Abstract
NEK7 is the smallest NIMA-related kinase (NEK) in mammals. The pathological and physiological roles of NEK7 have been widely reported in many studies. To date, the major function of NEK7 has been well documented in mitosis and NLRP3 inflammasome activation, but the detailed mechanisms of its regulation remain unclear. This review summarizes current advances in NEK7 research involving mitotic regulation, NLRP3 inflammasome activation, related diseases and potential inhibitors, which may provide new insights into the understanding and therapy of the diseases associated with NEK7, as well as the subsequent studies in the future.
Keywords: NEK7, mitosis, cellular homeostasis, NLRP3 inflammasome, inhibitors
Introduction
Mammalian NIMA-related kinases (NEKs) represent a family of serine/threonine kinases, named NEK1–NEK11, which are implicated in the control of several aspects of mitosis and are involved in non-mitotic functions (Forrest et al., 2003; O’Regan et al., 2007; Fry et al., 2012; Cullati et al., 2017). Members of the NEK family are conserved proteins in structure, sharing approximately 40–45% identity with NIMA within their C-terminal catalytic kinase domains (Kandli et al., 2000). Of these NEK family member, NEK7 is the smallest protein, composed of only a catalytic domain with a 30–40 amino acid N-terminal extension, which shares more than 85% sequence identity to NEK6 (Kandli et al., 2000; Kimura and Okano, 2001). Nevertheless, NEK7 and NEK6 show divergent cellular functions as a result of the differential spatiotemporal tissue distribution and enzymatic control (Belham et al., 2001; Kimura and Okano, 2001; Feige and Motro, 2002; Minoguchi et al., 2003; de Souza et al., 2014). Further analysis led to the discovery that NEK7 gene is on chromosome 1 and that its ORF encodes a 302-amino-acid polypeptide with a molecular mass of 34.5 kD (Kimura and Okano, 2001; Katoh and Katoh, 2004). NEK7 is widely expressed in various tissues, such as the heart, liver, lung, brain, muscle, testis, leukocyte, and spleen (Kimura and Okano, 2001). Accumulating evidence suggests that NEK7 is involved in mitosis regulation through an intricate mechanism (Belham et al., 2003; Forrest et al., 2003; Quarmby and Mahjoub, 2005; Fry et al., 2012).
The NLRP3 inflammasome is an intracellular multiprotein complex that assembles NLRP3, ASC, and pro-caspase-1, which leads to the activation of caspase-1, the cleavage and secretion of interleukin 1β (IL-1β) and interleukin 18 (IL-18) in response to diverse stimuli (He et al., 2016a; Mathur et al., 2017). With the inappropriate release of proinflammatory cytokines, the NLRP3 inflammasome is involved in various inflammatory diseases, such as atherosclerosis, type 2 diabetes, Alzheimer’s disease, gout, rheumatoid arthritis, and inflammatory bowel disease (Liu D. et al., 2020). In recent years, NEK7 has been demonstrated to be essential for canonical NLRP3 inflammasome activation by directly binding to the leucine-rich repeat (LRR) domain of NLRP3 (Shi et al., 2016; Sharif et al., 2019). Furthermore, increasing evidence suggests the important role of NEK7 in the development of NLRP3 inflammasome-related diseases (Xu et al., 2016; Nozaki and Miao, 2019). Therefore, the documented pathogenesis mechanism of NLRP3 inflammasome activation by NEK7 strongly indicates promising roles for targeting NEK7 in treating inflammation-related diseases (Xu et al., 2016).
Clearly, cell division and NLRP3 inflammasome activation are extremely important to normal cellular process and stress responses of organisms. NEK7 drew attention two decades ago, but its function has not been explored completely. Several reviews have summarized the partial role of NEK7 by focusing on single aspects of its function, especially NLRP3 inflammasome activation (Fry et al., 2012; He et al., 2016a; Xu et al., 2016; Liu G. et al., 2020). An extensive overview and detailed classification of the unique molecular mechanisms of NEK7, highlighting its potential as therapeutic targets, may provide novel insight into preventing and treating a host of related diseases.
NEK7 in the Regulation of Mitosis
To date, the role of NEK7 in mitotic progression has been the best characterized function, including centrosome enrichment and microtubule nucleation, which are essential for centriole duplication and centrosomal pericentriolar material (PCM) protein accumulation during interphase, centrosome separation in prophase and proper spindle assembly in metaphase (Yissachar et al., 2006; Kim et al., 2007; O’Regan and Fry, 2009; Salem et al., 2010; Sdelci et al., 2011). Nevertheless, NEKL-3 in Caenorhabditis elegans, although highly homologous to mammalian NEK6/NEK7, showed only molting functions, including the regulation of the apical extracellular matrix, intracellular trafficking and endocytosis, but not its role in mitosis (Yochem et al., 2015; Lazetic and Fay, 2017; Lazetic et al., 2018; Liu D. et al., 2020).
Emerging evidence indicates the possible involvement of NEK7 in centrosome formation and separation, microtube nucleation and spindle assembly (Yissachar et al., 2006; Kim et al., 2007; O’Regan and Fry, 2009; Salem et al., 2010; Sdelci et al., 2011). Endogenous NEK7 protein was initially shown to be enriched at the centrosome throughout all phases of the cell cycle (Kim et al., 2007). Additional studies revealed that the NEK7 signal was also detected temporally in the midbody, spindle poles and cytoplasm, in addition to the centrosome (Yissachar et al., 2006). Established works have demonstrated in detail that NEK7 is essential for centriole duplication and centrosomal accumulation of pericentriolar material proteins in interphase cells (Kim et al., 2011). Importantly, NEK7 deficiency results in a significant increase in the number of mitotic cells acquiring a multipolar or monopolar spindle phenotype and ultimately leads to cell arrest at G1 phase, prometaphase, metaphase, and cytokinesis of anaphase in the cell cycle (Kim et al., 2007). In addition, a decrease in centrosomal γ-tubulin levels and microtubule nucleation activity was observed in NEK7-suppressed cells (Kim et al., 2007; Cohen et al., 2013). Thus, microtubule regulation was thought to be the intermediate mechanism through which NEK7 regulated mitosis progression.
Microtubules are composed of highly dynamic filaments that connect kinetochores to the mitotic spindle poles critical for aligning and segregating chromosomes (Cohen et al., 2013). This precise network has long been suspected to be a major target of NEKs. NEK7 was initially found to be related to centrosomal γ-tubulin levels in mitotic cells in an earlier study (Kim et al., 2007). How γ-tubulin is regulated by NEK7 remains unclear. Another study provided a preliminary hint that Nercc1/NEK9 catalyzed the direct phosphorylation of prokaryotic recombinant NEK6 at Ser206 and probably participated in the regulation of NEK7 in a similar manner (Belham et al., 2003). Conformation and activity analyses showed that the C-terminal domain of NEK9 interacts with NEK6/NEK7 through the release of Tyr97 autoinhibition (Richards et al., 2009), a finding confirmed by a later study (Haq et al., 2015). However, the evidence that this function has an effect on mitotic progression is limited.
Several studies have found that Plk1 was likely the indispensable upstream activator of the NEK9/NEK6/NEK7 cascade in controlling early centrosome separation (Bertran et al., 2011; Sdelci et al., 2011, 2012; Dodson et al., 2013). Notably, one study found that NEK9 phosphorylation by CDK1 and Plk1 ultimately resulted in the phosphorylation of NEK6/NEK7 and the mitotic kinesin Eg5, which are necessary for normal centrosome separation during prophase (Bertran et al., 2011). In another study, DYNLL/LC8 increased the autophosphorylation of Ser944 of NEK9 by binding to a (K/R)XTQT motif adjacent to the NEK9 C-terminal coiled-coil motif, which directly interferes with NEK9 binding to its downstream partner NEK6/7 (Regue et al., 2011). This observation has been confirmed by a structural analysis in a later study by the same authors (Gallego et al., 2013). In addition, one study using Xenopus egg extracts and mammalian cells showed that NEK9 phosphorylated NEDD1 on Ser377, driving its recruitment of γ-tubulin to the centrosome by Plk1-dependent phosphorylation in mitotic cells (Sdelci et al., 2012). However, this study did not find evidence for a role of NEK7 in centriole duplication, in contrast to previous studies (Kim et al., 2011). However, in a recent study, a detailed investigation still showed the important impact of NEK7 depletion on contributions to centrosomal accumulation of the APC/C cofactor Cdh1, which negatively regulates centriole duplication (Gupta et al., 2017). Later, RGS2 was reported to be a novel interactor of NEK7. NEK7 binds to and phosphorylates RGS2 and leads to the localization of RGS2 to the mitotic spindle, which is required for proper mitotic spindle organization and spindle orientation (de Souza et al., 2015). In addition, NEK6 and NEK7 promote the dissociation of EML4 from microtubules in mitosis by phosphorylating the EML4 N-terminal domain at Ser144 and Ser146, which is required for efficient chromosome congression in interphase (Adib et al., 2019). As a potential function of NEK7 in microtube regulation (Cohen et al., 2013), NEK7 was identified to regulate dendrite growth and branching, as well as spine formation and morphology, in part through phosphorylation of the kinesin Eg5/KIF11 (Freixo et al., 2018). At approximately the same time, another study also found that NEK7 specifically controlled the shape and synaptic outputs of cortical parvalbumin interneurons (Hinojosa et al., 2018). However, these studies did not show the cell division-related role of NEK7. Summing up the above studies, the exact role of NEK7 in mitosis regulation needs to be further investigated.
NEK7 in the Regulation of NLRP3 Inflammasome Activation
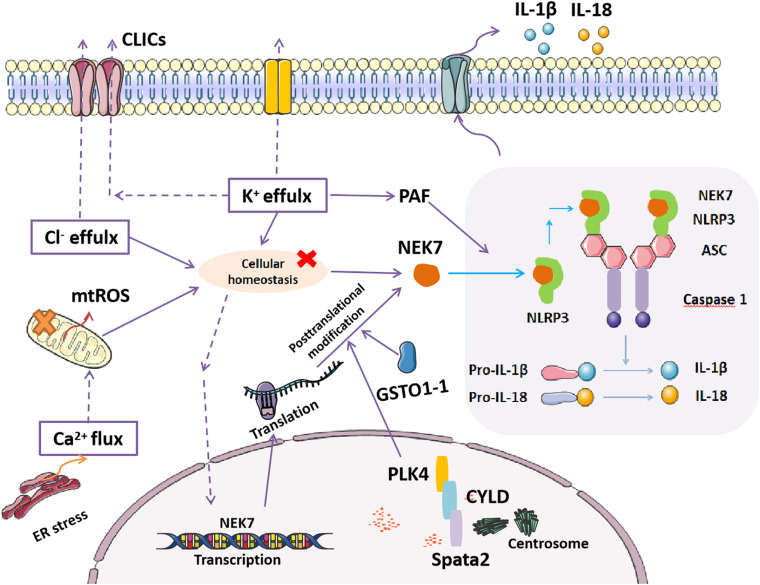
Generally, the activation of the NLRP3 inflammasome requires the synergistic effect of two signals (He et al., 2016a). Signal 1 (priming), such as the microbial component LPS and other endogenous cytokines, promotes the upregulation of inflammasome components as well as its newly discovered member, NEK7, as the protein levels of NLRP3 and NEK7 in resting cells are thought to be insufficient for NLRP3 activation (He et al., 2016a; Shi et al., 2016). Recent reports also showed that post-transcriptional regulation and post-translational modifications of NEK7 and NLRP3 were essential for inflammasome assembly (Song et al., 2017; Hughes et al., 2019; Gritsenko et al., 2020). Signal 2 (activation), such as ATP, pore-forming toxins, viral RNA, and particulate matter, accelerates the activation of the NLRP3 inflammasome, resulting in the activation of caspase 1 and the maturation and secretion of IL-1β and IL-18 (He et al., 2016a). In recent years, in addition to its mitotic function, NEK7 has been better understood for its direct binding with NLRP3, which is indispensable for NLRP3 inflammasome activation (He et al., 2016b; Shi et al., 2016; Nozaki and Miao, 2019; Figure 1).
FIGURE 1.
Mechanisms of NEK7-mediated NLRP3 inflammasome activation. NEK7 directly binds with NLRP3, which triggers canonical NLRP3 inflammasome activation, including the activation of caspase 1, cleavage of pro-IL-1β, and pro-IL-18, producing mature IL-1β and IL-18, which are secreted to the extracellular space as inflammatory effectors. Disordered cellular homeostasis, including K+ efflux, Ca2+ signaling, chloride efflux, and mitochondrial dysfunction (mtROS), are the upstream signals that regulate NEK7-mediated NLRP3 inflammasome activation by either upregulating NEK7 expression or inducing the binding of NEK7 to NLRP3. Post-translational modification of NEK7 regulated by centrosome proteins (spata, CYLD, and PLK4) and the deglutathionylating enzyme GSTO1-1 that inhibits or induces the binding of NEK7 to NLRP3, respectively, affect NLRP3 inflammasome activation.
Initially, through a forward genetic analysis, mice with a mutation in NEK7 were found to display diminished IL-1β secretion, and further evidence revealed that NEK7 binds directly to the leucine-rich repeat (LRR) domain of NLRP3 downstream of the induction of mitochondrial reactive oxygen species (ROS) (Shi et al., 2016). The interaction promotes the assembly and activation of the NLRP3 inflammasome, for the first time suggesting that NEK7 is a component of the NLRP3 inflammasome (Shi et al., 2016). Intriguingly, this study also showed that NEK7 cannot be available for inflammasome activation and mitosis simultaneously because of the limiting amount of it in cells (Shi et al., 2016). Later, compelling cumulative evidence confirms the direct binding of NEK7 and NLRP3, and their interaction in inflammasome activation is regulated under diverse conditions (Gross et al., 2016; Hoss et al., 2019; Nozaki and Miao, 2019; Sharif et al., 2019). Intriguingly, a recent study found that NEK7 was dispensable for NLRP3 inflammasome formation in human and murine cells under pro-inflammatory conditions (Schmacke et al., 2019).
The NLRP3 inflammasome is activated by diverse stimuli, including multiple microbial products, endogenous molecules, and particulate matter (Shi et al., 2016; Mathur et al., 2017; Liu D. et al., 2020). Furthermore, an increasing number of studies have demonstrated that disordered cellular homeostasis following these stimuli is the proximal upstream trigger of NLRP3 inflammasome activation (Shi et al., 2016). Cellular homeostasis are disrupted by various stimuli, including K+ efflux, Ca2+ signaling, chloride efflux, mitochondrial dysfunction, ROS, and lysosomal rupture, which are indispensable for the downstream interaction of NEK7 and NLRP3 (He et al., 2016b; Shi et al., 2016; Tang et al., 2017; Green et al., 2018). The phospholipid platelet-activating factor (PAF) activates the NLRP3 inflammasome in the presence of NEK7 in a mechanism that depends on calcium and potassium flux but not on ROS or cathepsin (Deng et al., 2019). However, as shown in an earlier study, cytochrome c released from stressed or damaged mitochondria negatively regulates NLRP3 inflammasome activation by competitively binding to the LRR domain of NLRP3, thus reducing the interaction between NLRP3 and NEK7 (Akiyama et al., 2016).
In addition, emerging studies have revealed that the transcriptional and post- transcriptional regulation of NEK7 and NLRP3 are also critical for the activation of the NLRP3 inflammasome. NF-kB has been previously reported to upregulate the expression of components of the NLRP3 inflammasome. Recently, a study found that RELA, a subunit of NF-kB, can also transcriptionally upregulate the expression of NEK7, thereby leading to the subsequent interaction of NEK7 and NLRP3 (Chen X. et al., 2019). Alternative splicing (AS) of pre-mRNA determines the generation of various proteins from individual gene with distinct functions. Evidence has indicated stochastic AS of the LRR domain in human NLRP3, but not mouse NLRP3, including the full-length variant and a variant lacking exon 5; the protein encoded by the latter variant cannot interact with NEK7 (Hoss et al., 2019). This finding suggests that LRR in NLRP3 that interacts with NEK7 is encoded by exon 5 (Hoss et al., 2019). At the time of this discovery, another study showed that the endogenous deglutathionylating enzyme GSTO1-1 is involved in the activation of the NLRP3 inflammasome through the mechanistic deglutathionylation of NEK7 of cysteine 253 (Hughes et al., 2019). However, a subsequent study found a novel complicated post-translation modification of NEK7. Specific centrosome-localized spata2 recruits CYLD for the deubiquitination of polo-like kinase 4 (PLK4), which further binds to and phosphorylates NEK7 at Ser204 (Yang et al., 2019). Thereby, this phosphorylation modification of NEK7 suppressed its binding with NLRP3 and inhibited NLRP3 inflammasome activation (Yang et al., 2019). In addition, HDAC6-mediated MTOC localization of NLRP3 may ensure the engagement of centrosomal kinase NEK7 (Magupalli et al., 2020). These studies suggest the important role of NEK7 in NLRP3 inflammasome activation. However, the mechanism by which NEK7 switches from a cell cycle regulator to an inflammasome regulator remains unclear (Yang et al., 2019). In conclusion, it is imperative to characterize the precise regulation of NEK7 involved in NLRP3 inflammasome activation for finding more refined therapeutic targets for related diseases.
Other Functions of NEK7
Other functions of NEK7, except for cell division and the NLRP3 inflammasome activation, have been uncovered in recent years. During the exploration of the protein kinases related to the formation of hippocampal long-term potentiation (LTP), NEK7 was found to be distributed in hippocampal areas and downregulated upon the induction of LTP (Li et al., 2014). In another similar study, NEK7 was found to be a candidate for the neurotransmitter system and immunomodulation by genome-wide association analysis (Gley et al., 2019). However, these studies are observational and the mechanisms remains obscure. In addition, the importance of NEK7 in maintaining telomere integrity has been studied (Tan et al., 2017). In response to damage, NEK7 is recruited to telomeres and directly phosphorylates TRF1 on Ser114, which prevents the subsequent proteasomal degradation (Tan et al., 2017). These studies have implied that NEK7 may be involved in other cellular processes in addition to the regulation of mitosis and the NLRP3 inflammasome. Therefore, further studies are needed to comprehensively understand the role of NEK7.
NEK7-Regulated Mitosis and NLRP3 Inflammasome in Diseases
Diseases Related to Mitosis
Established evidence shows that NEK7 is involved in regulating various aspects of microtubule stability, spindle formation and cytokinesis. Atypical expression or post-translation modification of NEK7 leads to spindle disorganization, cytokinesis disturbance, micronuclei formation, mitotic arrest, and even cell death. An early study reported that the absence of NEK7 leads to lethality in late embryogenesis or at early postnatal stages and to severe growth retardation in mice (Filonenko et al., 2007; Salem et al., 2010). Furthermore, the intimate connection between microtubule instability and unregulated cell division and cancer development suggests that NEK7 has a potential role in oncogenesis. Accordingly, in the last decade, a host of studies have demonstrated the potential role of NEK7 in the cancer development of various tissues. This evidence suggests that NEK7 is a potential therapeutic target for diseases related to mitotic regulation.
Initially, NEK7 was thought to be the downstream target of WHSC1L1 involved in human carcinogenesis through expression profile analysis in vitro using human bladder and lung cancer cell lines (Kang et al., 2013). Similarly, in a recent study, EML4-ALK variant 3(V3) was proposed to mediate microtubule stabilization through NEK7 and NEK9, accelerating cell migration in EML4-ALK lung cancer (O’Regan et al., 2020). Another study found NEK7 is probably a downstream target gene of WHSC1 in multiple squamous cell carcinoma of the head and neck (SCCHN) cell lines as indicated via microarray expression profile analysis (Saloura et al., 2015). Chromatin immunoprecipitation (ChIP) assays showed NEK7 was directly regulated by WHSC1 through H3K36me2 (Saloura et al., 2015). However, solid evidence is limited. Notably, in gallbladder cancer, a significant correlation was first observed between the increased protein expression of NEK7, FoxM1 and Plk1 and tumor differentiation, development and shorter overall survival time (Wang et al., 2013). However, how NEK7 is involved in gallbladder cancer needs to be further determined. Similarly, high NEK7 expression was also significantly correlated with hepatocellular carcinoma (HCC), with the degree of malignancy, as reflected in tumor numbers, tumor diameter, adjacent organ invasion, tumor grade, and TNM stage (Zhou et al., 2016). The downstream target was preliminarily focused on cyclin B1, as silencing of NEK7 resulted in decreased cyclin B1 levels both in vitro and in vivo (Zhou et al., 2016). Nevertheless, a later study demonstrated that silencing NEK7 resulted in reduced CDK2, cyclin D1, and cyclin E levels in vitro, which therefore significantly inhibited retinoblastoma cell (Y79, SO-RB50 and WERI-RB1) proliferation (Zhang J. et al., 2017). The role of NEK7 in prompting retinoblastoma progression was first discovered in a refined meta-analysis of retinoblastoma copy numbers (Krahe et al., 2016). However, the detailed mechanism is still unclear. A recent breast cancer-related study provides solid evidence that UNC45A nuclear localization promotes the expression of the mitotic kinase NEK7 and that the mitotic catastrophe resulting from UNC45A deficiency can be rescued by heterologous NEK7 expression (Eisa et al., 2019). Furthermore, detailed work including computational sequence analysis, RNA-seq data, ChIP-qPCR, and EMSAs have indicated that two novel glucocorticoid response elements (GREs) exist upstream of the NEK7 transcription start site (TSS) and that the glucocorticoid receptor (GR) is a positive regulator downstream of UNC45A that promotes NEK7 gene transcription (Eisa et al., 2019). However, these findings are based only on experiments performed in vitro, and in vivo studies are necessary to ultimately confirm the mechanisms (Eisa et al., 2019). Additionally, in a previous study, Anks3, an interactor of nephronophthisis (NPH, an autosomal recessive cystic kidney disease)-related gene Anks6, may contribute to the nuclear exclusion of NEK7 and prevent undesired re-entry of interphase cells into the cell cycle (Ramachandran et al., 2015). This is the only study of NEK7 in mitosis regulation related to kidney disease (Ramachandran et al., 2015). However, the detailed mechanism and spatiotemporal relationship remain unclear. Comprehensively summarizing the current knowledge of NEK7 function in various aspects of cell division and related diseases will highlight potential targets for effective therapeutic strategies.
Diseases Related to the NLRP3 Inflammasome
In recent years, NEK7-mediated NLRP3 inflammasome activation has been reported to be involved in various inflammatory diseases (Xu et al., 2016; Chen X. et al., 2019; Gomes Torres et al., 2019). A significant correlation was shown between gene polymorphisms in NEK7, TLR (toll-like receptors), NLR (nod-like receptors), and lipid and glucose parameters from healthy children and adolescents and adults (Gomes Torres et al., 2019). This finding suggests a role of NEK7 in metabolic and inflammatory disorders. A later study showed that NEK7 may also be involved in inflammatory bowel disease mediated by NLRP3 inflammasome activation, the mechanism of which has been described in the previous section (Chen X. et al., 2019). Similarly, several studies demonstrated that the development of neuroinflammation post-traumatic brain injury, ventilator-induced lung injury, diabetic periodontitis, DSS-induced ulcerative colitis, and endometritis in cattle display a significant correlation with NEK7-involved NLRP3 inflammasome activation (Chen Y. et al., 2019; Kelly et al., 2019; Liu et al., 2019; Liu H. et al., 2020; Zhou et al., 2019, 2020; Cao et al., 2020). In a previous study, NEK7 was predicted to be a target for miR-664 involved in the proinflammatory response to influenza A (H7N9) virus, but direct evidence was lacking (Chan et al., 2016). In contrast, clinical analysis in patients with systemic lupus erythaematosus (SLE) revealed a negative correlation between the levels of NEK7, NLPR3, and ASC and disease development, whereas a positive correlation was observed with IL-1β and IL-18 (Ma et al., 2018). This finding suggests that the NEK7-NLRP3 complex might play a protective role (Ma et al., 2018). However, the detailed mechanism remains unclear. As a mediator of NLRP3 inflammasome assembly and activation, NEK7 may also be associated with other related diseases resulting from the improper activation of the NLRP3 inflammasome, which requires further evidence.
Inhibitors and Their Application to Diseases
As a critical component of the NLRP3 inflammasome, NEK7 contributes to various pathologies of NLRP3 inflammasome-related diseases (Xu et al., 2016). Some promising selective inhibitors and medicines already used to treat other diseases are found to target various aspects of inflammasome activation regulated by NEK7. These inhibitors may disrupt the upstream events or directly regulate the expression or modification of NEK7 and NLRP3 thus effecting their interaction (Zhang Y. et al., 2017; He et al., 2018; Chiu et al., 2019; Wu et al., 2019; Liu D. et al., 2020; Shi et al., 2020).
Among these inhibitors, MCC950 is the best characterized for its potent effect in many diseases with NEK7-NLRP3-mediated inflammasome activation (Wu et al., 2019), including high glucose-induced human retinal endothelial cell dysfunction (diabetic retinopathy) (Zhang Y. et al., 2017), lung ischemia-reperfusion injury (Xu et al., 2018), endometritis in cattle and peritonitis (Kelly et al., 2019). Although some studies are limited to in vitro applications, MCC950 is still considered to be a promising treatment, as it selectively blocks the interaction between NEK7 and NLRP3 (Wu et al., 2019). In addition, several inhibitors target genes that have been found to be involved in the regulation of NEK7-mediated NLRP3 inflammasome activation, such as C1-27 (GSTO1-1 inhibitor) (Hughes et al., 2019), IAA94 (CLIC inhibitor) (Tang et al., 2017), and JSH-23 (p65 inhibitor) (Chen X. et al., 2019). These inhibitors display a prominent attenuating effect on NEK7-related NLRP3 inflammasome activation, as indicated by in vitro and in vivo experiments under certain conditions.
Importantly, a growing number of studies have suggested that some drugs, including natural products (traditional Chinese medicine) (He et al., 2018; Kim et al., 2019; Shi et al., 2020) and chemical medicines (Zhang et al., 2018; Chiu et al., 2019; Torp et al., 2019; Zhou et al., 2019), have emerging roles in inhibiting NEK7-related NLRP3 inflammasome activation with distinct regulatory mechanisms. The three natural products oridonin (He et al., 2018; Liu H. et al., 2020), artemisinin (Kim et al., 2019), and ginsenoside Rg3 (Shi et al., 2020) have been reported to have similar functions in abrogating NEK7-NLRP3 interaction, as corroborated by different mouse models (He et al., 2018; Kim et al., 2019; Shi et al., 2020). Seven chemical medicines, including glucosamine (Chiu et al., 2019), metformin (Zhou et al., 2019, 2020), glibenclamide (Liu H. et al., 2020), ALK inhibitors (ceritinib and lorlatinib) (Zhang et al., 2018), autophagy inhibitors (chloroquine and bafilomycin A1) (Torp et al., 2019), and 1,25(OH)2D3 (Cao et al., 2020), have also shown significant inhibitory effects on various aspects of the NEK7-NLRP3 interaction. To better understand the therapeutic effect of molecular inhibitors and medicines, more tightly controlled samples from different species urgently need to be included in future studies.
Conclusion and Perspectives
In summary, the biological functions of NEK7, including its pathological and physiological effects, have been explored extensively with various approaches from different organisms in the past 20 years. As explained above, NEK7 is mainly involved in various aspects of mitosis regulation and the activation of the NLRP3 inflammasome. The atypical expression and modification of NEK7 has been found to cause cellular oncogenicity and excessive inflammatory responses, thereby leading to the tumorigenesis of multiple organs and aggravating systemic inflammation. This review also summarizes the current inhibitors and medicines targeting NEK7-mediated diseases. However, the regulation mechanism and the effects of the treatment are neither consistent nor assured. Although some reported inhibitors have shown inhibition of NEK7-mediated inflammasome activation, no published works have shown screening of highly selective and potent inhibitors of NEK7. The latest research showed that use of conventional methods led to failed designs of NEK7 inhibitors owing to its unique conformation and high similarity to its paralog NEK6 (Byrne et al., 2020). Then, this group developed a novel strategy using SRS mutations of NEK7, which is a promising starting point for developing potent inhibitors targeting NEK7 (Byrne et al., 2020). However, the kinase activity inhibition of NEK7 might have no effect on NLRP3 inflammasome activation (Shi et al., 2016). Therefore, further studies unraveling detailed mechanism and spatiotemporal relationships and searches for more specific inhibitors will provide additional beneficial insights useful for developing more-effective and specific approaches to understanding and treating related diseases.
Author Contributions
ZS drafted the manuscript. ZJ, YZ, and WG revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- NLRP3
NLR Family Pyrin Domain Containing 3
- ORF
Open reading frame
- Plk1
Polo Like Kinase 1
- CDK1
Cyclin Dependent Kinase 1
- RGS2
Regulator Of G Protein Signaling 2
- EML4
EMAP Like 4
- ATP
adenosine triphosphate
- GSTO1-1
Glutathione S-Transferase Omega 1
- CYLD
CYLD Lysine 63 Deubiquitinase
- MTOC
microtubule-organizing center
- HDAC6
dynein adapter histone deacetylase 6
- LTP
Hippocampal long term potentiation
- TRF1
Telomeric Repeat Binding Factor 1
- WHSC1L1
Wolf-Hirschhorn Syndrome Candidate 1-Like 2
- EML4
EMAP Like 4
- FoxM1
Forkhead Box M1
- TNM
Tumor Node Metastasis
- DSS
Dextran Sulfate Sodium
- SRS
Strong R-spine.
Footnotes
Funding. Publication of this review and the authors’ research related to the topic were supported by grant from the National Natural Science Foundation of China (81700651).
References
- Adib R., Montgomery J. M., Atherton J., O’Regan L., Richards M. W., Straatman K. R., et al. (2019). Mitotic phosphorylation by NEK6 and NEK7 reduces the microtubule affinity of EML4 to promote chromosome congression. Sci. Signal. 12:eaaw2939. 10.1126/scisignal.aaw2939 [DOI] [PubMed] [Google Scholar]
- Akiyama T., Shi C.-S., Kehrl J. H. (2016). Cytochrome c negatively regulates NLRP3 inflammasomes. PLoS One 11:e0167636. 10.1371/journal.pone.0167636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belham C., Comb M. J., Avruch J. (2001). Identification of the NIMA family kinases NEK6/7 as regulators of the p70 ribosomal S6 kinase. Curr. Biol. 11 1155–1167. 10.1016/s0960-9822(01)00369-4 [DOI] [PubMed] [Google Scholar]
- Belham C., Roig J., Caldwell J. A., Aoyama Y., Kemp B. E., Comb M., et al. (2003). A mitotic cascade of NIMA family kinases. Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J. Biol. Chem. 278 34897–34909. 10.1074/jbc.M303663200 [DOI] [PubMed] [Google Scholar]
- Bertran M. T., Sdelci S., Regue L., Avruch J., Caelles C., Roig J. (2011). Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. EMBO J. 30 2634–2647. 10.1038/emboj.2011.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M. J., Nasir N., Basmadjian C., Bhatia C., Cunnison R. F., Carr K. H., et al. (2020). Nek7 conformational flexibility and inhibitor binding probed through protein engineering of the R-spine. Biochem. J. 477 1525–1539. 10.1042/BCJ20200128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R., Ma Y., Li S., Shen D., Yang S., Wang X., et al. (2020). 1,25(OH)2 D3 alleviates DSS-induced ulcerative colitis via inhibiting NLRP3 inflammasome activation. J. Leukoc Biol. 108 283–295. 10.1002/JLB.3MA0320-406RR [DOI] [PubMed] [Google Scholar]
- Chan M. C. W., Wolf S., Wu W., Jones C., Perwitasari O., Mahalingam S., et al. (2016). MicroRNA regulation of human genes essential for influenza A (H7N9) replication. PLoS One 11:e0155104. 10.1371/journal.pone.0155104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Liu G., Yuan Y., Wu G., Wang S., Yuan L. (2019). NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis. 10:906. 10.1038/s41419-019-2157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Meng J., Bi F., Li H., Chang C., Ji C., et al. (2019). Corrigendum: NEK7 Regulates NLRP3 inflammasome activation and neuroinflammation post-traumatic brain injury. Front. Mol. Neurosci. 12:247. 10.3389/fnmol.2019.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H. W., Li L. H., Hsieh C. Y., Rao Y. K., Chen F. H., Chen A., et al. (2019). Glucosamine inhibits IL-1beta expression by preserving mitochondrial integrity and disrupting assembly of the NLRP3 inflammasome. Sci. Rep. 9:5603. 10.1038/s41598-019-42130-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Aizer A., Shav-Tal Y., Yanai A., Motro B. (2013). Nek7 kinase accelerates microtubule dynamic instability. Biochim. Biophys. Acta 1833 1104–1113. 10.1016/j.bbamcr.2012.12.021 [DOI] [PubMed] [Google Scholar]
- Cullati S. N., Kabeche L., Kettenbach A. N., Gerber S. A. (2017). A bifurcated signaling cascade of NIMA-related kinases controls distinct kinesins in anaphase. J. Cell Biol. 216 2339–2354. 10.1083/jcb.201512055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza E. E., Hehnly H., Perez A. M., Meirelles G. V., Smetana J. H. C., Doxsey S., et al. (2015). Human Nek7-interactor RGS2 is required for mitotic spindle organization. Cell Cycle 14 656–667. 10.4161/15384101.2014.994988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza E. E., Meirelles G. V., Godoy B. B., Perez A. M., Smetana J. H., Doxsey S. J., et al. (2014). Characterization of the human NEK7 interactome suggests catalytic and regulatory properties distinct from those of NEK6. J. Proteome Res. 13 4074–4090. 10.1021/pr500437x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M., Guo H., Tam J. W., Johnson B. M., Brickey W. J., New J. S., et al. (2019). Platelet-activating factor (PAF) mediates NLRP3-NEK7 inflammasome induction independently of PAFR. J. Exp. Med. 216 2838–2853. 10.1084/jem.20190111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson C. A., Yeoh S., Haq T., Bayliss R. (2013). A kinetic test characterizes kinase intramolecular and intermolecular autophosphorylation mechanisms. Sci. Signal. 6:ra54. 10.1126/scisignal.2003910 [DOI] [PubMed] [Google Scholar]
- Eisa N. H., Jilani Y., Kainth K., Redd P., Lu S., Bougrine O., et al. (2019). The co-chaperone UNC45A is essential for the expression of mitotic kinase NEK7 and tumorigenesis. J. Biol. Chem. 294 5246–5260. 10.1074/jbc.RA118.006597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige E., Motro B. (2002). The related murine kinases. Nek6 and Nek7, display distinct patterns of expression. Mech. Dev. 110 219–223. 10.1016/s0925-4773(01)00573-1 [DOI] [PubMed] [Google Scholar]
- Filonenko E. S., Volchkov P., Mufazalov I. A., Kiselev S. L., Lagar’kova M. A. (2007). Protein kinases abundantly expressed in undifferentiated human ESC lines and derived embryoid bodies. Tsitologiia 49 561–565. [PubMed] [Google Scholar]
- Forrest A. R., Taylor D., Grimmond S., Group R. G., Members G. S. L. (2003). Exploration of the cell-cycle genes found within the RIKEN FANTOM2 data set. Genome Res. 13 1366–1375. 10.1101/gr.1012403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freixo F., Martinez Delgado P., Manso Y., Sanchez-Huertas C., Lacasa C., Soriano E., et al. (2018). NEK7 regulates dendrite morphogenesis in neurons via Eg5-dependent microtubule stabilization. Nat. Commun. 9:2330. 10.1038/s41467-018-04706-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A. M., O’Regan L., Sabir S. R., Bayliss R. (2012). Cell cycle regulation by the NEK family of protein kinases. J. Cell Sci. 125 4423–4433. 10.1242/jcs.111195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego P., Velazquez-Campoy A., Regue L., Roig J., Reverter D. (2013). Structural analysis of the regulation of the DYNLL/LC8 binding to Nek9 by phosphorylation. J. Biol. Chem. 288 12283–12294. 10.1074/jbc.M113.459149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gley K., Murani E., Haack F., Trakooljul N., Zebunke M., Puppe B., et al. (2019). Haplotypes of coping behavior associated QTL regions reveal distinct transcript profiles in amygdala and hippocampus. Behav. Brain Res. 372:112038. 10.1016/j.bbr.2019.112038 [DOI] [PubMed] [Google Scholar]
- Gomes Torres A. C. M. B., Leite N., Tureck L. V., de Souza R. L. R., Titski A. C. K., Milano-Gai G. E., et al. (2019). Association between Toll-like receptors (TLR) and NOD-like receptor (NLR) polymorphisms and lipid and glucose metabolism. Gene 685 211–221. 10.1016/j.gene.2018.11.065 [DOI] [PubMed] [Google Scholar]
- Green J. P., Yu S., Martin-Sanchez F., Pelegrin P., Lopez-Castejon G., Lawrence C. B., et al. (2018). Chloride regulates dynamic NLRP3-dependent ASC oligomerization and inflammasome priming. Proc. Natl. Acad. Sci. U.S.A. 115 E9371–E9380. 10.1073/pnas.1812744115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsenko A., Green J. P., Brough D., Lopez-Castejon G. (2020). Mechanisms of NLRP3 priming in inflammaging and age related diseases. Cytokine Growth Factor Rev. 55 15–25. 10.1016/j.cytogfr.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C. J., Mishra R., Schneider K. S., Medard G., Wettmarshausen J., Dittlein D. C., et al. (2016). K+ Efflux-Independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity 45 761–773. 10.1016/j.immuni.2016.08.010 [DOI] [PubMed] [Google Scholar]
- Gupta A., Tsuchiya Y., Ohta M., Shiratsuchi G., Kitagawa D. (2017). NEK7 is required for G1 progression and procentriole formation. Mol. Biol. Cell 28 2123–2134. 10.1091/mbc.E16-09-0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq T., Richards M. W., Burgess S. G., Gallego P., Yeoh S., O’Regan L., et al. (2015). Mechanistic basis of Nek7 activation through Nek9 binding and induced dimerization. Nat. Commun. 6:8771. 10.1038/ncomms9771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Jiang H., Chen Y., Ye J., Wang A., Wang C., et al. (2018). Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 9:2550. 10.1038/s41467-018-04947-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Hara H., Nunez G. (2016a). Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 41 1012–1021. 10.1016/j.tibs.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Zeng M. Y., Yang D., Motro B., Nunez G. (2016b). NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530 354–357. 10.1038/nature16959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa A. J., Deogracias R., Rico B. (2018). The microtubule regulator NEK7 coordinates the wiring of cortical parvalbumin interneurons. Cell Rep. 24 1231–1242. 10.1016/j.celrep.2018.06.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoss F., Mueller J. L., Rojas Ringeling F., Rodriguez-Alcazar J. F., Brinkschulte R., Seifert G., et al. (2019). Alternative splicing regulates stochastic NLRP3 activity. Nat. Commun. 10:3238. 10.1038/s41467-019-11076-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M. M., Hooftman A., Angiari S., Tummala P., Zaslona Z., Runtsch M. C., et al. (2019). Glutathione transferase Omega-1 Regulates NLRP3 inflammasome activation through NEK7 deglutathionylation. Cell Rep. 29 151.e5–161.e5. 10.1016/j.celrep.2019.08.072 [DOI] [PubMed] [Google Scholar]
- Kandli M., Feige E., Chen A., Kilfin G., Motro B. (2000). Isolation and characterization of two evolutionarily conserved murine kinases (Nek6 and nek7) related to the fungal mitotic regulator. NIMA. Genomics 68 187–196. 10.1006/geno.2000.6293 [DOI] [PubMed] [Google Scholar]
- Kang D., Cho H. S., Toyokawa G., Kogure M., Yamane Y., Iwai Y., et al. (2013). The histone methyltransferase Wolf-Hirschhorn syndrome candidate 1-like 1 (WHSC1L1) is involved in human carcinogenesis. Genes Chromosomes Cancer 52 126–139. 10.1002/gcc.22012 [DOI] [PubMed] [Google Scholar]
- Katoh M., Katoh M. (2004). Identification and characterization of Crumbs homolog 2 gene at human chromosome 9q33.3. Int. J. Oncol. 24 743–749. [PubMed] [Google Scholar]
- Kelly P., Meade K. G., O’Farrelly C. (2019). Non-canonical inflammasome-mediated IL-1β production by primary endometrial epithelial and stromal fibroblast cells Is NLRP3 and Caspase-4 dependent. Front. Immunol. 10:102. 10.3389/fimmu.2019.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kim S., Rhee K. (2011). NEK7 is essential for centriole duplication and centrosomal accumulation of pericentriolar material proteins in interphase cells. J. Cell Sci. 124 3760–3770. 10.1242/jcs.078089 [DOI] [PubMed] [Google Scholar]
- Kim S., Lee K., Rhee K. (2007). NEK7 is a centrosomal kinase critical for microtubule nucleation. Biochem. Biophys. Res. Commun. 360 56–62. 10.1016/j.bbrc.2007.05.206 [DOI] [PubMed] [Google Scholar]
- Kim S. K., Choe J. Y., Park K. Y. (2019). Anti-inflammatory effect of artemisinin on uric acid-induced NLRP3 inflammasome activation through blocking interaction between NLRP3 and NEK7. Biochem. Biophys. Res. Commun. 517 338–345. 10.1016/j.bbrc.2019.07.087 [DOI] [PubMed] [Google Scholar]
- Kimura M., Okano Y. (2001). Identification and assignment of the human NIMA-related protein kinase 7 gene (NEK7) to human chromosome 1q31.3. Cytogenetics Cell Genet. 94 33–38. 10.1159/000048779 [DOI] [PubMed] [Google Scholar]
- Krahe R., Kooi I. E., Mol B. M., Massink M. P. G., de Jong M. C., de Graaf P., et al. (2016). A meta-analysis of retinoblastoma copy numbers refines the list of possible driver genes involved in tumor progression. PLoS One 11:e0153323. 10.1371/journal.pone.0153323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazetic V., Fay D. S. (2017). Conserved ankyrin repeat proteins and their NIMA kinase partners regulate extracellular matrix remodeling and intracellular trafficking in Caenorhabditis elegans. Genetics 205 273–293. 10.1534/genetics.116.194464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazetic V., Joseph B. B., Bernazzani S. M., Fay D. S. (2018). Actin organization and endocytic trafficking are controlled by a network linking NIMA-related kinases to the CDC-42-SID-3/ACK1 pathway. PLoS Genet. 14:e1007313. 10.1371/journal.pgen.1007313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Wan J., Sase S., Gröger M., Pollak A., Korz V., et al. (2014). Protein kinases paralleling late-phase LTP formation in dorsal hippocampus in the rat. Neurochem. Int. 76 50–58. 10.1016/j.neuint.2014.05.014 [DOI] [PubMed] [Google Scholar]
- Liu D., Zeng X., Li X., Cui C., Hou R., Guo Z., et al. (2020). Advances in the molecular mechanisms of NLRP3 inflammasome activators and inacativators. Biochem. Pharmacol. 175:113863 10.1016/j.bcp.2020.113863 [DOI] [PubMed] [Google Scholar]
- Liu G., Chen X., Wang Q., Yuan L. (2020). NEK7: a potential therapy target for NLRP3-related diseases. Biosci. Trends 14 74–82. 10.5582/bst.2020.01029 [DOI] [PubMed] [Google Scholar]
- Liu H., Gu C., Liu M., Liu G., Wang Y. (2020). NEK7 mediated assembly and activation of NLRP3 inflammasome downstream of potassium efflux in ventilator-induced lung injury. Biochem. Pharmacol. 177:113998. 10.1016/j.bcp.2020.113998 [DOI] [PubMed] [Google Scholar]
- Liu H., Gu C., Liu M., Liu G., Wang D., Liu X., et al. (2019). Ventilator-induced lung injury is alleviated by inhibiting NLRP3 inflammasome activation. Mol. Immunol. 111 1–10. 10.1016/j.molimm.2019.03.011 [DOI] [PubMed] [Google Scholar]
- Ma Z. Z., Sun H. S., Lv J. C., Guo L., Yang Q. R. (2018). Expression and clinical significance of the NEK7-NLRP3 inflammasome signaling pathway in patients with systemic lupus erythematosus. J. Inflamm. 15:16. 10.1186/s12950-018-0192-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magupalli V. G., Negro R., Tian Y., Hauenstein A. V., Di Caprio G., Skillern W., et al. (2020). HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science 369:eaas8995. 10.1126/science.aas8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A., Hayward J. A., Man S. M. (2017). Molecular mechanisms of inflammasome signaling. J. Leukocyte Biol. 103 233–257. 10.1189/jlb.3MR0617-250R [DOI] [PubMed] [Google Scholar]
- Minoguchi S., Minoguchi M., Yoshimura A. (2003). Differential control of the NIMA-related kinases. Nek6 and Nek7, by serum stimulation. Biochem. Biophys. Res. Commun. 301 899–906. 10.1016/s0006-291x(03)00049-4 [DOI] [PubMed] [Google Scholar]
- Nozaki K., Miao E. A. (2019). A licence to kill during inflammation. Nature 570 316–317. 10.1038/d41586-019-01764-9 [DOI] [PubMed] [Google Scholar]
- O’Regan L., Barone G., Adib R., Woo C. G., Jeong H. J., Richardson E. L., et al. (2020). EML4-ALK V3 oncogenic fusion proteins promote microtubule stabilization and accelerated migration through NEK9 and NEK7. J. Cell Sci. 133:jcs241505. 10.1242/jcs.241505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Regan L., Blot J., Fry A. M. (2007). Mitotic regulation by NIMA-related kinases. Cell Divis. 2 25. 10.1186/1747-1028-2-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Regan L., Fry A. M. (2009). The Nek6 and Nek7 protein kinases are required for robust mitotic spindle formation and cytokinesis. Mol. Cell. Biol. 29 3975–3990. 10.1128/mcb.01867-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby L. M., Mahjoub M. R. (2005). Caught Nek-ing: cilia and centrioles. J. Cell Sci. 118 5161–5169. 10.1242/jcs.02681 [DOI] [PubMed] [Google Scholar]
- Ramachandran H., Engel C., Muller B., Dengjel J., Walz G., Yakulov T. A. (2015). Anks3 alters the sub-cellular localization of the Nek7 kinase. Biochem. Biophys. Res. Commun. 464 901–907. 10.1016/j.bbrc.2015.07.063 [DOI] [PubMed] [Google Scholar]
- Regue L., Sdelci S., Bertran M. T., Caelles C., Reverter D., Roig J. (2011). DYNLL/LC8 protein controls signal transduction through the Nek9/Nek6 signaling module by regulating Nek6 binding to Nek9. J. Biol. Chem. 286 18118–18129. 10.1074/jbc.M110.209080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M. W., O’Regan L., Mas-Droux C., Blot J. M., Cheung J., Hoelder S., et al. (2009). An autoinhibitory tyrosine motif in the cell-cycle-regulated Nek7 kinase is released through binding of Nek9. Mol. Cell 36 560–570. 10.1016/j.molcel.2009.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H., Rachmin I., Yissachar N., Cohen S., Amiel A., Haffner R., et al. (2010). Nek7 kinase targeting leads to early mortality, cytokinesis disturbance and polyploidy. Oncogene 29 4046–4057. 10.1038/onc.2010.162 [DOI] [PubMed] [Google Scholar]
- Saloura V., Cho H. S., Kiyotani K., Alachkar H., Zuo Z., Nakakido M., et al. (2015). WHSC1 promotes oncogenesis through regulation of NIMA-related kinase-7 in squamous cell carcinoma of the head and neck. Mol. Cancer Res. 13 293–304. 10.1158/1541-7786.MCR-14-0292-T [DOI] [PubMed] [Google Scholar]
- Schmacke N. A., Gaidt M. M., Szymanska I., O’Duill F., Stafford C. A., Chauhan D., et al. (2019). Priming enables a NEK7-independent route of NLRP3 activation. bioRxiv [Preprint]. 10.1101/799320 [DOI] [Google Scholar]
- Sdelci S., Bertran M. T., Roig J. (2011). Nek9, Nek6, Nek7 and the separation of centrosomes. Cell Cycle 10 3816–3817. 10.4161/cc.10.22.18226 [DOI] [PubMed] [Google Scholar]
- Sdelci S., Schutz M., Pinyol R., Bertran M. T., Regue L., Caelles C., et al. (2012). Nek9 phosphorylation of NEDD1/GCP-WD contributes to Plk1 control of gamma-tubulin recruitment to the mitotic centrosome. Curr. Biol. 22 1516–1523. 10.1016/j.cub.2012.06.027 [DOI] [PubMed] [Google Scholar]
- Sharif H., Wang L., Wang W. L., Magupalli V. G., Andreeva L., Qiao Q., et al. (2019). Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570 338–343. 10.1038/s41586-019-1295-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Wang Y., Li X., Zhan X., Tang M., Fina M., et al. (2016). NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 17 250–258. 10.1038/ni.3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wang H., Zheng M., Xu W., Yang Y., Shi F. (2020). Ginsenoside Rg3 suppresses the NLRP3 inflammasome activation through inhibition of its assembly. FASEB J. 34 208–221. 10.1096/fj.201901537R [DOI] [PubMed] [Google Scholar]
- Song N., Liu Z. S., Xue W., Bai Z. F., Wang Q. Y., Dai J., et al. (2017). NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol. Cell 68 185.e6–197.e6. 10.1016/j.molcel.2017.08.017 [DOI] [PubMed] [Google Scholar]
- Tan R., Nakajima S., Wang Q., Sun H., Xue J., Wu J., et al. (2017). Nek7 protects telomeres from oxidative DNA damage by phosphorylation and stabilization of TRF1. Mol. Cell 65 818.e5–831.e5. 10.1016/j.molcel.2017.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T., Lang X., Xu C., Wang X., Gong T., Yang Y., et al. (2017). CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat. Commun. 8:202. 10.1038/s41467-017-00227-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torp M. K., Yang K., Ranheim T., Huso Lauritzen K., Alfsnes K., Vinge L. E., et al. (2019). Mammalian target of rapamycin (mTOR) and the proteasome attenuates IL-1beta expression in primary mouse cardiac fibroblasts. Front. Immunol. 10:1285. 10.3389/fimmu.2019.01285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Song Y., Xu X., Wu Q., Liu C. (2013). The expression of Nek7, FoxM1, and Plk1 in gallbladder cancer and their relationships to clinicopathologic features and survival. Clin. Transl. Oncol. 15 626–632. 10.1007/s12094-012-0978-9 [DOI] [PubMed] [Google Scholar]
- Wu D., Chen Y., Sun Y., Gao Q., Li H., Yang Z., et al. (2019). Target of MCC950 in Inhibition of NLRP3 inflammasome activation: a literature review. Inflammation 43 17–23. 10.1007/s10753-019-01098-8 [DOI] [PubMed] [Google Scholar]
- Xu J., Lu L., Li L. (2016). NEK7: a novel promising therapy target for NLRP3-related inflammatory diseases. Acta Biochim. Biophys. Sin. 48 966–968. 10.1093/abbs/gmw080 [DOI] [PubMed] [Google Scholar]
- Xu K. Y., Wu C. Y., Tong S., Xiong P., Wang S. H. (2018). The selective Nlrp3 inflammasome inhibitor Mcc950 attenuates lung ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 503 3031–3037. 10.1016/j.bbrc.2018.08.089 [DOI] [PubMed] [Google Scholar]
- Yang X. D., Li W., Zhang S., Wu D., Jiang X., Tan R., et al. (2019). PLK4 deubiquitination by Spata2-CYLD suppresses NEK7-mediated NLRP3 inflammasome activation at the centrosome. EMBO J. 39:e102201. 10.15252/embj.2019102201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yissachar N., Salem H., Tennenbaum T., Motro B. (2006). Nek7 kinase is enriched at the centrosome, and is required for proper spindle assembly and mitotic progression. FEBS Lett. 580 6489–6495. 10.1016/j.febslet.2006.10.069 [DOI] [PubMed] [Google Scholar]
- Yochem J., Lažetić V., Bell L., Chen L., Fay D. (2015). C. elegans NIMA-related kinases NEKL-2 and NEKL-3 are required for the completion of molting. Dev. Biol. 398 255–266. 10.1016/j.ydbio.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Wei W., Qiu J. (2018). ALK is required for NLRP3 inflammasome activation in macrophages. Biochem. Biophys. Res. Commun. 501 246–252. 10.1016/j.bbrc.2018.04.226 [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang L., Zhang Y. (2017). Downregulation of NIMA-related kinase-7 inhibits cell proliferation by inducing cell cycle arrest in human retinoblastoma cells. Exp. Ther. Med. 15 1360–1366. 10.3892/etm.2017.5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lv X., Hu Z., Ye X., Zheng X., Ding Y., et al. (2017). Protection of Mcc950 against high-glucose-induced human retinal endothelial cell dysfunction. Cell Death Dis. 8:e2941. 10.1038/cddis.2017.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Wang Z., Xu X., Wan Y., Qu K., Fan H., et al. (2016). Nek7 is overexpressed in hepatocellular carcinoma and promotes hepatocellular carcinoma cell proliferation in vitro and in vivo. Oncotarget 7 18620–18630. 10.18632/oncotarget.7620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Wang Q., Nie L., Zhang P., Zhao P., Yuan Q., et al. (2020). Metformin ameliorates the NLPP3 inflammasome mediated pyroptosis by inhibiting the expression of NEK7 in diabetic periodontitis. Arch. Oral. Biol. 116:104763. 10.1016/j.archoralbio.2020.104763 [DOI] [PubMed] [Google Scholar]
- Zhou X., Zhang P., Wang Q., Ji N., Xia S., Ding Y., et al. (2019). Metformin ameliorates experimental diabetic periodontitis independently of mammalian target of rapamycin (mTOR) inhibition by reducing NIMA-related kinase 7(Nek7) expression. J. Periodontol. 90 1032–1042. 10.1002/jper.10311 [DOI] [PubMed] [Google Scholar]