Highlights
-
•
Low CD3+ TILs rate was associated with shorter OS in those with stage II colon cancer who did not receive adjuvant therapy.
-
•
CD3+ TILs rate was not prognostic for patients with stage II colon cancer who had adjuvant therapy.
-
•
Low CD3+ TILs rate may be an additional risk factor for stage II colon cancer patients who did not have adjuvant therapy yet.
Keywords: Colorectal cancer, Post-surgery treatment, Fluoropyrimidines, Prognostic marker, T-cells
Abstract
Background
High tumor infiltrating lymphocytes (TILs) density was previously shown to be associated with favorable prognosis for patients with colon cancer (CC). However, the impact of TILs on overall survival (OS) of stage II CC patients who received adjuvant chemotherapy (ADJ) or not (no-ADJ) is unknown. We assessed the prognostic value of CD3+ TILs in stage II CC patients according to whether they had ADJ or not.
Methods
Patients treated with curative surgery for stage II CC (2002–2013) were selected from the Santa Maria alle Scotte Hospital registry. TILs at the invasive front, center of tumor, and stroma were determined by immunohistochemistry and manually quantified as the rate of TILs/total tissue areas. High TILs (H-TILs) was defined as >20%. Patients were categorized as high or low TILs (L-TILs) and ADJ or no-ADJ.
Results
Of the 678 patients included, 137 (20%) received ADJ and 541 (80%) did not. The distribution of the 4 groups were: 16% (L-TIL/ADJ), 64% (L-TIL/no-ADJ), 5% (H-TIL/ADJ), 15% (H-TIL/no-ADJ). Compared to H-TILs/no-ADJ, ADJ patients showed a significantly increased OS (P<.01) regardless of the TILs rate whereas L-TILs/no-ADJ had significantly decreased OS and higher risk of death (HR=1.41; 95% CI, 1.06–1.88; P<.0001). On multivariable analysis, the unfavorable prognostic value of L-TILs (vs. H-TILs) for no-ADJ patients was confirmed (HR=1.36; 95% CI 1.02, 1.82; P=.0373).
Conclusion
Low CD3+ TILs rate was associated with shorter OS in those with stage II colon cancer who did not receive adjuvant therapy. Low CD3+ TILs could be considered an additional risk factor for still ADJ-untreated stage II CC patients, which could facilitate clinical decision making.
Introduction
At resection, 30–40% of all colon cancers (CC) are diagnosed as stage II disease per AJCC (American Join Committee on Cancer) [1,2]. Patients with stage II CC treated with curative surgery alone have a good prognosis, with a 5-year overall survival (OS) rate of 70–80%, and thus the usefulness of adjuvant chemotherapy (ADJ) in this setting is still controversial [2,3]. In the attempt to strike a balance between the small and yet significant OS benefit and the toxicity and costs associated with ADJ for stage II CC, the ASCO and ESMO international guidelines currently recommend its use only for patients with high risk of recurrence [[4], [5]–6]. Nevertheless, the commonly used risk stratification is still insufficient to accurately identify the population who would benefit more from ADJ [7]. In particular, although a wide range of clinicopathologic factors have been shown to be independently associated with the risk of post-surgery recurrence and survival, to date, there is no validated biomarker able to predict the efficacy of ADJ for patients with stage II CC [8]. Furthermore, the prognostic value of these factors was never investigated accounting for the use of ADJ. Analyses of risk factors including such categorization could elicit data that would indirectly inform us over the efficacy of ADJ in a population with a specific risk factor and thus facilitate clinical decision making.
Tumor infiltrating lymphocytes (TILs) were proven to play a key role in the host defense against colorectal cancer, among other malignancies, thereby influencing the disease progression and spread [9]. This observation suggested that an association between TILs and CC survival was plausible and may be an even better predictor of survival than the TNM classification [10]. In fact, several studies showed that high TILs density is a favorable prognostic factor for CC and a few of these analyses, albeit heterogenous in the methods of TILs evaluation and types of TILs and tumor area investigated, demonstrated TILs prognostic value for patients with stage II CC only [[11], [12]–14]. However, none of these reports assessed whether the impact of TILs on patients’ survival differs depending on the administration of ADJ or not (no-ADJ). In this retrospective analysis, we evaluated the prognostic value of CD3+ TILs for stage II CC patients according to whether they received ADJ or not.
Materials and methods
This study is written in compliance with the REMARK criteria [15] and is part of an extensive collaboration between University of Siena, Italy, and Mayo Clinic of Rochester (MN), USA, for the analysis of CC specimens with the aim of evaluating novel prognostic markers.
Patients
All patients treated with curative surgery for AJCC stage II CC between 2002 and 2013 were identified from the institutional review board approved registry of Santa Maria alle Scotte Hospital, University of Siena. Pathologic data comprising location of primary tumor, T category, differentiation grade, number and positivity of lymph nodes resected, perineural or lymphovascular invasion were collected from pathology and surgical reports. Clinical data at diagnosis (date of surgery) including age, sex, family history of colorectal cancer, occurrence of rupture or obstruction, as well as administration of ADJ and dates of disease recurrence, death, and last follow-up visit were extracted and reviewed from electronic and chart medical records. Exclusion criteria included use of neoadjuvant chemotherapy or chemoradiotherapy and suspicion of AJCC stage III or IV. All patients were followed up until death, last follow-up visit or May 2018, whichever occurred first. ADJ comprised fluoropyrimidine alone or with oxaliplatin, by physician's choice. Tumors were defined as right-sided if located within cecum and transversum and left-sided if resected in left colonic flexure, descending colon, or sigmoideum. Poorly differentiated (or high grade) tumors were defined as those with differentiation grade >2. Inadequacy of lymph node harvest was set as <12 lymph nodes resected. High risk of recurrence (high risk) was defined as presence of T4 tumor, lymphovascular invasion, perineural invasion, high grade histology, inadequate nodal harvest, and/or rupture or obstruction.
Methods
CD3+ TILs density was evaluated on hematoxylin and eosin (H-E) stained sections of formalin-fixed, paraffin-embedded tissue blocks selected by our experienced pathologist to represent the deepest invasive tumor margin. Serial 4-μm sections were cut from the selected tumor blocks and mounted on TOMO Adhesion Microscope Slides (Matsunami glass, USA). The slides were heated at 60 °C and deparaffinized in xylene and rehydrated through a graded series of alcohol (ethanol 70–100%). The tumor area presenting with greater content of mononuclear cells, including lymphocytes and excluding granulocytes and other polymorphonuclear leukocytes, plasma cells, and macrophages, was manually evaluated at high magnification (x40). TILs were identified at the invasive front, center of tumor, and stroma by the observer and confirmed by immunohistochemistry staining of CD3+ TILs (mucosa associated lymphoid tissue [MALT] present in the normal tissue of the tumor sections was used as positive/negative controls). For staining, the Ventana BenchMark ULTRA automated stainer (Ventana Roche diagnostics, Monza, Italy) was used. Antigen retrieval was performed using the CC1 pre-diluited cell conditioning solution (Ventana). The sections were washed twice in a wash buffer (Ventana) and then incubated with the primary antibodies at 37 °C. The primary antibody used was rabbit monoclonal anti-CD3 (2GV6, Ventana Roche, Monza, Italy) ready to use, incubated at 37 °C for 30 min. Sections were then counterstained with Hematoxylin II (Ventana). The bound antibodies were identified using Ultra View universal detection kit (Ventana) and visualized by DAB and chromogen. Each reaction was run with appropriate positive controls. Finally, the slides were cover-slipped with DAKO cover glass (DAKO, Glostrup, Denmark). Quantification of CD3+ TILs was performed by our pathologist manually evaluating in 5 independent microscopic fields (x40 objective) the average percentage of the area occupied by CD3+ TILs over the total tissue area analyzed (invasive front, center of tumor, and stroma). Tumor areas with crush artifacts, necrosis, or regressive hyalinization were excluded for accuracy purposes. TILs density was classified as high (H-TILs) or low (L-TILs) on the basis of a data driven cut-off value. H-TILs was defined as >20% and any lower rate as L-TILs.
Statistical analysis
Patients were categorized as H-TILs vs. L-TILs and ADJ vs. no-ADJ. The primary endpoint was OS which was defined as the time from date of curative surgery to date of death, last follow-up visit, or May 2018, whichever came first. The secondary endpoint was time to recurrence (TTR), defined as the time from date of curative surgery to date of pathologically and/or radiologically confirmed disease recurrence, whichever occurred first. Patients who died without disease recurrence were censored at death date. Baseline characteristics by frequency of TIL/ADJ groups were compared using the Kruskal–Wallis test and chi-square test for continuous and categorical variables, respectively. The Kaplan-Meier method was used to estimate the event rate at 7-years post curative surgery for time-to-event endpoints while log-rank test was used to examine potential differences between the TIL/ADJ groups. The Cox proportional hazards model was used to compare survival and recurrence estimates among the groups while using H-TILs/no-ADJ as the reference group. Covariates included in the multivariable Cox model are clinicopathological factors known to be associated with survival such as T4, inadequate nodal harvest, perineural invasion, lymphovascular invasion, poorly differentiated tumor, and tumor characteristics (obstruction, perforation, neither). All statistical tests were two-sided and significance was set as P-value ≤0.05. The statistical analysis was performed using the SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Of the 678 patients included in this analysis, 137 (20.2%) received ADJ and 541 (79.8%) did not. Median follow up was 8.5 years. The distributions of the 4 risk groups were as follows: 16% L-TIL/ADJ, 5% H-TIL/ADJ, 64% L-TIL/no-ADJ, 15% H-TIL/no-ADJ.
Patient characteristics
Patient clinical and pathologic characteristics are displayed in Table 1. Median age at time of curative surgery was 75 years (interquartile range, 67–81 years) and evenly distributed across the ADJ-treated cohorts, both with median age of 66 years, and no-ADJ treated cohorts, with median age of 77 and 75 years for L-TILs and H-TILs, respectively. All tumors except for one were adenocarcinomas. Males were slightly predominant (52.4%) and most patients (76.7%) did not report family history of colorectal cancer. T4 tumors were uncommon (6.2%) while poorly differentiated tumors occurred in almost half of the patients (47.2%). Inadequate nodal harvest was reported in 25.1% of patients. The majority of the patients (57.1%) did not have rupture or obstruction. The rate of tumors with lymphovascular invasion was minimal (3.7%) whereas perineural invasion was found in 52.5% of tumors. High risk of recurrence was highly prevalent overall (87.8%).
Table 1.
Patient characteristics.
| L-TILs/ADJ (n = 106) |
L-TILs /No-ADJ (n = 437) |
H-TILs/ADJ (n = 31) |
H-TILs/No-ADJ (n = 104) |
Total (n = 678) |
P-value | |
|---|---|---|---|---|---|---|
| Age at diagnosis, years | <0.00011 | |||||
| Median (IQR) | 66 (60–71) | 77 (71–83) | 66 (63–71) | 75 (67–81) | 75 (67–81) | |
| Range | 31–86 | 30–98 | 30–79 | 32–100 | 30–100 | |
| Sex, n (%) | 0.602 | |||||
| Female | 56 (52.8) | 203 (46.5) | 13 (41.9) | 51 (49.0) | 323 (47.6) | |
| Male | 50 (47.2) | 234 (53.5) | 18 (58.1) | 53 (51.0) | 355 (52.4) | |
| Family History CRC, n (%) | 0.0042 | |||||
| No | 61 (66.3) | 326 (77.4) | 19 (65.5) | 85 (86.7) | 491 (76.7) | |
| Yes | 31 (33.7) | 95 (22.6) | 10 (34.5) | 13 (13.3) | 149 (23.3) | |
| Missing | 14 | 16 | 2 | 6 | 38 | |
| T4 Tumor, n (%) | 0.132 | |||||
| No | 99 (93.4) | 413 (94.5) | 26 (83.9) | 98 (94.2) | 636 (93.8) | |
| Yes | 7 (6.6) | 24 (5.5) | 5 (16.1) | 6 (5.8) | 42 (6.2) | |
| Grade, n (%) | 0.232 | |||||
| 2 | 54 (50.9) | 226 (51.7) | 14 (45.2) | 64 (61.5) | 358 (52.8) | |
| 3 | 52 (49.1) | 211 (48.3) | 17 (54.8) | 40 (38.5) | 320 (47.2) | |
| Poorly differentiated (high grade), n (%) | 0.232 | |||||
| No | 54 (50.9) | 226 (51.7) | 14 (45.2) | 64 (61.5) | 358 (52.8) | |
| Yes | 52 (49.1) | 211 (48.3) | 17 (54.8) | 40 (38.5) | 320 (47.2) | |
| Nodes Examined | 0.0141 | |||||
| Median (IQR) | 18 (12–24) | 16 (11–22) | 22 (13–30) | 16 (11–23) | 16 (11–23) | |
| Range | 4–63 | 1–60 | 7–47 | 3–55 | 1–63 | |
| Inadequate nodal harvest, n (%) | 0.092 | |||||
| No | 81 (76.4) | 321 (73.5) | 29 (93.5) | 77 (74.0) | 508 (74.9) | |
| Yes | 25 (23.6) | 116 (26.5) | 2 (6.5) | 27 (26.0) | 170 (25.1) | |
| Sidedness, n (%) | 0.932 | |||||
| Right/Transverse | 60 (56.6) | 244 (55.8) | 18 (58.1) | 55 (52.9) | 377 (55.6) | |
| Left | 46 (43.4) | 193 (44.2) | 13 (41.9) | 49 (47.1) | 301 (44.4) | |
| Tumor characteristics, n (%) | 0.432 | |||||
| Neither | 58 (54.7) | 256 (58.6) | 19 (61.3) | 54 (51.9) | 387 (57.1) | |
| Rupture | 2 (1.9) | 12 (2.7) | 2 (6.5) | 6 (5.8) | 22 (3.2) | |
| Obstruction | 46 (43.4) | 169 (38.7) | 10 (32.3) | 44 (42.3) | 269 (39.7) | |
| High risk of recurrence, n (%) | 0.262 | |||||
| No | 14 (13.2) | 49 (11.2) | 2 (6.5) | 18 (17.3) | 83 (12.2) | |
| Yes | 92 (86.8) | 388 (88.8) | 29 (93.5) | 86 (82.7) | 595 (87.8) | |
| Lymphovascular invasion, n (%) | 0.632 | |||||
| No | 104 (98.1) | 418 (95.7) | 30 (96.8) | 101 (97.1) | 653 (96.3) | |
| Yes | 2 (1.9) | 19 (4.3) | 1 (3.2) | 3 (2.9) | 25 (3.7) | |
| Perineural invasion, n (%) | 0.0072 | |||||
| No | 45 (42.5) | 195 (44.6) | 20 (64.5) | 62 (59.6) | 322 (47.5) | |
| Yes | 61 (57.5) | 242 (55.4) | 11 (35.5) | 42 (40.4) | 356 (52.5) | |
| Adjuvant chemotherapy, n (%) | <0.00012 | |||||
| No | 0 (0.0) | 437 (100.0) | 0 (0.0) | 104 (100.0) | 541 (79.8) | |
| Yes | 106 (100.0) | 0 (0.0) | 31 (100.0) | 0 (0.0) | 137 (20.2) |
Abbreviations: ADJ, adjuvant chemotherapy; CRC, colorectal cancer; H-TILs, high tumor infiltrating lymphocytes density; L-TILs, low tumor infiltrating lymphocytes density; no-ADJ, no adjuvant chemotherapy.
1Kruskal–Wallis p-value; 2Chi-Square p-value.
OS and TTR
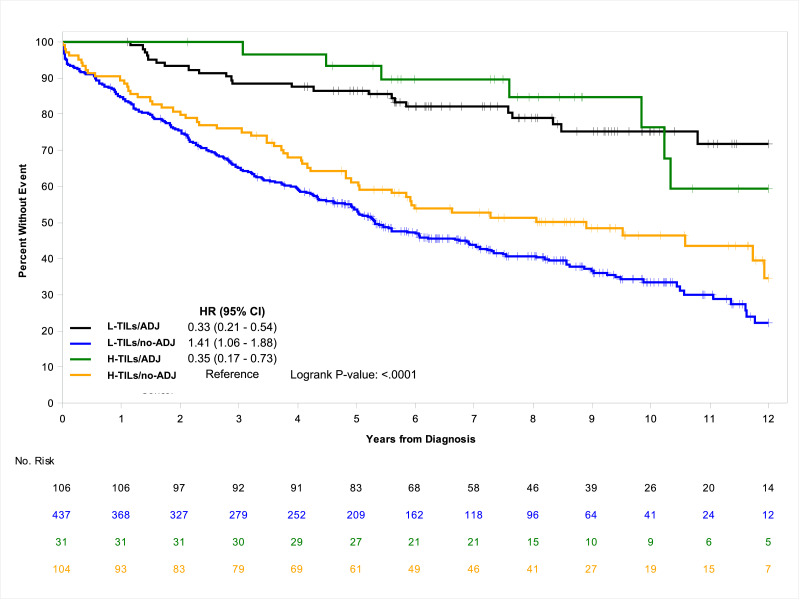
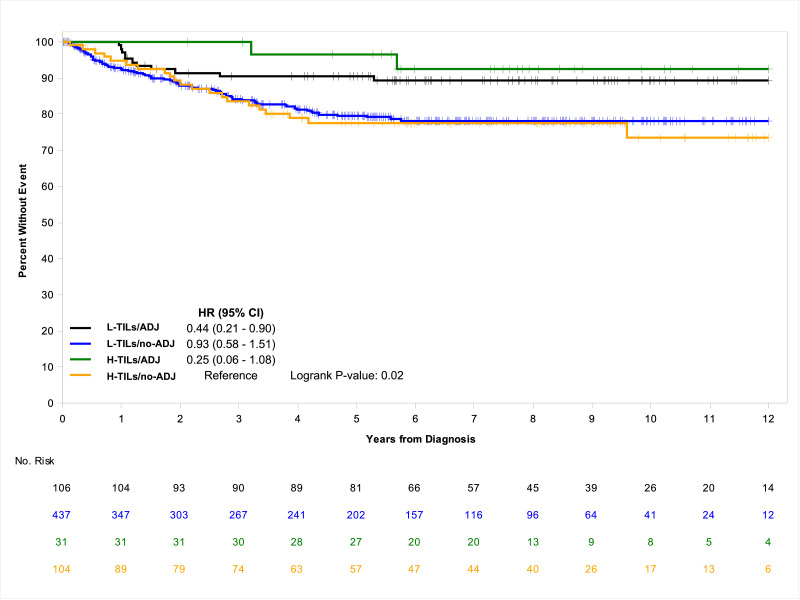
Total number of deaths was 356 (52.5%); 32 (23.4%) among those treated with ADJ and 324 (59.9%) among those untreated. In univariate analysis, compared to H-TILs/no-ADJ, the ADJ- treated population had a significantly improved median OS and a risk of death approximately reduced by two-thirds regardless of the TILs rate (HR=0.35; 95% CI, 0.17–0.73 for H-TILs/ADJ; HR=0.33; 95% CI, 0.21–0.54 for L-TILs/ADJ; P<.0001), whereas L-TILs/no-ADJ group had a significantly worsened median OS (5.3 years; 95% CI, 4.9–6.9) and higher risk of death (HR=1.41; 95% CI, 1.06–1.88; P<.0001) (Table 2). Similarly, the 7-year OS rate was lowest at 43.8% (95% CI, 39.2–49.0) for L-TILs/no-ADJ cohort, intermediate at 52.6% (95% CI, 43.7–63.4) for H-TILs/no-ADJ group and highest at 89.7% (95% CI, 79.4–100.0) and 82.0% (95% CI, 74.8–89.9) for H-TILs/ADJ and L-TILs/ADJ cohorts, respectively. The Kaplan-Meier curves further demonstrate the better prognostic outcome of the ADJ-treated patients, irrespective of the TILs density, and the worse prognostic value of L-TILs/no-ADJ, compared to H-TILs/no-ADJ (Fig. 1). During the follow-up period, 159 (23.5%) patients recurred and 146 of them (91.8%) did not receive ADJ. Median TTR was not reached in any risk group at the time of data cut-off. Compared to H-TILs/no-ADJ, both H-TILs/ADJ and L-TILs/ADJ cohorts had a significantly lower risk of recurrence (HR=0.25; 95% CI, 0.06–1.08 and HR=0.44; 95% CI, 0.21–0.90, respectively; P=.02) while that of L-TILs/no-ADJ, albeit significantly decreased, was only barely so (HR=0.93; 95% CI, 0.58–1.51). In a similar fashion, H-TILs/ADJ and L-TILs/ADJ populations showed greater 7-year TTR rates, at 92.5% (95% CI, 83.0–100.0) and 89.2% (95% CI, 83.4–95.5), respectively, compared to H-TILs/no-ADJ and L-TILs/no-ADJ groups that had comparable lower rates (77.6%; 95% CI, 69.4–86.8 and 78.2%; 95% CI, 73.9–82.8, respectively). The Kaplan-Meier estimates further highlight the improved TTR of the ADJ-treated population compared to that of the untreated, regardless of the TILs density (Fig. 2). In multivariable analysis, consistently with the results of the univariate model, the impact of L-TILs in patients without ADJ was confirmed independently associated with worse OS and TTR, along with tumor rupture, T4 tumor, and inadequate nodal harvest. Also poorly differentiated tumor and perineural invasion are associated with shorter OS but not TTR, while tumor obstruction was associated with shorter TTR but not OS (Table 3).
Table 2.
Overall survival and time to recurrence according to TILs rate and use of ADJ.
| Groups | Patients, n (Events, n) | Median OS, years (95% CI) | 7-year OS rate,% (95% CI) | HR (95% CI) # | P-value * |
|---|---|---|---|---|---|
| L-TILs/ADJ | 106 (24) | NE (13.0-NE) | 82.0 (74.8–89.9) | 0.33 (0.21–0.54) | <0.0001 |
| H-TILs/ADJ | 31 (8) | 13.3 (10.2-NE) | 89.7 (79.4–100.0) | 0.35 (0.17–0.73) | |
| L-TILs/no-ADJ | 437 (267) | 5.3 (4.9–6.9) | 43.8 (39.2–49.0) | 1.41 (1.06–1.88) | |
| H-TILs/no-ADJ | 104 (57) | 8.9 (5.6-NE) | 52.6 (43.7–63.4) | (Reference) | |
| Groups | Patients, n (Events, n) | Median TTR, years (95% CI) | 7-year TTR rate,% (95% CI) | HR (95% CI) # | P-value * |
|---|---|---|---|---|---|
| L-TILs/ADJ | 106 (11) | NE (NE-NE) | 89.2 (83.4–95.5) | 0.44 (0.21–0.90) | 0.02 |
| H-TILs/ADJ | 31 (2) | NE (NE-NE) | 92.5 (83.0–100.0) | 0.25 (0.06–1.08) | |
| L-TILs/no-ADJ | 437 (75) | NE (NE-NE) | 78.2 (73.9–82.8) | 0.93 (0.58–1.51) | |
| H-TILs/no-ADJ | 104 (71) | NE (NE-NE) | 77.6 (69.4–86.8) | (Reference) |
Abbreviations: ADJ, adjuvant chemotherapy; HR, hazard ratio; H-TILs, high tumor infiltrating lymphocytes density; L-TILs, low tumor infiltrating lymphocytes density; NE, not-estimated; no-ADJ, no adjuvant chemotherapy; OS, overall survival; TTR, time to recurrence.
* Log-rank test; # Unadjusted hazard ratio.
Fig. 1.
Associations of OS according to TILs rate and use of ADJ.
Abbreviations: ADJ, adjuvant chemotherapy; H-TILs, high tumor infiltrating lymphocytes density; l-TILs, low tumor infiltrating lymphocytes density; No, number; no-ADJ, no adjuvant chemotherapy; OS, overall survival.
Fig. 2.
Associations of TTR according to TILs rate and use of ADJ.
Abbreviations: ADJ, adjuvant chemotherapy; H-TILs, high tumor infiltrating lymphocytes density; l-TILs, low tumor infiltrating lymphocytes density; No, number; no-ADJ, no adjuvant chemotherapy; TTR, time to recurrence.
Table 3.
Multivariable analysis of OS and TTR using H-TILs/no-ADJ as reference.
| OS, HR (95% CI) | Pr > ChiSq # | P-value * | TTR, HR (95% CI) | Pr > ChiSq # | P-value * | |
|---|---|---|---|---|---|---|
| H-TILs/ADJ ^ | 0.32 (0.15–0.68) | 0.0030 | <0.0001 | 0.25 (0.06, 1.07) | 0.0618 | 0.0063 |
| L-TILs/ADJ ^ | 0.30 (0.19–0.50) | <0.0001 | 0.44 (0.21, 0.93) | 0.0310 | ||
| L-TILs/no-ADJ ^ | 1.36 (1.02–1.82) | 0.0373 | 0.99 (0.61, 1.61) | 0.9665 | ||
| Tumor Characteristics Rupture | 1.73 (1.00–3.00) | 0.0498 | 0.1095 | 3.44 (1.55, 7.61) | 0.0023 | 0.0075 |
| Tumor Characteristics Obstruction | 1.15 (0.93–1.43) | 0.1989 | 1.51 (1.01, 2.24) | 0.0422 | ||
| T4 Tumor | 1.96 (1.28–2.99) | 0.0018 | 2.53 (1.31, 4.90) | 0.0056 | ||
| Poorly differentiated (high grade) | 1.31 (1.05–1.62) | 0.0149 | 1.23 (0.84, 1.82) | 0.2906 | ||
| Perineural Invasion | 1.28 (1.02–1.61) | 0.0337 | 0.89 (0.59, 1.35) | 0.5915 | ||
| Lymphovascular Invasion | 0.83 (0.47–1.46) | 0.5211 | 1.79 (0.81, 3.95) | 0.1494 | ||
| Inadequate nodal harvest | 1.57 (1.25–1.98) | 0.0001 | 1.85 (1.23, 2.77) | 0.0030 |
Abbreviations: ADJ, adjuvant chemotherapy; HR, hazard ratio; H-TILs, high tumor infiltrating lymphocytes density; l-TILs, low tumor infiltrating lymphocytes density; no-ADJ, no adjuvant chemotherapy; OS, overall survival; TTR, time to recurrence.
Models were adjusted for: T4, inadequate nodal harvest, perineural invasion, lymphovascular invasion, poorly differentiated tumor, obstruction, rupture, and adjuvant chemotherapy, when applicable.
^ Reference group is H-TILs/no-ADJ; # P-value for each level compares to the reference; * P-value for difference across levels.
Discussion
The density of TILs in the tumor tissue has been demonstrated to be a reliable proxy of the immune defense of the host against colorectal cancer [9]. Although TILs rate was proven to be associated with survival in previous studies of colorectal cancer [11–14], the present population-based single institution study is the first to investigate the prognostic value of CD3+ TILs for stage II CC according to the use of ADJ. In our retrospective analysis, we demonstrated that L-TILs in the stroma, center of tumor, and invasive margin is an independent negative prognostic factor for those who did not receive ADJ. Several studies reported an impact of CD3+ TILs on survival of patients with colorectal cancer [16,17]. However, in these reports, patients with stage II CC were only a subgroup and CD3+ TILs were assessed at the tumor invasive front only. Conversely, in our study, CD3+ lymphocytes were quantified in the invasive margin, center of tumor, and stroma; the latter is an area typically rich of T-cells and thus should probably not be excluded from the evaluation. Our findings are also consistent with those of two retrospective analyses of exclusively stage II CC that documented the prognostic value of CD3+ TILs rate [13,14]. However, as opposed to our study, both these analyses were conducted on patients treated with curative surgery only. The decision to offer ADJ to patients with stage II CC is often difficult as it entails weighing up the risks of toxicity against the potential survival improvement. The risk factors currently used to select patients with stage II CC more at risk of recurrence, although associated with worse outcomes, are not predictive of improved efficacy of ADJ [7]. Given the lack of validated predictive biomarkers for ADJ in this setting, the discovery of biological or clinicopathological factors able to predict survival of patients treated with ADJ or not would be very informative and could facilitating clinical decision making. In this respect, the categorization of our population according to the administration of ADJ allowed us to show that TILs density is a valid prognostic biomarker only for those who did not receive ADJ, while patients receiving ADJ fared better regardless of the TILs rate. Therefore, L-TILs could be considered an additional risk factor that physicians should take into account when deciding whether to administer ADJ or not to a patient with stage II CC. Moreover, both the aforementioned studies used an automatic image analysis software to quantify TILs. Although computerized imaging programs are typically considered more accurate and error-free than the observer, they actually tend to be less efficient at counting TILs as the identification process hinges on the changes of the depth of color, disregarding shape, features, or location [18]. Extending the range of the color depth, the count of TILs could be overestimated since also non-inflammatory cells could be selected. Vice versa, decreasing the range of the color depth, TILs are likely to be underestimated as minor color variations exist even within each lymphocyte. As such, using automatic image analysis systems for TILs assessment may result in inaccuracies. In the present study, TILs were manually evaluated by a single pathologist. Of note, the reproducibility of the observers’ manual count of TILs and the interobserver agreement of TILs measurement were demonstrated in breast cancer and advocated by the international TILS working group [19]. Additionally, in the analysis by Lee et al., TILs density was assessed according to the tumor area (stromal or intraepithelial) [13]. In contrast, in our study T-cells were quantified in intratumoral and stromal areas as a whole. This approach is probably more balanced since we observed that TILs are typically more numerous in the stroma, as previously reported [13,14]. Furthermore, their analysis had a small sample size (n = 87) and CD3+ TILs was not showed independently associated with survival. Our study comprised a large population and the prognostic value of CD3+ TILs was demonstrated independent of T4, inadequate nodal harvest, perineural invasion, lymphovascular invasion, poorly differentiated tumor, obstruction, and rupture. Finally, in the report by Eriksen et al., TILs were classified by CD3+ or CD8+ [14]. Although the correlation between subtypes of TILs and prognosis of colorectal cancer has been documented in multiple analyses, we did not subtype TILs in our study [9,14]. As CD3 is a pan-T antigen, we used CD3+ staining only to facilitate the observer's manual determination of all T-cells in the tumoral and stromal areas. In this regard, it should be noted that the International TILs Working Group currently does not recommend the detection of specific subpopulations of TILs and suggests identification of all T-lymphocytes on H-E stained sections [20].
The better prognosis associated with a high rate of TILs for the no-ADJ population documented in our analysis could suggest that differences in TILs density can directly affect the patient's clinical outcomes. A plausible biological rationale is that the activated cytotoxic lymphocytes infiltrating the tumor may destroy cancer cells by releasing cytotoxic proteins such as perforin and granzymes or by a Fas/FasL-mediated mechanism [21]. Nonetheless, patients treated with ADJ showed better median OS and risk of recurrence irrespective of the TILs rate. In fact, L-TILs/no-ADJ cohort showed an approximately halved survival and 7-year OS rate, compared to the cohorts treated with ADJ. As expected of a population with stage II CC, only less than 1/4 (23.5%) of patients recurred within the follow-up period. As such, none of the risk groups reached median TTR and most patients did not recur within 7 years from diagnosis, no matter the risk group. However, the benefit of ADJ for our population was confirmed since 91.8% of relapsing patients had not received ADJ and rates of 7-year OS were higher for those who had ADJ. Nonetheless, it is noteworthy that the median age of the cohorts treated with ADJ was much younger (66 years) compared to that of the no-ADJ cohorts (74.5 years and 77 years for H-TILs and L-TILs, respectively). Age is a recognized prognostic factor and could have partly affected the survival differences. Besides, despite the lack of a proven robust correlation between risk factors and ADJ efficacy, it is interesting to note that the proportion of patients with one or more poor prognostic features was remarkably high (87.8%).
The present analysis documented that CD3+ L-TILs is independently associated with shorter survival and TTR for those with stage II CC not treated with ADJ. However, this conclusion should be cautiously interpreted due to certain study limitations. The chief one is the retrospective, single institution design, for which cohorts with ADJ vs. no-ADJ were numerically unbalanced and the cause of death of patients could not be safely ascertained to be related to the primary cancer. Also, physician's patient selection for ADJ may have been affected by confounding clinical factors which may have biased the results. Furthermore, the microsatellite instability (MSI) status was not investigated. MSI status is known to be strongly associated with prognosis. In particular, early CC patients with MSI tumors were shown to have increased survival [22]. However, it should be noted that studies investigating potential OS interactions between MSI status and CD3+ T-cells density were so far inconclusive [14,23]. Namely, Dahlin et al. observed no significant survival difference between patients with tumor MSI or microsatellite stability and similar CD3+ TILs density [23]. Although large-scale randomized clinical trials are warranted to validate our results, they suggest that evaluation of T-lymphocyte density could aid identification of high-risk patients with stage II CC for whom ADJ may be most beneficial. Because H-E stained sections are easy to make, immunohistochemistry is relatively quick and cost-effective to perform, and the experienced observer's T-cell count was shown reliable and reproducible, we recommend considering this method of TILs assessment in the clinicopathological routine to potentially determine an additional risk factor which could aid the physician in his decision of whether administering ADJ or not for early stage CC.
Conclusion
For the first time, the prognostic value of T-lymphocytes density was investigated in stage II CC patients according to the use of adjuvant chemotherapy. Low stromal and intraepithelial CD3+ TILs density showed an unfavorable impact on prognosis of patients with stage II CC who did not receive adjuvant chemotherapy. Despite the need of a validation in larger randomized trials, these results suggest that low CD3+ TILs rate could be an additional risk factor that may help identifying the best candidate for adjuvant chemotherapy.
CRediT authorship contribution statement
Edoardo Francini: Writing - original draft, Conceptualization, Data curation, Project administration. Fang-Shu Ou: Formal analysis, Conceptualization, Data curation, Writing - review & editing, Project administration. Stefano Lazzi: Conceptualization, Methodology, Data curation, Writing - review & editing. Roberto Petrioli: Conceptualization, Supervision, Writing - review & editing. Andrea G. Multari: Data curation, Project administration. Guido Pesola: Data curation, Project administration. Luciana Messuti: Data curation. Elena Colombo: Data curation. Virginia Livellara: Data curation. Serena Bazzurri: Data curation. Sara Cherri: Data curation. Salvatora T. Miano: Data curation, Project administration. Eric G. Wolfe: Formal analysis. Steven R. Alberts: Supervision, Writing - review & editing. Joleen M. Hubbard: Methodology, Supervision, Writing - review & editing. Harry H. Yoon: Conceptualization, Methodology, Supervision, Writing - review & editing. Guido Francini: Conceptualization, Methodology, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Ethics approval
The study was approved by the Santa Maria alle Scotte Hospital, University of Siena review board and all patients were de-identified.
Data availability
The database is available on demand.
Funding information
The authors declare no funding.
References
- 1.Jemal A., Tiwari R.C., Murray T., American Cancer Society Cancer statistics. CA Cancer J. Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Morris M., Platell C., McCaul K., Millward M., van Hazel G., Bayliss E. Survival rates for stage II colon cancer patients treated with or without chemotherapy in a population-based setting. Int. J. Colorectal Dis. 2007;22:887–895. doi: 10.1007/s00384-006-0262-y. [DOI] [PubMed] [Google Scholar]
- 3.Kannarkatt J., Joseph J., Kurniali P.C., Al-Janadi A., Hrinczenko B. Adjuvant chemotherapy for stage II colon cancer: a clinical dilemma. J. Oncol. Pract. 2017;13:233–241. doi: 10.1200/JOP.2016.017210. [DOI] [PubMed] [Google Scholar]
- 4.Sargent D., Sobrero A., Grothey A., O'Connell M.J., Buyse M., Andre T. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J. Clin. Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labianca R., Nordlinger B., Beretta G.D., Mosconi S., Mandalà M., Cervantes A. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013;24:64–72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 6.Benson A.B., III, Schrag D., Somerfield M.R., Cohen A.M., Figueredo A.T., Flynn P.J. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J. Clin. Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor E.S., Greenblatt D.Y., LoConte N.K., Gangnon R.E., Liou J., Heise C.P. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J. Clin. Oncol. 2011;29:3381–3388. doi: 10.1200/JCO.2010.34.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J.J., Chu E. Adjuvant chemotherapy for stage II colon cancer: the debate goes on. J. Oncol. Pract. 2017;13:245–246. doi: 10.1200/JOP.2017.022178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canna K., McArdle P., McMillan D., McNicol A., Smith G.W., McKee R.F. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br. J. Cancer. 2005;92:651–654. doi: 10.1038/sj.bjc.6602419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galon J., Pages F., Marincola F.M., Thurin M., Trinchieri G., Fox B.A. The immune score as a new possible approach for the classification of cancer. J. Transl. Med. 2012;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pagès F., Kirilovsky A., Mlecnik B., Asslaber M., Tosolini M., Bindea G. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J. Clin. Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 12.Yoonjin K., Jiwon K., Duck-Woo K., Kang S., Kim W.H., Lee H.S. Immunoscore encompassing CD3+ and CD8+ T cell densities in distant metastasis is a robust prognostic marker for advanced colorectal cancer. Oncotarget. 2016;7:81778–81790. doi: 10.18632/oncotarget.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee W.S., Park S., Lee W.Y., Chun H.K. Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer. 2010;116:5188–5199. doi: 10.1002/cncr.25293. [DOI] [PubMed] [Google Scholar]
- 14.Eriksen A.C., Flemming B.S., Lindebjerg J., Hager H., dePont Christensen R., Kjær-Frifeldt S. The prognostic value of tumor- infiltrating lymphocytes in stage II colon cancer. A nationwide population-based study. Transl. Oncol. 2018;11:979–987. doi: 10.1016/j.tranon.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McShane L.M., Altman D.G., Sauerbrei W., Taube S.E., Gion M., Clark G.M. REporting recommendations for tumour MARKer prognostic studies (REMARK) Br. J. Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laghi L., Bianchi P., Miranda E., Balladore E., Pacetti V., Grizzi F. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 17.Iseki Y., Shibutani M., Maeda K., Nagahara H., Fukuoka T., Shinji Matsutani S. A new method for evaluating tumor-infiltrating lymphocytes (TILs) in colorectal cancer using hematoxylin and eosin (H-E)-stained tumor sections. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng F., Miu D., Meng X., Kong L., Hui Zhu H., Liu S. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am. J. Cancer Res. 2015;5:2064–2074. [PMC free article] [PubMed] [Google Scholar]
- 19.Swisher S.K., Wu Y., Castaneda C.A., Lyons G.R., Yang F., Tapia C. Interobserver agreement between pathologists assessing tumor-infiltrating lymphocytes (TILs) in breast cancer using methodology proposed by the international TILs working group. Ann. Surg. Oncol. 2016;23:2242–2248. doi: 10.1245/s10434-016-5173-8. [DOI] [PubMed] [Google Scholar]
- 20.Salgado R., Denkert C., Demaria S., Sirtaine N., Klauschen F., Pruneri G., International TILs Working Group 2014 The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberg R.A. 2nd ed. Garland Science; New York: 2014. The Biology of Cancer; pp. 641–689. [Google Scholar]
- 22.Merok M.A., Ahlquist T., Royrvik E.C., Tufteland K.F., Hektoen M., Sjo O.H. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann. Oncol. 2013;24:1274–1282. doi: 10.1093/annonc/mds614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahlin A.M., Henriksson M.L., Van Guelpen B., Stenling R., Oberg A., Rutegård J. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod. Pathol. 2011;24:671–682. doi: 10.1038/modpathol.2010.234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The database is available on demand.