Abstract
Drinking water is frequently recontaminated during transport and storage when water is poured into jerrycans. To address this issue, three strategies aiming at reducing these recontamination risks were implemented at water kiosks in Eastern Uganda. In all three strategies, water at the kiosks was chlorinated to a free residual chlorine (FRC) concentration of 2 mg/L at the tap of the kiosk. In addition, water was collected in different containers for drinking water transport: a) uncleaned jerrycans, b) cleaned jerrycans, and c) cleaned improved containers with a wide mouth and a spigot. Water quality in the containers was compared to that of a control group collecting unchlorinated water in uncleaned jerrycans. Water samples were collected at the tap of the kiosk, from the containers of 135 households after they were filled at the tap, and from the same containers in the households after 24 h of water storage. The samples were analysed for counts of E. coli, total coliforms, and FRC. Household interviews and structured observations were conducted to identify confounding variables and to assess the influence of water, sanitation, and hygiene infrastructure and practices on recontamination.
All three intervention strategies contributed to significantly lower E. coli recontamination levels after 24 h than in the control group (Median (Mdn) = 9 CFU/100 mL, Interquartile Range (IQR) = 25). Median E. coli counts and mean FRC consumption were higher in uncleaned jerrycans (Median = 1 CFU/100 mL, IQR = 6, ΔFRC = 1.8 mg/L) than in cleaned jerrycans (Median = 0 CFU/100 mL IQR = 2, ΔFRC = 1.6 mg/L) and the lowest in cleaned improved containers (Median = 0 CFU/100 mL, IQR = 0, ΔFRC = 1.2 mg/L). The FRC concentration at the tap of 2 mg/L was too low to protect water from E. coli recontamination in uncleaned jerrycans over 24 h. Cleaning the jerrycans was inconvenient due to their small openings, therefore, sand was used. The cleaning with sand reduced recontamination with E. coli but did not reduce the count of total coliforms. Improved containers with a larger opening allowed for cleaning with a brush and showed the lowest levels of recontamination for both E. coli and total coliforms. In addition to the intervention strategies, households receiving a higher number of WASH education visits within the previous year had lower recontamination levels of E. coli in stored water (OR = 0.54, p = 0.003).
Keywords: Maximum 6): Chlorination, Drinking water treatment, Water kiosks, Safe storage, Drinking water recontamination, Hygiene
Highlights
-
•
86% of initially clean water samples contained E. coli after 24 h storage in jerrycans in Uganda.
-
•
Chlorination and cleaning of water containers reduced recontamination of E. coli.
-
•
FRC of 2 mg/L at the water kiosk did not prevent recontamination in uncleaned jerrycans.
-
•
Sand used to clean commonly used narrow-neck containers increased total coliforms.
-
•
Containers with a larger opening and a spigot had lowest recontamination levels.
1. Introduction
Sustainable Development Goal (SDG) 6.1 of the United Nations (UN) calls for access to safe drinking water and sanitation for all by 2030. Achieving SDG 6 is a global human right and supports good health and gender equality (WHO, 2015). Nevertheless, progress is slow, and access to safe water for everyone has yet to be achieved. Globally, at least 2.3 billion people used drinking water services that were not safely managed in 2017 (WHO, 2019). Contaminated water can transmit diseases, such as diarrhoea, cholera, dysentery, typhoid, and polio, and is estimated to cause 485′000 diarrhoeal deaths each year (Prüss-Ustün et al., 2019). The establishment of water kiosks is a strategy for improving access to safe drinking water at community level in remote rural areas (Thompson et al., 2000; Mcgranahan et al., 2006; Opryszko et al., 2009; Sima and Elimelech, 2013). Various technologies have been installed in water kiosks that mostly deliver high-quality water at the tap (Huttinger et al., 2015; Peter, 2015; Patrick et al., 2017). However, keeping water safe until consumption has been a challenge (Opryszko et al., 2013).
In Eastern Uganda, the majority of people living along the shores of Lake Victoria use untreated water from the lake as their drinking water source. To address this issue, Eawag, the Swiss Federal Institute of Aquatic Science and Technology, in collaboration with an NGO, Africa Water Solutions, established five water kiosks with gravity driven ultrafiltration membrane (GDM) technology in the Busia and Namayingo districts. An analysis revealed that the GDM system produces high-quality treated water (Peter et al., 2016). However, a follow-up study to check the quality of water from the kiosks in households showed that the water is often recontaminated during transport and storage in households (Meierhofer et al., 2017).
Recontamination after collection is a frequently reported concern (Opryszko et al., 2013; Wright et al., 2004; Mellor et al., 2013). Previous research has identified hands, utensils for fetching water, and transport and storage canisters as the main sources of water deterioration (Trevett et al., 2005; Opryszko et al., 2013; Mellor et al., 2013).
An effective, cost-efficient, and locally available method for ensuring safe storage of drinking water even under poor hygienic conditions is the addition of chlorine to the water. Chlorine provides protection against bacteria and viruses as long as sufficient free residual chlorine (FRC) is present (Murphy et al., 2016). Correct dosage is crucial to ensuring safe drinking water during storage. The WHO recommends dosing to a concentration of 2 mg/L of FRC for non-turbid water at the point of delivery. In stored water, a minimum chlorine concentration of 0.2 mg/L should remain to prevent recontamination (WHO, 2017). Container material can influence chlorine degradation. Clay pots were found to consume more chlorine than plastic ones; more organic materials were observed on the inner surface, and these probably consumed chlorine (Murphy et al., 2009).
In addition to the material the container is made from, its design has an impact on water quality. Containers are often kept at ground level, making them easily accessible to children and animals, which could be a reason for water deterioration (Steele et al., 2008). Hence, it is important to cover containers with lids and to store them above floor level. Water often deteriorates during extraction due to contact with contaminated hands, cups, and ladles (Jagals et al., 2003). Hands can be prevented from entering by integrating a spout for extraction; this solution was evaluated in a refugee camp in Malawi (Roberts et al., 2001). Several studies have evaluated the use of containers with narrow openings that prevent hands from entering the canister and found better water quality than containers with larger openings (Mintz et al., 1995; Reed et al., 2011; Mellor et al., 2013). Levy et al. (2014) found higher contamination levels in containers with openings larger than 8 cm. Although narrow openings impede contact with possible contamination sources, they also hinder systematic cleaning with a brush. The regular use of drinking water containers without proper cleaning leads to the formation of a biofilm on the container’s inner walls. This biofilm harbours bacterial colonies, provides them with food for growth, and protects them from disinfection, thus increasing the risk of contaminating water that is poured into the containers (Harris et al., 2013; Budeli et al., 2018; Jagals et al., 2003; Mellor et al., 2013). Hence, thorough cleaning of the containers may be a method for eliminating this source of recontamination.
Methods applied to remove the biofilm from the walls of containers with a narrow opening include scouring with sand and stones and disinfection with chlorine (Steele et al., 2008). Scouring with sand and stones, however, entails the risk of scratching the walls of the container, which would offer a niche for bacteria to grow (Van Der Merwe et al., 2012), whereas disinfection with chlorine supports the prevention of recontamination when the container is filled. However, relying solely on disinfecting the containers with chlorine was found to be insufficient to protecting the water during transport and storage (Steele et al., 2008).
As mentioned above, elements of poor household hygiene, such as contaminated hands, utensils, and containers, play a major role in recontamination pathways. Therefore, household factors, such as water handling and hygiene conditions, may increase the risk of recontaminating drinking water during storage in the household. A study in Ghana found that increased education was not related to improved drinking water quality and attributed this to a lack of water, sanitation and hygiene (WASH) training (Opryszko et al., 2013). A study in India failed to find any statistically significant correlation between recontamination of drinking water in households and demographics, sanitation, or household practices of water handling and hygiene, but it detected a slight correlation between increased contamination and a higher number of household members with lower average ages (Eshcol et al., 2008). Another study in Kenya linked a higher frequency of users cleaning the storage container to a higher water quality (Meierhofer et al., 2019). However, the practice of transferring water from smaller transport containers to larger storage containers was found to add another source of contamination (Opryszko et al., 2013).
The goal of this study was to evaluate whether systematic cleaning of water containers prior to filling them and the use of improved containers with a wide mouth and a spigot in addition to chlorinating water at the point of distribution can further reduce the recontamination risk while storing the water over 24 h in households. The study also assessed the impact of container handling and hygiene conditions in households on water quality changes during storage.
2. Methods
2.1. Study design
The study was implemented using a quasi-experimental design with the inclusion of intervention randomization at three sites where community-scale water treatment systems using GDM filtration provide water to the local communities through water kiosks: Lugala, Bulundira, and Bumeru in Eastern Uganda. Due to the small number of only five water kiosks in the area, a random selection of sites was not possible. A matching approach was applied to select three sites and reduce the potential confounding due to non-randomization. Variables defining the socio-economic conditions, water handling and hygiene conditions in the control and intervention sites were assessed and variables with statistically significant differences between the sites identified. During analysis, these potential confounders were included in the multivariate regression model to control for a potential bias. Details of the sites and analysis of difference are presented in Table A of the supplementary materials.
For all three sites, the water is sourced from Lake Victoria and pumped through pipelines to the water treatment system in the community. The treated water is provided to the local schools and can be purchased by households living in the community. Customers collect the water purchased at the kiosk’s taps in their own containers for a price of 0.026 USD per 20 L container. Each of the kiosks is run by a local operator, who is supported by a water management committee (Peter-Varbanets et al., 2017).
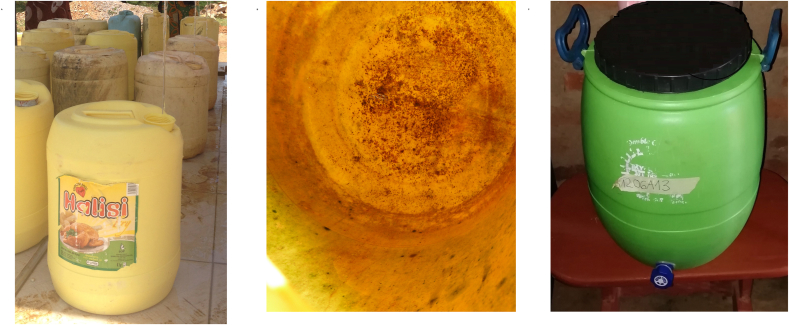
Three intervention strategies were implemented to reduce recontamination risks in stored drinking water. Water in the safe water tank of the kiosk was manually chlorinated each day under supervision of the research team with sodium hypochlorite to obtain a concentration of 2 mg/L of FRC at the tap at all sites except the control site. Different containers were used to collect and transport the drinking water at the intervention sites. Customers at the control site where water was not chlorinated used uncleaned common jerrycans. At site 1, customers used the same type of uncleaned jerrycans (strategy 1) while water was chlorinated at the water kiosk. Half of the customers at site 2 used cleaned jerrycans (strategy 2), and the other half used cleaned improved containers (strategy 3). At site 2 (strategy 2 and 3) all containers (jerrycans and improved containers) used by the customers were cleaned by the kiosk operator before filling them with water at the kiosk. The improved container had a larger opening to facilitate easy cleaning, a tap for drawing water during storage, and two handles for carrying (Fig. 1). The jerrycans were cleaned by introducing a handful of sand and some water into the jerrycans and shaking them for about 1 min. Afterwards, the sand was rinsed out with about 10 L of water. Then, the jerrycans were filled with 5 L of water mixed with liquid soap, shaken, and rinsed again with water. The improved containers were cleaned by inserting liquid soap and about 2 L of water and using a brush to remove the biofilm. Afterwards, they were rinsed with about 5 L of water. Chlorinated water from the water kiosk was used for all cleaning processes. There was no need to clean the improved containers with sand, because the larger opening allowed for a brush to enter and remove the biofilm from the inner walls. The jerrycans and improved containers were always washed by the same individuals to ensure homogenous washing practice. After cleaning, the improved containers were filled, and the people were instructed to release water only through the tap.
Fig. 1.
Commonly used jerrycan, inside of a jerrycan, and improved container.
The improved containers were purchased from the market in Kampala after a market survey for containers with a large mouth and spigot was done and were distributed to households a few months prior to the start of the study.
Improved containers were distributed to about half of the kiosk’s customers in site 2 after randomly selecting the names of recipients from a lottery box containing the names of all customers. Households at site 3, where water was not chlorinated at the kiosk tap, used uncleaned jerrycans and served as a control group.
The common jerrycans used in the study were reused vegetable oil canisters that are widely used in East Africa for the transport and storage of drinking water. Vegetable oil residuals in these containers might support bacterial growth and increase recontamination (Jagals et al., 2003). The jerrycans were of different ages and in different conditions. We were not able to quantify the age of the jerrycans used and some of the containers used could have been significantly older than the improved containers, which had been in use for five months. To reduce the bias of comparing very old jerrycans with newer containers, the containers were visibly inspected and heavily scratched, soiled and damaged jerrycans were excluded from the study.
Data were collected from November to December 2018 from a total of 135 households: 46 households using an uncleaned jerrycan at Site 1, 23 households using cleaned jerrycans and 23 households using cleaned improved containers at Site 2, and 43 households in the control group at Site 3. Nearly all customers of the water kiosks at the three sites were involved in the study. All customers that came to the kiosk for water collection during the days of data collection were invited to participate. The person mainly responsible for water handling in the household was informed about the goal, purpose, and methodology of the study and asked for informed consent. The study protocol was reviewed and approved by the Ethics Committee of Makerere University on July 18, 2018, the Uganda Nation Council for Science and Technology, and the Ethical Committee of Eawag, the Swiss Federal Institute of Aquatic Science and Technology on June 6, 2018.
During microbial water quality analysis, contamination levels with E. coli and total coliforms were assessed to evaluate the impact of the intervention strategies on recontamination. E. coli is commonly used as an indicator of faecal contamination, and total coliforms served as another indicator of recontamination (WHO, 2019; WHO, 2017). Water samples were taken daily during the days of data collection in the field from the kiosk tap, from the freshly filled containers of 135 participants, and from the same containers after 24 h of water storage in the households. Before samples were taken at the kiosk tap, water was left running for about 3 s. After the first sample was taken, the containers were marked and the participants’ name was documented. Then, the participants were accompanied to their homes and instructed to leave some water in the container for the household visit the next day. Twenty-four hours later, the same households were revisited, and samples were taken from the marked containers. Before the samples were taken, the containers were shaken well for around 10 s. Water samples of 100 mL were poured directly from the containers into sterile Nasco Whirl–pak Thio bags and stored on ice in cooler boxes for about 6 h prior to lab analysis. Samples were vacuum-filtered through 0.45 μm millipore cellulose membrane filters using sterilized filtration equipment and then placed on Nissui Pharmaceutical compact dry coli-scan plates. The dry plates were incubated at 35 +/− 2 °C for 24 h. Due to occasional electricity cuts, body incubation was also used: Instead of storing the dry plates in the incubator, they were kept in a bumbag close to the body. The temperature was monitored with a thermometer and was constantly kept at around 34–35 °C. After the incubation period, E. coli and total coliform colonies were counted visually up to 300 colony forming units (CFUs). A negative control using boiled water as field blank and a duplicate sample were analysed every 16th sample.
In all samples collected except in those from the control group, FRC concentrations were measured 30 min after collection with DPD Nr.1 tablets and a colorimeter from LaMotte or a pooltester (WHO, 2017).
During the second household visit after 24 h, structured, quantitative interviews were conducted with the person mainly responsible for water management to identify confounding variables and assess the influence of water, sanitation, and hygiene infrastructure and practice on recontamination. The questionnaire contained pretested questions, coded in ODK on tablets. Interviews were conducted in the local languages, Samia and Luganda, by two trained interviewers. The questionnaire comprised structured questions, most with categorical and Likert-scale answer categories, on household demographics, access to water, water handling practice, hygiene behaviour, and wealth indicators. In addition to questions, structured observations in the household gathered information on the condition and existence of storage and transport containers, hand-washing and sanitation facilities, and water treatment methods available in the household.
2.2. Data analysis
The data was analysed using IBM SPSS Version 25. Differences were calculated (i) between water quality at the tap and water quality after filling the container and (ii) between water quality at the tap and water quality after 24 h of storage to obtain information about the degree of recontamination. To improve visualization, recontamination was log-transformed, and for all zero recontamination values, 0.5 was added to allow logarithmic transformations. Descriptive statistics were calculated to assess central tendencies. Since the distribution of coliform counts was not normal, nonparametric Kruskal-Wallis and Mann-Whitney tests were conducted to assess differences between intervention strategies. A Bonferroni correction was applied, and the results are reported at a 0.0083 level of significance. To aid interpretation, the effect size of the Mann–Whitney test was calculated (Rosenthal, 1991).
The difference between the FRC concentration at filling and after 24 h of storage was calculated to obtain the FRC degradation. Then, t-tests were applied to compare the differences between FRC in uncleaned and cleaned jerrycans and between cleaned jerrycans and cleaned improved containers.
To investigate the impact of household factors on bacterial recontamination, univariate and multivariate logistic regression models were calculated. A dichotomous variable was formed by classifying counts of E. coli recontamination into uncontaminated (0 CFU/100 mL = 0) and contaminated (>0 CFU/100 mL = 1). The multivariate model (n = 135) was controlled for intervention assignment by forming three dummy variables: (a) water chlorinated at the kiosk (differentiating between the control site (=0) and sites that had received chlorination at the kiosk (=1) b) the use of a normal jerrycan cleaned at the kiosk (=1) versus a not cleaned jerrycan or an improved container (=0) and c) the use of a cleaned improved container (=1) versus a not cleaned jerrycan or a cleaned jerrycan (=0); water handling and hygiene factors that were significantly different between the intervention sites, factors known from literature to impact water quality during handling and factors that were significant at a p-value 0.2 during univariate analysis. The final model was then obtained using backward selection with the same level of 0.2. Associations were considered as statistically significant if p-values were <.05. The multivariate model included 11 predictors relying on Vittinghoff’s rule of thumb that the number of predictors in multiple logistic regression should not exceed a case number of 10 per predictor (Vittinghoff et al., 2005). Values of the Variance Inflating Factor (VIF) of all predictors were between 1.135 and 2.246 and Tolerance was between 0.445 and 0.881 confirming the validity of model assumptions for multi-collinearity. Chi-square and Spearman’s rho were used to assess correlations between predictors included in the model.
A wealth index, hand-washing index and toilet index were calculated using principal component analysis with orthogonal rotation (varimax). The wealth index was compiled in accordance with previously described procedures from educational level, money spent per week, and possessions available in the household: solar panel, radio, TV, phone, bicycle, motorbike, car, fridge, watch, fuel type, owning the house, number of rooms, wall, roof and floor type of the house (Rutstein, 2008; Krishnan, 2010). The hand-washing index included the presence of a hand–washing facility, the condition of the facility, and the availability of soap and water at the handwashing facility. The toilet index was formed on the basis of the toilet type and the cleanliness of the toilet in the household.
3. Results and discussion
3.1. Household demographics and water handling
Households across all study sites consisted on average of seven people (SD = 3), among them four children (SD = 3), of whom two were under the age of 5 (SD = 1). Seventy percent of the respondents reported having completed primary school, 16% had completed secondary school, and 14% were illiterate. The majority of respondents (96%) lived in their own house with an average of two rooms (SD = 1), and they worked in agriculture (92%). None of the respondents had an electricity connection in the home, but 55% used solar panels for light and charging their phones. The median expenditure per week and household was 15′000 UGX (∼4 USD; IQR = 20′000 UGX).
The average distance to the water kiosk was about 600 m (SD = 400 m), and the average time to collect water per day, including waiting time, was 130 min per person (SD = 113 min). Thirty-three percent of the respondents stated that they used other sources in addition to the water kiosk, with the lake being the main additional source (72%). The customers of the kiosk ranked the water quality at the kiosk as safe (77%) and perceived the taste of the water to be good (67%). This was reflected in the low number of additional household water treatment products in the households (11%) and shows that people are not aware of the risks of recontamination. The most commonly used method for household water treatment was filtration with a cloth (4.4%). The respondents reported cleaning their water transport container every second to third day on average. The majority used soap (63%) and a sponge (63%). Fifty-six percent stated that they used different containers for transport and storage. The use of an additional container for water storage bears the risk of further recontamination, as Harris et al. (2013) documented. The most common storage containers in use were clay pots. This study did not investigate the impact of transferring water into storage containers but only tested water quality changes if water was stored in the same container over 24 h.
Forty percent of the households had received an educational visit in the last year, providing information on WASH. Forty-five percent of households took part on average in two community meetings during the last year where WASH training was provided (SD = 2). Seventy-seven percent of the respondents lacked hand-washing facilities in the household and in only 32% of the households with handwashing facilities was soap present. Fifty-six percent of the respondents owned private pit latrines, and 29% shared pit latrines with neighbours. Twelve percent lacked sanitation facilities and practiced open defecation instead. Further details about the socio-economic conditions, water handling and hygiene at the three sites are presented in Table A of the supplementary materials.
3.2. The influence of the interventions on bacterial recontamination
Water quality tests revealed that none of the samples taken at the kiosk tap at any site, including the control site, contained E. coli. Total coliforms were detected at the control site (Median = 11 CFU/100 mL, Interquartile Range (IQR) = 9 CFU/100 mL). The kiosk taps at the intervention sites providing chlorinated water did not contain any total coliforms.
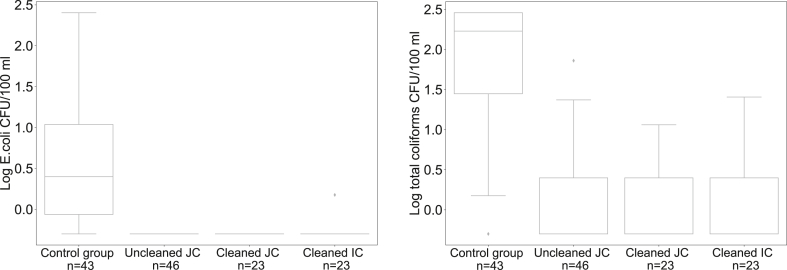
The presence of chlorine protected the water in all three interventions after filling the jerrycans, resulting in a median of zero E. coli and total coliforms. In the control group 74.4% of the containers contained E. coli immediately after filling, corresponding to a Median of 2 CFU/100 mL (IQR = 10) E. coli counts. This was significantly different from the three intervention groups, with Mann-Whitney U = 253, p < 0.0083, r = −0.61 for uncleaned jerrycans, U = 127, p < 0.0083, r = −0.46 for cleaned jerrycans, and U = 135, p < 0.0083, r = −0.45 for cleaned improved containers (see Fig. 2a). Similarly, the control group also contained a significantly higher recontamination level of total coliforms after container filling (Median = 169 CFU/100 mL, IQR = 260) than the intervention groups, with Mann-Whitney U = 213, p < 0.0083, r = −0.56 for uncleaned jerrycans, U = 100, p < 0.0083, r = −0.46 for cleaned jerrycans, and U = 104, p < 0.0083, r = −0.46 for cleaned improved containers (see Fig. 2b).
Fig. 2.
a) Recontamination with E. coli between tap and container filling b) Recontamination with total coliforms between tap and container filling (JC = jerrycan; IC = improved container) (Detection limit: 0 Log; −0.3 log = non-detects).
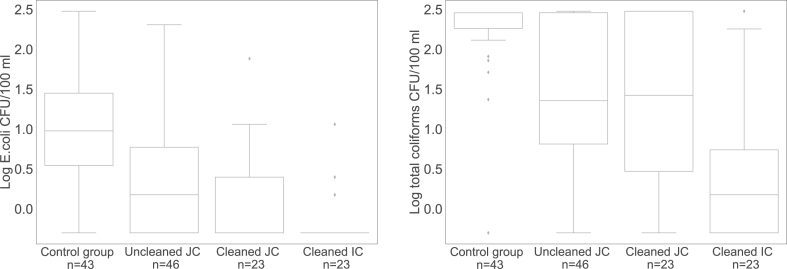
After 24 h of storage, E. coli was detected in 86% of the containers of the control group (Median = 9 CFU/100 mL, IQR = 25), 54% of the uncleaned jerrycans (Median = 1 CFU/100 mL, IQR = 6), 30% of the cleaned jerrycans (Median = 0 CFU/100 mL, IQR = 2) and 13% of the cleaned improved containers (Median = 0 CFU/100 mL, IQR = 0). The E. coli recontamination, calculated as the change in CFU/100 mL between the kiosk’s tap and 24 h storage in the container, in all three intervention groups differed significantly from that in the control group (Mann-Whitney U = 539, p < 0.0083, r = −0.32 for uncleaned jerrycans, Mann-Whitney U = 167, p < 0.0083, r = −0.39 for cleaned jerrycans and Mann-Whitney U = 100, p < 0.0083, r = −0.47 for improved cleaned containers (see Fig. 3a). Fewer samples recontaminated with E. coli were found if the jerrycans were cleaned, but the difference from uncleaned jerrycans was not statistically significant (U = 400, ns, r = −0.15). The use of cleaned improved containers reduced recontamination the most, but the difference from cleaned jerrycans was not statistically significant (U = 215, ns, r = −0.13). A significant difference in recontamination with E. coli was found between uncleaned jerrycans and cleaned improved containers, with Mann-Whitney U = 300, p < 0.0083, r = 0.28.
Fig. 3.
a) Recontamination with E. coli between tap and 24 h of storage in household b) Recontamination with total coliforms between tap and 24 h storage in household (JC = jerrycan; IC = improved container) (Detection limit: 0 Log; −0.3 log = non-detects).
Total coliform contamination after 24 h of storage was detected in 93% of the containers of the control group (Median = 287 CFU/100 mL, IQR = 104), 96% of the uncleaned jerrycans (Median = 23 CFU/100 mL, IQR = 282), 96% of the cleaned jerrycans (Median = 26 CFU/100 mL, IQR = 298), and 65% of the cleaned improved containers (Median = 1 CFU/100 mL, IQR = 5). Recontamination with total coliforms in the control group did not significantly differ from levels in both uncleaned and cleaned jerrycans (Mann-Whitney U = 686, ns, r = −0.22 and Mann-Whitney U = 381, ns, r = −0.14 respectively). This may be due to the effect of cleaning jerrycans with sand as the narrow neck of the jerrycans impedes cleaning with a brush. This procedure could have reintroduced total coliforms which also multiply in soil environments (WHO, 2017) into the container. Cleaning with sand also increases the risk of scratching the inner walls of containers and thus offering a niche for bacteria to grow (Van Der Merwe et al., 2012).
Only the improved containers that were cleaned with brushes instead of sand had significantly lower recontamination counts with total coliforms than the control group (U = 109, p < 0.0083, r = −0.46, see Fig. 3b). The cleaned improved containers also had significantly lower counts of total coliforms than uncleaned jerrycans and jerrycans cleaned with sand (U = 201; p < 0.0083; r = −0.0.36 and U = 118; p < 0.0083; r = −0.0.28). In addition to enhanced cleaning of the improved container, water extraction through the tap, instead of pouring water out from the opening in the case of the jerrycan, could also have contributed to a reduction of recontamination.
Ninety-six percent of the people who had received an improved container preferred it to a common jerrycan. The following reasons were given: the improved containers are easier to clean, they have a good cover and tap, and they can be used to fetch water from the lake. The last reason does not serve the purpose of the containers because lake water samples contained E. coli counts in the high-risk category (>100 CFU/100 mL), and improved containers should not facilitate access to lake water (WHO, 2017).
It was observed that after only five months of use, some of the improved containers were already broken. Leaking taps and broken handles and lids were observed. The tap with its exposed position near the bottom of the container is very vulnerable to damage and contamination during transport. The study demonstrated the positive effect improved containers with an opening that facilitates proper cleaning had on reducing recontamination, which suggests that a more robust container with such a design should be developed. A robust design that facilitates the daily use of containers was also highlighted by Reed et al. (2011).
3.3. The influence of container cleaning on FRC degradation
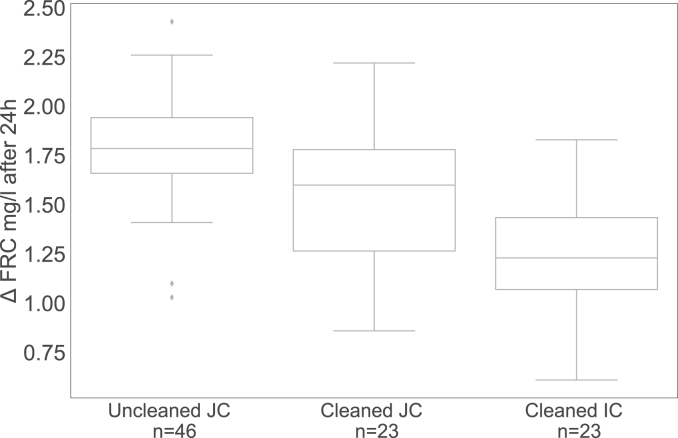
Recontamination of E. coli and total coliforms was significantly associated with the provision of chlorination at the water kiosk after membrane filtration (OR = 0.101; p = 0.001) (see Table 1). FRC concentrations measured immediately after filling the containers were on average 1.9 mg/L in uncleaned jerrycans (Standard Deviation (SD) = 0.3 mg/L), 2 mg/L in cleaned jerrycans (SD = 0.3 mg/L), and 2 mg/L (SD = 0.2 mg/L) in cleaned improved containers. The FRC degradation over 24 h in cleaned jerrycans (Mean of degradation = 1.6 mg/L, SD = 0.4 mg/L) was significantly less than in uncleaned jerrycans (Mean of degradation = 1.8 mg/L, SD = 0.3 mg/L, t(34) = 2.466, p < 0.05, r = 0.39). The lowest FRC degradation values over 24 h were found in cleaned improved containers (Mean of degradation = 1.2 mg/L, SD = 0.3 mg/L). They differed significantly from cleaned jerrycans (t(44) = 3.501, p < 0.05, r = 0.47; see Fig. 4).
Table 1.
Univariate and multivariate logistic regression of factors related with E.coli recontamination∗ in water stored for 24 h.
| Recontamination [N (cases) = 135] |
Univariate logistic regressiona |
Multivariate logistic regressionb |
||||
|---|---|---|---|---|---|---|
| n(0 CFU/100 mL) = 63; n(>0 CFU/100 mL) = 72 | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Intervention assignment | ||||||
| Water chlorinated at kiosk | 0.100 | 0.04–0.26 | <0.001 | 0.105 | 0.02–0.47 | 0.003 |
| Cleaned normal jerrycan | 0.316 | 0.12–0.83 | 0.019 | 0.180 | 0.05–0.66 | 0.009 |
| Cleaned improved container | 0.093 | 0.03–0.33 | <0.001 | 0.094 | 0.02–0.43 | 0.002 |
| Wealth index | 1.001 | 0.99–1.01 | 0.880 | |||
| Education level | 0.943 | 0.51–1.73 | 0.851 | 0.706 | 0.30–1.66 | 0.424 |
| Employed in agriculturec | 2.370 | 0.42–13.42 | 0.328 | |||
| Distance to water kiosk | 0.999 | 0.99–1.01 | 0.080 | 0.999 | 0.99–1.00 | 0.431 |
| Additional use of other drinking water source | 0.590 | 0.28–1.20 | 0.145 | 0.845 | 0.32–2.23 | 0.734 |
| Frequency of cleaning the container at home | 1.020 | 0.76–1.37 | 0.888 | |||
| Materials used to clean the container at home | ||||||
| Soap | 0.437 | 0.21–0.90 | 0.025 | 1.316 | 0.46–3.74 | 0.607 |
| Sand | 2.970 | 1.47–5.99 | 0.002 | 1.599 | 0.59–4.27 | 0.349 |
| Lantana kamara leaves | 1.350 | 0.57–3.18 | 0.491 | |||
| Sponge | 0.960 | 0.48–1.93 | 0.905 | |||
| Hand-Hygiene at household | ||||||
| Freq. hands washed yesterday with soap | 1.060 | 0.89–1.26 | 0.500 | |||
| Handwashing facility available | 1.082 | 0.48–2.42 | 0.848 | |||
| Handwashing facility with soap & water | 1.341 | 0.36–4.48 | 0.661 | |||
| Handwashing facility index | 1.000 | 0.98–1.01 | 0.969 | 0.992 | 0.98–1.01 | 0.399 |
| Sanitation | ||||||
| Toilet is clean | 0.954 | 0.45–1.99 | 0.900 | |||
| Household has no toilet | 1.532 | 0.52–4.49 | 0.436 | |||
| Household uses a shared toilet | 1.600 | 0.75–3.42 | 0.225 | |||
| Household has a private Pit-latrine | 0.651 | 0.33–1.29 | 0.220 | |||
| Toilet index | 0.772 | 0.53–1.11 | 0.166 | 0.722 | 0.41–1.26 | 0.253 |
| HH visit received | 1.409 | 0.70–2.83 | 0.335 | |||
| Nr of HH visits received | 0.862 | 0.68–1.09 | 0.223 | 0.535 | 0.35–0.81 | 0.003 |
∗ Recontamination included the absence (0 CFU/100 ml) or presence (>0 CFU/100 ml) of change in E.coli counts between source and 24 h of water storage.
a Odds ratios (OR) and p-values are based on the likelihood ratio test. Significant odds rations and corresponding p-values are indicated bold. CI= Confidence Interval.
b Model χ2 (11) = 62.12, p < 0.001; Cox & Snell R2 = 0.37; Nagelkerke R2 = 0.49.
Tolerance = 0.445–0.881; Variance Inflating Factor (VIF) = 1.135–2.246.
c All binary categorical variables were coded as present = 1/ absent = 0.
Fig. 4.
FRC degradation over 24 h in uncleaned and cleaned jerrycans (JC) and in improved containers (IC).
These results highlight that a thorough cleaning process is effective in removing organic material from the containers, thus contributing to the stabilization of FRC in the water that is kept in the containers.
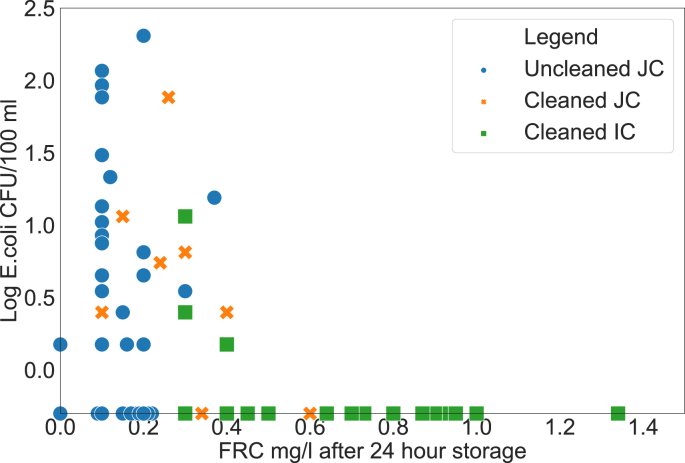
After 24 h, none of the uncleaned jerrycans contained FRC concentrations above 0.4 mg/L (mean = 0.2 mg/L, SD = 0.1 mg/L), cleaned jerrycans contained concentrations up to 1 mg/L (mean = 0.4 mg/L, SD = 0.2 mg/L), and cleaned improved containers had FRC concentrations up to 1.4 mg/L (mean = 0.7 mg/L, SD = 0.3 mg/L). Water quality in all jerrycans or containers containing FRC concentrations above 0.4 mg/L after 24 h of storage met WHO guidelines (zero E. coli CFU/100 mL) (WHO, 2017) (Fig. 5). Contrary to earlier recommendations, our findings suggest that FRC concentrations above 0.2 mg/L may be required to ensure that water from Lake Victoria is safe to drink water after 24 h of storage (WHO, 2017; Lantagne, 2008). Most of the samples in uncleaned jerrycans were recontaminated after 24 h, indicating that chlorination of about 2 mg/L was not sufficient to prevent recontamination in uncleaned jerrycans during 24 h of storage, while most households using cleaned jerrycans and improved cleaned containers still had water free of E. coli after 24 h of storage. A possible solution to prevent recontamination irrespective of the container used would be a higher chlorine dosage. However, higher concentrations of chlorine change the taste of treated water and thus could lead to its rejection by the user (Crider et al., 2018). A higher chlorine demand indicates higher concentrations of organic matter in the water, which could increase the risk of forming carcinogenic byproducts, such as trihalomethanes (Crittenden et al., 2012). The authors recommend that chlorination be combined with another intervention strategy, such as the use of properly cleaned containers, to reduce the amount of organic matter in the jerrycans.
Fig. 5.
Log–transformed counts of E. coli versus FRC (Detection limit: 0 Log; −0.3 log = non-detects).
3.4. Factors associated with the recontamination of water
Univariate and multivariate logistic regressions with presence or absence of E. coli recontamination in water stored for 24 h revealed that most water-handling and hygiene-related household factors did not significantly influence water quality. The results of the regression models using E. coli recontamination are presented in Table 1. Multivariate regression revealed that water chlorination after membrane filtration at the kiosk was significantly associated with lower E. coli recontamination (OR = 0.105, p = 0.003) after 24 h. Cleaning of the containers by the kiosk operator before filling them significantly reduced the risk of recontamination with E. coli (OR = 0.180, p = 0.009). Also, the use of improved containers significantly reduced the recontamination risk of E. coli (OR = 0.094, p = 0.002). These results correspond with the findings in section 3.2.
In addition to intervention strategies, the number of WASH education visits received by the household was associated with lower E. coli recontamination (OR = 0.54, p = 0.003). This is interesting as the number of WASH education visits received by the household was significantly correlated with the handwashing facility index (rs = 0.201; p = 0.019), the toilet index (rs = 0.223; p = 0.009) and with the use of sand to individually clean the containers at household level (rs = 0.290; p = 0.001). The effects of these correlations, however, are all quite small and did not violate the assumption of multi-collinearity in the regression model. A possible explanation for the association between the number of WASH education visits and recontamination in stored drinking water is that the WASH training may have improved the consistency of hygiene practices – a factor which is not captured in the data – and may therefore have improved general household hygiene.
4. Conclusions
Recontamination of drinking water provided at the tap of a kiosk treating water from Lake Victoria was observed after filling the jerrycan and during transport and storage. All three intervention strategies, comprising of chlorinating water at the tap after treating water from Lake Victoria with ultrafiltration, in combination with uncleaned jerrycans, cleaned jerrycans, or cleaned improved containers, significantly reduced E. coli recontamination during transport and storage over 24 h compared to the control group. Containers with FRC residuals of at least 0.4 mg/L after 24 h of storage did not exhibit recontamination with E. coli. An FRC concentration of 2 mg/L at the kiosk’s tap was not sufficient to obtain more than 0.4 mg/L FRC in uncleaned jerrycans after 24 h of storage. Cleaning the conventional jerrycans reduced the recontamination risks of E. coli but was inconvenient due to their having a small opening. The wider opening of improved containers enabled their cleaning with a brush and soap. Improved containers had the lowest recontamination levels for both E. coli and total coliforms after 24 h. Although former studies recommend small container openings to prevent recontamination (Levy et al., 2014), this study demonstrated a reduction of recontamination risks in containers with a wide opening that facilitated proper cleaning of the container’s inner walls.
In addition to the intervention strategies, higher numbers of household visits providing WASH education were associated with a lower risk of bacterial recontamination in stored drinking water, indicating the importance of adequate hygiene practices during safe water storage.
Declarations of interest
None.
Author agreement
RM, NG, LG, KW and HO designed the study. NG, LG and KW collected data. NG and RM analysed the data. RM and NG wrote and revised the article. LG, KW and HO commented on draft versions of the article. All authors have approved the final article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the households in the villages of Lugala, Bulundira, and Bumeru and the team at Africa Water solutions in Busia. This study was financed by Eawag. The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.wroa.2020.100079.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Budeli P., Moropeng R.C., Mpenyana-Monyatsi L., Ndombo Benteke Momba M. Inhibition of biofilm formation on the surface of water storage containers using biosand zeolite silver-impregnated clay granular and silver impregnated porous pot filtration systems. PloS One. 2018;13(4):e0194715. doi: 10.1371/journal.pone.0194715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider Y., Sultana S., Unicomb L., Davis J., Luby S.P., Pickering A.J. Can you taste it? Taste detection and acceptability thresholds for chlorine residual in drinking water in Dhaka, Bangladesh. Sci. Total Environ. 2018;613–614:840–846. doi: 10.1016/j.scitotenv.2017.09.135. [DOI] [PubMed] [Google Scholar]
- Crittenden J.C., Rhodes Trussell R., Hand D.W., Howe K.J., Tchobanoglous G. John Wiley & Sons, Inc; New Jersey: 2012. MWH’s Water Treatment - Principles and Design. [Google Scholar]
- Eshcol J., Mahapatra P., Keshapagu S. Is fecal contamination of drinking water after collection associated with household water handling and hygiene practices? A study of urban slum households in Hyderabad, India. J. Water Health. 2008;7:145–154. doi: 10.2166/wh.2009.094. [DOI] [PubMed] [Google Scholar]
- Harris A.R., Davis J., Boehm A.B. Mechanisms of post-supply contamination of drinking water in Bagamoyo, Tanzania. J. Water Health. 2013;11:543–554. doi: 10.2166/wh.2013.023. [DOI] [PubMed] [Google Scholar]
- Huttinger A., Dreibelbis R., Roha K., Ngabo F., Kayigamba F., Mfura L., Moe C. Evaluation of membrane ultrafiltration and residual chlorination as a decentralized water treatment strategy for ten rural healthcare facilities in Rwanda. 2015;12:13602–13623. doi: 10.3390/ijerph121013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagals P., Jagals C., Bokako T.C. The effect of container-biofilm on the microbiological quality of water used from plastic household containers. J. Water Health. 2003;1:101–108. [PubMed] [Google Scholar]
- Krishnan V. 2010. Constructing an Area-Based Socioeconomic Index: A Principal Components Analysis Approach. [Google Scholar]
- Lantagne D.S. American Water Works Association; 2008. Sodium Hypochlorite Dosage for Household and Emergency Water Treatment. [DOI] [PubMed] [Google Scholar]
- Levy K., Anderson L., A. Robb K., Cevallos W., Trueba G., Eisenberg J.N.S. Household effectiveness vs. laboratory. efficacy of point-of-use chlorination. 2014;54:69–77. doi: 10.1016/j.watres.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgranahan G., Njiru C., Albu M., Smith M., Mitlin D. WEDC at Loughborough University; 2006. How Small Water Enterprises Can Contribute to the Millennium Development Goals: Evidence from Dar Es Salaam, Nairobi, Khartoum And Accra, Loughborough. [Google Scholar]
- Meierhofer R., Rubli P., Dreyer K., Ouma H., Wanyama K., Peter-Varbanets M. 40th WEDC International Conference; Loughborough, UK: 2017. Membrane Filtration Reduces Recontamination Risk in Chlorinated Household Water Containers. [Google Scholar]
- Meierhofer R., Wietlisbach B., Matiko C. Influence of container cleanliness, container disinfection with chlorine, and container handling on recontamination of water collected from a water kiosk in a Kenyan slum. Water and Health. 2019;17:308–317. doi: 10.2166/wh.2019.282. [DOI] [PubMed] [Google Scholar]
- Mellor J.E., Smith J.A., Samie A., Dillingham R.A. Coliform sources and mechanisms for regrowth in household drinking water in limpopo, South Africa. J. Environ. Eng. 2013;139:1152–1161. doi: 10.1061/(ASCE)EE.1943-7870.0000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz E.D., Reiff F.M., Tauxe R.V. Safe water treatment and storage in the home: a practical new strategy to prevent waterborne disease. J. Am. Med. Assoc. 1995;273:948–953. [PubMed] [Google Scholar]
- Murphy H.M., Sampson M., Mcbean E., Farahbakhsh K. Influence of household practices on the performance of clay pot water filters in rural Cambodia. Desalination. 2009;248:562–569. [Google Scholar]
- Murphy J.L., Ayers T.L., Knee J., Oremo J., Odhiambo A., Faith S.H., Nyagol R.O., Stauber C.E., Lantagne D.S., Quick R.E. Evaluating four measures of water quality in clay pots and plastic safe storage containers in Kenya. Water Res. 2016;104:312–319. doi: 10.1016/j.watres.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opryszko M.C., Guo Y., Macdonald L., Macdonald L., Kiihl S., Schwab K.J. Impact of water-vending kiosks and hygiene education on household drinking water quality in rural Ghana. Am. J. Trop. Med. Hyg. 2013;88:651–660. doi: 10.4269/ajtmh.12-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opryszko M.C., Huang H., Soderlund K., Schwab K.J. Data gaps in evidence-based research on small water enterprises in developing countries. J. Water Health. 2009;7:609. doi: 10.2166/wh.2009.213. [DOI] [PubMed] [Google Scholar]
- Patrick M., Steenland M., Dismer A., Pierre-Louis J., Murphy J.L., Kahler A., Mull B., Etheart M.D., Rossignol E., Boncy J., Hill V., Handzel T. Assessment of drinking water sold from private sector kiosks in post-earthquake. Port-au-Prince, Haiti. 2017;97:84–91. doi: 10.4269/ajtmh.16-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter-Varbanets M., Dreyer K., Mcfadden N., Ouma H., Wanyama K., Etenu C., Meierhofer R. Loughborough; UK: 2017. Evaluating Novel Gravity-Driven Membrane (GDM) Water Kiosks in Schools. 40th WEDC International Conference. [Google Scholar]
- Peter M. Dübendorf; Eawag: 2015. Gravity-driven Membrane Disinfection for Household Water Treatment: Final Report: GDMD Project 2010-2014. [Google Scholar]
- Peter M., Meierhofer R., Ochieng J., Dreyer K., Mcfadden N., Ouma H. Dübendorf; 2016. Evaluation of GDM Filtration for Safe Water Provision in Schools in Uganda. [Google Scholar]
- Prüss-Ustün A., Wolfa J., Bartram J., Clasen T., Cumming O., Freeman M.C., Gordon B., Hunter P.R., Medlicott K., Johnston R. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: an updated analysis with a focus on low and middle-income countries. Int. J. Hyg Environ. Health. 2019;222:765–777. doi: 10.1016/j.ijheh.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B., Scott R., Skinner B., Jackson T. Loughborough University; 2011. Domestic Water Containers: an Engineer’s Guide. [Google Scholar]
- Roberts L., Chartier Y., Chartier O., Malenga G., Toole M., Rodka H. Keeping clean water clean in a Malawi refugee camp: a randomized intervention trial. Bull. World Health Organ. 2001;79:280–287. [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. SAGE; Newbury Park: 1991. Meta-Analytic Procedures for Social Research. [Google Scholar]
- Rutstein S.O. Demographic and Health Research Division; 2008. The DHS Wealth Index: Approaches for Rural and Urban Areas. [Google Scholar]
- Sima L.C., Elimelech M. More than a drop in the bucket: decentralized membrane-based drinking water refill stations in southeast asia. Environ. Sci. Technol. 2013;47:7580–7588. doi: 10.1021/es304384n. [DOI] [PubMed] [Google Scholar]
- Steele A., Clarke B., Watkins O. Impact of jerry can disinfection in a camp environment – experiences in an IDP camp in Northern Uganda. J. Water Health. 2008;6:559. doi: 10.2166/wh.2008.072. [DOI] [PubMed] [Google Scholar]
- Thompson J., Porras I.T., Wood E., Tumwine J.K., Mujwahuzi M.R., Katui-Katua M., Johnstone N. Vol. 12. 2000. pp. 37–52. (Waiting at the Tap: Changes in Urban Water Use in East Africa over Three Decades). [Google Scholar]
- Trevett A.F., Carter R.C., Tyrrel S.F. Mechanisms leading to post - supply water quality deterioration in rural Honduran communities. Int. J. Hyg Environ. Health. 2005;208:153–161. doi: 10.1016/j.ijheh.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Van Der Merwe V., Duvenage S., Korsten L. Comparison of biofilm formation and water quality when water from different sources was stored in large commercial water storage tanks. J. Water Health. 2012;11:30–40. doi: 10.2166/wh.2012.014. [DOI] [PubMed] [Google Scholar]
- Vittinghoff E., Glidden D.V., Shiboski S., Mcculloch C.E. Springer; New York: 2005. Regression Methods in Biostatistics. [Google Scholar]
- WHO Clean water and sanitation: why it matters. Sustainable Development Goal. 2015;6 [Google Scholar]
- WHO . World Health Organization; 2017. Guidelines for Drinking Water Quality. [PubMed] [Google Scholar]
- WHO U. 2019. WASH in Health Care Facilities: Global Baseline Report 2019. Geneva. [Google Scholar]
- Wright J., Gundry S., Conroy R. Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Trop. Med. Int. Health. 2004;9:106–117. doi: 10.1046/j.1365-3156.2003.01160.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.