Abstract
Background
Primary ciliary dyskinesia (PCD) is a genetically diverse disease which causes impaired mucociliary clearance, and results in pulmonary, otologic, and rhinologic disease in affected patients. Genetic mutations in multiple genes impair the ability of patients to clear mucous from the lungs, middle ear, and sinonasal cavity and lead to chronic pulmonary and sinonasal symptoms.
Methods
We identified 17 PCD patients who had available CT scans. Volumes for bilateral maxillary, sphenoid, and frontal sinuses were calculated. A control population of patients who had preoperative CT scans for endoscopic endonasal resection of skull base pathology without sinonasal cavity involvement was also identified.
Results
The mean age of PCD was 33 and ranged from 13 to 54 years. Patients were age- and gender-matched to a control group that underwent resection of anterior skull-base tumors and had a mean age of 35 that ranged between 17–53 years old. The volumes for all thee sinus cavities were significantly smaller (p < 0.007) compared to the control population. The average Lund-Mackay score was 10.6 in the PCD cohort (range 6–16) in comparison to an average of 0.7 in the control cohort (range 0–2).
Conclusions
Overall sinus volumes were smaller in patients with PCD compared to our control population. Future studies will be aimed at understanding defects in sinus development as a function of specific genetic mutations in PCD patients. Ultimately, a better understanding of the underlying pathophysiology of PCD will allow us to identify the optimal treatment practices for this unique patient group.
Keywords: hypoplastic sinus, primary ciliary dyskinesia, sinus development
Introduction
Primary ciliary dyskinesia (PCD) is a heterogeneous genetic disease in which defects in ciliary biogenesis, ultrastructure, or function result in chronic otologic, sinus, and pulmonary disease. 1 Inheritance of PCD is primarily autosomal recessive, and results from mutations in multiple genes. 2 Symptoms of PCD include year round ear infection, purulent rhinorrhea, and a wet cough, which are commonly observed in all children, yet the prevalence of PCD is rare, estimated to be only 1 in 15,000. 3 Accurately diagnosing PCD is a challenge due to its genetic heterogeneity, the prevalence of its symptoms, and the rarity of its occurrence. Although this challenge is somewhat alleviated in patients with classic Kartagener’s Syndrome, which exhibit situs inversus, bronchiectasis, and chronic sinusitis, this triad of characteristics is only observed in the minority of PCD patients.4,5 Thus, a better understanding of the sinonasal manifestations and sinus anatomy seen in PCD could help clinicians identify patients with PCD who have undiagnosed PCD, plan appropriate surgeries, and counsel patients regarding expectations of their sinonasal health.
Defective mucociliary clearance is a central feature of both PCD and cystic fibrosis (CF), with both diseases having high rates of chronic sinonasal symptoms despite the different underlying genetic and pathophysiologic causes of each disease. It is well-established that patients with CF frequently exhibit hypoplasia and a computed tomography (CT) scan often depicts agenesis of their sinuses.6–12 This was initially thought to be caused by the chronic sinusitis that CF patients experience; however, recent studies in rats 13 as well as pigs 14 have shown that hypoplasia precedes sinusitis and is present at birth.
In contrast, the literature describing sinus anatomy in PCD in sparse. A case report by Gomez et al. described a PCD patient who was noted to have agenesis of the frontal sinus. 15 In 2011, Pifferi et al, described a cohort of PCD patients who frequently had hypoplastic or aplastic sinuses, but no control group was included. 16 Therefore, to better characterize sinus development and size in a PCD cohort, we identified 17 patients in the UNC PCD cohort that had available CT scans, performed volumetric analysis of their sinuses, and compared these data to an age- and gender- matched cohort of patients with no evidence of sinus disease.
Materials and Methods
Patients older than 13 years of age with a diagnosis of PCD and available CT scans were included in the study (Table 1). The PCD diagnosis of all patients was classified as definite, made on the basis of a compatible clinical phenotype and a diagnostic abnormality in ciliary ultrastructure observed by electron microscopy (EM) and/or the presence of two disease-causing genetic mutations. CT scans were analyzed in iPlan software (BrainLab, Germany). Individual and total volume for bilateral maxillary, sphenoid, and frontal sinuses were calculated by the senior author (AJK) while blinded to the disease state. Using this software, the senior author, outlined each sinus in the axial plane and computationally expanded the volume in three dimensions. Then the 3D sinus reconstructions were adjusted in the coronal and sagittal plane to ensure accuracy and consistency. In addition to volumetric analysis, radiographic extent of disease was assessed by Lund-Mackay scores 17 that were calculated for each patient by the senior author.
Table 1.
Patient Demographics.
| No. | Age (Years) | Sex | Category | Method |
|---|---|---|---|---|
| 1 | 47 | F | Definite | EM/Genetics |
| 2 | 35 | F | Definite | Genetics |
| 3 | 38 | F | Definite | EM/Genetics |
| 4 | 26 | F | Definite | EM/Genetics |
| 5 | 42 | F | Definite | EM |
| 6 | 16 | F | Definite | EM |
| 7 | 24 | F | Definite | EM |
| 8 | 51 | M | Definite | Genetics |
| 9 | 31 | F | Definite | EM/Genetics |
| 10 | 13 | F | Definite | EM/Genetics |
| 11 | 54 | F | Definite | EM/Genetics |
| 12 | 21 | M | Definite | EM/Genetics |
| 13 | 38 | M | Definite | Genetics |
| 14 | 13 | F | Definite | Genetics |
| 15 | 34 | F | Definite | EM/Genetics |
| 16 | 34 | F | Definite | EM/Genetics |
| 17 | 38 | F | Definite | EM/Genetics |
Seventeen study patients were identified with a definite diagnosis of primary ciliary dyskinesia. nNO – nasal nitric oxide, EM – electron microscopy, F – female, M – males.
A control population of age- and gender-matched patients was selected from our UNC skull base database and consisted of those with preoperative CT scans obtained for image guidance during endoscopic endonasal resection of skull base pathology. Patients with acromegaly or erosion of the sella turcica were excluded. Paired age-matched controls were within four years of the study population. Sinus volumes and Lund-Mackay scores were computed as described above.
Statistical analysis was conducted using paired T-tests with GraphPad Prism v7 (GraphPad Software, Inc) with significance determined with p-values ≤0.05.
Results
Seventeen patients with available CT scans (14 female and 3 male) were identified. The mean age was 33, and ranged from 13–54 years of age (Table 1). The control group had a mean age of 35, with an age range of 17–53 years old.
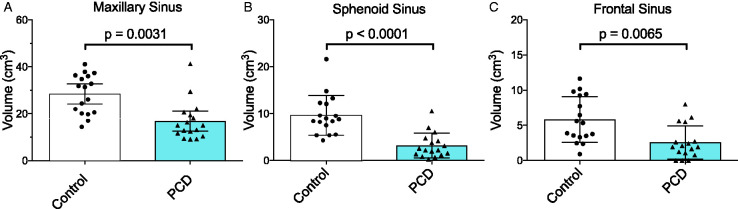
The mean volume of the maxillary sinus in the PCD group was 16.8 cm3 (range 9.1–41.3 cm3), which was significantly smaller (p = 0.00031) than that of the control group (28.5 cm3, range 14.4–41.1 cm3; Figure 1(A)). Similarly, the average volume of the sphenoid sinus in PCD patients was significantly smaller at 3.2 cm3 (range 0.25–10.54 cm3), relative to the control group at 9.6 cm3 (range 4.3–21.6 cm3; p < 0.0001; Figures 1(B) and 2). Lastly, the mean volume of the frontal sinus in PCD patients was 2.6 cm3 (range 0–7.99), which was also significantly smaller (p = 0.0065) than that observed in the control population (5.8 cm3, range 0.9–11.6 cm3; Figures 1(C) and 3).
Figure 1.
Sinus volumes in PCD patients are significantly smaller than in control patients. Volume of (A) maxillary, (B) sphenoid, and (C) frontal sinus cavities in the PCD or control cohorts. Column height is the mean of the cohort and error bars represent 95% confidence intervals.
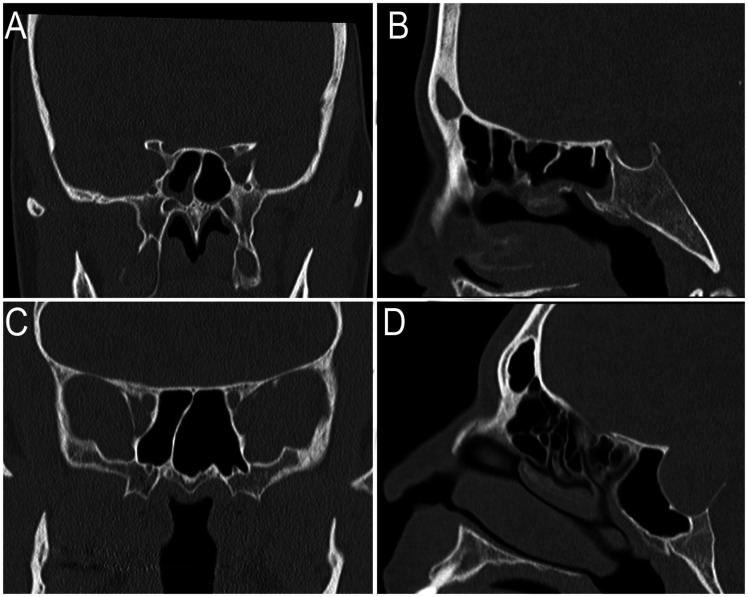
Figure 2.
Representative images of sphenoid volume in a PCD or control patient. The patients with the median sphenoid volume are shown for the PCD population (A and B) or the control population (C and D). PCD patient #9 has decreased pneumatization of their sphenoid sinus as shown on coronal (A) and sagittal (B) planes as compared to the control population (C and D).
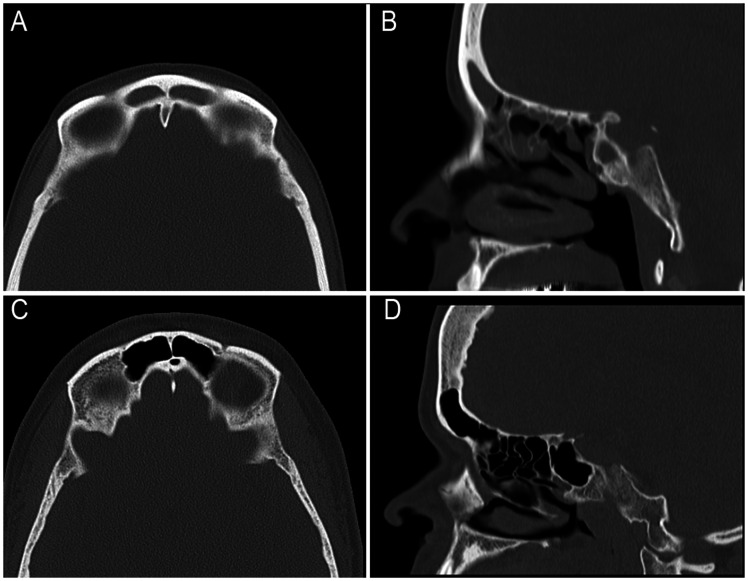
Figure 3.
Representative images of frontal sinus volume in a PCD or control patient. The patients with the median frontal sinus volume are shown for the PCD population (A and B) or the control population (C and D). PCD patient #3 has decreased pneumatization of their frontal sinus as shown on coronal (A) and sagittal (B) planes compared to the control patient (C and D).
In the PCD cohort, the minimum Lund-Mackay score was 6, the maximum score was 16, and the average score was 10.6. This was significantly different (p < 0.0001) compared to the control cohort, which had an average score of 0.7 (range 0–2; Figure 4).
Figure 4.

Patients with PCD have higher disease severity on CT scans relative to control patients. Lund-Mackay scores of PCD and control CT scans. The mean score in the PCD cohort was 10.6 with a range of 6 to 16. The mean Lund-Mackay score in the control population was 0.7 with a range of 0 to 2. Error bars represent the 95% confidence interval.
Discussion
Our study is the first to quantify sinus volume in PCD patients. We observed that the average volumes of the maxillary, sphenoid, and frontal sinuses were significantly smaller in the PCD population than in age- and gender-matched controls. Our control population had similar volumes as seen in other studies. 18 , 19 These results are in congruence with an earlier study that found that 53% of frontal sinuses and 46% of sphenoid sinuses in PCD patients were aplastic or hypoplastic, respectively. 16 While hypoplasia is more obvious on CT scans in the frontal and sphenoid sinuses, we noted that 88% (15/17) of patients in the PCD population had a maxillary sinus volume that was less than the average maxillary sinus volume in the control population. In addition, Lund-Mackay scoring indicated significantly worse sinus disease in the PCD population compared to the control cohort. Overall our study demonstrates that total sinus volume is lower in PCD patients and disease severity as assessed on CT scans is worse than in a control population.
The results of our study have important clinical implications. When performing endoscopic sinus surgery on PCD patients, surgeons should expect to encounter hypoplastic sinuses. Smaller sinus volumes restrict potential angles of approach and reduce room for instruments, making functional endoscopic sinus surgery in PCD patients more complicated. While the use of image guidance and pre-operative CT scans can minimize this risk, the complexities associated with hypoplastic sinuses should always be anticipated and considered before surgeons operate on a patient with PCD.
Nasal nitric oxide is released from sinonasal mucosa and has antimicrobial properties. 20 While the mechanism is unknown, low levels of nasal nitric oxide are nearly universal in patients with PCD and help support a diagnosis of PCD. 1 , 2 It is possible that this decreased nasal nitric oxide is related to the decrease in mucosal surface area; however, larger cohorts and in vitro studies will be necessary to understand the mechanism by which PCD results in low nasal nitric oxide.
In the absence of situs inversus, PCD is difficult to diagnose and there is no single test, genetic or otherwise, that captures all known PCD patients. Therefore, in patients with recalcitrant sinus disease, especially those that are younger, hypoplastic sinuses should raise the suspicion for PCD.
Conclusion
Patients with primary ciliary dyskinesia (PCD) exhibit significantly decreased pneumatization of their maxillary, sphenoid, and frontal sinuses, a difference that was observed across age and gender. Chronic rhinosinusitis is difficult to treat and our observations indicate that hypoplastic sinuses in PCD patients can complicate surgical management of the disease.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project described was supported by the National Center for Advancing Translational Sciences (NCATS) through grant KL2TR002490 to AJK. Support for MRK was from grant R01HL071798 from the National Heart Lung Blood institute. MRK, KMS, MWL were supported by the Genetic Disorders of Mucociliary Clearance (U54HL096458) a part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR), NCATS, funded through a collaboration between NCATS and NHLBI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ORCID iDs
Erin M. Lopez https://orcid.org/0000-0002-4636-055X
Adam J. Kimple https://orcid.org/0000-0003-1670-8401
References
- 1.Shapiro AJ, Zariwala MA, Ferkol T, et al. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr Pulmonol. 2016; 51(2):115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popatia R, Haver K, Casey A. Primary ciliary dyskinesia: an update on new diagnostic modalities and review of the literature. Pediatr Allergy Immunol Pulmonol. 2014; 27(2):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noone PG, Leigh MW, Sannuti A, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004; 169(4):459–467. [DOI] [PubMed] [Google Scholar]
- 4.Goutaki M, Meier AB, Halbeisen FS, et al. Clinical manifestations in primary ciliary dyskinesia: systematic review and meta-analysis. Eur Respir J. 2016; 48(4):1081–1095. [DOI] [PubMed] [Google Scholar]
- 5.Knowles MR, Zariwala M, Leigh M. Primary ciliary dyskinesia. Clin Chest Med. 2016; 37(3):449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggesbo HB, Eken T, Eiklid K.Kolmannskog, F. Hypoplasia of the sphenoid sinuses as a diagnostic tool in cystic fibrosis. Acta Radiol. 1999; 40(5):479–485. [DOI] [PubMed] [Google Scholar]
- 7.Eggesbo HB, Sovik S, Dolvik S.Kolmannskog F. CT characterization of developmental variations of the paranasal sinuses in cystic fibrosis. Acta Radiol. 2001; 42(5):482–493. [DOI] [PubMed] [Google Scholar]
- 8.Eggesbo HB, Sovik S, Dolvik S.Kolmannskog F. CT characterization of inflammatory paranasal sinus disease in cystic fibrosis. Acta Radiol. 2002; 43(1):21–28. [DOI] [PubMed] [Google Scholar]
- 9.Calton JB, Koripella PC, Willis AL.Le C, Chiu A, Chang E. Paranasal sinus size is decreased in CFTR heterozygotes with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017; 7(3):256–260. [DOI] [PubMed] [Google Scholar]
- 10.Orlandi RR, Wiggins RH. Radiological sinonasal findings in adults with cystic fibrosis. Am J Rhinol Allergy. 2009; 23(3): 307–311. [DOI] [PubMed] [Google Scholar]
- 11.Woodworth BA, Ahn C, Flume PA.Schlosser R. The delta F508 mutation in cystic fibrosis and impact on sinus development. Am J Rhinol. 2007; 21(1):122–127. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Friedman EM, Sulek M.McCluggage C. Paranasal sinus development in chronic sinusitis, cystic fibrosis, and normal comparison population: a computerized tomography correlation study. Am J Rhinol. 1997; 11(4):275–281. [DOI] [PubMed] [Google Scholar]
- 13.Grayson J, Tipirneni KE, Skinner DF, et al. Sinus hypoplasia in the cystic fibrosis rat resolves in the absence of chronic infection. Int Forum Allergy Rhinol. 2017; 7(9):904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang EH, Pezzulo AA, Meyerholz DK, et al. Sinus hypoplasia precedes sinus infection in a porcine model of cystic fibrosis. Laryngoscope. 2012; 122(9):1898–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez R, Perez Trullen A, Ruiz C.Suárez F, Ramón y Cajal S. [Primary ciliary dyskinesia with frontal sinus agenesis]. Acta Otorrinolaringol Esp. 1997; 48(4):315–316. [PubMed] [Google Scholar]
- 16.Pifferi M, Bush A, Caramella D, et al. Agenesis of paranasal sinuses and nasal nitric oxide in primary ciliary dyskinesia. Eur Respir J. 2011; 37(3):566–571. [DOI] [PubMed] [Google Scholar]
- 17.Lund VG, Mackay IS. Staging in rhinosinusitis. Rhinology. 1993; 31(4):183–184. [PubMed] [Google Scholar]
- 18.Ariji Y, Kuroki T, Moriguchi S.Kanda S. Age changes in the volume of the human maxillary sinus: a study using computed tomography. Dentomaxillofac Radiol. 1994; 23(3):163–168. [DOI] [PubMed] [Google Scholar]
- 19.Cohen O, Warman M, Fried M, et al. Volumetric analysis of the maxillary, sphenoid and frontal sinuses: a comparative computerized tomography based study. Auris Nasus Larynx. 2018; 45(1):96–102. [DOI] [PubMed] [Google Scholar]
- 20.Carey RM, Chen B, Adappa ND, et al. Human upper airway epithelium produces nitric oxide in response to Staphylococcus epidermidis. Int Forum Allergy Rhinol. 2016; 6(12):1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]