Abstract
Mitochondrial-derived peptides (MDPs) are small bioactive peptides encoded by short open-reading frames (sORF) in mitochondrial DNA that do not necessarily have traditional hallmarks of protein-coding genes. To date, eight MDPs have been identified, all of which have been shown to have various cyto- or metaboloprotective properties. The 12S ribosomal RNA (MT-RNR1) gene harbors the sequence for MOTS-c, whereas the other seven MDPs [humanin and small humanin-like peptides (SHLP) 1–6] are encoded by the 16S ribosomal RNA gene. Here, we review the evidence that endogenous MDPs are sensitive to changes in metabolism, showing that metabolic conditions like obesity, diabetes, and aging are associated with lower circulating MDPs, whereas in humans muscle MDP expression is upregulated in response to stress that perturbs the mitochondria like exercise, some mtDNA mutation-associated diseases, and healthy aging, which potentially suggests a tissue-specific response aimed at restoring cellular or mitochondrial homeostasis. Consistent with this, treatment of rodents with humanin, MOTS-c, and SHLP2 can enhance insulin sensitivity and offer protection against a range of age-associated metabolic disorders. Furthermore, assessing how mtDNA variants alter the functions of MDPs is beginning to provide evidence that MDPs are metabolic signal transducers in humans. Taken together, MDPs appear to form an important aspect of a retrograde signaling network that communicates mitochondrial status with the wider cell and to distal tissues to modulate adaptative responses to metabolic stress. It remains to be fully determined whether the metaboloprotective properties of MDPs can be harnessed into therapies for metabolic disease.
Keywords: aging, humanin, mitochondria, mitochondrial derived peptides, mitokine, MOTS-c, SHLP
INTRODUCTION
Traditionally, the identification of protein-coding open-reading frames (ORFs) within genomes has focused on sequences that are initiated with an AUG start codon, have conserved homologous amino acid sequences, and are >100 codons in length (2). However, there is growing evidence that short ORFs that lack these classical hallmarks of protein-coding genes can encode biologically active small peptides or micropeptides (<100 amino acids) and can be found in transcripts annotated for larger proteins or long noncoding RNAs (lncRNAs) (5). Although efforts are increasing to systematically identify functionally relevant nuclear short ORFs, several small regulatory peptides encoded by mitochondrial genome (mtDNA) short ORFs (collectively termed mitochondrial derived peptides) have been shown to have broad cellular cyto- and metaboloprotective properties (6, 13, 31).
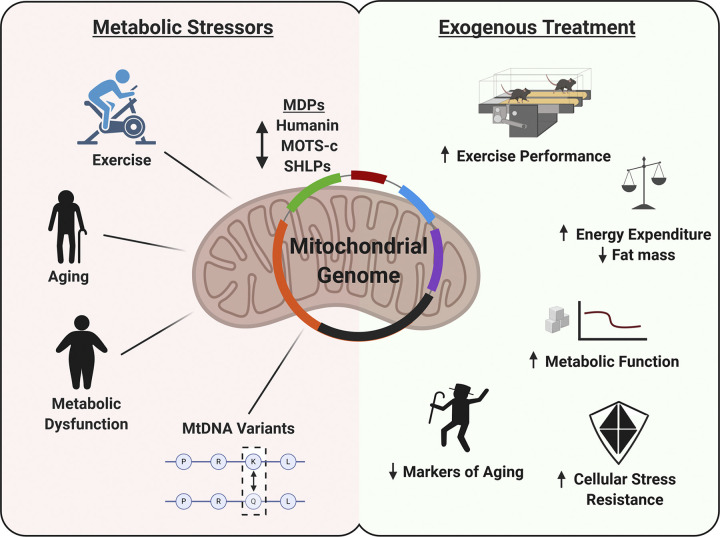
The mitochondria’s involvement in maintaining cellular homeostasis extends beyond that of energy production to include a regulatory role in a range of processes, including immune/inflammatory responses, proteostasis, adaptive stress responses, and apoptosis (43). To achieve this, the mitochondria have developed extensive retrograde signaling networks to communicate with the nuclear genome, other intracellular organelles, and potentially neighboring cells or organs (43), of which mitochondrial-derived peptides (MDPs) appear to form a critical aspect. The first described MDP, humanin, is encoded within the 16S ribosomal RNA gene (MT-RNR2) (15, 30), and more recently, the MT-RNR2 gene has been shown to harbor sequences for several additional small-humanin-like peptides (SHLP1–6) (6). Despite the mtDNA containing tens to hundreds of potential peptide-encoding short ORFs, the only other MDP to have been described is the mitochondrial open-reading frame of the 12S rRNA-c (MOTS-c), a 16-amino acid peptide transcribed from the 12S ribosomal RNA (MT-RNR1) gene that appears to have potent metabolic modulation properties (31). In this review, we argue that MDPs are metabolically active peptides by summarizing the evidence that they are endogenously responsive to metabolic stress and can promote an adaptive response to metabolic stressors, focusing on metabolic disease, aging, and exercise (Fig. 1).
Fig. 1.
Summary of metabolic stressors that modulate mitochondrial-derived peptide (MDP) expression and in vivo metabolic effects of MDP treatment in rodents.
MITOCHONDRIAL-DERIVED PEPTIDES IN METABOLIC DISEASE
Mitochondrial dysfunction is a key player in the pathophysiology of metabolic diseases, including obesity, insulin resistance, type 2 diabetes, and nonalcoholic fatty liver disease (NAFLD) (3). As such, it is not surprising that there is a growing number of studies that have assessed the effect of administering exogenous MDPs (or their analogs) on glucose and lipid metabolism of rodents under metabolically challenging conditions (Table 1). Of the known native MDPs, MOTS-c has most consistently been reported to have metaboloprotective properties in multiple models of metabolic dysfunction (Table 1). Initial reports by Lee et al. (31) showed that the stable overexpression of MOTS-c in cultured cells promotes glucose clearance and lactate accumulation in an AMP-activated protein kinase (AMPK) and sirtuin (SIRT) 1-dependent manner. Both of these proteins are nutrient sensors that have the ability to control cellular substrate utilization and energy metabolism in response to metabolic perturbations, and consistent with this, daily administration of MOTS-c increased glucose tolerance and insulin sensitivity of aged and diet-induced obese mice (31). Whether the enhanced skeletal muscle insulin sensitivity in mice was the direct result of MOTS-c-induced muscle AMPK activation and/or increased energy expenditure, in part, by increased lipid oxidation is not clear (31); two studies suggest that MOTS-c may increase the thermogenic capacity of white and brown fat in an AMPK-dependent manner, leading to weight loss (35, 36).
Table 1.
Metabolic outcomes of exogenous MDP treatment in vivo
| Reference(s) | Model | MDP | Dose | Response |
|---|---|---|---|---|
| Aging | ||||
| Kim et al. (23) | Aged mice | HNG | 5 mg·kg−1·day−1 | ↑Hippocampus Akt and ERK phosphorylation |
| Qin et al. (47) | Aged mice | HNG | 4 mg·kg−1·2× wk−1 | ↓Reduced myocardial fibrosis |
| Yen et al. (56) | Aged mice | HNG | 4 mg·kg−1·2× wk−1 | ↑Metabolic healthspan, ↔lifespan |
| Lee et al. (31) | Aged mice | MOTS-c | 5 mg·kg−1·day−1 | ↑Insulin sensitivity |
| Reynolds et al. (50) | Aged mice | MOTS-c | 15 mg·kg·3× wk | ↑Lifespan, ↓aging markers |
| Exercise | ||||
| Reynolds et al. (50) | Treadmill run (mice) | MOTS-c | 5–15 mg·kg−1·day−1 | ↑Performance |
| Cardiovascular | ||||
| Oh et al. (45), Zhang et al. (59) | apoE-deficient mice | HNGF6A | 0.4 mg·kg−1·day−1 | ↑Aortic function, ↓atherogenesis |
| Wei et al. (52) | Rat; vitamin D3 + nicotine | MOTS-c | 5 mg·kg−1·day−1 | ↓Vascular calcification |
| Metabolic | ||||
| Han et al. (14) | APP/PS1 mice | HNG | 50–100 μg·kg−1·day−1 | ↓IRS-1, ↑Akt phosphorylation in brain |
| Lu et al. (35) | Cold exposure (mice) | MOTS-c | 5 mg·kg−1·day−1 | ↑Cold adaptation (browning WAT and BAT response) |
| Li et al. (32) | d-Galactose-treated mice | MOTS-c | 10 mg·kg−1·day−1 | ↓Hepatic lipid accumulation |
| Gong et al. (12) | DIO mice | HNG | 2 mg·kg−1·day−1 | ↓Fat mass and tissue lipid, ↑glucose homeostasis |
| Mehta et al. (38), Kim et al. (24) | DIO mice | HNG, SHLP2, MOTS-c | 2.5 mg·kg−1·day−1 | ↓Metabolic disease metabolite signatures in blood |
| Lee et al. (31) | DIO mice | MOTS-c | 0.5–5 mg·kg−1·day−1 | ↑Insulin sensitivity, ↓fat mass and tissue lipid |
| Kuliawat et al. (27) | Rat | HNGF6A | 0.07 mg·kg−1·h−1 | ↑GSIS |
| Cobb et al. (6) | Rats and mice | SHLP2, SHLP3 | icv 0.16 μg·kg−1·min−1, 2 mg·kg−1·BID−1 | SHLP2 ↑insulin sensitivity, SHLP3 ↑IL-6 and MCP-1 |
| Muzumdar et al. (44) | Rat: icv and iv | HNGF6A | 20 μg icv, 0.05 mg·kg−1·h iv−1 | ↑Insulin sensitivity |
| Muzumdar et al. (44) | Diabetic rat | HNGF6A | 100 ug | ↓Blood glucose |
| Lu et al. (36) | Ovariectomy mice | MOTS-c | 5 mg·kg−1·day−1 | ↓Fat mass, ↓lipid, ↑glucose homeostasis |
APP/PS1, amyloid precursor protein/presenilin-1; BAT, brown adipose tissue; DIO, diet induced obesity; GSIS, glucose stimulated insulin secretion; HNG/HNGF6A, humanin analogs; icv, intracerebroventricular; iv, intravenous; MDP, mitochondrial-derived peptide WAT, white adipose tissue. ↑Increase; ↓decrease.
Systemic MOTS-c treatment has beneficial effects in multiple rodent models of metabolic stress, including attenuating ovariectomy-induced fat accumulation, insulin resistance (36), and bone loss (42), reducing d-galactose-induced peripheral lipid accumulation and mitochondrial dysfunction (32), and downregulating circulating metabolite profiles that are associated with type 2 diabetes and obesity (24). Consistent with these metaboloprotective effects of MOTS-c in rodents, circulating levels in humans have been reported to be reduced with obesity (9), insulin resistance (4), type 2 diabetes (48), chronic kidney disease (33), and endothelial function (Table 2) (47). Therefore, it will be interesting to determine whether restoring circulating MOTS-c levels in patients with metabolic dysfunction can improve clinical outcomes. Indeed, clinical trials on MOTS-c and a MOTS-c analog are underway, with indications for coronary artery disease in patients with type 2 diabetes (NCT04027712) and nonalcoholic hepatic steatosis and obesity (NCT03998514).
Table 2.
Endogenous MDP response to metabolic stressors in vivo
| Reference(s) | Metabolic Stress | MDP | Tissue | Effect |
|---|---|---|---|---|
| Aging | ||||
| Muzumdar et al. (44), Bachar et al. (1), D’Souza et al. (8) | Aging (human) | Humanin, MOTS-c | Blood | ↓ |
| Conte et al. (7) | Aging (human) | Humanin | Blood | ↑ |
| D’Souza et al. (8) | Aging (human) | MOTS-c | Muscle | ↑ |
| Muzumdar et al. (44), Cobb et al. (6), Lee et al. (31) | Aging (rodent) | Humanin, MOTS-c, SHLP2 | Blood, muscle, hypothalamus | ↓ |
| Exercise | ||||
| Woodhead et al. (54) | AEx (human) | Humanin | Muscle, blood | ↑,↑ |
| Woodhead et al. (54) | TRx (human) | Muscle, blood | ↔, ↓ | |
| Woodhead et al. (54) | AEx, TRx (human) | SHLP2 | Blood | ↔ |
| Woodhead et al. (54) | AEx, TRx (human) | SHLP6 | Blood | ↑, ↓ |
| Reynolds et al. (50) | AEx (human) | MOTS-c | Muscle, blood | ↑,↑ |
| Gidlund et al. (11) | TRx (human) | Humanin | Muscle, blood | ↑↔,↔ |
| Ramanjenaya et al. (49) | TRx (human) | MOTS-c | Blood | ↔ |
| Cardiovascular | ||||
| Widmer et al. (53), Zhloba et al. (60), Qin et al. (47) | Heart disease (human) | Humanin | Blood | ↓ |
| Mangkhang et al. (37) | Mitral valve disease (canine) | Humanin | Blood | ↓ |
| Metabolic disorders | ||||
| Ramanjaneya et al. (48) | Type 2 diabetes (human) | Humanin, MOTS-c | Blood | ↓ |
| Cataldo et al. (4) | IR (human) | MOTS-c | Blood | ↑ |
| Cataldo et al. (4) | Obesity (human) | MOTS-c | Blood | ↔ |
| Du et al. (9) | Obesity (human) | MOTS-c | Blood | ↓ |
| Liu et al. (33) | Kidney disease (human) | MOTS-c, Humanin | Muscle, blood Muscle, blood Muscle |
↓ ↓,↑ ↑# |
| Other | ||||
| Lu et al. (35) | Cold exposure (rodent) | MOTS-c | Blood | ↓ |
| Ramanjaneya et al. (49) | Intralipid infusion (human) | MOTS-c | Blood | ↑ |
| Kariya et al. (19), Kin et al. (25) | mtDNA-related diseases (human) | Humanin | Muscle | ↑ |
AEx, acute exercise bout; blood, serum or plasma; IR, insulin resistance; MDP, mitochondrial-derived peptide; TRx, exercise training. ↑, increase; ↓, decrease; ↔, no change;
Relative to mtDNA.
Humanin was first identified in a cDNA library screen derived from a surviving brain fraction of an individual with Alzheimer’s disease. Consistently, humanin has best been known for its neuroprotective effects, in part, by regulating pro-apoptotic pathways including Bax-related proteins (13) and insulin-like growth factor binding protein-3 (IGFBP-3) (17). Circulating levels of humanin have been shown to be reduced in several metabolic disorders (Table 2), including cardiovascular disease (53, 60) and diabetes (48). Interestingly, however, muscles of patients with the mitochondrial mutations that lead to MELAS (mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes) and CPEO (chronic progressive external ophthalmoplegia) have elevated humanin expression (19, 25), which could be a tissue-specific stress response aimed at restoring/repairing mitochondrial homeostasis, as humanin can enhance mitochondrial metabolism under metabolic stress (20, 51), and the expression of humanin in the muscle can increase in response to acute exercise stress (54).
Several humanin analogs have been manufactured with improved potency and stability, including HNG, which has a glycine-to-serine substitution at position 14, F6AHN with an alanine-to-phenylalanine substitution at position 6 (abrogates IGFBP-3 binding), and HNGF6A, which contains both substitutions. The infusion of humanin, its analogs, or SHLP2 centrally (intracerebroventricularly) enhances insulin sensitivity in rodents, as determined by euglycemic-hyperinsulinemic clamp studies (44). Similar effects were observed when F6AHN and HNGF6A were infused peripherally (44), whereas intraperitoneal injections attenuate high-fat diet-induced increases in fat mass and lipid accumulation, particularly in the liver (12). Humanin may partially act via the activation of hypothalamic STAT3 to promote the suppression of hepatic glucose production. However, this appears to occur in concert with improved peripheral tissue glucose uptake (44). Indeed, under cellular stress, humanin can activate the insulin-stimulated glucose transport pathway, including insulin receptor substrate 1 and Akt (14, 55).
The observation that a single dose of humanin can lower blood glucose in Zucker diabetic fatty (ZDF) rats by maintaining high insulin levels has led to the hypothesis that humanin may also be insulinotropic (44). Indeed, HNGF6A infusion can increase insulin levels during hyperglycaemic clamps in mice and promote glucose-stimulated insulin secretion of murine β-cells by enhancing the sensitivity of the β-cells to glucose (27). Furthermore, humanin treatment can delay the onset of type 1 diabetes in nonobese diabetic (NOD) mouse (16). Taken together, this suggests that the MDPs MOTS-c, humanin, and SHLP2 are metabolically active peptides that respond to metabolic stress (Table 2) and have the potential to modulate insulin sensitivity, secretion, and energy utilization pathways. The precise molecular networks that underlie these functions are currently active topics of investigation.
MITOCHONDRIAL-DERIVED PEPTIDES IN AGING
Aging is associated with a progressive loss of cellular homeostasis and resilience, increasing susceptibility to multiple chronic diseases, and is at least partially dependent on metabolism at multiple levels, as demonstrated by dietary (e.g., dietary restriction), genetic (e.g., insulin/insulin-like signaling), and pharmacological (e.g., rapamycin, metformin) interventions that extend a healthy lifespan (34). Mitochondrial-nuclear communication is considered key to cellular fitness and organismal healthspan (43), and although traditionally thought to be primarily mediated by nuclear-encoded proteins, transient molecules, and mitochondrial metabolites, mitochondrial-encoded factors are now also emerging as key players. Notably, under stress conditions, MOTS-c can translocate to the nucleus and regulate adaptive gene expression through interactions with stress-responsive transcription factors and chromatin binding (22). When delivered via intraperitoneal injection in rodents, MOTS-c and other MDPs can exert tissue specific effects, suggesting that they can also cross the extracellular space (Ref. 31 and Table 1).
The levels of MDPs have been reported to be age related (Table 2). In cross-sectional studies, plasma humanin levels were lower in aged mice (2 mo vs. 13 mo) and humans [45–65 vs. 65–80 vs. 81–110 yr (44); 39 vs. 60 yr (1)]. Furthermore, humanin and SHLP2 systemic and tissue levels were lower in older rodents compared with young (6, 44). In contrast, however, within a large cohort of 693 individuals of varying health status and age (21–113 yr), Conte et al. (7) reported a strong positive association between age and plasma humanin levels. These differences may be attributed to the larger sample size, greater age range, and differing individual characteristics; however, the latter cannot be assessed due to limited participant data being reported in the earlier studies (1, 44). Because humanin can extend metabolic healthspan (56), an increase in plasma humanin was interpreted as a hormetic response aimed at improving the ability of cells/tissues to cope with stress (7) and postulated to have beneficial or detrimental effects, depending on the levels of response (39). This is compatible with the finding that patients with chronic kidney disease have higher circulating humanin levels and lower skeletal muscle levels compared with healthy controls (33). As for MOTS-c, in cross-sectional studies, circulating levels were found to be reduced in aged mice (4 mo vs. 32 mo) (31) and in humans [18–30 vs. 45–55 vs. 70–81 yr (8); significant negative correlation (48)]. However, whereas MOTS-c levels in older skeletal muscle from mice are lower (4 mo vs. 32 mo) (31), levels are increased in aged men (18–30 vs. 45–55 vs. 70–81 yr) (8). This discrepancy may be because 32-mo-old mice from the National Institute on Aging (NIA) aged rodent colony represent an end-of-life morbid condition, whereas the human tissue donors were still active and healthy.
Humanin is heavily involved in the growth hormone (GH)/IGF-1 axis, one of the most prominent endocrine regulators of aging. GH-deficient Ames mice are long-lived and show higher circulating humanin levels, whereas short-lived GH-transgenic mice had lower humanin levels compared with their wild-type mice (29). Furthermore, intermittent MOTS-c treatment initiated later in life reversed age-dependent loss of physical capacity and improved aging metabolism in mice (50). Because aging is linked with a progressive decline in mitochondrial function and disruption of metabolic homeostasis (28), MDPs may play a key role in slowing down the rate of aging and delaying the onset of age-related diseases. Therefore, we suggest that an age-dependent decline in MDP expression and/or function may dampen mitochondrial communication and consequently reduce the cellular capacity to dynamically adapt to insults and the ever-shifting conditions.
MITOCHONDRIAL-DERIVED PEPTIDES IN EXERCISE
Regular exercise is a therapeutic and preventative measure for most metabolic diseases and increases the activity of the mitochondria to provide energy for the sustained contractile activity of muscle. Mitochondrial-derived peptides, particularly MOTS-c, activate similar signaling pathways to exercise and when administered exogenously promote exercise-like adaptations (22, 31), leading to speculation that MOTS-c may be an exercise mitokine. Investigations into exercise and MDPs have been relatively scarce. In a pre- versus posttraining study design, Gidlund et al. (11) reported that 12 wk of resistance training in middle-aged prediabetic men resulted in an increase in intramuscular humanin expression; however, similar responses were not seen following Nordic walking (11), 8 wk of aerobic training (plasma measures only) (49), or 2 wk of high-intensity interval training (54) in participants that ranged from young, healthy males (54) to middle-aged individuals with indications of metabolic disease (11, 49). Although it is difficult to discern the reasons for the inconsistent effect of exercise training on humanin levels, it is possible that muscle humanin is more responsive to resistance training (11).
More recently, MOTS-c and humanin have been observed to increase in muscle (11.9-fold) and plasma (1.5-fold) following acute high-intensity cycling exercise in healthy young men (50, 54), whereas plasma SHLP6 but not SHLP2 concentration also responds to exercise. In support of the hypothesis that skeletal muscle is a source of circulating MDPs during exercise, contraction of isolated mouse muscle rapidly (within 10 min) increases intramuscular humanin and MOTS-c expression (Ref. 54, and Woodhead JST and Merry TL, unpublished observations). Although it remains to be determined what intracellular signals regulate MDPs during exercise, the rapid increase in levels following the onset of exercise/contraction suggests a suppression of MDP degradation rather than an upregulation of transcription (54). Reactive oxygen species (ROS) increase during exercise and regulate adaptive responses to exercise training (39), and oxidative stress in cell culture promotes MOTS-c translocation to the nucleus and expression (22). The thiols in both humanin and MOTS-c sequences provide a potential mechanism through which oxidative stress may directly alter the stability of the peptides. However, the effects of oxidative and other metabolic stresses (metformin and serum/glucose restriction) on MOTS-c levels appear to be AMPK dependent (22). Because AMPK is a multifunctional and exercise-sensitive cellular energy sensor, it is possible that MDPs may be responsive to multiple signals associated with the change in energy status that occurs with exercise/contraction. Although the role and molecular targets of endogenously produced MDPs during exercise are yet to be identified, higher doses [15 mg·kg−1·day−1 as opposed to 5 mg·kg−1·day−1 used in obesity interventions (31)] of MOTS-c for as little as 2 wk can increase running capacity of both young and old mice (50). Mechanistically, MOTS-c treatment upregulated glycolytic and protein metabolism markers following exercise and led to an enrichment of genes associated with protein regulation/metabolism, cellular metabolism, and oxidative stress response, which are largely under the control of heat shock proteins (50). Therefore, it is tempting to speculate that MOTS-c may regulate adaptive responses to exercise-related stress conditions by forming an integral part of the mitochondrial retrograde signaling network activated during exercise (40). In support for MOTS-c potentially acting to facilitate nuclear genomic interactions that lead to enhanced metabolic flexibility (defined by the overall adaptive capacity to a shift in metabolic supply-demand equilibrium in response to a perturbation) (22, 50) as part of the exercise training response, a loss-of-function MOTS-c polymorphism (K14Q, MT-1382A>C) has been linked to increased type 2 diabetes susceptibility in those with low physical activity levels (58).
GENETIC VARIATIONS IN MITOCHONDRIAL DNA AND THEIR FUNCTIONAL IMPLICATION FOR MDPs
The human mitochondrial genome encodes 37 genes that are involved in oxidative phosphorylation, ribosomes, and translation within the mitochondria. As such, alteration of mtDNA copy number and mtDNA polymorphisms can affect mitochondrial function and cell metabolism to modify metabolic disease risk (58). Because mtDNA sequences are more varied by ethnicity compared with nuclear DNA sequences (41), studying mtDNA polymorphisms provides a unique insight into ethnicity-specific disease risk and functional significance of mtDNA regions. Indeed, mitochondrial genomic association studies are beginning to reveal the influence of mtDNA on metabolic disease, with mitochondrial variants MT-16320 being associated with blood glucose levels, MT-8706 and MT-8898 with waist/hip ratio (26), and MT-8414 and MT-16189 increasing the risk of type 2 diabetes in an ethnicity-specific manner (18, 46); however, the functional effects of the mtDNA polymorphisms remain relatively underexplored.
Sequence variation in mtDNA may result in differences in the function of classically recognized mtDNA-encoded proteins leading to alterations in mitochondrial respiration, reactive oxygen species (ROS) production, mitochondrial matrix pH, and intracellular calcium levels (18, 21). Equally, mtDNA polymorphisms could also impact the function of MDPs. Consistent with this, the MT-2706 variant in the humanin coding short ORF is associated with a decrease in circulating humanin levels and is associated with accelerated cognitive aging (57). More recently it has been recognized that 5–10% of people with East Asian ancestry have a variant (MT-1382) in the MOTS-c coding region that causes a K14Q amino acid replacement in the MOTS-c peptide (10). A meta-analysis of three independent Japanese cohorts (n = 27,527) demonstrated that the C allele of MT-1382 variant is associated with an increased risk of type 2 diabetes in males (when adjusted by for age and BMI), but not in females, and that this effect was dependent on physical activity levels (58). Importantly, treating high-fat-fed mice with K14Q MOTS-c does not confer the metabolic benefits associated with native MOTS-c administration (31, 58), suggesting that the MT-1382 variant results in inactive endogenous MOTS-c, which contributes to increased metabolic disease risk. Therefore, MDP treatment could potentially be a means of preventing metabolic dysfunction associated with this mtDNA variant that inactivates endogenous MDPs.
CONCLUSIONS AND FUTURE PERSPECTIVES
There is growing evidence that many MDPs are responsive to changes in metabolism in a tissue- and stress-specific manner and that exogenous humanin and MOTS-c protect against metabolic dysfunction associated with aging and energy imbalance through improving insulin sensitivity and activating energy-consuming pathways. However, the underlying mechanisms driving these responses are just beginning to be investigated, and there are still many open questions, including how MDP transcription is regulated and how transcripts from the mitochondria are exported and translated. Whereas CNTFR/GP130/WSX-1 and FPRL-1 are putative receptors for humanin (30), establishing whether other MDPs also have extracellular receptors or primarily exert their effects on energy metabolism by intracellular interactions (22) will increase our understanding of the biological significance of these peptides. The difficulty of editing the mitochondrial genome has slowed progress in this field; however, naturally occurring genetic variants within the mitochondrial genome (mitochondrial haplotypes) will likely be able to provide causative mechanistic insight into currently identified MDPs and perhaps guide which of the many additional short open-reading frames of mtDNA also encode biologically active MDPs. In addition, antagonists such as blocking antibodies to be developed against MDPs will further aid to define their endogenous role in health and disease. Understanding these aspects of MDP regulation will help elucidate how these new players in the mitochondrial retrograde signaling network modulate adaptative responses to metabolic stress and whether they can be harnessed to treat metabolic disease.
GRANTS
This work was funded by a Marsden Fast-start grant (T.L.M.). T.L.M. was supported by a Rutherford Discovery Fellowship. C.L. was supported by the NIA (R01AG052258), Ellison Medical Foundation (EMF), AFAR, and the Hanson-Thorell Family.
DISCLOSURES
C.L. is a consultant and shareholder of Cohbar, Inc. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
T.L.M. and J.S.T.W. prepared figures; T.L.M., A.C., J.S.T.W., J.C.R., H.K., S.K., and C.L. drafted manuscript; T.L.M., A.C., J.S.T.W., J.C.R., H.K., S.K., and C.L. edited and revised manuscript; T.L.M., A.C., J.S.T.W., J.C.R., H.K., S.K., and C.L. approved final version of manuscript.
REFERENCES
- 1.Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, Lerman LO, Pagano RE, Cohen P, Lerman A. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res 88: 360–366, 2010. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basrai MA, Hieter P, Boeke JD. Small open reading frames: beautiful needles in the haystack. Genome Res 7: 768–771, 1997. doi: 10.1101/gr.7.8.768. [DOI] [PubMed] [Google Scholar]
- 3.Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis 1863: 1066–1077, 2017. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cataldo LR, Fernández-Verdejo R, Santos JL, Galgani JE. Plasma MOTS-c levels are associated with insulin sensitivity in lean but not in obese individuals. J Investig Med 66: 1019–1022, 2018. doi: 10.1136/jim-2017-000681. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Brunner AD, Cogan JZ, Nuñez JK, Fields AP, Adamson B, Itzhak DN, Li JY, Mann M, Leonetti MD, Weissman JS. Pervasive functional translation of noncanonical human open reading frames. Science 367: 1140–1146, 2020. doi: 10.1126/science.aay0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobb LJ, Lee C, Xiao J, Yen K, Wong RG, Nakamura HK, Mehta HH, Gao Q, Ashur C, Huffman DM, Wan J, Muzumdar R, Barzilai N, Cohen P. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY) 8: 796–809, 2016. doi: 10.18632/aging.100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conte M, Ostan R, Fabbri C, Santoro A, Guidarelli G, Vitale G, Mari D, Sevini F, Capri M, Sandri M, Monti D, Franceschi C, Salvioli S. Human aging and longevity are characterized by high levels of mitokines. J Gerontol A Biol Sci Med Sci 74: 600–607, 2019. doi: 10.1093/gerona/gly153. [DOI] [PubMed] [Google Scholar]
- 8.D’Souza RF, Woodhead JST, Hedges CP, Zeng N, Wan J, Kumagai H, Lee C, Cohen P, Cameron-Smith D, Mitchell CJ, Merry TL. Increased expression of the mitochondrial derived peptide, MOTS-c, in skeletal muscle of healthy aging men is associated with myofiber composition. Aging (Albany NY) 12: 5244–5258, 2020. doi: 10.18632/aging.102944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du C, Zhang C, Wu W, Liang Y, Wang A, Wu S, Zhao Y, Hou L, Ning Q, Luo X. Circulating MOTS-c levels are decreased in obese male children and adolescents and associated with insulin resistance. Pediatr Diabetes 19: 1058–1064, 2018. doi: 10.1111/pedi.12685. [DOI] [PubMed] [Google Scholar]
- 10.Fuku N, Pareja-Galeano H, Zempo H, Alis R, Arai Y, Lucia A, Hirose N. The mitochondrial-derived peptide MOTS-c: a player in exceptional longevity? Aging Cell 14: 921–923, 2015. doi: 10.1111/acel.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gidlund EK, von Walden F, Venojärvi M, Risérus U, Heinonen OJ, Norrbom J, Sundberg CJ. Humanin skeletal muscle protein levels increase after resistance training in men with impaired glucose metabolism. Physiol Rep 4: e13063, 2016. doi: 10.14814/phy2.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong Z, Su K, Cui L, Tas E, Zhang T, Dong HH, Yakar S, Muzumdar RH. Central effects of humanin on hepatic triglyceride secretion. Am J Physiol Endocrinol Metab 309: E283–E292, 2015. doi: 10.1152/ajpendo.00043.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 423: 456–461, 2003. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 14.Han K, Jia N, Zhong Y, Shang X. S14G-humanin alleviates insulin resistance and increases autophagy in neurons of APP/PS1 transgenic mouse. J Cell Biochem 119: 3111–3117, 2018. doi: 10.1002/jcb.26452. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc Natl Acad Sci USA 98: 6336–6341, 2001. [Erratum in Proc Natl Acad Sci USA 98: 12854, 2001.] doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoang PT, Park P, Cobb LJ, Paharkova-Vatchkova V, Hakimi M, Cohen P, Lee KW. The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism 59: 343–349, 2010. doi: 10.1016/j.metabol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, Nishimoto I, Cohen P. Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA 100: 13042–13047, 2003. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang W, Li R, Zhang Y, Wang P, Wu T, Lin J, Yu J, Gu M. Mitochondrial DNA mutations associated with type 2 diabetes mellitus in Chinese Uyghur population. Sci Rep 7: 16989, 2017. doi: 10.1038/s41598-017-17086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kariya S, Hirano M, Furiya Y, Sugie K, Ueno S. Humanin detected in skeletal muscles of MELAS patients: a possible new therapeutic agent. Acta Neuropathol 109: 367–372, 2005. doi: 10.1007/s00401-004-0965-5. [DOI] [PubMed] [Google Scholar]
- 20.Kariya S, Hirano M, Furiya Y, Ueno S. Effect of humanin on decreased ATP levels of human lymphocytes harboring A3243G mutant mitochondrial DNA. Neuropeptides 39: 97–101, 2005. doi: 10.1016/j.npep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Kazuno AA, Munakata K, Nagai T, Shimozono S, Tanaka M, Yoneda M, Kato N, Miyawaki A, Kato T. Identification of mitochondrial DNA polymorphisms that alter mitochondrial matrix pH and intracellular calcium dynamics. PLoS Genet 2: e128, 2006. doi: 10.1371/journal.pgen.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KH, Son JM, Benayoun BA, Lee C. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab 28: 516–524.e7, 2018. doi: 10.1016/j.cmet.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SJ, Mehta HH, Wan J, Kuehnemann C, Chen J, Hu JF, Hoffman AR, Cohen P. Mitochondrial peptides modulate mitochondrial function during cellular senescence. Aging (Albany NY) 10: 1239–1256, 2018. doi: 10.18632/aging.101463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SJ, Miller B, Mehta HH, Xiao J, Wan J, Arpawong TE, Yen K, Cohen P. The mitochondrial-derived peptide MOTS-c is a regulator of plasma metabolites and enhances insulin sensitivity. Physiol Rep 7: e14171, 2019. doi: 10.14814/phy2.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kin T, Sugie K, Hirano M, Goto YI, Nishino I, Ueno S. Humanin expression in skeletal muscles of patients with chronic progressive external ophthalmoplegia. J Hum Genet 51: 555–558, 2006. doi: 10.1007/s10038-006-0397-2. [DOI] [PubMed] [Google Scholar]
- 26.Kraja AT, Liu C, Fetterman JL, Graff M, Have CT, Gu C, Yanek LR, Feitosa MF, Arking DE, Chasman DI, Young K, Ligthart S, Hill WD, Weiss S, Luan J, Giulianini F, Li-Gao R, Hartwig FP, Lin SJ, Wang L, Richardson TG, Yao J, Fernandez EP, Ghanbari M, Wojczynski MK, Lee WJ, Argos M, Armasu SM, Barve RA, Ryan KA, An P, Baranski TJ, Bielinski SJ, Bowden DW, Broeckel U, Christensen K, Chu AY, Corley J, Cox SR, Uitterlinden AG, Rivadeneira F, Cropp CD, Daw EW, van Heemst D, de Las Fuentes L, Gao H, Tzoulaki I, Ahluwalia TS, de Mutsert R, Emery LS, Erzurumluoglu AM, Perry JA, Fu M, Forouhi NG, Gu Z, Hai Y, Harris SE, Hemani G, Hunt SC, Irvin MR, Jonsson AE, Justice AE, Kerrison ND, Larson NB, Lin KH, Love-Gregory LD, Mathias RA, Lee JH, Nauck M, Noordam R, Ong KK, Pankow J, Patki A, Pattie A, Petersmann A, Qi Q, Ribel-Madsen R, Rohde R, Sandow K, Schnurr TM, Sofer T, Starr JM, Taylor AM, Teumer A, Timpson NJ, de Haan HG, Wang Y, Weeke PE, Williams C, Wu H, Yang W, Zeng D, Witte DR, Weir BS, Wareham NJ, Vestergaard H, Turner ST, Torp-Pedersen C, Stergiakouli E, Sheu WH, Rosendaal FR, Ikram MA, Franco OH, Ridker PM, Perls TT, Pedersen O, Nohr EA, Newman AB, Linneberg A, Langenberg C, Kilpeläinen TO, Kardia SLR, Jørgensen ME, Jørgensen T, Sørensen TIA, Homuth G, Hansen T, Goodarzi MO, Deary IJ, Christensen C, Chen YI, Chakravarti A, Brandslund I, Bonnelykke K, Taylor KD, Wilson JG, Rodriguez S, Davies G, Horta BL, Thyagarajan B, Rao DC, Grarup N, Davila-Roman VG, Hudson G, Guo X, Arnett DK, Hayward C, Vaidya D, Mook-Kanamori DO, Tiwari HK, Levy D, Loos RJF, Dehghan A, Elliott P, Malik AN, Scott RA, Becker DM, de Andrade M, Province MA, Meigs JB, Rotter JI, North KE. Associations of mitochondrial and nuclear mitochondrial variants and genes with seven metabolic traits. Am J Hum Genet 104: 112–138, 2019. doi: 10.1016/j.ajhg.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuliawat R, Klein L, Gong Z, Nicoletta-Gentile M, Nemkal A, Cui L, Bastie C, Su K, Huffman D, Surana M, Barzilai N, Fleischer N, Muzumdar R. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the β cell. FASEB J 27: 4890–4898, 2013. doi: 10.1096/fj.13-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lane RK, Hilsabeck T, Rea SL. The role of mitochondrial dysfunction in age-related diseases. Biochim Biophys Acta 1847: 1387–1400, 2015. doi: 10.1016/j.bbabio.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee C, Wan J, Miyazaki B, Fang Y, Guevara-Aguirre J, Yen K, Longo V, Bartke A, Cohen P. IGF-I regulates the age-dependent signaling peptide humanin. Aging Cell 13: 958–961, 2014. doi: 10.1111/acel.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee C, Yen K, Cohen P. Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol Metab 24: 222–228, 2013. doi: 10.1016/j.tem.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab 21: 443–454, 2015. doi: 10.1016/j.cmet.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Lu H, Hu G, Ye Z, Zhai D, Yan Z, Wang L, Xiang A, Lu Z. Earlier changes in mice after D-galactose treatment were improved by mitochondria derived small peptide MOTS-c. Biochem Biophys Res Commun 513: 439–445, 2019. doi: 10.1016/j.bbrc.2019.03.194. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Gidlund EK, Witasp A, Qureshi AR, Söderberg M, Thorell A, Nader GA, Barany P, Stenvinkel P, von Walden F. Reduced skeletal muscle expression of mitochondrial-derived peptides humanin and MOTS-C and Nrf2 in chronic kidney disease. Am J Physiol Renal Physiol 317: F1122–F1131, 2019. doi: 10.1152/ajprenal.00202.2019. [DOI] [PubMed] [Google Scholar]
- 34.López-Otín C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic Control of Longevity. Cell 166: 802–821, 2016. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Lu H, Tang S, Xue C, Liu Y, Wang J, Zhang W, Luo W, Chen J. Mitochondrial-derived peptide MOTS-c increases adipose thermogenic activation to promote cold adaptation. Int J Mol Sci 20: 2456, 2019. doi: 10.3390/ijms20102456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu H, Wei M, Zhai Y, Li Q, Ye Z, Wang L, Luo W, Chen J, Lu Z. MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J Mol Med (Berl) 97: 473–485, 2019. doi: 10.1007/s00109-018-01738-w. [DOI] [PubMed] [Google Scholar]
- 37.Mangkhang K, Punyapornwithaya V, Tankaew P, Pongkan W, Chattipakorn N, Boonyapakorn C. Plasma humanin as a prognostic biomarker for canine myxomatous mitral valve disease: a comparison with plasma NT-roBNP. Pol J Vet Sci 21: 673–680, 2018. doi: 10.24425/124305. [DOI] [PubMed] [Google Scholar]
- 38.Mehta HH, Xiao J, Ramirez R, Miller B, Kim SJ, Cohen P, Yen K. Metabolomic profile of diet-induced obesity mice in response to humanin and small humanin-like peptide 2 treatment. Metabolomics 15: 88, 2019. doi: 10.1007/s11306-019-1549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merry TL, Ristow M. Mitohormesis in exercise training. Free Radic Biol Med 98: 123–130, 2016. doi: 10.1016/j.freeradbiomed.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 40.Merry TL, Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and antioxidant response in mice. J Physiol 594: 5195–5207, 2016. doi: 10.1113/JP271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller B, Arpawong TE, Jiao H, Kim SJ, Yen K, Mehta HH, Wan J, Carpten JC, Cohen P. Comparing the utility of mitochondrial and nuclear DNA to adjust for genetic ancestry in association studies. Cells 8: 306, 2019. doi: 10.3390/cells8040306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ming W, Lu G, Xin S, Huanyu L, Yinghao J, Xiaoying L, Chengming X, Banjun R, Li W, Zifan L. Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation. Biochem Biophys Res Commun 476: 412–419, 2016. doi: 10.1016/j.bbrc.2016.05.135. [DOI] [PubMed] [Google Scholar]
- 43.Mottis A, Herzig S, Auwerx J. Mitocellular communication: Shaping health and disease. Science 366: 827–832, 2019. doi: 10.1126/science.aax3768. [DOI] [PubMed] [Google Scholar]
- 44.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, Budagov T, Cui L, Einstein FH, Poduval A, Hwang D, Barzilai N, Cohen P. Humanin: a novel central regulator of peripheral insulin action. PLoS One 4: e6334, 2009. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh YK, Bachar AR, Zacharias DG, Kim SG, Wan J, Cobb LJ, Lerman LO, Cohen P, Lerman A. Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice. Atherosclerosis 219: 65–73, 2011. doi: 10.1016/j.atherosclerosis.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poulton J, Luan J, Macaulay V, Hennings S, Mitchell J, Wareham NJ. Type 2 diabetes is associated with a common mitochondrial variant: evidence from a population-based case-control study. Hum Mol Genet 11: 1581–1583, 2002. doi: 10.1093/hmg/11.13.1581. [DOI] [PubMed] [Google Scholar]
- 47.Qin Q, Delrio S, Wan J, Jay Widmer R, Cohen P, Lerman LO, Lerman A. Downregulation of circulating MOTS-c levels in patients with coronary endothelial dysfunction. Int J Cardiol 254: 23–27, 2018. doi: 10.1016/j.ijcard.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Ramanjaneya M, Bettahi I, Jerobin J, Chandra P, Abi Khalil C, Skarulis M, Atkin SL, Abou-Samra AB. Mitochondrial-derived peptides are down regulated in diabetes subjects. Front Endocrinol (Lausanne) 10: 331, 2019. doi: 10.3389/fendo.2019.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramanjaneya M, Jerobin J, Bettahi I, Bensila M, Aye M, Siveen KS, Sathyapalan T, Skarulis M, Abou-Samra AB, Atkin SL. Lipids and insulin regulate mitochondrial-derived peptide (MOTS-c) in PCOS and healthy subjects. Clin Endocrinol (Oxf) 91: 278–287, 2019. doi: 10.1111/cen.14007. [DOI] [PubMed] [Google Scholar]
- 50.Reynolds J, Lai RW, Woodhead JST, Joly JH, Mitchell CJ, Cameron-Smith D, Lu R, Cohen P, Graham NA, Benayoun BA, Merry TL, Lee C. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis (Preprint). bioRxiv: 2019.2012.2022.886432, 2019. doi: 10.1101/2019.12.22.886432. [DOI] [PMC free article] [PubMed]
- 51.Thummasorn S, Apaijai N, Kerdphoo S, Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N. Humanin exerts cardioprotection against cardiac ischemia/reperfusion injury through attenuation of mitochondrial dysfunction. Cardiovasc Ther 34: 404–414, 2016. doi: 10.1111/1755-5922.12210. [DOI] [PubMed] [Google Scholar]
- 52.Wei M, Gan L, Liu Z, Liu L, Chang JR, Yin DC, Cao HL, Su XL, Smith WW. Mitochondrial-derived peptide MOTS-c attenuates vascular calcification and secondary myocardial remodeling via adenosine monophosphate-activated protein kinase signaling pathway. Cardiorenal Med 10: 42–50, 2020. doi: 10.1159/000503224. [DOI] [PubMed] [Google Scholar]
- 53.Widmer RJ, Flammer AJ, Herrmann J, Rodriguez-Porcel M, Wan J, Cohen P, Lerman LO, Lerman A. Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol 304: H393–H397, 2013. doi: 10.1152/ajpheart.00765.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woodhead JST, D’Souza RF, Hedges CP, Wan J, Berridge MV, Cameron-Smith D, Cohen P, Hickey AJR, Mitchell CJ, Merry TL. High-intensity interval exercise increases humanin, a mitochondrial encoded peptide, in the plasma and muscle of men. J Appl Physiol (1985) 128: 1346–1354, 2020. doi: 10.1152/japplphysiol.00032.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu X, Chua CC, Gao J, Chua KW, Wang H, Hamdy RC, Chua BH. Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Res 1227: 12–18, 2008. doi: 10.1016/j.brainres.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yen K, Mehta HH, Kim SJ, Lue Y, Hoang J, Guerrero N, Port J, Bi Q, Navarrete G, Brandhorst S, Lewis KN, Wan J, Swerdloff R, Mattison JA, Buffenstein R, Breton CV, Wang C, Longo V, Atzmon G, Wallace D, Barzilai N, Cohen P. The mitochondrial derived peptide humanin is a regulator of lifespan and healthspan. Aging (Albany NY) 12: 11185–11199, 2020. doi: 10.18632/aging.103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yen K, Wan J, Mehta HH, Miller B, Christensen A, Levine ME, Salomon MP, Brandhorst S, Xiao J, Kim SJ, Navarrete G, Campo D, Harry GJ, Longo V, Pike CJ, Mack WJ, Hodis HN, Crimmins EM, Cohen P. Humanin prevents age-related cognitive decline in mice and is associated with improved cognitive age in humans. Sci Rep 8: 14212, 2018. doi: 10.1038/s41598-018-32616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zempo H, Kim SJ, Fuku N, Nishida Y, Higaki Y, Wan J, Yen K, Miller B, Vicinanza R, Miyamoto-Mikami E, Kumagai H, Naito H, Xiao J, Mehta HH, Lee C, Hara M, Patel YM, Setiawan VW, Moore TM, Hevener AL, Sutoh Y, Shimizu A, Kojima K, Kinoshita K, Tanaka K, Cohen P. A Pro-diabetogenic mtDNA polymorphism in the mitochondrial-derived peptide, MOTS-c (Preprint). bioRxiv: 695585, 2019. doi: 10.1101/695585. [DOI] [PMC free article] [PubMed]
- 59.Zhang X, Urbieta-Caceres VH, Eirin A, Bell CC, Crane JA, Tang H, Jordan KL, Oh YK, Zhu XY, Korsmo MJ, Bachar AR, Cohen P, Lerman A, Lerman LO. Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE deficient mice. Life Sci 91: 199–206, 2012. doi: 10.1016/j.lfs.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhloba AA, Subbotina TF, Molchan NS, Polushin YS. [The level of circulating humanin in patients with ischemic heart disease]. Klin Lab Diagn 63: 466–470, 2018. doi: 10.18821/0869-2084-2018-63-8-466-470. [DOI] [PubMed] [Google Scholar]