Abstract
This study focused on the characterization of a novel cysteine proteinase inhibitor from Enterolobium contortisiliquum seeds targeting the inhibition of the growth of Callosobruchus maculatus larvae, an important cosmopolitan pest of the cowpea Vigna unguiculata during storage. The inhibitor was isolated by ion-exchange besides of size exclusion chromatography. EcCI molecular mass is 19,757 Da, composed of two polypeptide chains. It strongly inhibits papain (Kiapp 0.036 nM) and proteinases from the midguts of C. maculatus (80 μg mL−1, 60% inhibition). The inhibitory activity is reduced by 40% after a heat treatment at 100 °C for 2 h. The protein displayed noxious activity at 0.5% and 1% (w/w) when incorporated in artificial seeds, reducing larval mass in 87% and 92%, respectively. Treatment of C. maculatus larvae with conjugated EcCI-FIT and subsequent biodistribution resulted in high fluorescence intensity in midguts and markedly low intensity in malpighian tubules and fat body. Small amounts of labeled proteins were detected in larvae feces. The detection of high fluorescence in larvae midguts and low fluorescence in their feces indicate the retention of the FITC conjugated EcCI inhibitor in larvae midguts. These results demonstrate the potential of the natural protein from E. contortisiliquum to inhibit the development of C. maculatus.
Keywords: Bioinsecticide, Callosobruchus maculatus, Cysteine proteinase, Enterolobium contortisiliquum, Plant inhibitor
Highlights
-
•
A new cysteine proteinase inhibitor, named EcCI, was purified from Enterolobium contortisiliquum seeds.
-
•
The EcCI inhibitor is thermostable and strongly inhibited papain (Kiapp 0.036 nM).
-
•
EcCI has a detrimental effect on the development of C. maculatus larvae.
1. Introduction
Legumes have high levels of proteins, some of which are characterized as enzymes, storage proteins, and proteinase inhibitors [[1], [2], [3]]. Enterolobium contortisiliquum, a member of the Fabaceae family and Mimosoideae subfamily, are trees whose height exceeds 20 m and are commonly recognized as black ear due to the form of their fruits [4]. Its cotyledon has a high amount of proteins, several of which, including a serine proteinase inhibitor, have been characterized from seeds [[5], [6], [7], [8], [9], [10]]. Inhibitors can act as highly definite substrates for target enzymes by forming a very stable enzyme-inhibitor complex that dissociates significantly more slowly than enzyme-substrate or enzyme-product complexes [11].
Proteins isolated from plants that block the activities of proteolytic enzymes are grouped into distinct families based on their primary sequences, the similarity of disulfide bond locations, and the position of reactive sites. These families are named Bowman-Birk, Potato I, Potato II, Kunitz, squash, barley, cystatin, and miscellaneous [1,[11], [12], [13]].
The Kunitz inhibitors family, extracted mainly from leguminous seeds, is the best characterized among other plant inhibitor families because of their abundance in seeds [1,14]. Kunitz inhibitors purified from Leguminosae seeds show molecular masses about 20 kDa (180 amino acid residues, with four cysteines in two disulfide bonds) [11,13]. Oliva et al. [1] regrouped the plant Kunitz members in subgroups according to the presence of cysteine residues from 1 to 4. The inhibitor of serine proteinases found in E. contortisiliquum belongs to the Kunitz subgroup 4 with 2 S–S.
Proteinase inhibitors may play a role as reserve protein in plants [13,15] and may be implicated in plant defense mechanisms. These proteins may be produced during the normal development constitutively or induced in response to an injury [16,17]. The harmful action of these proteins on the growth and survival of insect larvae is well documented, parameters evaluated in experimental studies of insecticides [18,19].
Various insect orders contain chewing or sucking insects that are predators of legumes. Some, such as beetles or weevils from the Bruchidae family, Coleoptera order, have become skilled seed predators. The weevil Callosobruchus maculatus is a serious pest of several Vigna species, particularly Vigna unguiculata (L.) Walp. [20], attacking stored seeds and affecting crop quality and yield due to low seed germination rates. The reduction in potential seed germination in V. unguiculata is closely related to the degree of infestation by the weevil and can reach 100% in seeds with more than four holes, a situation which indicates the emergence of adult insects [21,22].
The use of proteinase inhibitors in the combating pests by targeting digestive enzymes of insects has received considerable attention. Bioassays and experiments have demonstrated that feeding insects with transgenic plants expressing these proteins delay insect growth and development and cause insect starvation and death [17,23,24].
This study characterized a novel cysteine proteinase inhibitor isolated from seeds of E. contortisiliquum, named EcCI, and evaluated its potential as a biological insecticide.
2. Materials and methods
2.1. Inhibitor purification
The purification of the inhibitors from E. contortisiliquum seeds followed to the procedure shown in Fig. 1S, Supporting Information. The seeds (40 g) were homogenized in 0.02 M Tris/HCl, pH 8.0 (1:40 w/v) using a blender, centrifuged at 4000×g, and the supernatant was fractionated with acetone at a final concentration of 80% v/v (80 mL of acetone and 20 mL of seed extract) at 4 °C. The precipitated protein was separated by centrifugation (30 min, 4 °C at 18,800×g), and maintained at 25 °C until complete elimination of the residual acetone, solubilized in 0.02 M Tris/HCl buffer, pH 8.0, and applied to a DEAE-Sepharose column (2 × 25 cm) with 0.1 M Tris/HCl buffer, pH 8.0. The non-adsorbed material was removed by washing the column with an equilibration buffer and bound protein was eluted with NaCl (0.15 M and 0.3 M) in the buffer above.
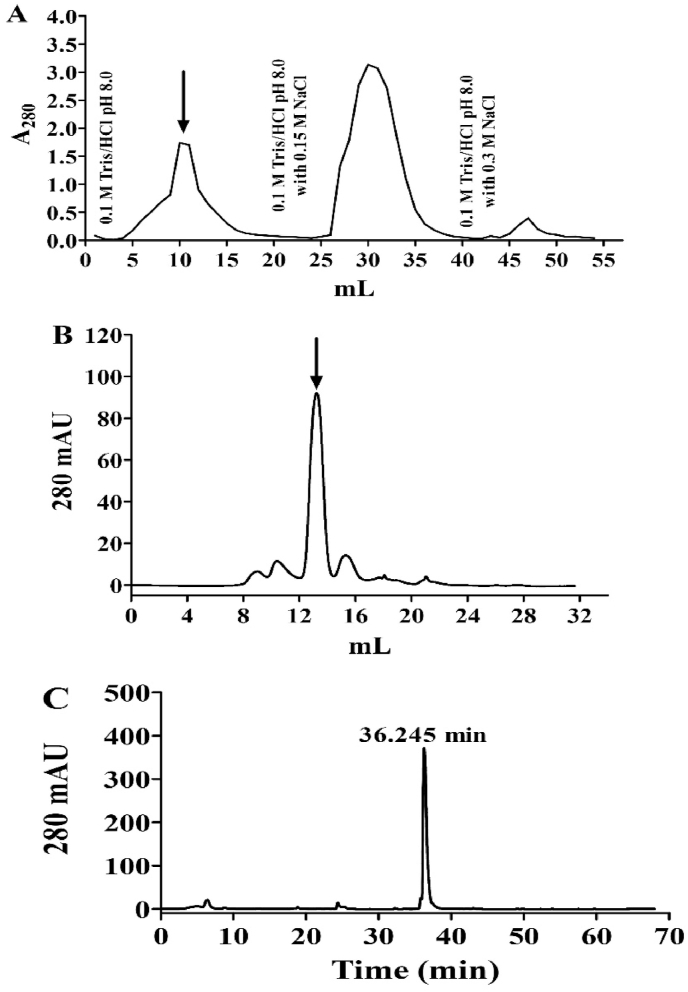
Fig. 1.
Purification of EcCI. (A) Chromatography (DEAE-Sepharose equilibrated with 0.1 M Tris/HCl pH 8.0); Two mL fractions were collected at the flow rate of 60 mL/h. (B) Size exclusion chromatography on Superdex 75 equilibrated with 0.05 M Tris/HCl pH 8.0 and 0.15 M NaCl at the flow rate of 30 mL/h. The arrow indicates inhibiting activity on the papain enzyme. (C) Reverse phase HPLC chromatography. The protein fraction was eluted with a linear gradient (5–100%) of 90% acetonitrile in 0.1% TFA in Milli-Q water (solvent B) at the flow rate of 42 mL/h (t = 0.1 min, 5% B; t = 5 min, 5% B; t = 60 min, 100% B, t = 68 min, 0% B).
Papain inhibition was measured by the inhibition of activity in the hydrolysis of 5 mM Z-Phe-Arg-p-Nan (Bachem, Bubendorf, Switzerland) as the substrate. A ÄKTA avant (GE Healthcare) was used in the process. Fractions from the DEAE-Sepharose column showing cysteine inhibitory activity (eluted with NaCl 0.15 M) were pooled and injected to a Superdex 75 column equilibrated with 0.05 M Tris/HCl buffer, pH 8.0. The fraction containing the inhibitor was then applied to a trypsin-Sepharose column with 0.1 M Tris/HCl buffer, pH 8.0. The non-bounded fraction in the resin in which the cysteine inhibitor was detected was subsequently analyzed by C18 Vyda protein/peptide reverse phase (15/0.46 cm) eluted at a flow rate of 0.7 mL min−1with an acetonitrile gradient (0–100%) in trifluoroacetic acid (TFA) (0.1%, v/v).
Protein quantification in each purification step was determined by the Folin Ciocalteu assay [25] using serum albumin (BSA) at 0–500 μg mL−1 as the standard curve.
2.2. Functional studies in the inhibitor
2.2.1. The inhibitory activity of EcCI on papain
The inhibition curve was prepared using 14 nM papain activated with 0.1 M NaH2PO4 pH 6.3, 0.01 M EDTA, 0.4 M NaCl, and 5 mM DTT at 40 °C for 10 min; this mixture was pre-incubated in the absence and presence of increasing concentrations of the inhibitor for 10 min at 40 °C, and subsequently added to the Z-Phe-Arg-pNAN substrate (0.4 mM) (Calbaiochem Ltda, Darmstadt, Germany); this mixture (final volume of 250 μL) was incubated for 30 min at 40 °C. The reaction was interrupted by adding 30 μL of 30% acetic acid (v/v). Hydrolysis of the substrate was checked by the absorbance of p-nitroaniline released at 405 nm in the Spectra max plus 384 (Molecular Devices). Inhibitory activity was determined by residual enzyme activity in the presence of EcCI compared to the control. The experiment was performed in duplicate.
2.2.2. Thermal stability of the inhibitor
Samples of EcCI in 10 mM PBA buffer (pH 7.0) were incubated at temperatures of 37 °C, 40 °C, 60 °C, 80 °C, and 100 °C for 30, 60, and 120 min. After heating, the samples were cool down to room temperature, and activity was measured and compared to the sample control that had been chilled on ice for 120 min. The inhibitory activity of papain was assayed as above.
2.2.3. Stability of EcCI at different pHs
EcCI was dialyzed against water and 500 μL aliquots were lyophilized. Lyophilized samples were reconstituted in 0.05 M sodium citrate buffer pH 3.0, 4.0, 5.0, and 6.0; 0.05 M sodium phosphate buffer pH 7.0; 0.05 M Tris/HCl buffer (pH 8.0 and pH 9.0); and 0.05 M sodium bicarbonate buffer pH 10.0.
After 3 h of incubation at 37 °C, the sample pH was fitted to 8.0, lyophilized, and reconstituted in 500 μL 0.05 M Tris/HCl, pH 8.0. The inhibitory activity of papain was subsequently determined.
2.2.4. Inhibitory activity of EcCI on cysteine proteinases present in the intestine of C. maculatus larvae
Larvae of C. maculatus (twenty-eighteen days old) were removed from infested seeds, placed on a stereomicroscope, and dissected with tweezers to isolate midguts. The intestines were macerated manually in 0.15 M NaCl solution under constant agitation for 1 h at 8 °C. The extract was centrifuged at 4000 g and 4 °C for 10 min. The collected supernatant was frozen at −20 °C.
This supernatant was 15-fold diluted in 0.1 M NaH2PO4 pH 6.3 buffer; 0.01 M EDTA; 0.4 M NaCl; and 5 mM DTT and incubated at 37 °C for 10 min to activate enzymes; 20 μL aliquots were incubated in the absence and presence of increasing concentrations of EcCI for 10 min at 37 °C. After this period, 20 μL of the Z-Phe-Arg-pNan substrate (5 mM) was added, and the final reaction volume was adjusted to 250 μL. Hydrolysis was monitored for 60 min, the reaction was stopped with 30 μL of acetic acid 40% (v/v), and absorbance was measured at 405 nm.
2.3. Structural characterization of EcCI
2.3.1. The N-terminal sequence identification
EcCI was denatured with 200 μL of 50 mM Tris/HCl buffer pH 8.5 containing 6.0 M guanidinium HCl, 1.0 mM EDTA, and 5.0 mM and reduced by dithiothreitol for 3 h at 37 °C. The S-pyridyl ethylation of cysteines was achieved by the addition of 5 μL 4-vinylpyrimidine for 3 h at 37 °C with incubation under a nitrogen atmosphere in the dark. After the incubate was applied onto a C18 and subsequently, the N-terminal sequences of the separated chains were determined by Edman degradation (PPSQ-23 Sequencer, Shimadzu, Tokyo, Japan) [26].
2.3.2. Mass spectrometry
The EcCI inhibitor molecular weight was determined by Liquid Chromatography coupled Electrospray Ionization Mass Spectrometric using the Waters 3100 apparatus attached to a Waters e 2695 separation module and a 2489 detector. A column (C18 2.1/150 mm, 60Å, 3.5 μM/Waters Nova-Park) was equilibrated with 0.1% trifluoroacetic (TFA) in Milli-Q water. The protein was eluted with 90% acetonitrile containing 0.1% TFA in Milli-Q water in a linear gradient (5–95%) for 30 min (wavelength 214 and 220 nm) at the flow rate of 0.4 mL min−1 in positive mode (ES+), and under the following conditions: mass range between 200 and 2000 m/z; nitrogen gas flow of 6.0 l/h; 4.0 kV capillary; 40 V-cone voltage; 3.0 V extractor; source heater at 120 °C; solvent heater at 400 °C, 1.0 V ion energy; and 500.85 V multiplier [27].
2.3.3. SDS – PAGE
Electrophoresis non-reducing, and reducing (dithiothreitol, 200 mg mL−1) conditions followed to the procedure described by Laemmli [28] using 4–20% separating gels, stained for 30 min with 0.25% (w/v) Coomassie Brilliant Blue R-250 and destained in 10% (v/v) acetic acid.
2.4. Spectroscopic measurements
Conformational studies of EcCI (0.1 mg mL−1) were carried out in a Jasco J-810 circular dichroism spectropolarimeter at 25 °C in a 1 mm path length cuvette. The CD spectrum was recorded in the 190–250 nm range as an average of eight scans. Data were expressed as the mean residue ellipticity [θ] [29]. The percentage of secondary structure was estimated by deconvoluting the CD spectrum using the CDPro software package, which contains three CD analysis programs: CONTINLL, SELCON3, and CDSSTR with a protein reference set of 37 proteins [30,31].
To analyze the pH effect on the secondary structure of EcCI, the protein (0.1 mg mL−1) was incubated in phosphate-borate-acetate buffer (PBA) at 10 mM during 3 h and pH values of 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0, and 12.0. CD measurements were recorded as describe above [29].
The effect of temperature on the secondary structure of EcCI was also analyzed. Protein samples in 10 mM Tris/HCl buffer pH 8.0 (0.1 mg mL−1) were heated at 25, 40, 60, 80, and 100 °C for 2 h. The CD spectrum in the 195–200 nm UV range was recorded as described above.
2.5. Bioassay
2.5.1. Insect
The C. maculatus colony was kept at the Proteins Chemistry and Function Laboratory of the Department of Biochemistry at the Federal University of São Paulo, São Paulo, SP, Brazil. Bruchids were raised on V. unguiculata host seeds (cv. Fradinho), acquired at city supermarkets, and maintained in glass bottles at 28 °C and the relative humidity was maintained at around 60–80% inside a B.O.D. incubator.
2.5.2. Artificial seeds
Artificial seeds (400 mg) prepared using a cylindrical brass mold filling up with fine flour of V. unguiculata cotyledons containing different concentrations (w/w) of EcCI were offered over 24 h to three C. maculatus females (2 days old) in the same conditions described in section 2.6.1. After 24 h, the females and excess eggs were removed to keep only four eggs on each seed. Artificial seeds containing only V. unguiculata flour were used as controls. Seeds were opened after 18 days of incubation, (28 °C and 60–80% relative humidity) larvae were weighed, and emergence numbers determined [29].
2.5.3. FITC (isothiocyanate fluorescein) conjugated to EcCI
FITC (50 mg mL −1 in DMSO) was diluted with 0.75 M bicarbonate buffer pH 9.5 and added to EcCI in a 1:1 (w/w) ratio. The tube was wrapped in aluminum foil and incubated at room temperature with rotation for 1 h, dialyzed against Milli-Q water for the elimination of the unconjugated FITC, and lyophilized for further incorporation into artificial seeds [29].
2.5.4. Artificial seeds containing EcCI coupled to FITC
FITC-EcCI (1.0% w/w) was mixed with cowpea flour compacted and inserted into gelatin capsules. Three C. maculatus larvae (fourth instar) present in the seeds of V. unguiculata were transferred to gelatin capsules to allow feeding movements. After a feeding period of 48 h, were dissected for analysis by confocal microscopy (Leica TCS SP8) of the midguts, Malpighian tubules, and fat bodies [29,32,33].
3. Results
3.1. Purification of E. contortisiliquum cysteine proteinase inhibitor (EcCI)
The saline extract of EcCI from E. contortisiliquum seeds was fractionated by acetone (80% v/v) and purified by ion-exchange chromatography over a DEAE-Sepharose column (Fig. 1A). The fraction exhibiting inhibitory activity toward the papain enzyme was pooled, lyophilized and onto to Superdex 75 column (Fig. 1B). The fraction containing the cysteine proteinase inhibitor was chromatographed on trypsin-Sepharose to reduce the contamination of the serine proteinase inhibitor (data not shown). The purification phases are in Table 1. C18 reverse phase chromatography was performed to analyze the homogeneity of the preparation and the N-terminus sequence determine (Fig. 1C).
Table 1.
Purification of cysteine proteinase inhibitor from E. contortisiliquum seeds.
| Steps | Volume (mL) | Protein (mg/mL) | Total protein (mg) | aUI/mL | bUI/total | cEA | dPurif. | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| Precipitation at 80% acetone | 400 | 2.9 | 1160 | 50 | 20,000 | 17.24 | 1 | 100 |
| DEAE-Sepharose | 132 | 1.6 | 211.2 | 37.5 | 4950 | 23.44 | 1.36 | 24.75 |
| Superdex 75 | 120 | 0.04 | 4.8 | 10 | 1.200 | 250 | 14.5 | 6 |
Inhibition Unit per mL.
Total Inhibition Unit (UI/mL x total volume).
Specific activity.
Fold purification.
3.2. Functional studies of the EcCI inhibitor
3.2.1. Inhibitory activity of EcCI on papain
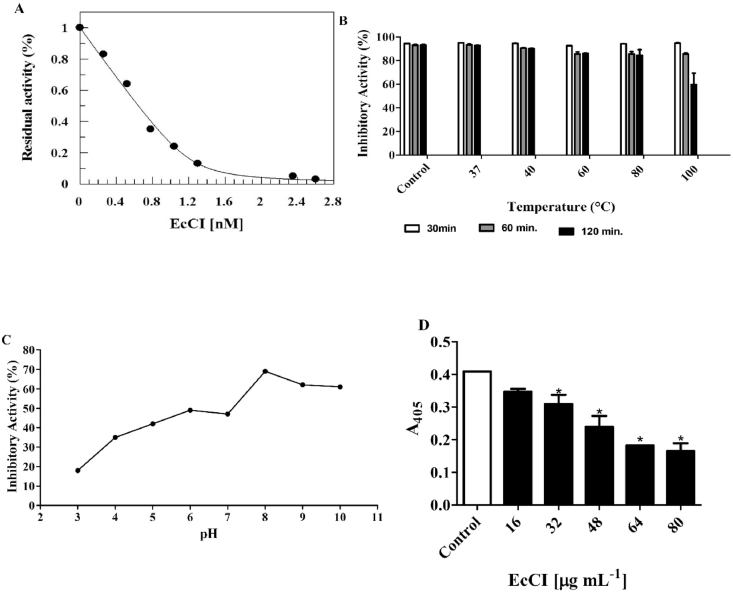
EcCI inhibits the cysteine proteinase papain with an apparent dissociation constant (Kiapp) of 0.036 nM (Fig. 2A). The inhibitor loses 40% of its activity after exposure to 100 °C for 120 min in 10 mM PBA buffer, pH 7.0 (Fig. 2B); its functionality is best maintained within the pH range of 8.0 and 9.0 (Fig. 2C).
Fig. 2.
Functional studies in the EcCI inhibitor. (A) EcCI inhibition curve on papain. Papain (1 nM) was pre-incubated at 40 °C for 10 min with various EcCI concentrations in 0.1 M NaH2PO4 pH 6.3 buffer; 0.01 M EDTA; 0.4 M NaCl; and 5 mM DTT. (B) EcCI thermal stability. EcCI was subjected to thermal treatment at different temperatures for 30, 60, and 120 min. (C) EcCI inhibitory activity after treatment at different pH values. EcCI was incubated for 180 min at different pH values, and activity was evaluated on papain hydrolysis of the Z-Phe-Arg-pNan substrate (0.4 mM) for 30 min at 40 °C. (D) EcCI inhibition curve in the gut extract of C. maculatus larvae. Increasing concentrations of EcCI were incubated for 10 min at 37 °C with 20 μL larvae gut extract in 0.1 M NaH2PO4 buffer pH 6.3; 0.01 M EDTA; 0.4 M NaCl; and 5 mM DTT. The Z-Phe-Arg-pNAn substrate (5 mM) was added, and hydrolysis followed for 1 h *Statistical significance compared to control (p-value ˂ 0.05 by One-way ANOVA).
3.2.2. Inhibitory activity of EcCI on cysteine proteinases from intestinal extracts of C. maculatus larvae
The inhibitory effect of EcCI was tested on enzymes extracted from the C. maculatus larvae midgut. EcCI (80 μg mL−1) inhibited 60% of proteolytic activity present in the larval extract (Fig. 2D).
3.3. Structural characterization of the EcCI inhibitor
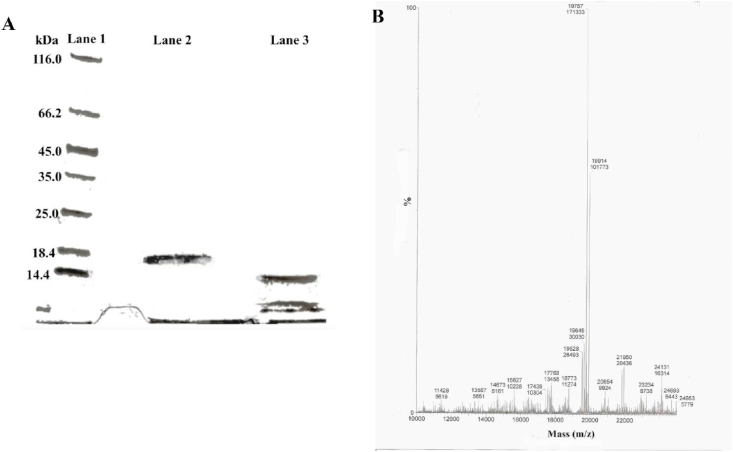
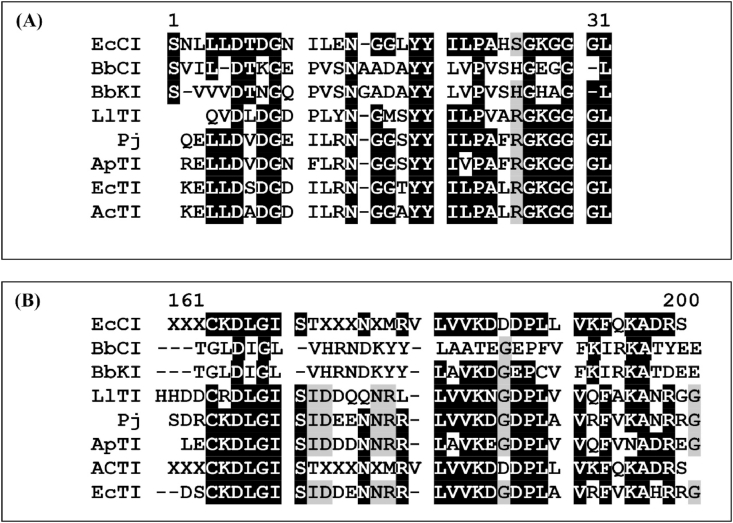
SDS-PAGE electrophoresis of EcCI showed an apparent molecular mass of approximately 18.0 kDa and reducing conditions showed two polypeptides chains (Fig. 3A). The exact EcCI molecular mass is 19,757 Da, as determined by mass spectrometry (Fig. 3B). The N-terminal sequence of the isolated alfa chain SNLLLDTDGNILENGGLYYILPAHSGKGGGL determined by the Edman degradation as well some amino acids residues from the isolated B chain (Fig. 4A and B) compared to other sequences deposited in the protein database using the Blast protein program available at http://blast.ncbi.nlm.nih.gov/have similarity to Kunitz inhibitors, such as the inhibitors purified from Prosopis juliflora [34], Adenatera pavonina [35], Copaifera langsdorffii [36], E. contortisiliquum [7] and Bauhinia bauhinioides [37] among others.
Fig. 3.
Determination of EcCI molecular weight. (A) 4–20% SDS-PAGE; lane 1 represents the kDa molecular weight marker, lane 2 and 3 represent EcCI (10 μg) heated at 100 °C for 10 min in the absence and presence of DTT, respectively. (B) Mass spectra of EcCI by LC/ESI-MS.
Fig. 4.
EcCI N-terminal sequences in comparison with other inhibitors. (A) Alignment of the EcCI A chain; (B) alignment of the EcCI B chain with BbCI, cysteine proteinase inhibitor from B. bahinioides [37], LlTI, L. leucocephala trypsin inhibitor [51]; Pj from P. juliflora [34]; ApTI, A. pavonina trypsin inhibitor [35]; EcTI, E. contortisiliquum trypsin inhibitor [7,10]; and AcTI, A. confuse trypsin inhibitor [52]; Dashes indicate gaps that were introduced for optimal alignment and maximum similarity in the MULTALIN program. Residues identical to EcCI are displayed in black boxes.
3.4. Spectroscopic measurements
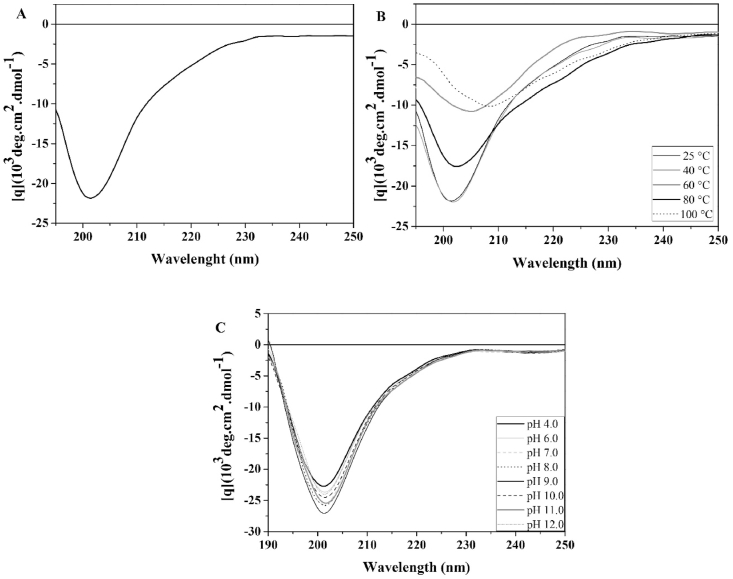
CD spectroscopy was used to characterize EcCI secondary structure. The spectrum showed a negative band at 201.5 nm. Based on CD, the EcCI secondary structure was estimated to be composed of 19% α-helices, 26% β-sheets, 24% β-turns, and 31% unordered structure (Fig. 5A). Three programs (CONTIN, SELCON3, and CDSSTR) were used to improve the reliability of the protein CD analysis, which resulted in less than 2.0% deviation.
Fig. 5.
Spectroscopic measurements. (A) EcCI Circular dichroism (CD) spectrum. Measurements recorded with 0.1 mg mL−1 EcCI in 10 mM acetate borate-phosphate buffer pH 8.0, (B) EcCI structural stability according to temperature. CD of EcCI(0.1 mg mL−1) was obtained after incubation for 120 min at various temperatures; the protein was cooled to room temperature (25 °C). (C) Structural stability at different pH values. EcCI (0.1 mg mL−1) was subjected to various pH values in 0.01 M acetate borate-phosphate buffer at 25 °C for 3 h.
EcCI thermal stability was also analyzed by CD. The EcCI CD spectrum showed approximately 46% decrease in the intensity of ellipticity at 201.5 nm with an associated displacement of the band at 208.5 nm in the temperature range from 25 to 100 °C demonstrating that the EcCI conformation is stable in the temperature range from 25 °C to 40 °C (Fig. 5B).
In contrast, extreme pHs have little effects on the EcCI conformation because no change to its secondary structure was observed after exposure to different pHs (Fig. 5C).
3.5. EcCI inhibitor effects on C. maculatus larval development
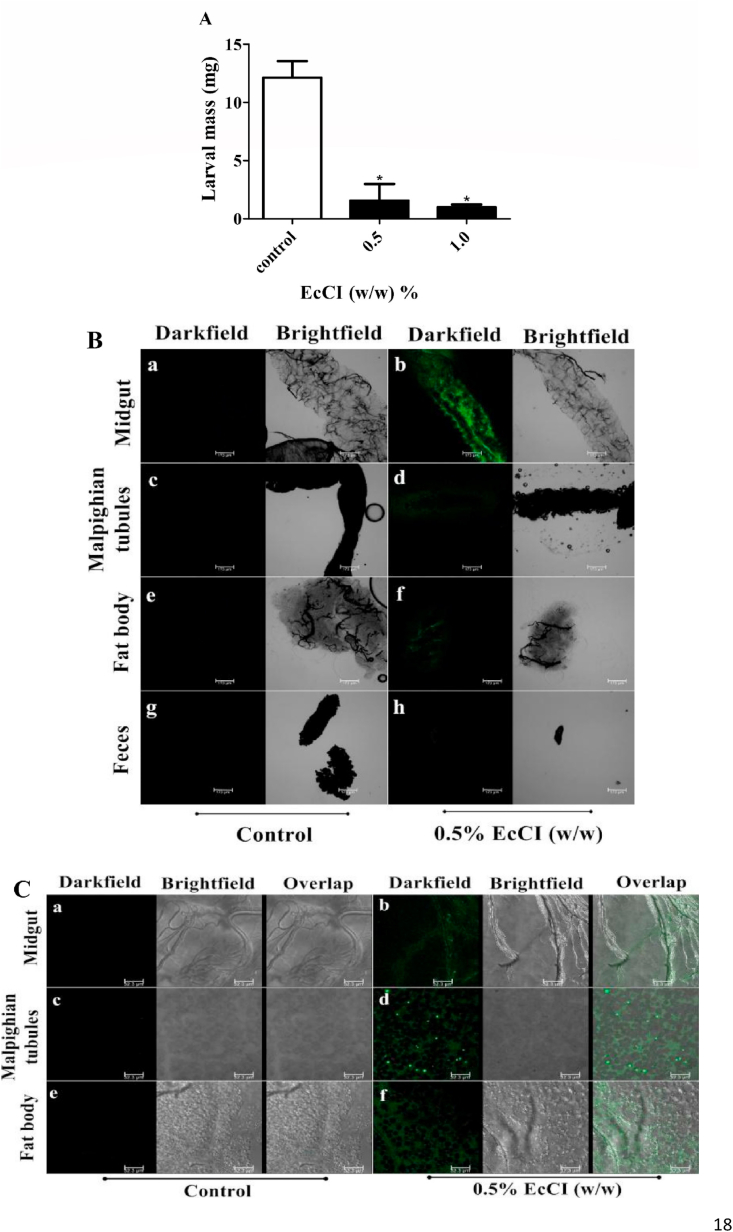
The EcCI inhibitor was toxic to C. maculatus larvae. Fig. 6A shows that the inhibitor reduces larvae body mass by 87% and 92% at concentrations of 0.5% and 1.0% (w/w), respectively when compared to control larvae.
Fig. 6.
Bioassay. (A) EcCI inhibitory effect on the development of larvae. Effect of EcCI on hatched larvae mass. The inhibitor was incorporated into artificial seeds of V. ungiculata at concentrations of 0.5% and 1.0% (w/w). N = 20 larvae. *Statistical significance compared to the control. The data represent means ± SEM (p-value ˂ 0.05 by One-way ANOVA). (B) EcCI-FITC conjugate in C. maculatus larvae, visualization with 10× magnification. a-b midgut, c-d Malphigian tubules, e-f fat body, g-h feces. Bar = 173 μm. (C) EcCI-FITC conjugate in C. maculatus larvae, visualization at 63× magnification. a-b midgut, c-d Malphigian tubules, e-f fat body. Bar = 32.3 μm.
3.6. Effects of conjugated EcCI-FIT on C. maculatus larvae
Treatment of C. maculatus larvae with conjugated EcCI-FIT and subsequent biodistribution resulted in high fluorescence intensity in the midgut of larvae (Fig. 6B a-b), and markedly lower intensity in the Malpighian tubules (Fig. 6B c-d) and fat body (Fig. 6B e-f). Few labeled proteins were detected in larvae feces (Fig. 6B g-h).
EcCI protein was visualized on the cellular surface of the midgut (Fig. 6C a-b) using microscopic analysis at 63× magnification (Fig. 6C); however increase in EcCI protein in malpighian tubules (Fig. 6C c-d) and fat bodies (Fig. 6C e-f) was observed around to the cells, indicating that EcCI was not internalized.
4. Discussion
A large number of inhibitors have been purified from several families of legumes such as Mimosoideae [7,38], Caesalpinioideae [39,40], and Papilionoideae [41,42]. Two inhibitors have been described in E. contortisiliquum seeds, the serine proteinase inhibitor EcTI [7] and a high molecule weight cysteine proteinase inhibitor [5] which is distinct from the inhibitor characterized in this study.
The initial purification steps (extraction, precipitation, and ion exchange) followed the procedures described by Batista et al. [7] for the isolation of the EcTI serine proteinase inhibitor. Precipitation of the saline extract in acetone 80% concentrated its inhibitory activity against papain and reduced the existing pigment.
Papain inhibitory activity (75%) was recovered from fractions obtained from ion-exchange chromatography on DEAE-Sepharose; separation of EcCI from the other cysteine proteinase inhibitor was achieved by size exclusion chromatography on Superdex 75. Although a single elution peak was obtained by reverse phase HPLC chromatography, we suspected that the preparation could be contaminated with the serine proteinase inhibitor, or that both activities could be attributed to the same protein since the molecular weight of EcTI (19.851.5 Da) is similar to EcCI. To distinguish between the two inhibitors, the sample was purified on a trypsin-Sepharose column until no protein was adsorbed in the matrix. The N-terminal sequences of the two chains also indicated differences between the two inhibitors regardless of their observed high similarity.
Other studies show that serine proteinase inhibitors correspond to an important fraction of inhibitors in seeds [[43], [44], [45]]. This is also true in E. contortisiliquum seeds since approximately 12% is responsible for serine proteinase inhibition and 6% to cysteine proteinase inhibition. Nevertheless, even at small concentrations, the high affinity of EcCI for papain (Kiapp 0.036 nM), an enzyme model of the cysteine proteinase, makes it an interesting tool for investigating models in systems in which this enzyme class plays an important role.
EcCI papain inhibition is more effective than other plant inhibitors such as the canecystatin recombinant inhibitor [46], cystatin extracted from apples [16], the P. juliflora inhibitor [47], and the ApTI inhibitor from A. pavonina [19] with Ki's of 3.3 nM, 0.21 nM, 0.59 nM, and 1.0 μM, respectively.
The EcCI amino-terminal region is not similar to phytocystatins, the archetype of plant cysteine proteinase inhibitors [48,49], but is similar to BbCI, fitting the classical plant Kunitz inhibitor family, mainly characterized from seeds of Fabaceae, and the Mimosoideae, Papilionoideae, and Caesalpinioideae taxonomic subfamilies. The EcCI N-terminal region shows similarity with purified proteins from the Cesalpinoideae subfamily such as Bauhinia bauhinioides [37], Copaifera langsdorffii [36], and Caesalpinia echinata [50], and the following Mimosoideae subfamily members: Adenathera pavonina [35], Enterolobium contortisiliquum [7], Leucaena leucocephala [51], Acacia confused [52] and Prosopis juliflora [34]. These groups include proteins with a molecular weight of approximately 20–22 kD, consisting of one or two polypeptide chains and generally four cysteine residues that form two disulfide bonds (Cys39-Cys85 and Cys136-Cys145, in comparison of the soybean trypsin inhibitor) [11,13]. However, the BbCI inhibitor is devoid of cysteine residue [37].
The EcCI molecular mass of 19,757 kDa is comparable with other Kunitz inhibitors [1] but differs from EcTI (19,851 kDa) [10]. It should be noted that inhibitors isolated from species in the Mimosoideae subfamily, including EcCI, are constituted by two polypeptide chains with a long chain (designated A or α) of approximately 13–16 kDa, an intra-chain disulfide bridge, and a small chain (designated B or β) of around 5–6 kDa that binds alpha chain by a disulfide bridge.
Although no structural similarity was detected between EcCI and classical inhibitors of cysteine proteinases, the amino-terminal region showed similarity to proteins such as BbCI [37], ApTI [19,53], and the inhibitor extracted from Prosopis juliflora [47], which are described as inhibitors of this class of enzymes. Secondary structure analysis shows a high proportion of β-structures and disordered structures indicating that EcCI belongs to the class of β proteins and confirming its similarity to the Kunitz family of inhibitors whose structural feature is the high percentage of betas and disordered structures [52,[54], [55]].
EcCI, unlike EcTI, is thermostable: its inhibitory activity against papain decreases by only 40% after 2 h exposure to 100 °C, while EcTI is less heat resistant and shows total decreased functionality after 10 min of exposure to heat [7]. The loss of functionality of proteins in even short treatment periods at extreme pH conditions is common. However, EcCI was shown to be functional (30–60%) after long-term treatment in acidic and basic pH conditions. This property indicates that the inhibitory function of this molecule is still partially preserved in the acidic conditions of the digestive tract of C. maculatus larvae with pH around 5.0–6.0 [56]. The best functional efficiency of EcCI is at pH 6.0 to 8.0, similar to most inhibitors isolated from this plant genus [7,[57], [58], [59]]. The EcCI preservation of conformational structure in extreme pH values was confirmed by the circular dichroism spectrum; however, this did not occur with heat treatment, indicating that this protein is heat-labile.
The frequency of proteinase inhibitors in the seeds of many plant species has raised attention in their physiological functions and suggests a role in nutrient storage and, especially, a protective action against the attack of predators [15,18,[60], [61], [62], [63], [64]]. Cowpea is an important nutritional source for people around the world, especially poor populations. Infestations of insects causing “bean weevil” by Zabrotes subfasciatus and C. maculatus severely affect harvest quality and yield and are of concern to farmers and scholars seeking non-toxic control alternatives. Several studies show the detrimental effect on the development and survival of insect larvae caused by proteinase inhibitors, especially those obtained from edible seeds. The mechanism by which proteinase inhibitors interfere with the digestive process of insects is due to decreased absorption of nutrients. When insects are subjected to an artificial diet containing major classes of proteinase inhibitors specific for their guts, they display altered growth and development that lead to significant mortality. Development and survival are parameters frequently explored in studies of insecticide activity [19,29,32,65,66].
The evaluation of larvicidal activity through the incorporation of EcCI into artificial seeds shows that, although there was no larval mortality during the study period, EcCI had a major deleterious effect in reducing larval weight and affecting their development. EcCI (1% w/w) inhibited the development of larvae body mass by 92%, which is a result similar to that observed for the cysteine proteinase inhibitor ApTI [19]. The EcCI activity was similar to that of BrTI (Bauhinia rufa Trypsin inhibitor) purified from seeds of Bauhinia rufa [33], although the inhibition specificity of these proteins is dissimilar. In the case of BrTI, the authors attributed the deleterious effect to not only its inhibitory function but also to an additional sequence (RGE) which can induce cell death.
Detection of high fluorescence in the midgut of larvae and low fluorescence intensity in their feces suggest retention of FITC conjugated EcCI inhibitor in the larvae midgut. This fact was also observed by De Sá et al. [67] in which C. maculatus larvae were artificially fed with seeds containing tegument of P. vulgaris conjugated with FITC.
5. Conclusion
This study reports a novel cysteine proteinase inhibitor purified from E. contortisiliquumm seeds, EcCI that blocks cysteine proteinases from the intestinal extract of C. maculatus larvae, a pest of great economic importance in agriculture. The protein showed evidence of the larvicidal potential, as its incorporation in artificial seeds results in a detrimental effect on the development of the larvae.
Author contributions
Natalia N. S. Nunes performed the experimental work and data analysis. Leonardo F. R. de Sá and A. E. de Oliveira supervised the C. mauculatus experiments. Rodrigo S. Ferreira performed CD experiments and analysis. MLVO designed the experiments, contribute to discussing reviewing, and approving the final version of the manuscript for publication. All authors read and approved the final manuscript.
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [2017/07972-9 and 2017/06630-7]; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [401452/2016-6]. M.L.V.O. received a Research fellowship from CNPq, Brazil.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
This manuscript was reviewed by a professional science editor and a native English-speaking editor to improve readability. Nice Shindo, Ph.D. and Rita J Gray, MSc. (niceshindo@gmail.com and rita.j.gray@gmail.com).
References
- 1.Oliva M.L., Silva M.C., Sallai R.C., Brito M.V., Sampaio M.U. A novel subclassification for Kunitz proteinase inhibitors from leguminous seeds. Biochimie. 2010;92:1667–1673. doi: 10.1016/j.biochi.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Oddepally R., Gopi S., Guruprasad L. Purification and characterization of a stable Kunitz trypsin inhibitor from Trigonella foenum-gaecum (fenugreek) seeds. Phytochemistry. 2013;96:26–36. doi: 10.1016/j.phytochem.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Macedo M.L.R., Ribeiro S.F.F., Taveira G.B., Gomes V.M., de Barros K.M.C.A., Maria-Neto S. Antimicrobial activity of ILTI, a Kunitz-type trypsin inhibitor from Inga laurina (SW.) willd. Curr. Microbiol. 2016;72:538–544. doi: 10.1007/s00284-015-0970-z. [DOI] [PubMed] [Google Scholar]
- 4.Araújo A.P., Sobrinho S.P. Germinação e produção de mudas de tamboril (Enterolobium contortisiliquum) (Vell) morong) em diferentes substrates. Rev. Árvore. 2011;35:581–588. [Google Scholar]
- 5.Oliva M.L., Sampaio U.M., Sampaio C.A. Serine and SH-proteinase inhibitor from Enterolobium contortisiliquum bean. Purification and preliminary characterization. Braz. J. Med. Biol. Res. 1987;20:767–770. [PubMed] [Google Scholar]
- 6.Silva G.A., Oliva M.L., Sampaio M.U., Araújo M.S., Sampaio C.A. Isolation and partial characterization of an endopeptidase from Enterolobium contortisiliquum seeds. Braz. J. Med. Biol. Res. 1994;27:1299–1310. [PubMed] [Google Scholar]
- 7.Batista I.F.C., Oliva M.L.V., Araújo M.S., Sampaio M.U., Richardson M., Fritz H., Sampaio C.A. Primary structure of Kunitz-type trypsin inhibitor from Enterolobium contortisiliquum seeds. Phytochemistry. 1996;41940:1017–1022. doi: 10.1016/0031-9422(95)00710-5. [DOI] [PubMed] [Google Scholar]
- 8.Lima M.R., Zanotta P.J.P., Ricart C.A.O., Souza M.V. Presence of the cytolytic protein enterolobin in different developmental stages of Enterolobium contortisiliquum seeds. Braz. J. Plant Physiol. 2007;19:163–170. [Google Scholar]
- 9.Moura F.T., Oliveira A.S., Macedo L.L.P., Vianna A.L.B.R., Andrade L.B.S., Martins-Miranda A.S., Oliveira J.T.A., Santos E.A., Sales M.P. Effects of chitin-binding vicilin from Enterolobium contortisiliquum seeds on bean bruchid pests (Callosobruchus maculatus and Zabrotes subfasciatus) and phytopathogenic fungi (Fusarium solani and Colletrichum lindemuntianum) J. Agric. Food Chem. 2007;55:260–266. doi: 10.1021/jf061623k. [DOI] [PubMed] [Google Scholar]
- 10.Zhou D., Lobo Y.A., Batista I.F.C., Marque-Porto R., Gustchina A., Oliva M.L.V., Wlodawer A. Crystal structure of a plant trypsin inhibitor from Enterolobium contortisiliquum (EcTI) and of its complex with bovine trypsin. PloS One. 2013;8 doi: 10.1371/journal.pone.0062252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birk Y. In: Plant Proteinase Inhibitors: Significance in Nutrition, Plant Protection, Cancer Prevention and Genetic Engineering. Berlin, editor. Springer-Verlag.; 2003. pp. 1–126. [Google Scholar]
- 12.Laskowski M., Jr., Kato I. Protein inhibitor of proteinases. Ann. Rev. biochem. 1980;49:685–993. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- 13.Richardson M. Seed storage proteins: the enzyme inhibitors. Methods Plant Biochem. 1991;5:259–305. [Google Scholar]
- 14.Hansen D., Macedo-Ribeiro S., Veríssimo P., Yoo I.S., Sampaio U.M., Oliva M.L.V. Crystal structure of a novel cysteinless plant Kunitz-type proteinase inhibitor. Biochem. Biophys. Res. Commun. 2007;360:735–740. doi: 10.1016/j.bbrc.2007.06.144. [DOI] [PubMed] [Google Scholar]
- 15.Mosolov V.V., Valueva T.A. Proteinase inhibitors and their function in plants: a review. Prikl. Biokhim. Mikrobiol. 2005;41:261–282. [PubMed] [Google Scholar]
- 16.Ryan C.A., Pearce G. Systemin: a polypeptide signal for plant defensive genes. Annu. Rev. Cell Dev. Biol. 1998;14:1–17. doi: 10.1146/annurev.cellbio.14.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Mosolov V.V., Grigor’eva L.I., Valueva T.A. Involvement of proteolytic enzymes and their inhibitors in plant protection (review) Appl. Biochem. Microbiol. 2001;37:115–123. [PubMed] [Google Scholar]
- 18.Carlini C.R., Grossi-de-Sá M.F. Plant toxic proteins with insecticidal properties. A review on their potentialities as bioinsecticides. Toxicon. 2002;40:1515–1539. doi: 10.1016/s0041-0101(02)00240-4. [DOI] [PubMed] [Google Scholar]
- 19.Macedo M.L.R., Sá C.M., Freire M.G.M., Parra J.R.P.A. Kunitz-Type inhibitor of Coleopteran proteinases, isolated from Adenanthera pavonina L. seeds and its effect on Callosobruchus maculatus. J. Agric. Food Chem. 2004;52:2533–2540. doi: 10.1021/jf035389z. [DOI] [PubMed] [Google Scholar]
- 20.Tiroesele B., Thomas K., Seketeme S. Control of cowpea weevil, Callosobruchus maculatus (F.) (Coleoptera: bruchidae), using natural products. Insects. 2015;6:77–84. doi: 10.3390/insects6010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Southgate B.J. Biology of the bruchidae. Annu. Rev. Entomol. 1979;24:449–473. [Google Scholar]
- 22.Dongre T.K., Pawar S.E., Thakare R.G., Harwalkar M.R. Identification of resistant sources to cowpea weevil (Callosobruchus maculatus (F.)) in Vigna sp. and inheritance of their resistance in black gram (Vigna mungo var. mungo) J. Stored Prod. Res. 1996;32:201–204. [Google Scholar]
- 23.Koiwa H., Shade R.E., Zhu-Salzman K., Subramanian L., Murdock L.L., Nielsen S.S., Bressan R.A., Hasegawa P.M. Phage display selection can differentiate insecticidal activity of soybean cystatins. Plant J. 1998;14:371–379. doi: 10.1046/j.1365-313x.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- 24.Schuler T.N., Poppy G.M., Kerry B.R., Denholm I. Insect-resistant transgenic plants. Trends Biotechnol. 1998;16:168–175. doi: 10.1016/S0167-7799(98)01298-0. [DOI] [PubMed] [Google Scholar]
- 25.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Edman P., Högfeldt E., Sillén L.G., Kinell P.-O. Method for determination of the amino acid sequence in peptides. Acta Chem. Scand. 1950;4:283–293. doi: 10.3891/acta.chem.scand.04-0283. [DOI] [Google Scholar]
- 27.Silva M.C.C., Santana L.A., Mentele R., Ferreira R.S., de Miranda A., Silva-Lucca R.A., Sampaio M.U., Correia M.T.S., Oliva M.L.V. Purification, primary structure and potential functions of a novel lectin from Bauhinia forficata seeds. Process Biochem. 2012;47:1049–1059. [Google Scholar]
- 28.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Nunes N.N.S., Ferreira R.S., Silva-Lucca R.A., de Sá L.F.R., Oliveira A.E.A., Correia M.T.S., Paiva P.M.G., Wlodawer A., Oliva M.L.V. Potential of the lectin/inhibitor isolated from Crataeva tapia bark (CrataBL) for controlling Callosobruchus maculatus larva development. J. Agric. Food Chem. 2015;63:10431–10436. doi: 10.1021/acs.jafc.5b03634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sreerama N., Woody R.W. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 2000;287:252–260. doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
- 31.Sreerama N., Venyaminov S.Y., Woody R.W. Analysis of protein circular dichroism spectra based on the tertiary structure classification. Anal. Biochem. 2001;299:271–274. doi: 10.1006/abio.2001.5420. [DOI] [PubMed] [Google Scholar]
- 32.Uchoa A.F., Da Matta R.A., Retamal C.A., Albuquerque-Cunha J.M., Souza S.M., Samuels R.I., Silva C.P., Xavier-Filho J. Presence of the storage seed protein vicilin in internal organs of larval Callosobruchus maculatus (Coleoptera: bruchidae) J. Insect Physiol. 2006;52:169–178. doi: 10.1016/j.jinsphys.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Sumikawa J.T., Brito M.V., Macedo M.L.R., Uchoa A.F., Miranda A., Araújo A.P.U., Silva-Luca R.A., Sampaio U.M., Oliva M.L.V. The defensive functions of plant inhibitors are not restricted to insect inhibition enzyme. Phytochemistry. 2010;71:214–220. doi: 10.1016/j.phytochem.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Negreiros A.N., Carvalho M.M., Xavier-Filho J., Blanco-Labra A., Shewry P.R., Richardson M. The complete amino acid sequence of the major Kunitz trypsin inhibitor from the seeds of Prosopsis juliflora. Phytochemistry. 1991;30:2829–2833. doi: 10.1016/s0031-9422(00)98207-4. [DOI] [PubMed] [Google Scholar]
- 35.Richardson M., Campos F.A.P., Xavier-Filho J., Macedo M.L.R., Maia G.M.C., Yarwood A. The amino acid sequence and reactive (inhibitory) site of the major trypsin isoinhibitor (DE5) isolated from seeds of the Brazilian Carolina tree (Adenanthera pavonina L.) Biochim. Biophys. Acta. 1986;872:134–140. [Google Scholar]
- 36.Silva J.A., Macedo M.L., Novello J.C., Marangoni S. Biochemical characterization and N-terminal sequences of two new trypsin inhibitors from Copaifera langsdorffii seeds. J. Protein Chem. 2001;20:1–7. doi: 10.1023/a:1011053002001. [DOI] [PubMed] [Google Scholar]
- 37.De Oliveira C., Santana L.A., Carmona A.K., Cezari M.H., Sampaio M.U., Sampaio C.A., Oliva M.L. Structure of cruzipain/cruzain inhibitors isolated from Bauhinia bauhinioides seeds. Biol. Chem. 2001;382:847–852. doi: 10.1515/BC.2001.103. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharyya A., Mazumdar S., Mazumdar-Leighton S., Babu C.R.A. Kunitz proteinase inhibitor from Archidendron ellipticum seeds: purification characterization and kinetic properties. Phytochemistry. 2006;67:232–241. doi: 10.1016/j.phytochem.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Pando S.C., Oliva M.L., Sampaio C.A., Di Ciero L., Novello J.C., Marangoni S. Primary sequence determination of a Kunitz inhibitor isolated from Delonix regia seeds. Phytochemistry. 2001;57:625–631. doi: 10.1016/s0031-9422(01)00080-2. [DOI] [PubMed] [Google Scholar]
- 40.Araujo A.P., Hansen D., Vieira D.F., Oliveira C., Santana L.A., Beltramini L.M., Sampaio C.A., Sampaio U.M., Oliva M.L. Kunitz-type Bauhinia bauhinioides inhibitors devoid of disulfide bridges: isolation of cDNAs, heterologous expression and structural studies. Biol. Chem. 2005;386:561–568. doi: 10.1515/BC.2005.066. [DOI] [PubMed] [Google Scholar]
- 41.Pando L.A., Di Ciero L., Novello J.C., Oliveira B., Weder J.K., Marongoni S. Isolation and characterization of a new trypsin inhibitor from Crotalaria pulina seeds. IUBMB Life. 1999;48:519–523. doi: 10.1080/713803553. [DOI] [PubMed] [Google Scholar]
- 42.Gomes C.E.M., Barbosa A.E.A.D., Macedo L.L.P., Pitanga J.C.M., Moura F.T., Oliveira A.S., Moura R.M., Queiroz A.F.S., Macedo F.P., Andrade L.B.S., Vidal M.S., Sales M.P. Effect of trypsin inhibitor from Crotalaria pallida seeds on Callosobruchus maculatus (cowpea weevil) and Ceratitis capitata (Fruit fly) Plant Physiol. Biochem. 2005;43:1095–1102. doi: 10.1016/j.plaphy.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Koepke J., Ermler U., Warkentin E., Wenzl G., Flecker P. Crystal structure of cancer chemopreventive Bowman-Birk inhibitor in ternary complex with bovine trypsin at 2.3 Å resolution. Structural basis of janus-faced serine proteinase inhibitor specificity. J. Mol. Biol. 2000;298:477–491. doi: 10.1006/jmbi.2000.3677. [DOI] [PubMed] [Google Scholar]
- 44.Kowalska J., Zablocka A., Wilusz T. Isolation and primary structures of seven serine proteinase inhibitors from Cyclanthera pedata seeds. Biochim. Biophys. Acta. 2006;1760:1054–1063. doi: 10.1016/j.bbagen.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Konarev A.V., Lovegrove A., Sheury P.R. Serine proteinase inhibitors in seeds of Cycas siamensis and other gymnosperms. Phytochemistry. 2008;69:2482–2489. doi: 10.1016/j.phytochem.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Oliva M.L.V., Carmona A.K., Andrade S.S., Cotrin S.S., Soares-Costa A., Henrique-Silva F. Inhibitory selectivity of canecystatin: a recombinant cysteine peptidase inhibitor from sugarcane. Biochem. Biophys. Res. Commun. 2004;320:1082–1086. doi: 10.1016/j.bbrc.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 47.Oliveira A.S., Pereira R.A., Lima L.M., Morais A.H.A., Melo F.R., Franco O.L., Bloch J.R.C., Grossi-De-Sá M.F., Sales M.P. Activity toward bruchid pest of a Kunitz-type inhibitor from seeds of the algarroba tree (Prosopis juliflora D.C.) Pestic. Biochem. Physiol. 2002;72:122–132. [Google Scholar]
- 48.Chu M.-H., Liu K.-L., Wu H.-Y., Yeh K.-W., Cheng Y.-S. Crystal structure of tarocystatin-papain complex: implications for the inhibition property of group-2 phytocystatins. Planta. 2011;234:243–254. doi: 10.1007/s00425-011-1398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irene D., Chung T.-Y., Chen B.-J., Liu T.-H., Li F.-Y., Tzen J.T.C., Wang C., Chyan C.-L. Solution structural of a phytocystatin from Ananas comosus and its molecular interaction with papain. PloS One. 2012;7 doi: 10.1371/journal.pone.0047865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cruz-Silva I., Gozzo A.J., Nunes V.A., Carmona A.K., Faljoni-Alario A., Oliva M.L., Sampaio M.U., Sampaio C.A., Araujo M.S. A proteinase inhibitor from Caesalpinia echinata (pau-brasil) seeds for plasma kallikrein, plasmin and fator XIIa. Biol. Chem. 2004;385:1083–1086. doi: 10.1515/BC.2004.140. [DOI] [PubMed] [Google Scholar]
- 51.Oliva M.L., Souza-Pinto J.C., Batista I.F., Araújo M.S., Silveira V.F., Auerswald E.A., Mentele R., Eckerskorn C., Sampaio M.U., Sampaio C.A. Leucena leucocephala serine proteinase inhibitor: primary structure and action on blood coagulation, kinin release and rat paw edema. Biochim. Biophys. Acta. 2000;1477:64–74. doi: 10.1016/s0167-4838(99)00285-x. [DOI] [PubMed] [Google Scholar]
- 52.Wu H.C., Lin J.Y. The complete amino acid sequence of Kunitz family trypsin inhibitor from seeds of Acacia confuse. J. Biochem. 1993;113:258–263. doi: 10.1093/oxfordjournals.jbchem.a124036. [DOI] [PubMed] [Google Scholar]
- 53.Migliolo L., Oliveira A.S., Santos E.A., Franco O.L., Sales M.P. Structural and mechanistic insights into a novel non-competitive Kunitz trypsin inhibitor from Adenanthera pavonina L. seeds with double activity toward serine and cysteine-proteinases. J. Mol. Graph. Model. 2010;29:148–156. doi: 10.1016/j.jmgm.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Azarkan M., Dibiani R., Goormaghtigh E., Raussens V., Baeyens-Volant D. The papaya Kunitz-type trypsin inhibitor is a highly stable beta-sheet glycoprotein. Biochim. Biophys. Acta. 2006;1764:1063–1072. doi: 10.1016/j.bbapap.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 55.Bhattacharyya A., Babu C.R. Purification and biochemical characterization of serine proteinase inhibitor from Derris trifoliate Lour.Seeds: insight into structural and antimalarial features. Phytochemistry. 2009;70:703–712. doi: 10.1016/j.phytochem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Silva C.P., Terra W.R., Xavier-Filho J., de Sá M.F.G., Lopes A.R., Pontes E.G. Digestion in larvae of Callosobruchus maculatus and Zabrotes subfasciatus (Coleoptera: bruchidae) with emphasis on α-amylases and oligosaccharidases. Insect Biochem. Mol. Biol. 1999;29:355–366. [Google Scholar]
- 57.Oliva M.L., Sallai R.C., Sampaio C.A., Fritz H., Auerswald E.A., Tanaka A.S., Torquato R.J., Sampaio M.U. Bauhinia serine proteinase inhibitors: effect on factor X, factor XII and plasma kallikrein. Immunopharmacology. 1996;32:85–87. doi: 10.1016/0162-3109(95)00058-5. [DOI] [PubMed] [Google Scholar]
- 58.Oliva M.L., Andrade S.A., Batista I.F., Sampaio M.U., Juliano M., Fritz H., Auerswald E.A., Sampaio C.A. Human plasm kallikrein and tissue kallikrein binding to substrate based on the reactive site of a factor Xa inhibitor isolated from Bauhinia ungulata seeds. Immunopharmacology. 1999;45:145–149. doi: 10.1016/s0162-3109(99)00146-0. [DOI] [PubMed] [Google Scholar]
- 59.Oliva M.L., Mendes C.R., Juliano M.A., Chagas J.R., Rosa J.C., Greene L.J., Sampaio M.U., Sampaio C.A. Characterization of a tissue kallikrein inhibitor isolated from Bauhinia bauhinioides seeds: inhibitor of the hydrolysis of kininogen related substrates. Immunopharmacology. 1999;45:163–169. doi: 10.1016/s0162-3109(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 60.Lawrence P.K., Koundal K.R. Plant protease inhibitor in control of phytophagous insects. Electron. J. Biotechnol. 2002;5:93–109. [Google Scholar]
- 61.Barrette-Ng I.H., Ng K.K., Cherney M.M., Pearce G., Ghani U., Ryan C.A., James M.N. Unbound form of tomato inhibitor-II reveals interdomain flexibility and conformational variability in the reactive site loops. J. Biol. Chem. 2003;278:1391–31400. doi: 10.1074/jbc.M304562200. [DOI] [PubMed] [Google Scholar]
- 62.Breiteneder H., Radauer C. A classification of plant food allergens. J. Allergy Clin. Immunol. 2004;113:821–830. doi: 10.1016/j.jaci.2004.01.779. [DOI] [PubMed] [Google Scholar]
- 63.Chye M., Sin S., Xu Z., Yeung E. Serine proteinase inhibitor proteins: exogenous and endogenous functions, in Vitro Cell. Dev. Biol. Plant. 2006;42:100–108. [Google Scholar]
- 64.Oliva M.L., Ferreira R.S., Ferreira J.G., de Paula C.A., Salas C.E., Sampaio M.U. Structural and functional properties of Kunitz proteinase inhibitors from leguminosae: a mini review. Curr. Protein Pept. Sci. 2011;12:348–357. doi: 10.2174/138920311796391061. [DOI] [PubMed] [Google Scholar]
- 65.Sales M.P., Gehardt I.R., Grossi-De-Sá M.F., Xavier-Filho J. Do legumes storage protein play a role in defending seeds against bruchids? Plant Physiol. 2000;124:515–522. doi: 10.1104/pp.124.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Souza A.J., Ferreira A.T.S., Perales J., Beghini D.G., Fernandes K.V.S., Xavier-Filho J., Venâncio T.M., Oliveira A.E.A. Identification of Albizia lebbeck seed coat chitin-binding vicilins (7s globulins) with high toxicity to larvae of bruchid Callosobruchus maculatus. Braz. J. Med. Biol. Res. 2012;45:118–124. doi: 10.1590/S0100-879X2012007500008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Sá L.F., Wermelinger T., Ribeiro E.S., Gravina G.A., Fernandes K.V.S., Xavier-Filho J., Venâncio T.M., Resende G.L., Oliveira A.E.A. Effects of Phaseolus vulgaris (Fabaceae) seed coat on the embryonic and larval development of cowpea weevil Callosobruchus maculatus (Coleoptera: bruchidae) J. Insect Physiol. 2014;60:50–57. doi: 10.1016/j.jinsphys.2013.10.004. [DOI] [PubMed] [Google Scholar]