Highlights
-
•
A machine learning model predicted body congruence after cross-sex hormone therapy.
-
•
Predictive features included clinical metrics and network functional connectivity.
-
•
The most predictive networks were fronto-parietal and cingulo-opercular.
-
•
Functional connectivity may provide insights into body-brain effects of hormones.
-
•
Methods could be used to enhance personalized therapies.
Keywords: Gender incongruence, Cross-sex hormone therapy, Machine learning, LASSO, Transgender, Gender dysphoria, Prediction
Abstract
Individuals with gender incongruence (GI) experience serious distress due to incongruence between their gender identity and birth-assigned sex. Sociological, cultural, interpersonal, and biological factors are likely contributory, and for some individuals medical treatment such as cross-sex hormone therapy and gender-affirming surgery can be helpful. Cross-sex hormone therapy can be effective for reducing body incongruence, but responses vary, and there is no reliable way to predict therapeutic outcomes. We used clinical and MRI data before cross-sex hormone therapy as features to train a machine learning model to predict individuals’ post-therapy body congruence (the degree to which photos of their bodies match their self-identities). Twenty-five trans women and trans men with gender incongruence participated. The model significantly predicted post-therapy body congruence, with the highest predictive features coming from the cingulo-opercular (R2 = 0.41) and fronto-parietal (R2 = 0.30) networks. This study provides evidence that hormone therapy efficacy can be predicted from information collected before therapy, and that patterns of functional brain connectivity may provide insights into body-brain effects of hormones, affecting one's sense of body congruence. Results could help identify the need for personalized therapies in individuals predicted to have low body-self congruence after standard therapy.
1. Introduction
Awareness of gender incongruence has climbed sharply in recent years. Issues related to self-identity, body image, and medical interventions in individuals with a gender incongruence diagnosis (GI) are challenges for the 21st century, particularly given the high suicide risk associated with GI (Clements-Nolle et al., 2006, Maguen and Shipherd, 2010, Mueller et al., 2017, Narang et al., 2018), possibly due to their core dysphoria related to gender incongruence and/or psychiatric conditions such as anxiety (Bouman et al., 2017a) and depressive disorders (Witcomb et al., 2018). These psychiatric conditions in many could be the result of stigmatization and childhood maltreatment (Guss et al., 2019, Yang et al., 2015). Gender incongruence in ICD-11 (World Health Organization, 1992) or gender dysphoria, in DSM-5 (American Psychiatric Association, 2013), refers to significant distress and/or impairment due to a feeling of incongruence between a person’s experienced gender and their birth-assigned sex. A subset of those who identify as transgender suffer from GI (van de Grift et al., 2016a, van de Grift et al., 2016b, van de Grift et al., 2016c, though not all GI-experiencing people identify as transgender (Safer and Tangpricha, 2019). This implies that being transgender does not equate with having a mental health condition.
GI is often treated with cross-sex hormone therapy (Dhejne et al., 2016, Nguyen et al., 2018; Solomon et al., 2019), in many cases followed by gender-affirming surgery (van de Grift et al., 2018). In a non-trivial proportion, therapy has had limited success as evidenced by the high heterogeneity in quality of life outcomes across studies (Murad et al., 2010, Nobili et al., 2018), as well as variable improvements in body image (van de Grift et al., 2016b, van de Grift et al., 2017). This may be due to inter-individual biological variability, contributions from sociological or cultural factors, or a combination of these factors (Cooper et al., 2020). The desired therapy outcome is improved congruence between one’s gender identify and the sex-related physical characteristics of one’s body. This outcome is a conscious experience and has a prominent basis in gender roles, psychological and sociological structures, and in neural function. The medical profession currently lacks the ability to predict who will respond well to therapy and who will not - a critical piece in moving towards personalized, evidence-based medicine to optimize clinical outcomes and efficacy of therapy. This needs to be considered in the context of rapidly changing societal notions of gender identity. The current study is specifically focused on constructing a preliminary prediction model of cross-sex hormone therapy response.
Overall there have been few studies that have examined predictors of clinical outcomes for cross-sex hormone or gender-affirming surgical treatments. A recent study of hormone and surgical treatments (van de Grift et al., 2017) found that that body dissatisfaction pre-therapy predicted body dissatisfaction post-therapy (p < 0.001), but there was no predictive value of birth-assigned sex (p = 0.83), age (p = 0.23), nor by clinicians valuation at first sight that the person’s gender is their experienced gender (p = 0.50), sometimes referred to by the (contested) term “passing.” A study of surgical treatments found better outcomes were predicted by pre-treatment lower dissatisfaction with secondary sex characteristics (p < 0.001) and less psychopathology (p < 0.028), as well as being homosexual (p < 0.002) (defined in relationship to birth-assigned sex) (Smith et al., 1999).
We recently proposed a hypothesis that GI is characterized by a functional disconnection between systems in the brain that process the perception of self (“self-referential”) and those that mediate own-body perception (Majid et al., 2020, Manzouri et al., 2017). Self-referential systems include the default mode network, particularly medial prefrontal cortical regions such as the dorsal and pregenual anterior cingulate cortex (Northoff et al., 2006), and the salience network, particularly the insular cortex (Craig, 2010, Uddin, 2015). Involved in own-body perception are the temporoparietal junction (Blanke et al., 2005) and the extrastriate and fusiform body areas (Vocks et al., 2010). We proposed that differences in coordinated activation and connections between own-body and self-perception networks could explain the discomfort with their bodies reported by individuals with GI (Majid et al., 2020, Manzouri et al., 2017).
To better understand relationships between body perception and gender-related self-identity, we previously designed a “body morph task” (Feusner et al., 2016), specifically to test the degree of incongruence between self own-body identification and one’s body sex characteristics. Studies using the body morph task (Burke et al., 2018, Feusner et al., 2016, Kilpatrick et al., 2019) have shown that this task may provide an indication of body-related and gender-specific self-identity pre- and post-therapy using a metric calculated from the body morph task, the body index. In the current study we used the body index as our main outcome variable to quantify an individual’s body congruence.
In alignment with our previous studies of structural and functional brain systems in GI, we focused on seven brain networks as potential predictive features. Previous studies have shown differences in transgender compared with cisgender individuals in brain activation (Burke et al., 2019), cortical thickness (Kilpatrick et al., 2019), or in connectivity (Feusner et al., 2017, Uribe et al., 2020). To capture these regions, we included the (i) salience and (ii) default mode networks (Manzouri and Savic, 2019), as well as the (iii) fronto-parietal and (iv) cingulo-opercular task control networks because they include the dorsal and pregenual anterior cingulate cortices, which are implicated in the perception of self (Northoff et al., 2006). We additionally included the (v) dorsal and (vi) ventral attention networks because they include the temporal parietal junction and surrounding cortices important in own body perception (Blanke et al., 2005). Finally, we included the (vii) memory retrieval network, as it includes midline portions of the posterior cingulate shown to be important for self-perception and which in our earlier studies showed greater cortical thickness compared to (Manzouri et al., 2017, Manzouri and Savic, 2019). All network-defined regions of interest (ROIs) were derived using a brain parcellation from Power (Power et al., 2011) who partitioned the brain into functional networks based on resting-state connectivity data. (See Fig. S1 for node locations of a priori networks.)
For the current study, we used this knowledge of underlying biology to build a set of features we hypothesized should be capable of predicting therapeutic outcomes for individuals diagnosed with GI within a machine-learning framework. We focused on resting-state fMRI connectivity measures before cross-sex hormone therapy, combined with clinical data - pre-therapy body index ratings, body mass index (BMI), therapy duration, and a (simplified model) of sexual orientation (Kinsey scores) - to train and test a penalized regression model for predicting post-hormone therapy body congruence, measured by the post-hormone therapy body index scores.
2. Materials and methods
2.1. Participants
Participants were recruited in Stockholm, Sweden by the Gender Team of the Center for Andrology and Sexual Medicine at Karolinska University Hospital, a center specializing in the evaluation and GI therapy. Adults aged 18 to 50 who were diagnosed with “Transsexualism” based on ICD-10 diagnostic criteria (F64.0, World Health Organization, 1992) (note, this term is currently outdated) and sought gender-affirming medical interventions were invited to enter the study. Because accepted nomenclature has changed since the study began (Bouman et al., 2017b), and to be in line with ICD-11 terminology, we will refer to participants henceforth as “participants with gender incongruence” (GI). Participants identifying as nonbinary, or identifying other than transgender were not enrolled. None of the trans women or trans men had received hormonal therapy at the time of the first scanning session, or gender-affirming surgery at the time of scanning sessions. Participants were excluded for previous or current hormonal therapy, any known chromosomal or hormonal disorder, or any concurrent psychiatric disorder (determined by the Mini International Neuropsychiatric Interview, MINI, Sheehan (Sheehan and Lecrubier, 2010), neurological or other medical disorders including autism spectrum disorder, substance abuse, or the use of psychoactive medications. All participants provided full informed consent in accordance with the Karolinska Institute ethical committee (Application # Dnr 2011/281–31/4). See Supplementary Materials for details of hormone therapy and gender identity.
2.2. Data acquisition
Participants underwent an MRI scan and were evaluated with psychometric tools prior to hormonal therapy at session 1 (S1/pre-therapy). The participants were scanned and evaluated at session 2 (S2/post-therapy), on average 14 months later. We used S1 clinical measures and resting state functional connectivity (FC) as inputs to our machine learning algorithms to predict metrics of body satisfaction at S2, with the goal of determining which patients will benefit from hormone therapy - prior to undergoing hormone therapy.
2.3. Body morph task and body index (BI)
Details of the body morph task can be found in (Burke et al., 2019). Each participant was dressed in a tight, full-body unitard to provide an accurate representation of their body shape without the discomfort of being nude. Hands, feet, and head were cropped from the photos, and both front and side views were taken. Each participant’s picture was morphed towards those pictures of five different female-presenting and five different male-presenting individuals at degree intervals of 20%, using FantaMorph Software, version 5.0 (Abrosoft http://www.fantamorph.com/). Eleven morph conditions resulted, ranging between −100% morphed completely to a picture of a cisgender person with opposite birth-assigned sex to +100% morphed completely towards a picture of a cisgender person with same birth-assigned sex as the participant. Thus, 0% referred to the original unmorphed own-body image of the participant. A set of 62 images (using a randomized order and number of repetitions of the body image morphs and unmorphed own-body image) were presented for two different viewing conditions (short duration = 0.5 s and long duration = 2 s), totaling 128 trials.
These images were presented using MATLAB 2012a, on a laptop computer. Each trial consisted of the image (presented for either 0.5 or 2 s) followed by a 1 s response screen with button press options, followed by a fixation cross. Participants were instructed to respond as quickly as possible to the question “To what degree is this picture you?” on a 4-point scale (1: 0–25% “me”, 2: 25–50% “me”, 3: 50–75% “me”, and 4: 75–100% “me”). Before the task, participants engaged in a practice session to ensure task comprehension. We calculated the “body index” (BI): the perception of the degree of self, represented by an index calculated from ratings across all of the morphed bodies presented. The body index provides an indication of an individual’s maximal perception of ‘self’ on a continuum from traditionally feminine to traditionally masculine appearances. The clinical body index (Feusner et al., 2017) was subsequently employed as a predictive feature in our machine learning algorithms.
2.4. Demographics and psychometrics
Clinical metrics collected at S1 were used as features or covariates of non-interest: body mass index (BMI), age, therapy duration (in months from initiation of cross-sex hormone therapy) birth-assigned sex, and Kinsey sexual orientation score (Kinsey score range is 0–6, with 0 being exclusively heterosexual and 6 being exclusively homosexual. Heterosexual and homosexual are arbitrarily in reference to birth-assigned sex, (Kinsey et al., 2003)). The predicted clinical measure was the body index score, calculated from the body morph task described below (Fig. 1). The body morph task data were collected on a laptop, prior to the resting state MRI acquisition.
Fig. 1.
The body morph task asks subjects to rate morphed and unmorphed own-body images. Shown are examples of a front view photograph of a participant who was assigned male sex at birth morphed by 20, 40, 60, 80, and 100% to a photograph of a female (top), and male (bottom) sex-assigned-at-birth cisgender individual. Morphing to same and opposite sex-assigned-at-birth photographs are denoted (arbitrarily) by positive and negative morph degrees, respectively. Note that 100% photographs were unaltered images of another person. The 0% image is the unaltered, unmorphed own-body photograph of the participant.
To calculate the body index, we first multiplied each degree (1–4) of “self” rated for each morph with the degree of each morph. These weighted values were averaged for each participant across ratings for all images and then divided by the number of rated images, providing an average index of self-perception for each participant, weighted by how close or far from the actual self-photograph the image was morphed, and in which direction. Positive values of the body index represent ratings toward birth-assigned sex (incongruent), while negative values represent ratings toward gender (congruent).
2.5. MR data acquisition
MRI data was acquired on a 3 Tesla MRI scanner (Discovery 3T GE-MR750, General Electric, Milwaukee, Wisconsin) using a 32-channel head coil. Resting-state functional MRI data were acquired with a gradient echo pulse sequence with: voxel size of 2.25 × 2.25 × 3 mm, TR/TE = 2500/30 ms, FOV = 28.8 cm, 45 interleaved axial slices, 90 flip angle. Each resting-state scan totaled 7 min 35 s and participants were instructed to rest with eyes closed, to remain as still as possible, and not to sleep while the sequence was acquired. Structural data, 3D T1-weighted Spoiled Gradient Echo pulse sequence, were acquired with voxel size 0.94 × 0.94 × 1 mm, TR/TE = 7.91/3.06 ms, TI = 450 ms, FOV = 24 cm, 176 axial slices, and 12 flip angle.
2.6. Data analysis
MRI analysis was performed using FEAT (fMRI Expert Analysis Tool) version 5.0.8, part of FSL (FMRIB Software Library http://www.fmrib.ox.ac.uk/fsl, (Jenkinson et al., 2012). Bold sequences were motion-corrected (FMRIB linear image registration tool MCFLIRT), without spatial smoothing, and individual participants’ resting state data were denoised using FSL’s AROMA tool (non-aggressive denoising option). Functional images were registered to their respective T1-weighted images (FMRIB non-linear image registration tool, FNIRT) after brain extraction using FSL’s BET, and then to the MNI-152 brain for functional connectivity analysis. Two participants (of 27 recruited) were excluded due to head motion greater than 1.5 mm, resulting in 25 participants included in these analyses.
For the LASSO and ridge analyses, we leveraged MATLAB Version R2015b, Mathworks, Inc., Natick, MA) as part of the Statistics and Machine Learning Toolbox. The scripts are available at this link (Open Science Framework): https://osf.io/zn2ev/.
2.7. ROI selection
We used a functionally-defined set of ROIs (10 mm diameter spheres using FSL’s fslmaths command with the roi argument) that have been previously mapped to functional networks (Jenkinson et al., 2012, Power et al., 2011, Reggente et al., 2018). We narrowed our scope to seven a priori networks that covered regions and networks with functional and/or structural differences between cisgender or transgender individuals: default mode, fronto-parietal, dorsal attention, salience, cingulo-opercular, memory retrieval, and ventral attention. This resulted in 264 ROIs, each of which was tagged with one of seven functional-network identities.
2.8. ROI correlation matrices
We collected resting-state data from each participant prior to cross-sex hormone therapy and used the denoised images to determine connectivity among the ROIs. For each participant we computed the mean BOLD activity within each of the ROIs at every time point (every 2.5 s), resulting in a time course of mean ROI activity. We then computed a pairwise Pearson correlation matrix for each mean time course resulting in 264 × 264 matrices containing the pairwise functional connectivity values (r-values) across all ROIs.
2.9. Feature creation
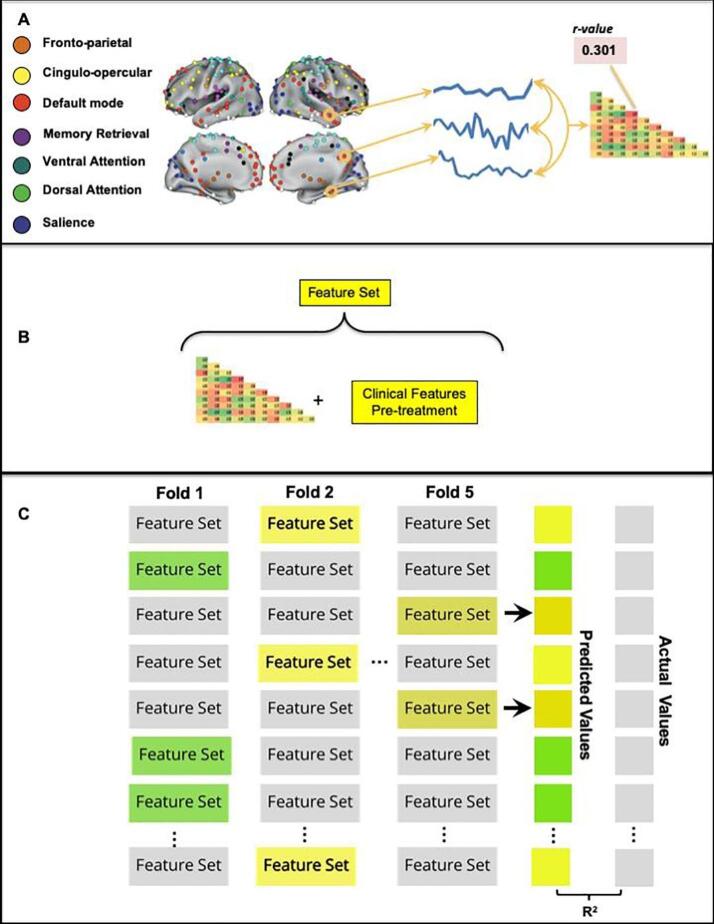
We indexed each correlation matrix depending on network identity, extracted the lower diagonal of each matrix and then identified the rows and columns corresponding to the functional connectivity across ROIs within a specific network. The resultant values constituted the functional connectivity (FC) feature sets. We included these feature sets plus clinical data, as the “hand-selected” features that we tested in the machine learning algorithms. See Fig. 2 for a flow chart of the machine learning analysis.
Fig. 2.
Analysis flow chart. (A) The average resting-state activity within ROIs from seven functional brain networks defined by Power (30) was used to create a mean BOLD time course. Pairwise Pearson correlations of these time courses resulted in a functional connectivity matrix specific for each network. (B) The lower diagonal of each participant’s network-specific matrix was concatenated with the participant’s pre-therapy clinical features scores to create a feature set for that participant. (C) The LASSO regression model was trained on n − 5 participants’ feature sets and their associated post-therapy body index scores and used to predict each of the left-out participant’s post-therapy body index scores. Left-out participants are denoted as highlighted feature sets (only three shown here). This process was repeated until all participants had been left out in a fold of the cross-validation and had been assigned a predicted post-therapy body index score. We correlated the array of predicted values (ŷ) with the actual values (y), resulting in Pearson’s r, and R2 a measure of our model’s feature-dependent ability to capture the outcome variable variance across participants. Note that due to our participant sample size (n = 25), one-fold of the cross-validation left out five participants.
2.10. Machine learning regression analysis predicting post-therapy body index using least absolute shrinkage and selection operator (LASSO)
We built a least absolute shrinkage and selection operator (LASSO) (Reggente et al., 2018, Tibshirani, 1996); regression model whose regularization parameter (lambda, λ = 5.0) was optimized using the least angle regression (LARS) algorithm (Efron et al., 2004; Tibshirani et al., 2004) on an N − 1 cross-validation that maximized the Pearson correlation between actual and predicted post-therapy body index scores. To minimize overfitting, we used these optimized model parameters in an N − 5 cross-validation: a random subset of 5 participants (20%) were left out and the model was trained on the remaining participants and tested on the left-out subjects; this process was repeated until each participant was left out of the training set once. We did 100 iterations of this procedure and averaged over the 100 iterations for the final prediction values.
LASSO was chosen as the regression model of choice due to its ability to handle large feature sets, impose a self-directed feature selection, and output continuous variables. Using each trained model’s intercept term and beta coefficients, we calculated a predicted measure of interest from each left-out participant’s feature set. After obtaining a prediction for each participant (ŷ), we correlated the array of predicted values with the actual values (y) to quantify the model’s feature-dependent ability to capture the variance in clinical measures across participants. Although the sample size was small due to the select nature of this this treatment-seeking population, adequate sample sizes for machine learning approaches using regularization are challenging to determine and depend on the strength of the relationships between predictive features and the outcome of interest, which in this first-of-its-kind study were not known a priori.
Clinical features included pre-therapy body index rating, therapy duration, BMI, and Kinsey scores. Kinsey scores were included to avoid a confound of sexual orientation on sexual dimorphism in brain networks in transgender individuals (Manzouri and Savic, 2019). Age was treated as a covariate of non-interest and iteratively regressed out of each feature in the feature set prior to the machine learning regression. We did not include birth-assigned sex as a covariate, as it is considered within the body index ratings. For predicting body index for short duration trials, we used the pre-therapy body index ratings for short duration trials as a feature, likewise, for predicting pre-therapy body index for long duration trials, we used the body index ratings for long duration trials as a feature. In a preliminary analysis, we examined the effect of therapy duration in our analyses by including therapy duration either as a covariate or as a clinical feature. For six of the seven networks, using therapy duration as a feature was more predictive than using therapy duration as a covariate (Table S1); therefore, results reported for body index are for therapy duration as a feature.
2.11. Machine learning prediction of post-therapy body index using ridge regression
To provide a robustness check on our LASSO predictions, we re-ran the above machine learning analysis using ridge (Marquardt and Snee, 1975) regression. Ridge regression is appropriate when the predictor features potentially have collinearity. (See Table S2, also see Table S3 for additional post hoc tests of specificity.)
2.12. Statistical methods
We report results of Pearson’s correlations as R2 values and r-values, to provide a measure of the variance explained by the model and to allow assessment of effect sizes. According to Cohen (Cohen, 2013, Cohen et al., 2017, Lachenbruch and Cohen, 1989), the effect size is low if the values of r-values are around 0.1, medium if r-values are around 0.3, and large if r-values are more than 0.5.
Unless otherwise indicated, all predictions were tested for significance of p < 0.05 and were subjected to the stringent Bonferroni method to correct for multiple comparisons. The Bonferroni-adjusted p-value, pbf ≤ 0.006 (p < 0.05/9), was adjusted for a total of 9 comparisons: the seven networks in our a priori hypotheses, one combination of all seven networks, and one combination of the fronto-parietal and cingulo-opercular networks. For an additional statistical validation, and to examine the distribution of all our statistical results (to determine if the distribution is by chance and whether or not our results-of-interest are by chance), we used the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995). See Supplemental Materials S7 for those statistical results that substantiate the results of using the Bonferroni method for multiple comparisons.
3. Results
3.1. Participants
Twenty-five adults ages 18–50, mean years 25.2 (SD 7.8), with GI participated in the study. Data from 16 women, assigned male at birth, and 9 men, assigned female at birth, were combined for all analyses. See Table 1 for demographics.
Table 1.
Demographics, clinical values and ratings of the participants (N = 25).
| Characteristic | Value | SD | P value T-test | P value correlation |
|---|---|---|---|---|
| Trans women/Trans men | 16/9 | |||
| Age | 25.2 | 7.8 | ||
| BMI | 24.1 | 5.5 | ||
| Kinsey scores | 4.0 | 2.0 | ||
| Years of education | 13.3 | 1.9 | ||
| Therapy duration (months) | 14.3 | 5.4 | ||
| Body index pre-therapy short duration trials | −10.4 | 21.8 | ||
| Body index post-therapy short duration trials | −23.1 | 25.7 | p = 0.002* | p < 0.001+ |
| Body index pre-therapy long duration trials | −11.1 | 33.5 | ||
| Body index post-therapy long duration trials | −21.3 | 31.7 | p = 0.040* | p < 0.001+ |
Negative values of the body index represent (arbitrarily) ratings toward pictures of opposite sex-assigned-at-birth individuals (congruent with sense of self in those diagnosed with gender incongruence).
Paired one-tailed, t-test, comparing pre- versus post-hormone therapy.
Correlation, comparing pre- versus post-hormone therapy.
3.2. Body congruence changes
For short duration trials, body index scores changed in the direction of increased congruence pre- to post hormone-therapy in 18 of 25 participants (72%), see Fig. S2. The pre-therapy mean was −10.4 (SD, 21.8); the post-therapy mean was −23.1 (SD, 25.7), t24 = 3.1, p = 0.002, 1-tailed. Similarly, for long duration trials, change in the direction of increased congruence was observed in 15 of 25 participants (60%). The pre-therapy mean was −11.1 (SD, 33.5); the post-therapy mean was −21.3 (SD, 31.7), t24 = 1.8, p = 0.04, 1-tailed (Table 2). More-negative scores on the body index are indicative of greater congruence of their body with their gender identity. There were associations in a comparison of pre- and post-therapy values of body index scores: body index ratings for short duration trials (R2 = 0.41, r = 0.64, p = 0.006) and body index ratings for long duration trials (R2 = 0.39, r = 0.63, p = 0.008). There was a trend for an association between therapy duration and body index ratings after hormone therapy for short duration trials, (R2 = 0.11, r = 0.33, p = 0.11), see Table S4.
Table 2.
Associations between predicted post-therapy body congruence for seven brain functional connectivity networks, combined with clinical features, using multivariate analysis.
| Network | R2 Functional connectivity and clinical features | r-value Pearson’s Correlation |
|---|---|---|
| Cingulo-opercular | 0.41* | 0.64* |
| Fronto-parietal | 0.30* | 0.54* |
| Memory Retrieval | 0.20 | 0.45 |
| Salience | 0.19 | 0.43 |
| Dorsal Attention | 0.09 | 0.31 |
| Ventral Attention | 0.06 | 0.24 |
| Default Mode | 0.02 | 0.14 |
| All 7 networks | 0.09 | 0.30 |
| Exploratory post-hoc | ||
| Cingulo-opercular & Fronto-parietal | 0.33* | 0.57* |
Clinical features alone were not significant, R2 = 0.24, r = 0.49. Pearson’s r-values are provided for an estimate of effect sizes. Results are for short-duration trials.
for pbf ≤ 0.006, Bonferroni-corrected significance level.
3.3. Body congruence prediction
3.3.1. Predicting post-therapy body index ratings using LASSO machine learning regression
For these analyses, we first examined functional connectivity (FC) from resting-state fMRI data between all nodes of each a priori network separately. Next, we examined FC between all nodes (combining all seven a priori networks). Finally, as a post-hoc analysis, we examined FC between the nodes resulting from the combination of our two most predictive networks, the cingulo-opercular and fronto-parietal networks.. Short-duration trials predicted post-therapy body congruence (Table 2), but long-duration trials were not significantly predictive (Table S5). Clinical data points that were also leveraged alongside the FC values in the feature set included pre-therapy body index ratings, sexual orientation (Kinsey scores), BMI, and time from initiation of therapy. See Table S6 for similar results, without considering therapy duration.
3.3.2. Individual a priori networks
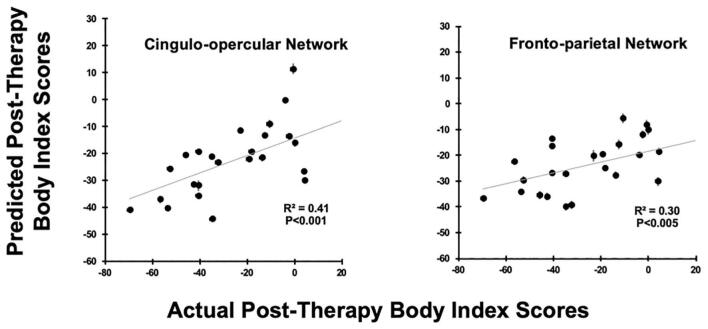
The associations between the algorithm’s predicted post-therapy body index ratings (ŷ) and the actual ratings (y) was statistically significant when using two of the seven a priori networks, the fronto-parietal network and the cingulo-opercular network, alongside the clinical features. When only the clinical features were considered in the model, there was not an association between predicted (ŷ) and actual (y) body index values (R2 = 0.24, r = 0.49, p = 0.013); however, when the model included both the clinical features and the network-defined functional connectivity, there were associations for the fronto-parietal network (R2 = 0.30, r = 0.54, p < 0.005) and for the cingulo-opercular network (R2 = 0.41, r = 0.64, p < 0.006), for Bonferroni corrected, pbf ≤ 0.006. See Table 2 and Fig. 3.
Fig. 3.
Associations between the distributions of body index predictions and actual post-therapy values are shown in scatter plots. These LASSO cross-validation models used feature sets that included pre-therapy functional connectivity from the cingulo-opercular network (Left) and the fronto-parietal network (Right), in addition to clinical features. Error bars are standard-errors across the 100 cross-validation predictions for each individual. The Bonferroni-corrected significance level is pbf ≤ 0.006.
3.3.3. Aggregated a priori networks
Including all seven of the a priori networks in the model along with pre-therapy clinical features resulted in a lower predictive power (R2 = 0.09, r = 0.30, p = 0.149). However, combining the two networks (cingulo-opercular and fronto-parietal) that showed significant associations between predicted and actual body index ratings above, with clinical features, resulted in a significant account of the variance (R2 = 0.32, r = 0.57, p = 0.003).
3.3.4. Predicting post-therapy body index ratings using ridge machine learning regression
We additionally tested predictions using ridge regression. The results when including the same clinical and network features were similar to those using the LASSO models, showing predictive power when leveraging functional connectivity within the cingulo-opercular (R2 = 0.41, r = 0.64, p = 0.001) and fronto-parietal networks (R2 = 0.32, r = 0.57, p = 0.003), and when combining the two networks (R2 = 0.32, r = 0.57, p = 0.003), all significant at a Bonferroni corrected p-value threshold pbf ≤ 0.006, Table S2. Clinical features alone were not predictive, (R2 = 0.25, r = 0.50, p = 0.010).
3.3.5. Post hoc prediction of pre-therapy body index ratings
To examine whether the same networks that predicted post-therapy body congruence were also associated with pre-therapy body congruence, we combined network connectivity and clinical features in a model to predict pre-therapy body congruence. The association between the predicted pre-therapy body index ratings and actual pre-therapy ratings for short duration trials was not significant when using clinical features and the FC of any of our a priori networks.
4. Discussion
This study in individuals with GI tested whether multivariate pattern recognition using neurobiological features from resting state brain connectivity along with clinical features could be used to predict therapeutic response to cross-sex hormone therapy. The goal was to predict, on an individual basis, the important clinical outcome of body congruence in those with GI after hormone therapy by using brain functional connectivity data from a short (7.5 min) MRI scan, BMI, and body congruence ratings before hormone therapy. Multivariate connectivity in the cingulo-opercular and fronto-parietal networks before hormone therapy explained a high proportion of the variance in individual body congruence after hormone therapy. Clinical variables alone were not able to explain body congruence using the body index ratings. These findings have implications for identifying those who will benefit more or less from hormone therapy. Furthermore, these results support our previous finding using anatomical metrics (Kilpatrick et al., 2019, Manzouri and Savic, 2019), and contribute to identifying the specific brain networks in GI, prior to therapy, whose connectivity patterns are critical with respect to hormone therapy effects.
4.1. Multivariate approach provides novel insights
The predictive model that we built and tested was able to explain 41% of the variance in body congruence subsequent to cross-sex hormone therapy when using pre-therapy patterns of resting-state functional connectivity. The predictive power of multivariate connectivity was substantiated by the overlapping results of LASSO and ridge machine learning algorithms, which converged to provide evidence that functional connectivity from cingulo-opercular and fronto-parietal networks can be used prior to initiation of hormone therapy to predict body congruence after hormone therapy. Exploiting multivariate techniques may provide additional insight into not only the neurobiological bases but also the sociological, cultural, and psychological bases of gender and body satisfaction. A recent study (Clemens et al., 2020) employed machine learning, based on functional connectivity, to predict self-reported gender identity in four groups (trans/cis, women/men). Including therapy duration in our analyses generates a model that assigned beta weights for each feature included in the model. Therefore, if a new person came into a clinic, entering a specific future time point after therapy initiation, e.g. 6 months, or 1 year, could provide an estimate of that person's body congruence at that future time point. The algorithm that did not include therapy duration was similarly predictive (Table S6).
4.2. Network connectivity predicts outcome of cross-sex hormone therapy
This work can be appreciated in the context of the evolving concept of using a functional connectome as a “fingerprint” that indexes highly individualized latent neural organization (Finn et al., 2015). This latent neural organization is linked to response tendencies, processing of stimuli, multisensory integration, and patterns of conscious and unconscious thinking (Finn et al., 2015). The most predictive networks in the current study, the fronto-parietal and cingulo-opercular networks, comprise important regions implicated in self-identity, self-referential thinking, as well as supporting top-down control of executive functioning (Herold et al., 2016, Seeley et al., 2007). While the fronto-parietal and cingulo-opercular networks are largely intraconnected and separable, they also appear to communicate, or perhaps compete, for control functions (Dosenbach et al., 2007). Others (Koush et al., 2019) found that the superior frontal gyrus (within the fronto-parietal network) modulates self-referential processes in the temporal parietal junction as well as affective valuation in ventromedial prefrontal cortex - which in turn is an important hub of the default mode network.
In the current study the fronto-parietal and cingulo-opercular networks were predictive of post-therapy, but not pre-therapy body congruence. One explanation is that these networks might be more involved in cognitive reorganization as a result of hormone therapy. In future studies, pre-therapy connectivity in these networks could be investigated as markers for potential brain network reorganization due to hormonal milieu changes. As these are cognitive control networks it might point to the directed control of self-referential thought processes with body self-awareness. This is potentially informative of the neurological underpinnings of gender identity in relation to body and hormonal status among transgender individuals as they transition.
The observation that these networks that significantly predicted post-therapy body congruence were not also associated with pre-therapy body congruence suggests that these networks may be more specifically involved in cognitive reorganization occurring with hormone therapy. One speculation, for example, is that this may identify those individuals whose multivariate connectivity pattern may index better or worse ability to bring their experience of their gender identity in line with the perception of their post-hormone bodies.
Connectivity before hormone therapy within the fronto-parietal and cingulo-opercular networks was most predictive of body congruence for short duration trials. It is not clear why ratings of short-duration trials were more predictive of body congruence than ratings of long-duration trials. One possibility is that the longer two-second trials allow rumination that interferes with the “truer” reflexive responses required by the half-second trials. Related to this, some of the ratings for long duration trials may have been influenced by individuals’ difficulty viewing the body images for longer times because of continued dysphoria triggered by viewing the images. This is in addition to longstanding patterns of avoidance of viewing their bodies, leading to ratings that may have been made in a cursory way and thereby not reflecting their true degree of congruence.
The observation that two of the seven networks that we had hypothesized a priori were significantly predictive is an indication of the specificity of these results and therefore a lower likelihood of overfitting. Other studies have also shown specificity of predictive networks, some conforming and some not conforming to a-priori hypotheses (Christov-Moore et al., 2020, Khosla et al., 2019, Lehmann et al., 2015, Seeley et al., 2007).
4.3. Body index ratings as a measure of body congruence
The body index has been used in other studies (Burke et al., 2018, Feusner et al., 2016, Kilpatrick et al., 2019, Majid et al., 2020) as a metric of body congruence. Another scale measuring body congruence is the self-report Transgender Congruence Scale (TCS) (Kozee et al., 2012). We did not have TCS scores for most of the participants in this study so did not include that metric in this analysis; however, we have examined TCS scores in two ongoing datasets and found trends for positive associations between TCS scores and the body index in transgender individuals (Supporting Information). This, in addition to significant changes in the body index pre- to post-therapy and an association between therapy duration and changes in body index (Fig. S3) lends support for the body index as a measure of therapy-sensitive body congruence.
4.4. Potential clinical implications
The aim of the current work was to investigate the therapeutic outcome of cross-sex hormone therapy for people diagnosed with gender incongruence. As some members of the transgender community seek medical help, and do suffer from gender incongruence, medical treatment is a significant need. Some patients can be helped with cross-sex hormone therapy, whereas for others this treatment shows poor clinical outcome. Therefore, prediction tools could support and inform medical professionals whether or not to treat individuals with cross-sex hormone therapy.
Consequently, such a prediction tool could reduce substantial harm in terms of physical and psychological impact of subjecting the patients to this time-intensive and slowly progressive treatment in a subgroup of patients who might receive little benefit from it. Hormone therapy is expensive and requires years of commitment in most cases. If these results are replicated in other larger samples, such an algorithm potentially could be used to identify individuals for whom it may be critical to apply other treatments in addition to hormones to optimize body congruence - such as gender-affirming surgeries. In addition, the algorithm possibly could identify those who may need alternate types or regimens of sex hormones, hormone blockers, or no sex hormones.
4.5. Limitations and future work
A limitation of the current study is the modest sample size, however see (Zandvakili et al., 2019, Zheng et al., 2017). The cross-validation approach of leaving out 20% of the participants for model testing reduced the likelihood of overfitting. Future studies with larger samples will be required to replicate these findings before applying this approach in practice. Larger datasets would provide the opportunity to split the participants into training and testing groups for a more robust validation. In addition, validation in fully independent test sets, ideally in different settings, would determine if the results may be generalizable to other populations with GI in different geographical locations and cultural and societal environments.
Further, our results are limited to binary-identifying individuals and thus cannot necessarily be generalized to nonbinary and differently gendered populations. Importantly, the use of more complex models that include other variables, for example minority stress, societal attitudes, a more nuanced characterization of gender identities, and sociological factors, would more comprehensively capture likely contributing factors to treatment outcomes, and might result in a better-performing algorithm. Due to sample size limitations we were not able to consider trans men and trans women separately in this study. Future work should do so, since a recent report (Majid et al., 2020) has shown that trans women generally had lower body index ratings than trans men for short duration trials and trans men rated images morphed opposite to their birth-assigned sex slightly higher than trans women. This is in line with other work (van de Grift et al., 2016a) that found trans men had a more positive body image than trans women. Another limitation is that the MINI diagnostic evaluation may have missed the presence of certain psychiatric conditions.
Future investigations of the mechanisms underlying the regions within the fronto-parietal and cingulo-opercular networks that drive the results seen here are warranted. In addition, while the current investigation adds to evidence that hormone therapy may enhance body congruence in GI, changes in gonadal steroid levels have been shown to affect mood and cognition (Epperson et al., 1999, Wierckx et al., 2013). Future larger studies could also allow for determination of relative weights of specific regions within the fronto-parietal and cingulo-opercular networks found to be predictive, including the dorsal and pregenual anterior cingulate regions that could represent important nodes involved in perception of self. In the future larger studies could explore cross-network interactions between these cognitive control networks and networks that include nodes involved in body perception such as the dorsal and ventral attention (overlaps with the temporoparietal junction), memory retrieval, salience (includes the anterior insula) and visual networks (includes the extrastriate body area).
5. Conclusion
This study illustrates the potential for predicting hormone therapy responsiveness in transgender individuals with GI. Results from the study could help us understand what pre-therapy brain networks may be involved in post-therapy body congruence and point to potential biomarkers that could be used to develop novel ways of improving body congruence.
Credit authorship contribution statement
Teena D Moody: Conceptualization, Methodology, Software, Validation, Writing - original draft, Writing - review & editing. Jamie D. Feusner: Conceptualization, Methodology, Writing - original draft, Writing - review & editing, Supervision. Nicco Reggente: Conceptualization, Methodology, Software, Validation, Writing - review & editing. Jojo Vanhoecke: Validation, Writing - review & editing. Mats Holmberg: Investigation, Writing - review & editing. Amirhossein Manzouri: Investigation. Behzad Sorouri Khorashad: Investigation. Ivanka Savic: Conceptualization, Methodology, Writing - review & editing, Supervision.
Acknowledgments
Acknowledgement
We thank Natalie Rotstein for assistance with data validation.
Funding
This work was supported by the Swedish Science Council (Dnr 200 7-3107 to I.S) and the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (1R01HD087712 to I.S. and J.F.). J.V. was supported by the Belgian American Educational Foundation and the Vocatio Foundation (Belgium).
Disclosure statement
The authors declare no competing interests.
Ethics
The Karolinska Institute ethical committee (Application # Dnr 2011/281-31/4) approved this study. All participants gave informed consent at the beginning of the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102517.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders: DSM-5, 5th ed. American Psychiatric Association, Washington, DC.
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc.: Ser. B (Methodol.) 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Blanke O., Mohr C., Michel C.M., Pascual-Leone A., Brugger P., Seeck M., Landis T., Thut G. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. J. Neurosci. 2005;25:550–557. doi: 10.1523/JNEUROSCI.2612-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouman W.P., Claes L., Brewin N., Crawford J.R., Millet N., Fernandez-Aranda F., Arcelus J. Transgender and anxiety: a comparative study between transgender people and the general population. Int. J. Transgenderism. 2017;18(1):16–26. doi: 10.1080/15532739.2016.1258352. [DOI] [Google Scholar]
- Bouman W.P., Schwend A.S., Motmans J., Smiley A., Safer J.D., Deutsch M.B., Adams N.J., Winter S. Language and trans health. Int. J. Transgenderism. 2017;18(1):1–6. doi: 10.1080/15532739.2016.1262127. [DOI] [Google Scholar]
- Burke, S.M., Manzouri, A.H., Dhejne, C., Bergström, K., Arver, S., Feusner, J.D., Savic-Berglund, I., 2018. Testosterone Effects on the Brain in Transgender Men. Cereb. Cortex 28, 1582–1596. DOI:10.1093/cercor/bhx054. [DOI] [PMC free article] [PubMed]
- Burke S.M., Majid D.S.A., Manzouri A.H., Moody T., Feusner J.D., Savic I. Sex differences in own and other body perception. Hum. Brain Mapp. 2019;40(2):474–488. doi: 10.1002/hbm.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov-Moore L., Reggente N., Douglas P.K., Feusner J.D., Iacoboni M. Predicting empathy from resting state brain connectivity: a multivariate approach. Front. Integr. Neurosci. 2020;14:3. doi: 10.3389/fnint.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens, B., Derntl, B., Smith, E., Junger, J., Neulen, J., Mingoia, G., Schneider, F., Abel, T., Bzdok, D., Habel, U., 2020. Predictive Pattern Classification Can Distinguish Gender Identity Subtypes from Behavior and Brain Imaging. Cereb. Cortex 30, 2755–2765. DOI:10.1093/cercor/bhz272. [DOI] [PubMed]
- Clements-Nolle K., Marx R., Katz M. Attempted suicide among transgender persons: the influence of gender-based discrimination and victimization. J. Homosex. 2006;51(3):53–69. doi: 10.1300/J082v51n03_04. [DOI] [PubMed] [Google Scholar]
- Cohen J. Routledge; 2013. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Cohen, L., Manion, L., Morrison, K., 2017. Statistical significance, effect size and statistical power. Research Methods in Education. DOI:10.4324/9781315456539-39.
- Cooper K., Russell A., Mandy W., Butler C. The phenomenology of gender dysphoria in adults: a systematic review and meta-synthesis. Clin. Psychol. Rev. 2020;80:101875. doi: 10.1016/j.cpr.2020.101875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. The sentient self. Brain Struct. Funct. 2010;214(5-6):563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Dhejne C., Van Vlerken R., Heylens G., Arcelus J. Mental health and gender dysphoria: a review of the literature. Int. Rev. Psychiatry. 2016;28(1):44–57. doi: 10.3109/09540261.2015.1115753. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A.T., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson C.N., Wisner K.L., Yamamoto B. Gonadal steroids in the treatment of mood disorders. Psychosom. Med. 1999;61(5):676–697. doi: 10.1097/00006842-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Feusner J.D., Dervisic J., Kosidou K., Dhejne C., Bookheimer S., Savic I. Female-to-male transsexual individuals demonstrate different own body identification. Arch. Sex Behav. 2016;45(3):525–536. doi: 10.1007/s10508-015-0596-z. [DOI] [PubMed] [Google Scholar]
- Feusner J.D., Lidström A., Moody T.D., Dhejne C., Bookheimer S.Y., Savic I. Intrinsic network connectivity and own body perception in gender dysphoria. Brain Imaging Behav. 2017;11(4):964–976. doi: 10.1007/s11682-016-9578-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S., Shen X., Scheinost D., Rosenberg M.D., Huang J., Chun M.M., Papademetris X., Constable R.T. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015;18(11):1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guss C.E., Woolverton G.A., Borus J., Austin S.B., Reisner S.L., Katz-Wise S.L. Transgender adolescents' experiences in primary care: a qualitative study. J. Adolesc. Health. 2019;65(3):344–349. doi: 10.1016/j.jadohealth.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold D., Spengler S., Sajonz B., Usnich T., Bermpohl F. Common and distinct networks for self-referential and social stimulus processing in the human brain. Brain Struct. Funct. 2016;221:3475–3485. doi: 10.1007/s00429-015-1113-9. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. Fsl. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Khosla M., Jamison K., Ngo G.H., Kuceyeski A., Sabuncu M.R. Machine learning in resting-state fMRI analysis. Magn. Reson. Imaging. 2019;64:101–121. doi: 10.1016/j.mri.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick L.A., Holmberg M., Manzouri A., Savic I. Cross sex hormone treatment is linked with a reversal of cerebral patterns associated with gender dysphoria to the baseline of cisgender controls. Eur. J. Neurosci. 2019;50:3269–3281. doi: 10.1111/ejn.14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey A.C., Pomeroy W.R., Martin C.E. Sexual behavior in the human male. Am. J. Public Health. 2003;93:894–898. doi: 10.2105/AJPH.93.6.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koush Y., Pichon S., Eickhoff S.B., Van De Ville D., Vuilleumier P., Scharnowski F. Brain networks for engaging oneself in positive-social emotion regulation. NeuroImage. 2019;189:106–115. doi: 10.1016/j.neuroimage.2018.12.049. [DOI] [PubMed] [Google Scholar]
- Kozee H.B., Tylka T.L., Bauerband L.A. Measuring transgender individuals’ comfort with gender identity and appearance: development and validation of the transgender congruence scale. Psychol. Women Q. 2012;36:179–196. doi: 10.1177/0361684312442161. [DOI] [Google Scholar]
- Lachenbruch P.A., Cohen J. Statistical power analysis for the behavioral sciences (2nd ed.) J. Am. Stat. Assoc. 1989;84:1096. doi: 10.2307/2290095. [DOI] [Google Scholar]
- Lehmann M., Madison C., Ghosh P.M., Miller Z.A., Greicius M.D., Kramer J.H., Coppola G., Miller B.L., Jagust W.J., Gorno-Tempini M.L., Seeley W.W., Rabinovici G.D. Loss of functional connectivity is greater outside the default mode network in nonfamilial early-onset Alzheimer's disease variants. Neurobiol. Aging. 2015;36:2678–2686. doi: 10.1016/j.neurobiolaging.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguen S., Shipherd J.C. Suicide risk among transgender individuals. Psychol. Sexuality. 2010;1:34–43. doi: 10.1080/19419891003634430. [DOI] [Google Scholar]
- Majid, D.S.A., Burke, S.M., Manzouri, A., Moody, T.D., Dhejne, C., Feusner, J.D., Savic, I., 2020. Neural Systems for Own-body Processing Align with Gender Identity Rather Than Birth-assigned Sex. Cereb. Cortex. DOI:10.1093/cercor/bhz282. [DOI] [PMC free article] [PubMed]
- Manzouri, A., Savic, I., 2019. Possible Neurobiological Underpinnings of Homosexuality and Gender Dysphoria. Cereb. Cortex 29, 2084–2101. DOI:10.1093/cercor/bhy090. [DOI] [PMC free article] [PubMed]
- Manzouri A., Kosidou K., Savic I. Anatomical and functional findings in female-to-male transsexuals: testing a new hypothesis. Cereb. Cortex. 2017;27:998–1010. doi: 10.1093/cercor/bhv278. [DOI] [PubMed] [Google Scholar]
- Marquardt D.W., Snee R.D. Ridge regression in practice. Am. Statistician. 1975;29:3–20. doi: 10.1080/00031305.1975.10479105. [DOI] [Google Scholar]
- Mueller S.C., De Cuypere G., T’Sjoen G. Transgender research in the 21st Century: a selective critical review from a neurocognitive perspective. AJP. 2017;174:1155–1162. doi: 10.1176/appi.ajp.2017.17060626. [DOI] [PubMed] [Google Scholar]
- Murad, M.H., Elamin, M.B., Garcia, M.Z., Mullan, R.J., Murad, A., Erwin, P.J., Montori, V.M., 2010. Hormonal therapy and sex reassignment: a systematic review and meta-analysis of quality of life and psychosocial outcomes. Clinical Endocrinology. DOI:10.1111/j.1365-2265.2009.03625.x. [DOI] [PubMed]
- Narang, P., Sarai, S.K., Aldrin, S., Lippmann, S., 2018. Suicide Among Transgender and Gender-Nonconforming People. Prim. Care Companion CNS Disord. 20. DOI:10.4088/PCC.18nr02273. [DOI] [PubMed]
- Nguyen H.B., Chavez A.M., Lipner E., Hantsoo L., Kornfield S.L., Davies R.D., Epperson C.N. Gender-affirming hormone use in transgender individuals: impact on behavioral health and cognition. Curr. Psychiatry Rep. 2018;20 doi: 10.1007/s11920-018-0973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili A., Glazebrook C., Arcelus J. Quality of life of treatment-seeking transgender adults: a systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2018;19:199–220. doi: 10.1007/s11154-018-9459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Power J., Cohen A., Nelson S., Wig G., Barnes K., Church J., Vogel A., Laumann T., Miezin F., Schlaggar B., Petersen S. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggente N., Moody T.D., Morfini F., Sheen C., Rissman J., O’Neill J., Feusner J.D. Multivariate resting-state functional connectivity predicts response to cognitive behavioral therapy in obsessive–compulsive disorder. Proc. Natl. Acad. Sci. U.S.A. 2018;115(9):2222–2227. doi: 10.1073/pnas.1716686115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan, D., Lecrubier, Y., 2010. Mini international neuropsychiatric interview version 6.0 (MINI 6.0)--The international standard (MINI 6.0).
- Smith Y.L.S., Van Goozen S.H.M., Kuiper A.J., Cohen-Kettenis P.T. Sex reassignment: outcomes and predictors of treatment for adolescent and adult transsexuals. Psychol. Med. 1999;35:89–99. doi: 10.1017/S0033291704002776. [DOI] [PubMed] [Google Scholar]
- Solomon C.G., Safer J.D., Tangpricha V. Care of transgender persons. N. Engl. J. Med. 2019;381:2451–2460. doi: 10.1056/NEJMcp1903650. [DOI] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. J. Roy. Stat. Soc.: Ser. B (Methodol.) 1996;58:267–288. doi: 10.1111/j.2517-6161.1996.tb02080.x. [DOI] [Google Scholar]
- Tibshirani R., Johnstone I., Hastie T., Efron B. Least angle regression. Ann. Statist. 2004;32(2):407–499. doi: 10.1214/009053604000000067. [DOI] [Google Scholar]
- Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Uribe C., Junque C., Gómez-Gil E., Abos A., Mueller S.C., Guillamon A. Brain network interactions in transgender individuals with gender incongruence. NeuroImage. 2020;211:116613. doi: 10.1016/j.neuroimage.2020.116613. [DOI] [PubMed] [Google Scholar]
- van de Grift T.C., Cohen-Kettenis P.T., Elaut E., De Cuypere G., Richter-Appelt H., Haraldsen I.R., Kreukels B.P.C. A network analysis of body satisfaction of people with gender dysphoria. Body Image. 2016;17:184–190. doi: 10.1016/j.bodyim.2016.04.002. [DOI] [PubMed] [Google Scholar]
- van de Grift T.C., Cohen-Kettenis P.T., Steensma T.D., De Cuypere G., Richter-Appelt H., Haraldsen I.R.H., Dikmans R.E.G., Cerwenka S.C., Kreukels B.P.C. Body satisfaction and physical appearance in gender dysphoria. Arch. Sex Behav. 2016;45(3):575–585. doi: 10.1007/s10508-015-0614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Grift T.C., Kreukels B.P.C., Elfering L., Özer M., Bouman M.-B., Buncamper M.E., Smit J.M., Mullender M.G. Body image in transmen: multidimensional measurement and the effects of mastectomy. J. Sex. Med. 2016;13:1778–1786. doi: 10.1016/j.jsxm.2016.09.003. [DOI] [PubMed] [Google Scholar]
- van de Grift T.C., Elaut E., Cerwenka S.C., Cohen-Kettenis P.T., De Cuypere G., Richter-Appelt H., Kreukels B.P.C. Effects of medical interventions on gender dysphoria and body image: a follow-up study. Psychosom. Med. 2017;79(7):815–823. doi: 10.1097/PSY.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Grift T.C., Elaut E., Cerwenka S.C., Cohen-Kettenis P.T., Kreukels B.P.C. Surgical satisfaction, quality of life, and their association after gender-affirming surgery: a follow-up study. J. Sex Marital Ther. 2018;44(2):138–148. doi: 10.1080/0092623X.2017.1326190. [DOI] [PubMed] [Google Scholar]
- Vocks S., Busch M., Grönemeyer D., Schulte D., Herpertz S., Suchan B. Differential neuronal responses to the self and others in the extrastriate body area and the fusiform body area. Cognitive, Affective, Behav. Neurosci. 2010;10:422–429. doi: 10.3758/CABN.10.3.422. [DOI] [PubMed] [Google Scholar]
- Wierckx, K., Elaut, E., Declercq, E., Heylens, G., De Cuypere, G., Taes, Y., Kaufman, J.M., T’Sjoen, G., 2013. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case–control study. European Journal of Endocrinology. DOI:10.1530/eje-13-0493. [DOI] [PubMed]
- Witcomb G.L., Bouman W.P., Claes L., Brewin N., Crawford J.R., Arcelus J. Levels of depression in transgender people and its predictors: results of a large matched control study with transgender people accessing clinical services. J. Affect. Disord. 2018;235:308–315. doi: 10.1016/j.jad.2018.02.051. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 1992. The ICD-10 classification of mental and behavioural disorders : clinical descriptions and diagnostic guidelines. World Health Organization.
- Yang M.-F., Manning D., van den Berg J.J., Operario D. Stigmatization and mental health in a diverse sample of transgender women. LGBT Health. 2015;2:306–312. doi: 10.1089/lgbt.2014.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandvakili A., Philip N.S., Jones S.R., Tyrka A.R., Greenberg B.D., Carpenter L.L. Use of machine learning in predicting clinical response to transcranial magnetic stimulation in comorbid posttraumatic stress disorder and major depression: a resting state electroencephalography study. J. Affect. Disord. 2019;252:47–54. doi: 10.1016/j.jad.2019.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z.S., Reggente N., Lutkenhoff E., Owen A.M., Monti M.M. Disentangling disorders of consciousness: Insights from diffusion tensor imaging and machine learning. Hum. Brain Mapp. 2017;38:431–443. doi: 10.1002/hbm.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.