Abstract
Oxidative stress (OS) and inflammation are often present in polycystic ovary syndrome (PCOS). We examined the effects of salsalate treatment on nutrient-induced OS and inflammation, ovarian androgen secretion, ovulation, and insulin sensitivity in PCOS. Eight lean insulin-sensitive women with PCOS and eight age- and body composition-matched ovulatory controls for baseline comparison participated in the study. The women with PCOS underwent a 12-wk treatment of salsalate, a nonsteroidal anti-inflammatory drug, at a dose of 3 g daily. Markers of OS and inflammation were quantified in mononuclear cells (MNC) and plasma from blood drawn fasting and 2 h after saturated fat ingestion before and after treatment. Ovarian androgen secretion was assessed from blood drawn fasting and 24, 48, and 72 h after human chorionic gonadotropin (HCG) administration before and after treatment. Ovulation was documented based on biphasic basal body temperatures and luteal range progesterone elevations. A two-step pancreatic clamp was performed pre- and posttreatment to measure basal endogenous glucose production (EGP) and the steady-state glucose disposal rate (GDR) during the euglycemic phase and markers of OS and inflammation in MNC and plasma during the hyperglycemic phase. Salsalate administration suppressed lipid- and glucose-stimulated reactive oxygen species generation, activated nuclear factor-κB and circulating tumor necrosis factor-α, normalized basal androgen levels, and lowered HCG-stimulated androgen secretion without altering EGP or GDR. Four salsalate-treated subjects responded with two consecutive ovulations. We conclude that in PCOS, salsalate-induced suppression of OS and inflammation ameliorates ovarian androgen hypersecretion and may induce ovulation while maintaining insulin action.
Keywords: inflammation, nutrients, ovarian hyperandrogenism, oxidative stress, polycystic ovary syndrome, salicylates
INTRODUCTION
Hyperandrogenism, chronic oligo-anovulation, and polycystic ovaries are diagnostic features of polycystic ovary syndrome (PCOS) (20). Oxidative stress (OS), chronic low-grade inflammation, and insulin resistance are frequent findings in PCOS that are augmented by concomitant obesity (16, 21, 27). Chronic low-grade inflammation in particular is manifested by persistent activity of peripheral blood mononuclear cells (MNC) (22).
Our work has shown that MNC of women with PCOS exhibit increased sensitivity to nutrient intake not evident in healthy ovulatory women, thereby generating mediators of OS and inflammation even in the absence of obesity (28, 30, 39, 40). In response to glucose or saturated fat ingestion, lean women with PCOS exhibit increases in MNC-derived reactive oxygen species (ROS) generation and the gene expression of p47phox, a key cytosolic component of NADPH oxidase, the ROS-producing enzyme (35, 39). ROS-induced oxidative stress promotes inflammation (9, 19). In response to glucose or saturated fat ingestion, lean women with PCOS also exhibit increases in activation of the cardinal inflammatory transcription factor nuclear factor κB (NF-κB) in MNC, decreases in NFκB cytoplasmic inhibitory protein inhibitory-κBα (IκBα), and increases in gene transcription of the NF-κB p50 and p65 DNA-binding subunits, resulting in TNFα and suppressor of cytokine 3 (SOCS3) production (34, 38, 40). TNFα mediates insulin resistance by upregulating SOCS3, which attenuates facilitative glucose transport by truncating insulin signaling (9, 18, 55). Thus, nutrient-induced inflammation may be an important contributor to insulin resistance in PCOS, and the nutrient-stimulated, pro-oxidant, proinflammatory response can provide a useful physiological measure of chronic low-grade inflammation status in the disorder.
The cause of hyperandrogenism in PCOS is poorly understood. Theca cell androgen production capacity is increased within the polycystic ovary (49). Ovarian androgen hypersecretion occurs in women with PCOS in response to HCG administration irrespective of weight class (38, 44). The compensatory hyperinsulinemia of insulin resistance is considered a primary driver of hyperandrogenism in PCOS, with the thought that insulin may serve as a cogonadotropin that amplifies luteinizing hormone (LH)-mediated androgen synthesis (4). Nevertheless, physiological insulin infusion alone does not increase circulating androgens (17). As many as 30–50% of women with PCOS are lean without insulin resistance (15), raising the prospect that another modulator is involved in driving ovarian androgen hypersecretion in PCOS. TNFα stimulates theca-cell proliferation (56) and promotes serine phosphorylation, which may increase the 17,20-lyase activity of P450c17 (62). Our work has consistently shown that in PCOS, nutrient-induced increases in markers of OS and inflammation are linked to basal and HCG-stimulated ovarian androgen secretion (29, 30, 34, 35, 39, 40). Furthermore, chronic androgen suppression does not decrease inflammation in PCOS (33), whereas prooxidant and proinflammatory stimuli increase theca-cell androgen production in vitro (23, 52). Thus, nutrient-induced inflammation may directly stimulate ovarian androgen hypersecretion in PCOS.
Salicylates are a class of nonsteroidal anti-inflammatory drugs that directly target OS and inflammation by inducing antioxidant enzymes and inhibiting the activity of inhibitory-κB kinase-β, the central integrator of the inflammation pathway, thereby preventing NF-κB activation (2, 61). Salicylates have been shown to increase insulin sensitivity with associated beneficial effects on glucose metabolism, but only when used at high doses (24, 25, 53). Nonacetylated salicylates such as salsalate are safe to use at high doses since they have no effect on platelets and bleeding time and are less likely to cause gastric irritation or gastrointestinal bleeding with long-term use (26, 48, 54, 57). Salsalate administration in particular has been shown to modestly increase insulin sensitivity and improve glycemic status in patients with type 2 diabetes mellitus (26). However, an increase in insulin sensitivity may not be required to elicit a potential reduction in hyperandrogenism if the beneficial anti-inflammatory effect of salsalate is on ovarian theca-cell function. Given that salsalate simultaneously alters inflammation and insulin sensitivity, documentation of changes in insulin sensitivity is necessary to dissect the role of inflammation independent of insulin sensitivity. Interestingly, salsalate treatment causes a physiological increase in glucose-stimulated insulin secretion because salicylates are known to decrease insulin clearance (26, 42). Because hepatic and peripheral insulin action is dependent upon systemic insulin levels, indices of insulin sensitivity or the standard hyperinsulinemic euglycemic clamp cannot accurately assess insulin sensitivity following salsalate treatment. In fact, post-salsalate circulating insulin elevations can suppress endogenous glucose production (EGP), leading to inaccurate interpretation of alterations in hepatic insulin resistance (5).
We examined the effect of salsalate administration on nutrient-stimulated pro-oxidant, proinflammatory responses from MNC, HCG-stimulated androgen secretion, and ovulation in lean insulin-sensitive women with PCOS. We also examined this effect on peripheral and hepatic insulin sensitivity using a pancreatic clamp that matched peripheral and hepatic insulin levels via somatostatin-induced endogenous hormone suppression and exogenous weight-based insulin infusion. The metabolic and endocrine characteristics of our highly selected cohort of women with PCOS were compared at baseline with age- and body composition-matched ovulatory women serving as controls. We hypothesized that in subjects with PCOS, salsalate administration suppresses nutrient-stimulated prooxidant, proinflammatory responses from MNC, reduces HCG-stimulated androgen secretion, and induces ovulation without altering insulin sensitivity.
MATERIALS AND METHODS
Participants
We recruited eight lean insulin-sensitive women with PCOS 19 to 35 yr of age and eight age- and body composition-matched ovulatory women 20–36 yr of age to serve as control subjects. Some of the control subjects in the current study were involved in our previous work on PCOS and abdominal adiposity (34). All subjects were required to have a normal body mass index (BMI) between 18 and 25 kg/m2, the absence of abdominal adiposity based on having a percent ratio of truncal fat to total body fat (% TF/TBF) measured by dual X-ray energy absorptiometry (DEXA) that was <42%, as previously established (8, 28, 30, 35), and normal insulin sensitivity based on an insulin area under the curve of <7,000 over 120 min (insulin AUC0–120) of an oral glucose tolerance test (OGTT), which is highly predictive to screen for insulin resistance in PCOS (10). The diagnosis of PCOS was based on the presence of secondary amenorrhea and hyperandrogenemia after excluding nonclassic congenital adrenal hyperplasia, Cushing syndrome, hyperprolactinemia, and thyroid disease. All of the women with PCOS had polycystic ovaries on ultrasound. All control subjects had regular menses every 25 to 35 days and a luteal range serum progesterone level consistent with ovulation (>5 ng/mL), along with normal serum androgen levels, and no evidence of androgen excess skin manifestations or polycystic ovaries on ultrasound.
Five women with PCOS and four control subjects had a family history of type 2 diabetes. Exclusion criteria included diabetes, inflammatory illnesses, tobacco smoking, or medication use impacting carbohydrate metabolism or immune function for ≥6 wk before study participation. All subjects were weight stable within 5 lbs. and either sedentary or lightly active during the 6 mo before beginning the study. Both study groups had similar prestudy physical activity levels. This research protocol involving human subject studies was reviewed and approved by the Indiana University Institutional Review Board before the study was started, and all subjects provided written, informed consent.
Study Design
The women with PCOS received salsalate (Amneal Pharmaceuticals, Bridgewater, NJ), 3 g daily, in two divided doses 12 h apart for 12 wk while using nonhormonal barrier contraception. All women with PCOS underwent a human chorionic gonadotropin stimulation test (HCG-ST) over 4 days, followed by a cream challenge test (CCT) on the last day of the HCG-ST before and after salsalate treatment. The pretreatment HCG-ST was begun on day 3 after the onset of a progestin-induced withdrawal bleed. Five of the eight women with PCOS underwent a two-step pancreatic clamp 2 days after the CCT before and after salsalate treatment. Five of the eight women with PCOS, including two who underwent clamps, also underwent a posttreatment OGTT within 2 to 3 days after the CCT.
An overnight fast for ∼12 h was required before each of the HCG-ST blood draws and before undergoing an OGTT, a CCT, or a pancreatic clamp. All of the women with PCOS were provided with a healthy diet consisting of 50% carbohydrate, 35% fat, and 15% protein for 3 consecutive days before the CCT and for the day and a half before the pancreatic clamp upon completing the CCT. They also received extensive counseling by a trained dietician for consumption of a healthy diet throughout the 12 wk of salsalate treatment. Diet compliance was confirmed by a review of 24-h food records and documentation of weight stability within 5 lbs. in each subject at 4, 8, and 12 wk of treatment. A fasting blood sample was obtained to measure a salicylate level at 0, 4, 8, and 12 wk of treatment to assess for compliance and efficacy of salsalate use. The women with PCOS maintained a basal body temperature (BBT) chart throughout the 12 wk of salsalate treatment. A serum progesterone level to detect ovulation was drawn on the 7th day of temperature elevation of ≥0.75°F (0.42°C) from baseline in subjects whose BBT chart demonstrated a biphasic curve.
To reduce the possibility of unintentional bias during data generation, the investigators who measured analytes in plasma, serum, and cell culture supernatants, quantified gene expression by RT-PCR and performed the clamps and calculations of insulin sensitivity and clearance (methods described below) were blinded to the study protocol.
Eligibility Testing
Body composition assessment.
Height without shoes was measured to the nearest 1.0 cm, and body weight was measured to the nearest 0.1 kg to calculate BMI. A DEXA scan was performed on all subjects using a QDR 4500 Elite model scanner (Hologic Inc., Waltham, MA) to determine the percentage of total body fat and truncal fat as well as R1 central abdominal fat, as described previously (8, 28).
Oral glucose tolerance test.
All subjects consumed a 75-g glucose beverage and underwent blood sampling in the fasting state and 30, 60, 90, 120, and 180 min after glucose ingestion to measure glucose and insulin. In control subjects, serum and plasma were isolated from the fasting sample and stored at −80°C until being assayed for hormone levels and TNFα, respectively. Insulin AUC0–120 was calculated at baseline using the trapezoidal rule to assess insulin sensitivity for study eligibility (60). A secondary measure of baseline insulin sensitivity was also derived from the OGTT (ISOGTT) using the Matsuda index (47). In the women with PCOS who also underwent an OGTT after salsalate treatment, glucose AUC0–180 and insulin AUC0–180 were calculated using the trapezoidal rule to assess for treatment-related alterations (60).
Study Testing
HCG stimulation test.
As described by Koivunen et al. (44), women with PCOS received an intramuscular injection of 5,000 IU of HCG (Pregnyl; Merck & Co., Whitehouse Station, NJ). Blood was sampled at 8 AM as a baseline and 24, 48, and 96 h after the HCG injection. Serum isolated from these samples was stored at −80°C until being assayed for testosterone, androstenedione, and baseline sex hormone-binding globulin (SHBG). The AUC for these androgens was calculated using the trapezoidal rule (60). The free androgen index (FAI) was calculated using the following equation: 100 × baseline testosterone (nmol/L) ÷ SHBG.
Cream challenge test.
As adapted from Deopurkar et al. (14), women with PCOS consumed 100 mL of dairy cream (gourmet heavy whipping cream; Land O Lakes Inc., Arden Hills, MN) composed in volume of 70% saturated fat content, 28% unsaturated fat, <2% protein, and 0% glucose. Blood was sampled in the fasting state and 2 h after cream ingestion to quantify OS and inflammation markers from MNC that were isolated as described previously (36, 37). Plasma was isolated from these same blood samples and stored at −80°C until being assayed for TNFα.
Two-step pancreatic clamp.
A forearm vein was cannulated at 5 AM to begin a primed, continuous infusion of [3-3H]glucose (30 µCi prime, 0.2 µCi/min; Perkin Elmer, Waltham, MA) to measure basal and steady-state EGP, with the latter representing hepatic insulin sensitivity. A contralateral dorsal hand vein was cannulated at 7:30 AM and kept warm with a heating pad (55°C) applied to the hand to obtain arterialized venous blood. An infusion of somatostatin analog (60 ng·kg−1·min−1, octreotide acetate; Sun Pharmaceutical Industries, Princeton, NJ), insulin (0.78 mU/kg-lean body mass/min; Humulin R, Eli Lily and Co., Indianapolis, IN), glucagon (0.65 ng·kg−1·min−1, GlucaGen; Bedford Laboratories, Bedford, OH), and growth hormone (3 ng·kg−1·min−1, Genotropin; Pfizer Pharmaceutical Co., New York, NY) was started at 8 AM and continued throughout the test to maintain steady-state hormone concentrations. A 20% glucose infusion enriched with [3-3H]glucose was also initiated at 8 AM, with ongoing changes to the infusion rate based on immediate plasma glucose measurements from blood drawn every 5 min to maintain euglycemia (phase 1, 90 mg/dL) for the next 180 min. At 11 AM, the glucose infusion was increased to maintain a modest hyperglycemia (phase 2, 130 mg/dL) for the next 120 min. Plasma isolated from the frequent euglycemic phase blood samples was stored at −80°C until being assayed for insulin and assessed for [3-3H]glucose enrichment (46). Blood samples were drawn at 11 AM (euglycemia) and 1 PM (hyperglycemia) to isolate MNC, as previously described (34, 35), and to quantify markers of OS and inflammation. Whole body glucose disposal rate (GDR) representing peripheral insulin sensitivity was calculated at the end of phase 1 by steady-state kinetics using the updated Steele formula with adjustment for non-steady-state shifts (11). The insulin clearance rate was calculated as the insulin infusion rate divided by the increase in steady-state plasma insulin concentration above baseline (13). All infusions were discontinued at 3 PM, and the subject was fed a standard meal.
Oxidative stress and inflammation assays.
The superoxide ROS was detected in MNC via chemiluminescence as a measure of respiratory burst activity, as previously described (31), with a validated variation in ROS generation in humans using this method of <8% over a 2-wk period (12). Nuclear-bound NF-κB was quantified in MNC nuclear extracts by electrophoretic mobility shift assay (EMSA), as previously described (31). The specificity of the EMSA bands for intranuclear activated NF-κB was verified by supershift and cold competition experiments. For the supershift experiments, a nuclear extract sample was preincubated with antibodies against p50 (sc-7178), p65 (sc-7151), or early growth response-1 (sc-110), the last of which served as a nonspecific antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). For the competition experiment, a nuclear extract sample was preincubated with either an unlabeled NF-κB oligonucleotide consensus sequence (sc-2505; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) serving as a specific cold competitor or with an unlabeled activator protein-1 oligonucleotide consensus sequence (sc-2501; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) serving as a nonspecific cold competitor. The mRNA content of p47phox, NF-κB p105 (p50 gene precursor) and p65 subunits and TNFα was quantified by real-time PCR, as previously described (7, 32, 39). The protein content of p47phox, IκBα and actin was quantified by Western blotting, as previously described using a monoclonal antibody against p47phox subunit (610354; BD Transduction Laboratories, San Diego, CA), IκBα (12500-01; United States Biological Corp., Salem, MA), or actin (sc-69879; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a 1:1,000 dilution (1, 39, 40), and all densitometry values for p47phox and IκBα were corrected for loading using those obtained for actin. MNC were cultured for 24 h, as previously described (37), and the supernatants were collected and stored at −80°C until assayed for secreted TNFα.
Plasma, serum and MNC supernatant measurements.
Plasma levels of glucose, insulin, and TNFα, MNC-derived TNFα concentrations, and serum levels of LH, androstenedione, and dehydroepiandrosterone-sulfate (DHEA-S) were measured as previously described (8, 36). Serum testosterone levels were measured by a radioimmunoassay (sensitivity, 5 ng/dL, 0.1 ng/mL; intra-assay CV 6.8%; interassay CV 11.2%; Siemens, Los Angeles, CA) that demonstrates good correlation with commercial liquid chromatography-tandem mass spectrometry (45). Serum SHBG was measured using an electrochemiluminescence immunoassay (Elecsys, Modular E170 automatic analyzer, sensitivity, 0.74 nmol/L; intraassay CV 4.0%; interassay CV 2.9%; Roche Diagnostics, Indianapolis, IN). Serum progesterone was measured using a chemiluminescence immunoassay (sensitivity, 0.1 ng/ml; intraassay CV 7.3%; interassay CV 6.6%; DXI 800 automatic analyzer; Beckman Coulter, Inc., Brea, CA). Plasma salicylate levels were measured using a homogenous enzyme immunoassay (sensitivity, 3.0 mg/dL; intraassay CV 5.1%; interassay CV 4.0%, Emit tox, AU680 automatic analyzer; Beckman Coulter, Inc., Brea, CA). All samples from each subject were measured in duplicate in the same assay at the end of the study.
Statistics
The StatView software package (SAS Institute, Cary, NC) was used to perform the statistical analysis. All values were initially examined graphically for departure from normality. The presence or absence of normality was subsequently confirmed using the Shapiro-Wilk test. The natural logarithm transformation was applied to LH before the analysis, since this value was not normally distributed. For each participant, the percent change from baseline was used to determine treatment effects on OS and inflammation markers due to intersubject variability. Unpaired Student’s t tests were used to compare baseline data between women with PCOS and control subjects. Paired Student’s t tests were used to compare variables before and after salsalate treatment. Pearson product moment correlation coefficients were calculated for correlation analyses of the pre- and post-salsalate absolute differences in the percent change from baseline for the key lipid-stimulated markers of OS (ROS generation) and inflammation (activated NF-κB, plasma TNFα) with those for basal and HCG-stimulated androgens. Data are presented as means ± SE, and results with a two-tailed α-level of 0.05 were considered to be significant.
RESULTS
Age and Baseline Body Composition, Metabolic and Endocrine Parameters
Age, height, weight, BMI, total body fat, truncal fat, %TF/TBF, R1 fat, systolic and diastolic blood pressures, plasma TNFα levels, and measures of glycemia and insulin sensitivity were similar in women with PCOS and control subjects (Table 1). Serum levels of LH, testosterone, androstenedione, and DHEA-S were significantly (P < 0.03) higher in women with PCOS compared with control subjects, although mean DHEA-S levels in both groups were clinically normal based on age-related assay cutoffs (Table 1).
Table 1.
Age, body composition, and baseline metabolic and endocrine parameters of subjects
| PCOS (n = 8) | Controls (n = 8) | |
|---|---|---|
| Age, yr | 26 ± 2 | 26 ± 2 |
| Height, cm | 159.0 ± 2.0 | 163.8 ± 1.1 |
| Body weight, kg | 57.0 ± 2.5 | 60.0 ± 1.9 |
| Body mass index, kg/m2 | 22.5 ± 0.7 | 22.1 ± 0.5 |
| Total body fat, g | 18,410 ± 1,349 | 17,378 ± 1,419 |
| Truncal fat, g | 6,986 ± 545 | 6,589 ± 549 |
| Truncal fat/total body fat, % | 37.8 ± 0.8 | 37.9 ± 0.5 |
| Central fat (R1), g | 739 ± 51 | 727 ± 46 |
| Systolic blood pressure, mmHg | 107 ± 4 | 108 ± 4 |
| Diastolic blood pressure, mmHg | 65 ± 3 | 61 ± 4 |
| TNFα, pg/mL | 1.0 ± 0.1 | 0.9 ± 0.1 |
| Fasting glucose, mg/dL | 87 ± 3 | 84 ± 2 |
| 2-h Glucose, mg/dL | 102 ± 9 | 111 ± 8 |
| Glucose AUC0–120 | 13,249 ± 949 | 15,276 ± 910 |
| Fasting insulin, µU/mL | 5.5 ± 0.4 | 5.7 ± 1.0 |
| Insulin AUC0–120 | 5,455 ± 481 | 4,151 ± 488 |
| ISOGTT | 8.0 ± 0.5 | 9.4 ± 1.1 |
| LH, mIU/mL | 15.7 ± 1.1a | 4.2 ± 0.8 |
| Testosterone, ng/dL | 65.8 ± 4.8a | 35.9 ± 2.8 |
| Androstendione, ng/mL | 4.2 ± 0.4a | 1.6 ± 0.1 |
| DHEA-S, µg/dL | 267 ± 30b | 161 ± 30 |
Values are expressed as means ± SE. AUC0–120, area under the curve from 0 to 120 min of the oral glucose tolerance test (OGTT); DHEA-S, dehydroepiandrosterone-sulfate; ISOGTT, insulin sensitivity derived from the OGTT; LH, luteinizing hormone; PCOS, polycystic ovary syndrome; TNFα, tumor necrosis factor-α. Conversion factors to SI units: testosterone × 3.467 (nmol/L), androstenedione × 3.492 (nmol/L), DHEA-S × 0.002714 (µmol/L), glucose × 0.0551 (mmol/L), and insulin × 7.175 (pmol/L). PCOS vs. controls (unpaired Student’s t test).
P < 0.0001;
P < 0.03.
Salicylate Levels During Treatment
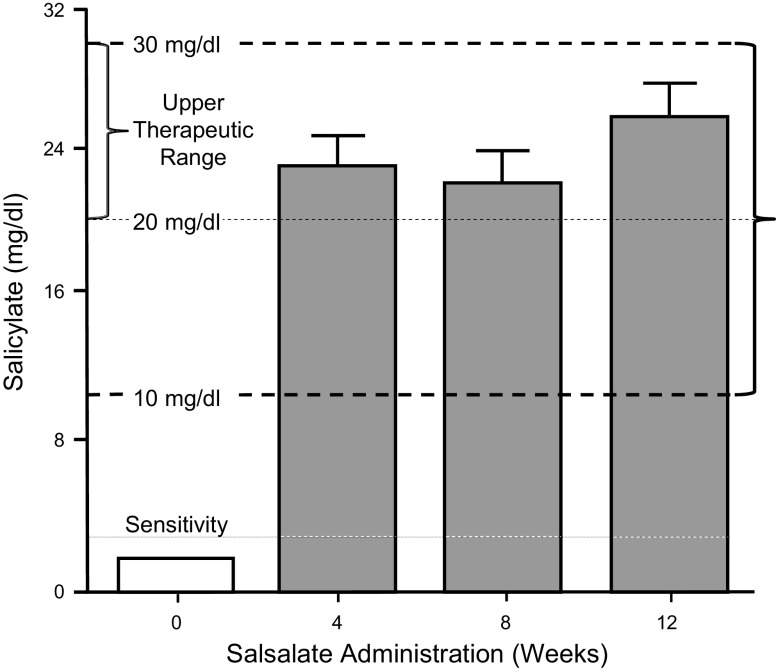
Plasma salicylate levels were undetectable (<3 mg/dL) before salsalate treatment began in all women with PCOS (Fig. 1). The mean plasma salicylate level rose to the upper therapeutic range (20–30 mg/dL) after 4 wk and remained in this range throughout treatment. The range of salicylate levels after 4 and 8 wk was 15–29 mg/dL and after 12 wk was 16–30 mg/dL. Moreover, at each of the three monthly checks during treatment, two completely different subjects of the total of eight had salicylate levels in the lower therapeutic range (i.e., 15–19 mg/dL).
Fig. 1.
Plasma salicylate levels in women with polycystic ovary syndrome (PCOS) were undetectable (<3 mg/dL) at baseline. The mean salicylate level rose to the upper therapeutic range after 4 wk and remained in this range at 8 and 12 wk of salsalate treatment.
OS and Inflammation Responses in MNC and Plasma
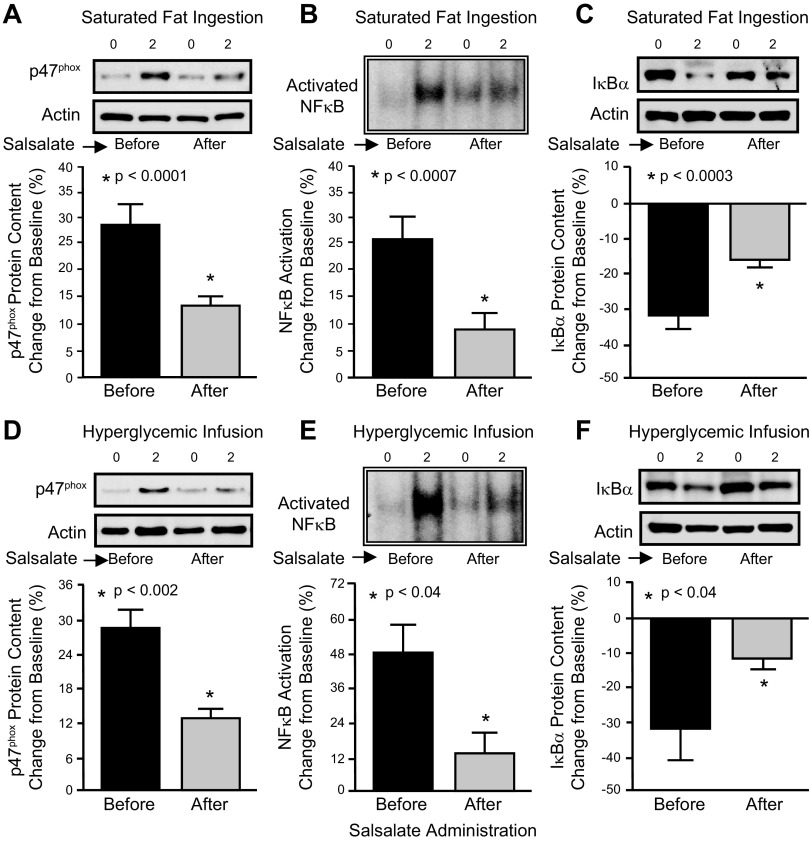
In response to salsalate administration, the change from baseline in ROS generation, activated NF-κB, p47phox, p105, p65 and TNFα mRNA content, p47phox protein content, TNFα secretion, and plasma TNFα significantly (P < 0.03) decreased and the change from baseline in IκBα protein content significantly (P < 0.04) increased following saturated fat ingestion and the hyperglycemic phase of the pancreatic clamp compared with the pretreatment response (Table 2 and Fig. 2).
Table 2.
Change from baseline (%) in oxidative stress and inflammation markers before and after salsalate treatment
| Before | After | |
|---|---|---|
| Saturated fat ingestion (n = 8) | ||
| ROS generation | 89 ± 13 | 42 ± 13a |
| p47phox mRNA content | 29 ± 5 | 11 ± 2a |
| p105 mRNA content | 30 ± 3 | 12 ± 3a |
| p65 mRNA content | 28 ± 3 | 11 ± 2a |
| TNFα mRNA content | 18 ± 4 | 5 ± 2a |
| TNFα secretion | 24 ± 12 | 3 ± 6a |
| Plasma TNFα | 19 ± 4 | 7 ± 1a |
| Hyperglycemic infusion (n = 5) | ||
| ROS generation | 56 ± 9 | 23 ± 11b |
| p47phox mRNA content | 27 ± 5 | 10 ± 2b |
| p105 mRNA content | 28 ± 4 | 12 ± 6b |
| p65 mRNA content | 23 ± 5 | 8 ± 4b |
| TNFα mRNA content | 36 ± 6 | 13 ± 3b |
| TNFα secretion | 26 ± 8 | 4 ± 7b |
| Plasma TNFα | 34 ± 7 | 16 ± 4b |
Values are expressed as means ± SE. mRNA, messenger ribonucleic acid; ROS, reactive oxygen species; TNFα, tumor necrosis factor-α. Significantly decreased after salsalate treatment (paired Student’s t test),
P < 0.02;
P < 0.03.
Fig. 2.
Comparison of the change from baseline (%) in mononuclear cell (MNC)-derived markers of oxidative stress and inflammation in women with PCOS before and after salsalate treatment in response to saturated fat ingestion (n = 8) [p47phox protein content (A), activated NF-κB (B), and IκBα protein content (C)] and in response to the hyperglycemic phase of the pancreatic clamp (n = 5) [p47phox protein content (D), activated NF-κB (E), and IκBα (F) protein content]. Representative Western blots in A, C, D, and F show the change in quantity of p47phox and IκBα in MNC homogenates, and the representative electrophoretic mobility shift assay bands in B and E show the change in quantity of NF-κB in nuclear extracts from MNC in samples collected at 0 and 2 h after the respective pro-oxidant or proinflammatory triggers. The samples used to quantify p47phox and IκBα protein content and activated NF-κB by densitometry were run on the same gel. Data are presented as means ± SE. *Pretreatment significantly different compared with posttreatment (paired Student’s t test); P < 0.0001 (A), P < 0.0007 (B), P < 0.0003 (C), P < 0.002 (D), and P < 0.04 (E) and (F).
Metabolic Responses During the OGTT and Pancreatic Clamp
In response to salsalate administration, the insulin clearance rate significantly (P < 0.006) decreased by 31%, with a corresponding significant (P < 0.003) increase in the insulin AUC1–180 by 38% and a significant (P < 0.04) decrease in the glucose AUC1–180 by 18% during the OGTT (Table 3). Basal EGP and GDR during the steady-state insulin infusion remained unaltered compared with pretreatment values. EGP exhibited complete suppression after 120 min of insulin infusion and remained suppressed throughout the subsequent 60 min of steady-state maintenance before and after salsalate treatment.
Table 3.
Metabolic, endocrine and general health parameters before and after salsalate treatment
| Before | After | |
|---|---|---|
| Metabolic parameters (n = 5) | ||
| OGTT glucose AUC0–180 | 19,065 ± 1,447 | 15,560 ± 1,813a |
| OGTT insulin AUC0–180 | 6,917 ± 242 | 9,434 ± 159b |
| Basal EGP, mg·kg−1·min−1 | 3.3 ± 0.4 | 2.9 ± 0.4 |
| Steady state GDR, mg·kg−1·min−1 | 5.0 ± 0.2 | 4.6 ± 0.4 |
| Insulin clearance rate, mL/min | 1,760 ± 185 | 1,223 ± 220c |
| Endocrine and general health parameters (n = 8) | ||
| SHBG, nmol/L | 64.9 ± 13.8 | 62.7 ± 12.9 |
| Free androgen index | 4.79 ± 1.13 | 2.58 ± 0.82d |
| WBC count, 1,000/µL | 6.4 ± 0.7 | 5.8 ± 0.4 |
| Platelet count, 1,000/µl | 274 ± 15 | 269 ± 12 |
| ALT, U/L | 15 ± 1 | 14 ± 3 |
| AST, U/L | 18 ± 2 | 18 ± 2 |
| ALP, U/L | 56 ± 8 | 53 ± 5 |
| BUN, mg/dl | 12 ± 1 | 14 ± 2 |
| Creatinine, mg/dL | 0.71 ± 0.04 | 0.81 ± 0.06 |
Values are expressed as means ± SE. Conversion factors to SI units: testosterone × 3.467 (nmol/L) and androstenedione × 3.492 (nmol/L). ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC0–180, area under the curve from 0 to 180 min of the OGTT; BUN, blood urea nitrogen; EGP, endogenous glucose production; GDR, glucose disposal rate; OGTT, oral glucose tolerance test; SHBG, sex hormone binding globulin; WBC, white blood cell. Significantly different after salsalate treatment (paired Student’s t test),
P < 0.04;
P < 0.003;
P < 0.006;
P < 0.02.
Ovarian Androgen and Ovulation Responses
In response to salsalate administration, basal levels of testosterone and androstenedione significantly (P < 0.03) decreased and became similar to baseline levels observed in control subjects, the AUC for the HCG-stimulated responses of testosterone and androstenedione significantly (P < 0.03) decreased by 42–48% (Fig. 3), and the FAI significantly (P < 0.03) decreased by 46% as SHBG levels remained unaltered (Table 3). Four of the eight previously anovulatory salsalate-treated women with PCOS responded with at least two consecutive ovulations based on biphasic BBT charting and luteal range serum progesterone levels (>5 ng/dL).
Fig. 3.
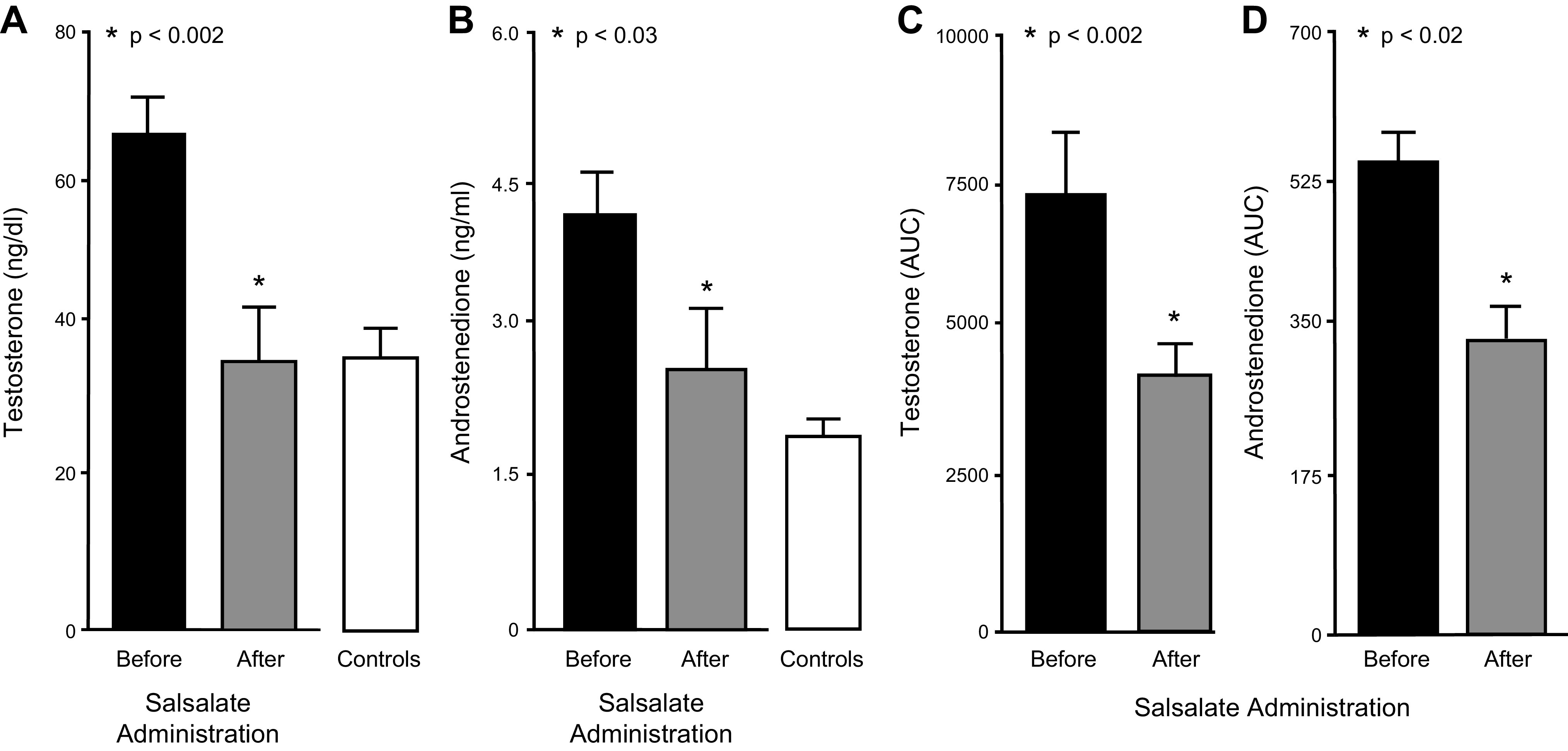
Basal levels of testosterone (A) and androstenedione (B) in women with polycystic ovary syndrome (PCOS) before and after salsalate treatment (n = 8) and in control subjects (n = 8) at baseline. Area under the curve (AUC) for human chorionic gonadotropin (HCG)-stimulated testosterone (C) and androstenedione (D) secretion in women with PCOS before and after salsalate treatment. *Significantly decreased after treatment (paired Student’s t test); P < 0.002 (A) and (C), P < 0.03 (B), and P < 0.02 (D).
Correlations
The pre- and post-salsalate absolute differences in the lipid-stimulated responses of key OS and inflammation markers were positively correlated with those for the basal levels of testosterone (ROS generation: r = 0.82, P = 0.04; activated NF-κB: r = 0.81, P = 0.03; plasma TNFα: r = 0.78, P = 0.04) and androstenedione (plasma TNFα: r = 0.82, P = 0.04) and the HCG-stimulated AUC for testosterone (ROS generation: r = 0.83, P = 0.01) and androstenedione (ROS generation: r = 0.76, P = 0.03).
Adverse Treatment Effects
Two women with PCOS reported tinnitus, an expected adverse effect of high-dose salicylate use, at 2 and 4 wk after starting salsalate treatment, respectively. The tinnitus resolved in both of these subjects within 3 days of lowering the salsalate dose upon report to 2.5 g daily for the remainder of the study. There were no complaints from the other six salsalate-treated subjects. White blood cell and platelet counts and liver and renal function tests were clinically normal and remained unaltered after salsalate treatment (Table 3).
DISCUSSION
Our data provide promising evidence that in PCOS, salicylate-induced suppression of OS and inflammation normalizes basal ovarian androgen levels and ameliorates ovarian androgen hypersecretion. In response to high-dose salsalate treatment for 12 wk, lean insulin-sensitive women with PCOS exhibit decreases in lipid- and glucose-stimulated pro-oxidant, proinflammatory responses manifested by profound suppression of MNC-derived NF-κB activation and TNFα secretion in particular, along with increases in IκBα protein. Importantly, the suppression of inflammation in our select cohort goes hand in hand with decreases in basal testosterone and androstenedione and HCG-stimulated secretion of these androgens. These findings coupled with evidence of ovulation during salsalate treatment in some of our previously anovulatory subjects with PCOS suggest that inflammation is an important driver of ovarian dysfunction in PCOS independent of insulin resistance and adiposity. Further support for this concept is provided by the positive association between the decline in key OS and inflammation markers and the decline in basal and HCG-stimulated androgen secretion.
Comparison of baseline data from the women with PCOS selected for our study and age- and body composition-matched control subjects confirms that in PCOS, adiposity and insulin resistance are not required to manifest the classic well-described diagnostic features of this disorder (20). Compared with control subjects, the lean-insulin sensitive women with PCOS have higher circulating androgen levels and are anovulatory at baseline. However, the normal basal circulating TNFα levels are expected in these subjects with PCOS who also lack abdominal adiposity. We have previously reported that basal circulating TNFα elevations in lean women with PCOS are evident only in those with abdominal adiposity implicating adipose tissue as the source of these elevations (34). Although adiposity and insulin resistance may incite or exacerbate the signs and symptoms of PCOS (4, 51), another factor must contribute to the ovarian dysfunction. Interestingly, our previous reports show that OS and inflammation are present in PCOS completely independent of adiposity and are highly correlated with circulating androgens (34, 35, 37). Thus, the current study is the logical next step in exploring the possibility that inflammation plays a role in promoting ovarian dysfunction in PCOS.
The high dose of salsalate selected for this study adequately maintains the mean salicylate level in the upper therapeutic range throughout the 12 wk of treatment in lean women with PCOS. Interestingly, the selected dose is the maximum recommended in the salsalate package insert yet is well tolerated with prompt resolution of tinnitus in two subjects by modestly lowering the salsalate dose without loss of efficacy, as previously reported (26). Most importantly, the selected dose effectively suppresses lipid- and glucose-induced NF-κB activation from MNC, as previously reported (25), along with a corresponding increase in IκB protein and decreases in lipid- and glucose-induced ROS generation and the expression of p47phox, NF-κB and TNFα, as well as MNC-derived TNFα secretion and circulating TNFα levels. In contrast, to basal circulating TNFα elevations, it is likely that the nutrient-triggered responses in circulating TNFα originate from MNC. Thus, high-dose salsalate administration achieves the intended goal of suppressing OS and inflammation stimulated by nutrients at the molecular, cellular, and systemic level.
The decrease in insulin clearance known to occur with salsalate administration is not the primary cause for the reduction in nutrient-stimulated OS and inflammation in women with PCOS (29, 55). In the current study, plasma insulin levels after an oral glucose challenge exhibit a greater physiological increase following salsalate administration, as expected after a salsalate-induced decrease in insulin clearance (26, 42, 43). The higher insulin levels can account for the lower glucose levels that can attenuate the proinflammatory effect of glucose ingestion (30, 34, 36, 37). In contrast, a decrease in the prooxidant, proinflammatory response is observed in the face of constant postprandial range hyperglycemia created by the glucose infusion during the second phase of the clamp and following saturated fat ingestion that can also contribute to decreased insulin clearance (59). Thus, the suppression of nutrient-triggered inflammation in our subjects with PCOS is through the anti-inflammatory effect of salsalate.
Hepatic and peripheral insulin sensitivity remained unaltered by salsalate administration in subjects with PCOS. These findings are unencumbered by the higher posttreatment insulin levels based on our experimental approach that inhibits endogenous insulin secretion using somatostatin while replacing it with weight-based insulin to match hepatic and peripheral insulin levels before and after salsalate administration. Although salsalate has been shown to increase insulin sensitivity in insulin-resistant individuals (26), the unaltered insulin sensitivity is not unexpected given that the pretreatment clamp studies confirm that our select cohort is already insulin sensitive (mean basal EGP <4.0 mg·kg−1·min−1, mean steady state GDR >4.5 mg·kg−1·min−1) in concurrence with the initial screening (10, 41). In fact, the complete suppression of EGP under steady state conditions before and after salsalate administration reaffirms the adequacy of insulin action unaffected by salsalate in subjects with PCOS (6).
Salsalate administration ameliorates ovarian dysfunction in PCOS. Basal ovarian androgen levels decline to normal in conjunction with a formidable decrease in the FAI and HCG-stimulated androgen secretion in the salsalate-treated women with PCOS. Although hyperinsulinemia has been shown to lower SHBG in insulin-resistant women with PCOS and obesity (50), the physiological insulin elevation induced by salsalate administration had no effect on SHBG in our lean insulin-sensitive study cohort. The reason for the lack of SHBG alteration is unclear but may be related to the difference in PCOS phenotype or insufficient treatment duration. Furthermore, consecutive ovulations occurred in half of these subjects during salsalate administration. These findings combined with the positive association between the decline in key OS and inflammation markers and the decline in basal and HCG-stimulated androgen secretion provide the first evidence that inflammation may directly impair ovarian function in PCOS. MNC-derived macrophages increase within the ovary in response to saturated fat ingestion (58). Macrophage TNFα promotes theca-cell proliferation (56) and induces serine phosphorylation, which may increase 17,20-lyase activity (62). In corroboration, treatment with the antioxidant resveratrol lowers circulating androgens in women with PCOS (3). Thus, lipid and glucose intake may increase ovarian androgen production in PCOS by accelerating MNC migration into the polycystic ovary and inciting OS and a local inflammatory response to enhance thecal cell proliferation and steroidogenic activity.
The modest sample size is a limitation of our study in our initial step to explore the role of inflammation in promoting ovarian dysfunction in PCOS. A larger double-blind placebo-controlled study that also includes group-stratified insulin-resistant women with PCOS with and without obesity is merited to confirm the study findings.
In conclusion, chronic salsalate-induced suppression of lipid- and glucose-stimulated OS and inflammation manifested by a reduction in ROS generation and a profound decrease in NF-κB activation and TNFα secretion normalizes basal ovarian androgen levels, ameliorates ovarian androgen hypersecretion, and may induce ovulation while maintaining insulin action in lean insulin-sensitive women with PCOS. These findings suggest that in PCOS, inflammation may directly promote hyperandrogenism and chronic anovulation even in the absence of insulin resistance and adiposity.
GRANTS
This research was supported by grant R01-DK-107605 to F.G. from the National Institutes of Health, the Indiana Clinical and Translational Sciences Institute Clinical Research Center, which is funded in part by grant UL1-TR-002529 from the National Institutes of Health, the National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award, and the Indiana University Center for Diabetes and Metabolic Diseases funded by Grant P30-DK-097512 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.G. and K.J.M. conceived and designed research; F.G., K.J.M., O.A.A., and A.J.A. performed experiments; F.G. and K.J.M. analyzed data; F.G. and R.V.C. interpreted results of experiments; F.G. prepared figures; F.G. drafted manuscript; F.G., K.J.M., R.V.C., O.A.A., and A.J.A. edited and revised manuscript; F.G., K.J.M., R.V.C., O.A.A., and A.J.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the nursing staff of the Indiana Clinical and Translational Sciences Institute Clinical Research Center for supporting the implementation of the study and assisting with data collection. We gratefully acknowledge Merck Sharp & Dohme for generously donating the Pregnyl used in this study.
This paper was presented in part at the 71st meeting of the American Society for Reproductive Medicine, Baltimore, MD, October 17–21, 2015, and the 98th meeting of the Endocrine Society, Boston, MA, April 1–4, 2016 (ClinicalTrials.gov NCT01489319; registered 9 December 2011).
REFERENCES
- 1.Aljada A, Ghanim H, Dandona P. Translocation of p47phox and activation of NADPH oxidase in mononuclear cells. Methods Mol Biol 196: 99–103, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Ayyadevara S, Bharill P, Dandapat A, Hu C, Khaidakov M, Mitra S, Shmookler Reis RJ, Mehta JL. Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid Redox Signal 18: 481–490, 2013. doi: 10.1089/ars.2011.4151. [DOI] [PubMed] [Google Scholar]
- 3.Banaszewska B, Wrotyńska-Barczyńska J, Spaczynski RZ, Pawelczyk L, Duleba AJ. Effects of resveratrol on polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab 101: 4322–4328, 2016. doi: 10.1210/jc.2016-1858. [DOI] [PubMed] [Google Scholar]
- 4.Barbieri RL, Makris A, Ryan KJ. Effects of insulin on steroidogenesis in cultured porcine ovarian theca. Fertil Steril 40: 237–241, 1983. doi: 10.1016/S0015-0282(16)47243-2. [DOI] [PubMed] [Google Scholar]
- 5.Basu R, Basu A, Johnson CM, Schwenk WF, Rizza RA. Insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production differ in nondiabetic humans and are abnormal in people with type 2 diabetes. Diabetes 53: 2042–2050, 2004. doi: 10.2337/diabetes.53.8.2042. [DOI] [PubMed] [Google Scholar]
- 6.Båvenholm PN, Pigon J, Ostenson CG, Efendic S. Insulin sensitivity of suppression of endogenous glucose production is the single most important determinant of glucose tolerance. Diabetes 50: 1449–1454, 2001. doi: 10.2337/diabetes.50.6.1449. [DOI] [PubMed] [Google Scholar]
- 7.Bell LN, Cai L, Johnstone BH, Traktuev DO, March KL, Considine RV. A central role for hepatocyte growth factor in adipose tissue angiogenesis. Am J Physiol Endocrinol Metab 294: E336–E344, 2008. doi: 10.1152/ajpendo.00272.2007. [DOI] [PubMed] [Google Scholar]
- 8.Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, Di Fede G, Rini G. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab 92: 2500–2505, 2007. doi: 10.1210/jc.2006-2725. [DOI] [PubMed] [Google Scholar]
- 9.Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol 165: 1013–1021, 2000. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 10.Ciampelli M, Leoni F, Cucinelli F, Mancuso S, Panunzi S, De Gaetano A, Lanzone A. Assessment of insulin sensitivity from measurements in the fasting state and during an oral glucose tolerance test in polycystic ovary syndrome and menopausal patients. J Clin Endocrinol Metab 90: 1398–1406, 2005. doi: 10.1210/jc.2004-0410. [DOI] [PubMed] [Google Scholar]
- 11.Cobelli C, Mari A, Ferrannini E. Non-steady state: error analysis of Steele’s model and developments for glucose kinetics. Am J Physiol Endocrinol Metab 252: E679–E689, 1987. doi: 10.1152/ajpendo.1987.252.5.E679. [DOI] [PubMed] [Google Scholar]
- 12.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 86: 3257–3265, 2001. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 237: E214–E223, 1979. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 14.Deopurkar R, Ghanim H, Friedman J, Abuaysheh S, Sia CL, Mohanty P, Viswanathan P, Chaudhuri A, Dandona P. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care 33: 991–997, 2010. doi: 10.2337/dc09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 83: 1454–1460, 2005. doi: 10.1016/j.fertnstert.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 16.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 33: 981–1030, 2012. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 38: 1165–1174, 1989. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 18.Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem 276: 47944–47949, 2001. doi: 10.1074/jbc.M104602200. [DOI] [PubMed] [Google Scholar]
- 19.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23: 599–622, 2002. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 20.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 97: 28–38.e25, 2012. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril 80: 123–127, 2003. doi: 10.1016/S0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- 22.Fleit HB Chronic inflammation In: Pathology of Human Disease – A Dynamic Encyclopedia of Disease Mechanisms, edited by McManus L, Mitchell RN. Cambridge, MA: Academic Press, 2014, p. 300–214. [Google Scholar]
- 23.Fox CW, Zhang L, Sohni A, Doblado M, Wilkinson MF, Chang RJ, Duleba AJ. Inflammatory stimuli trigger increased androgen production and shifts in gene expression in theca-interstitial cells. Endocrinology 160: 2946–2958, 2019. doi: 10.1210/en.2019-00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giugliano D, Sacca L, Scognamiglio G, Ungaro B, Torella R. Influence of acetylsalicylic acid on glucose turnover in normal man. Diabete Metab 8: 279–282, 1982. [PubMed] [Google Scholar]
- 25.Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, Shoelson SE. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci 1: 36–43, 2008. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, Shoelson SE; Targeting Inflammation Using Salsalate in Type 2 Diabetes Study Team . Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med 159: 1–12, 2013. doi: 10.7326/0003-4819-159-1-201307020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González F, Thusu K, Abdel-Rahman E, Prabhala A, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor α in normal-weight women with polycystic ovary syndrome. Metabolism 48: 437–441, 1999. doi: 10.1016/S0026-0495(99)90100-2. [DOI] [PubMed] [Google Scholar]
- 28.González F, Minium J, Rote NS, Kirwan JP. Hyperglycemia alters tumor necrosis factor-α release from mononuclear cells in women with polycystic ovary syndrome. J Clin Endocrinol Metab 90: 5336–5342, 2005. doi: 10.1210/jc.2005-0694. [DOI] [PubMed] [Google Scholar]
- 29.González F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab 91: 336–340, 2006. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- 30.González F, Rote NS, Minium J, Kirwan JP. Increased activation of nuclear factor kappaB triggers inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab 91: 1508–1512, 2006. doi: 10.1210/jc.2005-2327. [DOI] [PubMed] [Google Scholar]
- 31.González F, Rote NS, Minium J, O’Leary VB, Kirwan JP. Obese reproductive-age women exhibit a proatherogenic inflammatory response during hyperglycemia. Obesity (Silver Spring) 15: 2436–2444, 2007. doi: 10.1038/oby.2007.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.González F, Nair KS, Daniels JK, Basal E, Schimke JM. Hyperandrogenism sensitizes mononuclear cells to promote glucose-induced inflammation in lean reproductive-age women. Am J Physiol Endocrinol Metab 302: E297–E306, 2012. doi: 10.1152/ajpendo.00416.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.González F, Sia CL, Stanczyk FZ, Blair HE, Krupa ME. Hyperandrogenism exerts an anti-inflammatory effect in obese women with polycystic ovary syndrome. Endocrine 42: 726–735, 2012. doi: 10.1007/s12020-012-9728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.González F, Sia CL, Shepard MK, Rote NS, Minium J. Inflammation in response to glucose ingestion is independent of excess abdominal adiposity in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab 97: 4071–4079, 2012. doi: 10.1210/jc.2012-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González F, Sia CL, Shepard MK, Rote NS, Minium J. Hyperglycemia-induced oxidative stress is independent of excess abdominal adiposity in normal-weight women with polycystic ovary syndrome. Hum Reprod 27: 3560–3568, 2012. doi: 10.1093/humrep/des320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.González F, Kirwan JP, Rote NS, Minium J. Glucose ingestion stimulates atherothrombotic inflammation in polycystic ovary syndrome. Am J Physiol Endocrinol Metab 304: E375–E383, 2013. doi: 10.1152/ajpendo.00491.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.González F, Sia CL, Shepard MK, Rote NS, Minium J. The altered mononuclear cell-derived cytokine response to glucose ingestion is not regulated by excess adiposity in polycystic ovary syndrome. J Clin Endocrinol Metab 99: E2244–E2251, 2014. doi: 10.1210/jc.2014-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.González F, Considine RV, Abdelhadi OA, Acton AJ. Saturated fat ingestion promotes lipopolysaccharide-mediated inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab 104: 934–946, 2019. doi: 10.1210/jc.2018-01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.González F, Considine RV, Abdelhadi OA, Acton AJ. Oxidative stress in response to saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab 104: 5360–5371, 2019. doi: 10.1210/jc.2019-00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González F, Considine RV, Abdelhadi OA, Acton AJ. Inflammation triggered by saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab 105: e2152–e2167, 2020. doi: 10.1210/clinem/dgaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henry RR, Mudaliar S, Ciaraldi TP, Armstrong DA, Burke P, Pettus J, Garhyan P, Choi SL, Jacober SJ, Knadler MP, Lam EC, Prince MJ, Bose N, Porksen N, Sinha VP, Linnebjerg H. Basal insulin peglispro demonstrates preferential hepatic versus peripheral action relative to insulin glargine in healthy subjects. Diabetes Care 37: 2609–2615, 2014. doi: 10.2337/dc14-0210. [DOI] [PubMed] [Google Scholar]
- 42.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest 109: 1321–1326, 2002. doi: 10.1172/JCI0214955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SH, Liu A, Ariel D, Abbasi F, Lamendola C, Grove K, Tomasso V, Ochoa H, Reaven G. Effect of salsalate on insulin action, secretion, and clearance in nondiabetic, insulin-resistant individuals: a randomized, placebo-controlled study. Diabetes Care 37: 1944–1950, 2014. doi: 10.2337/dc13-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koivunen RM, Morin-Papunen LC, Ruokonen A, Tapanainen JS, Martikainen HK. Ovarian steroidogenic response to human chorionic gonadotrophin in obese women with polycystic ovary syndrome: effect of metformin. Hum Reprod 16: 2546–2551, 2001. doi: 10.1093/humrep/16.12.2546. [DOI] [PubMed] [Google Scholar]
- 45.Legro RS, Schlaff WD, Diamond MP, Coutifaris C, Casson PR, Brzyski RG, Christman GM, Trussell JC, Krawetz SA, Snyder PJ, Ohl D, Carson SA, Steinkampf MP, Carr BR, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Myers ER, Santoro N, Eisenberg E, Zhang M, Zhang H; Reproductive Medicine Network . Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab 95: 5305–5313, 2010. doi: 10.1210/jc.2010-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mather KJ, Paradisi G, Leaming R, Hook G, Steinberg HO, Fineberg N, Hanley R, Baron AD. Role of amylin in insulin secretion and action in humans: antagonist studies across the spectrum of insulin sensitivity. Diabetes Metab Res Rev 18: 118–126, 2002. doi: 10.1002/dmrr.263. [DOI] [PubMed] [Google Scholar]
- 47.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 48.Mielants H, Veys EM, Verbruggen G, Schelstraete K. Comparison of serum salicylate levels and gastro-intestinal blood loss between salsalate (Disalcid)and other forms of salicylates. Scand J Rheumatol 10: 169–173, 1981. doi: 10.3109/03009748109095292. [DOI] [PubMed] [Google Scholar]
- 49.Nelson VL, Legro RS, Strauss JF III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol 13: 946–957, 1999. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 50.Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, Clore JN, Blackard WG. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 72: 83–89, 1991. doi: 10.1210/jcem-72-1-83. [DOI] [PubMed] [Google Scholar]
- 51.Pasquali R, Gambineri A, Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG 113: 1148–1159, 2006. doi: 10.1111/j.1471-0528.2006.00990.x. [DOI] [PubMed] [Google Scholar]
- 52.Piotrowski PC, Rzepczynska IJ, Kwintkiewicz J, Duleba AJ. Oxidative stress induces expression of CYP11A, CYP17, STAR and 3βHSD in rat theca-interstitial cells. J Soc Gynecol Investig 12, Suppl: 319A, 2005. [Google Scholar]
- 53.Reid J, MacDougall AI, Andrews MM. Aspirin and diabetes mellitus. BMJ 2: 1071–1074, 1957. doi: 10.1136/bmj.2.5053.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roth S, Bennett R, Caldron P, Hartman R, Mitchell C, Doucette M, Ekholm B, Goldlust B, Lee E, Wilson R. Reduced risk of NSAID gastropathy (GI mucosal toxicity) with nonacetylated salicylate (salsalate): an endoscopic study. Semin Arthritis Rheum 19, Suppl 2: 11–19, 1990. [PubMed] [Google Scholar]
- 55.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 277: 42394–42398, 2002. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 56.Spaczynski RZ, Arici A, Duleba AJ. Tumor necrosis factor-alpha stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod 61: 993–998, 1999. doi: 10.1095/biolreprod61.4.993. [DOI] [PubMed] [Google Scholar]
- 57.Sweeney JD, Hoernig LA. Hemostatic effects of salsalate in normal subjects and patients with hemophilia A. Thromb Res 61: 23–27, 1991. doi: 10.1016/0049-3848(91)90165-S. [DOI] [PubMed] [Google Scholar]
- 58.Thornton K, Asemota O, Jindal S, Charron M, Buyuk E. High fat diet and aging are associated with macrophage infiltration in mice ovaries. Fertil Steril 104, Suppl: e104–e105, 2015. doi: 10.1016/j.fertnstert.2015.07.322. [DOI] [Google Scholar]
- 59.Xiao C, Giacca A, Carpentier A, Lewis GF. Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia 49: 1371–1379, 2006. doi: 10.1007/s00125-006-0211-x. [DOI] [PubMed] [Google Scholar]
- 60.Yeh ST Using a trapezoidal rule for the area under a curve calculation – SAS advanced tutorial (Abstract 229) Proceedings of the 27th Annual Conference of SAS Users Group International, Orlando, FL, 2002. [Google Scholar]
- 61.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(κ)B kinase-β. Nature 396: 77–80, 1998. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 62.Zhang LH, Rodriguez H, Ohno S, Miller WL. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci USA 92: 10619–10623, 1995. doi: 10.1073/pnas.92.23.10619. [DOI] [PMC free article] [PubMed] [Google Scholar]