Abstract
In the future, humans may live in space because of global pollution and weather fluctuations. In microgravity, convection does not occur, which may change the amyloidogenicity of proteins. However, the effect of gravity on amyloid fibril formation is unclear and remains to be elucidated. Here, we analyzed the effect of microgravity on amyloid fibril formation of amyloidogenic proteins including insulin, amyloid β42 (Aβ42), and transthyretin (TTR). We produced microgravity (10−3 g) by using the gravity controller Gravite. Human insulin, Aβ42, and human wild-type TTR (TTRwt) were incubated at pH 3.0, 7.0, and 3.5 at 37 °C, respectively, in 1 g on the ground or in microgravity. We measured amyloidogenicity via the thioflavin T (ThT) method and cell-based 1-fluoro-2,5-bis[(E)-3-carboxy-4-hydroxystyryl]benzene (FSB) assay. ThT fluorescence intensity and cell-based FSB assay results for human insulin samples were decreased in microgravity compared with results in 1 g. Aβ42 samples did not differ in ThT fluorescence intensity in microgravity and in 1 g on the ground. However, in the cell-based FSB assay, the staining intensity was reduced in microgravity compared with that on 1 g. Human TTRwt tended to form fewer amyloid fibrils in ThT fluorescence intensity and cell-based FSB assays in microgravity than in 1 g. Human insulin and Aβ42 showed decreased amyloid fibril formation in microgravity compared with that in 1 g. Human TTRwt tended to form fewer amyloid fibrils in microgravity. Our experiments suggest that the earth's gravity may be an accelerating factor for amyloid fibril formation.
Keywords: Insulin, Aβ42, TTR, Microgravity, Amyloidosis, Amyloid fibrils
Abbreviations: Aβ, amyloid β; DMSO, dimethyl sulfoxide; FSB, 1-fluoro-2,5-bis[(E)-3-carboxy-4-hydroxystyryl]benzene; PBS, phosphate-buffered saline; ThT, thioflavin T; TTR, transthyretin; TTRwt, wild-type transthyretin
Highlights
-
•
Soon, humans may live in space where gravity is less than the ground.
-
•
In microgravity, amyloidogenic proteins did not form much amyloid fibrils.
-
•
Amyloidosis patients are beneficial to live in space.
1. Introduction
In amyloidosis, is protein metabolism disorder, soluble proteins are deposited as abnormal insoluble fibrils in tissues. Amyloid fibrils are formed by polymerization of the β-sheet conformation of amyloidogenic proteins. Amyloid fibrils are similar to nylon fibrils.
Amyloidosis is a group of hereditary or nonhereditary diseases that are distinguished by extracellular deposition of insoluble amyloid fibrils derived from different proteins in organs such as the eyes, nerves, heart, liver, kidneys [[1], [2], [3]]. So far, 37 amyloidogenic proteins have been identified as disease-causing molecules in amyloid-related illnesses such as prion disease, Alzheimer's disease, AA amyloidosis, systemic immunoglobulin light-chain amyloidosis, hereditary transthyretin amyloidosis ATTRv (familial amyloid polyneuropathy), and wild-type transthyretin amyloidosis ATTRwt (senile systemic amyloidosis) [[4], [5], [6]]. Aging, genetic mutations, inflammation, obesity, neoplastic disorders, and medical treatments reportedly affect amyloidosis development by means of misfolding, overproduction, and decreased clearance of disease-specific amyloid-related proteins and by proteolysis [2,3,7]. In addition, the protein-protein interaction of amyloidogenic proteins may be important in amyloid fibril formation.
Space medicine has made remarkable progress, and many reports on the effects of gravity on healthy astronauts have been published [8]. In space, microgravity influences human physiology and pathological conditions including blood pressure, osteogenesis, muscle wasting, obstructive stress, vestibular organs, and radiation effects. However, the relationship of gravity with various diseases has not been clarified. In the amyloid formation mechanism, protein-protein polymerization is indispensable for forming amyloid fibrils. No convection exists in zero gravity and under such conditions, the frequency of the protein-protein interaction may change; microgravity may thus affect amyloidogenicity.
Recently, abnormal weather has been occurring throughout the world; in the future, human beings may live in space. Because of that possibility, we should investigate whether various amyloidoses may be affected by the gravity changes in space.
In this study, we analyzed the effects of microgravity on amyloid fibril formation of amyloidogenic proteins including human insulin, amyloid β42 (Aβ42), and human transthyretin (TTR) by means of the gravity controller Gravite, which produces microgravity in a laboratory on the ground.
2. Materials and methods
2.1. Amyloid fibril formation of human insulin
Recombinant human insulin solution (Eli Lilly and Company, Indiana, USA) was mixed with an equal volume of 1 mol/L glycine-HCl buffer at pH 3.0 and the 10 μM of insulin was incubated at 37 °C with agitation for 96 h in microgravity controlled by Gravite [9] and in 1 g on the ground.
2.2. Amyloid fibril formation of Aβ42
Samples of 2 μM Aβ42 (human, 1–42) (Peptide Institute, Inc., Osaka, Japan) were incubated in phosphate-buffered saline (PBS) at 37 °C for 24 h in microgravity controlled by Gravite [10] and in 1 g on the ground.
2.3. Amyloid fibril formation of human wild-type TTR (TTRwt)
To make acid-induced amorphous aggregates of human TTRwt, we incubated 20 μM recombinant full-length human TTRwt (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) at pH 3.5 in 50 mM sodium acetate buffer containing 50 mM NaCl for 48 h at 37 °C, according to methods used in our previous study [3,11,12], with samples being incubated in 1 g on the ground and in microgravity.
2.4. Microgravity production
To produce microgravity in the laboratory at 1 g on the ground, we used the gravity controller Gravite (Space Bio-Laboratories Co., Ltd., Hiroshima, Japan) [13]. This device produces microgravity similar to gravity in space. The principles of the instrument are summarized briefly as follows: microgravity (10−3 g) was generated by rotating a sample around two axes and integrating the gravity vector and the temporal axis. In 8 min, these specific conditions create a environment of 10−3 g that is measured by the gravity acceleration sensor; and this environment was defined as simulated microgravity [[13], [14], [15]].
2.5. Thioflavin T (ThT) fluorescence assay
During in vitro incubations of human insulin, Aβ42, or human TTR under each gravity condition, we analyzed ThT fluorescence with a spectrofluorometer (F-2700; Hitachi High Technologies, Tokyo, Japan) and using a quartz cuvette with a 10-mm path length under excitation and emission wavelengths of 445 and 490 nm (insulin and Aβ42) and 442 and 489 nm (TTR), respectively. Since each amyloidogenic protein and peptide have different molecular structure, it is more appropriate that TTR experiment should be performed in different excitation and emission lengths. Samples (each 3 μL) were mixed with 600 μL of 5 μM ThT in 50 mM glycine/NaOH buffer, pH 9.5, and were used for measurements, obtained at room temperature [3]. We obtained three scans per sample.
2.6. Cell-based 1-fluoro-2,5-bis[(E)-3-carboxy-4-hydroxystyryl]benzene (FSB) assay
In the cell-based FSB assay, we seeded glomotel cells in half-area 96-well culture plates (Greiner Bio-One, Kremunster, Austria) [3]. After 24 h, we added human insulin, Aβ42, or TTR to the cell culture medium (DMEM, Thermo Fisher Scientific, Massachusetts, USA) and incubated the samples at 37 °C for 24 h. We fixed the cultured cells with 10% formaldehyde neutral buffer solution (Nacalai Tesque, Kyoto, Japan) for 30 min. After we washed the fixed cells with PBS, we stained the cells with 0.0001% FSB in 50% EtOH for 30 min. We washed FSB-stained cells with 50% EtOH once, followed by washing with PBS three times. We analyzed the amount of amyloid in deposits by using the IN Cell Analyzer 2200 (GE Healthcare).
2.7. Statistics
We used the Student t-test to identify significant differences between two conditions. P values less than 0.01 or 0.05 were considered to be significant. Data are shown as means + SEM.
3. Results
3.1. Effect of microgravity on amyloid fibril formation of human insulin
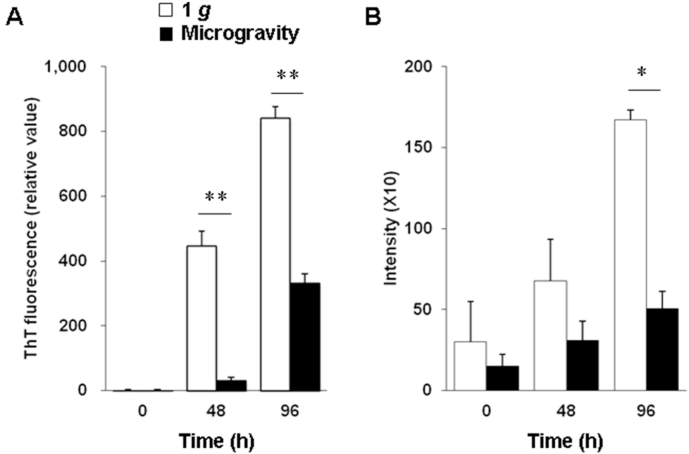
To examine the effect of microgravity on amyloid fibril formation of human insulin, we incubated the protein at pH 3.0 and at 37 °C in 1 g on the ground or in microgravity (10−3 g). The human insulin samples increased in ThT fluorescence intensity in a time-dependent manner when incubated in 1 g. In contrast, intensity values decreased in microgravity (Fig. 1A). In the cell-based FSB assay, the staining intensity was also reduced in microgravity compared with that in 1 g (Fig. 1B).
Fig. 1.
Amyloid fibril formation of human insulin in microgravity and in 1 g on the ground. (A) ThT fluorescence intensity of human insulin. (B) Cell-based FSB assay of human insulin. White and black bars indicate 1 g and microgravity, respectively. Data represent means + SEM. **P < 0.01, *P < 0.05, vs. the 1 g group.
3.2. Effect of microgravity on amyloid fibril formation of Aβ42
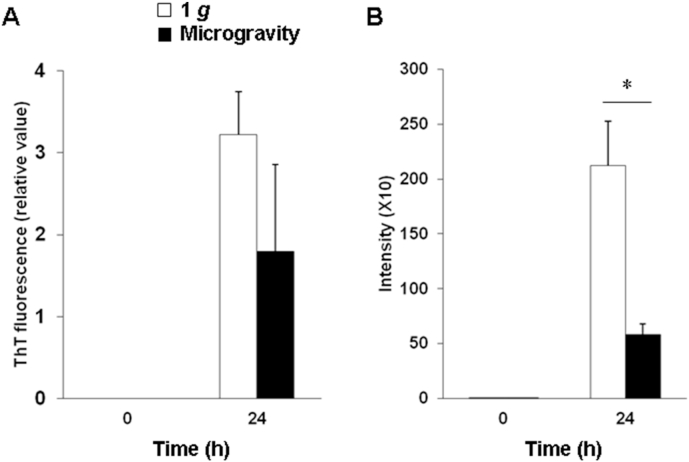
Samples of Aβ42 were incubated at pH 7.0 at 37 °C in 1 g on the ground or in microgravity. The ThT fluorescence intensity of the peptide samples did not differ significantly at 24 h in microgravity compared with that in 1 g (Fig. 2A). However, in the cell-based FSB assay, the staining intensity was significantly reduced at 24 h in microgravity compared with that in 1 g (Fig. 2B). After 24 h of incubation, the peptide could not be measured because it formed aggregates in the solution.
Fig. 2.
Amyloid fibril formation of Aβ42 in microgravity and in 1 g on the ground. (A) ThT fluorescence intensity of Aβ42. (B) Cell-based FSB assay of Aβ42. White and black bars indicate 1 g and microgravity, respectively. Data represent means + SEM. *P < 0.05 vs. the 1 g group.
3.3. Effect of microgravity on amyloid fibril formation of human TTRwt
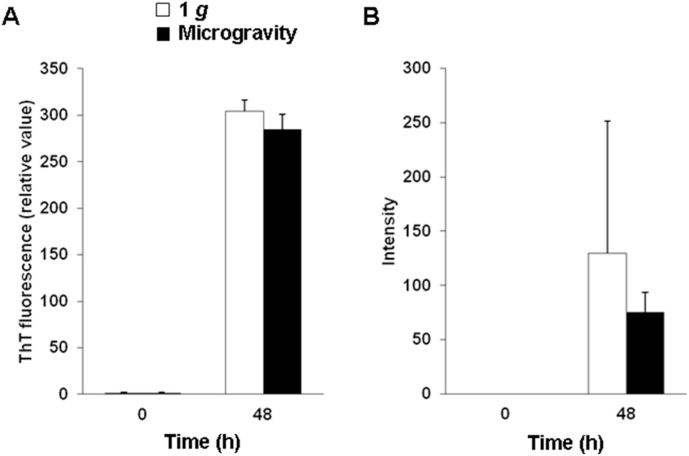
Human TTRwt samples were incubated at pH 3.5 at 37 °C in 1 g on the ground or in microgravity. The protein tended to form fewer amyloid fibrils in the ThT fluorescence intensity and cell-based FSB assays in microgravity compared formation in 1 g (Fig. 3). At 24 and 96 h of incubation, no obvious differences were observed for results in 1 g and in microgravity (data not shown).
Fig. 3.
Amyloid fibril formation of human TTRwt in microgravity and in 1 g on the ground. (A) ThT fluorescence intensity of human TTR. (B) Cell-based FSB assay of human TTRwt. White and black bars indicate 1 g and microgravity, respectively. Data represent means + SEM.
4. Discussion
That microgravity can be produced either by space flight or by free fall has been well documented. However, such conditions cannot reproduced in test tube experiments in the laboratory. Fortunately, the development of the gravity controller Gravite has allowed production of microgravity conditions in a typical laboratory in 1 g on the ground. Therefore, we were able to investigate the effect of microgravity on amyloid fibril formation in amyloidogenic proteins including human insulin, Aβ42, and human TTRwt.
The present study provided the following important findings: (1) Human insulin and Aβ42 showed significantly suppressed amyloid fibril formation in microgravity compared with that in 1 g on the ground. (2) Human TTRwt tended to form fewer amyloid fibrils in microgravity compared with formation in 1 g. To form amyloid fibrils, the following conditions are required: induction of structural changes via mutation of amyloidogenic proteins, and a protein-protein (peptide-peptide) interaction, which is indispensable. Our experiments confirmed that microgravity suppressed amyloid fibril formation by reducing the protein-protein interaction via decreased convection.
Why human TTRwt in microgravity alone did not show a significant difference in amyloid formation between 1 g and microgravity, compared with data for human insulin and Aβ42, is unknown. Because human TTR forms tetramers, the TTR tetramers must dissociate into monomers before amyloid formation of monomeric TTR. TTRwt may be protected against amyloid fibril formation as tetramers even in microgravity. In cell based assay, decrease in TTR aggregation in microgravity tended to be more obvious, suggesting effect of medium, such as albumin may influence the result.
Ueda et al. [3] recently demonstrated that the C-terminal fragment of TTR81-127 did form tetramers and amyloid fibrils at neutral pH. In addition, the variant TTR ATTR V30 M is less stable in tetrameric form than TTRwt [16]. Therefore, experiments with variant TTR or ATTR V30 M may provide improved results.
Human insulin was able to form amyloid fibrils in 1 g because it exists in monomeric form in water-soluble solutions. Our results clearly indicate that microgravity suppressed the amyloid fibril formation, ThT fluorescence intensity, and cell-based FSB assay results of insulin (Fig. 1AB).
Aβ42 did not show significant differences for 1 g and microgravity in the ThT fluorescence intensity assay (Fig. 2A) because Aβ42 is hydrophobic and dissolves poorly in water-soluble solutions. Furthermore, Aβ42 forms aggregates during prolonged incubations. Therefore, we could not extend experiments to more than 24 h of incubation. Recently, a cell-based assay was used to elucidate the effects of amyloid toxicity on cell viability [3]. Aβ42 suppressed the staining intensity of the cell-based FSB assay in microgravity compared with 1 g (Fig. 2B). We thus suggest that microgravity reduced the toxicity of Aβ42 through the process of amyloid formation.
In summary, gravity may changed amyloid fibril formation. In microgravity, amyloid fibril formation may be suppressed, and as the results showed, the cytotoxicity of amyloid fibrils may be reduced. We preliminary performed electron microscopic studies for the samples subjected to these experiments. Unfortunately, differences were not obvious, compared to biochemical studies. There is a possibility that biochemical changes may be more sensitive than those of morphology. Further studies are needed. Our experiments suggest that amyloidogenic proteins form fewer amyloid fibrils in microgravity and that patients with amyloidosis may be more suitable than healthy people to live in space.
Author statement
Hiroaki Matsushita, data collection and writing a paper. Aito Isoguchi, data collection. Masamitsu Okada, data collection. Teruaki Masuda, data analysis and interpretation. Yohei Misumi, data analysis and interpretation. Yuko Ichiki, data analysis and interpretation. Mitsuharu Ueda, research ideas and designs. Yukio Ando, research ideas and approval of final draft.
Funding
This work was supported by Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (KAKENHI grant number 19H03565).
Ethical approval
Not required.
Guarantor
HM.
Contributorship
Conception and design of study: HM, AI, Acquisition of data: MO, TM, YM, YI, Drafting the manuscript: MU, and YA.
Declaration of Competing interest
The authors have no conflicts of interest relevant to the content of this article.
Acknowledgment
This work was supported by Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (KAKENHI grant number 19H03565).
Contributor Information
Hiroaki Matsushita, Email: hiroakim@niu.ac.jp.
Yukio Ando, Email: andoy430@niu.ac.jp.
References
- 1.Benson M.D., Buxbaum J.N., Eisenberg D.S. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. 2018;25:215–219. doi: 10.1080/13506129.2018.1549825. [DOI] [PubMed] [Google Scholar]
- 2.Chiti F., Dobson C.M. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu. Rev. Biochem. 2017;86:27–68. doi: 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- 3.Ueda M., Okada M., Mizuguchi M. A cell-based high-throughput screening method to directly examine transthyretin amyloid fibril formation at neutral pH. J. Biol. Chem. 2019;294:11259–11275. doi: 10.1074/jbc.RA119.007851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueda M., Horibata Y., Shono M. Clinicopathological features of senile systemic amyloidosis: an ante- and post-mortem study. Mod. Pathol. 2011;24:1533–1544. doi: 10.1038/modpathol.2011.117. [DOI] [PubMed] [Google Scholar]
- 5.Ueda M., Ando Y. Recent advances in transthyretin amyloidosis therapy. Transl. Neurodegener. 2014;3:19. doi: 10.1186/2047-9158-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperry B.W., Reyes B.A., Ikram A. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J. Am. Coll. Cardiol. 2018;72:2040–2050. doi: 10.1016/j.jacc.2018.07.092. [DOI] [PubMed] [Google Scholar]
- 7.Ando Y., Coelho T., Berk J.L. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J. Rare Dis. 2013;8:31. doi: 10.1186/1750-1172-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams D.R. The biomedical challenges of space flight. Annu. Rev. Med. 2003;54:245–256. doi: 10.1146/annurev.med.54.101601.152215. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura M., Misumi Y., Nomura T. Extreme adhesion activity of amyloid fibrils induces subcutaneous insulin resistance. Diabetes. 2019;68:609–616. doi: 10.2337/db18-0846. [DOI] [PubMed] [Google Scholar]
- 10.Shin W.S., Di J., Cao Q. Amyloid β-protein oligomers promote the uptake of tau fibril seeds potentiating intracellular tau aggregation. Alzheimer's Res. Ther. 2019;11:86. doi: 10.1186/s13195-019-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai Z., Colón W., Kelly J.W. The acid-mediated denaturation pathway of transthyretin yields a conformational intermediate that can self-assemble into amyloid. Biochemistry. 1996;35:6470–6482. doi: 10.1021/bi952501g. [DOI] [PubMed] [Google Scholar]
- 12.Misumi Y., Ando Y., Ueda M. Chain reaction of amyloid fibril formation with induction of basement membrane in familial amyloidotic polyneuropathy. J. Pathol. 2009;219:481–490. doi: 10.1002/path.2618. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa T., Tanimoto K., Fukazawa T. Simulated microgravity attenuates myogenic differentiation via epigenetic regulations. NPJ Microgravity. 2018;4:11. doi: 10.1038/s41526-018-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawahara Y., Manabe T., Matsumoto M. LIF-free embryonic stem cell culture in simulated microgravity. PloS One. 2009;4:e6343. doi: 10.1371/journal.pone.0006343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitsuhara T., Takeda M., Yamaguchi S. Simulated microgravity facilitates cell migration and neuroprotection after bone marrow stromal cell transplantation in spinal cord injury. Stem Cell Res. Ther. 2013;4:35. doi: 10.1186/scrt184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tojo K., Sekijima Y., Kelly J.W. Diflunisal stabilizes familial amyloid polyneuropathy-associated transthyretin variant tetramers in serum against dissociation required for amyloidogenesis. Neurosci. Res. 2006;56:441–449. doi: 10.1016/j.neures.2006.08.014. [DOI] [PubMed] [Google Scholar]