Abstract
Purpose
Examine the use of systemic phosphodiesterase inhibition (sildenafil) to clear central serous chorioretinopathy (CSCR).
Observations
In a long-standing CSCR patient, sildenafil produced a rapid resolution. When discontinued (dechallenge) the CSCR returned. When rechallenged, the CSCR again rapidly disappeared and did not recur in 5 months of continued therapy.
Conclusions
AND IMPORTANCE: Systemic sildenafil can cause rapid clearance of CSCR and can augment or replace other treatments.
Keywords: PDE5/6 inhibitor, Sildenafil, Central serous chorioretinopathy
1. Introduction
Central serous chorioretinopathy (CSCR) is a disease characterized by macular serous retinal detachment, first described by Von Graefe in 1866.1 It has had numerous definitions of causation and may be accompanied by a detachment of the pigment epithelium (PED).
The prognosis is often quite good with no treatment and resolution is common within 3–6 months although visual disturbances that interfere with reading tasks may persist. More severe or recurrent cases can produce significant visual symptoms. Yannuzzi and colleagues reported a subset of CSCR patients where 25% had visual acuity of 20/200 or worse.2
The two main pathophysiological changes in CSCR relate to a primary disease of the RPE or a psychogenic component that ultimately affects, among other things, the choriocapillaris. Clearly the disease is multifactorial, and stress appears to be a significant risk factor. Treatment is presently quite varied and includes mineralocorticoid antagonism, beta adrenergic antagonism, or laser therapy with variable results. A landmark summary of the clinical characterization and the treatment panorama is provided in a recent paper on evidence based treatment guidelines.3
The present report describes a treatment using sildenafil to increase blood flow to the choroid via production of phosphodiesterase 5 and 6 (PDE5/PDE6) inhibition. PDE6 inhibition decreases Warburg-like glycolysis in rod photoreceptors and promotes oxidative metabolism while PDE5 inhibition increases nitric oxide perfusion and waste removal.4 This last reference specifically describes improvement in photoreceptor neuronal function possibility due to enhanced mitochondrial ATP production.
This case describes a long standing CSCR which was treated with a good result. When treatment stopped, CSCR recurred; when treatment restarted, CSCR again rapidly resolved (challenge, dechallenge, rechallenge). Sildenafil citrate (Viagra®, Pfizer, New York, NY, USA) is primarily indicated and FDA-approved for erectile dysfunction (ED), and has become one of the most popular drugs globally. Through selective inhibition of the cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase types 5 inherently present in all vascular tissues, the medication improves erectile function in men with ED by enhancing the smooth muscle relaxant effects of nitric oxide.5 From this increased blood flow effect, sildenafil has also demonstrated use for other conditions such as cardiac ischemia6 and pulmonary hypertension.7 Sildenafil also inhibits PDE6 in rod and cone photoreceptors. PDE6 is a homolog of PDE5.
This case report is part of an IRB-approved study of sildenafil treatment of macular degenerations and dystrophies.
2. Case report
A 50 year old male noted recurrent metamorphopsia of the central vision in his right eye occurring during periods of stress in his writing career. Both his ability to function and his ability to maintain concentration on his work were seriously affected. Optical coherence tomography (OCT) showed serous elevation of the para-macular area. He agreed to a trial of sildenafil as part of a Columbia University Medical Center Institutional Review Board (IRB) approved study of sildenafil for treatment of macular degenerations and dystrophies. Sixty milligrams of oral sildenafil was prescribed (40 mg in the morning and 20 mg at bedtime).
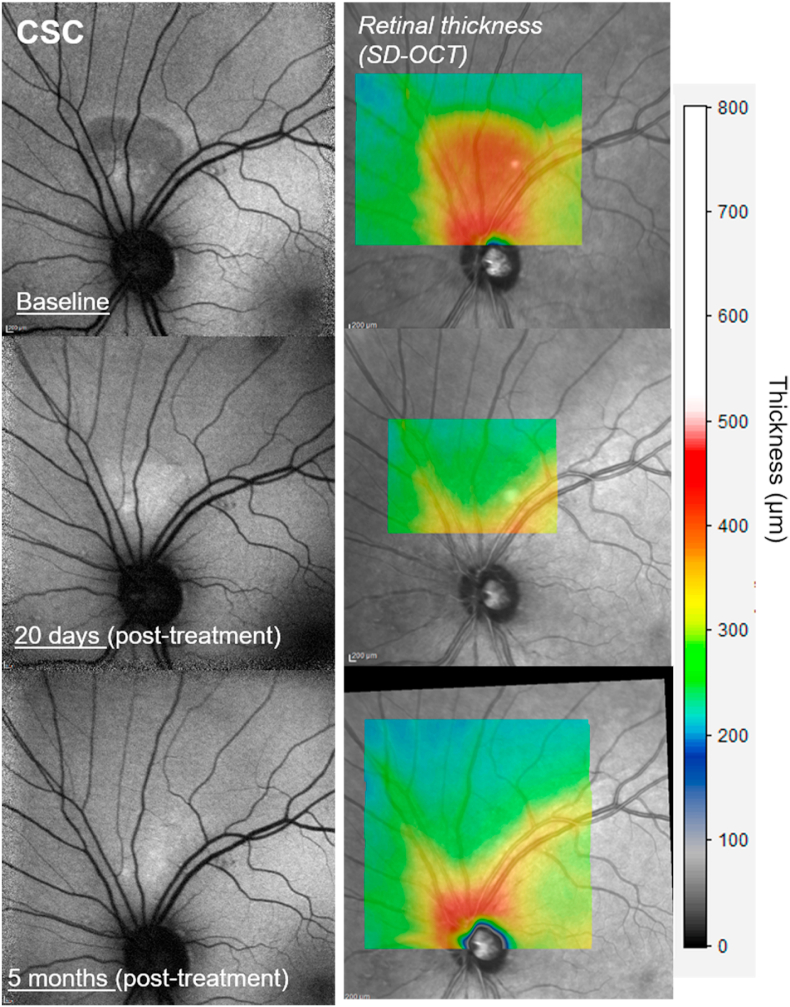
Despite his symptoms, the visual acuity remained 20/20 in both eyes prior to and throughout treatment. Vision returned to normal with no serous fluid on OCT within 2 weeks and sildenafil was discontinued (dechallenge). Three weeks later, he again became symptomatic, serous fluid recurred, and sildenafil was resumed (rechallenge). Within 2 weeks serous fluid disappeared and he remained symptom-free while on sildenafil for 5 months (Fig. 1).
Fig. 1.
Central serous chorioretinopathy. Spectral domain optical coherence tomography of serous elevation of macula demonstrating the effects of challenge, dechallenge, and rechallenge. The elevations were mild but the vision change was disconcerting to the patient. The retreatment was continued for 5 months. No further recurrences have been observed in this patient. Infrared reflectance image (left) registered to the horizontal SD-OCT indicates the horizontal axis of the SD-OCT image (green line). . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Serous fluid was also observed superior to the optic nerve in the fellow (left) eye, although no visual symptoms were described for this eye (Fig. 2). In this eye fundus hypoautofluorescence (hypo-AF) was followed by hyperautofluorescence (hyper-AF) after treatment.
Fig. 2.
Central serous chorioretinopathy. (Left) Short wavelength fundus autofluorescence images of the fellow eye showing subretinal fluid superior to the optic nerve at baseline and after treatment. (Right) Macular thickness measurements acquired superior to the optic disk.
3. Discussion
Despite evidence of increased choroidal blood flow with sildenafil intake in normal, healthy patients,7,8,9 very few investigations regarding potential therapeutic effects of this medication in eyes with macular disease, such as central serous chorioretinopathy (CSCR) have been done. Reasons include reports of CSCR linked to sildenafil usage within large databases,10 and rare instances of other possible ophthalmic toxicities.11 Several cases analyzed by Fraunfelder and Fraunfelder10 suggested that CSCR activity was temporally linked with the medication, either with subretinal fluid present during sildenafil intake (positive challenge) or resolution with its cessation (positive dechallenge). However, a post-marketing surveillance survey demonstrated lack of a significant association between sildenafil usage and CSCR,12 and toxicity has instead primarily been found in the setting of excessive dosage or medication impurity from non-regulated sources.13 Sildenafil is not recommended for patients on nitrates as it may reduce blood pressure significantly.
Our hypothesis is that select patients with macular disease including CSCR may benefit from increased choroidal blood flow afforded by sildenafil.14,15,16 We anticipate that sildenafil, administered with attention to “challenge” and “dechallenge” settings - that is, continued treatment beyond the initial resolution of serous fluid - should produce clinical improvement for these patients. The use of spironolactone – and eplerenone to a lesser extent - most closely parallels the present treatment but has shown slower treatment response and greater side effects.17,18
We note that in short-wavelength fundus autofluorescence (SW-AF) images (Fig. 2) the serous retinal detachment associated with CSCR initially presented with a focal area of hypo-SW-AF superior to the optic disc in the fellow eye. The reduced autofluorescence (AF) is likely due to blockage of the transmission of fundus AF by subretinal fluid, as previously suggested.19, 20, 21, 22 Subsequently, however, this site was marked by increased SW-AF presenting with a speckled pattern. This aberrant SW-AF signal could represent a window defect created by reduced photopigment in the CSCR lesion. Alternatively, the SW-AF intensity at this location could reflect an actual elevation in SW-AF due to increased bisretinoid lipofuscin formation within damaged photoreceptor cells.
4. Conclusions
This case represents a positive response to PDE5/6 inhibition of central serous chorioretinopathy using an oral medication with minimal to no adverse effects. The use of the challenge-dechallenge-rechallenge paradigm strengthens the validity of the use of sildenafil as a viable treatment option in these patients.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
Consent to publish this report has been obtained from this patient. This report does not contain any personal identifying information.
Funding
This study was support, in part, by grants from the National Eye Institute/NIH (Bethesda, Maryland) EY024091, Triad Foundation, Ithaca, New York and the St. Giles Foundation, New York, New York. Jonas Children's Vision Care is supported by the National Institute of Health 5P30CA013696, U01 EY030580, U54OD020351, R24EY028758, R24EY027285, 5P30EY019007, R01EY018213, R01EY024698, R01EY026682, R21AG050437, the Schneeweiss Stem Cell Fund, New York State [SDHDOH01-C32590GG-3450000 ], the Foundation Fighting Blindness New York Regional Research Center Grant [PPA-1218-0751-COLU], Nancy & Kobi Karp, the Crowley Family Funds, The Rosenbaum Family Foundation, Alcon Research Institute, the Gebroe Family Foundation, the Research to Prevent Blindness (RPB) Physician-Scientist Award, unrestricted funds from RPB, New York, NY, USA.
Conflict of interest
The authors do not have any proprietary interests in the materials described in the article.
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Intellectual property
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Research ethics
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
IRB approval was obtained (required for studies and series of 3 or more cases).
Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient(s) or their legal guardian(s).
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
The International Committee of Medical Journal Editors (JCMJE) recommends that.
Authorship be based on the following four criteria:
-
1.
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
2.
Drafting the work or revising it critically for important intellectual content; AND
-
3.
Final approval of the version to be published; AND
-
4.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
All those designated as authors should meet all four criteria for authorship and all who meet the four criteria should be identified as authors. For more information on authorship, please see http://icjmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of -authors-and-contributors.html#two.
All listed authors meet the lCMJE criteria. We attest that all authors contributed significantly to the creation of this manuscript, each having fulfilled criteria as established by the ICMJE.
One or more listed authors do(es) not meet the lCMJE criteria.
Funding
Supported by the National Eye Institute/National Institutes of Health Grant EY024091 and unrestricted grants from the Triad Foundation, Ithaca, New York and St. Giles Foundation, New York, New York.
Declaration of competing interest
The following authors have no financial disclosures: DJC, WL, SD, MPB, JS, SHT.
Acknowledgements
None.
References
- 1.von Graefe A. Central recurrent retinitis. Graefes Arch Clin Exp Ophthal. 1866;12:211–215. [Google Scholar]
- 2.Yannuzzi L.A., Shakin J., Fisher Y. Peripheral retinal detachment and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984;91:1554–1572. doi: 10.1016/s0161-6420(84)34117-3. [DOI] [PubMed] [Google Scholar]
- 3.vanRijssen T.J., vanDijk E.H.C., Yzer S. Central serous retinopathy: towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019 doi: 10.1016/j.preteyeres.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Lin M.K., Kim S.H., Zhagn L., Tsai Y.T., Tsang S.H. Rod metabolic demand drives progress in retinopathies. Taiwan J Ophthalmolo. 2015;5:105–108. doi: 10.1016/j.tjo.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard S.A., Gingell C.J., Tang K. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitreo and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- 6.Kukreja R.C. Sildenafil and cardioprotection. Curr Pharm Des. 2013;19(39):6842–6847. doi: 10.2174/138161281939131127110156. [DOI] [PubMed] [Google Scholar]
- 7.Vitulo P., Stanziola A., Confalonieri M. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Heart Lung Transplant. 2017 Feb;36(2):166–174. doi: 10.1016/j.healun.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Kim D.Y., Silverman R.H., Chan R.V.P. Measurement of choroidal perfusion and thickness following systemic sildenafil (Viagra®) Acta Ophthalmol. 2013 Mar;91(2):183–188. doi: 10.1111/j.1755-3768.2011.02305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vance S.K., Imamura Y., Freund K.B. The effects of sildenafil citrate on choroidal thickness as determined by enhanced depth imaging optical coherence tomography. Retina. 2011 Feb;31(2):332–335. doi: 10.1097/IAE.0b013e3181eef0ae. [DOI] [PubMed] [Google Scholar]
- 10.Fraunfelder F.W., Fraunfelder F.T. Central serous chorioretinopathy associated with sildenafil. Retina. 2008 Apr;28(4):606–609. doi: 10.1097/IAE.0b013e31815ec2c8. [DOI] [PubMed] [Google Scholar]
- 11.Coca M.N., Morgan M.L., Gupta P., Elkeeb A., Lee A.G. Bilateral posterior ischemic optic neuropathy associated with the use of sildenafil for pulmonary hypertension. Can J Ophthalmol. 2016 Jun;51(3):e96–e99. doi: 10.1016/j.jcjo.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 12.French D.D., Margo C.E. Central serous chorioretinopathy and phosphodiesterase-5 inhibitors: a case-control postmarketing surveillance study. Retina. 2010 Feb;30(2):271–274. doi: 10.1097/IAE.0b013e3181b7740f. [DOI] [PubMed] [Google Scholar]
- 13.Yanoga F., Gentile R.C., Chui T.Y.P. Sildenafil citrate induced retinal toxicity – electroretinogram, optical coherence tomography, and adaptive optics findings. Retin Cases Brief Rep. 2018;12(Suppl 1):S33–S40. doi: 10.1097/ICB.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman D.J., Silverman R.H., Rondeau M.J. Age-related macular degeneration: choroidal ischaemia? Br J Ophthalmol. 2013;97:1020–1023. doi: 10.1136/bjophthalmol-2013-303143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman D.J., Lee W., Chang S. Treatment of macular degeneration with sildenafil: results of a two-year trial. Ophthalmologica. 2018;240(1):45–54. doi: 10.1159/000486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yui G., Vuong V.S., Tran S. Vascular response to sildenafil citrate in aging and age-related macular degeneration. Sci Rep. 2019;9(5049):1–9. doi: 10.1038/s41598-019-41509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dariuch A., Matet A., Dirani A. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi: 10.1016/j.preteyeres.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Lotery A., Sivaprasad S., O'Connell A. Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395:294–303. doi: 10.1016/S0140-6736(19)32981-2. [DOI] [PubMed] [Google Scholar]
- 19.Sekiryu T., Iida T., Maruko I., Saito K., Kondo T. Infrared fundus autofluorescence and central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2010;51:4956–4962. doi: 10.1167/iovs.09-5009. [DOI] [PubMed] [Google Scholar]
- 20.Dinc U.A., Tatlipinar S., Yenerel M., Gorgun E., Ciftci F. Fundus autofluorescence in acute and chronic central serous chorioretinopathy. Clin Exp Optom. 2011;94:452–457. doi: 10.1111/j.1444-0938.2011.00598.x. [DOI] [PubMed] [Google Scholar]
- 21.Ayata A., Tatlipinar S., Kar T., Unal M., Ersanli D., Bilge A.H. Near-infrared and short-wavelength autofluorescence imaging in central serous chorioretinopathy. Br J Ophthalmol. 2009;93:79–82. doi: 10.1136/bjo.2008.141564. [DOI] [PubMed] [Google Scholar]
- 22.Framme C., Walter A., Gabler B., Roider J., Sachs H.G., Gabel V.P. Fundus autofluorescence in acute and chronic-recurrent central serous chorioretinopathy. Acta Ophthalmol Scand. 2005;83:161–167. doi: 10.1111/j.1600-0420.2005.00442.x. [DOI] [PubMed] [Google Scholar]