Highlights
-
•
High-dose RT upregulated pDCs within the tumor microenvironment.
-
•
The administration of intratumoral TLR9 agonist (CMP-001) after stereotactic RT significantly enhanced the anti-tumor immune response both locally and at secondary tumor site.
-
•
CMP-001 Post-RT delayed the abscopal tumor growth and extended the survival rate via increasing the percentages of activated CD4+ and CD8+ T-cells within the tumor microenvironment.
-
•
The treatment proved efficacious in both lung adenocarcinoma and colon carcinoma syngeneic models used.
Keywords: Cancer, Radiotherapy, Abscopal effect, TLR9 agonist, CMP-001
Abstract
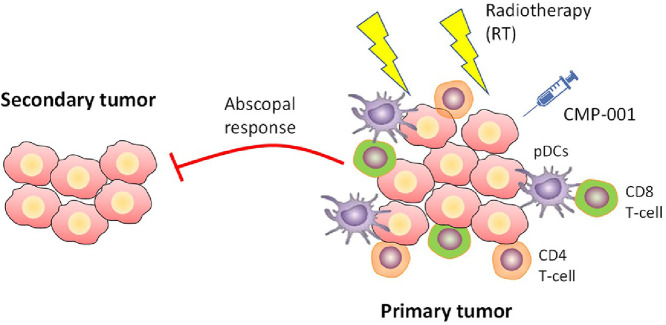
Radiotherapy (RT) has been used to control tumors by physically damaging DNA and inducing apoptosis; it also promotes antitumor immune responses via neoantigens release and augmenting immune-oncology agents to elicit systemic response. Tumor regression after RT can recruit inflammatory cells, such as tumor-associated macrophages and CD11b+ myeloid cell populations, a major subset of which may actually be immunosuppressive. However, these inflammatory cells also express Toll-like receptors (TLRs) that can be stimulated to reverse suppressive characteristics and promote systemic antitumor outcomes. Here, we investigated the effects of adding CMP-001, a CpG-A oligodeoxynucleotide TLR9 agonist delivered in a virus-like particle (VLP), to RT in two murine models (344SQ metastatic lung adenocarcinoma and CT26 colon carcinoma). High-dose RT (12Gy x 3 fractions) significantly increased the percentages of plasmacytoid dendritic cells within the tumor islets 3- and 5-days post-RT; adding CMP-001 after RT also enhanced adaptive immunity by increasing the proportion of CD4+ and CD8+ T cells. RT plus CMP-001-mediated activation of the immune system led to significant inhibition of tumor growth at both primary and abscopal tumor sites, thereby suggesting a new combinatorial treatment strategy for systemic disease.
Graphical Abstract
1. Introduction
Radiotherapy (RT) is an effective primary treatment modality in about 50% of patients with cancer [1]. However, various neoplasms do not respond to radiation, in part owing to the influence of the tumor stroma [2,3] and the upregulation of regulatory T cells (Tregs) [4] that inhibit antitumor immune responses [5]. High doses of radiation also increase myeloid-derived suppressor cells (MDSCs) and polarize macrophages to the M2 (anti-inflammatory) phenotype in the tumor microenvironment [6]. Another determinant of the radio-sensitivity of particular tumor cells is their susceptibility to radiation-induced apoptosis [7]. We previously showed that combining certain therapeutic agents with radiation, such as Treg-targeting antibodies [8] or agents that repolarize macrophages to the M1 (pro-inflammatory) phenotype, could abolish RT-induced immunosuppressive effects and enhance systemic antitumor immune responses [9].
Another mechanism whereby RT can paradoxically enhance tumor growth and invasion is through the recruitment of inflammatory cells (e.g., tumor-associated macrophages and CD11b+ myeloid cells) by tumors regressing following RT [10]. These cells express TLRs and can be taken advantage of by using endogenous or exogenous ligands to activate them, and therefore revert the inhibitory tumor microenvironment and limit tumor growth. TLRs have been generally characterized into subtypes that recognize extracellular pathogens (TLR1/2, TLR4, TLR5) and TLRs that recognize nucleic acids from cells or viruses (TLR3, TLR7, TLR8, and TLR9) [11]. TLRs can be activated by damage-associated molecular patterns (DAMPs), including intracellular molecules released from necrotic cells [12], or by pathogen-associated molecular patterns (PAMPs), including lipopolysaccharides and peptidoglycans [13]. Recognition of PAMPs by TLRs initiates inflammatory responses and activates TLR-expressing innate immune cells. One such cell type is plasmacytoid dendritic cells (pDCs) [14] that express high levels of TLR9 and are specialized in generating innate immune responses against intracellular pathogens, resulting in rapid production of type-I interferons, chemokines and cytokines, to ultimately activate the adaptive immune response [15], [16], [17]. In cancer settings, pDCs are frequently present in tumors in an inactivated state, supporting regulatory T-cell activity and immune tolerance, while inhibiting CD8+ T-cell immunity within the tumor microenvironment [16,[18], [19], [20], [21]]. Synthetic CpG-A oligodeoxynucleotides engage TLR9 and activate tumor-associated pDCs to secrete type I interferons and stimulate CD4+ and CD8+ T cells in vitro [22], [23], [24]. Therefore, we hypothesized that intratumoral injection of the TLR9 agonist CMP-001, a CpG-A oligodeoxynucleotide packaged in a virus-like particle (VLP), may convert immunologically cold tumors into hot, especially when combined with RT.
The TLR9 agonist CMP-001, is composed of a viral capsid (Qβ) that engulfs around 20% of the active ingredient CpG-A. Pre-sensitizing the mice with a subcutaneous dose of CMP-001 prior to tumor inoculation, generates antibodies against the viral capsid. When the actual CMP-001 is given after RT, these antibodies bind to CMP-001 molecule and help its internalization into pDCs that express high levels of TLR9. In human trials, it has been shown that CMP-001 can overcome resistance to PD1 inhibition in melanoma that has progressed on prior anti-PD1 therapy; this combination was well tolerated and found to promote systemic antitumor immune responses [25,26]. Although other groups have used other TLR9 agonists (e.g., SD-101, CpG) with RT [27,28], the optimal timing and route of administration for TLR9 agonists with radiation have yet to be elucidated. Herein, we tested whether three doses of CMP-001, given after local tumor irradiation, could generate antitumor immunity as indicated by inhibiting primary and abscopal tumors, stimulating pDCs, and increasing the proportions of CD4+ and CD8+ T cells at tumor sites.
2. Materials and methods
2.1. Cell lines and antibodies
Two cell lines were used for these studies: 344SQ cells, a murine metastatic adenocarcinoma NSCLC cell line derived from a spontaneous subcutaneous metastatic lesion in p53R172HΔg/+ KrasLA1/+ mice [29] (kindly provided by Dr. Johnathan Kurie of MD Anderson Cancer Center); and CT26 WT, an N-nitroso-N-methylurethane−induced undifferentiated colon carcinoma cell line (obtained from the American Type Culture Collection, Manassas, VA, USA). Cells were cultured in RPMI-1640 supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% heat-activated fetal bovine serum and incubated at 37°C in a 5% CO2 atmosphere. CMP-001, a CpG-A ODN formulated within a virus-like particle, was obtained from Checkmate Pharmaceuticals (Cambridge, MA, USA) and was prepared for injection in phosphate-buffered saline at pH 7.4. The human anti-PDL1 monoclonal antibody (mAb) durvalumab was provided by AstraZeneca (Cambridge, UK). Anti-PD1 (RMP1-14) and α-OX40 (OX86) antibodies were provided by BioXcell (West Lebanon, NH, USA).
2.2. Mice
Eight- to 12-week-old male 129Sv/Ev mice (for the NSCLC model) and female BALB/c mice (for the CT26 colon cancer model) were purchased from Taconic Biosciences (Rensselaer, NY, USA) and maintained by the Department of Veterinary Medicine and Surgery at The University of Texas MD Anderson Cancer Center. Protocols for animal use, treatment, and euthanasia were approved by the Institutional Animal Care and Use Committee of MD Anderson Cancer Center. All animal procedures were conducted in accordance with the ethical guidelines of the IACUC committee at MD Anderson Cancer Center (protocol# 00001018RN02).
2.3. Tumor establishment and treatments
Mice were inoculated by subcutaneous injection of tumor cells into the right hind leg to establish “primary” tumors (to be irradiated) and in the left leg to establish “secondary” (abscopal) tumors. Tumor diameters were measured twice a week with digital calipers. Primary tumors were irradiated on days 6,7,8 or 7,8,9 when the tumors had reached about 7 mm in diameter, using a CT-guided XRAD225 linear accelerator. Tumor-bearing mice were injected intratumorally with CMP-001 at the primary tumor site (100 µg/100 µl injection) on days 11, 15, and 20 (i.e., after RT). Mice treated with CMP-001 combinations did receive a subcutaneous pre-sensitization dose 7 days before the tumor inoculation to help generate antibodies against the VLP-Qβ portion of the molecule. This step augments with the uptake of subsequent CMP-001 doses by pDCs. Anti-PD1 mAb (200 µg/injection) or α-PDL1 mAb (200 µg/injection) were given intraperitoneally on days 5, 8, 12, and 16. Anti-OX40 was given in the same schedule and route of administration as CMP-001. Mice were euthanized when the average tumor diameter reached 14 mm in any dimension or if the tumors became ulcerated. Lungs were also harvested where indicated, stained with Bouin's fixative solution, then enumerated and reported accordingly.
2.4. Tumor processing and flow cytometry
Tumors were harvested and tissues digested with 250 µg/mL of Liberase (Roche, Basel, Switzerland) and incubated for 30 min at 37 °C with shaking at 105 rpm. Fetal bovine solution was added to stop the digestion reaction, samples were filtered, and TILs were enriched by using Histopaque 1077 (Sigma, St. Louis, MO, USA, Cat. #H8889). Cells were then blocked with anti-CD16/CD32 before being stained for flow cytometry. Stains included fluorochrome-conjugated anti-CD4 BV510 (Cat. #100449), anti-CD8 PercpCy5.5 (Cat. #100734), anti-CD45 Pacific blue (Cat. #103126), anti-CD49b FITC (Cat. #108905), anti-CD44 APC (Cat. #103012), anti-CD11c PE (Cat. #117308), anti-CD103 PE-Cy7 (Cat. #121426), anti-B220 FITC (Cat. #103206), anti-PDCA-1 APC (Cat. #127016), anti-IFNg Alexa 488 (Cat. #505813), and anti-Granzyme B Pacific blue (Cat. #515407), all from BioLegend (San Diego, CA, USA). Flow cytometry data were analyzed with FlowJo software (Ashland, OR, USA).
2.5. Immunohistochemical staining and analysis
Tumors were harvested 7 days after the last fraction of radiation. Tumor samples were fixed in 10% neutral buffered formalin for 24 h and then washed in phosphate-buffered saline at room temperature. The antibodies used were mouse anti-CD4 (Cat. #25229T), and mouse anti-CD8 (Cat. #98941T). Tumors were processed and stained at MD Anderson's Research Histology Core Laboratory (NCI grant P30 CA016672). Digital quantification was done by using the Aperio Digital Pathology Platform (Leica Biosystems) and evaluated by the Department of Veterinary Pathology at MD Anderson.
2.6. Statistics
All statistical analyses were done with GraphPad Prism 8 software. Mouse survival was analyzed by the Kaplan–Meier method, with curves compared with log-rank tests. Statistical significance was defined as p ≤ 0.05. Student t-tests were conducted to compare two groups where indicated.
3. Results
3.1. High-dose RT significantly increases the percentages of tumor-associated plasmacytoid dendritic cells, CD4+ T cells, and NK cells in the 344SQ NSCLC tumor model
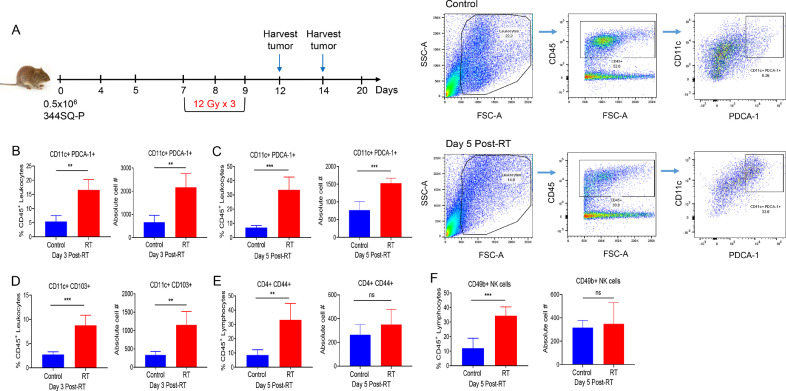
The schema for our mouse model experiments is illustrated in Fig. 1A. On day 0, mice were inoculated with 5 × 105 344SQ cells in their right hind legs (to establish the “primary” tumor), which was then irradiated on days 7−9 with high-dose RT (36 Gy given in three 12-Gy fractions). Primary tumors were then harvested on days 12 or 14, and infiltrating lymphocytes/leukocytes were isolated and analyzed by flow cytometry, with CD11c+ PDCA-1+ used to stain pDCs and CD11c+ CD103+ to stain DCs. High-dose RT greatly increased the percentage of pDCs within the tumor islets at both measurement times, i.e., at 3 days (p = 0.0002) and at 5 days (p = 0.0002) after RT (Fig. 1, B−C; Fig. S1, A-B). Similarly, the absolute cell numbers of pDCs were significantly increased both 3 days (p = 0.0010) and 5 days (p = 0.0009) after High-dose RT. Moreover, we observed an increase in the percentage of DCs at 3 days after RT (p = 0.0003) (Fig. 1D) and in the percentage of CD4+ CD44+ T cells (p = 0.0018) (Fig. 1E) and CD49b+ NK cells (p = 0.0007) at 5 days after RT (Fig. 1F). However, the absolute numbers for CD4+ CD44+ and NK cell-populations did not achieve significance.
Fig. 1.
High-dose radiation (three 12-Gy fractions) led to increased numbers of plasmacytoid dendritic cells (pDCs) at tumor sites in a mouse model of lung cancer. (A) Mice were inoculated in the right hind legs with 344SQ non-small cell lung adenocarcinoma cells to establish tumors and then deliver high-dose radiotherapy (RT) as shown. Tumors were harvested, processed, and analyzed by flow cytometry at 3 days (n = 5 samples) and 5 days (n = 5 samples) after the final fraction of RT (arrows). (B, C) Percentages and absolute cell numbers of pDCs were analyzed by gating on CD45+ population, followed by CD11c+ PDCA-1+ subpopulation, as also shown by the dot plots. (D) Percentages and absolute numbers of DCs were analyzed using CD11c+ CD103+ markers. (E, F) Percentages and absolute numbers of CD4+ T cells and CD49b+ natural killer (NK) cells were analyzed. Student t-tests were conducted to compare statistical significance between groups. *p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
3.2. Injection of CMP-001 after high-dose RT extends survival, inhibits tumor growth, and decreases pulmonary metastases in the 344SQ NSCLC model
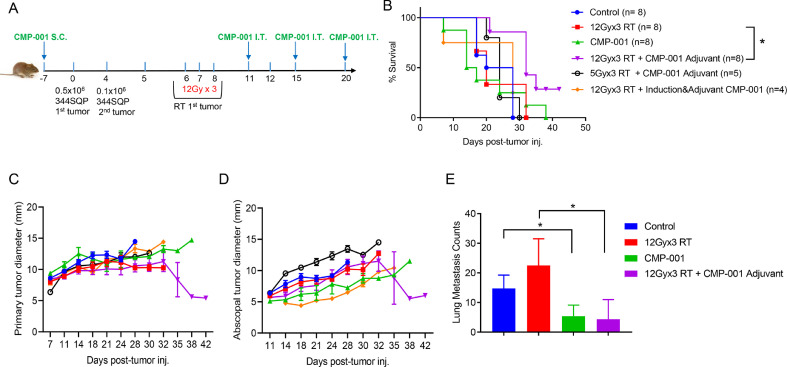
Seven days before tumors inoculation, a subcutaneous pre-sensitizing dose of CMP-001 was given to induce the production of drug-augmenting anti-Qβ antibodies. Mice were then inoculated with 5 × 105 344SQ tumor cells in their right hind legs (Fig. 2A). Four days later, 1 × 105 344SQ cells were injected in the left leg to establish the site of abscopal tumors. CMP-001, given as three intratumoral doses on days 11, 15, and 20 after high-dose RT (Adjuvant treatment), was found to extend mouse survival time to day 42, beyond that of other groups (Fig. 2B); to suppress the growth of both primary (irradiated and injected) and abscopal (unirradiated and noninjected) tumors (Fig. 2, C−D); and to reduce the number of lung metastases relative to RT only (p = 0.0330) (Fig. 2E). In contrast, lower-dose RT (three 5-Gy fractions, for a total dose of 15 Gy) followed by CMP-001 did not delay the growth of primary (Fig. 2C) or abscopal (Fig. 2D) tumors. Moreover, adding an induction dose of CMP-001 before tumor irradiation did not suppress the growth of irradiated (primary) (Fig. 2C) or unirradiated (abscopal) (Fig. 2D) tumors. To further study the sequencing of CMP-001 with RT in the context of a checkpoint inhibitor, intratumoral CMP-001 was given either as two doses post-RT (Adjuvant) or single dose with the first fraction of RT (Concurrent) or both. Adjuvant injection of CMP-001 after RT with a backbone of α-PDL1 (given systemically on days 5, 8, 12, and 16) led to better survival to day 38 (p = 0.0177 vs RT + α-PDL1 only) (Fig. S2, A), with a trend of slowing both primary and secondary tumors growth versus all other groups (Fig. S2, B−C). On the other hand, Concurrent administration of CMP-001 with radiation diminished the survival benefit that was observed with Adjuvant CMP-001 (Fig. S2, A). It is also worth noting that the addition of α-PDL1 backbone did not result in an enhanced anti-tumor efficacy beyond what was observed with stereotactic RT + Adjuvant CMP-001 in Fig. 2B, at least in this particular model.
Fig. 2.
Radiation followed by CMP-001 led to inhibited tumor growth, improved survival rates, and reduced lung metastases in a mouse model of lung cancer. (A) Mice were inoculated in the hind legs with 344SQ cancer cells, with the right leg considered the “primary” tumor (and therefore irradiated with high-dose radiotherapy [RT]) and the left leg considered the abscopal tumor (and therefore left unirradiated). Mice were treated with RT, CMP-001, or RT+ CMP-001 as shown (B) Kaplan-Meier survival curves indicated prolonged survival of mice treated with high-dose radiotherapy (RT; three 12-Gy fractions on days 6, 7, and 8) followed by three IT doses of CMP-001 given on days 11, 15, and 20. (C and D) Growth curves for “primary” (right leg, irradiated) tumors (C) and “secondary” (abscopal, unirradiated) tumors (D), showed that high-dose RT followed by adjuvant CMP-001 had the greatest antitumor effect of the indicated treatments. (E) High-dose RT followed by adjuvant CMP-001 led to decreased numbers of lung metastases. Data are expressed as mean diameters from each mouse at each time point and reported as Mean ± SEM. Experiments were conducted twice, and data was pooled for survival and tumor growth.
3.3. High-dose RT followed by CMP-001 increases activated CD4 and CD8 T-cell populations in primary tumors of 344SQ NSCLC model
Next, for phenotyping purposes, the 344SQ tumors were bilaterally established in the hind legs of 129Sv/Ev mice. RT was delivered to primary tumors on days 6,7,8 and intratumoral CMP-001 was given on days 11,14,19. Primary tumors were harvested on day 20, weighed, and processed for TILs phenotyping using flow cytometry (Fig. S3). T-cell activation was measured by CD44 marker expression, and T-cell functionality was assessed by Granzyme B production. RT alone or RT + CMP-001 significantly increased the percentages of activated CD4 T cells over control group (Fig. S3, A) as well as numbers/mg tumor (Fig. S3, B). More importantly, RT + CMP-001 increased the percentages of functional CD8+ GranzymeB+ T cells over RT group (p = 0.05) and control group (p = 0.0426) (Fig. S3, A). Similarly, RT + CMP-001 combination significantly increased the numbers of CD8+ GranzymeB+ T cells/mg tumor as compared to RT alone (p = 0.0499) or control (p = 0.0055) groups (Fig. S3, B).
3.4. A modified radiation schedule enhances the response with CMP-001 in CT26 colon carcinoma model
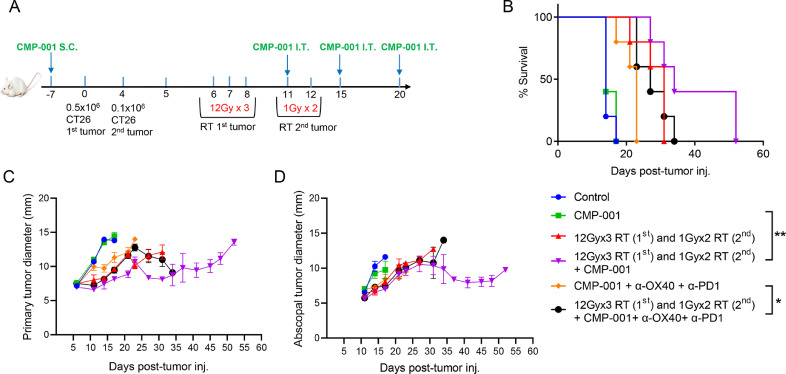
CT26 colon carcinoma cells were injected subcutaneously in the hind legs of BALB/c mice as shown in Fig. 3A. The “primary” tumors (from 5 × 105 cells in the right leg, implanted on day 0) were treated with high-dose RT (three 12-Gy fractions) on days 7, 8, and 9. The “secondary” or “abscopal” tumors (from 1 × 105 cells in the left leg, implanted on day 4), were treated to low-dose non-ablative RT (two 1-Gy fractions) on days 11 and 12 to modulate the tumor stroma and accentuate systemic outcomes (Fig. 3A). The addition of this combinatorial high-dose/low-dose RT to CMP-001 and α-OX40 and α-PD1 extended mouse survival relative to CMP-001 + α-OX40 + α-PD1 (p = 0.0291). Furthermore, the combination of high-dose/low-dose RT with CMP-001 extended survival over that of RT-only (p = 0.0797, n.s.) and that of CMP-001-only (p = 0.0023) (Fig. 3B). However, the quadruple combination (high-dose/low-dose RT + CMP-001 + α-OX40 + α-PD1) did not seem to suppress growth of both primary and abscopal tumors (Fig. 3, C−D) as compared to CMP-001 post-RT treatment.
Fig. 3.
Radiation followed by CMP-001 led to inhibited tumor growth and improved survival rates in a colon carcinoma mouse model. (A) Mice (n = 5/group) were inoculated in the hind legs with CT26 cancer cells, with the right leg considered the “primary” tumor (and therefore irradiated with high-dose radiotherapy [RT]) and the left leg considered the abscopal tumor (and therefore irradiated with low-dose RT). Mice were treated with RT, CMP-001, or RT+ CMP-001 as shown. (B) Survival of mouse treatment groups indicated prolonged survival after RT (three 12-Gy fractions to the primary tumor and two 1-Gy fractions to the abscopal tumor) followed by adjuvant CMP-001) (p = 0.0797, n.s. vs RT only) and (p = 0.0023 vs CMP-001 only group). (C, D) Tumor growth curves indicated that RT (three 12-Gy fractions to the primary tumor and two 1-Gy fractions to the abscopal tumor) followed by adjuvant CMP-001 had superior antitumor activity relative to the other treatment conditions. The addition of α-PD1 and α-OX40 did not extend survival of high dose + low dose RT + CMP-001 treatment. However, high dose + low dose RT led to significant delay in tumor growth when added to triple immunotherapy (CMP-001 + α-OX40 + α-PD1) vs. triple immunotherapy alone (p = 0.0291). Data are expressed as mean diameters from each mouse at each time point, and reported as Mean ± SEM.
3.5. High-dose RT and Adjuvant CMP-001 increases the percentage of CD4+ and CD8+ T cells within the microenvironment of CT26 tumors
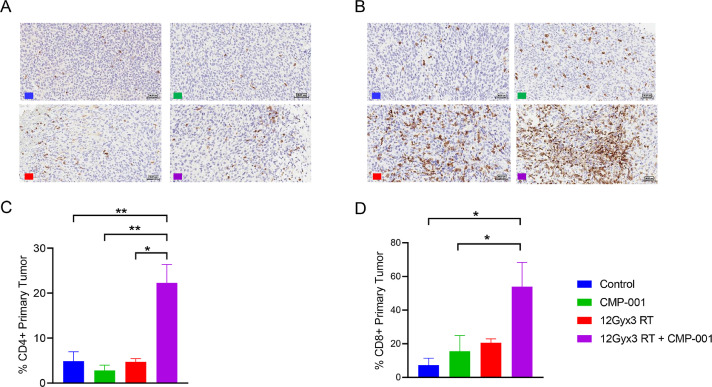
To explore the mechanism underlying the suppression of tumor growth in the CT26 model, we established primary tumors in the right legs of mice as described earlier, and subjected them to high-dose RT (three 12-Gy fractions) followed by three doses of CMP-001. Tumor tissues were collected from the following study groups: control, CMP-001-only, RT-only, and RT followed by CMP-001 and then sectioned for immunohistochemical staining to detect tumor-infiltrating immune cells (Fig. 4, A−B). The combination of high-dose RT and CMP-001 led to significant increases in the numbers of CD4+ T cells versus all other treatment conditions and the numbers of CD8+ T cells relative to CMP-001-only (p = 0.0194) (Fig. 4, C−D).
Fig. 4.
Radiation followed by CMP-001 increased the percentages of CD4+ and CD8+ T cells within the primary tumor in a colon carcinoma model. BALB/c mice (3 per group) were inoculated in the right hind legs with CT26 cancer cells (“primary” tumors) and then treated with high-dose radiotherapy (RT; three 12-Gy fractions), CMP-001, or high-dose RT followed by CMP-001. (A, B) Tumors were harvested on experimental day 21 and then sectioned for immunohistochemical staining (IHC). The digitized IHC images were then annotated for tumor areas and analysis was performed with Aperio Digital Pathology Platform. (C, D) Percentage analysis of tumor infiltrating CD4+ T cells and CD8+ T cells, respectively.
3.6. Injection of intratumoral CMP-001 versus subcutaneous administration after RT
Keeping clinical applications in mind, we sought to compare the efficacy of subcutaneous CMP-001 administration to the more challenging intratumoral route in combination with RT. Here we followed the same schedule as the previous experiments. Basically, mice were inoculated with 5 × 105 344SQ tumor cells in their right hind legs, and 1 × 105 344SQ cells were injected in the left leg to establish the site of primary and abscopal tumors, respectively. CMP-001 was given either as three intratumoral doses or three subcutaneous doses on days 11, 15, and 20, or single intratumoral dose on day 11. Anti-PDL1 was given systemically to all groups on days 5, 9, 13, and 17. We found that three IT doses of CMP-001 after radiotherapy led to slightly better survival (Fig. S4, A) and control of primary tumors (Fig. S4, B) as compared to three SC doses in this aggressive lung adenocarcinoma model used. However, larger sample sizes are needed to assess for significance, and additional human safety and efficacy data needs to be acquired to support the conclusions with radiation.
4. Discussion
Radiation has traditionally been used for local tumor control [30]. The recent addition of immunotherapy to radiation, with or without chemotherapy, was done in an attempt to improve abscopal (systemic) response rates, which have been rare [31]. Our own preclinical studies have shown that RT can overcome resistance to some immune checkpoint inhibitors (e.g., α-PD1) through upregulation of MHC I on the tumor cells [32]. Nevertheless, disease recurrence is still common, in part because of infiltration of immunosuppressive cells such as Tregs and M2 macrophages in addition to CD8+ and CD4+ T-cell exhaustion in cancer [33]. The antitumor effects of RT are also mediated by the upregulation of co-inhibitory receptors on the surfaces of T cells (e.g., BTLA, PD1) [33] and signal-regulatory protein-α [34]. Regressing tumors have also been found to recruit tumor-associated macrophages and CD11b+ myeloid cells [10]. Some have suggested that the addition of novel innate cell-based therapies could overcome radiation-induced immunosuppressive effects [35] through their activation of Toll-like receptors. One such receptor, TLR9, is expressed in pDCs and stimulates antitumor immunity by producing IFNs [36]. Mature pDCs are also known to prime antigen-specific T-cell immunity through response to a variety of DNA and RNA viruses [37]. TLR9 agonists such as CpG ODN (oligodeoxynucleotide) have been tested for their ability to enhance the radio-sensitivity of A549 lung carcinoma cells and with mice bearing fibrosarcoma (FSa) tumors by activating immune responses [28,38]. The addition of α-OX40 and α-PD1 has also been suggested to increase the response to TLR9 agonists [39].
On the basis of these findings, we explored the potential therapeutic effects of CMP-001, a virus-like particle composed of the Qβ bacteriophage capsid protein encapsulating an immunostimulatory CpG-A ODN CMP-001 [40], in two mouse models of cancer. Researchers have shown that activation of TLR9 with CMP-001, in the presence of opsonizing Qβ antibodies, leads to the maturation of pDCs and production of type I interferons [40]. We found that adding high-dose RT (three 12-Gy fractions) led to increased levels of pDCs in the tumor microenvironment (Fig. 1, Fig. S1), and that adding CMP-001 after high-dose RT led to significant delay in primary and abscopal tumor growth. We further found that relatively lower RT doses (e.g., three 5-Gy fractions) did not delay tumor growth (Fig. 2, C−D). Our findings suggest that delivering CMP-001 in repeated intratumoral injections after RT allowed RT to prime the immune system, promote abscopal responses, and enhance survival among tumor-bearing mice (Fig. 2, Fig. S2). We further showed that adding α-PD1 and α-OX40 did not extend survival in CT26 colon carcinoma model. However, irradiating “secondary” (abscopal) tumors with two 1-Gy fractions plus CMP-001 did control the growth of those tumors (Fig. 3).
The RT-induced immune response in our study was manifested as an increased influx of dendritic cells, NK cells, and CD4+ T cells into the tumor microenvironment. We further found that CMP-001 post-RT enhanced the adaptive immune response by significantly increasing the activation status (Fig. S3) and numbers of CD4+ and CD8+ T cells (Fig. 4), the presence of which may indicate primary and abscopal tumor response [41], [42], [43].
Combinatorial treatment strategies with both TLR9 agonists and radiotherapy have been reported in a limited manner in the clinical setting. The Pilot study, a phase I/II trial reported in 2010, evaluated 15 patients with relapsed/refractory low-grade B cell lymphoma and in situ injection of the TLR9 agonist PF-3512676 [44]. Low-dose (4 Gy in 2 fractions) RT was applied owing to the exquisite radiosensitivity of lymphomas. Treatment was tolerated very well, as there was no grade 3+ adverse events. The out-of-field objective response rate was 27%, likely as a result of the relapsed/refractory nature of the cohort; however, response and outcomes were higher in patients who did not induce higher levels of Treg cells, indicating that consequences of combinatorial therapy must be further addressed going forward. Next, a phase I/II trial of SD-101 with the same RT dose was done in 29 patients with previously untreated B cell lymphomas [27]. Although there were 8 cases of grade 3 events, 24 patients experienced a clinical response in unirradiated sites. Although these sites were not histologically examined, the primary site was associated with CD4 and CD8 cell infiltration along with relatively few Treg cells, the latter of which could in part (along with the lack of relapsed/refractory cases) explain the discrepancy in response rate as compared to the Pilot trial. Moreover, two phase I/II trials of PF-3512676 with low-dose RT have been reported for previously treated mycosis fungoides, the most common form of cutaneous T cell lymphoma [45,46]. Both trials showed very few grade 3+ toxicities, and response rates between 33-37%. Importantly, pDCs were upregulated in the treated areas, thus recapitulating results found herein. Lastly, the only NSCLC trial (NCT03438318) is studying combined CMP-001 and atezolizumab, and RT is used in part B of the study to determine the effect of adding radiation therapy to the combined treatment. Similar to our preclinical design, a priming SC dose of CMP-001 is given to generate anti-Qβ drug-augmenting antibodies. Three IT doses are then given after RT once a week, followed by dosing every 3 weeks thereafter (either SC or IT) until discontinuation of treatment.
Our results from this translational study may be of significant value for patients with disease that has progressed on checkpoint inhibitors such as α-PD1 or α-PDL1. These patients could be treated with various combinations of RT and CMP-001, and therefore mediate an adaptive immune response that could overcome tumor resistance to checkpoint treatments.
5. Conclusion
We propose the following explanation for the results of the current study: High-dose RT upregulated pDCs within the tumor microenvironment. The administration of intratumoral TLR9 agonist (CMP-001) after stereotactic RT significantly enhanced the anti-tumor immune response both locally and at secondary tumor site. Our results demonstrated that CMP-001 Post-RT delayed the abscopal tumor growth and extended the survival rate via increasing the percentages of activated CD4+ and CD8+ T-cells within the tumor microenvironment. The treatment proved efficacious in both lung adenocarcinoma and colon carcinoma syngeneic models used.
Author Contributions
Writing the manuscript, AIY, HBB and VV; generating and interpreting data: HBB, AIY and DS; reviewing and proofreading the manuscript: AIY, HBB, DS, VV, YH, RP, MW, JD, KH, DC, HM, SN, FM, MG, LY, PN, MAC and JWW; supervision and oversight: MAC and JWW.
Declaration of competing Interest
Dr. Welsh serves on the science advisory board for RefleXion Medical, Checkmate Pharmaceuticals, and Alpine Immune Sciences; he is the founder of Healios Oncology, Molecular Match, and OncoResponse; and has research support in collaborations with BMS, Merck, Mavu Pharma, Checkmate Pharmaceuticals, and Nanobiotix. All other authors declare no conflicts of interest.
Acknowledgments
This work was supported and funded by Checkmate Pharmaceuticals, and further supported by the family of M. Adnan Hamed, the Susan and Peter Goodwin Foundation, the Orr Family Foundation (to MD Anderson Cancer Center's Thoracic Radiation Oncology program). We also have Cancer Center Support (Core) Grant P30 CA016672 from the National Cancer Institute, National Institutes of Health (to The University of Texas MD Anderson Cancer Center). We would like to thank Art Krieg, Aaron Morris, and Christine F. Wogan for reviewing and editing the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.100983.
Appendix. Supplementary materials
References
- 1.Bernier J., Hall E.J., Giaccia A. Radiation oncology: a century of achievements. Nat. Rev. Cancer. 2004;4:737–747. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 2.Karar J., Maity A. Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol. Ther. 2009;8:1994–2001. doi: 10.4161/cbt.8.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menon H., Ramapriyan R., Cushman T.R., Verma V., Kim H.H., Schoenhals J.E., Atalar C., Selek U., Chun S.G., Chang J.Y., Barsoumian H.B., Nguyen β.N., Altan M., Cortez M.A., Hahn S.M., Welsh J.W. Role of radiation therapy in modulation of the tumor stroma and microenvironment. Front. Immunol. 2019;10:193. doi: 10.3389/fimmu.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y., Auh S.L., Wang Y., Burnette B., Wang Y., Meng Y., Beckett M., Sharma R., Chin R., Tu T., Weichselbaum R.R., Fu Y.X. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt A., Oberle N., Krammer P.H. Molecular mechanisms of treg-mediated T cell suppression. Front. Immunol. 2012;3:51. doi: 10.3389/fimmu.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang C.S., Fu S.Y., Wang S.C., Yu C.F., Chen F.H., Lin C.M., Hong J.H. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front. Oncol. 2012;2:89. doi: 10.3389/fonc.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunne A.L., Price M.E., Mothersill C., McKeown S.R., Robson T., Hirst D.G. Relationship between clonogenic radiosensitivity, radiation-induced apoptosis and DNA damage/repair in human colon cancer cells. Br. J. Cancer. 2003;89:2277–2283. doi: 10.1038/sj.bjc.6601427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoenhals J.E., Cushman T.R., Barsoumian H.B., Li A., Cadena A.P., Niknam S., Younes A.I., Caetano M.D.S., Cortez M.A., Welsh J.W. Anti-glucocorticoid-induced Tumor Necrosis Factor-Related Protein (GITR) therapy overcomes radiation-induced treg immunosuppression and drives abscopal effects. Front. Immunol. 2018;9:2170. doi: 10.3389/fimmu.2018.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caetano M.S., Younes A.I., Barsoumian H.B., Quigley M., Menon H., Gao C., Spires T., Reilly T.P., Cadena A.P., Cushman T.R., Schoenhals J.E., Li A., Nguyen Q.N., Cortez M.A., Welsh J.W. Triple Therapy with MerTK and PD1 inhibition plus radiotherapy promotes abscopal antitumor immune responses. Clin. Cancer Res. 2019;25:7576–7584. doi: 10.1158/1078-0432.CCR-19-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uematsu S., Akira S. Toll-Like receptors (TLRs) and their ligands. Handb. Exp. Pharmacol. 2008:1–20. doi: 10.1007/978-3-540-72167-3_1. [DOI] [PubMed] [Google Scholar]
- 12.Piccinini A.M., Midwood K.S. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010 doi: 10.1155/2010/672395. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guery L., Hugues S. Tolerogenic and activatory plasmacytoid dendritic cells in autoimmunity. Front. Immunol. 2013;4:59. doi: 10.3389/fimmu.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgerald-Bocarsly P., Dai J., Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008;19:3–19. doi: 10.1016/j.cytogfr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegal F.P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P.A., Shah K., Ho S., Antonenko S., Liu Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 17.Cella M., Jarrossay D., Facchetti F., Alebardi O., Nakajima H., Lanzavecchia A., Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 18.Demoulin S., Herfs M., Delvenne P., Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J. leukocyte Biol. 2013;93:343–352. doi: 10.1189/jlb.0812397. [DOI] [PubMed] [Google Scholar]
- 19.Di Domizio J., Demaria O., Gilliet M. Plasmacytoid dendritic cells in melanoma: can we revert bad into good? J. Invest. Dermatol. 2014;134:1797–1800. doi: 10.1038/jid.2014.155. [DOI] [PubMed] [Google Scholar]
- 20.Lombardi V.C., Khaiboullina S.F., Rizvanov A.A. Plasmacytoid dendritic cells, a role in neoplastic prevention and progression. Eur. J. Clin. Invest. 2015;45(Suppl 1):1–8. doi: 10.1111/eci.12363. [DOI] [PubMed] [Google Scholar]
- 21.Swiecki M., Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmann E., Wollenberg B., Rothenfusser S., Wagner M., Wellisch D., Mack B., Giese T., Gires O., Endres S., Hartmann G. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003;63:6478–6487. [PubMed] [Google Scholar]
- 23.Labidi-Galy S.I., Sisirak V., Meeus P., Gobert M., Treilleux I., Bajard A., Combes J.D., Faget J., Mithieux F., Cassignol A., Tredan O., Durand I., Menetrier-Caux C., Caux C., Blay J.Y., Ray-Coquard I., Bendriss-Vermare N. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res. 2011;71:5423–5434. doi: 10.1158/0008-5472.CAN-11-0367. [DOI] [PubMed] [Google Scholar]
- 24.Sisirak V., Faget J., Gobert M., Goutagny N., Vey N., Treilleux I., Renaudineau S., Poyet G., Labidi-Galy S.I., Goddard-Leon S., Durand I., Le Mercier I., Bajard A., Bachelot T., Puisieux A., Puisieux I., Blay J.Y., Menetrier-Caux C., Caux C., Bendriss-Vermare N. Impaired IFN-alpha production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012;72:5188–5197. doi: 10.1158/0008-5472.CAN-11-3468. [DOI] [PubMed] [Google Scholar]
- 25.M. Milhem, R. Gonzales, T. Medina, J.M. Kirkwood, E. Buchbinder, I. Mehmi, J. Niu, M. Shaheen, R. Weight, K. Margolin, J. Luke, A. Morris, D. Mauro, A.M. Krieg, A. Ribas, Abstract CT144: Intratumoral toll-like receptor 9 (TLR9) agonist, CMP-001, in combination with pembrolizumab can reverse resistance to PD-1 inhibition in a phase Ib trial in subjects with advanced melanoma, 78 (2018) CT144-CT144.
- 26.Milhem M., Zakharia Y., Davar D., Buchbinder E., Medina T., Daud A., Ribas A., Niu J., Gibney G., Margolin K., Olszanski A., Mehmi I., Sato T., Shaheen M., Morris A., Mauro D., Campbell K., Bao R., Weiner G., Luke J., Krieg A., Kirkwood J. O85 Durable responses in anti-PD-1 refractory melanoma following intratumoral injection of a toll-like receptor 9 (TLR9) agonist. CMP-001, in Comb. Pembrolizumab. 2020;8:A2–A3. [Google Scholar]
- 27.Frank M.J., Reagan P.M., Bartlett N.L., Gordon L.I., Friedberg J.W., Czerwinski D.K., Long S.R., Hoppe R.T., Janssen R., Candia A.F., Coffman R.L., Levy R. In Situ Vaccination with a TLR9 agonist and local low-dose radiation induces systemic responses in untreated indolent Lymphoma. Cancer Discov. 2018;8:1258–1269. doi: 10.1158/2159-8290.CD-18-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milas L., Mason K.A., Ariga H., Hunter N., Neal R., Valdecanas D., Krieg A.M., Whisnant J.K. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res. 2004;64:5074–5077. doi: 10.1158/0008-5472.CAN-04-0926. [DOI] [PubMed] [Google Scholar]
- 29.Gibbons D.L., Lin W., Creighton C.J., Rizvi Z.H., Gregory P.A., Goodall G.J., Thilaganathan N., Du L., Zhang Y., Pertsemlidis A., Kurie J.M. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes. Dev. 2009;23:2140–2151. doi: 10.1101/gad.1820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mavragani I.V., Nikitaki Z., Kalospyros S.A., Georgakilas A.G. Ionizing radiation and complex DNA damage: from prediction to detection challenges and biological significance. Cancers (Basel) 2019;11 doi: 10.3390/cancers11111789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dagoglu N., Karaman S., Caglar H.B., Oral E.N. Abscopal effect of radiotherapy in the immunotherapy era: systematic review of reported cases. Cureus. 2019;11:e4103. doi: 10.7759/cureus.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X., Schoenhals J.E., Li A., Valdecanas D.R., Ye H., Zang F., Tang C., Tang M., Liu C.G., Liu X., Krishnan S., Allison J.P., Sharma P., Hwu P., Komaki R., Overwijk W.W., Gomez D.R., Chang J.Y., Hahn S.M., Cortez M.A., Welsh J.W. Suppression of Type I IFN signaling in tumors mediates resistance to Anti-PD-1 treatment that can be overcome by radiotherapy. Cancer Res. 2017;77:839–850. doi: 10.1158/0008-5472.CAN-15-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haymaker C., Wu R., Bernatchez C., Radvanyi L. PD-1 and BTLA and CD8(+) T-cell "exhaustion" in cancer: "Exercising" an alternative viewpoint. Oncoimmunology. 2012;1:735–738. doi: 10.4161/onci.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers L.M., Tal M.C., Torrez Dulgeroff L.B., Carmody A.B., Messer R.J., Gulati G., Yiu Y.Y., Staron M.M., Angel C.L., Sinha R., Markovic M., Pham E.A., Fram B., Ahmed A., Newman A.M., Glenn J.S., Davis M.M., Kaech S.M., Weissman I.L., Hasenkrug K.J. A functional subset of CD8(+) T cells during chronic exhaustion is defined by SIRPalpha expression. Nat. Commun. 2019;10:794. doi: 10.1038/s41467-019-08637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z., Wang Z., Li B., Wang S., Chen T., Ye Z. Innate Immune Cells: A Potential and Promising Cell Population for Treating Osteosarcoma. Front. Immunol. 2019;10:1114. doi: 10.3389/fimmu.2019.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monti M., Consoli F., Vescovi R., Bugatti M., Vermi W. Human plasmacytoid dendritic cells and cutaneous melanoma. Cells. 2020;9 doi: 10.3390/cells9020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koyama M., Hashimoto D., Aoyama K., Matsuoka K., Karube K., Niiro H., Harada M., Tanimoto M., Akashi K., Teshima T. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113:2088–2095. doi: 10.1182/blood-2008-07-168609. [DOI] [PubMed] [Google Scholar]
- 38.Li R., Song Y., Chen W. Enhancing radiosensitivity of human pulmonary adenocarcinoma cell line A549 by CpG ODN1826. Cancer Biother. Radiopharm. 2011;26:69–76. doi: 10.1089/cbr.2010.0849. [DOI] [PubMed] [Google Scholar]
- 39.Sagiv-Barfi I., Czerwinski D.K., Levy S., Alam I.S., Mayer A.T., Gambhir S.S., Levy R. Eradication of spontaneous malignancy by local immunotherapy. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemke-Miltner C.D., Blackwell S.E., Yin C., Krug A.E., Morris A.J., Krieg A.M., Weiner G.J. Antibody Opsonization of a TLR9 agonist-containing virus-like particle enhances in situ immunization. J. Immunol. 2020;204:1386–1394. doi: 10.4049/jimmunol.1900742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shankaran V., Ikeda H., Bruce A.T., White J.M., Swanson P.E., Old L.J., Schreiber R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 42.Fumet J.D., Richard C., Ledys F., Klopfenstein Q., Joubert P., Routy B., Truntzer C., Gagne A., Hamel M.A., Guimaraes C.F., Coudert B., Arnould L., Favier L., Lagrange A., Ladoire S., Saintigny P., Ortiz-Cuaran S., Perol M., Foucher P., Hofman P., Ilie M., Chevrier S., Boidot R., Derangere V., Ghiringhelli F. Prognostic and predictive role of CD8 and PD-L1 determination in lung tumor tissue of patients under anti-PD-1 therapy. Br J Cancer. 2018;119:950–960. doi: 10.1038/s41416-018-0220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu-Trantien C., Loi S., Garaud S., Equeter C., Libin M., de Wind A., Ravoet M., Le Buanec H., Sibille C., Manfouo-Foutsop G., Veys I., Haibe-Kains B., Singhal S.K., Michiels S., Rothe F., Salgado R., Duvillier H., Ignatiadis M., Desmedt C., Bron D., Larsimont D., Piccart M., Sotiriou C., Willard-Gallo K. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Invest. 2013;123:2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brody J.D., Ai W.Z., Czerwinski D.K., Torchia J.A., Levy M., Advani R.H., Kim Y.H., Hoppe R.T., Knox S.J., Shin L.K., Wapnir I., Tibshirani R.J., Levy R. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J. Clin. Oncol. 2010;28:4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Y.H., Girardi M., Duvic M., Kuzel T., Link B.K., Pinter-Brown L., Rook A.H. Phase I trial of a Toll-like receptor 9 agonist, PF-3512676 (CPG 7909), in patients with treatment-refractory, cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 2010;63:975–983. doi: 10.1016/j.jaad.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y.H., Gratzinger D., Harrison C., Brody J.D., Czerwinski D.K., Ai W.Z., Morales A., Abdulla F., Xing L., Navi D., Tibshirani R.J., Advani R.H., Lingala B., Shah S., Hoppe R.T., Levy R. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119:355–363. doi: 10.1182/blood-2011-05-355222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.