Abstract
SRT1720, a sirtuin1-activator, and metformin (MET), an antidiabetic drug, confer health and life-span benefits when administered individually. It is unclear whether combination of the two compounds could lead to additional benefits. Groups of 56-week-old C57BL/6J male mice were fed a high-fat diet (HFD) alone or supplemented with either SRT1720 (2 g/kg food), a high dose of MET (1% wt/wt food), or a combination of both. Animals were monitored for survival, body weight, food consumption, body composition, and rotarod performance. Mice treated with MET alone did not have improved longevity, and life span was dramatically reduced by combination of MET with SRT1720. Although all groups of animals were consuming similar amounts of food, mice on MET or MET + SRT1720 showed a sharp reduction in body weight. SRT1720 + MET mice also had lower percent body fat combined with better performance on the rotarod compared to controls. These data suggest that co-treatment of SRT1720 with MET is detrimental to survival at the doses used and, therefore, risk-benefits of combining life-span-extending drugs especially in older populations needs to be systematically evaluated.
Keywords: Sirtuin, SRT1720, Metformin, Combination, Life span
Pharmacological stimulation of sirtuins (a family of NAD+-dependent deacetylases) elicits several benefits for life-span and health extension (1). Sirtuin 1 (SIRT1) activation extends life span and health in model organisms (2,3). Small molecules such as resveratrol activate SIRT1 and trigger cellular signaling leading to improved mitochondrial biogenesis (4) and reduced inflammation (5). Studies have shown that SRT2104, an allosteric SIRT1 activator, preserves bone and muscle mass and extends survival of male mice on standard diet (SD) (6). SRT1720, another SIRT1 activator, improves healthspan and increases survival of middle-aged mice on SD (7) and on high-fat diet (HFD) (8). SRT1720 also improves glucose homeostasis and insulin sensitivity in mouse and rat models of type 2 diabetes (T2D) (3). Some of these SIRT1 activators have also been tested in clinical trials, mostly for metabolic conditions such as T2D, with some encouraging results (9), making the use of SIRT1 activators clinically relevant.
Metformin (MET) is a biguanide commonly used as the first line of treatment for T2D, as it reduces hepatic gluconeogenesis and increases insulin sensitivity and glycolysis. MET has also been proposed for weight loss in people that are overweight who do not have T2D (10). We previously showed that daily treatment of aged mice fed SD with 0.1% MET elicited a small but significant extension in longevity, whereas 1% MET (high dose, ~500 mg/kg body weight) resulted in a marked reduction in survival (11). We also showed that intermittent consumption of 1% MET improved metabolic parameters in SD-fed middle-aged mice without reduction in life span, although the MET-treated mice still exhibited some kidney issues (12). Within the interventions testing program, 0.1% MET lead to a nonsignificant 7% increase in median life span in only male mice when data from multiple study sites were combined (13). Other reports have shown that 100 mg MET/kg body weight (~0.2% MET) mediates beneficial geroprotective effects in female mice (14). Even though 1% MET resulted in life-span reduction, it strongly mimics transcriptomic changes elicited by caloric restriction (CR) (11,15), which prompted the investigation of alternative strategies to circumvent adverse effects of high dose MET.
Combining small molecules has been a successful strategy to obtain better efficacy, decreased toxicity, and reduced drug resistance (16). Mixtures and combinations of two or more compounds have been proposed as anti-aging interventions (17). Based on this, we tested the hypothesis that the combination of SRT1720 with MET may alleviate some of the adverse outcomes of 1% MET, while conferring synergistic benefits on health and survival in a cohort of aged mice fed HFD.
Method
Animals and Diets
Male C57BL/6J mice at 15 weeks of age were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were kept on a standard mouse diet (cat. #2018SX, Envigo, Frederick, MD) with ad libitum access to food and water, and group-housed in conventional micro-isolator cages (Lab Products, Seaford, DE) on a light:dark 12:12-h schedule and maintained between 20 and 22°C at 30%–70% humidity. At 56 weeks age, mice were randomized into experimental groups: (a) HFD (AIN-93G containing 60% total calories from fat (carbohydrate:protein:fat ratios of 16:23:61 percent of kcal), n = 106; (b) HFD with SRT1720 (2 g/kg chow or ~100 mg/kg body weight) (HFD + SRT1720), n= 107; (c) HFD with 1% MET (10 g/kg chow or ~500 mg/kg body weight) (HFD + MET), n = 94; and (d) HFD with the combination (HFD + SRT1720 + MET), n = 108. Data obtained for HFD and HFD + SRT1720 mice were previously published in Minor and colleagues (8), but all experiments were conducted simultaneously as part of a larger study and divided up only for the purpose of reporting. The large original study was conducted as a way to conserve animal numbers in control groups which can be found in Mitchell and colleagues (7) and Minor and colleagues (8). Diets were purchased from Dyets, Inc. (Bethlehem, PA). SRT1720 was provided by Sirtris Pharmaceuticals (Cambridge, MA) and MET was obtained from Farmhispania (Farmhispania S.A., Barcelona, Spain). Body weight and food intake were measured every other week. All animal protocols were approved by the Animal Care and Use Committee (352-LEG-2012) of the National Institute on Aging, NIH.
Survival Study
Mice were examined daily for signs of health issues, and deaths and euthanasia of moribund mice were recorded. Every mouse found dead or euthanized was necropsied. Criteria for euthanasia were based on an independent assessment by a veterinarian according to AAALAC guidelines. Only cases where the condition of mice was considered incompatible with continued survival are represented as deaths in the curves. Animals euthanized for reasons not related to incompatible survival were censored. The number of censored animals by group is shown in Supplementary Table S1.
Body Composition
Measurements of body fat and lean mass in live mice were acquired by nuclear magnetic resonance (NMR) spectroscopy with the Minispec LF90 (Burker Optics, Billerica, MA) (n = 15 mice per group; 8 weeks on diet; 64 weeks old).
Rotarod
All mice were acclimated for 15 minutes before testing. Mice were tested at the same time every day after a habituation trial at a constant speed of 4 rpm for 1 minute before the first trial. On the same day, mice were given three trials on the accelerating rotarod, during which the rotarod accelerated from 4 to 40 rpm over a period of 5 minutes. Each trial was separated by a 30-minute rest period. The latency to fall was recorded and averaged over the three trials (n = 9 mice per group; 12 weeks on diet, 68 weeks of age).
Data Analysis and Statistics
Investigators were not blinded during experiments/assessment. Log-rank test was used to compare the differences in Kaplan–Meier survival curves. Maximum life span was defined as 10th percentile of mice still alive. Number of animals used for this study were based on our previous experience as well 80% power, α = .05 for 10% effect size (18). Data are expressed as mean ± SEM and two-way analysis of variance with Tukey’s multiple comparison post hoc test was performed as it allowed to additionally assess the effect of MET alone, SRT1720 alone, and their interaction. No interaction between MET and SRT1720 was found. Analyses were performed using Excel 2010 (Microsoft Corp., Redmond, WA) and Graph Pad Prism version 8 (San Diego, CA, USA). A p value of less than .05 was considered statistically significant.
Results
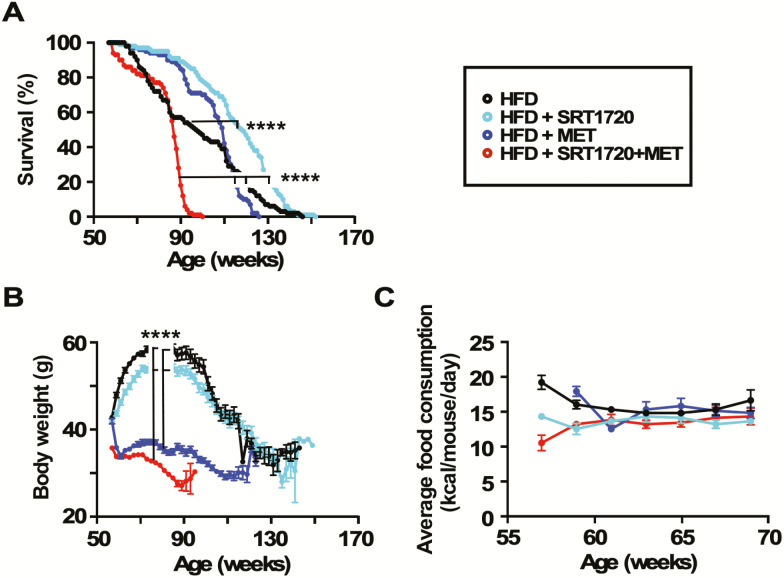
Treatment with SRT1720 significantly improved median survival (103 weeks vs 115 weeks, p < .0001) without maximum life-span extension, while MET alone had no impact on life-span in HFD-fed mice (Figure 1A; Supplementary Table S1). Mice on the combination MET + SRT1720 exhibited a dramatic reduction both in median (84 weeks) and maximum (~100–105 weeks) life span, with a ~35% reduction in maximum life span compared to HFD controls (Figure 1A). Glomerulonephrosis (characterized by enlarged, discolored, and lumpy kidneys) was observed in 24% of the MET-treated mice during necropsy as per previous reports about high doses of MET associating with renal problems in mice (12). Glomerulonephrosis incidence was markedly higher in the combination group (Supplementary Figure S1), although pathological characterization of these kidneys was not performed.
Figure 1.
Effects of SRT1720 and metformin (MET) supplementation on survival, bodyweight, and food consumption in high-fat diet (HFD)-fed mice. (A) Kaplan–Meier survival curves for mice fed HFD alone or supplemented with SRT1720, MET, or the combination SRT1720 + MET. n = 94–108 mice per group. See also Supplementary Table 1. ****p < .0001 combination group compared to MET alone, ****p < .0001 combination group compared to SRT1720 alone, ****p < .0001 combination group compared to control, ****p < .0001 SRT1720 alone compared to control (Mantel–Cox test). (B) Bodyweight trajectories. ****p < .0001 combination group compared to control and SRT1720 alone; ****p < .0001 MET alone compared to control and SRT1720 alone (two-way analysis of variance with Tukey post hoc analysis). (C) Trajectories of daily food intake per mouse over a period of 12 weeks. Data in (B) and (C) are means ± SEM.
Compared to controls, mice on SRT1720 exhibited a modest reduction in body weight gain in the early phase of the protocol, whereas MET and MET + SRT1720 mice steadily lost weight (Figure 1B). Body weight of mice in the combination group at the beginning was slightly lower than the other groups in the study, an effect we believe to be random but warrants acknowledgment. No significant differences in food consumption were observed (Figure 1C). Food consumption was not measured beyond 70 weeks as mice in the combination group started to rapidly die and measurements during this time would have been directly impacted by moribundity.
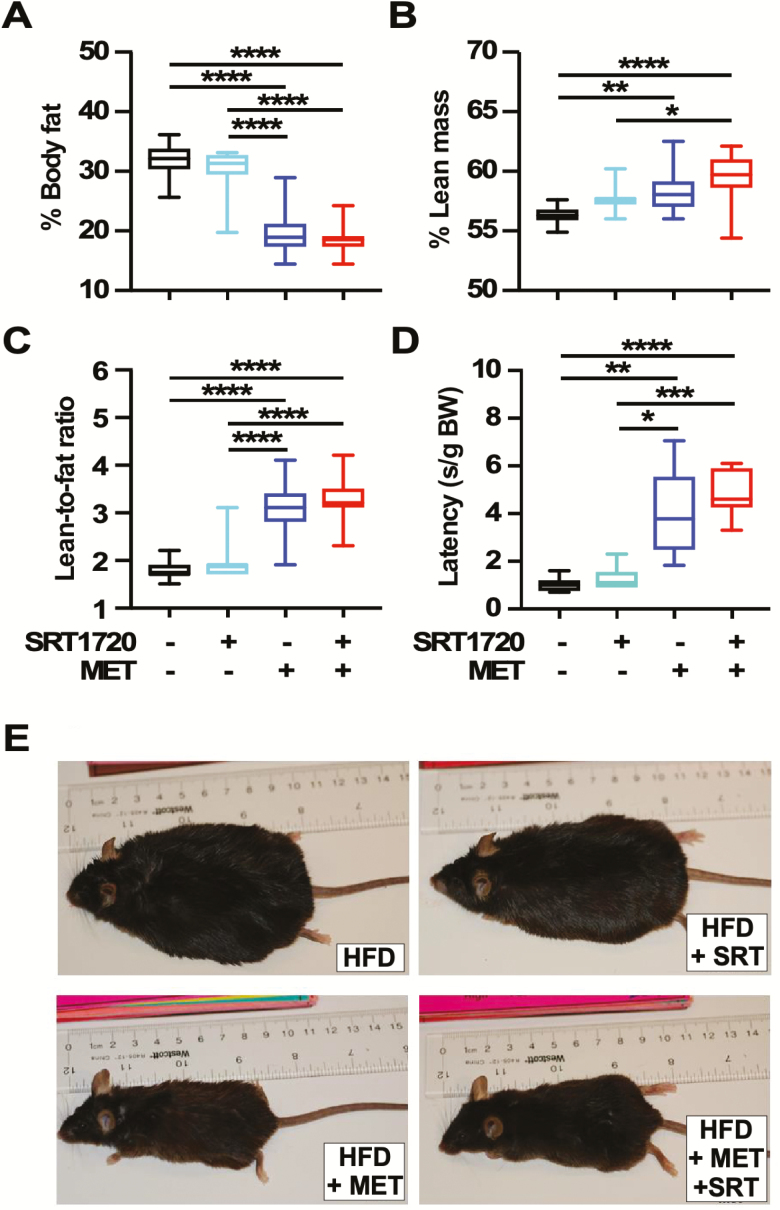
Several functional measurements were performed to characterize health status. Testing was done only at 64 and 68 weeks of age to limit stress imposed on them. Amount of whole-body fat and lean mass were obtained from NMR spectroscopy in live animals after 8 weeks on diet (64 weeks old). SRT1720 mice had a similar percent body fat and lean mass as controls while mice on MET alone or combined with SRT1720 showed a significant reduction in percent body fat (Figure 2A), coincident with an increase in percent lean mass (Figure 2B) and lean-to-fat ratio (Figure 2C). Motor coordination was measured using the accelerating rotarod at 68 weeks of age, where results indicated that SRT1720 had no beneficial effects while MET alone and the combination SRT1720 + MET significantly improved performance versus HFD controls (Figure 2D). Control and SRT1720-treated mice on HFD became obese in comparison to those maintained on MET alone or combination, with the latter groups of mice remaining lean after 26 weeks on diet (82 weeks old) (Figures 1B and 2E).
Figure 2.
Impact of SRT1720 and metformin (MET) supplementation on whole-body physiology and performance in high-fat diet (HFD)-fed mice. (A, B) Estimates of percent body fat and lean mass content as determined by nuclear magnetic resonance (NMR). (C) Lean-to-fat ratio calculated from (A) and (B). Measures for (A), (B), and (C) were done at the same time on n =15 mice per group, 8 weeks on diet (64 weeks old). (D) Time to fall from an accelerating rotarod in seconds standardized to body weights. n = 9 mice per group, 12 weeks on diet (68 weeks old). (E) Representative images of mice after 26 weeks on diet (82 weeks old). Data are represented as box and whisker plots (A–D) and analyzed using two-way analysis of variance with Tukey’s post hoc analysis. *p < .05; **p < .01; ***p < .001; ****p < 0.0001.
Discussion
Over the past decades, different pharmacologic agents have shown to exert prolongevity benefits through a variety of related but distinct mechanisms. As such, combination of different life-span-extending compounds is regarded as a promising way to achieve effective anti-aging. However, we found that mice fed an obesogenic diet supplemented with MET + SRT1720 died prematurely with a severe drop in body weight without loss of appetite. It is not clear whether these dramatic differences in body weight are due to changes in heat production, ambulatory activity, gut absorption, or a number of other factors that could be involved in promoting these effects. No signs of diarrhea, enhanced anxiety, or activity were observed in these animals during this experiment. Although detrimental to survival, mice on MET + SRT1720 were leaner and performed significantly better on the accelerating rotarod than their age-matched controls or SRT1720 mice, suggesting that the drug combination, at the administered doses, were effective at conferring a strong biological response.
This work was an extension of one of our previous studies testing SRT1720 in HFD-fed aged mice (8). MET has proven to be beneficial for life-span and health extension in several animal models (19) and in humans (20), although adverse effects on life span were reported in male C57BL/6J mice fed SD with 1% MET (11). In the present study, 1% MET in HFD did not alter median life span and only showed a modest reduction in maximum life span. Therefore, it is likely that dietary composition (eg, high fat) itself plays a role in the outcomes observed. Our data clearly show that combining 1% MET with SRT1720 was detrimental to the survival of HFD-fed mice. Whether the increased mortality occurred due to exacerbation of kidney pathology was not examined in depth. Visual inspection of the kidneys at necropsy revealed high number of glomerulonephrosis cases in the combination group that suggests widespread kidney pathogeneses in these mice. A more in-depth quantitation must be conducted in future studies. The importance of age of onset in the long-term benefits of MET has been previously reported (14). Starting treatment at 3 months of age increased mean life span by 14% and maximum life span by 1 month, whereas treatment initiated at 9 and 15 months of age was ineffective. Whether combining MET with SRT1720 would result in a different outcome in younger mice is unknown. Given that MET is commonly prescribed to adults that are metabolically challenged, we explored the effects of the SRT1720 + MET combination within an obesogenic context.
Gradual changes in body weight and food consumption over the life course pose a challenge to achieving accurate dosing in life-span studies, which is further complicated when the treatment itself impacts body weight. Using MET doses that are more “translational” and reflective of changing body weights and food consumption patterns should therefore be a focus of future mouse aging intervention studies using MET. Measuring circulating levels of MET (11) at select points of the study could be helpful for dose modification. Conducting dose–response experiments with various MET + SRT1720 combinations would allow to better understand what doses synergize or antagonize. In addition to these considerations, emerging research also shows that MET plays an important role in gut microbiome composition (21,22) and epigenetic changes (23). Whether such changes could contribute to the functional and phenotypic alterations seen here is unknown.
There are some limitations in the current study. First, the study was carried out in C57BL/6J male mice only and did not account for mouse genetic background and sex, two variables that contribute to variance in longevity. This is especially important considering that sex differences may significantly contribute to the manifestation of adverse effects of MET in humans (24) and prior demonstrations of sex- and strain-specific prolongevity effects of MET (25). Second, lower doses of MET should be tested because 0.1% MET extends life span in some studies and is devoid of adverse side effects (11). Third, the rapid death rate of the MET + SRT1720 mice made it unfeasible to conduct repeated testing of their physical performance throughout life span but must be included in follow-up experiments. Yet, significant phenotypic differences were observed between the groups even as early as 8 weeks on diets. Fourth, as the primary objective of this study was to assess the outcome on life span, mechanisms leading to the observed phenotypes were not investigated and must be a focus of future studies. Collectively, studying the effects of combinations of compounds having geroprotective properties is not straightforward and requires that randomized preclinical and clinical studies be performed in order to evaluate the occurrence of unforeseen adverse events. This work highlights the necessity of these studies as it demonstrates that drug combinations that may be expected to work in a synergistic manner can in fact lead to serious adverse outcomes and must be carefully evaluated.
Supplementary Material
Acknowledgments
The authors would like to thank Dawn Nines, Dawn Phillips, and Justine Lucas for their excellent animal care. This research was conducted under a Cooperative Research and Development Agreement (CRADA) between Glaxo Smith-Kline and the National Institute on Aging, National Institutes of Health (NIA/NIH). The authors would also like to thank Dr. Christopher Morrell (Loyola University, MD) and Dr. Eric Shiroma (Laboratory of Epidemiology and Population Sciences, NIA) for their input on statistical analyses.
Funding
Funding was provided by the Intramural Research Program of the NIA/NIH. S.J.M. was supported by a National Medical Health and Research Council of Australia CJ Martin Early Career Fellowship (RGMS ID 2010-01671).
Author Contributions
D.L.P.: Data curation and analysis, writing original draft, review, and editing; R.K.M. and S.J.M.: Design, investigation, and methodology; H.H.P., J.J.L., T.M.W., and G.A.: Investigation and methodology; P.E., C.W., J.L.E., and D.A.S.: Data interpretation; N.L.P.: Writing—review and editing; M.B.: Data curation and analysis, writing— review and editing, and figure creation; R.d.C.: Conceptualization, design, resources, supervision, methodology, and writing—review and editing.
Conflicts of Interest
P.E., C.W., and J.L.E. were employed by Sirtris, a GSK company that had a commercial interest in developing SIRT1 activators. D.A.S. consulted for Sirtris. The remaining authors report no conflicts of interest.
References
- 1. Dai H, Sinclair DA, Ellis JL, Steegborn C. Sirtuin activators and inhibitors: promises, achievements, and challenges. Pharmacol Ther. 2018;188:140–154. doi: 10.1016/j.pharmthera.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35:146–154. doi: 10.1016/j.tips.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Price NL, Gomes AP, Ling AJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mercken EM, Mitchell SJ, Martin-Montalvo A, et al. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell. 2014;13:787–796. doi: 10.1111/acel.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell SJ, Martin-Montalvo A, Mercken EM, et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014;6:836–843. doi: 10.1016/j.celrep.2014.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minor RK, Baur JA, Gomes AP, et al. SRT1720 improves survival and healthspan of obese mice. Sci Rep. 2011;1:70. doi: 10.1038/srep00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baksi A, Kraydashenko O, Zalevkaya A, et al. A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes. Br J Clin Pharmacol. 2014;78:69–77. doi: 10.1111/bcp.12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nieuwenhuis-Ruifrok AE, Kuchenbecker WK, Hoek A, Middleton P, Norman RJ. Insulin sensitizing drugs for weight loss in women of reproductive age who are overweight or obese: systematic review and meta-analysis. Hum Reprod Update. 2009;15:57–68. doi: 10.1093/humupd/dmn043 [DOI] [PubMed] [Google Scholar]
- 11. Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alfaras I, Mitchell SJ, Mora H, et al. Health benefits of late-onset metformin treatment every other week in mice. NPJ Aging Mech Dis. 2017;3:16. doi: 10.1038/s41514-017-0018-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strong R, Miller RA, Antebi A, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15:872–884. doi: 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anisimov VN, Berstein LM, Popovich IG, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY). 2011;3:148–157. doi: 10.18632/aging.100273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spindler SR. Use of microarray biomarkers to identify longevity therapeutics. Aging Cell. 2006;5:39–50. doi: 10.1111/j.1474-9726.2006.00194.x [DOI] [PubMed] [Google Scholar]
- 16. Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 2015;3:e00149. doi: 10.1002/prp2.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blagosklonny MV. From rapalogs to anti-aging formula. Oncotarget. 2017;8:35492–35507. doi: 10.18632/oncotarget.18033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ackert-Bicknell CL, Anderson LC, Sheehan S, et al. Aging research using mouse models. Curr Protoc Mouse Biol. 2015;5:95–133. doi: 10.1002/9780470942390.mo140195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song J, Jiang G, Zhang J, et al. Metformin prolongs lifespan through remodeling the energy distribution strategy in silkworm, Bombyx mori. Aging (Albany NY). 2019;11:240–248. doi: 10.18632/aging.101746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31–44. doi: 10.1016/j.arr.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 21. Hine C, Zhu Y, Hollenberg AN, Mitchell JR. Dietary and endocrine regulation of endogenous hydrogen sulfide production: implications for longevity. Antioxid Redox Signal. 2018;28:1483–1502. doi: 10.1089/ars.2017.7434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bauer PV, Duca FA, Waise TMZ, et al. Metformin alters upper small intestinal microbiota that impact a glucose-SGLT1-sensing glucoregulatory pathway. Cell Metab. 2018;27:101–117.e5. doi: 10.1016/j.cmet.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 23. Yu X, Mao W, Zhai Y, et al. Anti-tumor activity of metformin: from metabolic and epigenetic perspectives. Oncotarget. 2017;8:5619–5628. doi: 10.18632/oncotarget.13639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Vries ST, Denig P, Ekhart C, Mol PGM, van Puijenbroek EP. Sex differences in adverse drug reactions of metformin: a longitudinal survey study. Drug Saf. 2020;43:489–495. doi: 10.1007/s40264-020-00913-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weiss R, Fernandez E, Liu Y, Strong R, Salmon AB. Metformin reduces glucose intolerance caused by rapamycin treatment in genetically heterogeneous female mice. Aging (Albany NY). 2018;10:386–401. doi: 10.18632/aging.101401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.