture) which is an important molecule in many biological processes (Gruber and Haferkamp, 2019, Major et al., 2017, Murakawa and Takahashi, 1978, Zhou et al., 2011).
Figure 3.
Accumulation of NAD(P)H in the ECM is dependent on light and the connection between the cells and the electrochemical system
2D-FM spectra of the ECM of Syn after incubation in test tubes or during CA measurements on the electrodes in dark or under illumination.
(A) In test tubes in the dark.
(B) In test tubes under illumination.
(C) On the electrodes after 100 s of CA measurement in the dark.
(D) On the electrodes after 100 s of CA measurement under illumination. The inset in (B) displays NAD(P)H concentrations which were calculated based on the fluorescence intensities at (λ(ex) 350 = nm, λ(em) = 450 nm). The error bars in the insert of (B) represents the standard deviation over 6 independent measurements. The fluorescence intensities in the color bar are displayed as normalized fluorescence (N.F).
However, when the cells become a component in a bio-electrochemical cell, they export more NADPH. Activation of the BPEC changes the distribution of the ions (including protons) that are associated with the cyanobacterial cell wall, as well as on the surface of the anode. Such ionic changes on the cell surface may impact the permeability of the outer cell wall (Vaara, 1992). In order to determine the NAD(P)H concentration in the Syn ECM, a calibration curve of NADH concentration vs. fluorescence intensity was prepared based on NADH fluorescence (Figure S3). The calibration assumes that the 2D-FM of NADH and NADPH is spectrally identical under these conditions of measurement, as has been described by others (Blacker et al., 2014). While addition of DCMU to the Syn cells increases the current 2-fold (Figure S4), the change in the concentration of NADPH in the ECM was beyond the resolution of the 2D-FM method.
In order to estimate the ability of exogenous NADPH to produce photocurrent in the BPEC, 1μM NADPH was added with or without Syn cells (Figure S5A). In both measurements, a fast increase of about 4 μA was obtained followed by a fast decrease of the current to the same level as it was prior to the addition within a few seconds. To evaluate the kinetics of the decay in measured current, we performed fitting analysis to the curve decay. The analysis showed that the mathematical decay function is exponential (Figure S5B). The fast current decay suggests that in order to maintain the current formation, cyanobacteria needs to continuously secrete NADPH to the ECM, whereas the active portion of NADPH that reaches the anode to reduce it is about 1 μM/sec at the maximal current values.
NAD(P)H concentrations were calculated for ECM of Syn solutions which were illuminated or not – illuminated in test tubes or on electrodes during CA measurements as shown in the insert in Figure 3B. As can be seen, illumination in the BPEC results with the accumulation of about ∼2 μM NAD(P)H in the ECM. This concentration is about ten times greater than the amount of NAD(P)H that accumulates without any connection to the electrochemical system and about four times greater than accumulated without illumination. These results showed that the connection between the active electrochemical system and live Syn cells enhances secretion and accumulation of NAD(P)H in the ECM that can reduce the anode and produce electric current. Under illumination, the concentration of NAD(P)H in the cells is increased further; there is a concomitant increase in NAD(P)H release to the ECM and more current is produced. In the absence of connection to the electrochemical system, the release of NAD(P)H is low, and no significant difference can be observed between cells which were illuminated or not illuminated. These results show that in the absence of the electrochemical system, NAD(P)H release is not favored by the cells.
Under illumination, NADPH accumulates in the Syn ECM more than NADH
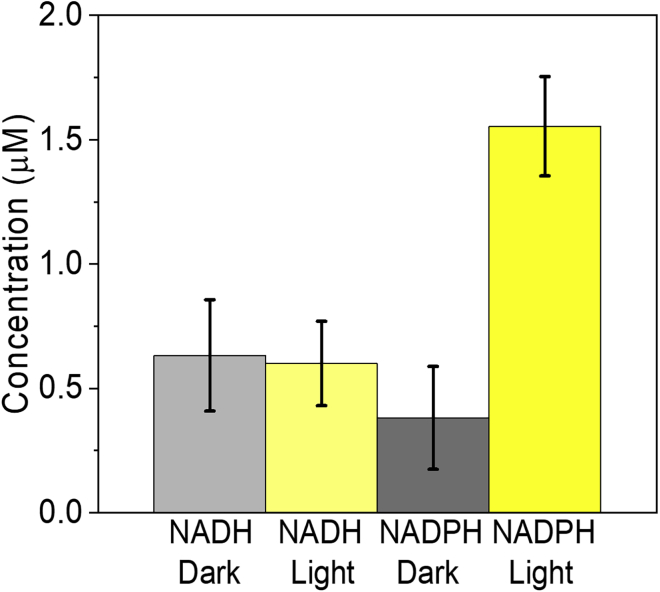
Enzymatic kits were used for quantification and differentiation between NADH and NADPH in the Syn ECM (see Transparent Methods for details). Both enzymatic assays were applied to the ECM of Syn after 100 s of CA measurements in either dark or under illumination. The presence of both NADH and NADPH was detected. No significant difference was found between NADH concentrations in the ECM of cells which were illuminated or not illuminated and was found to be about 0.6 μM (Figure 4). However, the concentration of NADPH in the ECM, following illumination, was higher, increasing from about 0.4 to 1.6 μM (Figure 4). The sum of NADH + NADPH concentrations is about 2 μM which is in agreement with the NAD(P)H concentration which was calculated based on the 2D-FMs. This result indicates that NADPH, rather than NADH, accumulates in the ECM in the light and suggests that this molecule may serve as the primary mediator of the light-dependent current. In contrast with NADPH formation by photosynthesis which increases during illumination, the concentration of NADH is not changed during illumination and any NADH found in the ECM is probably not due to the formation of new NADH molecules by a light-related process.
Figure 4.
When illuminated, NADPH accumulates in the Syn ECM more than NADH
Quantification of NADH and NADPH concentrations in the ECM of Syn, after 100 s of CA measurement with or without illumination, was done using a chemical enzymatic assay. The error bars represent the standard deviation over 9 independent measurements.
Addition of ferredoxin NADP+ reductase eliminates both the light-dependent electric current and the NAD(P)H accumulation in the ECM
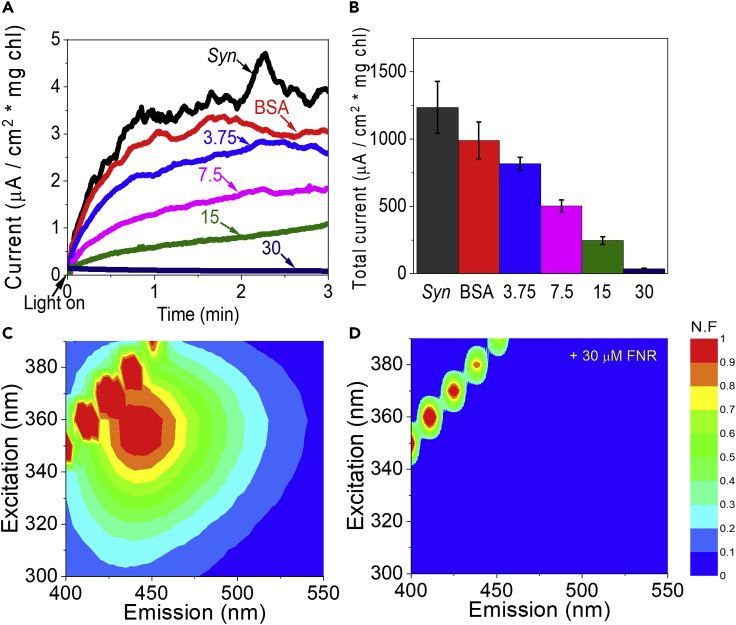
Ferredoxin NADP+ reductase (FNR) binds both NAD(P)+ and NAD(P)H (Batie and Kamin, 1986). In order to further support our identification of NAD(P)H as the main endogenous electron mediator, increasing concentrations of the enzyme FNR were added to Syn cells and CA was measured. We observed that the current was reduced as function of the increasing FNR concentrations and was almost totally abrogated at FNR concentration of 30 μM.
In order to make sure that the current was abrogated because of the specific binding activity of FNR and not by generic protein binding or changes in the conductance and rigidity of the electrolyte, the CA of Syn was measured with 1 mg/mL of bovine serum albumin (BSA). Addition of BSA did not significantly affect the photocurrent (Figure 5A). In addition, the experiment was repeated with 15 μM of a recombinant FNR enzyme whose origin is the green algae C. reinardtii. Addition of the CrFNR also abrogated the photocurrent (Figure S6).
Figure 5.
Addition of FNR that binds NAD(P)H eliminates both the light-dependent electric current and the NAD(P)H accumulation in the ECM
(A) CA of Syn (black) and Syn + BSA (red, negative control), and Syn + 3.75, 7.5, 15, and 30 μM FNR (light blue, magenta, green, and dark blue, respectively).
(B) Calculations of the current sum that was accumulated during 3 min in CA measurements of Syn (black), + Syn + BSA (red), and Syn + 3.75, 7.5, 15 and 30 μM FNR (light blue, magenta, green, and dark blue, respectively). The error bars represent the standard deviation over 3 independent measurements.
(C) 2D-FM of Syn filtrate.
(D) 2D-FM of Syn + FNR filtrate. The ECMs of Syn, with and without the addition of FNR at the concentration of 1 mg/mL, were filtrated through a 3 kD filter and measured with 2D-FMs. The fluorescence intensities in the color bar are displayed as normalized fluorescence (N.F).
In order to assess further whether the reduction in current was caused by FNR binding of NADPH and preventing it from being the mediator, Syn cells were incubated for 30 min with or without 1 mg/mL FNR which is a ∼10-fold molar excess to that of the calculated NADP(H) concentration. The samples were then centrifuged in ultrafiltration devices with a 3kD filter. 2D-FMs (λ(ex) = 260–400 nm, λ(em) = 400–520 nm) of the filtrates were measured. The spectral fingerprint NAD(P)H was observed only in the filtrates of samples which did not contain FNR, indicating that NAD(P)H molecules were bound to FNR and therefore did not appear in the filtrate (Figures 5C and 5D). Since the addition of FNR almost completely abolished both photocurrent and the 2D-FM signal of NAD(P)H, these results imply that NADPH is the primary mediator of the electrons from Syn PSI to the graphite anode when generating the light-dependent electric current in the BPEC. As an additional control, we performed CA in the presence of exogenous NADPH added in excess of the concentration of the added FNR, and as expected, the current was not abrogated (Figure S7). Addition of DCMU, shown to increase the measured current from Syn cells, does not prevent the abrogation of the photocurrent by FNR (Figure S8)
Addition of an exogenous NADP+ to the ECM enhances photocurrent production
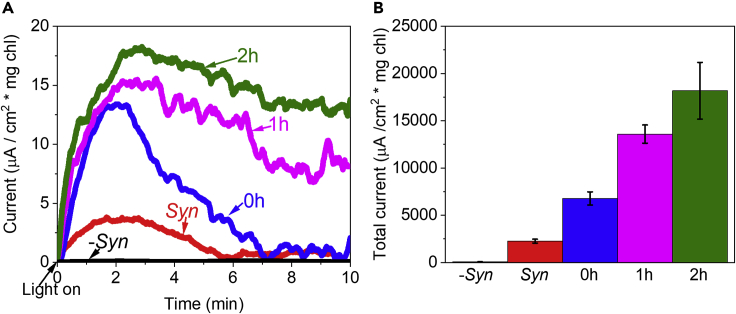
There are two scenarios whereby NADPH can serve as the mediator in MET-type current production. In the first, NADPH is secreted but following electron transfer is not uptaken by the cells for additional cycles. In the second scenario, following oxidation, NADP+ is uptaken for further cycles. To differentiate between these two scenarios, we performed a pre-incubation of Syn with exogenously added NADP+ to observe whether indeed more NADPH would be produced under illumination resulting in an elevated electric current. We measured the BPEC photocurrent with 5 mM NADP+ in the absence of cells or in the presence of Syn at different pre-incubation times (0–2 hr). In the absence of cells, addition of NADP+ did not produce any significant photocurrent. In the presence of Syn cells, addition of NADP+ significantly increased the photocurrent. The longevity, maximum, and total current all increased in correlation with the pre-incubation time (Figure 6A).
Figure 6.
Addition of NADP+ enhances photocurrent production
(A) CA measurement of Syn cells with or without pre-incubation with 5 mM NADP+ for 0, 1, and 2 hr under continuous illumination. NADP+ without cells (-Syn, black), Syn without NADP+ (red), Syn after 0, 1, and 2 hr incubation with NADP+ (blue, magenta, and green respectively).
(B) Calculations of the current sum that was accumulated during 10 min in CA measurements of NADP+ without cells (-Syn, black), Syn without NADP+ (red), Syn after 0, 1, and 2 hr incubation with NADP+ (blue, magenta, and green respectively). The error bars represent the standard deviation over 3 independent measurements.
This result implies a mechanism whereby NADP+ can enter and exit the cells and mediate electrons from the photosynthetic system (PSI) and the BPEC anode. Therefore, the addition of NADP+ at a concentration higher than what is released from the cells can significantly increase the photocurrent. Although Syn does not produce current in dark, the possibility that addition of exogenous NADP+ can produce dark current by mediating electrons was examined (Figure S9). CA of Syn, pre-incubated for 2 hr with 5mM NADP+, was measured in dark and light. While current was obtained in the light, no photocurrent was produced in the dark. We postulate that in the absence of light, the uptake of exogenous NADP+ into the cytoplasm may be inhibited or that lack of photosynthetic activity prevents NADP+ reduction. Addition of exogenous NADP+ in the light also significantly increased the current when measured with Syn cells in the large 50 mL BPEC previously described (Figure S10) (Saper et al., 2018).
Two additional cyanobacterial species export NAD(P)H and generate photocurrent
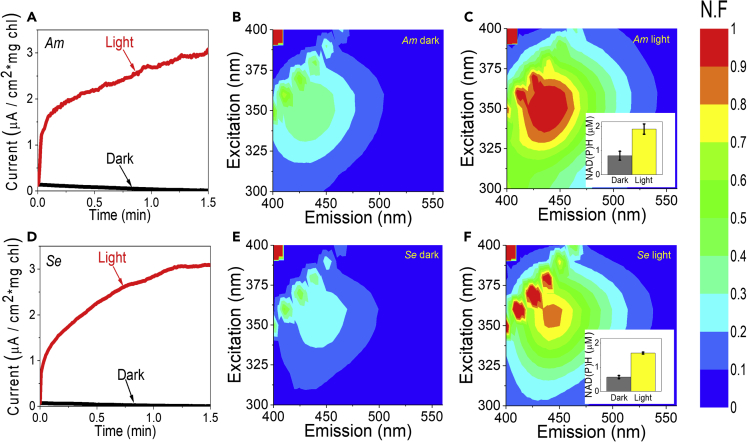
Our previous studies which showed that photocurrent can be produced without addition of an external electron mediator were focused on Syn. We wished to screen additional species in our mini-BPEC to assess whether the ability to produce photocurrent by secretion of an endogenous electron mediator is specific to Syn or a more general phenomenon in cyanobacteria. In order to address this question, two additional cyanobacterial species were studied: Acaryochloris marina MBIC 11017 (Am) and Synechococcus elongatus PCC 7942 (Se). Similar to Syn, the native habitat of Se is fresh water. However, Se has an elongated filament shape which is different than the round cells of Syn. The native habitat of Am is the oceanic salt water. Moreover, Am contains chl d which enables it to absorb wavelength beyond 700 nm for efficient photosynthesis.
2D-FMs of the ECMs of Am and Se after a 100 s of CA measurement in dark or under illumination were measured. NAD(P)H concentrations were calculated based on the fluorescence intensities and compared to standards of known concentrations. The results revealed that both strains exhibit light-induced accumulation of NAD(P)H in their ECM (Figure 7). In addition, the difference between light and dark incubation of both strains was similar to that shown for Syn.
Figure 7.
Am and Se exhibit light-driven current and export NADP(H) into the BPEC ECM similar to Syn
CA of Am and Se cells was measured for 100 s in dark and under illumination. The cells were filtrated immediately following the measurement, and 2D-FM spectra of their ECMs were measured.
(A) CA of Am cells in dark (black) and under illumination (red).
(B) 2D-FM spectra of the ECM of Am after measurements in dark.
(C) 2D-FM spectra of the ECM of Am after measurements under illumination.
(D) CA of Se cells in dark (black) and under illumination (red).
(E) 2D-FM spectra of the ECM of Se after measurements in dark.
(F) 2D-FM spectra of the ECM of Se after measurements under illumination. The inset in (C) and (F) displays NAD(P)H concentrations which were calculated based on the fluorescence intensities at (λ(ex) = 340 nm, λ(em) = 450 nm) after incubation in dark and under illumination. The error bars represent the standard deviation over 6 independent measurements. The fluorescence intensities in the color bar are displayed as normalized fluorescence (N.F).
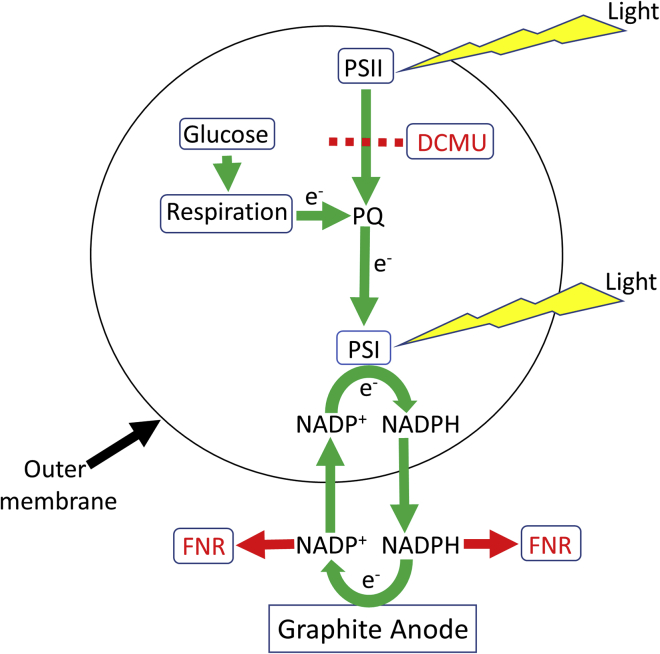
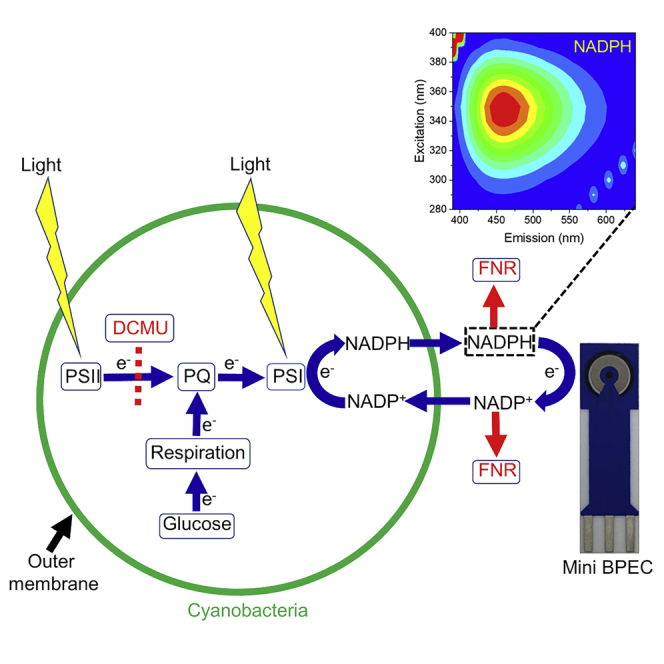
As shown above for Syn, addition of exogenous NADP+ or glucose significantly increases the photocurrent obtained from these two species (Figure S11). Addition of DCMU has a similar effect on the photocurrent than seen for Syn, while addition of FNR completely abrogates the photocurrent (Figure S11). Based on our findings and in agreement with our previous work (Saper et al., 2018), we suggest a model for the mechanism of the electron transport in the BPEC in which the electron source starts from the respiratory pathway and continues through the plastoquinone pool to PSI which reduces NADP+ to NADPH. The link to the electrochemical component of the BPEC induces the release of NADPH, which reduces the anode and re-enters the cyanobacterial cell to accept additional electrons from PSI (Figure 8). Another minor electron donor is NADH which was also found to exit the cell and functions similar to NADPH. Several options exist for the final electron acceptor which is being reduced by the cathode: reduction of H+ or water hydrolysis to form H2, reduction of metabolites in the ECM, or direct reduction of the cells.
Figure 8.
Schematic depiction of the main electron transport pathway in cyanobacterial BPECs
The electron transport originates from the respiratory pathway which reduces the PQ pool and continues downstream to PSI which under illumination reduces NADP + to NADPH. NADPH exits the stroma (cytoplasm) and outer membrane to the ECM and reduces the graphite anode. It then re-enters the cyanobacterial cells to accept more electrons from PSI. Yellow flash shapes indicate the light illumination which is absorbed in the photosystems. Green arrows represent the directionality of the electron transport pathway. Black arrows indicate the internal and outer cell membranes. A dashed line represents the electron blockage between PSII and the plastoquinone pool (PQ) in the presence of DCMU. Red arrows represent the binding of NADP+ and NADPH to exogenous FNR that prevent them from reducing the anode or re-entering the cell and in this manner abrogate the current production.
The actual capacity of photosynthetic organisms to perform efficient solar energy conversion has been a subject of debate for the past 20 years since the first studies of attempts to utilize photosynthesis (in the form of live cells, membranes, or isolated complexes) were reported. Comparisons with solid-state or dye-sensitized solar cells are seldom performed using the same metrics (Blankenship et al., 2011). Overall, photosynthetic efficiency is typically seen as low since much of the chemical output (in the form of sugars and ATP) goes toward cell growth and control. Comparison of PV-driven electrolysis vs. biomass production is for instance greatly in favor of PV. This of course does not take into account the energy expended in mining the raw materials (or used in their synthesis) or the carbon footprint of moving raw materials to sites of synthesis/assembly and transportation of completed panels to their site of use. When comparing pure quantum efficiency (photons absorbed to electrons transferred), PSII and PSI have been shown to be highly efficient comparable to photovoltaic/Dye-sensitized solar cells (PV/DSSC) devices. Translating the maximal electron flux through PSII (with water as the donor) or PSI (with a sacrificial electron donor), one can obtain sustained currents of 10-3000 mA mg chl−1 (depending on organism source and level of isolation) for saturating illumination in the range of 400-700 nm. Rates of PSI reduction of NADP+ are comparable. We estimate that a two-dimensional 1 cm2 solar panel based on photosynthetic material would require ∼100 μg chl (in cells or thylakoid membranes) or about 10 μg chl (in isolated PSII/PSI) to operate. This is about the amount of material that can be isolated from a few spinach leaves or 1 gram (dry weight) of cyanobacterial cells. This current is on the same order of current that is obtained from commercial DSSC solar cells. Use of isolated components (membranes or complexes) requires systematic replacement due to irreparable damage due to photoinhibition but can provide elevated rates of electron transfer. Cells continually self-repair damaged systems; however, if dependent on the release of endogenous electron mediators, the currents will be an order of magnitude smaller. For this reason, we have identified the mediator to see if we can improve our BPEC that utilizes cells. Our identification of NADPH as a major mediating molecule is thus important since this natural molecule has many of the characteristics one would hope to use in a BPEC device: it is non-toxic, water soluble, redox active, and relatively stable. Developing methods where a single addition of NADP+ to the ECM of a system based on live cells that can operate during a single day would be a tremendous advancement toward the future use of such a BPEC in the future for small-scale local solar energy conversion.
Limitations of the study
We show here that live cyanobacterial cells use endogenous NADPH as an electron mediator in a BPEC. NADPH is a redox active metabolite that is found in these cells at relatively high concentration (>15 nmol/mg chl) (Kauny and Sétif, 2014) and is continually produced by the coupled respiration and photosynthesis processes in active cells. Taken with our previous studies (Saper et al., 2018), these observations indicate that use of easily grown cells can provide current with the potential for either fuel (H2) production or power storage for the entire length of a day without addition of polluting chemicals. In the future, we may see the production of sufficient energy to run a small local agricultural device using such a clean energy system; however, there are many technical obstacles, including the recycling of the live cells, maintaining such cultures during the night hours, and avoiding contaminations by other species.
Resource availability
Lead contact
Noam Adir (noam@ch.technion.ac.il)
Materials availability
Materials are available from the corresponding authors on request or are commercially available.
Data and code availability
This study did not generate computer code. All data and analytical methods are available in the main text or in Supplemental information section.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Funding for this study was provided by a “Nevet” grant from the Grand Technion Energy Program (GTEP) and a Technion VPR Berman Grant for Energy Research. Some of the results reported in this work were obtained using central facilities at the Technion's Hydrogen Technologies Research Laboratory (HTRL) supported by the Nancy & Stephen Grand Technion Energy Program (GTEP), the ADELIS Foundation, and the Solar Fuels I-CORE. We thank Dr. Yifat Nakibly for technical support. We thank Prof. Rachel Nechushtai and Prof. Iftach Yacoby for supplying biological materials. We thank Shira Bar-Zvi, Sonya Copperstein, and Lee Keysar for their assistance. Y.S. and T.üN.T.ó are supported by fellowships of the Nancy & Stephen Grand Technion Energy Program (GTEP) and by a Schulich Graduate fellowship.
Authors contribution
Y.S., G.S., and N.A. conceived the idea. Y.S., G.S., and N.A. designed the experiments. Y.S. performed the main experiments. B.E., T.N.T., and G.L. assisted in performing the experiments. Y.S., G.S., and N.A. wrote the paper. G.S. and N.A. supervised, administrated, and acquired the funding for the entire research.
Declaration of interests
The authors declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101892.
Contributor Information
Gadi Schuster, Email: gadis@technion.ac.il.
Noam Adir, Email: noam@ch.technion.ac.il.
Supplemental information
References
- Batie C.J., Kamin H. Association of ferredoxin-NADP+ reductase with NADP(H) specificity and oxidation-reduction properties. J. Biol. Chem. 1986;261:11214–11223. [PubMed] [Google Scholar]
- Bergel A., Féron D., Mollica A. Catalysis of oxygen reduction in PEM fuel cell by seawater biofilm. Electrochem. Commun. 2005;7:900–904. [Google Scholar]
- Blacker T.S., Mann Z.F., Gale J.E., Ziegler M., Bain A.J., Szabadkai G., Duchen M.R. Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nat. Commun. 2014;5:3936. doi: 10.1038/ncomms4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship R.E., Tiede D.M., Barber J., Brudvig G.W., Fleming G., Ghirardi M., Gunner M.R., Junge W., Kramer D.M., Melis A. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science. 2011;332:805–809. doi: 10.1126/science.1200165. [DOI] [PubMed] [Google Scholar]
- Bombelli P., Müller T., Herling T.W., Howe C.J., Knowles T.P.J. A high power-density, mediator-free, microfluidic biophotovoltaic device for cyanobacterial cells. Adv. Energy Mater. 2015;5:1–6. doi: 10.1002/aenm.201401299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond D.R., Holmes D.E., Tender L.M., Lovley D.R. Electrode-reducing microorganisms that harvest energy from marine sediments. 2002;295:483–485. doi: 10.1126/science.1066771. [DOI] [PubMed] [Google Scholar]
- Bond D.R., Lovley D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003;69:1548–1555. doi: 10.1128/AEM.69.3.1548-1555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins J.O., Umasankar Y., O’Neill H., Ramasamy R.P. High photo-electrochemical activity of thylakoid–carbon nanotube composites for photosynthetic energy conversion. Energy Environ. Sci. 2013;6:1891–1900. [Google Scholar]
- Cereda A. A bioelectrochemical approach to characterize extracellular electron transfer by Synechocystis sp. PCC6803. PLoS One. 2014;9:e91484. doi: 10.1371/journal.pone.0091484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri S.K., Lovley D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003;21:1229–1232. doi: 10.1038/nbt867. [DOI] [PubMed] [Google Scholar]
- Choi Y., Jung E., Kim S., Jung S. Membrane fluidity sensoring microbial fuel cell. Bioelectrochemistry. 2003;59:121–127. doi: 10.1016/s1567-5394(03)00018-5. [DOI] [PubMed] [Google Scholar]
- Dartnell L.R., Storrie-Lombardi M.C., Ward J.M. Complete fluorescent fingerprints of extremophilic and photosynthetic microbes. Int. J. Astrobiol. 2010;9:245–257. [Google Scholar]
- Deng M.D., Coleman J.R. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol. 1999;65:523–528. doi: 10.1128/aem.65.2.523-528.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrati A., Lu C.H., Michaeli D., Nechushtai R., Alsaoub S., Schuhmann W., Willner I. Assembly of photo-bioelectrochemical cells using photosystem I-functionalized electrodes. Nat. Energy. 2016;1:1–7. 15021. [Google Scholar]
- Efrati A., Tel-Vered R., Michaeli D., Nechushtai R., Willner I. Cytochrome c-coupled photosystem I and photosystem II (PSI/PSII) photo-bioelectrochemical cells. Energy Environ. Sci. 2013;6:2950–2956. [Google Scholar]
- Fang X., Kalathil S., Reisner E. Semi-biological approaches to solar-to-chemical conversion. Chem. Soc. Rev. 2020;49:4926–4952. doi: 10.1039/c9cs00496c. [DOI] [PubMed] [Google Scholar]
- Gizzie E.A., Niezgoda J.S., Robinson M.T., Harris A.G., Jennings G.K., Rosenthala S.J., Cliffel D.E. Photosystem I-polyaniline/TiO2 solid-state solar cells: simple devices for biohybrid solar energy conversion. Energy Environ. Sci. 2015;8:3572–3576. [Google Scholar]
- Grattieri M. Purple bacteria photo-bioelectrochemistry: enthralling challenges and opportunities. Photochem. Photobiol. Sci. 2020;19:424–435. doi: 10.1039/c9pp00470j. [DOI] [PubMed] [Google Scholar]
- Grattieri M., Beaver K., Gaffney E.M., Minteer S.D. Tuning purple bacteria salt-tolerance for photobioelectrochemical systems in saline environments. Faraday Discuss. 2019;215:15–25. doi: 10.1039/c8fd00160j. [DOI] [PubMed] [Google Scholar]
- Grattieri M., Rhodes Z., Hickey D.P., Beaver K., Minteer S.D. Understanding biophotocurrent generation in photosynthetic purple bacteria. ACS Catal. 2019;9:867–873. [Google Scholar]
- Gruber A., Haferkamp I. Nucleotide transport and metabolism in diatoms. Biomolecules. 2019;9:761. doi: 10.3390/biom9120761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory K.B., Bond D.R., Lovley D.R. Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 2004;6:596–604. doi: 10.1111/j.1462-2920.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- Hartmann V., Harris D., Bobrowski T., Ruff A., Frank A., Günther Pomorski T., Rögner M., Schuhmann W., Adir N., Nowaczyk M.M. Improved quantum efficiency in an engineered light harvesting/photosystem II super-complex for high current density biophotoanodes. J. Mater. Chem. A. 2020;8:14463–14471. [Google Scholar]
- Hartshorne R.S., Jepson B.N., Clarke T.A., Field S.J., Fredrickson J., Zachara J., Shi L., Butt J.N., Richardson D.J. Characterization of Shewanella oneidensis MtrC: a cell-surface decaheme cytochrome involved in respiratory electron transport to extracellular electron acceptors. J. Biol. Inorg. Chem. 2007;12:1083–1094. doi: 10.1007/s00775-007-0278-y. [DOI] [PubMed] [Google Scholar]
- Hasan K., Milton R.D., Grattieri M., Wang T., Stephanz M., Minteer S.D. Photobioelectrocatalysis of intact chloroplasts for solar energy conversion. ACS Catal. 2017;7:2257–2265. [Google Scholar]
- Heidary N., Kornienko N., Kalathil S., Fang X., Ly K.H., Greer H.F., Reisner E. Disparity of cytochrome utilization in anodic and cathodic extracellular electron transfer pathways of Geobacter sulfurreducens biofilms. J. Am. Chem. Soc. 2020;142:5194–5203. doi: 10.1021/jacs.9b13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L.F., Lin J.Y., Pan K.Y., Huang C.K., Chu Y.K. Overexpressing ferredoxins in Chlamydomonas reinhardtii increase starch and oil yields and enhance electric power production in a photo microbial fuel cell. Int. J. Mol. Sci. 2015;16:19308–19325. doi: 10.3390/ijms160819308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieropoulos I., Greenman J., Melhuish C., Hart J. Comparative study of three types of microbial fuel cell. Enzyme Microb. Technol. 2005;37:238–245. [Google Scholar]
- Kaiser B.K., Carleton M., Hickman J.W., Miller C., Lawson D., Budde M., Warrener P., Paredes A., Mullapudi S., Navarro P. Fatty aldehydes in cyanobacteria are a metabolically flexible precursor for a diversity of biofuel products. PLoS One. 2013;8:e58307. doi: 10.1371/journal.pone.0058307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauny J., Sétif P. NADPH fluorescence in the cyanobacterium Synechocystis sp. PCC 6803: a versatile probe for in vivo measurements of rates, yields and pools. Biochim. Biophys. Acta. 2014;1837:792–801. doi: 10.1016/j.bbabio.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Keren N., Kidd M.J., Penner-Hahn J.E., Pakrasi H.B. A light-dependent mechanism for massive accumulation of manganese in the photosynthetic bacterium Synechocystis sp. PCC 6803. Biochemistry. 2002;41:15085–15092. doi: 10.1021/bi026892s. [DOI] [PubMed] [Google Scholar]
- Kim B.H., Kim H.J., Hyun M.S., Park D.H. Direct electrode reaction of Fe(III)-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 1999;9:127–131. [Google Scholar]
- Kowata H., Tochigi S., Takahashi H., Kojima S. Outer membrane permeability of cyanobacterium Synechocystis sp. strain PCC 6803: studies of passive diffusion of small organic nutrients reveal the absence of classical porins and intrinsically low permeability. J. Bacteriol. 2017;199 doi: 10.1128/JB.00371-17. e00371–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler C., Lis H., Finkel O.M., Schmetterer G., Shaked Y., Keren N. Coordinated transporter activity shapes high-affinity iron acquisition in cyanobacteria. ISME J. 2014;8:409–417. doi: 10.1038/ismej.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J.C.W., Raman K., Huang C.M., Chang C.M. The impact of monochromatic blue and red LED light upon performance of photo microbial fuel cells (PMFCs) using Chlamydomonas reinhardtii transformation F5 as biocatalyst. Biochem. Eng. J. 2013;78:39–43. [Google Scholar]
- Laohavisit A., Anderson A., Bombelli P., Jacobs M., Howe C.J., Davies J.M., Smith A.G. Enhancing plasma membrane NADPH oxidase activity increases current output by diatoms in biophotovoltaic devices. Algal Res. 2015;12:91–98. [Google Scholar]
- Larom S., Kallmann D., Saper G., Pinhassi R., Rothschild A., Dotan H., Ankonina G., Schuster G., Adir N. The Photosystem II D1-K238E mutation enhances electrical current production using cyanobacterial thylakoid membranes in a bio-photoelectrochemical cell. Photosynth. Res. 2015;126:161–169. doi: 10.1007/s11120-015-0075-3. [DOI] [PubMed] [Google Scholar]
- Larom S., Salama F., Schuster G., Adir N. Engineering of an alternative electron transfer path in photosystem II. Proc. Natl. Acad. Sci. 2010;107:9650–9655. doi: 10.1073/pnas.1000187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawaetz A.J., Stedmon C.A. Fluorescence intensity calibration using the Raman scatter peak of water. Appl. Spectrosc. 2009;63:936–940. doi: 10.1366/000370209788964548. [DOI] [PubMed] [Google Scholar]
- Lee S.A., Choi Y., Jung S., Kim S. Effect of initial carbon sources on the electrochemical detection of glucose by Gluconobacter oxydans. Bioelectrochemistry. 2002;57:173–178. doi: 10.1016/s1567-5394(02)00115-9. [DOI] [PubMed] [Google Scholar]
- Li J., Feng X., Fei J., Cai P., Huang J., Li J. Integrating photosystem II into a porous TiO2 nanotube network toward highly efficient photo-bioelectrochemical cells. J. Mater. Chem. A. 2016;4:12197–12204. [Google Scholar]
- Liu Z.D., Lian J., Du Z.W., Li H.R. Construction of sugar-based microbial fuel cells by dissimilatory metal reduction bacteria. Chin. J. Biotechnol. 2006;22:131–137. doi: 10.1016/s1872-2075(06)60010-1. [DOI] [PubMed] [Google Scholar]
- Longatte G., Sayegh A., Delacotte J., Rappaport F., Wollman F.A., Guille-Collignon M., Lemaître F. Investigation of photocurrents resulting from a living unicellular algae suspension with quinones over time. Chem. Sci. 2018;9:8271–8281. doi: 10.1039/c8sc03058h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012;66:391–409. doi: 10.1146/annurev-micro-092611-150104. [DOI] [PubMed] [Google Scholar]
- Lovley D.R., Coates J.D., Blunt-Harris E.L., Phillips E.J.P., Woodward J.C. Humic substances as electron acceptors for microbial respiration. Nature. 1996;382:445–448. [Google Scholar]
- Lovley D.R., Holmes D.E., Nevin K.P. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 2004;49:219–286. doi: 10.1016/S0065-2911(04)49005-5. [DOI] [PubMed] [Google Scholar]
- Major P., Embley T.M., Williams T.A. Phylogenetic diversity of NTT nucleotide transport proteins in free-living and parasitic bacteria and Eukaryotes. Genome Biol. Evol. 2017;9:480–487. doi: 10.1093/gbe/evx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick A.J., Bombelli P., Bradley R.W., Thorne R., Wenzele T., Howe C.J. Biophotovoltaics: oxygenic photosynthetic organisms in the world of bioelectrochemical systems. Energy Environ. Sci. 2015;8:1092–1109. [Google Scholar]
- McCormick A.J. Hydrogen production through oxygenic photosynthesis using the cyanobacterium Synechocystis sp. PCC 6803 in a bio-photoelectrolysis cell (BPE) system. Energy Environ. Sci. 2013;6:2682–2690. [Google Scholar]
- McCormick A.J., Bombelli P., Scott A.M., Philips A.J., Smith A.G., Fisher A.C., Howe C.J. Photosynthetic biofilms in pure culture harness solar energy in a mediatorless bio-photovoltaic cell (BPV) system. Energy Environ. Sci. 2011;4:4699–4709. [Google Scholar]
- Menicucci J., Beyenal H., Marsili E., Veluchamy Demir G., Lewandowski Z. Procedure for determining maximum sustainable power generated by microbial fuel cells. Environ. Sci. Technol. 2006;40:1062–1068. doi: 10.1021/es051180l. [DOI] [PubMed] [Google Scholar]
- Mershin A., Matsumoto K., Kaiser L., Yu D., Vaughn M., Nazeeruddin M.K., Bruce B.D., Graetzel M., Zhang F. Self-assembled photosystem-I biophotovoltaics on nanostructured TiO2 and ZnO. Sci. Rep. 2012;2:234. doi: 10.1038/srep00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B., Cheng S., Logan B.E. Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 2005;39:1675–1686. doi: 10.1016/j.watres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Murakawa S., Takahashi T. Stimulation of glutamate uptake by NADH and NADPH in intact cells of Escherichia coli mutant defective in Mg2+, Ca2+-ATPase. Agric. Biol. Chem. 1978;42:1803–1804. [Google Scholar]
- Nevin K.P., Richter H., Covalla S.F., Johnson J.P., Woodard T.L., Orloff A.L., Jia H., Zhang M., Lovley D.R. Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environ. Microbiol. 2008;10:2505–2514. doi: 10.1111/j.1462-2920.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- Ochiai H., Shibata H., Sawa Y., Shoga M., Ohta S. Properties of semiconductor electrodes coated with living films of cyanobacteria. Appl. Biochem. Biotechnol. 1983;8:289–303. [Google Scholar]
- Park D., Zeikus J. Impact of electrode composition on electricity generation in a single-compartment fuel cell using Shewanella putrefaciens. Appl. Microbiol. Biotechnol. 2002;59:58–61. doi: 10.1007/s00253-002-0972-1. [DOI] [PubMed] [Google Scholar]
- Park D.H., Laivenieks M., Guettler M.V., Jain M.K., Zeikus J.G. Microbial utilization of electrically reduced neutral red as the sole electron donor for growth and metabolite production. Appl. Environ. Microbiol. 1999;65:2912–2917. doi: 10.1128/aem.65.7.2912-2917.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D.H., Zeikus J.G. Electricity generation in microbial fuel cells using neutral red as an electronophore. Appl. Environ. Microbiol. 2000;66:1292–1297. doi: 10.1128/aem.66.4.1292-1297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D.H., Zeikus J.G. Utilization of electrically reduced neutral red by Actinobacillus succinogenes: physiological function of neutral red in membrane-driven fumarate reduction and energy conservation. J. Bacteriol. 1999;181:2403–2410. doi: 10.1128/jb.181.8.2403-2410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham C.A., Jung S.J., Phung N.T., Lee J., Chang I.S., Kim B.H., Yi H., Chun J. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol. Lett. 2003;223:129–134. doi: 10.1016/S0378-1097(03)00354-9. [DOI] [PubMed] [Google Scholar]
- Pinhassi R.I., Kallmann D., Saper G., Dotan H., Linkov A., Kay A., Liveanu V., Schuster G., Adir N., Rothschild A. Hybrid bio-photo-electro-chemical cells for solar water splitting. Nat. Commun. 2016;7:12552. doi: 10.1038/ncomms12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinhassi R.I., Kallmann D., Saper G., Larom S., Linkov A., Boulouis A., Schöttler M.A., Bock R., Rothschild A., Adir N., Schuster G. Photosynthetic membranes of Synechocystis or plants convert sunlight to photocurrent through different pathways due to different architectures. PLoS One. 2015;10:e0122616. doi: 10.1371/journal.pone.0122616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaey K., Boon N., Höfte M., Verstraete W. Microbial phenazine production enhances electron transfer in biofuel cells. Environ. Sci. Technol. 2005;39:3401–3408. doi: 10.1021/es048563o. [DOI] [PubMed] [Google Scholar]
- Rabaey K., Boon N., Siciliano S.D., Verhaege M., Verstraete W. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl. Environ. Microbiol. 2004;70:5373–5382. doi: 10.1128/AEM.70.9.5373-5382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads A., Beyenal H., Lewandowski Z. Microbial fuel cell using anaerobic respiration as an anodic reaction and biomineralized manganese as a cathodic reactant. Environ. Sci. Technol. 2005;39:4666–4671. doi: 10.1021/es048386r. [DOI] [PubMed] [Google Scholar]
- Ringeisen B.R., Henderson E., Wu P.K., Pietron J., Ray R., Little B., Biffinger J.C., Jones-Meehan J.M. High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10. Environ. Sci. Technol. 2006;40:2629–2634. doi: 10.1021/es052254w. [DOI] [PubMed] [Google Scholar]
- Saper G., Kallmann D., Conzuelo F., Zhao F., Tóth T.N., Liveanu V., Meir S., Szymanski J., Aharoni A., Schuhmann W. Live cyanobacteria produce photocurrent and hydrogen using both the respiratory and photosynthetic systems. Nat. Commun. 2018;9:2168. doi: 10.1038/s41467-018-04613-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa M., Fantuzzi A., Bombelli P., Howe C.J., Hellgardt K., Nixon P.J. Electricity generation from digitally printed cyanobacteria. Nat. Commun. 2017;8:1327. doi: 10.1038/s41467-017-01084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmetterer G., Alge D., Gregor W. Deletion of cytochrome c oxidase genes from the cyanobacterium Synechocystis sp. PCC6803: evidence for alternative respiratory pathways. Photosynth. Res. 1994;42:43–50. doi: 10.1007/BF00019057. [DOI] [PubMed] [Google Scholar]
- Sekar N., Jain R., Yan Y., Ramasamy R.P. Enhanced photo-bioelectrochemical energy conversion by genetically engineered cyanobacteria. Biotechnol. Bioeng. 2016;113:675–679. doi: 10.1002/bit.25829. [DOI] [PubMed] [Google Scholar]
- Sekar N., Umasankar Y., Ramasamy R.P. Photocurrent generation by immobilized cyanobacteria via direct electron transport in photo-bioelectrochemical cells. Phys. Chem. Chem. Phys. 2014;16:7862–7871. doi: 10.1039/c4cp00494a. [DOI] [PubMed] [Google Scholar]
- Shi L., Rosso K.M., Zachara J.M., Fredrickson J.K. Mtr extracellular electron-transfer pathways in Fe(III)-reducing or Fe(II)-oxidizing bacteria: a genomic perspective. Biochem. Soc. Trans. 2012;40:1261–1267. doi: 10.1042/BST20120098. [DOI] [PubMed] [Google Scholar]
- Simoska O., Sans M., Eberlin L.S., Shear J.B., Stevenson K.J. Electrochemical monitoring of the impact of polymicrobial infections on Pseudomonas aeruginosa and growth dependent medium. Biosens. Bioelectron. 2019;142:111538. doi: 10.1016/j.bios.2019.111538. [DOI] [PubMed] [Google Scholar]
- Stingaciu L.R., O’Neill H.M., Liberton M., Pakrasi H.B., Urban V.S. Influence of chemically disrupted photosynthesis on cyanobacterial thylakoid dynamics in Synechocystis sp. PCC 6803. Sci. Rep. 2019;9:5711. doi: 10.1038/s41598-019-42024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Kashiwagi N., Ogawa T. Effects of light on the electrical output of bioelectrochemical fuel-cells containing Anabaena variabilis M-2: mechanism of the post-illumination burst. J. Chem. Technol. Biotechnol. 1988;42:235–240. [Google Scholar]
- Tanaka K., Tamamushi R., Ogawa T. Bioelectrochemical fuel-cells operated by the cyanobacterium, anabaena variabilis. J. Chem. Technol. Biotechnol. Biotechnol. 1985;35 B:191–197. [Google Scholar]
- Tanaka K., Vega C., Tamamushi R. Mediating effects of ferric chelate compound in microbial fuel cells. J. Electroanal. Chem. 1983;156:135–143. [Google Scholar]
- Thirumurthy M.A., Hitchcock A., Cereda A., Liu J., Chavez M.S., Doss B.L., Ros R., El-Naggar M.Y., Heap J.T., Bibby T.S. Type IV pili-independent photocurrent production by the cyanobacterium synechocystis sp. PCC 6803. Front. Microbiol. 2020;11:1344. doi: 10.3389/fmicb.2020.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston C.F., Bennetto H.P., Delaney G.M. Glucose metabolism in a microbial fuel cell. Stoichiometry of product formation in a thionine-mediated Proteus vulgaris fuel cell and its relation to coulombic yields. J. Gen. Microbiol. 1985;131:1393–1401. [Google Scholar]
- Torimura M., Miki A., Wadano A., Kano K., Ikeda T. Electrochemical investigation of cyanobacteria Synechococcus sp. PCC7942-catalyzed photoreduction of exogenous quinones and photoelectrochemical oxidation of water. J. Electroanal. Chem. 2001;496:21–28. [Google Scholar]
- Tschörtner J., Lai B., Krömer J.O. Biophotovoltaics: green power generation from sunlight and water. Front. Microbiol. 2019;10:866. doi: 10.3389/fmicb.2019.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. IEA . 2011. Annual Energy Outlook 2011. [Google Scholar]
- Vaara M. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Lee H., Choi S. Biopower generation in a microfluidic bio-solar panel. Sens. Actuators B Chem. 2016;228:151–155. [Google Scholar]
- Yagishita T., Horigome T., Tanaka K. Effects of light, CO2 and inhibitors on the current output of biofuel cells containing the photosynthetic organism Synechococcus sp. J. Chem. Technol. Biotechnol. 1993;56:393–399. [Google Scholar]
- Yehezkeli O., Tel-Vered R., Wasserman J., Trifonov A., Michaeli D., Nechushtai E., Willner I. Integrated photosystem II-based photo-bioelectrochemical cells. Nat. Commun. 2012;3:742. doi: 10.1038/ncomms1741. [DOI] [PubMed] [Google Scholar]
- Yi H., Nevin K.P., Kim B.C., Franks A.E., Klimes A., Tender L.M., Lovley D.R. Selection of a variant of Geobacter sulfurreducens with enhanced capacity for current production in microbial fuel cells. Biosens. Bioelectron. 2009;24:3498–3503. doi: 10.1016/j.bios.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Zhang L., Selão T.T., Pisareva T., Qian J., Sze S.K., Carlberg I., Norling B. Deletion of Synechocystis sp. PCC 6803 leader peptidase LepB1 affects photosynthetic complexes and respiration. Mol. Cell. Proteomics. 2013;12:1192–1203. doi: 10.1074/mcp.M112.022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Conzuelo F., Hartmann V., Li H., Nowaczyk M.M., Plumeré N., Rögner M., Schuhmann W. Light induced H2 evolution from a biophotocathode based on photosystem 1–Pt nanoparticles complexes integrated in solvated redox polymers films. J. Phys. Chem. B. 2015;119:13726–13731. doi: 10.1021/acs.jpcb.5b03511. [DOI] [PubMed] [Google Scholar]
- Zhao F., Sliozberg K., Rögner M., Plumeré N., Schuhmann W. The role of hydrophobicity of Os-complex-modified polymers for photosystem 1 based photocathodes. J. Electrochem. Soc. 2014;161:H3035–H3041. [Google Scholar]
- Zhou Y., Wang L., Yang F., Lin X., Zhang S., Zhao Z.K. Determining the extremes of the cellular NAD(H) level by using an Escherichia coli NAD+-auxotrophic mutant. Appl. Environ. Microbiol. 2011;77:6133–6140. doi: 10.1128/AEM.00630-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Pisciotta J., Baskakov I.V.( Nanostructured polypyrrole-coated anode for sun-powered microbial fuel cells. Bioelectrochemistry. 2010;79:50–56. doi: 10.1016/j.bioelechem.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Zou Y., Pisciotta J., Billmyre R.B., Baskakov I.V.( Photosynthetic microbial fuel cells with positive light response. Biotechnol. Bioeng. 2009;104:939–946. doi: 10.1002/bit.22466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate computer code. All data and analytical methods are available in the main text or in Supplemental information section.