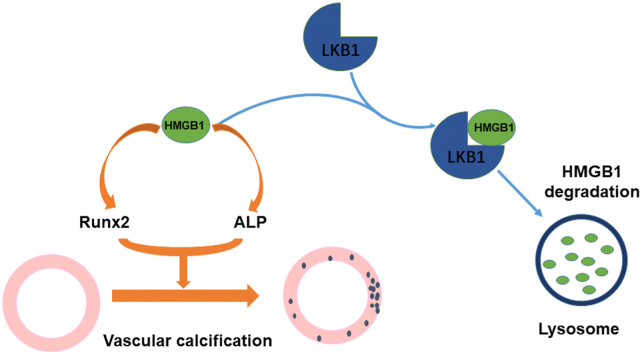
Abstract
Vascular calcification is a common pathological feature of atherosclerosis, chronic kidney disease, vascular injury, and aging. Liver kinase B1 (LKB1) plays pivotal roles in cellular processes such as apoptosis, metabolism, and cell cycle regulation. In addition, growing evidence has indicated that LKB1 functions as a tumor suppressor gene. However, its role in vascular calcification has not been reported. LKB1flox/flox mice were hybridized with SM22-CreERT2 transgenic mice and adult mice received tamoxifen to obtain smooth muscle-specific LKB1-knockout (LKB1SMKO) mice. LKB1 expression was decreased under calcifying conditions, and LKB1 overexpression had a protective effect on vascular calcification. However, high mobility group box 1 (HMGB1) overexpression partially counteracted the promotion of vascular calcification induced by LKB1 overexpression. Mechanically, LKB1 could bind to HMGB1 to promote HMGB1 degradation. Furthermore, LKB1SMKO mice showed intensified vascular calcification, which was alleviated by treatment with the HMGB1 inhibitor glycyrrhizic acid. Based on our results, LKB1 may inhibit vascular calcification via inhibiting HMGB1 expression.
Keywords: Vascular calcification, LKB1, HMGB1, Runx2
Abbreviations: high mobility group box 1-HMGB1, Liver kinase B1-LKB1
Graphical abstract
Highlights
-
•
LKB1 expression was reduced under calcifying conditions.
-
•
LKB1 overexpression had a protective effect on vascular calcification.
-
•
Binding of LKB1 to HMGB1 promoted HMGB1 degradation.
-
•
LKB1 may inhibit vascular calcification by inhibiting HMGB1 expression.
1. Introduction
Vascular calcification is a key contributor to the high incidence and mortality of cardiovascular and cerebrovascular diseases in chronic kidney disease (CKD) [[1], [2], [3]]. It is mainly manifested as increased stiffness and decreased compliance of the vascular wall, leading to myocardial ischemia and heart failure [4,5]. Traditionally, vascular calcification is considered a passive, progressive, and irreversible terminal process. Nevertheless, in recent years, a number of basic and clinical studies have shown that vascular calcification, similar to the processes of bone development and cartilage formation, is an active, controllable, preventable, and treatable process [[6], [7], [8]].
The transformation of vascular smooth muscle cells (VSMCs) from a contraction phenotype to an osteoblastic phenotype is considered a strictly regulated process similar to bone mineralization [9,10]. Osteogenic differentiation of VSMCs is characterized by the upregulation of bone-related molecules such as osteocalcin [11], alkaline phosphatase (ALP), runt-related transcription factor 2 (Runx2), osteocalcin, and Msh homeobox 2 (MSX2) [8,12,13]. Moreover, increasing evidence has indicated that the Wnt/β-Catenin pathway, including Wnt3a and Wnt7a, promotes VSMC calcification and differentiation to an osteogenic phenotype [14,15].
Vascular calcification can be regulated by proteins such as high mobility group box 1 (HMGB1). HMGB1 facilitates the calcification and osteoblastic differentiation of VSMCs via Toll-like receptor 4-Janus kinase–nuclear factor-κB signaling [16] or the Wnt/β-Catenin pathway [17]. Moreover, many studies have indicated that both extracellular and intracellular HMGB1 have roles in promoting calcification. These roles include the promotion of osteochondrogenic differentiation, cell apoptosis, the release of calcific extracellular vesicles via transforming growth factor β/bone morphogenic protein, inflammation, oxidative stress, and autophagy signaling [18]. However, the molecular mechanism involved in regulating HMGB1 requires further exploration.
Liver kinase B1 (LKB1) was first identified as a tumor suppressor in Peutz–Jeghers syndrome, and is a serine/threonine protein kinase widely expressed in mammalian cells [19,20]. LKB1 is considered the regulatory center of cell polarity and energy metabolism, carrying out its role via phosphorylation and activation of the AMP kinase (AMPK) family of proteins [21,22]. Globally, LKB1 knockout has been shown to confer significant embryonic lethality in mice, and has been associated with severe defects in cardiovascular development [23]. LKB1 therefore plays a significant role in the cardiovascular system. In macrophages, LKB1 alleviated atherosclerosis by inhibiting scavenger receptor A expression and foam cell formation [24]. Endothelial-specific LKB1 knockout in mice caused hypertension and endothelial dysfunction in vivo [25]. Moreover, LKB1 may inhibit angiogenesis and lower the expression of vascular endothelial growth factor [26]. However, the role of LKB1 in vascular calcification remains unclear.
In this study, we used VSMCs with overexpressed LKB1 and smooth muscle-specific LKB1-knockout mice to examine whether LKB1 could inhibit vascular calcification via HMGB1.
2. Material and methods
2.1. Reagents
Adenoviruses overexpressing LKB1, HMGB1, and green fluorescent protein (GFP) were purchased from Vigenebio (Rockville, MD, USA). Chloroquine (CQ) was purchased from MCE (Monmouth Junction, NJ, USA), and 3-Methyladenine (3-MA) and MG-132 were purchased from Selleck (Houston, TX, USA). Dexamethasone and d-glucose were purchased from Sigma-Aldrich (St. Louis, MO, USA), and aldosterone was purchased from Solarbio (Beijing, China).
2.2. Mice
LKB1flox/flox mice were hybridized with SM22-CreERT2 transgenic mice expressing a tamoxifen-inducible Cre recombinase under the control of the SM22 promoter. LKB1flox/flox mice were purchased from Jackson Laboratory (Stock No. 014143) [27], and SM22- CreERT2 mice were a kind gift from Dr. Robert Feilin [28]. At 6 weeks of age, the LKB1flox/flox/Cre + mice received continuous intraperitoneal injection of tamoxifen (1 mg/day) for 5 days to obtain LKB1SMKO mice. Littermate LKB1flox/flox/Cre-mice were used as controls. The mice in this study were all adult 8–10-week-old males with no statistically significant differences in weight. The researchers were not blinded during allocation, mice handling, and endpoint measurements. All animals were housed under a 12-h day/night cycle at 25 °C. All experiments were conducted under protocols approved by the Animal Care and Use Committee of Shandong University.
2.3. Mouse model of vitamin D-mediated vascular calcification
Vascular calcification was caused by excess vitamin D treatment, as previously described [13]. Adult male control and LKB1SMKO mice were subcutaneously injected with vitamin D at 500,000 IU/kg/day for 4 consecutive days; sterile water containing 5% ethanol was used as a vehicle control. Animals were monitored daily and sacrificed 10 days after injection. In the experiment with the direct HMGB1 inhibitor glycyrrhizic acid (GA), mice were intraperitoneally injected with GA at 20 mg/kg/day, followed by injection with vitamin D 8 h later.
As accidental death of the mice may occur during injection, it is better to keep the mice under anesthesia than awake. In addition, due to the severe degree of calcification and high mortality in the LKB1SMKO group during the late induction period, we reduced the induction period to 10 days.
2.4. High phosphate-induced calcification in VSMCs
VSMCs were isolated from C57BL/6J mice and cultured in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (HyClone, Logan, UT, USA), 100 μg/mL streptomycin, and 100 U/mL penicillin. Cells were cultured in DMEM containing 2.6 mM Na2HPO4/NaH2PO4 solution for 6 days to induce calcification [29,30]. Medium was replaced every second day.
2.5. Primary culture of VSMCs
At 8 weeks of age, the mice were anaesthetized and dissected. The excised aortas were immediately transferred to a sterile Petri dish containing phosphate buffered saline (PBS), cut into pieces after removing the outer membrane, and then uniformly applied to the bottom of the culture bottle. The tissues were incubated at 37 °C for 2 h and mixed with culture medium containing 15% fetal bovine serum, 100 μg/mL streptomycin, and 100 U/mL penicillin at 37 °C for at least 5 days.
2.6. Plasmid transfection
Lipofectamine2000® (Thermo, USA) was used for plasmid transfection. These plasmids were synthesized by Biosune Biotechnology (Shanghai, China) and transfected into HEK293T cells according to the manufacturer's instructions, and the follow-up operation was performed 48 h later.
2.7. Calcification analysis
Tissues were incubated overnight at 37 °C in 0.6 M HCl and lysed with NP40 (Beyotime, P0013F). Total protein concentration was measured using a BCA assay (Solarbio, PC0020). The calcium content in the supernatant was determined by using a calcium colorimetric assay kit (Sigma-Aldrich, MAK022) according to the manufacturer's protocol. The serum calcium content was detected with the same kit.
VSMCs were decalcified for 24 h at 4 °C in 0.6 M HCl and lysed with RIPA buffer (Solarbio, R0010), and total protein concentration was measured using a BCA assay. Calcium content was determined with the calcium colorimetric assay kit. Calcium content was normalized to total protein concentration.
2.8. Alizarin Red S staining
VSMCs were washed with PBS, fixed with 4% paraformaldehyde, and stained with 1% Alizarin Red S staining solution (Solarbio, G1452) for 10 min. After washing, photographs were taken under a digital microscope.
Tissues were fixed in 10% paraformaldehyde, embedded in paraffin, and then sectioned at a thickness of 5 μm. After conventional dehydration, sections were stained with 1% Alizarin Red S stain solution for 5 min and washed with PBS. After xylene treatment, slides were sealed with gel resin. Photographs were taken under a digital microscope.
2.9. Von Kossa staining
An amount of 1% silver nitrate (Solarbio, G5491) was placed onto the sections before exposure under UV light for 20 min. The tissue slices were then rinsed in distilled water and incubated with 5% sodium thiosulfate for 2 min. Finally, samples were dehydrated and mounted, and images were taken under a digital microscope.
2.10. Western blot analysis
Proteins were extracted from cells or tissues with RIPA buffer (Solarbio, R0010), fractionated by SDS-PAGE, and transferred to PVDF membranes. The membranes were blocked in 5% non-fat dried milk/Tween 20-tris buffered saline (TBST) for 1 h and incubated overnight at 4 °C with a primary antibody. After three cycles of cleaning with TBST, membranes were incubated in appropriate horseradish peroxidase-conjugated secondary antibodies and observed by enhanced chemiluminescence (Pierce). ImageJ software was used for analysis. All experiments were performed at least three times.
2.11. Immunohistochemical analyses
Briefly, sections were dewaxed, rehydrated, blocked by endogenous peroxidase activity and nonspecific binding, and incubated overnight with primary antibodies at 4 °C. After three cycles of washing with PBS, sections were incubated with secondary antibody at 37 °C for 30 min. Bound secondary antibodies were detected by using DAB solution (Zhong Shan Golden Bridge Biological Technology, Beijing, China). Tissue sections were observed under a confocal FV 1000 SPD laser scanning microscope (Olympus, Japan).
2.12. ALP activity and HMGB1 level measurement
ALP levels in VSMCs, serum, and tissues were analyzed by using commercially available kits (Jiancheng Biology Engineering Institute, Nanjing, China). HMGB1 levels in serum and cell supernatant were detected by using the mouse HMGB1 ELISA Kit (CSB-E08225 m; Cusabio).
2.13. Quantitative real-time PCR (qPCR)
According to the instructions, total RNA was extracted from VSMCs and mouse tissues with Tri reagent (Ambion, Austin, TX, USA), and 1 μg RNA was reverse-transcribed into complementary DNA by using a iScriptcDNA synthesis kit (Bio-Rad, Hercules, CA, USA). PCR amplification was conducted using the SYBR PCR mix (Bio-Rad). The oligonucleotide primer sequences were as follows: for Lkb1, 5′-CCGACAGATTAGGCAGCACA-3′ and 5′-GGCTTGGTGGGATAGGTACG-3′; ALP ligand (ALPL): 5′-TTGTGCCAGAGAAAGAGAGAGA-3′ and 5′-GTTTCAGGGCATTTTTCAAGGT-3′; Runt-related transcription factor 2 (Runx2): 5′-AGAGTCAGATTACAGATCCCAGG-3′ and 5′-AGGAGGGGTAAGACTGGTCATA-3′; Msh homeobox 2 (Msx2): 5′-TTCACCACATCCCAGCTTCTA-3′ and 5′-TTGCAGTCTTTTCGCCTTAGC-3′; Wnt3a: 5′-AATTTGGAGGAATGGTCTCTCGG-3′ and 5′-CAGCAGGTCTTCACTTCACAG-3′; Wnt7a: 5′-GGCTTCTCTTCGGTGGTAGC-3′ and 5′-TGAAACTGACACTCGTCCAGG-3′; Hmbg1: 5′-CACCCGGATGCTTCTGTCAA-3′ and 5′- GAAGAAGGCCGAAGGAGGC-3′; and Gapdh: 5′-AGGTCGGTGTGAACGGATTTG-3′ and 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
2.14. Immunoprecipitation
All immunoprecipitation tests were performed by using commercially available kits (#635696; Takara).
2.15. Human samples
Human coronary arteries were obtained from brain-dead multi-organ donors, consisting of five controls and five patients with end-stage chronic kidney disease (CKD). Blood was collected from CKD patients before dialysis and healthy volunteers as controls, and serum was obtained by centrifugation at 3000 rpm for 15 min. VSMCs were in a serum starvation state for 24 h before treatment. Uremic serum (15%) from hemodialysis patients or control serum from matched healthy individuals (normal serum) was collected as previously described [8], followed by Western blot and RT-PCR analyses. The use of human tissues was approved by the Medical Institutional Ethics Committee of Qilu Hospital, Shandong University, China, and all donors provided informed consent.
2.16. Statistical analysis
All data analyses were conducted using GraphPad Prism 6.0, and the data are presented as the mean ± SEM. Comparisons between two groups were performed by Student's t-test, and comparisons between more than two groups were conducted via one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
3. Results
3.1. Vascular LKB1 expression was decreased under calcifying conditions
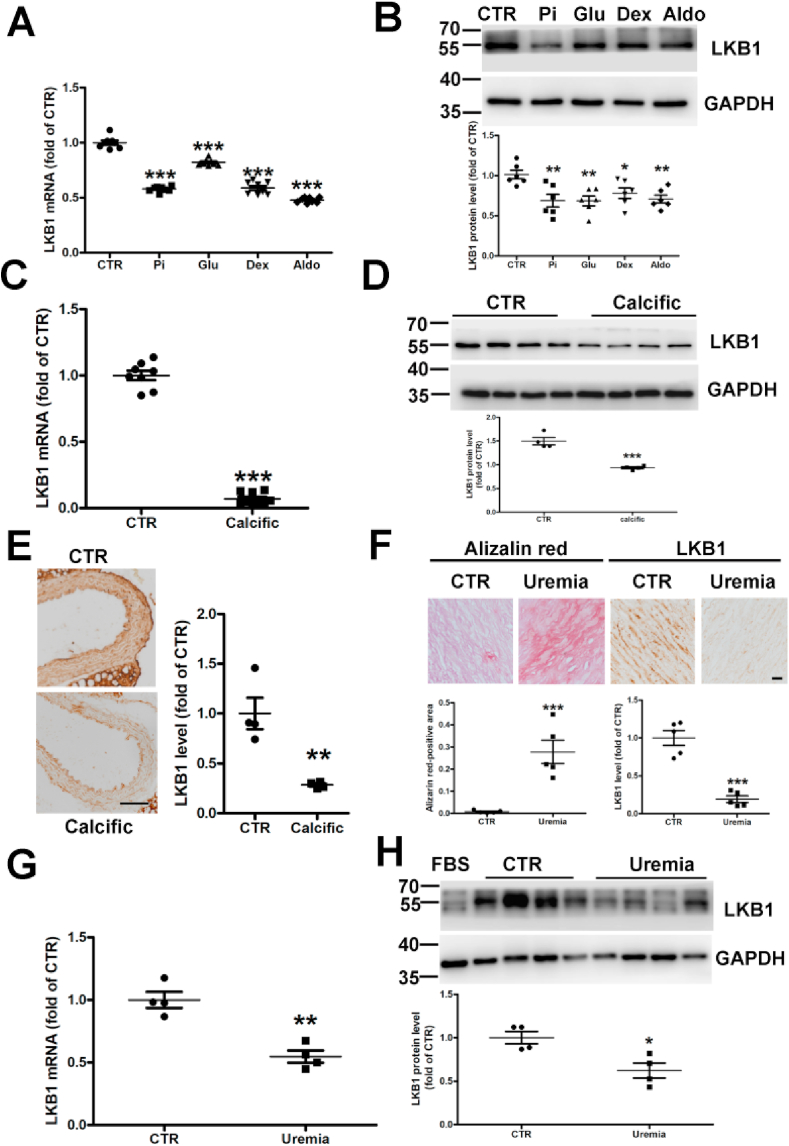
We explored the effect of known osteocyte/chondrocyte transdifferentiation and VSMC calcification triggers on the expression of vascular LKB1. After treatment with phosphate, glucose, dexamethasone, and aldosterone, the mRNA and protein levels of LKB1 were significantly downregulated in VSMCs compared to the control (Fig. 1 and 1B). Similarly, after treatment with vitamin D, the mRNA and protein levels of LKB1 were reduced in the aortic tissue of calcified mice compared to in that of control mice (Fig. 1C and D). Moreover, immunohistochemistry revealed reduced LKB1 levels in calcified coronary arteries in calcified mice (Fig. 1E and Supplementary Fig. 5A) and CKD patients compared with in the controls (Fig. 1F and Supplementary Fig. 1). Furthermore, after treating with serum from patients with advanced uremia, VSMCs calcification was induced (Supplementary Fig. 2), the mRNA and protein levels of LKB1 in VSMCs were remarkably reduced compared to the control group (Fig. 1G and H). Thus, LKB1 levels were negatively correlated with calcification.
Fig. 1.
Liver kinase B1 (LKB1) expression decreased under calcifying conditions. A. Vascular smooth muscle cells (VSMCs) were treated with control (CTR) or high phosphate (Pi) and dexamethasone (Dex) or glucose aldosterone (Aldo) for 48 h. RT-PCR analysis was conducted to determine the relative mRNA expression of LKB1 (n = 8). B. Western blot analysis of LKB1 protein levels (n = 6). C and D. Mice were treated with vehicle (CTR) or vitamin D (Calcific) to induce vascular calcification. The mRNA and protein levels of vascular LKB1 were determined by RT-PCR (n = 8) (C) and Western blot analysis (n = 4) (D). E. Immunohistochemistry of aortic LKB1 expression in control and calcified mice (n = 4). Scale bar: 50 μm. F. Representative histological images showing LKB1 expression and ectopic calcification by Alizarin Red staining in coronary artery sections from controls and end-stage chronic kidney disease (CKD) patients (n = 5). Scale bar: 20 μm. G and H. mRNA and protein expression of LKB1, as detected by RT-PCR (n = 4) (G) and Western blot analysis (n = 4) (H) in VSMCs supplemented with serum from patients with uremia (n = 4) and controls (CTR) (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 vs. CTR. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. LKB1 overexpression inhibits high phosphate-induced calcification in VSMCs
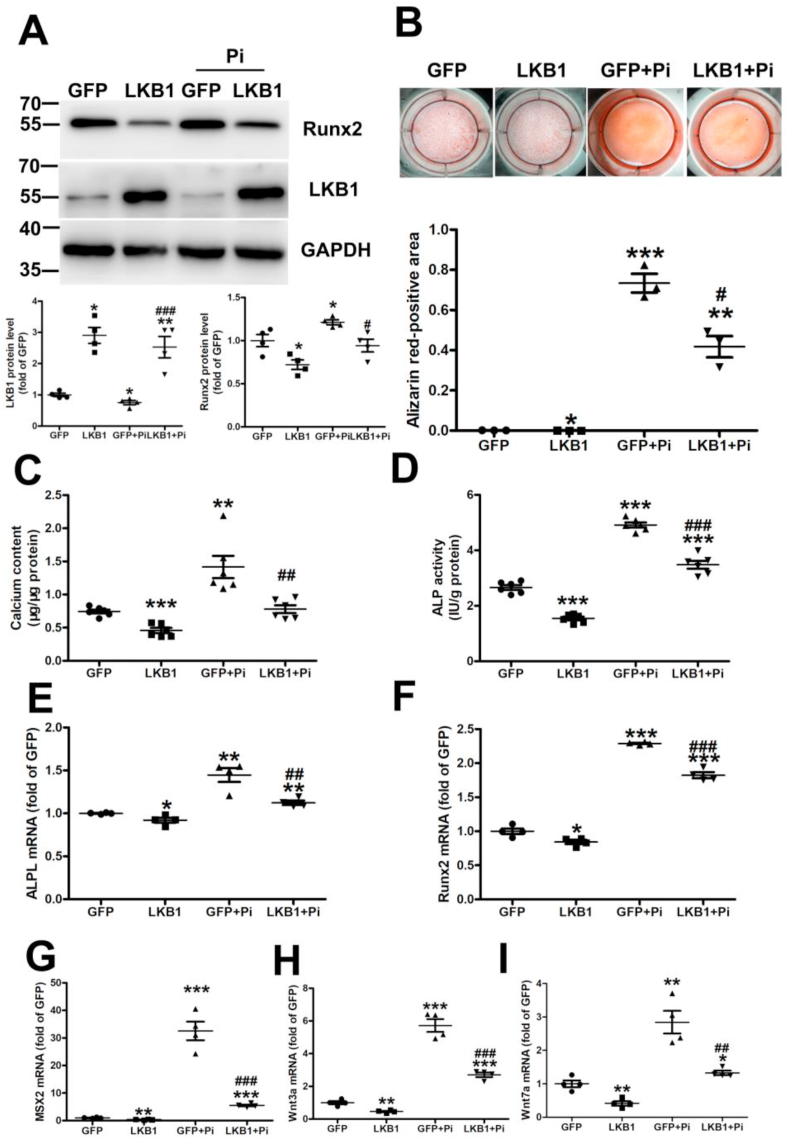
To illuminate the role of LKB1 in vascular calcification, we infected VSMCs with an adenovirus expressing GFP or LKB1, followed by treatment with high phosphate for 6 days. Runx2 is a major transcription factor that plays a vital role in the osteogenic differentiation of VSMCs [31]. Runx2 expression was significantly increased under high phosphate treatment in VSMCs, and this could be alleviated by LKB1 overexpression (Fig. 2A). Similarly, Alizarin Red staining showed that LKB1 overexpression relieved calcium deposition in calcified VSMCs with high phosphate treatment (Fig. 2B). In addition, LKB1 overexpression weakened the high phosphate induction of calcium content and ALP activity in VSMCs (Fig. 2C and D) and reduced the mRNA expression of ALPL, Runx2, Msx2, Wnt3a, and Wnt7a in high phosphate-induced VSMCs (Fig. 2E–I). Thus, LKB1 overexpression could inhibit high phosphate-induced calcification of VSMCs.
Fig. 2.
Liver kinase B1 (LKB1) overexpression inhibits high phosphate-induced calcification in vascular smooth muscle cells (VSMCs). VSMCs were infected with adenovirus-expressing green fluorescent protein (GFP) or LKB1 and treated with high phosphate for 6 days. A. Protein levels of Runx2 and LKB1, as detected by Western blot analysis (n = 4). B. Representative original images of Alizarin Red staining in VSMCs (n = 3). C. Total calcium content in VSMCs (n = 6). D. Alkaline phosphatase ligand (ALPL) activity in VSMCs (n = 6). E–I. mRNA levels of ALPL (E), Runx2 (F), Msx2 (G), Wnt3a (H), and Wnt7a (I), as detected by RT-PCR (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 vs. GFP; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. GFP + phosphate (Pi). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
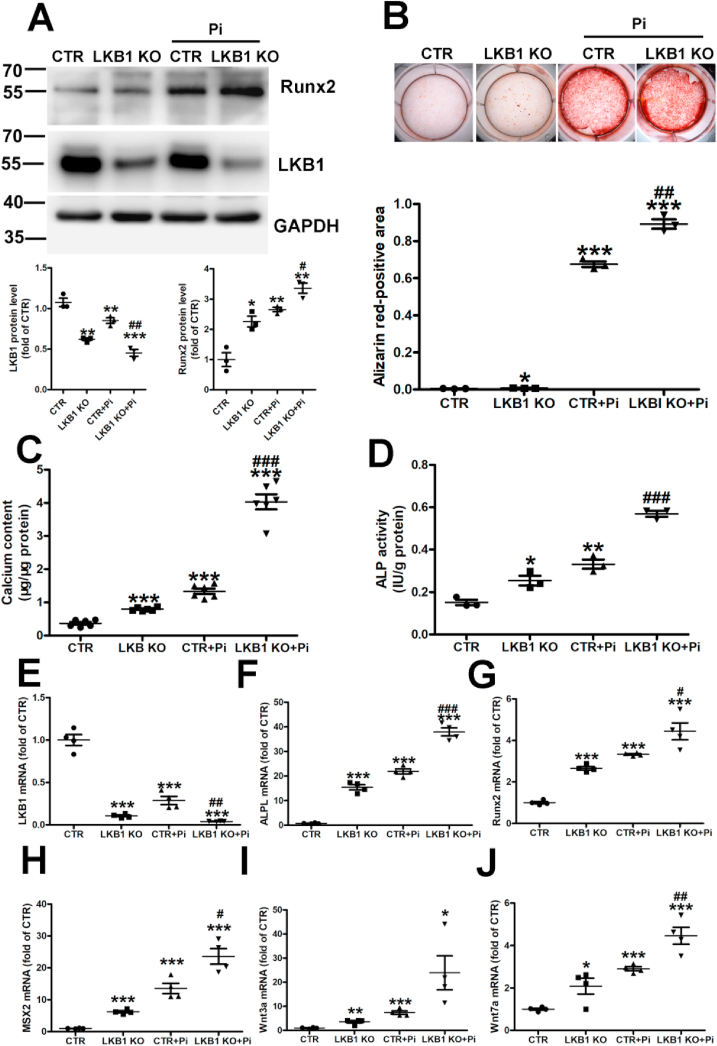
3.3. LKB1 deficiency aggravates high phosphate-induced calcification in VSMCs
To further verify the relationship between LKB1 and vascular smooth muscle calcification, the primary VSMCs from control and LKB1SMKO mice were incubated with high phosphate for 6 days. The Runx2 expression increased by high phosphate was magnified in the VSMCs of LKB1SMKO mice compared with in the controls (Fig. 3A). Alizarin Red staining revealed significant calcium deposition, that was amplified in the VSMCs of LKB1SMKO mice, but not in those of the controls (Fig. 3B). Similarly, calcium content and high phosphate-induced ALP activity intensified with LKB1 deficiency (Fig. 3C and D). In addition, LKB1 deficiency significantly increased the mRNA levels of ALPL, Runx2, Msx2, Wnt3a, and Wnt7a in high phosphate-induced VSMCs (Fig. 3E–J). Thus, LKB1 deficiency aggravated high phosphate-induced calcification in VSMCs.
Fig. 3.
Liver kinase B1 (LKB1) deficiency aggravates high phosphate-induced calcification in vascular smooth muscle cells (VSMCs). Primary VSMCs from control (CTR) and LKB1SMKO (LKB1 KO) mice were treated with high phosphate for 6 days. A. Protein levels of Runx2 and LKB1, as detected by Western blot analysis (n = 3). B. Representative Alizarin Red staining in VSMCs (n = 3). C. Total calcium content in VSMCs (n = 6). D. Alkaline phosphatase ligand (ALPL) activity in VSMCs (n = 3). E–J. mRNA levels of LKB1 (E), ALPL (F), Runx2 (G), Msx2 (H), Wnt3a (I), and Wnt7a (J) detected by RT-PCR (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 vs. CTR; #P < 0.05, ##P < 0.01, ###P < 0.01 vs. CTR + Pi. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. LKB1 binds with HMGB1 and promotes its degradation
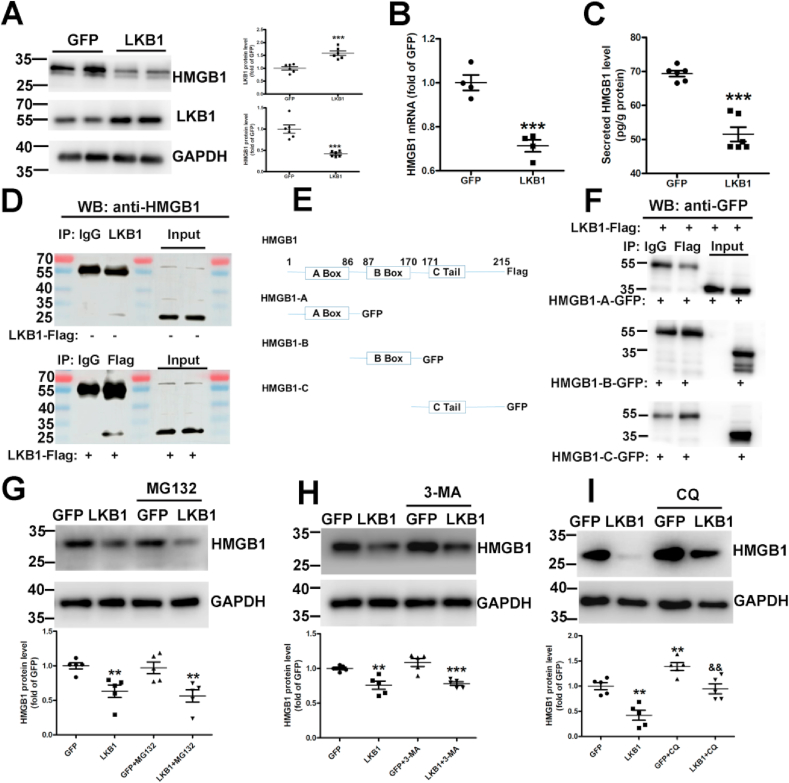
To study the molecular mechanism of LKB1 in vascular calcification, we examined a variety of molecules and pathways. LKB1 overexpression reduced the protein and mRNA levels of HMGB1 in VSMCs (Fig. 4A and 4B) and decreased secreted HMGB1 levels in cell culture medium (Fig. 4C). Thus, LKB1 could inhibit HMGB1 levels. Moreover, immunoprecipitation revealed that LKB1 could bind with HMGB1 both endogenously and exogenously (Fig. 4D). To explore the specific binding region of LKB1 and HMGB1, we constructed HMGB1 plasmids (Fig. 4E). Immunoprecipitation showed that HMGB1-A, HMGB1-B, and HMGB1-C could not combine with LKB1 alone, and only full-length HMGB1 could combine with LKB1 (Fig. 4D and 4F).
Fig. 4.
Liver kinase B1 (LKB1) binds with high mobility group box 1 (HMGB1) and promotes its degradation. A and B. Vascular smooth muscle cells (VSMCs) were infected with adenovirus-expressing green fluorescent protein (GFP) or LKB1, and results were determined using Western blot analysis (n = 6) (A) and RT-PCR analysis (n = 4) (B). C. HMGB1 levels in cell medium, as detected via ELISA (n = 6). D. VSMC (without LKB1 overexpression) lysates were immunoprecipitated with IgG or LKB1 antibody, and VSMC (with LKB1 overexpression) lysates were immunoprecipitated with IgG or Flag antibody. Results were determined via Western blot analysis with anti-HMGB1 antibody. E. Schematic diagram of the construction of HMGB1-truncated plasmids. F. HEK293T cells were transfected with LKB1-Flag and HMGB1-A- or HMGB1-B- or HMGB1-C-GFP plasmids, followed by immunoprecipitation with anti-Flag antibody and Western blot analysis with anti-GFP antibody. G–I. VSMCs were infected with adenovirus-expressing GFP or LKB1 and treated with proteasome inhibitor 1 μM MG-132 (n = 5) (G), 10 mM autophagic inhibitor 3-MA (n = 5) (H), or 10 μM lysosomal inhibitor CQ (n = 5) (I). Western blot analysis was performed to detect HMGB1 levels. *P < 0.05, **P < 0.01, ***P < 0.001 vs. GFP; && P < 0.01 vs. LKB1. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The three ways to promote protein degradation include the autophagy, proteasome, and lysosomal pathways. VSMCs were pretreated with the autophagy inhibitor 3-MA, proteasome inhibitor MG132, or lysosomal inhibitor CQ to explore the HMGB1 degradation pathway. LKB1 overexpression-induced HMGB1 degradation could be attenuated by CQ, but not by MG132 or 3-MA (Fig. 4G–I and Supplementary Fig. 3), which suggests that LKB1 promoted HMGB1 degradation via the lysosomal pathway. To further explore the mechanism of action of LKB1 on HMGB1, GPS5.0 software was used to predict phosphorylation sites where LKB1 may act on HMGB1. However, the results of immunoprecipitation did not show that LKB1 could phosphorylate HMGB1 (data not shown). Hence, LKB1 can bind to HMGB1 and promote its degradation via the lysosomal pathway.
3.5. LKB1 inhibits high phosphate-induced calcification via HMGB1
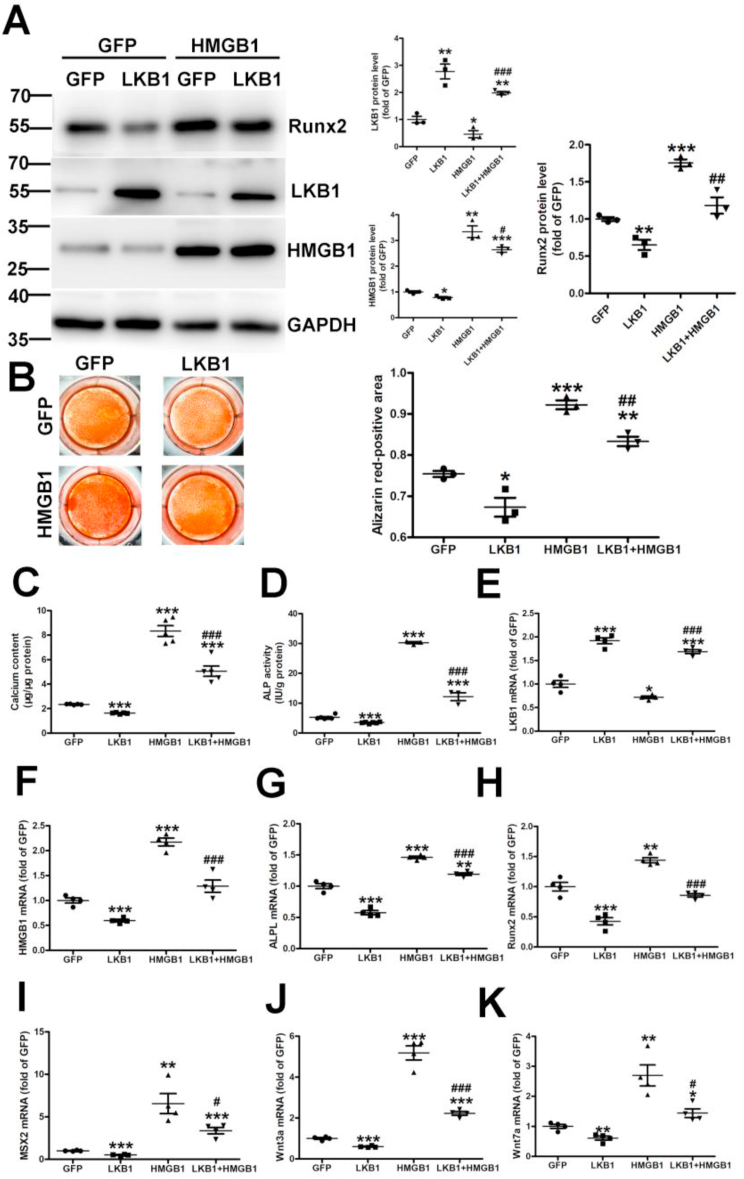
To further investigate whether LKB1 regulates vascular calcification via HMGB1, VSMCs were infected with adenovirus overexpressing LKB1 and/or HMGB1, followed by treatment with high phosphate for 6 days. HMGB1 overexpression could reverse the inhibition of Runx2 and calcium deposition induced by LKB1 overexpression (Fig. 5A and B). Also, LKB1 overexpression reduced calcium content and alkaline phosphatase activity in VSMCs, but HMGB1 overexpression could partially offset this trend (Fig. 5C and D). Consistently, HMGB1 overexpression heightened the downregulation effect of LKB1 on ALPL, Runx2, MSX2, Wnt3a, and Wnt7a (Fig. 5E–K). Hence, LKB1 may inhibit high phosphate-induced calcification by interacting with HMGB1.
Fig. 5.
Liver kinase B1 (LKB1) inhibits high phosphate-induced calcification via HMGB1. Vascular smooth muscle cells (VSMCs) were infected with adenovirus-expressing green fluorescent protein (GFP), LKB1, and/or HMGB1, then treated with high phosphate for 6 days. A. Protein levels of Runx2, LKB1, and HMGB1, as detected by Western blot analysis (n = 3). B. Representative Alizarin Red staining in VSMCs (n = 3). C. Total calcium content in VSMCs (n = 5). D. Alkaline phosphatase (ALP) activity in VSMCs (n = 6/GFP and LKB1 groups, n = 3/HMGB1 and LKB1+HMGB1 groups). E–K. mRNA levels of LKB1 (E), HMGB1 (F), alkaline phosphatase ligand (ALPL) (G), Runx2 (H), Msx2 (I), Wnt3a (J), and Wnt7a (K), as detected by RT-PCR (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 vs. GFP; #P < 0.05, ##P < 0.01, ###P < 0.01 vs. HMGB1. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Considering that LKB1 is an important upstream regulator of AMPKα activity, we detected whether LKB1 regulates calcification through AMPK. VSMCs infected with adenovirus overexpressing GFP or LKB1 were treated with the AMPK inhibitor Compound C and then incubated with high phosphate for 6 days. The inhibitory effect of LKB1 overexpression on Runx2, calcium content, and alkaline phosphatase activity could not be reversed by Compound C (Supplementary Fig. 4A–4D). Consistently, Compound C could not alter the downregulation effect of LKB1 on ALPL and MSX2 (Supplementary Fig. 4E–4F). Therefore, LKB1 inhibits high phosphate-induced calcification independent of AMPK.
3.6. LKB1 deficiency in smooth muscle increases HMGB1 levels
LKB1SMKO mice were generated according to Mendelian frequency, with no significant difference in growth between the control group and LKB1SMKO mice (data not shown). LKB1 protein expression was remarkably decreased in primary VSMCs from LKB1SMKO mice compared to the control group (Fig. 3A) and aortas from LKB1SMKO mice (Fig. 6C). Based on these results, we can conclude that smooth muscle-specific LKB1 knockout was effective.
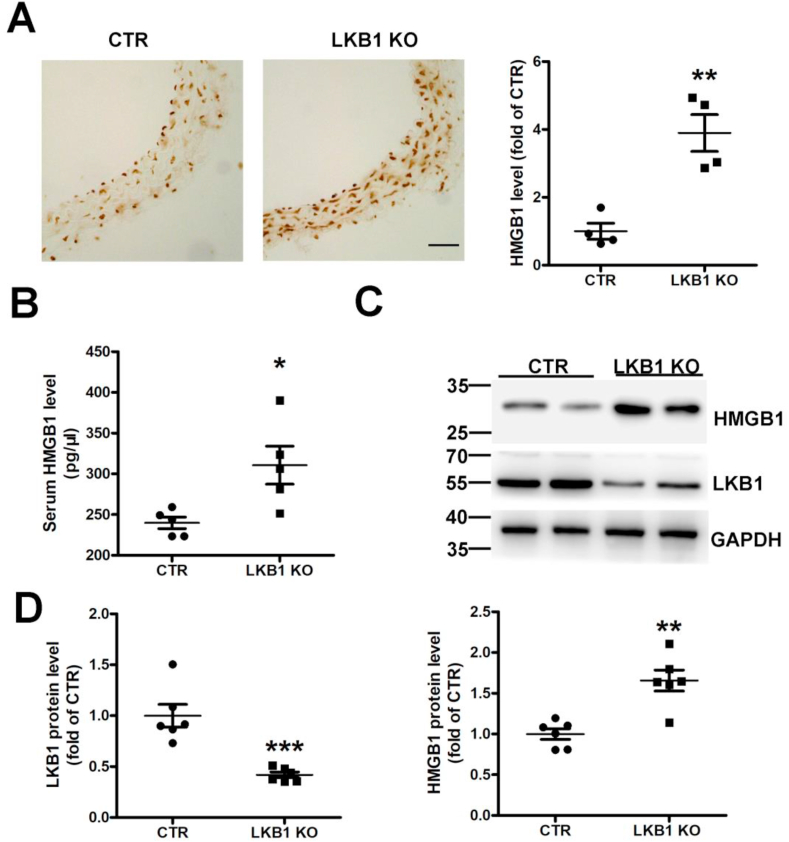
Fig. 6.
Liver kinase B1 (LKB1) deficiency in smooth muscle increases HMGB1 levels. A. Immunohistochemistry of HMGB1 in aortas from control (CTR) and LKB1SMKO (LKB1 KO) mice (n = 4). Scale bar: 20 μm. B. Serum HMGB1 measured by ELISA (n = 5). C. Western blot analysis of aortic HMGB1 and LKB1 protein levels from control (CTR) and LKB1SMKO (LKB1 KO) mouse aortas. D. Quantification of protein levels of LKB1 (n = 6) and HMGB1 (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 vs. CTR.
We further verified the correlation between LKB1 and HMGB1. Immunohistochemistry indicated that HMGB1 levels were significantly higher in aortas from LKB1SMKO mice than in those of the controls (Fig. 6A and Supplementary Fig. 5B). Similarly, LKB1 deficiency elevated serum HMGB1 levels in LKB1SMKO mice in comparison to the controls (Fig. 6B). HMGB1 protein levels were also higher in the aortas of LKB1SMKO mice than in those of the controls (Fig. 6C and D). Therefore, LGL1 deficiency in smooth muscle increased HMGB1 levels.
Smooth muscle-specific LKB1 deletion exacerbated vitamin D-induced vascular calcification.
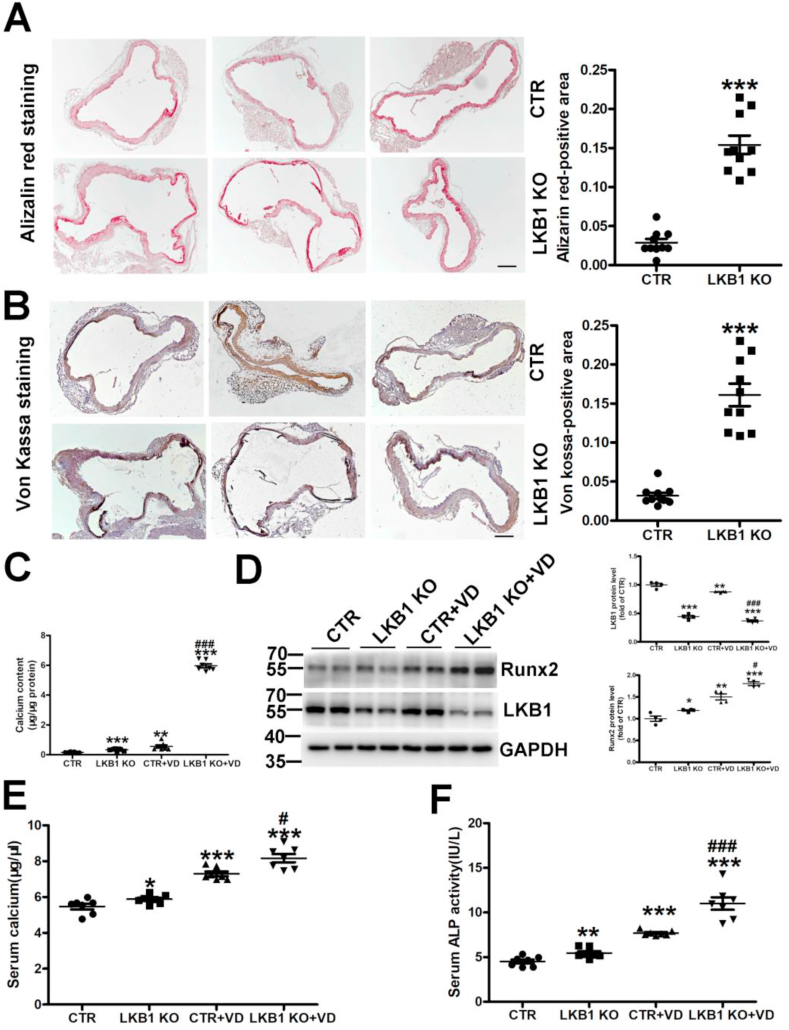
As aforementioned, vitamin D injection induced vascular calcification in control and LKB1SMKO mice. LKB1 deletion remarkably increased matrix mineral deposition, as assessed by Alizarin Red and Von Kossa staining (Fig. 7A and B). Moreover, calcium content and Runx2 expression were elevated in the aortas of LKB1SMKO mice compared to in those of the controls (Fig. 7C and D). Furthermore, as compared with the controls, LKB1SMKO mice showed significantly increased serum calcium content and ALP activity with or without vitamin D-induced calcification (Fig. 7E and F). Thus, the absence of smooth muscle specificity of LKB1 exacerbated vascular calcification.
Fig. 7.
Smooth muscle-specific deletion of liver kinase B1 (LKB1) aggravates vitamin D-induced vascular calcification. A–B. Vascular calcification was induced in control (CTR) and LKB1SMKO (LKB1 KO) mice by administering vitamin D (VD). The matrix mineral deposition in aortic tissues was assessed by Alizarin Red (n = 10) (A) and Von Kossa staining (n = 10) (B). Scale bar: 100 μm. C. Aortic calcium content in control (CTR) and LKB1SMKO (LKB1 KO) mice (n = 7). D. Protein level of aortic Runx2, as assessed by Western blot analysis (n = 4). E–F. Serum calcium content (n = 7) (E) and alkaline phosphatase (ALP) activity (n = 7) (F) in mice. *P < 0.05, **P < 0.01, ***P < 0.001 vs. CTR; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. CTR + VD. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.7. HMGB1 inhibitor alleviates deteriorated vascular calcification in LKB1SMKO mice
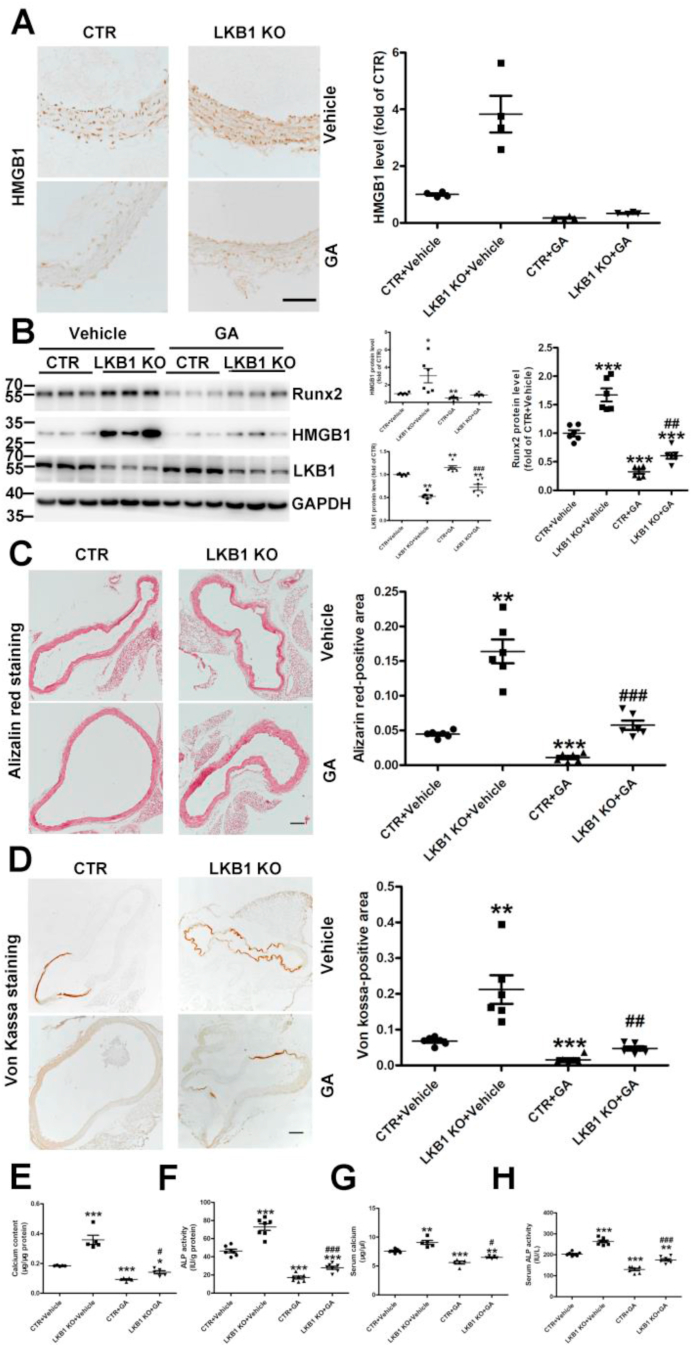
To verify that LKB1 alters vascular calcification via HMGB1 in vivo, we treated control and LGL1SMKO mice with the direct HMGB1 inhibitor GA and induced calcification with vitamin D. GA treatment significantly inhibited the increased expressions of HMGB1 and Runx2 caused by LKB1 deficiency (Fig. 8A–B and Supplementary Fig. 5C). Likewise, GA treatment attenuated the increased calcium deposition, as assessed by Alizarin Red and Von Kossa staining in LKB1SMKO mice (Fig. 8C–D). The increased calcium content and ALP activity in aortas and serum from LKB1SMKO mice further demonstrated this effect (Fig. 8E–H). Therefore, inhibiting HMGB1 alleviated the deteriorating vascular calcification in LKB1SMKO mice.
Fig. 8.
HMGB1 inhibitor attenuates aggravated vascular calcification in liver kinase B1 (LKB1SMKO) mice. A. HMGB1 inhibitor glycyrrhizic acid (GA) (20 mg/kg) was administered daily to (CTR) and LKB1SMKO (LKB1 KO) mice 8 h before injection of vitamin D to the control. Vascular calcification was then induced. The HMGB1 level in aortic tissues was assessed by immunohistochemistry (n = 4). Scale bar: 100 μm. B. Protein levels of LKB1, HMGB1 and Runx2 in aortic tissues, as detected by Western blot analysis (n = 6). C–D. Matrix mineral deposition in aortic tissues assessed by Alizarin Red staining (n = 6) (C) and Von Kossa staining (D). Scale bar: 100 μm. E–F. Aortic calcium content (n = 5) (E) and alkaline phosphatase (ALP) activity (n = 7) (F) in mice. G–H. Serum calcium content (n = 5) (G) and ALP activity (n = 7) (H) in mice. *P < 0.05, **P < 0.01, ***P < 0.001 vs. CTR + Vehicle; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. CTR + GA. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
To explore the role of LKB1 in vascular calcification, we used smooth muscle-specific LKB1 knockout mice. In aortic smooth muscle cells, loss of LKB1 induced Runx2 and ALP upregulation. Moreover, LKB1 deficiency enhanced HMGB1 expression and vascular calcification, which was confirmed by Alizarin Red staining, Von Kossa staining, and calcium content detection. In vitro, we cultured VSMCs that overexpressed LKB1. LKB1 overexpression in VSMCs downregulated bone formation genes such as Runx2 and ALP, reduced the severity of calcification (as demonstrated by Alizarin Red staining and calcium content detection), and decreased HMGB1 expression. Meanwhile, the degree of calcification and HMGB1 expression was significantly increased in VSMCs from LKB1SMKO mouse aortas compared with in those of the controls. Furthermore, HMGB1 overexpression in VSMCs could counteract the inhibitory effect of LKB1 overexpression on calcification. LKB1 could bind to HMGB1 endogenously and exogenously, and this binding depends on the structural integrity of HMGB1. Taken together, our results revealed that LKB1 inhibited vascular calcification by binding full-length HMGB1 and decreasing its expression via the lysosomal pathway.
A large number of studies have shown that LKB1 plays a vital role in cardiovascular diseases [24,[32], [33], [34]]. Cardiac-specific LKB1 deletion leads to cardiac hypertrophy and dysfunction [35]. In our previous study, absence of smooth muscle-specific LKB1 exacerbated angiotensin II-induced abdominal aortic aneurysm in mice [36]. However, no association between LKB1 and vascular calcification has been reported. In this study, we found that LKB1 inhibits vascular calcification by binding HMGB1 and promoting its degradation. Although AMPK is the most important substrate of LKB1, we demonstrated that LKB1 inhibits high phosphate-induced calcification independent of AMPK. Our study further elucidated the mechanism by which calcification occurs and improved understanding of the biological function of LKB1.
Vascular calcification refers to an increase in calcium and phosphorus deposition in cardiovascular tissue. It is a complex process that involves the migration and apoptosis of VSMCs and osteogenic differentiation, as well as the increased release of matrix vesicles [4]. The mechanism of vascular calcification regulation mainly includes VSMC apoptosis, increased osteogenic differentiation, and increased release of inflammatory factors [37,38]. As mentioned earlier, the inflammatory response promotes calcification [5,39,40], whereas osteogenic differentiation is an indicator of calcification [41,42]. TNF ligand-related molecule 1A inhibits vascular calcification by inhibiting osteogenic differentiation [43]. Moreover, the G protein signaling pathway could be implicated in the osteogenic differentiation of VSMCs during vascular calcification [44].
HMGB1 regulates vascular calcification though the Wnt/β-catenin signaling pathway [45] and the transforming growth factor-β/bone morphogenic protein signaling pathway [18]. HMGB1 can be adjusted by transcription factors such as Krüppel-like factor 4 [46] and bromodomain 4 [47] and microRNAs such as mir-142–3p and mir-129–5p [48]. E3 ligase FBXW7 [49] could also promote the degradation of HMGB1 protein. Our results suggest that LKB1 regulates vascular calcification by binding to full-length HMGB1. Also, the HMGB1 inhibitor GA attenuated the aggravated vascular calcification in LGL1SMKO mice, which suggests that HMGB1 may be a potential target in preventing and treating vascular calcification.
Vascular calcification is strongly linked to atherosclerosis, hypertension, vascular injury, chronic kidney disease, senility, and other common diseases. Therefore, understanding the mechanism of vascular calcification is crucial for preventing and treating these diseases.
In this study, vitamin D was used to construct an animal calcification model. In this model, the degree of calcification in the LKB1SMKO group was very severe, the health status of the mice decreased sharply, and induced mortality increased in the later period. We reduced the induction days to increase the survival rate, which led to an insignificant degree of calcification in the control group. However, this further suggested that smooth muscle-specific LKB1 deletion exacerbated vitamin D-induced vascular calcification. This study also had other limitations. First, there are no appropriate antibodies to explore the colocalization of LKB1 and HMGB1 in lysosomes. Second, according to group-based phosphorylation site predicting and scoring (GPS) 5.0, there is no phosphorylation site for LKB1 in HMGB1 (data not shown). Thus, the molecular mechanism by which LKB1 promotes HMGB1 degradation needs to be further explored in future studies.
Overall, this study confirmed that LKB1 inhibits vascular calcification via HMGB1. LKB1 expression was significantly reduced in the arteries of calcified mice and patients with CKD. We provide a new molecular mechanism for the occurrence and development of vascular calcification and new prospects for the translation of this knowledge to clinical practice.
Declaration of competing interest
None.
Acknowledgements
TZ conceived the research concept and design and conducted experimental operations, data analysis, and manuscript writing. HL, CO, GC, JG, JW, NY and JY performed a variety of data collection procedures and helped modify the manuscript. QM, CZ, and WZ directed the research, data analysis, and manuscript writing. All authors read and approved the final manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.redox.2020.101828.
Contributor Information
Qing Min, Email: baimin0628@hbust.edu.cn.
Cheng Zhang, Email: zhangc@sdu.edu.cn.
Wencheng Zhang, Email: zhangwencheng@sdu.edu.cn.
Funding
This work was supported by the National Natural Science Foundation of China (no. 81770473, 81970198, 81970366); the Taishan Scholar Project of Shandong Province of China (no. tsqn20161066); the Natural Science Foundation for Distinguished Young Scholars of Shandong Province (ZR201910230308).
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Rennenberg R.J., Kessels A.G., Schurgers L.J., van Engelshoven J.M., de Leeuw P.W., Kroon A.A. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc. Health Risk Manag. 2009;5(1):185–197. doi: 10.2147/vhrm.s4822. Epub 2009/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han X., Wang L.Y., Diao Z.L., Liu W.H. Apelin: a novel inhibitor of vascular calcification in chronic kidney disease. Atherosclerosis. Jan. 2016;244:1–8. doi: 10.1016/j.atherosclerosis.2015.10.102. Epub 2015/11/19. [DOI] [PubMed] [Google Scholar]
- 3.Mizobuchi M., Towler D., Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J. Am. Soc. Nephrol. : JASN (J. Am. Soc. Nephrol.) 2009;20(7):1453–1464. doi: 10.1681/ASN.2008070692. Epub 2009/05/30. [DOI] [PubMed] [Google Scholar]
- 4.Shanahan C.M., Crouthamel M.H., Kapustin A., Giachelli C.M. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ. Res. 2011;109(6):697–711. doi: 10.1161/CIRCRESAHA.110.234914. Epub 2011/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ewence A.E., Bootman M., Roderick H.L., Skepper J.N., McCarthy G., Epple M. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ. Res. 2008;103(5):e28–34. doi: 10.1161/CIRCRESAHA.108.181305. Epub 2008/08/02. [DOI] [PubMed] [Google Scholar]
- 6.Li Y., Wang W., Chao Y., Zhang F., Wang C. official publication of the Federation of American Societies for Experimental Biology; 2019. CTRP13 Attenuates Vascular Calcification by Regulating Runx2. FASEB Journal. fj201900293RRR. Epub 2019/05/31. [DOI] [PubMed] [Google Scholar]
- 7.Pescatore L.A., Gamarra L.F., Liberman M. Arteriosclerosis, thrombosis, and vascular biology; 2019. Multifaceted Mechanisms of Vascular Calcification in Aging. Atvbaha118311576. Epub 2019/05/31. [DOI] [PubMed] [Google Scholar]
- 8.Voelkl J., Luong T.T., Tuffaha R., Musculus K., Auer T., Lian X. SGK1 induces vascular smooth muscle cell calcification through NF-kappaB signaling. J. Clin. Invest. 2018;128(7):3024–3040. doi: 10.1172/JCI96477. Epub 2018/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou M., Song Y., Li Z., Luo C., Ou J.S., Yu H. Curcumin attenuates osteogenic differentiation and calcification of rat vascular smooth muscle cells. Mol. Cell. Biochem. 2016;420(1–2):151–160. doi: 10.1007/s11010-016-2778-y. Epub 2016/08/10. [DOI] [PubMed] [Google Scholar]
- 10.Evrard S., Delanaye P., Kamel S., Cristol J.P., Cavalier E. Vascular calcification: from pathophysiology to biomarkers. Clinica chimica acta; international journal of clinical chemistry. 2015;438:401–414. doi: 10.1016/j.cca.2014.08.034. Epub 2014/09/23. [DOI] [PubMed] [Google Scholar]
- 11.Millar S.A., John S.G., McIntyre C.W., Ralevic V., Anderson S.I., O'Sullivan S.E. An investigation into the role of osteocalcin in human arterial smooth muscle cell calcification. Front. Endocrinol. 2020;11:369. doi: 10.3389/fendo.2020.00369. Epub 2020/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanahan C.M., Cary N.R., Salisbury J.R., Proudfoot D., Weissberg P.L., Edmonds M.E. Medial localization of mineralization-regulating proteins in association with Monckeberg's sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. Nov. 1999;23(21):2168–2176. doi: 10.1161/01.cir.100.21.2168. Epub 1999/11/26. [DOI] [PubMed] [Google Scholar]
- 13.Lin M.E., Chen T., Leaf E.M., Speer M.Y., Giachelli C.M. Runx2 expression in smooth muscle cells is required for arterial medial calcification in mice. Am. J. Pathol. 2015;185(7):1958–1969. doi: 10.1016/j.ajpath.2015.03.020. Epub 2015/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rong S., Zhao X., Jin X., Zhang Z., Chen L., Zhu Y. Vascular calcification in chronic kidney disease is induced by bone morphogenetic protein-2 via a mechanism involving the Wnt/β-catenin pathway. Cell. Physiol. Biochem. : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2014;34(6):2049–2060. doi: 10.1159/000366400. Epub 2015/01/07. [DOI] [PubMed] [Google Scholar]
- 15.Cai T., Sun D., Duan Y., Wen P., Dai C., Yang J. WNT/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp. Cell Res. 2016;345(2):206–217. doi: 10.1016/j.yexcr.2016.06.007. Epub 2016/06/21. [DOI] [PubMed] [Google Scholar]
- 16.Wang B., Li F., Zhang C., Wei G., Liao P., Dong N. High-mobility group box-1 protein induces osteogenic phenotype changes in aortic valve interstitial cells. J. Thorac. Cardiovasc. Surg. 2016;151(1):255–262. doi: 10.1016/j.jtcvs.2015.09.077. Epub 2015/10/31. [DOI] [PubMed] [Google Scholar]
- 17.Jin X., Rong S., Yuan W., Gu L., Jia J., Wang L. High mobility group box 1 promotes aortic calcification in chronic kidney disease via the Wnt/β-catenin pathway. Front. Physiol. 2018;9:665. doi: 10.3389/fphys.2018.00665. Epub 2018/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Q., Wang Z.Y., Chen L.Y., Hu H.Y. Roles of high mobility group box 1 in cardiovascular calcification. Cell. Physiol. Biochem. : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;42(2):427–440. doi: 10.1159/000477591. Epub 2017/06/02. [DOI] [PubMed] [Google Scholar]
- 19.Giardiello F.M., Trimbath J.D. Peutz-Jeghers syndrome and management recommendations. Clin Gastroenterol Hepatol. Apr. 2006;4(4):408–415. doi: 10.1016/j.cgh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Katajisto P., Vallenius T., Vaahtomeri K., Ekman N., Udd L., Tiainen M. The LKB1 tumor suppressor kinase in human disease. Biochimica et biophysica acta. Jan. 2007;1775(1):63–75. doi: 10.1016/j.bbcan.2006.08.003. Epub 2006/10/03. [DOI] [PubMed] [Google Scholar]
- 21.Alessi D.R., Sakamoto K., Bayascas J.R. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 22.Shackelford D.B., Shaw R.J. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nature reviews Cancer. Aug. 2009;9(8):563–575. doi: 10.1038/nrc2676. Epub 2009/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ylikorkala A., Rossi D.J., Korsisaari N., Luukko K., Alitalo K., Henkemeyer M. Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science (New York, NY) 2001;293(5533):1323–1326. doi: 10.1126/science.1062074. Epub 2001/08/18. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z., Zhu H., Dai X., Wang C., Ding Y., Song P. Macrophage liver kinase B1 inhibits foam cell formation and atherosclerosis. Circ. Res. 2017;121(9):1047–1057. doi: 10.1161/CIRCRESAHA.117.311546. Epub 2017/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W., Wang Q., Wu Y., Moriasi C., Liu Z., Dai X. Endothelial cell-specific liver kinase B1 deletion causes endothelial dysfunction and hypertension in mice in vivo. Circulation. 2014;129(13):1428–1439. doi: 10.1161/CIRCULATIONAHA.113.004146. Epub 2014/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W., Ding Y., Zhang C., Lu Q., Liu Z., Coughlan K. Deletion of endothelial cell-specific liver kinase B1 increases angiogenesis and tumor growth via vascular endothelial growth factor. Oncogene. Jul 27 2017;36(30):4277–4287. doi: 10.1038/onc.2017.61. Epub 2017/03/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakada D., Saunders T.L., Morrison S.J. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468(7324):653–658. doi: 10.1038/nature09571. Epub 2010/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhbandner S., Brummer S., Metzger D., Chambon P., Hofmann F., Feil R. Temporally controlled somatic mutagenesis in smooth muscle. Genesis. 2000;28(1):15–22. doi: 10.1002/1526-968x(200009)28:1<15::aid-gene20>3.0.co;2-c. New York, NY. Epub 2000/10/06. [DOI] [PubMed] [Google Scholar]
- 29.Kim H., Kim H.J., Lee K., Kim J.M., Kim H.S., Kim J.R. alpha-Lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J Cell Mol Med. Feb. 2012;16(2):273–286. doi: 10.1111/j.1582-4934.2011.01294.x. Epub 2011/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai X.Y., Zhao M.M., Cai Y., Guan Q.C., Zhao Y., Guan Y. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int. 2013;83(6):1042–1051. doi: 10.1038/ki.2012.482. Epub 2013/02/01. [DOI] [PubMed] [Google Scholar]
- 31.Vimalraj S., Arumugam B., Miranda P.J., Selvamurugan N. Runx2: structure, function, and phosphorylation in osteoblast differentiation. Int. J. Biol. Macromol. 2015;78:202–208. doi: 10.1016/j.ijbiomac.2015.04.008. Epub 2015/04/18. [DOI] [PubMed] [Google Scholar]
- 32.Tang X., Chen X.F., Wang N.Y., Wang X.M., Liang S.T., Zheng W. SIRT2 acts as a cardioprotective deacetylase in pathological cardiac hypertrophy. Circulation. Nov. 2017;21(21):2051–2067. doi: 10.1161/CIRCULATIONAHA.117.028728. Epub 2017/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S.G., Kim J.R., Choi H.C. Quercetin-induced AMP-activated protein kinase activation attenuates vasoconstriction through LKB1-AMPK signaling pathway. Journal of medicinal food. Feb. 2018;21(2):146–153. doi: 10.1089/jmf.2017.4052. Epub 2017/10/17. [DOI] [PubMed] [Google Scholar]
- 34.Moral-Sanz J., Lewis S.A., MacMillan S., Ross F.A., Thomson A., Viollet B. The LKB1-AMPK-α1 signaling pathway triggers hypoxic pulmonary vasoconstriction downstream of mitochondria. Sci. Signal. 2018;11(550) doi: 10.1126/scisignal.aau0296. Epub 2018/10/04. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda Y., Sato K., Pimentel D.R., Sam F., Shaw R.J., Dyck J.R. Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. J. Biol. Chem. 2009;284(51):35839–35849. doi: 10.1074/jbc.M109.057273. Epub 2009/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H., Qin X., Yang J., Ouyang C., Wu J., Jiang X. Smooth muscle-specific LKB1 deletion exaggerates angiotensin II-induced abdominal aortic aneurysm in mice. J. Mol. Cell. Cardiol. 2019;130:131–139. doi: 10.1016/j.yjmcc.2019.03.021. Epub 2019/04/03. [DOI] [PubMed] [Google Scholar]
- 37.Parhami F., Bostrom K., Watson K., Demer L.L. Role of molecular regulation in vascular calcification. J. Atherosclerosis Thromb. 1996;3(2):90–94. doi: 10.5551/jat1994.3.90. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 38.Proudfoot D., Davies J.D., Skepper J.N., Weissberg P.L., Shanahan C.M. Acetylated low-density lipoprotein stimulates human vascular smooth muscle cell calcification by promoting osteoblastic differentiation and inhibiting phagocytosis. Circulation. 2002;106(24):3044–3050. doi: 10.1161/01.cir.0000041429.83465.41. Epub 2002/12/11. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Duffhues G., Garcia de Vinuesa A., van de Pol V., Geerts M.E., de Vries M.R., Janson S.G. Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J. Pathol. 2019;247(3):333–346. doi: 10.1002/path.5193. Epub 2018/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadra I., Mason J.C., Philippidis P., Florey O., Smythe C.D., McCarthy G.M. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ. Res. 2005;96(12):1248–1256. doi: 10.1161/01.RES.0000171451.88616.c2. Epub 2005/05/21. [DOI] [PubMed] [Google Scholar]
- 41.Steitz S.A., Speer M.Y., Curinga G., Yang H.Y., Haynes P., Aebersold R. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001;89(12):1147–1154. doi: 10.1161/hh2401.101070. Epub 2001/12/12. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi K., Saga H., Chimori Y., Kimura K., Yamanaka Y., Sobue K. Differentiated phenotype of smooth muscle cells depends on signaling pathways through insulin-like growth factors and phosphatidylinositol 3-kinase. J. Biol. Chem. 1998;273(44):28860–28867. doi: 10.1074/jbc.273.44.28860. Epub 1998/10/24. [DOI] [PubMed] [Google Scholar]
- 43.Zhao D., Li J., Xue C., Feng K., Liu L., Zeng P. TL1A inhibits atherosclerosis in apoE-deficient mice by regulating the phenotype of vascular smooth muscle cells. J. Biol. Chem. 2020 Nov 27;295(48):16314–16327. doi: 10.1074/jbc.RA120.015486. Epub 2020 Sep 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gambardella J., Wang X., Mone P., Khondkar W., Santulli G. Genetics of adrenergic signaling drives coronary artery calcification. Atherosclerosis. 2020;310:88–90. doi: 10.1016/j.atherosclerosis.2020.07.025. Epub 2020/08/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin X., Rong S., Yuan W., Gu L., Jia J., Wang L. High mobility group box 1 promotes aortic calcification in chronic kidney disease via the Wnt/beta-catenin pathway. Front. Physiol. 2018;9:665. doi: 10.3389/fphys.2018.00665. Epub 2018/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav S.S., Kumar M., Varshney A., Yadava P.K. KLF4 sensitizes the colon cancer cell HCT-15 to cisplatin by altering the expression of HMGB1 and hTERT. Life sciences. Mar. 2019;1:169–176. doi: 10.1016/j.lfs.2019.02.005. Epub 2019/02/05. [DOI] [PubMed] [Google Scholar]
- 47.Jiang Y., Zhu L., Zhang T., Lu H., Wang C., Xue B. BRD4 has dual effects on the HMGB1 and NF-kappaB signalling pathways and is a potential therapeutic target for osteoarthritis. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2017;1863(12):3001–3015. doi: 10.1016/j.bbadis.2017.08.009. Epub 2017/08/29. [DOI] [PubMed] [Google Scholar]
- 48.Liu K., Huang J., Ni J., Song D., Ding M., Wang J. MALAT1 promotes osteosarcoma development by regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell cycle (Georgetown, Tex. 2017;16(6):578–587. doi: 10.1080/15384101.2017.1288324. Epub 2017/03/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C., Chen F., Feng L., Shan Q., Zheng G.H., Wang Y.J. FBXW7 suppresses HMGB1-mediated innate immune signaling to attenuate hepatic inflammation and insulin resistance in a mouse model of nonalcoholic fatty liver disease. Molecular medicine (Cambridge, Mass) 2019;25(1):29. doi: 10.1186/s10020-019-0099-9. Epub 2019/06/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.