Abstract
Testosterone supplementation during energy deficit promotes whole body lean mass accretion, but the mechanisms underlying that effect remain unclear. To elucidate those mechanisms, skeletal muscle molecular adaptations were assessed from muscle biopsies collected before, 1 h, and 6 h after exercise and a mixed meal (40 g protein, 1 h postexercise) following 14 days of weight maintenance (WM) and 28 days of an exercise- and diet-induced 55% energy deficit (ED) in 50 physically active nonobese men treated with 200 mg testosterone enanthate/wk (TEST) or placebo (PLA) during the ED. Participants (n = 10/group) exhibiting substantial increases in leg lean mass and total testosterone (TEST) were compared with those exhibiting decreases in both of these measures (PLA). Resting androgen receptor (AR) protein content was higher and fibroblast growth factor-inducible 14 (Fn14), IL-6 receptor (IL-6R), and muscle ring-finger protein-1 gene expression was lower in TEST vs. PLA during ED relative to WM (P < 0.05). Changes in inflammatory, myogenic, and proteolytic gene expression did not differ between groups after exercise and recovery feeding. Mechanistic target of rapamycin signaling (i.e., translational efficiency) was also similar between groups at rest and after exercise and the mixed meal. Muscle total RNA content (i.e., translational capacity) increased more during ED in TEST than PLA (P < 0.05). These findings indicate that attenuated proteolysis at rest, possibly downstream of AR, Fn14, and IL-6R signaling, and increased translational capacity, not efficiency, may drive lean mass accretion with testosterone administration during energy deficit.
Keywords: androgen receptor, inflammation, muscle mass, myonuclear accretion, negative energy balance, translational capacity
INTRODUCTION
The effects of testosterone administration on lean body mass accretion are well documented (7–9) and suggest testosterone supplementation is a viable strategy for preserving muscle mass in populations exposed to extreme stress. United States military personnel, in particular, endure high physical demands, sleep deprivation, and sustained periods of severe unavoidable energy deficit during training and combat operations. Those stressors, especially energy deficit, may alter molecular regulation of muscle mass [i.e., mechanistic target of rapamycin (mTOR)-mediated anabolic signaling; see Ref. 35], attenuate skeletal muscle and whole body anabolism and increase catabolism (12), and result in lean body mass losses (4, 36, 37). Loss of lean mass under these conditions is likely accelerated by the concomitant suppression of endogenous testosterone synthesis (25, 29) and may therefore be attenuated with supplemental testosterone (42, 43).
The anabolic effects of supplemental testosterone have been attributed to androgen receptor (AR)-dependent and -independent regulation of muscle protein synthesis and breakdown (47), and satellite cell and muscle pluripotent stem cell commitment and differentiation (49, 51). Testosterone treatment in older men was shown to increase skeletal muscle AR expression (23, 27). Upregulation of anabolic signaling through the mTOR pathway (i.e., translational efficiency; see Refs. 3 and 58) and AR-dependent downregulation of ubiquitin-mediated proteolysis (64) has also been observed in vitro following testosterone administration in cultured rodent muscle cells. A persistent increase in muscle protein synthesis and decrease in muscle protein breakdown would lead to muscle mass accrual over time. Furthermore, testosterone-related increases in the fusion of activated satellite cells to existing muscle fibers has been hypothesized to contribute to greater muscle volume (13, 52). Activation, proliferation, and differentiation of normally quiescent muscle satellite cells and pluripotent stem cells occur with the sequential expression of specific myogenic regulatory factors [i.e., myogenic differentiation-1 (MyoD), paired box 7 (Pax7), myogenic factor 5 (Myf5), myogenic factor 6 (Myf6), and myogenin]. Testosterone supplementation in humans increases the number of proliferating satellite cells and expression of myogenin, indicating testosterone promotes cell cycle entry and later stages of myogenesis (51). Testosterone-mediated increases in satellite cell number and their fusion with existing fibers may be regulated through nongenomic AR-independent pathways (26) and AR-dependent signaling (32, 49, 50). Additionally, the potential anti-inflammatory effect of exogenous testosterone administration (1, 15, 54) may promote myogenesis and decrease proteolysis by attenuating excessive inflammation (10, 18, 31).
To our knowledge, the molecular effects of exogenous testosterone administration during exercise- and diet-induced energy deficits remain unexplored. Whether testosterone supplementation alters intramuscular signaling at rest or after exercise and recovery feeding is also unclear. Understanding the effect of testosterone on intracellular signaling pathways regulating muscle mass under these conditions may facilitate the development of androgen-targeted therapies for mitigating muscle loss during situations of severe energy deficit. Given the well-established intramuscular anabolic, proteolytic, myogenic, and inflammatory signaling responses to exercise and feeding (33, 57, 61), the possibility exists that testosterone administration promotes lean mass accretion by modulating these pathways under postexercise and postfeeding conditions. For example, testosterone may potentiate the synergistic effect of high-protein feeding and exercise on stimulating rates of muscle protein synthesis by improving the reutilization of amino acids (24). Testosterone-mediated increases in myogenesis may also enhance the repair, replacement, and remodeling of mechanical stress-induced muscle fiber damage during postexercise recovery to facilitate muscle mass maintenance (11). Therefore, the objective of this study was to determine the effect of testosterone (200 mg testosterone enanthate/wk, TEST) or placebo (PLA) supplementation during 28 days of a severe exercise- and diet-induced energy deficit (ED; ∼55% deficit) on AR protein content, mTOR-mediated anabolic signaling (translational efficiency), muscle total RNA content (translational capacity), ubiquitin-mediated proteolysis, myogenesis, and muscle inflammation relative to a period of weight maintenance (WM) under fasted rested conditions and in recovery from exercise and a protein-containing meal. We hypothesized that TEST vs. PLA supplementation would increase resting AR expression and attenuate intramuscular inflammation, resulting in greater myogenesis, enhanced efficiency of anabolic signaling, and lower proteolysis at rest and after exercise and recovery feeding during ED relative to WM.
MATERIALS AND METHODS
Participants.
Participants were part of a larger proof-of-concept, single center, randomized, double-blind, placebo-controlled trial that assessed the effects of exogenous testosterone administration during 28 days of a severe exercise- and diet-induced energy deficit on changes in body composition (43). Participant eligibility and recruitment details have been reported previously (42). Briefly, 50 young (18–39 yr) physically active (≥2 days/wk aerobic and/or resistance exercise) men who met age-specific U.S. Army body composition standards (55) and had total testosterone concentrations within the normal physiological range (300–1,000 ng/dL) were recruited locally from the Baton Rouge, LA, area. Data in this manuscript are presented for 20 of those 50 participants who were dichotomized into two groups characterized by increases (TEST, n = 10) or decreases (PLA, n = 10) in both leg lean mass and total testosterone during energy deficit. This study was approved by the Institutional Review Board at the Pennington Biomedical Research Center (PBRC, Baton Rouge, LA) and by the Human Research Protection Office of the U.S. Army Medical Research and Development Command (Ft. Detrick, Fredericksburg, MD). All participants provided written informed consent and the study is registered with ClinicalTrials.gov as NCT02734238.
Experimental design.
This study involved 14 days of weight maintenance (WM) followed by 28 days of a highly controlled exercise- and diet-induced energy deficit (ED) (Fig. 1). Skeletal muscle inflammatory, myogenic, synthetic, and proteolytic signaling pathways were assessed in vastus lateralis biopsies following WM (day 14) and ED (day 42) at rest and after exercise and a protein-containing mixed meal. Before WM, participants wore an accelerometer for 7 days, recorded physical activity for 3 days, and completed a 3-day food log to assess habitual diet and physical activity patterns. Participants subsequently maintained their habitual physical activity during the 14-day free-living WM phase as verified by daily activity records and accelerometry. Participants also began a controlled eucaloric diet providing 1.6 g protein·kg−1·day−1, 30% energy intake from fat, and remaining calories from carbohydrates during WM. This macronutrient distribution was maintained throughout the study. Energy requirements for WM were individualized using the Mifflin St. Jeor Equation with an activity factor of 1.3, as well as the 7-day accelerometer and 3-day activity log data obtained during screening visits (42, 43). Compliance with diet instructions during WM was verified by research dietitians and by measuring seminude body weight daily with a calibrated digital scale (GSE Inc. model 450; GSE Scale Systems, Novi, MI) after an overnight fast and morning void (43). Caloric adjustments were made if necessary to maintain body mass within ±2%.
Fig. 1.
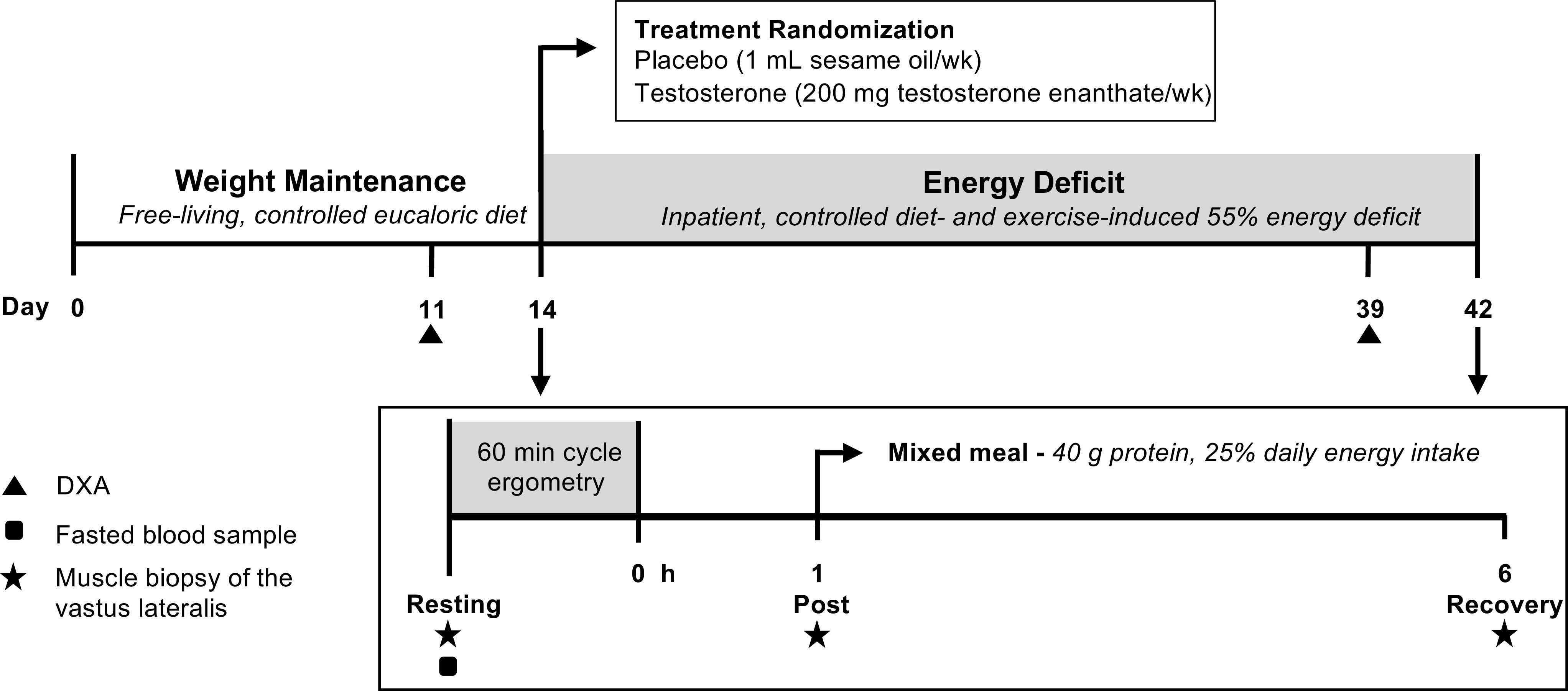
Experimental design. The current analysis was part of a larger study assessing the effects of exogenous testosterone administration on changes in body composition after a 28-day exercise- and diet-induced energy deficit (ED) designed to be 45% of total energy needs (43). Biopsies were collected at the end of weight maintenance (WM) and ED before (Resting) and 1 (Post) and 6 (Recovery) h after exercise (1 h cycle ergometry), with a mixed meal (40 g protein) consumed following the first postexercise biopsy. Steady-state aerobic exercise bouts were matched between WM and ED for each participant based on power output (124 ± 22 W) and total work performed (448 ± 77 kJ). DXA, dual-energy X-ray absorptiometry.
Participants were admitted to an inpatient unit at PBRC at the end of WM to begin the 28-day (days 15–42) exercise- and diet-induced ED. They were randomized (1:1 ratio) at the beginning of this phase to receive weekly intramuscular injections of 200 mg of testosterone enanthate (TEST) or 1 mL of sesame oil placebo (PLA). Participants were allocated to groups using a computer-generated randomization plan (version 9.4; SAS Institute, Cary, NC) with a permuted-block method (n = 4/block) and age stratification (<29 or ≥29 yr; see Ref. 43). The dose of testosterone administered on days 15, 21, 28, and 35 was chosen based on previous dose-response studies (9, 14, 30) to maintain normal testosterone concentrations during the severe energy deficit while minimizing the potential for secondary health effects (42). As previously described (43), participants performed ∼3.5 sessions of varied-intensity (40–85% of predetermined V̇o2peak) aerobic-type exercise per day to increase exercise-induced energy expenditure (EIEE) from habitual levels and elevate total daily energy expenditure (TDEE) by 50% from WM [i.e., ED EIEE = WM EIEE + (0.5 × WM TDEE)]. The 55% energy deficit was established by setting energy intake at 45% of the elevated TDEE. TDEE was increased during ED using discrete bouts of steady-state aerobic-type exercise in an effort to reflect the aerobic-type physical work performed during strenuous military training and operations. Exercise modalities used to increase EIEE have been reported previously (43) and included elliptical, stationary bike, and treadmill or outdoor walking, running, and load carriage (weighted backpack ∼30% of body mass). Exercise intensity was verified biweekly using open circuit indirect calorimetry (ParvoMedics TruOne 2400, East Sandy, UT) and adjusted when needed to maintain the prescribed EIEE (43). Light calisthenics were also incorporated every 3–4 days during ED to decrease the monotony of the prescribed aerobic exercise and better simulate field operations. Calisthenics were not performed within 48 h of exercise testing and muscle biopsies and integrated into individual exercise prescriptions to meet target energy expenditure.
Body composition.
Body composition was determined using a three-compartment model (lean body mass, fat mass, bone mineral content) derived from dual-energy X-ray absorptiometry (DXA; Lunar iDXA, GE Healthcare, Madison, WI). Scans were analyzed using the Lunar Encore software (version 13.6). Total body mass, lean body mass, and fat mass were primary outcomes of the parent study (43) and are reported in this manuscript for the involved participants as a change from WM to ED. Lean body mass was calculated as total body mass minus fat mass and bone mineral content. DXA scans were conducted by trained personnel on days 11 and 39 after an overnight fast and morning void using the participant positioning standards reported previously (43). Water intake was monitored, and conditions for the DXA were controlled across time and participants.
Endocrine profile.
Endocrine profiles were analyzed from fasted blood samples collected before the first muscle biopsy procedure on days 14 and 42 and have been presented previously for all 50 participants (43). In brief, an Immulite 2000 system (Siemens, Llanberis, UK) was used to analyze blood samples for follicle-stimulating hormone [FSH, analytical range of 0.1–170 mIU/mL, and intra-assay coefficient of variation (CV) of 3.2%], luteinizing hormone (LH, 0.05–200 mIU/mL and 3.2%), total testosterone (20–1,600 ng/dL and 8.3%), estradiol (E2; 20–2,000 pg/mL and 6.4%), sex-hormone binding globulin (SHBG; 0.02–180 nmol/L and 2.5%), and insulin (2–150 μIU/mL and 3.8%). Glucose (10–600 mg/dL and 2.0%) was analyzed on a Beckman DXC 600 Pro (Brea, CA). An enzyme-linked immunoassay (ALPCO, Salem, NH) with an analytical sensitivity of 0.09 ng/mL and intra-assay CV of 6.65% was used to analyze IGF-I. All samples were analyzed in duplicate. Blood was sampled between 0600 and 0900 h given the diurnal variation of total testosterone in young men (56). Hormone data in the current manuscript are presented for the involved participants as a change from WM to ED.
Participant stratification.
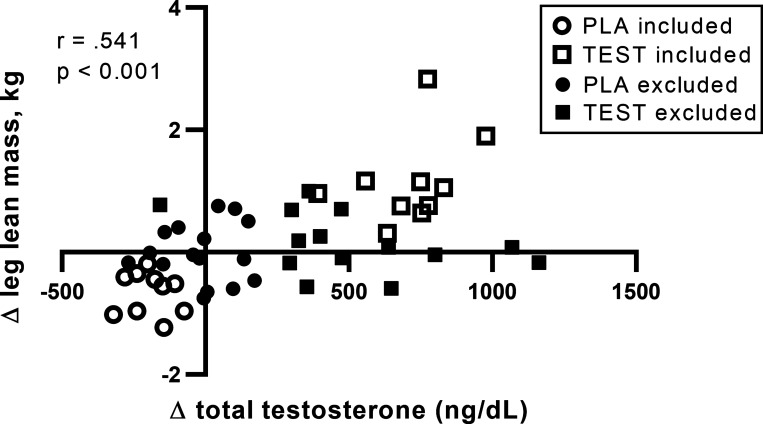
Changes in leg lean mass and total testosterone from WM to ED were positively associated for all 50 participants (r = 0.541, P < 0.001, Fig. 2). These changes remained close to zero for several individuals in both groups, however, suggesting intramuscular adaptations in TEST participants with substantial increases in both total testosterone and leg lean mass, and PLA participants whose total testosterone and leg lean mass markedly decreased, may best explain the mechanisms driving differences in lean mass with testosterone vs. placebo administration during energy deficit (43). Therefore, muscle biopsies from a subset of participants exhibiting substantial increases in leg lean mass and total testosterone (TEST, n = 10) or decreases in both of these measures (PLA, n = 10) were assayed to assess phosphorylation status, total protein and gene expression. This method of participant stratification allowed exclusion of individuals who, for example, had large increases in total testosterone but no change in leg lean mass during the intervention (i.e., nonresponders), as well as those with minimal changes in both of these parameters. This approach has been implemented previously by studies investigating differences in high vs. low responders to resistance exercise (16, 39, 41), which allocate participants who fall into extremes for study outcomes (i.e., greatest increase or decrease in muscle cross-sectional area, muscle mass, etc.) into subsets and evaluate group differences. Available muscle tissue was also a consideration when selecting individuals for analysis. A TEST participant with large increases in total testosterone (474 ng/dL) and leg lean mass (0.70 kg) was excluded, since there was insufficient muscle tissue for multiple muscle biopsy time points.
Fig. 2.
Participant stratification according to leg lean mass and total testosterone. Changes in leg lean mass (kg) and total testosterone (ng/dL) were positively associated for all 50 participants. A subset of individuals exhibiting marked increases (TEST, n = 10) or decreases (PLA, n = 10) in both leg lean mass and total testosterone were included in all analyses.
Experimental exercise bout and muscle biopsies.
Percutaneous muscle biopsies of the vastus lateralis were collected at rest and after a steady-state aerobic exercise bout on day 14 of WM and following the 28-day ED on day 42. The exercise bout included 60 min of cycle ergometry (Lode Excalibur Sport, Lode B.V., Groningen, the Netherlands) with exercise intensity matched between ED and WM for each participant based on power output (124 ± 22 W) and total work performed (448 ± 77 kJ). An absolute intensity was used to limit the confounding effects of weight loss on relative exercise intensity and standardize the absolute stress. Workloads for the experimental exercise bouts were determined during the 1st wk of WM using intermittent indirect calorimetry assessment of oxygen kinetics throughout a familiarization ride on the cycle ergometer. A total of three muscle biopsies were collected from one incision on one leg per biopsy protocol day using a 5-mm Bergström needle with manual suction (22) and under local anesthesia (1% lidocaine). The biopsy needle was inserted at different angles to separate sample sites by ∼5 cm and limit excessive trauma or inflammation. Muscle biopsies were snap-frozen in liquid nitrogen and stored at −80°C until further analysis. Muscle biopsies were collected under fasted rested conditions (Resting) and again after the cycle ergometry bout at 1 h (Post) and 6 h (Recovery) postexercise. Participants also consumed a standardized meal after the 1-h postexercise biopsy providing 25% of daily energy intake, 40 g of protein from animal sources, and 30% of kcal from fat (Table 1). The high protein content of the meal (∼40 g) was chosen to ensure maximal stimulation of the postexercise synthetic response (59), especially since a portion of dietary protein may be oxidized for fuel rather than support protein synthesis under energy deficit conditions (5).
Table 1.
Macronutrient composition of the postexercise meal in TEST vs. PLA at weight maintenance and energy deficit
| Absolute Intake | WM |
ED |
||||
|---|---|---|---|---|---|---|
| TEST | PLA | P Value | TEST | PLA | P Value | |
| Energy, kcal | 675 ± 122 | 640 ± 101 | 0.498 | 390 ± 58 | 376 ± 45 | 0.545 |
| Carbohydrate, g | 81 ± 23 | 75 ± 19 | 0.511 | 29 ± 11 | 26 ± 8 | 0.533 |
| Protein, g | 40 ± 0 | 40 ± 0 | 0.821 | 40 ± 0 | 40 ± 0 | 0.780 |
| Fat, g | 23 ± 4 | 21 ± 3 | 0.483 | 13 ± 2 | 13 ± 1 | 0.522 |
Values are means ± SD. Unpaired t tests were used to compare testosterone (TEST, n = 10) and placebo (PLA, n = 10) within weight maintenance (WM) and energy deficit (ED) phases.
mRNA expression and total RNA content.
Total RNA was extracted from ∼15 mg of muscle using a TRIzol/ethanol precipitation method. The resulting RNA pellet was resuspended in 50 µL of nuclease-free water and assessed for quality and quantity using a Nanodrop ND-2000 spectrophotometer (NanoDrop, Wilmington, DE). The muscle total RNA concentration (µg RNA/mg muscle) was assessed to provide insights on muscle translational capacity, since ribosomal RNA comprises the majority of cellular RNA (>85%; see Ref. 63). This was calculated based on the total RNA yield and the weight of the analyzed muscle sample [RNA concentration (µg/µL) × solution volume (50 µL) × muscle weight (mg)−1]. Equal amounts of total RNA (500 µg) were reverse-transcribed into cDNA using High-Capacity cDNA RT Kits (Applied Biosystems, Foster City, CA) and a T100 Thermal Cycler (Bio-Rad, Hercules, CA). Transcript levels of select genes linked to skeletal muscle inflammation [IL-6, IL-6 receptor (IL-6R), TNF-α, TNF-α receptor (TNFα-R), TNF-like weak inducer of apoptosis (TWEAK), and fibroblast growth factor-inducible 14 (Fn14)], myogenesis [MyoD, myogenin, Pax7, Myf5, and Myf6], and protein breakdown [muscle atrophy F-box (MAFbx) and muscle ring-finger protein-1 (MuRF1)] were determined using commercially available TaqMan probes (Applied Biosystems). Samples were run in 10-µL reactions in duplicate using TaqMan fast advanced master mix with a Step One Plus Real-Time PCR system (Applied Biosystems). Data were normalized to the geometric mean of glucuronidase-beta (GUSB) and tubulin beta class I (TUBB) mRNA, and fold changes were calculated using the ΔΔCT method (45). Resting gene expression during energy deficit was expressed as a fold change relative to WM for TEST and PLA. Gene data were also expressed as a fold change from resting values within each treatment (TEST and PLA) and phase (WM and ED) to evaluate the response to exercise and feeding. There was insufficient sample for one PLA participant at Post during WM and one TEST participant at Resting and one PLA participant at Post during ED (n = 9 for these time points). One Fn14 and IL-6 data point for a PLA participant at Post and Recovery during WM, and at Resting during ED, were considered outliers and removed given their values were greater than 3 SDs from the mean.
Intracellular signaling.
Total protein content of AR and the relative abundance and phosphorylation state of proteins involved in mTOR-mediated anabolic signaling were determined using standard SDS-PAGE and Western blot analysis. Approximately 15 mg of muscle were homogenized in ice-cold lysis buffer with protease and phosphatase inhibitors. Homogenized samples were snap-frozen in liquid nitrogen, thawed on ice, and centrifuged for 15 min at 10,000 g (4°C). Supernatant (lysate) was subsequently collected, and protein concentrations were determined using a 660-nm Protein Assay (ThermoScientific, Rockford, IL). Muscle lysates were solubilized in Laemmli buffer and loaded in equal amounts (i.e., 15 µg/lane) in precast Tris·HCl gels (Bio-Rad). Proteins were then separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes (Bio-Rad) that were incubated overnight at 4°C with commercially available primary antibodies specific to total ribosomal protein S6 (rpS6; Abcam, Cambridge, MA), p-rpS6Ser240/244, total mTOR, p-mTORSer2448, total p70 ribosomal protein S6 kinase (p70S6K), p-p70S6KSer424/Thr421, and total AR (Cell Signaling Technology, Danvers, MA). Labeling was performed using horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology), and signals were detected using a ChemiDoc XRS system (Bio-Rad) with Image Laboratory software (Bio-Rad) following application of chemiluminescent reagent (Pierce, Rockford, IL). Heat-shock protein 90 (HSP90) was used to confirm that equal amounts of protein were loaded per well. Phosphorylation status was expressed relative to totals of each protein, and total protein content was expressed relative to HSP90. Resting phosphorylation status and total protein content during ED are displayed as a fold change from WM for TEST and PLA. Protein phosphorylation status was also expressed as a fold change from resting values within each treatment (TEST and PLA) and phase (WM and ED) to evaluate the response to exercise and feeding. There was insufficient sample for one TEST participant at Recovery during WM and at Resting during ED (n = 9), one PLA participant at Resting and Post during WM (n = 9), and two PLA participants at Post during ED (n = 8).
Statistical analysis.
Differences between TEST and PLA in the composition of the postexercise meal and dietary intake during WM and ED, change (ED − WM) in body composition and endocrine profile, and participant characteristics were analyzed using unpaired t tests. We were also interested in the intramuscular molecular response to exercise and recovery feeding in TEST vs. PLA within WM and ED phases. Mixed-model repeated-measures ANOVA was therefore used to examine changes in phosphorylation status and mRNA expression following exercise and a high-protein mixed meal (Resting, Post, and Recovery) within each treatment (TEST and PLA) and phase (WM and ED). Bonferroni adjustments were performed for multiple comparisons if significant main effects or interactions were observed. This analysis did not examine the effect of treatment on molecular outcomes at rest, since the Resting time points were used as the control within each phase. Therefore, differences between TEST and PLA for fold change relative to WM in phosphorylation status, total protein content, and gene expression were evaluated at Resting time points using unpaired t tests. We have used similar methods previously to separately examine molecular adaptations at rest and changes in the response to exercise and recovery feeding (35). Associations between changes in Resting AR total protein content, total RNA, Fn14, IL-6R, and MuRF1 gene expression were examined using Pearson’s correlation. Normality was assessed using Shapiro-Wilk tests for dependent variables, and mRNA data were log2 transformed given several mRNA end points were not normally distributed. These data were presented as fold change means ± SD in Figs. 3 and 4 and Table 3 for clarity. Gene and protein data in correlations were log2 transformed and presented as such, since negative fold change means are on a scale of 0–1 while positive data are >1, resulting in uneven scales. All data within text, Figs. 1–5 and Tables 1–3 are presented as means ± SD. The α level of significance for all statistical tests was two-tailed and set at P < 0.05. Data were analyzed using IBM SPSS Statistics for Windows version 26 (IBM, Armonk, NY).
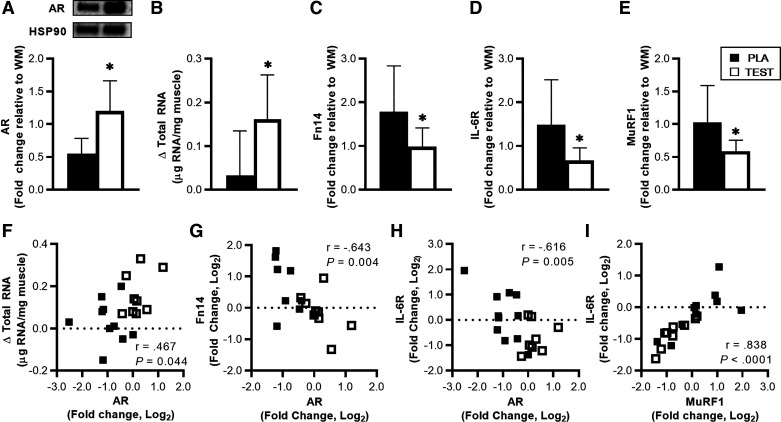
Fig. 3.
Resting androgen receptor (AR) protein content (A), muscle total RNA content (B), fibroblast growth factor-inducible 14 (Fn14, C), IL-6 receptor (IL-6R, D), and muscle ring-finger protein-1 (MuRF1, E) gene expression during energy deficit (ED) relative to weight maintenance (WM) and associations between AR, total RNA, Fn14, IL-6R, and MuRF1 changes relative to WM (F–I) in subjects receiving 200 mg testosterone enanthate/wk (TEST) or placebo (PLA). Total AR was normalized to heat shock protein 90 (HSP90), and muscle total RNA concentrations (µg RNA/mg muscle) were calculated based on muscle sample total RNA yield relative to muscle weight. Gene data were normalized to the geometric mean of GUSB and TUBB, and fold changes were calculated using the ΔΔCT method (45). Differences between TEST and PLA were examined at each time point using unpaired t tests, and associations were examined using Pearson’s correlation. Values are means ± SD [TEST, n = 9 and PLA, n = 10 (n = 9 for Fn14)]. *TEST different from PLA, P < 0.05.
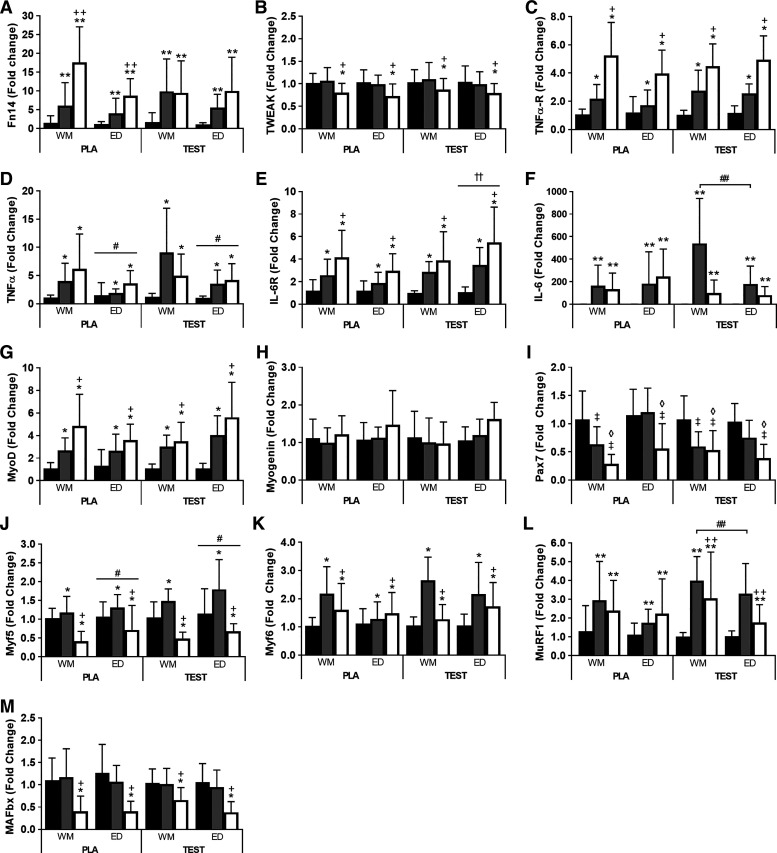
Fig. 4.
Within [Resting (black bars), Post (gray bars), and Recovery (white bars)]- and between [weight maintenance (WM) and energy deficit (ED)]-study phase responses to exercise and a high-protein mixed meal for fibroblast growth factor-inducible 14 (Fn14, A), TNF-like weak inducer of apoptosis (TWEAK, B), TNF-α receptor (TNFα-R, C), TNF-α (D), IL-6 receptor (IL-6R, E), IL-6 (F), myogenic differentiation-1 (MyoD, G), myogenin (H), paired box 7 (Pax7, I), myogenic factor 5 (Myf5, J), myogenic factor 6 (Myf6, K), muscle ring-finger protein-1 (MuRF1, L), and muscle atrophy F-box (MAFbx, M) in subjects receiving 200 mg testosterone enanthate/wk (TEST) or placebo (PLA). Data were normalized to the geometric mean of GUSB and TUBB, and fold changes were calculated using the ΔΔCT method (45). Changes in gene expression were evaluated using mixed-model repeated-measure ANOVA [n = 10 except Post for PLA during WM and ED (n = 9) and Resting for TEST during ED (n = 9); Fn14 and IL-6 Post for PLA during WM (n = 8), Recovery for PLA during WM (n = 9) and Resting for PLA during ED (n = 9). *Different from Resting, time main effect, P < 0.05. +Different from Post, time main effect, P < 0.05. **Different from Resting, time-by-treatment interaction, P < 0.05. ++Different from Post, time-by-treatment interaction, P < 0.05. #Different from WM, phase main effect, P < 0.05. ††Different from PLA, phase-by-treatment interaction, P < 0.05. ##Different from PLA, time-by-treatment interaction, P < 0.05. ‡Different from Resting, time-by-phase interaction, P < 0.05. ◊Different from Post, time-by-phase interaction, P < 0.05.
Table 3.
Inflammatory, myogenic, proteolytic, and anabolic signaling under fasted rested conditions during ED relative to WM
| PLA | TEST | P Value | |
|---|---|---|---|
| TNF-α | 1.21 ± 1.78 | 1.13 ± 0.36 | 0.303 |
| TNFα-R | 1.00 ± 0.90 | 0.80 ± 0.34 | 0.550 |
| TWEAK | 0.98 ± 0.26 | 0.99 ± 0.33 | 0.993 |
| IL-6 | 0.87 ± 0.59 | 1.21 ± 0.78 | 0.413 |
| Myf5 | 0.66 ± 0.24 | 0.72 ± 0.41 | 0.963 |
| Myf6 | 0.99 ± 0.47 | 0.68 ± 0.26 | 0.125 |
| Myogenin | 0.98 ± 0.41 | 0.75 ± 0.25 | 0.173 |
| Pax7 | 0.54 ± 0.21 | 0.57 ± 0.18 | 0.519 |
| MAFbx | 0.92 ± 0.47 | 0.75 ± 0.29 | 0.911 |
| p-mTORSer2448 | 1.09 ± 0.80 | 1.27 ± 0.77 | 0.613 |
| Total mTOR | 0.96 ± 0.51 | 1.41 ± 0.46 | 0.062 |
| p-p70S6KSer424/Thr421 | 0.74 ± 0.55 | 1.17 ± 0.68 | 0.146 |
| Total p70S6K | 0.83 ± 0.33 | 0.88 ± 0.39 | 0.780 |
| p-rpS6Ser240/244 | 0.89 ± 1.09 | 0.73 ± 0.29 | 0.655 |
| Total rpS6 | 1.12 ± 0.48 | 1.40 ± 0.88 | 0.402 |
Values are fold change means ± SD; n = 9 for testosterone (TEST) and n = 10 for placebo (PLA) (n = 9 for PLA IL-6). ED, energy deficit; MAFbx, muscle atrophy F-box; mTOR, mechanistic target of rapamycin; Myf5, myogenic factor 5; Myf6, myogenic factor 6; Pax7, paired box 7; p70S6K, p70 ribosomal protein S6 kinase; TNFα-R, TNF-α-receptor; TWEAK, TNF-like weak inducer of apoptosis; rpS6, ribosomal protein S6; WM, weight maintenance. ED data are expressed as a fold change relative to WM under fasted rested conditions (Resting) in TEST and PLA. Gene data were normalized to the geometric mean of GUSB and TUBB, and fold changes were calculated using the ΔΔCT method (45). Protein phosphorylation status expressed relative to totals of each protein and total protein content normalized to heat shock protein 90. Differences between TEST and PLA were examined at each time point using unpaired t tests.
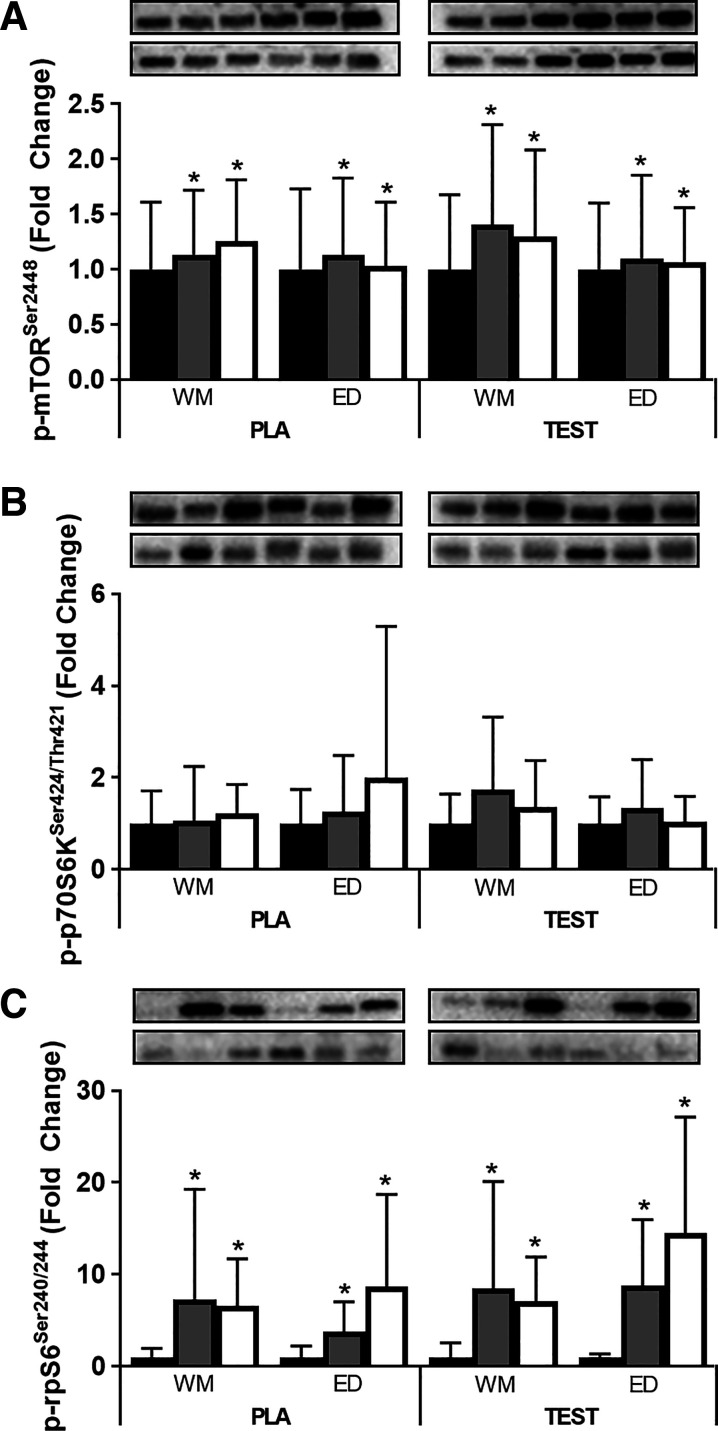
Fig. 5.
Within [Resting (black bars), Post (gray bars), and Recovery (white bars)]- and between [weight maintenance (WM) and energy deficit (ED)]-study phase responses to exercise and a high-protein mixed meal for phosphorylated (p) mechanistic target of rapamycin (mTOR)Ser2448 (A), p-p70 ribosomal protein S6 kinase (p70S6K)Ser424/Thr421 (B), and p-ribosomal protein S6 (rpS6)Ser240/244 (C) in subjects receiving 200 mg testosterone enanthate/wk (TEST) or placebo (PLA). For A–C, a representative band for the target phosphorylation site is on top and a representative band for total protein is on bottom. Protein phosphorylation status was expressed relative to totals of each protein, and changes were examined using mixed-model repeated-measure ANOVA. [n = 10 except WM Recovery (n = 9) and ED Resting (n = 9) for TEST and WM Resting (n = 9), WM Post (n = 9), and ED Post (n = 8) for PLA]. *Different from Resting, time main effect, P < 0.05.
RESULTS
Participants in the TEST and PLA groups did not differ in age (22 ± 3 vs. 25 ± 6 y), body mass index (25 ± 3 vs. 23 ± 2 kg/m2), or dietary intake during WM [energy (33.5 ± 3.1 vs. 36.5 ± 5.3 kcal·kg−1·day−1), carbohydrate (4.4 ± 0.5 vs. 4.9 ± 0.9 g·kg−1·day−1), protein (1.7 ± 0.1 vs. 1.7 ± 0.1 g·kg−1·day−1), and fat (1.1 ± 0.1 vs. 1.2 ± 0.1 g·kg−1·day−1)] and ED [energy (25.0 ± 4.0 vs. 24.0 ± 4.0 kcal·kg−1·day−1), carbohydrate (2.7 ± 0.5 vs. 2.6 ± 0.6 g·kg−1·day−1), protein (1.8 ± 0.3 vs. 1.6 ± 0.2 g·kg−1·day−1), and fat (0.8 ± 0.1 vs. 0.8 ± 0.1 g·kg−1·day−1)] (TEST vs. PLA, P > 0.05). Absolute energy and macronutrient content of the postexercise meal were also similar for TEST and PLA within WM and ED phases (Table 1). Adverse events were reported previously and not different between treatment groups (43).
Body composition and endocrine profile changes during the 28-day ED differed between groups (Table 2). Loss of total body mass and total leg mass was less for TEST than PLA (P < 0.05). Lean body mass and leg lean mass changes were more positive for TEST than PLA (P < 0.05). Changes in total testosterone, free testosterone, and E2 were more positive and changes in FSH and LH were more negative in TEST than PLA (P < 0.05). Change in SHBG was also lower for TEST than PLA following ED relative to WM (P < 0.05).
Table 2.
Body composition and endocrine profile changes in TEST and PLA following energy deficit
| ED – WM, Δ |
|||
|---|---|---|---|
| TEST | PLA | P Value | |
| Body composition | |||
| Body mass, kg | |||
| Total | −0.9 ± 1.9 | −5.1 ± 1.4 | <0.0001 |
| Lean | 3.8 ± 1.2 | −0.9 ± 1.0 | <0.0001 |
| Fat | −4.7 ± 1.6 | −4.1 ± 1.2 | 0.348 |
| Leg mass, kg | |||
| Total | −0.3 ± 0.7 | −1.8 ± 0.6 | <0.0001 |
| Lean | 1.2 ± 0.7 | −0.7 ± 0.4 | <0.0001 |
| Fat | −1.5 ± 0.4 | −1.3 ± 0.3 | 0.063 |
| Trunk mass, kg | |||
| Total | −0.3 ± 1.4 | −2.2 ± 0.9 | 0.003 |
| Lean | 2.4 ± 0.8 | 0.4 ± 0.7 | <0.0001 |
| Fat | −2.7 ± 1.1 | −2.6 ± 1.0 | 0.798 |
| Endocrine profile | |||
| TT, ng/dL | 711.9 ± 159.3 | −193.4 ± 77.8 | <0.0001 |
| FT, ng/dL | 19.0 ± 3.7 | −6.0 ± 1.7 | <0.0001 |
| FSH, mIU/mL | −3.2 ± 1.2 | −0.6 ± 1.0 | 0.007 |
| E2, pg/mL | 39.7 ± 17 | −9.1 ± 8.8 | <0.0001 |
| SHBG, µg/mL | 3.7 ± 9.3 | 18.3 ± 9.7 | 0.003 |
| LH, mIU/L | −2.8 ± 1.0 | −1.1 ± 1.5 | 0.009 |
| IGF-I, ng/mL | −82.7 ± 85.6 | −120.8 ± 77.3 | 0.310 |
| Glucose, mg/dL | −10.2 ± 8.8 | −8.5 ± 14.7 | 0.757 |
| Insulin, µIU/mL | −7.4 ± 8.5 | −7.0 ± 11.3 | 0.925 |
| Cortisol, µg/dL | 4.7 ± 4.3 | 6.0 ± 4.5 | 0.494 |
Values are means ± SD. Data were expressed as energy deficit (ED) minus weight maintenance (WM), and differences between subjects receiving 200 mg testosterone enanthate/wk (TEST, n = 10) or placebo (PLA, n = 10) were analyzed using unpaired t tests. TT, total testosterone; FT, free testosterone; FSH, follicle-stimulating hormone; E2, estradiol; SHBG, sex-hormone binding globulin; LH, luteinizing hormone.
Resting AR total protein content was higher for TEST than PLA during ED relative to WM (P < 0.05, Fig. 3A). Change in Resting total RNA was higher for TEST than PLA (P < 0.05, Fig. 3B). Resting Fn14, IL-6R, and MuRF1 gene expression was lower for TEST than PLA during ED relative to WM (P < 0.05, Fig. 3, C–E). Additional markers of inflammation, myogenesis, and proteolysis were not different between groups at rest (Table 3). Changes in Resting AR total protein were positively associated with changes in Resting total RNA (r = 0.467, P < 0.05, Fig. 3F), and negatively associated with Fn14 and IL-6R (r = −0.643 and −0.616, P < 0.05, Fig. 3, G and H). Changes in Resting IL-6R and MuRF1 were also positively associated (r = 0.838, P < 0.05, Fig. 3I).
Fn14 was greater than Resting at Post and Recovery independent of phase in both groups, with differences between Post and Recovery evident only in PLA (P < 0.05, time-by-treatment interaction; Fig. 4A). TWEAK and MAFbx were lower than Resting and Post at Recovery independent of phase and treatment (P < 0.05, time main effect, Fig. 4, Band M). TNFα-R, IL-6R, MyoD, and Myf6 were greater than Resting at Post and greater than Resting and Post at Recovery independent of phase and treatment (P < 0.05, time main effect, Fig. 4, C, E, and G, and K). IL-6R expression was also greater during ED in TEST vs. PLA independent of time (P < 0.05, phase-by-treatment interaction, Fig. 4E). Myf5 was greater during ED vs. WM independent of time and treatment (P < 0.05, phase main effect), greater than Resting at Post, and lower than Resting and Post at Recovery independent of phase and treatment (P < 0.05, time main effect, Fig. 4J). TNF-α was lower during ED than WM independent of time and treatment (P < 0.05, phase main effect) and greater than Resting at Post and Recovery independent of treatment and phase (P < 0.05, time main effect, Fig. 4D). IL-6 was greater than Resting at Post and Recovery and greater in TEST vs. PLA at Post independent of phase (P < 0.05, time-by-treatment interaction, Fig. 4F). MuRF1 was greater than Resting at Post and Recovery in both groups, with lower expression at Recovery than Post in TEST, and greater expression at Post in TEST vs. PLA independent of phase (P < 0.05, time-by-treatment interaction, Fig. 4L). Pax7 was lower than Resting at Post during WM and lower than Resting and Post at Recovery during WM and ED independent of treatment (P < 0.05, time-by-phase interaction, Fig. 4I). Myogenin was not different at any time point (Fig. 4H).
Phosphorylation status and total protein of mTOR, p70S6K, and rpS6 were not different during ED relative to WM under fasted rested conditions (Fig. 3). Post and Recovery p-mTORSer2448 and p-rpS6Ser240/244 were greater than Resting independent of treatment and phase (P < 0.05, time main effect; Fig. 5, A and C), whereas p-p70S6KSer424/Thr421 was similar at all time points (Fig. 5B).
DISCUSSION
The primary observation of this study is that AR protein content was higher and Fn14, IL-6R, and MuRF1 gene expression was lower at rest in TEST compared with PLA during ED relative to WM. Levels of mTOR-mediated anabolic signaling (i.e., translational efficiency) did not differ between groups at any time point; however, the greater increase in muscle total RNA content for TEST than PLA during ED supports an enhanced translational capacity. Molecular responses to an exercise bout and high-protein mixed meal were also similar in TEST and PLA. These novel findings suggest that, in addition to altered translational capacity, testosterone administration during a severe exercise- and diet-induced energy deficit attenuates proteolytic gene expression at rest, possibly via upstream AR, Fn14, and IL-6R signaling.
The anabolic effect of supplemental testosterone during energy deficit may be mediated by AR signaling and its downstream effect on proteolytic activity. Higher AR total protein content was observed at rest in TEST vs. PLA during ED relative to WM. This is consistent with previous work showing increases in skeletal muscle AR gene expression and protein abundance with testosterone administration in older men (23, 27). Likewise, castration-induced testosterone deficiency was shown to increase muscle proteolytic gene expression in mice (48, 58), whereas testosterone administration in C2C12 cells represses MAFbx expression through AR-dependent signaling (64). Recent work from Muta et al. (40) also showed that a selective AR agonist decreased expression of MAFbx and MuRF1 in cultured C2C12 myotubes. Although MAFbx did not differ between groups at rest, these findings are consistent with lower abundance of Resting MuRF1 in TEST than PLA during ED relative to WM, which may result from upstream changes in AR abundance.
Lower expression of the inflammatory markers Fn14 and IL-6R at rest in TEST vs. PLA during ED relative to WM may also mediate changes in proteolytic activity. Levels of Fn14 are generally low in healthy tissues, and therefore the induction of Fn14 expression is tied to TWEAK/Fn14 pathway activity (21). Fn14 expression is substantially increased under several catabolic conditions in mice (i.e., denervation, starvation), leading to muscle loss through downstream activation of MuRF1 (38, 44). Heightened activation of this pathway following TWEAK administration to cultured myotubes similarly increased MuRF1 and MAFbx expression (19). Likewise, inhibiting IL-6R decreased MuRF1 but not MAFbx expression and prevented disuse-induced muscle atrophy in mice subjected to hindlimb unloading (60). This potential relationship between IL-6R and MuRF1 is consistent with the highly associated changes in IL-6R and MuRF1 expression during ED relative to WM in the current study (r = 0.838, P < 0.0001). These data collectively indicate that greater AR protein content and lower Fn14 and IL-6R expression at rest in TEST vs. PLA during ED relative to WM may be tied to decreased MuRF1 expression in these individuals.
Lower Resting Fn14 expression in TEST vs. PLA during ED relative to WM may be regulated by AR activity. Yin et al. (62) reported an inverse relationship between Fn14 expression and AR signaling output (i.e., mRNA signature of AR target genes) in a microarray data set composed of 131 primary and 19 metastatic prostate cancer samples. Predicting androgen response elements in Fn14 promoter regions and subsequent analyses revealed that AR binding to the Fn14 enhancer decreased its expression (62). An inverse association between Fn14 expression and AR protein content in the current study supports a similar mechanism of action in human skeletal muscle. Participants with greater changes in AR protein content also had lower IL-6R. Although a potential mechanism underlying this relationship is less clear, Maggio et al. (34) similarly reported an inverse association between total testosterone and soluble IL-6R in a population of older men, suggesting increases in testosterone may act to suppress production of the receptor. The possibility exists that AR-induced attenuation of Fn14 expression and decreases in IL-6R expression downstream of AR signaling mediate the lower MuRF1 expression under resting fasted conditions with testosterone supplementation during ED relative to WM. These findings suggest testosterone supplementation may protect muscle mass by attenuating proteolysis at rest. It must be noted, however, that changes in proteolytic gene expression do not always translate to changes in protein abundance or rates of muscle protein breakdown (28), suggesting dynamic measures of proteolysis (i.e., stable isotope methodology) may be necessary to confirm this effect.
Markers of myogenesis were not different between TEST and PLA at rest or following exercise and recovery feeding despite the hypothesized effect of testosterone administration on the myogenesis. While exogenous testosterone administration in vitro and in humans enhanced proliferation and differentiation of myogenic progenitor cells (26, 51) and increased commitment of muscle pluripotent stem cells to the myogenic lineage (49), Englund et al. (20) recently challenged whether the consequent fusion of activated satellite cells to existing muscle fibers (i.e., myonuclear accretion) drives testosterone-induced skeletal muscle hypertrophy. Muscle fiber cross-sectional area was increased with testosterone administration in satellite cell-depleted mice, suggesting muscle hypertrophy following testosterone administration does not require an increase in satellite cell abundance or myonuclear accretion (20). Although it is unclear if these findings extend to humans, our work suggests increases in muscle mass with testosterone supplementation during energy deficit also occur independent of changes in the myogenesis.
Interestingly, we observed no differences in mTOR signaling between groups at rest or in response to exercise, suggesting changes in translation initiation had a limited role, at the selected sampling time points, in mediating the anabolic effect of supplemental testosterone during ED. These results are contrary to in vitro studies implicating the mTOR pathway in testosterone-induced increases in myotube hypertrophy (3, 58), as well as literature reporting altered synthetic rates with testosterone administration and androgen withdrawal in humans and mice, respectively (24, 53, 58). This apparent discrepancy may be attributed to energy status (i.e., energy balance vs. energy deficit) and differences in timing of biopsies and metabolic state (i.e., fed vs. fasted). The 6-h postexercise biopsy (5 h postfeeding) may have occurred too late to determine whether maximal activation of mTOR signaling during ED differed in TEST vs. PLA relative to WM, since peak stimulation of this pathway has been observed 1–2 h after feeding (2, 57). Additionally, while testosterone-mediated changes in protein synthetic rates have been observed in humans in the postabsorptive state (24, 53), the role of mTOR signaling in regulating protein synthesis under these conditions remains unclear (17). Reidy et al. (46) found that increased postabsorptive muscle protein synthesis following resistance exercise occurred with concomitant increases in translational capacity, whereas mTOR-dependent and -independent regulation of translation initiation (i.e., translational efficiency) did not change. We therefore measured total RNA content of skeletal muscle (µg RNA/mg muscle) during ED relative to WM to examine changes in translational capacity, since ribosomal RNA comprises the majority of cellular RNA (>85%; see Ref. 63). A more positive change in total RNA from WM to ED was observed in TEST vs. PLA. These findings are consistent with work in older men demonstrating increases in muscle total RNA content with testosterone vs. placebo administration during a resistance exercise training program (27). Positive associations between changes in total RNA and AR protein content suggest alterations in translational capacity may occur downstream of AR activity.
Testosterone treatment during ED had limited effects on changes in molecular markers of inflammation, myogenesis, and proteolysis following exercise and recovery feeding. Although MuRF1 and IL-6 were greater in TEST than PLA at Post, and Fn14 was greater at Recovery than Post in PLA but not TEST, these treatment effects occurred independent of phase (WM or ED) and are likely the result of baseline (WM) participant differences in the molecular response to exercise that persisted during ED rather than an effect of the TEST vs. PLA treatment. Increases in IL-6R expression with exercise and recovery feeding were also greater in TEST vs. PLA during ED. However, the physiological relevance of this finding is unclear, since there do not appear to be related effects on other markers of inflammation, myogenesis, or proteolysis (i.e., TWEAK, TNFα-R, TNF-α, MyoD, myogenin, Pax7, Myf5, Myf6, and MAFbx). Greater increases in IL-6R expression may also be a compensatory response resulting from lower baseline IL-6R mRNA in TEST vs. PLA. These findings collectively suggest that, despite the hypothesized effect of testosterone on the molecular response to an exercise bout and high-protein mixed meal, lean mass differences in TEST vs. PLA appear predominantly driven by adaptations under resting conditions rather than changes in the acute response to exercise and recovery feeding.
Some limitations must be acknowledged when interpreting these findings and their potential implications. First is the dichotomization of individuals into groups characterized by substantial increases in leg lean mass and total testosterone (TEST) or decreases in both of these measures (PLA). The analysis was conducted in this manner given observed differences in the anabolic response to supplemental testosterone during energy deficit (i.e., no change in leg lean mass or total testosterone for some individuals) and to understand the influence of molecular adaptations in two distinct groups of volunteers. The possibility exists that molecular adaptations (i.e., changes in resting AR, total RNA content, Fn14, IL-6R, and MuRF1) during ED relative to WM may have been similar in all individuals receiving testosterone regardless of whether they were included or excluded from the analysis. However, excluding individuals whose leg lean mass or total testosterone did not change was preferable to optimally evaluate potential mechanisms of testosterone-mediated lean mass accretion during energy deficit. Similar approaches have been effectively used to identify intracellular mechanisms underlying low vs. high muscle hypertrophic responses to resistance exercise training (16, 39, 41). Our study design and muscle biopsy time points also precluded us from examining nongenomic actions of testosterone in muscle. This signaling occurs within seconds to minutes of testosterone administration in vitro, although the physiological relevance is humans has not been fully elucidated. Nongenomic actions of testosterone may influence gene expression and cellular processes, as testosterone promotes myogenesis independent of AR via G protein-coupled receptors in L6 cells (26). Whether nongenomic actions of testosterone contributed to changes in gene expression observed in the current study is unknown and should be an area of future work.
In conclusion, the current study presents novel data reflecting skeletal muscle molecular adaptations to supplemental testosterone during a severe exercise- and diet-induced energy deficit. Given the similar molecular responses to an exercise bout and protein-containing mixed meal, lean mass differences in TEST vs. PLA appear predominantly driven by adaptations under resting fasted conditions. Testosterone vs. placebo administration during ED increased AR protein content and attenuated Fn14, IL-6R, and MuRF1 gene expression at rest. These findings suggest that AR, Fn14, and IL-6R signaling and subsequent alterations in downstream proteolytic activity may contribute to changes in lean mass. Additionally, mTOR pathway activation did not differ between groups at rest or in response to exercise; however, a more positive change in skeletal muscle total RNA content at rest in TEST compared with PLA suggests increased translational capacity, not efficiency, drives lean mass accretion in response to testosterone supplementation during ED.
GRANTS
This work was supported by the Collaborative Research to Optimize Warfighter Nutrition II and III projects, the Defense Health Program Joint Program Committee-5, and appointment to the U.S. Army Research Institute of Environmental Medicine administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Army Medical Research and Development Command.
DISCLAIMERS
The views and assertions expressed herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Army or the Department of Defense. Any citations of commercial organization and trade names in this report do not constitute an official Department of the Army endorsement of approval of the products or services of these organizations.
DISCLOSURES
J.C.R. and K.M.G. reported that their institution received funding from the US Department of Defense for work associated with this publication. H.R.L. reported receiving personal fees from Pfizer, Inc., for work outside this publication. All remaining authors declare no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
S.M.P., C.E.B., J.P.K., H.R.L., L.M.M., A.J.Y., M.A.M., W.J.E., K.M.G., and J.C.R. conceived and designed research; E.E.H., N.M.J., M.N.H., J.C.R., S.M.P., and L.M.M. performed experiments; E.E.H., S.M.P., L.M.M., and N.R.R. analyzed data; E.E.H., S.M.P., and L.M.M. interpreted results of experiments; E.E.H. prepared figures; E.E.H., S.M.P., and L.M.M. drafted manuscript; E.E.H., N.M.J., K.M.G., M.N.H., J.C.R., S.M.P., L.M.M., C.E.B., H.R.L., J.P.K., A.J.Y., M.A.M., W.J.E., and N.R.R. edited and revised manuscript; E.E.H., N.M.J., K.M.G., M.N.H., J.C.R., S.M.P., L.M.M., C.E.B., H.R.L., J.P.K., A.J.Y., M.A.M., W.J.E., and N.R.R. approved final version of manuscript.
REFERENCES
- 1.Altuwaijri S, Lin HK, Chuang KH, Lin WJ, Yeh S, Hanchett LA, Rahman MM, Kang HY, Tsai MY, Zhang Y, Yang L, Chang C. Interruption of nuclear factor kappaB signaling by the androgen receptor facilitates 12-O-tetradecanoylphorbolacetate-induced apoptosis in androgen-sensitive prostate cancer LNCaP cells. Cancer Res 63: 7106–7112, 2003. [PubMed] [Google Scholar]
- 2.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92: 1080–1088, 2010. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- 3.Basualto-Alarcón C, Jorquera G, Altamirano F, Jaimovich E, Estrada M. Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med Sci Sports Exerc 45: 1712–1720, 2013. doi: 10.1249/MSS.0b013e31828cf5f3. [DOI] [PubMed] [Google Scholar]
- 4.Berryman CE, Sepowitz JJ, McClung HL, Lieberman HR, Farina EK, McClung JP, Ferrando AA, Pasiakos SM. Supplementing an energy adequate, higher protein diet with protein does not enhance fat-free mass restoration after short-term severe negative energy balance. J Appl Physiol (1985) 122: 1485–1493, 2017. doi: 10.1152/japplphysiol.01039.2016. [DOI] [PubMed] [Google Scholar]
- 5.Berryman CE, Young AJ, Karl JP, Kenefick RW, Margolis LM, Cole RE, Carbone JW, Lieberman HR, Kim IY, Ferrando AA, Pasiakos SM. Severe negative energy balance during 21 d at high altitude decreases fat-free mass regardless of dietary protein intake: a randomized controlled trial. FASEB J 32: 894–905, 2018. doi: 10.1096/fj.201700915R. [DOI] [PubMed] [Google Scholar]
- 7.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab 82: 407–413, 1997. doi: 10.1210/jc.82.2.407. [DOI] [PubMed] [Google Scholar]
- 8.Bhasin S, Storer TW, Javanbakht M, Berman N, Yarasheski KE, Phillips J, Dike M, Sinha-Hikim I, Shen R, Hays RD, Beall G. Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. JAMA 283: 763–770, 2000. doi: 10.1001/jama.283.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 281: E1172–E1181, 2001. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 10.Bhatnagar S, Mittal A, Gupta SK, Kumar A. TWEAK causes myotube atrophy through coordinated activation of ubiquitin-proteasome system, autophagy, and caspases. J Cell Physiol 227: 1042–1051, 2012. doi: 10.1002/jcp.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burd NA, De Lisio M. Skeletal muscle remodeling: interconnections between stem cells and protein turnover. Exerc Sport Sci Rev 45: 187–191, 2017. doi: 10.1249/JES.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 12.Carbone JW, McClung JP, Pasiakos SM. Recent advances in the characterization of skeletal muscle and whole-body protein responses to dietary protein and exercise during negative energy balance. Adv Nutr 10: 70–79, 2019. doi: 10.1093/advances/nmy087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Zajac JD, MacLean HE. Androgen regulation of satellite cell function. J Endocrinol 186: 21–31, 2005. doi: 10.1677/joe.1.05976. [DOI] [PubMed] [Google Scholar]
- 14.Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 93: 914–919, 2008. doi: 10.1210/jc.2007-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Agostino P, Milano S, Barbera C, Di Bella G, La Rosa M, Ferlazzo V, Farruggio R, Miceli DM, Miele M, Castagnetta L, Cillari E. Sex hormones modulate inflammatory mediators produced by macrophages. Ann NY Acad Sci 876: 426–429, 1999. doi: 10.1111/j.1749-6632.1999.tb07667.x. [DOI] [PubMed] [Google Scholar]
- 16.Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol (1985) 110: 309–317, 2011. doi: 10.1152/japplphysiol.00901.2010. [DOI] [PubMed] [Google Scholar]
- 17.Dickinson JM, Drummond MJ, Fry CS, Gundermann DM, Walker DK, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin does not affect post-absorptive protein metabolism in human skeletal muscle. Metabolism 62: 144–151, 2013. doi: 10.1016/j.metabol.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dogra C, Changotra H, Mohan S, Kumar A. Tumor necrosis factor-like weak inducer of apoptosis inhibits skeletal myogenesis through sustained activation of nuclear factor-kappaB and degradation of MyoD protein. J Biol Chem 281: 10327–10336, 2006. doi: 10.1074/jbc.M511131200. [DOI] [PubMed] [Google Scholar]
- 19.Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J 21: 1857–1869, 2007. doi: 10.1096/fj.06-7537com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Englund DA, Peck BD, Murach KA, Neal AC, Caldwell HA, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Resident muscle stem cells are not required for testosterone-induced skeletal muscle hypertrophy. Am J Physiol Cell Physiol 317: C719–C724, 2019. doi: 10.1152/ajpcell.00260.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enwere EK, Lacasse EC, Adam NJ, Korneluk RG. Role of the TWEAK-Fn14-cIAP1-NF-κB signaling axis in the regulation of myogenesis and muscle homeostasis. Front Immunol 5: 34, 2014. doi: 10.3389/fimmu.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14: 101–102, 1982. [PubMed] [Google Scholar]
- 23.Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282: E601–E607, 2002. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 24.Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol Endocrinol Metab 275: E864–E871, 1998. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- 25.Friedl KE, Moore RJ, Hoyt RW, Marchitelli LJ, Martinez-Lopez LE, Askew EW. Endocrine markers of semistarvation in healthy lean men in a multistressor environment. J Appl Physiol (1985) 88: 1820–1830, 2000. doi: 10.1152/jappl.2000.88.5.1820. [DOI] [PubMed] [Google Scholar]
- 26.Fu R, Liu J, Fan J, Li R, Li D, Yin J, Cui S. Novel evidence that testosterone promotes cell proliferation and differentiation via G protein-coupled receptors in the rat L6 skeletal muscle myoblast cell line. J Cell Physiol 227: 98–107, 2012. doi: 10.1002/jcp.22710. [DOI] [PubMed] [Google Scholar]
- 27.Gharahdaghi N, Rudrappa S, Brook MS, Idris I, Crossland H, Hamrock C, Abdul Aziz MH, Kadi F, Tarum J, Greenhaff PL, Constantin-Teodosiu D, Cegielski J, Phillips BE, Wilkinson DJ, Szewczyk NJ, Smith K, Atherton PJ. Testosterone therapy induces molecular programming augmenting physiological adaptations to resistance exercise in older men. J Cachexia Sarcopenia Muscle 10: 1276–1294, 2019. doi: 10.1002/jcsm.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 295: E595–E604, 2008. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henning PC, Scofield DE, Spiering BA, Staab JS, Matheny RW Jr, Smith MA, Bhasin S, Nindl BC. Recovery of endocrine and inflammatory mediators following an extended energy deficit. J Clin Endocrinol Metab 99: 956–964, 2014. doi: 10.1210/jc.2013-3046. [DOI] [PubMed] [Google Scholar]
- 30.Lakshman KM, Kaplan B, Travison TG, Basaria S, Knapp PE, Singh AB, LaValley MP, Mazer NA, Bhasin S. The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. J Clin Endocrinol Metab 95: 3955–3964, 2010. doi: 10.1210/jc.2010-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langen RC, Van Der Velden JL, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J 18: 227–237, 2004. doi: 10.1096/fj.03-0251com. [DOI] [PubMed] [Google Scholar]
- 32.Lee DK Androgen receptor enhances myogenin expression and accelerates differentiation. Biochem Biophys Res Commun 294: 408–413, 2002. doi: 10.1016/S0006-291X(02)00504-1. [DOI] [PubMed] [Google Scholar]
- 33.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol (1985) 103: 1744–1751, 2007. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 34.Maggio M, Basaria S, Ceda GP, Ble A, Ling SM, Bandinelli S, Valenti G, Ferrucci L. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 28: 116–119, 2005. [PubMed] [Google Scholar]
- 35.Margolis LM, Carbone JW, Berryman CE, Carrigan CT, Murphy NE, Ferrando AA, Young AJ, Pasiakos SM. Severe energy deficit at high altitude inhibits skeletal muscle mTORC1-mediated anabolic signaling without increased ubiquitin proteasome activity. FASEB J fj201800163RR, 2018. doi: 10.1096/fj.201800163RR. [DOI] [PubMed] [Google Scholar]
- 36.Margolis LM, Murphy NE, Martini S, Gundersen Y, Castellani JW, Karl JP, Carrigan CT, Teien HK, Madslien EH, Montain SJ, Pasiakos SM. Effects of supplemental energy on protein balance during 4-d arctic military training. Med Sci Sports Exerc 48: 1604–1612, 2016. doi: 10.1249/MSS.0000000000000944. [DOI] [PubMed] [Google Scholar]
- 37.Margolis LM, Murphy NE, Martini S, Spitz MG, Thrane I, McGraw SM, Blatny JM, Castellani JW, Rood JC, Young AJ, Montain SJ, Gundersen Y, Pasiakos SM. Effects of winter military training on energy balance, whole-body protein balance, muscle damage, soreness, and physical performance. Appl Physiol Nutr Metab 39: 1395–1401, 2014. doi: 10.1139/apnm-2014-0212. [DOI] [PubMed] [Google Scholar]
- 38.Mittal A, Bhatnagar S, Kumar A, Lach-Trifilieff E, Wauters S, Li H, Makonchuk DY, Glass DJ, Kumar A. The TWEAK-Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J Cell Biol 188: 833–849, 2010. doi: 10.1083/jcb.200909117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morton RW, Sato K, Gallaugher MPB, Oikawa SY, McNicholas PD, Fujita S, Phillips SM. Muscle androgen receptor content but not systemic hormones is associated with resistance training-induced skeletal muscle hypertrophy in healthy, young men. Front Physiol 9: 1373, 2018. doi: 10.3389/fphys.2018.01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muta Y, Tanaka T, Hamaguchi Y, Hamanoue N, Motonaga R, Tanabe M, Nomiyama T, Nawata H, Yanase T. Selective androgen receptor modulator, S42 has anabolic and anti-catabolic effects on cultured myotubes. Biochem Biophys Rep 17: 177–181, 2019. doi: 10.1016/j.bbrep.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogasawara R, Akimoto T, Umeno T, Sawada S, Hamaoka T, Fujita S. MicroRNA expression profiling in skeletal muscle reveals different regulatory patterns in high and low responders to resistance training. Physiol Genomics 48: 320–324, 2016. doi: 10.1152/physiolgenomics.00124.2015. [DOI] [PubMed] [Google Scholar]
- 42.Pasiakos SM, Berryman CE, Karl JP, Lieberman HR, Orr JS, Margolis LM, Caldwell JA, Young AJ, Montano MA, Evans WJ, Vartanian O, Carmichael OT, Gadde KM, Harris M, Rood JC. Physiological and psychological effects of testosterone during severe energy deficit and recovery: a study protocol for a randomized, placebo-controlled trial for Optimizing Performance for Soldiers (OPS). Contemp Clin Trials 58: 47–57, 2017. doi: 10.1016/j.cct.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Pasiakos SM, Berryman CE, Karl JP, Lieberman HR, Orr JS, Margolis LM, Caldwell JA, Young AJ, Montano MA, Evans WJ, Vartanian O, Carmichael OT, Gadde KM, Johannsen NM, Beyl RA, Harris MN, Rood JC. Effects of testosterone supplementation on body composition and lower-body muscle function during severe exercise- and diet-induced energy deficit: A proof-of-concept, single centre, randomised, double-blind, controlled trial. EBioMedicine 46: 411–422, 2019. doi: 10.1016/j.ebiom.2019.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paul PK, Bhatnagar S, Mishra V, Srivastava S, Darnay BG, Choi Y, Kumar A. The E3 ubiquitin ligase TRAF6 intercedes in starvation-induced skeletal muscle atrophy through multiple mechanisms. Mol Cell Biol 32: 1248–1259, 2012. doi: 10.1128/MCB.06351-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfaffl MW A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reidy PT, Borack MS, Markofski MM, Dickinson JM, Fry CS, Deer RR, Volpi E, Rasmussen BB. Post-absorptive muscle protein turnover affects resistance training hypertrophy. Eur J Appl Physiol 117: 853–866, 2017. doi: 10.1007/s00421-017-3566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossetti ML, Steiner JL, Gordon BS. Androgen-mediated regulation of skeletal muscle protein balance. Mol Cell Endocrinol 447: 35–44, 2017. doi: 10.1016/j.mce.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serra C, Sandor NL, Jang H, Lee D, Toraldo G, Guarneri T, Wong S, Zhang A, Guo W, Jasuja R, Bhasin S. The effects of testosterone deprivation and supplementation on proteasomal and autophagy activity in the skeletal muscle of the male mouse: differential effects on high-androgen responder and low-androgen responder muscle groups. Endocrinology 154: 4594–4606, 2013. doi: 10.1210/en.2013-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 144: 5081–5088, 2003. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 50.Singh R, Bhasin S, Braga M, Artaza JN, Pervin S, Taylor WE, Krishnan V, Sinha SK, Rajavashisth TB, Jasuja R. Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/ beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology 150: 1259–1268, 2009. doi: 10.1210/en.2008-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab 91: 3024–3033, 2006. doi: 10.1210/jc.2006-0357. [DOI] [PubMed] [Google Scholar]
- 52.Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab 285: E197–E205, 2003. doi: 10.1152/ajpendo.00370.2002. [DOI] [PubMed] [Google Scholar]
- 53.Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol Endocrinol Metab 269: E820–E826, 1995. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 54.Urban RJ, Dillon EL, Choudhary S, Zhao Y, Horstman AM, Tilton RG, Sheffield-Moore M. Translational studies in older men using testosterone to treat sarcopenia. Trans Am Clin Climatol Assoc 125: 27–42, 2014. [PMC free article] [PubMed] [Google Scholar]
- 55.US Department of the Army The Army Body Composition Program: Army Regulation 600–9 (Online). https://api.army.mil/e2/c/downloads/566071.pdf.
- 56.Welliver RC Jr, Wiser HJ, Brannigan RE, Feia K, Monga M, Köhler TS. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol 192: 165–169, 2014. doi: 10.1016/j.juro.2014.01.085. [DOI] [PubMed] [Google Scholar]
- 57.West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr 94: 795–803, 2011. doi: 10.3945/ajcn.111.013722. [DOI] [PubMed] [Google Scholar]
- 58.White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol 365: 174–186, 2013. doi: 10.1016/j.mce.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 99: 86–95, 2014. doi: 10.3945/ajcn.112.055517. [DOI] [PubMed] [Google Scholar]
- 60.Yakabe M, Ogawa S, Ota H, Iijima K, Eto M, Ouchi Y, Akishita M. Inhibition of interleukin-6 decreases atrogene expression and ameliorates tail suspension-induced skeletal muscle atrophy. PLoS One 13: e0191318, 2018. doi: 10.1371/journal.pone.0191318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol (1985) 98: 1745–1752, 2005. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]
- 62.Yin J, Liu YN, Tillman H, Barrett B, Hewitt S, Ylaya K, Fang L, Lake R, Corey E, Morrissey C, Vessella R, Kelly K. AR-regulated TWEAK-FN14 pathway promotes prostate cancer bone metastasis. Cancer Res 74: 4306–4317, 2014. doi: 10.1158/0008-5472.CAN-13-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young V The role of skeletal and cardiac muscle in the regulation of protein metabolism In: Mammalian Protein Metabolism, edited by Munro HM New York: Academic, 1970, p. 585–674. [Google Scholar]
- 64.Zhao W, Pan J, Wang X, Wu Y, Bauman WA, Cardozo CP. Expression of the muscle atrophy factor muscle atrophy F-box is suppressed by testosterone. Endocrinology 149: 5449–5460, 2008. doi: 10.1210/en.2008-0664. [DOI] [PubMed] [Google Scholar]