Abstract
HIV cure is thwarted by the presence of quiescent yet replication competent HIV-1 (HIV). Antiretroviral therapy (ART) is unable to eradicate reservoirs, and upon cessation of ART, HIV will rebound. This review encompasses the curative strategies of HIV in the context of NF-κB sub-pathways that are currently exploited and demonstrate promise in the disruption of latent HIV. Canonical NF-κB signaling has long been established to drive HIV proviral expression while noncanonical NF-κB signaling, a novel and perhaps more desirable mechanism of latency reversal due to its unique characteristics, has recently been shown to also promote HIV expression from latency. Furthermore, we discuss the previously unrecognized upstream signaling of NF-κB as a new avenue for exploration of a functional cure of HIV.
Keywords: HIV latency, Canonical NF-κB, Noncanonical NF-κB, PEBP1, Raf1, HIV cure
1. Introduction
Antiretroviral therapy (ART), a first-line defense against human immunodeficiency virus-1 (HIV) replication, has decreased HIV incidence and transmission. Roughly 38 million people worldwide are living with HIV. Despite the declining rate of HIV infection, only 25.4 million people had access to ART in 2019, which indicates limitations that must be addressed on a global scale [1,2]. This includes socioeconomic barriers from costly treatment, social stigma, pill fatigue, patient non-adherence, drug resistance and age-related co-morbidities along with potential drug interactions from treatment of underlying diseases [2]. When ART is interrupted, HIV rebounds which promotes transmission and progresses to AIDS [3], indicating that while ART is able to suppress viral replication, replication competent HIV remains in a persistent state, otherwise known as the latent HIV reservoir [4].
Latent HIV reservoirs are products of active CD4+ T cells that have transitioned to a state of quiescence after stable viral integration. The state of quiescence achieved by HIV is a result of manipulations to mechanisms of transcription [5]. Once the HIV-infected cells are in the resting state, there is minimal transcription whereas the infected cells persist as memory resting CD4+ T cells, leading to reservoirs with transcriptionally silent HIV provirus. Through quantitative viral outgrowth assay (QVOA), it was previously determined that the half-life of replication-competent HIV is ~3.6–3.7 years and would require consistent treatment for at least 60 years to purge infected cells while natural decay would take approximately 73 years [4,6]. However, this underestimates the size of the latent reservoir because the sensitivity of QVOA only reflects inducible intact proviruses [7,8]. One example is that some proviruses cannot be effectively reactivated unless there is a second round of stimulation [9]. It has been shown that defective proviruses produce viral proteins [10], indicating a need for improved characterization of the latent reservoir. Intact proviral DNA assay (IPDA) was designed to overcome the limitations of QVOA and PCR of total HIV DNA where IPDA maximally quantifies replication-competent proviruses by analysis of amplicons in env and packaging signal regions [11]. Peluso and colleagues used IPDA to determine that the half-life of HIV reservoir was 4.0 years from initiation of ART to year 7, and jumps to 18.7 years after year 7 where intact proviruses decay at a faster rate than defective proviruses [12]. In addition, it has been shown that cells harboring defective virus can be recognized by HIV-1-specific cytotoxic T lymphocytes while cells harboring replication-competent HIV seem resistant to CD8+ T cells that may need to be addressed to cure infection [13], [14], [15]. These issues raise challenges for the eradication of HIV reservoirs.
2. To “shock and kill” or “block and lock”
Two central therapeutic approaches, i.e. “block and lock” and “shock and kill”, have been proposed for a cure of HIV. The “block and lock” strategy aims to suppress HIV transcriptional machinery to induce a deep silent state, followed by anticipated epigenetic modifications of HIV promoter for induction of a permanently silent transcriptional state so that viral rebound cannot occur or is significantly delayed if ART is ceased [16,5,17]. It is not known whether deep latency can be achieved and whether provirus integration sites impact the induction of deep latency as interestingly observed in the elite controllers [18]. In contrast, the “shock and kill” strategy utilizes latency reversal agents (LRAs) to reactivate latent HIV through host-dependent mechanisms where cell-induced apoptosis is then elicited by either immune-mediated clearance or by viral-mediated cytopathic effect [19,20].
Among these LRAs, NF-κB stimulators such as PKC agonists (PKCa) have been efficacious in latency reversal both in vitro and ex vivo [21], [22], [23]. Some recent in vivo studies demonstrated that the use of PKCa, such as ingenol mebutate (PEP005), Kansui and SMAC mimetics, may be a suitable clinical approach by targeting NF-κB signaling pathway [22,[24], [25], [26]] (Fig. 1b-c). PKCa displayed activity of latency reversal to a broad range of memory CD4+ T cell subsets compared with other LRAs [27]. These important and perhaps surprising findings urge us to look further into NF-κB signaling. Although NF-κB pathway has been extensively investigated in the regulation of HIV transcription and latency, a revisit of this molecular signaling pathway may be timely in our current efforts for a cure of HIV.
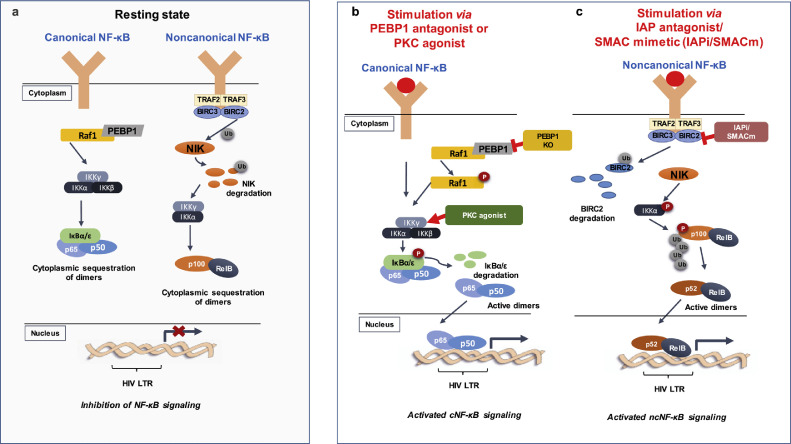
Fig. 1.
NF-κB signaling pathway is involved in the transcription of HIV which can be exploited for HIV cure studies. A. Canonical and noncanonical NF-κB subpathways at their resting states. B. Canonical NF-κB activation after PEBP1 knockdown (KO) leads to phosphorylation of Raf1 or PKC agonist acts on IKKγ to enable IκBα/ε degradation, leading to the activation of HIV transcription or latency reversal. C. Noncanonical NF-κB signaling is activated by IAPi/SMACm such as birinapant or AZD5582 via p100 cleavage into p52 for subsequent HIV transcription or latency reversal.
3. Molecular mechanism of HIV transcription and latency
Many gene components are essential for HIV replication in host immune cells, including its long terminal repeat (LTR) located at the 5′ end. After HIV cDNA integrates into the host genome in CD4+ T cells, some of these cells remain in a quiescent state, leading to the establishment of latent reservoirs unless reactivated [28]. At LTR where HIV transcription is initiated, there are binding sites of many transcription factors such as NF-κB, nuclear factor of activated T cells (NFAT), and Sp1 on the U3 region [29]. The expression and recruitment of these transcription factors into HIV LTR are essential for the initiation of HIV transcription while HIV Tat, when in the super elongation complex, is critical for efficient transcriptional elongation of HIV [5]. P-TEFb, an enzymatic complex comprised of CyclinT1 and CDK9, regulates Tat transactivation for transcriptional elongation of HIV mRNA [30]. Impaired expression or mis-translocation of these HIV transcription factors is associated with HIV latency [31,32].
HIV transcription is also controlled at the chromatin level. Histone acetylation and methylation are two established posttranslational modifications (PTMs) of the epigenetic landscape, which target histone tails of HIV LTR. Histone methylation or deacetylation at HIV LTR is related to the establishment and/or maintenance of HIV latency, which enforces a closed chromatin state to silence proviral HIV [33]. Histone deacetylase (HDAC) [34,35] and histone methyltransferase inhibitors [36,37] have been exhibited to reactivate latent HIV. Recently, histone lysine crotonylation (Kcr) was identified as an evolutionarily conserved PTM among several eukaryotic species [38]. Tan and colleagues identified and validated the presence of Kcr on several of the core histones where enrichment of Kcr was observed at sites of active promoters and potential enhancers [38] This has been linked to the regulation of HIV transcription where ACSS2 provides crotonyl-CoA to histones at the HIV LTR for PTM writers of histone crotonylation, such as p300 [39]. When histone decrotonylation occurs at the HIV LTR through ACSS2 suppression, HIV tends quiescence [39].
4. NF-κB signaling sub-pathways: Canonical, noncanonical and atypical
NF-κB, a family of evolutionarily conserved transcription factors, is a master regulator of the inflammatory and immune responses. In response to foreign stimuli, NF-κB recruits adaptive and innate immune cells such as macrophages and neutrophils to the infection site and triggers inflammation [40], which can be resolved thereafter. For example, the activation of NF-κB provokes antiviral transcription of interferon and interferon-stimulating genes, which provides protection against bacterial and viral pathogens [28]. However, dysregulated inflammatory responses can also contribute acute or chronic inflammatory diseases, inducing tissue damage. In addition, NF-κB promotes immune cell proliferation, differentiation, and inhibition of apoptosis by proapoptotic or antiapoptotic gene expression [40].
There are five constituents in the NF-κB family: NF-κB 1 (p50), NF-κB 2 (p52), p65 (RelA), RelB, and c-Rel [40]. They are structurally similar in that the members have a Rel homology domain (RHD), a domain responsible for the binding of DNA, dimerization and inhibitor of κB (IκB) interaction [41]. These five constituents are sequestered in the cytoplasm by upstream inhibitory proteins of the IκB family by which NF-κB activity is regulated [40]. Vital proteins of the IκB family contains an ankyrin repeat domain, a repeating structural motif made of roughly 33 amino acids, which facilitates the IκB interaction with dimers for the activation of the signaling pathways [31].
Two NF-κB pathways exist: canonical and noncanonical NF-κB signaling, which generally cooperate with each other. However, distinct features, such as required stimuli, protein synthesis and other functions, distinguish between the canonical and noncanonical signaling pathways [42]. In canonical NF-κB (cNF-κB) signaling, once phosphorylation of IκB kinase occurs, ubiquitination and proteasomal degradation of IκBα releases p65/p50 from the cytoplasm for nuclear translocation, leading to p65/p50 binding to gene promoter for transcription [28]. Noncanonical NF-κB (ncNF-κB) signaling responds to a subset of tumor necrosis factor receptor (TNFR) superfamily members that activates NF-κB-inducing kinase (NIK), which further phosphorylates IKKα, leading to the phosphorylation of p100 followed by its cleavage into p52 and nuclear translocation of p52/RelB heterodimer to drive its target gene transcription [43]. Vpr, a vital gene for HIV virulence, is associated with NF-κB signaling by which Vpr hijacks both NF-κB pathways via IκB kinase (IKK)α/β enhancement for productive viral infection [44].
NF-κB exhibits crosstalk with other signaling molecules resulting in atypical signaling [41,45]. A well-known example is the positive upregulation of p100 in ncNF-κB during the induction of cNF-κB signaling [46]. Unlike canonical or noncanonical NF-κB signaling whose activation occurs in membrane-bound receptors, atypical NF-κB signaling is triggered by genotoxic stress (DNA damage) after the activation of nuclear factor κB essential modulator (NEMO or IKKγ). NEMO initially translocates into nucleus, where it is sumoylated (sumoylation) and subsequently ubiquitinated (ubiquitination) via PTM. These sequential PTMs are regulated by the ataxia telangiectasia mutated (ATM) checkpoint kinase. Then, unbiquitinated NEMO/ATM complex returns to the cytosol and phosphorylates IKKβ for the subsequent activation of atypical NF-κB [41,43,47]. This is achieved via the phosphorylation of IκB, followed by its protein degradation and RelA/p50 nuclear translocation to activate its target gene transcription.
5. Targeting canonical NF-κB in HIV cure through PKC agonists
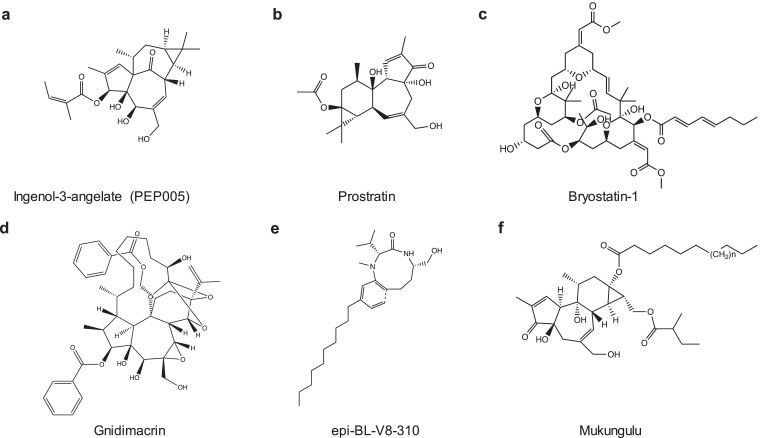
The cNF-κB pathway is well established in HIV transcription where the degradation of IκBα triggers the cNF-κB signaling cascade for translocation of p65/p50 to the nucleus, leading to the initiation of HIV transcription (Fig. 1b). In contrast, low expression of cNF-κB or impaired translocation of cNF-κB into nucleus is associated with HIV latency [31]. When IκBs were knocked down, latent HIV was reactivated [48,49], indicating that this pathway can be modulated to achieve latency disruption. By mimicking the physiologic ligand diacylglycerol, protein kinase C agonist binds to the regulatory domains of PKC isoforms, targets PKC/cNF-κB pathway to degrade IκBs, and activates HIV transcription from latency [31]. PKC agonist includes ingenol derivatives such as ingenol mebutate (PEP005) and ingenol B, phorbol esters such as phorbol 12-myristate 13-acetate (PMA), prostratin, bryostatin-1 and others [50] (Fig. 2 and Table 1), which have been tested in BLT humanized mice model and ART-suppressed HIV-positive individuals in vivo and shows some promise in executing the “shock and kill” strategy.
Fig. 2.
Pharmacological compounds for the activation of canonical NF-κB. Chemical structures of PKC agonists were shown. These PKC agonists have been tested in HIV cure studies. While latency reversal efficacy has been shown in vitro and ex vivo, in vivo evidence of latency disruption is lacking.
Table 1.
Targeting canonical and noncanonical NF-κB signaling pathways through pharmacological compounds.
| Pathway Target | Pharmacological compounds | Model system | References |
|---|---|---|---|
| Canonical NF-κB | Bryostatin-1 and analogs (bryologs) | A, B, E, F, H (NCT02269605) | DeChristopher et al., 2012; Gutierrez et al., 2016; Marsden et al., 2017; Marsden et al., 2018 |
Phorbol esters
|
B, D, E A, B, C, D, E |
Beans et al., 2013; Marsden et al., 2018 De la Torre-Tarazona et al., 2020 |
|
| Gnidimacrin | A, D | Huang Li et al., 2011; Lai W, et al., 2015 | |
| Ingenol-3-hexanoate (IngB) Ingenol-3-angelate (PEP005) Ingenol-3,20-dibenzoate (IDB) and other synthesized ingenol derivatives |
A, B, C, E A, C, E, H A, E |
José et al., 2014; Jiang et al., 2014 Jiang et al., 2015; Jiang et al., 2019 Spivak et al., 2015; Spivak AM et al., 2018 |
|
| Benzolactam derivatives | A, B, E | Matsuda et al., 2019 | |
| Mukungulu | A, H | Tietjen et al., 2018 | |
| Kansui and its derivatives | A, D, E, H (NCT02531295) | Cary, Fujinaga, and Peterlin, 2016; Lee et al., 2019; Yang et al., 2019 | |
| Noncanonical NF-κB |
SMAC mimetics (SMACm)
|
A, C, D, E, F E, F, G A, C C A, C A, C A, E |
Bobardt et al., 2019; Bobardt et al., 2020 Pache et al., 2015; Sampey et al., 2018; Nixon et al., 2020 Pache et al., 2015; Pache et al., 2015; Hattori et al., 2018; Campbell et al., 2018; Sampey et al., 2018 |
|
Experimental model system: A – cell line of HIV latency, in vitro B – PBMCs isolated from healthy blood donors, ex vivo C – Primary CD4+ T cells isolated from healthy blood donors, ex vivo D – PBMCs isolated from HIV-positive patients, ex vivo E – Primary CD4+ T cells isolated from HIV-positive patients, ex vivo F – BLT (bone marrow, liver, thymus) humanized mouse model, in vivo G – ART-suppressed SIV-infected rhesus macaques, in vivo H – ART-suppressed HIV-positive individuals, in vivo | |||
Prostratin is an active ingredient from plant derivatives such as Homalanthus nutans which was used by Samoan healers for non-HIV related conditions such as hepatitis. One issue with plant derivatives is the ability to quantify the active ingredient accurately as content varies between sections of the plant [51]. Wender and colleagues report a practical synthesis of prostratin, which can yield milligrams to grams of the active ingredient as a resolution [52]. Soon after, Beans and colleagues synthesized prostratin analogs that elicited latent HIV with 100-fold potency [53]. The practical synthesis proposed by Wender and colleagues provides insight into extending similar syntheses to other PKCa that are low yield such as bryostatin-1. Bryostatin-1 has been tested in a clinical trial. While it was safe in low dosage, it failed to reactivate latent HIV in vivo, indicating that higher doses may be needed [54,55]. To investigate this issue, Marsden and colleagues have generated bryologs, which have been successful in latency reversal in a J-Lat cell line model of HIV latency, CD4+ T cells isolated from ART-suppressed HIV-infected individuals, and BLT-humanized mice [56].
Ingenol mebutate, a compound derived from Euphorbia peplus sap, is a PKC agonist and is highly potent when used singly or combined with other known LRAs, such as JQ1, a small molecule of BET bromodomain inhibitor, the reactivation of latent HIV in vitro and ex vivo resulted in a synergy compared to single treatment [57]. Jiang and colleagues conducted a study to examine cutaneous administration of PICATO, an FDA-approved gel, in vivo. PICATO utilizes ingenol mebutate (PEP005) as its active ingredient where short dosing of PICATO effectively clears actinic keratosis, a pre-malignant and common skin lesion often diagnosed in older HIV-infected individuals [58]. The administration of ingenol mebutate in ART-suppressed HIV-infected individuals cleared actinic keratosis and reversed transcriptional blocks of latent HIV without adverse effects [22]. Skin inflammation at administration site and site-associated mild pain were observed, however, actinic keratosis was resolved, and symptoms subsided [22]. Throughout treatment, a decrease in blocks to stages of HIV transcription was observed in both peripheral CD4+ T cells and skin biopsies. The reactivation of latent HIV by PICATO did not cause systemic immune activation, signifying that ingenol mebutate, the active ingredient, is able to reactivate latent HIV in vivo. However, many compounds of PKCa are toxic in vivo. To this end, Cao et al., recently formulated hybrid nanocarriers for ingenol mebutate to not only maintain the efficacy of ingenol mebutate in latency reversal but also reduce its side effect in vivo [59].
In addition, Euphorbia Kansui (EK), which contains many novel ingenol derivatives [29,60] is undergoing a safety trial in the form of a tea for the reactivation of latent HIV [61]. EK is a traditional Chinese medicine used for thousands of years as treatments for fluid retention and cancer. A plethora of individual compounds have been screened from EK extracts for latency reversal by which nanomolar concentrations of the compounds were able to induce HIV via PKC/cNF-κB signaling in vitro and ex vivo [60,62]. These studies may ensure an alternative direction to apply PKCa to the cure of HIV in future.
Recent studies started to uncover the preference of PKCa to different subset of CD4+ T cells for latency reversal. PKC agonists are effective in inducing NF-κB phosphorylation in the naïve (TN) and central memory CD4+ T cells (TCM) cells (TN>TCM>transitional memory CD4+ T cells> effecter memory CD4+ T cells (TEM)) while they prefer to activate P-TEFb in the TEM cells [63]. HDAC inhibitor (HDACi), such as panobinostat and romidepsin, had a limited effect on latency reversal. Instead, ingenol mebutate (PEP005) induced HIV transcription from latency in all tested samples [63]. Ingenol mebutate displayed activity to a broad range of memory CD4+ T cell subsets compared with bryostatin-1 which preferred to disrupt latent HIV in the TEM cells [27]. When in combination with HDAC inhibitor, ingenol mebutate significantly enhanced latency reversal in TCM cells [63]. Similarly, the combination of romidepsin and ingenol was able to increase HIV RNA+ cells in most of the CD4+ T cell subsets. Interestingly, only the combination of panobinostat and bryostatin-1 was able to significantly increase HIV RNA+ cells in the population of memory stem cells [64]. These studies indicate that the cellular HIV reservoir is heterogeneous and responds differentially to different classes of LRAs, and combination therapy is required in order to achieve optimal latency reversal.
6. Targeting canonical NF-κB in HIV cure through PEBP1/Raf1 signaling
Although much is known upon the downstream NF-κB signaling, less of the upstream NF-κB signaling has been investigated in the regulation of HIV transcription. We recently discovered that phosphatidylethanolamine binding protein 1 (PEBP1), a Raf1 kinase inhibitor protein (RKIP), is implicated in the induction of HIV latency through a genome-wide library screening [65] (Fig. 1b). Raf1 is involved in IKK regulation via the Ras/Raf/MEK/ERK signaling pathway where phosphorylation of Raf1 leads to IKK activation in the NF-κB signaling pathway, thus releasing the sequestration of p65 in the cytosol and allowing nuclear translocation [66,67]. PEBP1 knockout in C11 cell model of HIV latency demonstrated an enhanced MAPK/IKK activation and latency reversal, indicating that PEBP1 inhibits upstream phosphorylation of Raf1 kinase, which thwarts the activation of the NF-κB signaling cascade, thereby establishing HIV latency. Yang and colleagues’ working model proposes PEBP1 as a suppressor of HIV transcription where PEBP1 induction inactivates NF-κB signaling by dephosphorylation of Raf1 or by protein complex formation with Raf1 or IKK, thereby establishing HIV latency [65,68,69]. Interestingly, PEBP1 inducer, such as epigallocatechin-3-gallate (EGCG) or dihydroartemisinin (DHA), inhibited α-CD3/CD28 reversal of latent HIV in resting CD4+ T cells isolated from ART-suppressed HIV-infected individuals [65]. An interaction between PEBP1 and Nef has been reported, however, it is unclear whether this is related to PEBP1 induction of latency [70]. Considering that PEBP1 is an antagonist of NF-κB signaling to establish HIV latency, upstream NF-κB signaling may be of interest for curative strategies of HIV.
7. Understanding the importance of noncanonical NF-κB in HIV cure
Given that NF-κB is a master regulator of inflammatory and immune response, it is important to explore other approaches to prevent global T cell activation or hypercytokinemia that PKCa application may trigger, due to the broad specificity induced by PKCa. NcNF-κB signaling juxtaposes cNF-κB signaling by acting as a slow, persistent and stimulus-selective mechanism. Noncanonical NF-κB is favored for its stimulus-selective mechanism where fewer genes are induced compared to cNF-κB stimulated by PKCa. This reinforces the activation of the noncanonical signaling pathway to ensure minimization of off-target effects [25,42], unique from cNF-κB signaling which permits a broad inflammatory response through tumor necrosis factor receptors [43] (Fig. 1c).
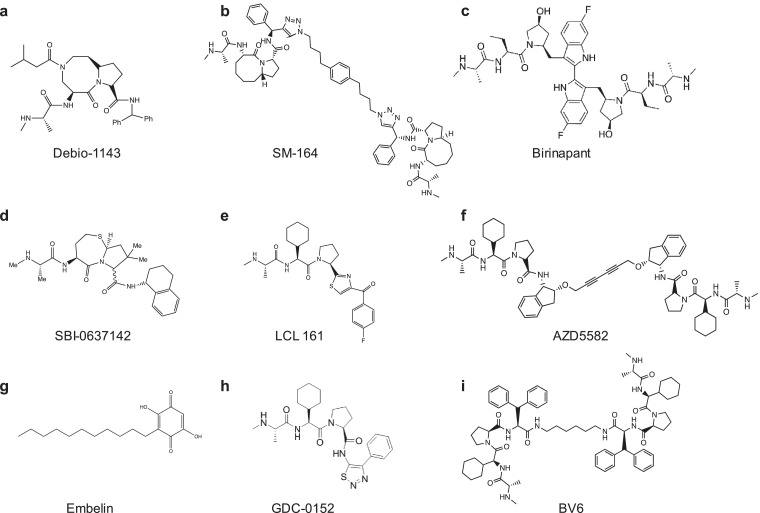
Pache et al. found that an ubiquitin ligase, Baculoviral IAP repeat-containing protein 2 (BIRC2)/cIAP1 (inhibitor of apoptosis protein-1), acts as a repressor of the ncNF-κB pathway to negatively regulate HIV transcription [42]. This study uncovered a family of IAP inhibitors (IAPi) or mimetics of the second mitochondrial-derived activator of caspases (SMACm) as a new class of LRAs. IAPi/SMACm modulates tumor activity and is known to induce ncNF-κB through cIAP1/cIAP2 degradation [71]. Similarly, Bobardt and colleagues found that another SMACm, Debio 1143, (Fig. 3) reverses latent HIV through the degradation of BIRC2/cIAP1 to permit cleavage of p100 into p52 for activation of ncNF-κB signaling in latently infected cell models of HIV latency, in ART-suppressed BLT humanized mice and ART-suppressed patient resting CD4+ T cells [24] (Table 1). When a proteasome inhibitor MG132 was included during Debio 1143 treatment, latency reversal was inhibited [24]. Inhibition of BIRC2 ultimately allows the degradation of a complex comprised of BIRC2, BIRC3, TRAF2 and TRAF3, protecting NIK and activating ncNF-κB. Interestingly, there was no observed increase in cytokine induction when Debio 1143 was administered in nanomolar concentrations to PBMCs and BLT humanized mice, highlighting its desirable potency as a potential LRA. The efficacy of IAPi is currently in clinical trials with cancer patients, which will provide future information about its safety in vivo [72,73]. IAPi exhibited synergism with HDACis such as vorinostat in vitro, which favors the use of IAPi in conjugation with other LRAs to exploit diverse mechanisms for elimination of latent HIV reservoirs. The structure-activity relationship analysis indicates that bivalent molecule of IAPi is more potent than monovalent compound where its potency may be enhanced by the two binding motifs of BIR domains in cIAP protein [74] (Fig. 3).
Fig. 3.
Pharmacological compounds for the activation of noncanonical NF-κB. Chemical structures of SMACm were shown. Many monovalents and divalents of SMACm have been tested in HIV cure studies, which displayed potency and safety in vitro, ex vivo and in vivo.
Another IAPi/SMACm that has garnered interest as a tool to disrupt latency via ncNF-κB signaling is AZD5582. In vitro, AZD5582 degrades BIRC2/cIAP1 for activation of NF-κB, similar to other IAPi such as birinapant and Debio 1143. Further, Nixon and colleagues revealed AZD5582 therapy in the ART-suppressed SIV-infected animal model of AIDS resulted in the induction of SIV RNA from latency in lymph node tissues of rhesus macaques. Remarkably, latent HIV in the resting CD4+ T cells was reactivated in the ART-suppressed HIV-infected BLT humanized mouse model, signifying the presence of latency reversal as a result of AZD5582. Additionally, the safety of AZD5582 was tested where both models showed that AZD5582 was efficacious and did not increase cytokine production. The ART-suppressed SIV-infected rhesus macaques tolerated AZD5582 intravenous injections over an 8-week period where 2 of the 12 rhesus macaques exhibited adverse reactions. In ART-suppressed HIV-infected mice, levels of alanine aminotransferase and aspartate aminotransferase were mildly elevated. Together, these studies support that IAPi/SMACm could be further developed to directly activate ncNF-κB signaling as a new tool to disrupt HIV latency [25].
8. Outstanding questions
NF-κB is a desirable target due to its biological function as a regulator of HIV transcription and other signaling pathways [40]. However, concerns of toxicity using PKCa to disrupt latency remains. Discovery of effective and safe PKCa is still urgently needed and an ongoing safety trial of Euphorbia Kansui may provide us some guidelines of use. PEBP1, an inhibitor of Raf1 kinase in the upstream cNF-κB signaling, is a new inducer of HIV latency, which could be developed for cure of HIV [65].
Noncanonical NF-κB is implicated in the establishment of HIV latency, however, the underlying molecular mechanisms of latency and latency disruption are not fully understood. However, it may be a more favorable pathway as it is a slow and persistent process that does not lead to global T cell activation. Tolerability of IAPi for the induction of ncNF-κB in both ART-suppressed HIV-infected BLT humanized mice and SIV-infected rhesus macaques provides some insight for curative HIV strategies in vivo [25]. However, a recent study applying AZD5582 in the SHIV model of HIV latency failed to induce latency reversal nor reservoir reduction in combination with clearance tool of bispecific HIVxCD3 DART molecules [75], indicating that latency reversal by IAPi alone may be not potent enough to present the viral antigens for immune clearance and our understanding of underlying in vivo mechanism of latency reversal is not complete.
An optimal combination strategy with other LRAs may lead to a more robust latency disruption than single LRA. It is quite possible that optimization of current IAPi is needed in order to find potent and safe IAPi combination strategy in the disruption of latency in patients. In addition, how ncNF-κB signaling is regulated in HIV transcription and latency is not clear. P100 cleavage into p52 is one of the essential steps in activation of ncNF-κB signaling. Many proteins, such as NIK/IKK and β-TrCP ubiquitination complex, are involved in this cleavage process, indicating that fine-tuning this step is essential for its biological functions. Lastly, the induction of apoptosis is a suitable aim as programmed cell death prevents viruses from propagation, thereby eradicating HIV reservoir. It has been reported that the induction of ncNF-κB signaling causes cell death in memory CD4+ T cells where latent HIV is harbored [76]. Whether this is specific for cells with reactivated HIV is a very interesting question, which is intriguing for the field. Therefore, much work remains in the understanding of NF-κB signaling subpathways and developing effective and safe IAPi for latency reversal.
Search strategy and selection criteria
Data search and selection occurred through searches from PubMed with the following search terms: “HIV reservoir”, “HIV latency”, “HIV deep latency”, “shock and kill”, “block and lock”, “canonical NF-κB” and “noncanonical NF-κB”. Selection of articles particularly emphasized articles between 2015 and 2020 with several distinguished papers as early as the 1990s.
Contributors
GJ generated the initial concepts of this review article. LMW and GJ wrote the article and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare no conflicts of interests.
Acknowledgments
We apologize for not being able to cite all the excellent studies and reviews due to space limitations. GJ is supported by Qura Therapeutics funding, University of North Carolina at Chapel Hill Center for AIDS Research (P30AI50410), and NIAID/CARE (1UM1AI126619). The funders have no roles in experimental design, data collection and analysis, interpretation of the data, or writing of this paper.
References
- 1.UNAIDS. Fact sheet - latest global and regional statistics on the status of the AIDS epidemic. 2020.
- 2.Siefried K.J., Kerr S., Richardson R. Socioeconomic and psychosocial factors are associated with poor treatment outcomes in Australian adults living with HIV: a case-control study. Sex Health. 2019;16(6):548–553. doi: 10.1071/SH18138. [DOI] [PubMed] [Google Scholar]
- 3.Sneller M.C., Huiting E.D., Clarridge K.E. Kinetics of plasma HIV rebound in the era of modern antiretroviral therapy. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finzi D., Blankson J., Siliciano J.D. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5(5):512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 5.Elsheikh M.M., Tang Y., Li D., Jiang G. Deep latency: a new insight into a functional HIV cure. EBioMedicine. 2019;45:624–629. doi: 10.1016/j.ebiom.2019.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soriano-Sarabia N., Bateson R.E., Dahl N.P. Quantitation of replication-competent HIV-1 in populations of resting CD4+ T cells. J Virol. 2014;88(24):14070–14077. doi: 10.1128/JVI.01900-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton K.M., Palmer S.E. How to define the latent reservoir: tools of the trade. Curr HIV/AIDS Rep. 2016;13(2):77–84. doi: 10.1007/s11904-016-0304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho Y.C., Shan L., Hosmane N.N. Replication-competent non-induced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155(3):540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosmane N.N., Kwon K.J., Bruner K.M. Proliferation of latently infected CD4(+) T cells carrying replication-competent HIV-1: potential role in latent reservoir dynamics. J Exp Med. 2017;214(4):959–972. doi: 10.1084/jem.20170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imamichi H., Smith M., Adelsberger J.W. Defective HIV-1 proviruses produce viral proteins. Proc Natl Acad Sci U S A. 2020;117(7):3704–3710. doi: 10.1073/pnas.1917876117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruner K.M., Wang Z., Simonetti F.R. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature. 2019;566(7742):120–125. doi: 10.1038/s41586-019-0898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peluso M.J., Bacchetti P., Ritter K.D. Differential decay of intact and defective proviral DNA in HIV-1-infected individuals on suppressive antiretroviral therapy. JCI Insight. 2020;5(4) doi: 10.1172/jci.insight.132997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollack R.A., Jones R.B., Pertea M. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T lymphocytes, which shape the proviral landscape. Cell Host Microbe. 2017;21(4):494–506. doi: 10.1016/j.chom.2017.03.008. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S.H., Ren Y., Thomas A.S. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J Clin Invest. 2018;128(2):876–889. doi: 10.1172/JCI97555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y., Anderson J.L., Lewin S.R. Getting the "kill" into "shock and kill": strategies to eliminate latent HIV. Cell Host Microbe. 2018;23(1):14–26. doi: 10.1016/j.chom.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahlenstiel C.L., Symonds G., Kent S.J., Kelleher A.D. Block and lock HIV cure strategies to control the latent reservoir. Front Cell Infect Microbiol. 2020;10:424. doi: 10.3389/fcimb.2020.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mousseau G., Kessing C.F., Fromentin R., Trautmann L., Chomont N., Valente S.T. The tat inhibitor didehydro-cortistatin a prevents HIV-1 reactivation from latency. MBio. 2015;6(4):e00465. doi: 10.1128/mBio.00465-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang C., Lian X., Gao C. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature. 2020;585(7824):261–267. doi: 10.1038/s41586-020-2651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehrman G., Hogue I.B., Palmer S. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366(9485):549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abner E., Jordan A. HIV "shock and kill" therapy: in need of revision. Antiviral Res. 2019;166:19–34. doi: 10.1016/j.antiviral.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Brogdon J., Ziani W., Wang X., Veazey R.S., Xu H. In vitro effects of the small-molecule protein kinase C agonists on HIV latency reactivation. Sci Rep. 2016;6:39032. doi: 10.1038/srep39032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang G., Maverakis E., Cheng M.Y. Disruption of latent HIV in vivo during the clearance of actinic keratosis by ingenol mebutate. JCI Insight. 2019;4(7) doi: 10.1172/jci.insight.126027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vemula S.V., Maxwell J.W., Nefedov A. Identification of proximal biomarkers of PKC agonism and evaluation of their role in HIV reactivation. Antiviral Res. 2017;139:161–170. doi: 10.1016/j.antiviral.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Bobardt M., Kuo J., Chatterji U. The inhibitor apoptosis protein antagonist Debio 1143 Is an attractive HIV-1 latency reversal candidate. PLoS ONE. 2019;14(2) doi: 10.1371/journal.pone.0211746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nixon C.C., Mavigner M., Sampey G.C. Systemic HIV and SIV latency reversal via non-canonical NF-kappaB signalling in vivo. Nature. 2020;578(7793):160–165. doi: 10.1038/s41586-020-1951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prins J.M., Jurriaans S., van Praag R.M. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS. 1999;13(17):2405–2410. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- 27.Baxter A.E., Niessl J., Fromentin R. Single-cell characterization of viral translation-competent reservoirs in HIV-infected individuals. Cell Host Microbe. 2016;20(3):368–380. doi: 10.1016/j.chom.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng L., Zeng Q., Wang M. Suppression of NF-kappaB activity: a viral immune evasion mechanism. Viruses. 2018;10(8) doi: 10.3390/v10080409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cary D.C., Fujinaga K., Peterlin B.M. Molecular mechanisms of HIV latency. J Clin Invest. 2016;126(2):448–454. doi: 10.1172/JCI80565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Couturier J., Orozco A.F., Liu H. Regulation of cyclin T1 during HIV replication and latency establishment in human memory CD4 T cells. Virol J. 2019;16(1):22. doi: 10.1186/s12985-019-1128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang G., Dandekar S. Targeting NF-kappaB signaling with protein kinase C agonists as an emerging strategy for combating HIV latency. AIDS Res Hum Retrovirus. 2015;31(1):4–12. doi: 10.1089/aid.2014.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karn J. The molecular biology of HIV latency: breaking and restoring the Tat-dependent transcriptional circuit. Curr Opin HIV AIDS. 2011;6(1):4–11. doi: 10.1097/COH.0b013e328340ffbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindqvist B., Svensson Akusjarvi S., Sonnerborg A., Dimitriou M., Svensson J.P. Chromatin maturation of the HIV-1 provirus in primary resting CD4+ T cells. PLoS Pathog. 2020;16(1) doi: 10.1371/journal.ppat.1008264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Archin N.M., Bateson R., Tripathy M.K. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J Infect Dis. 2014;210(5):728–735. doi: 10.1093/infdis/jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Archin N.M., Espeseth A., Parker D., Cheema M., Hazuda D., Margolis D.M. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009;25(2):207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouchat S., Gatot J.S., Kabeya K. Histone methyltransferase inhibitors induce HIV-1 recovery in resting CD4(+) T cells from HIV-1-infected HAART-treated patients. AIDS. 2012;26(12):1473–1482. doi: 10.1097/QAD.0b013e32835535f5. [DOI] [PubMed] [Google Scholar]
- 37.Imai K., Togami H., Okamoto T. Involvement of histone H3 lysine 9 (H3K9) methyltransferase G9a in the maintenance of HIV-1 latency and its reactivation by BIX01294. J Biol Chem. 2010;285(22):16538–16545. doi: 10.1074/jbc.M110.103531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan M.J., Luo H., Lee S. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146(6):1015–1027. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang G., Nguyen D., Archin N.M. HIV latency is reversed by ACSS2-driven histone crotonylation. J Clin Invest. 2018;128(3):1190–1198. doi: 10.1172/JCI98071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu T., Zhang L., Joo D., Sun S.C. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoesel B., Schmid J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pache L., Dutra M.S., Spivak A.M. BIRC2/cIAP1 is a negative regulator of HIV-1 transcription and can be targeted by smac mimetics to promote reversal of viral latency. Cell Host Microbe. 2015;18(3):345–353. doi: 10.1016/j.chom.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun S.C. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011;21(1):71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu R., Tan J., Lin Y. HIV-1 Vpr activates both canonical and noncanonical NF-kappaB pathway by enhancing the phosphorylation of IKKalpha/beta. Virology. 2013;439(1):47–56. doi: 10.1016/j.virol.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee N., Houston T.J., Cardenas E., Ghosh R. To be an ally or an adversary in bladder cancer: the NF-kappaB story has not unfolded. Carcinogenesis. 2015;36(3):299–306. doi: 10.1093/carcin/bgu321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dejardin E., Droin N.M., Delhase M. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17(4):525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 47.Huang T.T., Wuerzberger-Davis S.M., Wu Z.H., Miyamoto S. Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell. 2003;115(5):565–576. doi: 10.1016/s0092-8674(03)00895-x. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez G., Zaikos T.D., Khan S.Z., Jacobi A.M., Behlke M.A., Zeichner S.L. Targeting IkappaB proteins for HIV latency activation: the role of individual IkappaB and NF-kappaB proteins. J Virol. 2013;87(7):3966–3978. doi: 10.1128/JVI.03251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan S.Z., Gasperino S., Zeichner S.L. Nuclear transit and HIV LTR binding of NF-kappaB subunits held by IkappaB proteins: implications for HIV-1 activation. Viruses. 2019;11(12) doi: 10.3390/v11121162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spivak A.M., Planelles V. Novel latency reversal agents for HIV-1 cure. Annu Rev Med. 2018;69:421–436. doi: 10.1146/annurev-med-052716-031710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson H.E., Banack S.A., Cox P.A. Variability in content of the anti-AIDS drug candidate prostratin in Samoan populations of Homalanthus nutans. J Nat Prod. 2008;71(12):2041–2044. doi: 10.1021/np800295m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wender P.A., Kee J.M., Warrington J.M. Practical synthesis of prostratin, DPP, and their analogs, adjuvant leads against latent HIV. Science. 2008;320(5876):649–652. doi: 10.1126/science.1154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beans E.J., Fournogerakis D., Gauntlett C. Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc Natl Acad Sci U S A. 2013;110(29):11698–11703. doi: 10.1073/pnas.1302634110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gutierrez C., Serrano-Villar S., Madrid-Elena N. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS. 2016;30(9):1385–1392. doi: 10.1097/QAD.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 55.Bryostatin-1 effect on HIV-1 latency and reservoir in HIV-1 infected patients receiving antiretroviral treatment. 2014. https://ClinicalTrials.gov/show/NCT02269605.
- 56.Marsden M.D., Loy B.A., Wu X. In vivo activation of latent HIV with a synthetic bryostatin analog effects both latent cell “kick” and “kill” in strategy for virus eradication. PLoS Pathog. 2017;13(9) doi: 10.1371/journal.ppat.1006575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang G., Mendes E.A., Kaiser P. Synergistic reactivation of latent HIV expression by ingenol-3-angelate, PEP005, targeted NF-kB signaling in combination with JQ1 induced p-TEFb activation. PLoS Pathog. 2015;11(7) doi: 10.1371/journal.ppat.1005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fidler B., Goldberg T. Ingenol mebutate gel (picato): a novel agent for the treatment of actinic keratoses. P T. 2014;39(1):40–46. [PMC free article] [PubMed] [Google Scholar]
- 59.Cao S., Slack S.D., Levy C.N. Hybrid nanocarriers incorporating mechanistically distinct drugs for lymphatic CD4(+) T cell activation and HIV-1 latency reversal. Sci Adv. 2019;5(3):eaav6322. doi: 10.1126/sciadv.aav6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang H., Li X., Yang X. Dual effects of the novel ingenol derivatives on the acute and latent HIV-1 infections. Antiviral Res. 2019;169 doi: 10.1016/j.antiviral.2019.104555. [DOI] [PubMed] [Google Scholar]
- 61.Lee S., Kesmy K., Bacchetti P. Kansui, an ingenol-containing herbal supplement, safely induced CD8, NK, and monocyte activation in three ART-suppressed SIVmac251-infected rhesus macaques. J Virus Erad. 2019;5:12–13. [Google Scholar]
- 62.Wang P., Lu P., Qu X. Reactivation of HIV-1 from latency by an ingenol derivative from euphorbia kansui. Sci Rep. 2017;7(1):9451. doi: 10.1038/s41598-017-07157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pardons M., Fromentin R., Pagliuzza A., Routy J.P., Chomont N. Latency-reversing agents induce differential responses in distinct memory CD4 T cell subsets in individuals on antiretroviral therapy. Cell Rep. 2019;29(9) doi: 10.1016/j.celrep.2019.10.101. 2783-95 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grau-Exposito J., Luque-Ballesteros L., Navarro J. Latency reversal agents affect differently the latent reservoir present in distinct CD4+ T subpopulations. PLoS Pathog. 2019;15(8) doi: 10.1371/journal.ppat.1007991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang X., Wang Y., Lu P. PEBP1 suppresses HIV transcription and induces latency by inactivating MAPK/NF-kappaB signaling. EMBO Rep. 2020:e49305. doi: 10.15252/embr.201949305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang F., Steelman L.S., Shelton J.G. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review) Int J Oncol. 2003;22(3):469–480. [PubMed] [Google Scholar]
- 67.Tsolou A., Liousia M., Kalamida D., Pouliliou S., Giatromanolaki A., Koukourakis M. Inhibition of IKK-NFkappaB pathway sensitizes lung cancer cell lines to radiation. Cancer Biol Med. 2017;14(3):293–301. doi: 10.20892/j.issn.2095-3941.2017.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Granovsky A.E., Clark M.C., McElheny D. Raf kinase inhibitory protein function is regulated via a flexible pocket and novel phosphorylation-dependent mechanism. Mol Cell Biol. 2009;29(5):1306–1320. doi: 10.1128/MCB.01271-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yeung K.C., Rose D.W., Dhillon A.S. Raf kinase inhibitor protein interacts with NF-kappaB-inducing kinase and TAK1 and inhibits NF-kappaB activation. Mol Cell Biol. 2001;21(21):7207–7217. doi: 10.1128/MCB.21.21.7207-7217.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kammula E.C., Motter J., Gorgels A., Jonas E., Hoffmann S., Willbold D. Brain transcriptome-wide screen for HIV-1 Nef protein interaction partners reveals various membrane-associated proteins. PLoS ONE. 2012;7(12):e51578. doi: 10.1371/journal.pone.0051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dueber E.C., Schoeffler A.J., Lingel A. Antagonists induce a conformational change in cIAP1 that promotes autoubiquitination. Science. 2011;334(6054):376–380. doi: 10.1126/science.1207862. [DOI] [PubMed] [Google Scholar]
- 72.A dose-finding study of the second mitochondrial activator of caspases (SMAC) Mimetic Debio 1143 when given in combination with avelumab to participants with advanced solid malignancies and to participants with advanced or metastatic non-small cell lung cancer (NSCLC) after platinum-based therapy. 2017. https://ClinicalTrials.gov/show/NCT03270176.
- 73.Debio 1143-201 Dose-finding and Efficacy Phase I/II Trial. 2013. https://ClinicalTrials.gov/show/NCT02022098.
- 74.Pache L., Marsden M.D., Teriete P. Pharmacological activation of non-canonical NF-κB signaling activates latent HIV-1 reservoirs in vivo. Cell Rep Med. 2020;1(3):1–11. doi: 10.1016/j.xcrm.2020.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dashti A., Waller C., Mavigner M. SMAC mimetic plus triple combination bispecific HIVxCD3 DART(R) molecules in SHIV.C.CH505-infected, ART-suppressed rhesus macaques. J Virol. 2020 doi: 10.1128/JVI.00793-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campbell G.R., Spector S.A. DIABLO/SMAC mimetics selectively kill HIV-1-infected resting memory CD4(+) T cells: a potential role in a cure strategy for HIV-1 infection. Autophagy. 2019;15(4):744–746. doi: 10.1080/15548627.2019.1569950. [DOI] [PMC free article] [PubMed] [Google Scholar]