Highlights
-
•
Previous studies have shown automatic vOT activation when processing spoken language.
-
•
vOT is organized from small onset to large rime grain size.
-
•
Higher fluency in 7−8 year olds only correlated with rhyme processing in anterior vOT.
-
•
Results suggest that these children have transitioned to larger grain size.
-
•
Exploratory analyses show reliance on phonology in STG for processing onsets.
Abstract
Previous studies have shown that reading skill in 3- to 6-year-old children is related to the automatic activation of the posterior left ventral occipitotemporal cortex (vOT) during spoken language processing, whereas 8- to 15-year-old children and adult readers activate the anterior vOT. However, it is unknown how children who are between these two age groups automatically activate orthographic representations in vOT for spoken language. In the current study, we recruited 153 7- to 8-year-old children to fill the age gap from previous studies. Using functional magnetic resonance imaging (fMRI), we measured children’s reading-related skills and brain activity during an auditory phonological task with both a small (i.e. onset) and a large (i.e. rhyme) grain size condition. We found that letter fluency, but not reading accuracy, was correlated with activation in the anterior vOT for the rhyme condition. There were no reading-related skill correlations for the posterior vOT or for activation during the onset condition in this age group. Our findings reveal that automatic activation in the anterior vOT during spoken language processing already occurs in higher skilled 7- to 8-year-old children. In addition, increases in naming automaticity is the primary determinant of the engagement of vOT during phonological awareness tasks.
t1 Introduction
The left ventral occipitotemporal cortex (vOT) is an orthographic processing region activated during visual word recognition (e.g. see meta-analysis in Taylor et al., 2013; Martin et al., 2015). However, studies have often found that the left vOT is automatically activated during spoken language tasks (e.g. Yoncheva et al., 2009; Dehaene et al., 2010). Dehaene et al. (2010) recruited adults with different levels of literacy and found that only literate adults showed activation in the anterior vOT during an auditory lexical decision task, whereas illiterate adults did not. They suggested that reading acquisition drives the connection between written and oral language, eliciting automatic orthographic activation in the brain during spoken language tasks even though spelling information is not required for performance. Although this study with adults provides compelling evidence for the effect of reading skill on the automatic activation of spelling patterns when listening to words, it is unclear how reading development in children affects the involvement of automatic orthographic processing during spoken language tasks.
With reading development, there appears to be a transition in automatic orthographic activation during spoken language processing from the posterior vOT to the anterior vOT. Previous studies on preschoolers/kindergarteners aged 3–6 showed that the posterior vOT was involved during spoken language tasks and that greater activation in this region was associated with a better home literacy environment or reading potential (Raschle et al., 2012; Hutton et al., 2015; Powers et al., 2016). However, these previous studies did not directly measure reading skills. Wang et al. (2018) measured reading accuracy in 5-to 6-year-old children with a letter word identification test and measured brain activation during an auditory phonological task with both small (i.e. onset) and large grain size (i.e. rhyme) phonological conditions. They found that reading skill was only correlated with brain activation in the posterior vOT for the onset compared to the rhyme condition. In contrast to research on preschool/kindergarten children, studies on children in elementary/middle school aged 9–15 showed that the anterior vOT was involved in auditory rhyming tasks (Cone et al., 2008; Desroches et al., 2010), and that activation in this region was correlated with reading ability as measured by a pseudoword reading accuracy test (Desroches et al., 2010). Therefore, a transition of automatic orthographic activation during spoken language processing appeared to occur from posterior to anterior vOT.
According to the local combination detector model (Dehaene et al., 2005), the posterior left vOT specializes in processing smaller grain sizes like singular letters, whereas the anterior left vOT specializes in processing larger grain sizes like bigrams and trigrams. The transition of automatic activation in vOT from posterior to anterior during spoken language processing is probably due to reading development. According to one prominent theory of reading acquisition (Frith, 1985), children begin reading at the alphabetic stage using small grain letter-to-sound mapping, and progress to the orthographic stage employing large grain orthography-to-phonology mapping. In support of this, studies have also shown that beginning readers aged 5–6 recruit the posterior vOT, whereas older children and adults activate the anterior vOT, during visual word reading (Brem et al., 2009, 2010). The fact that younger children automatically activate the posterior vOT during spoken language tasks (e.g. Raschle et al., 2012; Hutton et al., 2015; Powers et al., 2016; Wang et al., 2018) is probably due to them being in the alphabetic stage of reading. Older elementary/middle school children may automatically activate their anterior vOT in auditory rhyming tasks (e.g. Cone et al., 2008; Desroches et al., 2010) because their reading skills have progressed to the stage of large grain size mapping.
Previous studies on older elementary/middle school children (Cone et al., 2008; Desroches et al., 2010) have only used rhyming judgements, which is a large grain phonological task, making it difficult to determine whether the involvement of the anterior, but not the posterior vOT, was due to a task requirement or due to reading development. Dębska et al. (2019) recruited Polish children aged 8–13 and used multiple phonological tasks at different grain sizes, including a pseudoword matching task, a rhyming task, and a first phoneme matching task. They measured children’s reading ability using one reading accuracy test and two reading speed tests, and created a composite score. They found that reading ability was only correlated with activation in the anterior vOT during large grain phonological processing (i.e. pseudoword matching) but not during smaller grain phonological processing (i.e. rhyme or first phoneme matching). This result suggests that reading skills in 8- to 13-year-old Polish children drives the connection between orthography and phonology only at large grain sizes. However, Dębska et al. (2019) found no correlation between reading skills and activation in the left vOT during rhyming judgment, a relatively large grain size task. This result is probably because Polish is a transparent language with regular mappings between letters and phonemes. Children may apply the same phonemic processing regardless of grain size. English is a less transparent language with irregular mapping between letters and phonemes and is relatively more consistent in mapping from spelling to pronunciation at the rhyme level than at the phoneme level (Treiman et al., 1995). The effect of grain sizes on the activation of vOT in English-speaking children are likely to be different from that of Polish-speaking children due to cross-linguistic differences in the nature of the writing systems, but this has yet to be investigated.
In the current study, we aimed to address the age gap in previous studies by examining if automatic activation of orthographic representations in the anterior vOT occurs during an auditory phonological task as early as 7–8 years old. Our study was novel in a variety of ways. First, we recruited 7- to 8-year-old English-speaking children, which filled the age gap in previous studies on English-speaking children aged 3–6 (e.g. Raschle et al., 2012; Hutton et al., 2015; Powers et al., 2016; Wang et al., 2018) versus children aged 9–15 (e.g. Cone et al., 2008; Desroches et al., 2010). According to the stages of reading development by Chall (1996), reading development in 7- to 8-year-old children is a pivotal period referred to as the confirmation and fluency stage in which readers confirm what is already known for decoding in order to develop their reading fluency. This is in contrast with children younger than 7, who are either pre-literate or early readers beginning to learn basic sound-symbol correspondence to establish decode ability. Second, the age range in our study was narrower than previous studies on older children (Desroches et al., 2010; Dębska et al., 2019). This allowed us to control for the confounding variable of age. Third, we designed both onset and rhyme conditions to examine small versus large grain sizes. Fourth, we measured both reading accuracy and letter fluency to examine which reading-related skill was associated with the automatic activation of orthography in vOT. Previous studies either only measured reading accuracy (e.g. Wang et al., 2018; Desroches et al., 2010) or used a combination of reading accuracy and speed (e.g. Dębska et al., 2019). Thus, they were unable to determine whether it is accuracy or fluency that affects the automatic orthographic activation in vOT for spoken words. Based on previous studies on preschool and kindergarten children (e.g. Raschle et al., 2012; Powers et al., 2016; Wang et al., 2018), we hypothesized that, if 7- to 8-year-old children were still at the alphabetic stage of reading, there should be a correlation between reading-related skill and brain activation in the posterior vOT only for onset but not rhyme. However, if 7- to 8-year-old children have transitioned to the orthographic stage of reading, according to previous studies on older elementary and middle school children (e.g. Desroches et al., 2010; Dębska et al., 2019), we hypothesized a correlation between reading-related skills and the automatic activation of orthography in the anterior vOT only for rhyme but not onset. Because children first develop the ability to accurately read words and then to read them fluently (Norton and Wolf, 2012), we expected reading accuracy to show more robust correlations if the children were at the alphabetic stage of reading. However, if children were at the orthographic stage of reading, then we expected letter fluency to show more robust correlations.
2. Experimental procedures
2.1. Participants
In this study, 153 monolingual English-speaking children (mean age = 7.6, SD = 0.4, range 7.1–8.5 years-old, 86 girls) were included in our final analysis. Children were recruited from the Austin, Texas metropolitan area. Informed consent forms were obtained from parents. The Institutional Review Board approved all the following procedures.
Participants were given developmental history questionnaires completed by their parents and a series of screening tests. The screening tests included 5-handedness questions in which the children needed to pretend they write, erase, pick, open and throw something, and the Diagnostic Evaluation of Language Variation (DELV) Part I Language Variation Status (Seymour et al., 2003). All the children met the following criteria: (1) Primarily right-handed, defined as performing at least 3 out of 5 items using their right hand; (2) Mainstream English speakers, defined as scoring 9 or more (out of 15) for 7 year-old children and 11 or more (out of 15) for 8-year-old children for the mainstream English items on the DELV. (3) No diagnosis of Attention Deficit Hyperactivity Disorder (ADHD), neurological disease, psychiatric disorders, learning or language disorder as reported in developmental history questionnaire completed by their parents; (4) normal hearing and normal or corrected-to-normal vision as reported by their parents.
Standardized testing was then administered to measure IQ, language, and reading skills. The Kaufman Brief Intelligence Test, Second Edition (KBIT-2, Kaufman and Kaufman, 2004), and the Clinical Evaluation of Language Fundamentals, Fifth Edition (CELF-5, Wiig et al., 2013) were used to assess children’s IQ and language. Children who scored less than a standard score of 80 (9th percentile) on KBIT-2 non-verbal subtest and CELF-5 core language score were excluded. The Letter-Word Identification subtest (word ID) on the Woodcock-Johnson III Test of Achievement (WJ-III, Woodcock et al., 2001) was used as a measure of reading accuracy. In this test, children were required to read out loud the presented letters and words with increasing difficulty (i.e. longer words and lower frequency). The Rapid Letter Naming (rapid LN) on the Comprehensive Test of Phonological Processing (CTOPP-2, Wagner et al., 1999) was used as a measure of letter fluency. Children were required to read visually presented letters as quickly and accurately as possible. The Rapid Digit Naming (rapid DN) in CTOPP-2 was used to control for individual differences in general processing speed. The Phonological Awareness (PA) composite score in CTOPP-2, which included elision, blending, and phoneme isolation subtests, was used in our follow-up analyses.
There were originally 232 children who participated in our study. Thirty-nine participants were excluded because of excessive movement (see movement criteria in the data analysis section). Eighteen participants were excluded because of poor performance inside the scanner (see accuracy criteria in the auditory phonological task description section). One subject was excluded due to a time gap greater than 6 months between the two runs. Thirteen participants were excluded because of low scores on the CELF-5 core language or KBIT-2 non-verbal. Eight participants were excluded due to the absence of reading tests. After these screening criteria, we had 153 subjects in our final data analysis with one subject missing a PA score. Table 1 shows the mean, standard deviation, and the range for each test for these 153 subjects.
Table 1.
Descriptive statistics for each standardized test (n = 153).
| Mean (SD) | Range | |
|---|---|---|
| Non-verbal Score in KBIT-2 (standardized scores) | 110 (16) | 80−147 |
| Core Language Score in CELF-5 (standardized scores) | 104 (13) | 80−139 |
| Word ID in WJ-III (number correct) | 44 (9) | 18−63 |
| Rapid LN in CTOPP-2 (in seconds) | 23 (5) | 15−43 |
| Rapid DN in CTOPP-2 (in seconds) | 21 (5) | 13−39 |
| PA in CTOPP-2 (composite score) n = 152 | 32 (6) | 17−46 |
2.2. Auditory phonological task
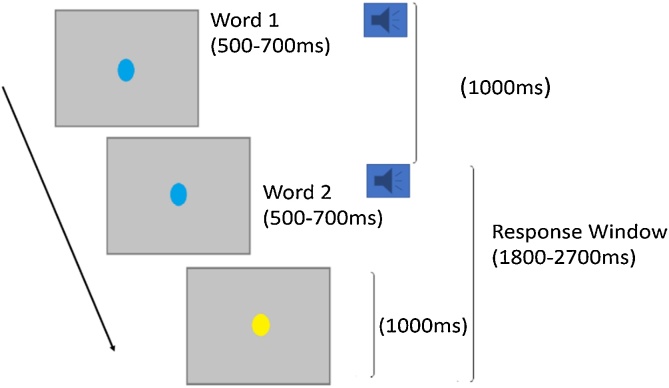
Our task used an event related design. On each trial, children heard two sequentially presented auditory words binaurally through earphones. There were four conditions: onset, rhyme, non-match, and perceptual control (see Table 2 for examples). Children were asked to judge “do the two words have any of the same sounds”. They were instructed to press the button with their right index finger for a yes response in the onset and rhyme conditions, and with the right middle finger for a no response in the non-match condition. Because the perceptual control condition serves as a baseline for the onset and rhyme conditions, children were also instructed to press the button with their right index finger for a yes response in this condition. For each trial procedure (see Fig. 1), the first auditory word was presented with a blue circle appearing on the screen. A second word then appeared 1000 milliseconds (ms) after the onset of the first word followed by a response interval of 1800 ms. The duration of each word was between 500 and 700 ms. The blue circle turned to yellow 1000 ms before the end of the trial to remind participants to respond timely. There were 24 trials for each condition, divided into two runs. The four conditions were pseudo-randomized so there were no more than 5 of the same response in a row. To aid in convolution of the hemodynamic response, inter-trial intervals were jittered by randomly adding 0, 450 or 900 ms to each trial, in equal proportions for the first run. For the second run, jitters of 0, 375 or 750 ms were similarly added to the trials. Each run lasted about 3 min.
Table 2.
Auditory Stimuli Conditions and Examples.
| Conditions | Response | Brief Explanation | Example |
|---|---|---|---|
| Onset | Yes | The two words start with the same sound | Coat – Cup |
| Rhyme | Yes | The two words rhyme | Wide – Ride |
| Non-match | No | The two words have no same sounds | Zip – Cone |
| Perceptual | Yes | Frequency modulated noise | Sh – Sh |
Fig. 1.
Experimental paradigm used in the current study.
The three word-conditions were designed according to the following standards. For the rhyme condition, the word pairs shared the same vowel and final phoneme/cluster (corresponding to 2–3 letters at the end of its written form). For the onset condition, the word pairs only shared the same initial phoneme (corresponding to one letter of its written form). For the non-match condition, there were no shared phonemes (or letters of its written form). All the words were monosyllabic. Every paired word had no semantic association based on the University of South Florida Free Association Norms (Nelson et al., 1998). There were no significant differences between conditions in phonotactic frequency (Vitevitch and Luce, 2004), word frequency (Balota et al., 2007), part of speech (Balota et al., 2007), and the phonological and orthographic consistency (Bolger et al., 2008). Neither irregular forms nor inflected forms of words were used. The auditory perceptual control was made of frequency-modulated noise .
Participants who scored within an acceptable accuracy range and had no response bias were included in our analysis. Acceptable accuracy was defined as the accuracy of the perceptual control condition being greater than 50 % to be confident that the children were engaged in the task. The lack of a response bias was indicated by the accuracy difference between the rhyme and non-match conditions being lower than 50 %. Prior to taking part in the fMRI scanning session, participants were required to complete the same task with different stimuli in the mock scanner and also before scanning in the practice room to make sure they understood the task and to acclimatize themselves to the scanner environment. However, we did not collect data from outside the scanner to confirm that children’s poorer performance on the onset than the rhyme condition was due to difficulty rather than scanner noise.
2.3. Data acquisition
Participants lay in the scanner with a response button box placed in their right hand. Visual stimuli were projected onto a screen, viewed via a mirror attached to the inside of the head coil. Participants wore earphones to hear the auditory stimuli and two ear pads were used to attenuate the scanner noise. The two phonological task runs were counterbalanced across participants.
Images were acquired using 3.0 T Skyra Siemens scanner with a 64-channel head coil. The blood oxygen level dependent (BOLD) signal was measured using a susceptibility weighted single-shot echo planar imaging (EPI) method. Functional images were acquired with multiband EPI (TE = 30 ms, flip angle = 80, matrix size = 128 × 128, FOV = 256 mm, slice thickness = 2 mm without gaps, number of slices = 56, TR = 1250 ms, Multi-band accel.factor = 4, voxel size = 2 × 2×2). A high resolution, T1 weighted 3D image was acquired. The following scan parameters were used: TR = 1900 ms, TE = 2.34 ms, matrix size = 256 × 256, field of view = 256 mm, slice thickness = 1 mm, number of slices = 192.
2.4. Data analysis
The SPM12 toolbox (Statistical Parametric Mapping) (http://www.fil.ion.ucl.ac.uk/spm) was used to analyze the data. First, all functional images were realigned to their mean functional image across runs. Then, the anatomical image was segmented and warped to the pediatric tissue probability map template (Wilke, et al., 2017) to obtain the transformation field. An anatomical brain mask was created by combining three segmentation products (i.e. grey, white and cerebrospinal fluid), and then applied to its original anatomical image to produce a skull-stripped image. After that, we co-registered the mean functional image and all functional images to the skull-stripped anatomical image. All the functional images were then normalized to the pediatric template by applying the transformation field and re-sampled with a voxel size of 2 × 2 × 2 mm in the standard space. Finally, all the normalized functional images were smoothed using a 6 mm isotropic Gaussian kernel.
We have created the pediatric tissue probability map template by using CerebroMatic (Wilke, et al., 2017), a tool that makes SPM12 compatible pediatric templates with user-defined age, gender, and magnetic field. We chose the unified segmentation parameters estimated from 1919 participants described in the Wilke et al. (2017) paper (parameters downloaded from https://www.medizin.uni-tuebingen.de/kinder/en/research/neuroimaging/software/). We defined ages as 5.5–8.5 years old with one-month interval, two females and two males at each age interval with 3 T scans, resulting in a sample size of 148 for our pediatric template.
Art-Repair (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html) was used to correct for the effect of motion on brain signal. The outlier volumes, defined as those with volume-to-volume head movement exceeding 1.5 mm in any direction, head movement greater than 5 mm in any directions from the mean functional image or deviations of more than 4% from the mean global signal, were repaired by interpolating using the adjacent non-outlier volumes. Participants having more than 10 % or more than 6 consecutive outlier volumes in each run were excluded from the current study.
First level statistical analysis was calculated using an event-related design with the four experimental conditions as conditions of interest. Six motion parameters estimated in the realignment step were entered in the first level modeling as regressors of no interest and the repaired volumes were de-weighted (Mazaika et al., 2009). A high pass filter with a cutoff of 128 s and the mask threshold of 0.5 were applied. All experimental trials were included in the analysis. Word and perceptual control pairs were treated as individual events for analysis and modeled using a canonical hemodynamic response function (HRF). Contrast maps were generated for onset versus perceptual and rhyme versus perceptual for each individual. Then, a one-sample t-test was used for group level analysis to display the activation maps for onset and rhyme processing at whole brain level. Cluster significance at the whole brain level was determined using a voxel-wise p < 0.001 uncorrected, cluster-wise p < 0.05 family wise error (FWE) corrected threshold in SPM12.
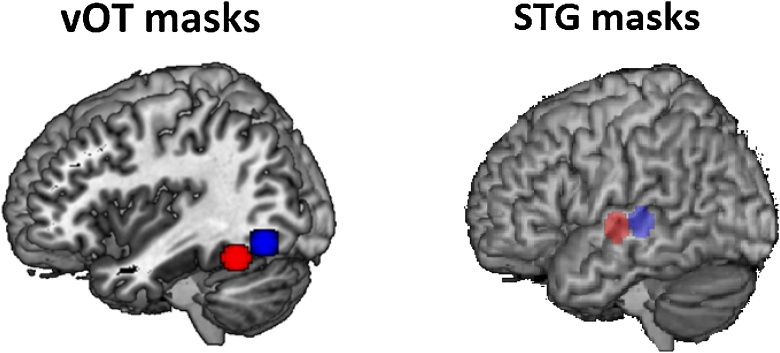
We then used ROI analyses to explore the relation between reading-related skills and brain activation in the anterior and posterior vOT for either onset or rhyme processing to examine our main hypotheses. According to the combination detector model by Dehaene et al. (2005), the posterior vOT is a letter sensitive region and the anterior vOT is a bigram/trigram sensitive region. We selected the classical visual word form area coordinate (-46, -53, -20 in MNI) from McCandliss et al. (2003) and the letter sensitive region coordinate (-36, -68, -12 in MNI) from Dehaene et al. (2004) as the centers for the anterior and the posterior vOT and then drew spheres around them with a radius of 7.5 mm. In this way, we created two adjacent anterior and posterior left vOT as our regions of interest without overlap, which should be sensitive to either bigrams/trigrams or letters (see Fig. 2).
Fig. 2.
The ventral occipitotemporal (vOT) masks and the superior temporal gyrus (STG) masks. The anterior part is colored in red, and the posterior in blue.
The top 100 most activated voxels for onset > perceptual within the anterior or the posterior vOT masks were selected based on the t values of that contrast for each individual, then beta values associated with each condition were extracted using MarsBar (http://marsbar.sourceforge.net/). The brain activation of onset > perceptual within the anterior or posterior vOT was calculated as the betas for the onset condition minus the betas for the perceptual control condition. The brain activation for the rhyme > perceptual within the anterior or posterior vOT was also extracted in the same way. This approach allowed us to select voxels that were most responsive to the experimental manipulation and were thus more sensitive in detecting brain effects. In support of this idea, Fedorenko et al. (2010) found that defining ROIs functionally in each individual showed greater specificity than using a group ROI. Tong et al. (2016) showed that selecting the top activated voxels for each individual within an anatomical ROI as compared to other methods was more sensitive in finding group differences. In addition to our current study, several previous studies have also used this approach to examine brain-behavioral correlations (Suárez‐Pellicioni and Booth, 2018; Younger et al., 2019; Wang et al., 2020).
After extracting the brain activation for onset > perceptual and rhyme > perceptual, we used SPSS to calculate the correlations between reading-related skills (i.e. reading accuracy and letter fluency) and brain activations for onset > perceptual or rhyme > perceptual in either the anterior or posterior vOT. Bonferroni correction (p equals to 0.05/4 = 0.0125) was used to account for multiple comparisons in each region of interest.
In addition to ROI analyses, we conducted a voxel-wise regression analysis to explore if reading-related skills were related to activation in any other brain regions outside the anterior and posterior vOT. The regression analyses were only conducted for the onset > perceptual and rhyme > perceptual contrasts. We used the regression analysis implemented within the SPM12 to analyze the relation between reading accuracy and brain activation because reading accuracy followed a normal distribution. However, because letter fluency violated the normal distribution, we used the multi-subject simple regression analysis with the Statistical nonParametric Mapping toolbox (SnPM13, http://www.nisox.org/Software/SnPM) to analyze the relation. We used an updated version of AFNI’s 3dClustSim, following the suggestion by Eklund et al. (2016), to calculate the number of voxels needed for a significant cluster within the whole brain mask after excluding the anterior and posterior vOT (140,132 voxels). Averaged ACF values (0.47, 4.56, 12.76) calculated by 3dFWHMx from each participant were entered into 3dClustSim (Cox et al., 2016). The voxel size was 2 × 2 × 2 mm. A significant cluster required more than 79 voxels at a voxel-wise threshold at p < 0.001 and cluster-wise threshold at the corrected level of p < 0.05.
Because we did not find any reading-related skill correlations with brain activation for onset > perceptual in vOT, we hypothesized that children may rely more on their refined phonological representation in the left superior temporal gyrus (STG), a phonological processing region, to solve small grain problems. Therefore, we conducted a follow-up correlational analysis to examine if phonological awareness (PA) skill was more related to brain activation for onsets in the left STG as compared to rhymes. We defined ROIs in STG according to the hierarchical structure of STG identified by Dewitt and Rauschecker (2012) where the mid-STG is sensitive to smaller grain phonemes, whereas the anterior STG is sensitive to larger grain phonology such as whole words. We selected two peaks (mid STG peak at -57, -25, 1; anterior STG peak at -57, -17, -1) from Dewitt et al. (2012). However, we shifted the y coordinates 3 mm backwards or forwards (peaks at -47, -28, 1 and -57, -14, -1) to separate the centers for mid STG and anterior STG so regions did not overlap. We then drew spheres around the two centers with a radius of 7.5 mm. In this way, we created two adjacent phonological processing masks, i.e. the mid and anterior STG (see Fig. 2 on the right).
The top 100 most activated voxels for onset > perceptual within the anterior or the mid STG masks were selected based on the t values of that contrast for each individual. Beta values associated with each condition were then extracted using MarsBar (http://marsbar.sourceforge.net/). The brain activation of onset > perceptual within anterior or mid STG was calculated as the betas for the onset condition minus the betas for the perceptual control condition. The brain activation for the rhyme > perceptual within anterior or mid STG was also extracted in the same way. We used SPSS to calculate the correlations between phonological awareness (PA) and brain activations for onset > perceptual or rhyme > perceptual in either anterior or mid STG. The Bonferroni correction (p equals to 0.05/2 = 0.025) was used to account for multiple comparisons in each region of interest.
3. Results
3.1. Behavioral results
Table 3 represents the accuracies for the auditory phonological task and their correlations with two reading-related skills (i.e. reading accuracy and letter fluency) and a control variable (i.e. a general processing speed measure). Reading accuracy was measured by the Woodcock Johnson Letter Word Identification (Word ID) test and letter fluency was measured by the CTOPP-2 Rapid Letter Naming (Rapid LN) test. The CTOPP-2 Rapid Digit Naming (Rapid DN) test was used as a control variable for general processing speed. For the analyses of reading accuracy, which followed a normal distribution, we used the Pearson correlational analysis. For the analyses of letter fluency, which violated a normal distribution, we used the non-parametric Spearman correlational analysis. We found that reading-related skills as measured by both reading accuracy and letter fluency were significantly correlated with accuracies in the onset, rhyme, and non-match conditions during the in-scanner auditory phonological task. These correlations suggest that children with higher reading-related skills had better phonological processing.
Table 3.
Mean accuracies for each condition in the auditory phonological awareness task and their correlations with reading-related skills (n = 153).
| Conditions | Mean (SD) | Range | Word ID |
Rapid LN |
Rapid DN |
||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | ||||
| Onset (%) | 68.0 (16.0) | 21−96 | .231 | .002 | -.205 | .006 | -.310 | <.001 | |
| Rhyme (%) | 84.8 (13.1) | 38−100 | .181 | .013 | -.187 | .010 | -.128 | .057 | |
| Non-match (%) | 80.2 (14.5) | 46−100 | .241 | .001 | -.215 | .004 | -.180 | .013 | |
| Perceptual (%) | 95.4 (6.1) | 75−100 | .053 | .259 | .105 | .098 | .010 | .451 | |
| Word ID | – | – | – | – | -.337 | <.001 | -.339 | <.001 | |
| Rapid LN | – | – | – | – | – | – | .663 | <.001 | |
Note: Word ID refers to the Woodcock Johnson Letter Word Identification and served as a measure of reading accuracy. Rapid LN refers to CTOPP-2 Rapid Letter Naming and served as a measure of letter fluency with lower scores indicating better fluency.
3.2. Brain results
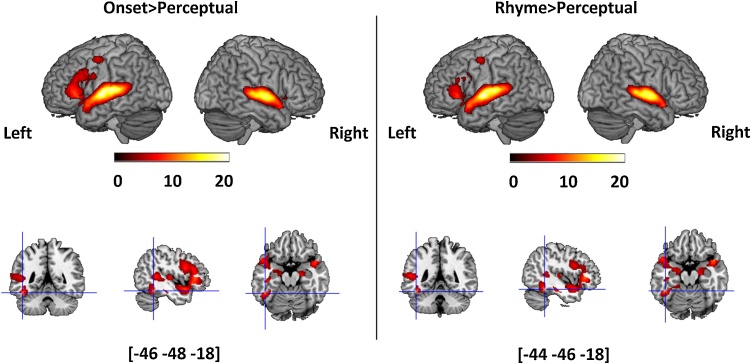
Fig. 3 and Table 4 show the significant clusters found for onset > perceptual and rhyme > perceptual at whole brain level. The activation maps for onset and rhyme processing were very similar. Both onset and rhyme processing activated the anterior vOT regions and the bilateral superior temporal gyrus (STG), which extended into the left inferior frontal gyrus (IFG).
Fig. 3.
Brain activation for onset > perceptual and rhyme > perceptual at whole brain level at a threshold of voxel-wise p < 0.001 uncorrected, cluster-wise p < 0.05 FWE-corrected. The top row shows activation in a rendered brain surface for each contrast. The bottom row with the blue crossing shows the location of a significantly activated cluster in the anterior vOT for each contrast. Color Bar shows the t values for the contrast.
Table 4.
Significant clusters for onset > perceptual and rhyme > perceptual at whole brain level.
| Region | Hemisphere | Brodmann Area | Peak coordinates [x y z] |
Cluster size (2 mm voxels) | T (peak) |
|---|---|---|---|---|---|
| Onset > Perceptual | |||||
| Superior Temporal Gyrus/ Inferior Frontal Gyrus | L | 22/47/45/44 | −62 -8 0 | 6534 | 20.00 |
| Superior Temporal Gyrus | R | 22 | 66 -10 -2 | 2595 | 17.08 |
| Inferior Temporal Gyrus | L | 20/37 | −46 -48 -18 | 568 | 10.12 |
| Caudate | L | – | −10 12 8 | 1140 | 8.57 |
| Supplementary Motor | L | 8 | −4 18 50 | 586 | 8.22 |
| Precentral Gyrus | L | 6 | −54 -4 50 | 148 | 6.16 |
| Insula | R | 13 | 34 20 0 | 167 | 6.16 |
| Rhyme > Perceptual | |||||
| Superior Temporal Gyrus /Inferior Frontal Gyrus | L | 22/47/45/44 | −62 -8 0 | 5153 | 19.66 |
| Superior Temporal Gyrus | R | 22 | 66 -6 -2 | 2493 | 16.69 |
| Fusiform Gyrus | L | 37 | −44 -46 -18 | 377 | 10.61 |
| Caudate | L | – | −16 6 4 | 523 | 6.47 |
| Precentral Gyrus | L | 6 | −52 -4 50 | 124 | 6.07 |
| Caudate | R | – | 16 8 2 | 234 | 5.46 |
Correlational analyses were conducted to examine whether brain activation for the different grain sizes of phonology in either anterior or posterior vOT were related to reading-related skills. In the anterior vOT, we failed to find that reading accuracy, as measured by word ID, correlated with brain activation for either onset [r(151) = 0.084, p = 0.151] or rhyme [r(151) = 0.052, p = 0.262] processing. However, we found that letter fluency, as measured by Rapid LN, was significantly correlated with brain activation in the anterior vOT only for rhyme [r(151) =-0.214, p = 0.004] but not onset processing [r(151)=-0.103, p = 0.103]. Fig. 4 shows the scatterplot of the correlation between rapid LN and brain activation in the anterior vOT for rhyme processing (left) and for onset processing (right). This correlation was still significant after partialing general processing speed as measured by Rapid Digit Naming [r(151) = -0.151, p = 0.031], suggesting that this relation was mainly due to reading-related skills and not general processing speed or phonological coding. In regard to the posterior vOT, we found that neither reading accuracy nor letter fluency was correlated with brain activation for either onset [reading accuracy: r(151) = 0.034, p = 0.338; letter fluency: r(151)=-0.037, p = 0.325] or rhyme processing [reading accuracy: r(151)=-0.108, p = 0.092; letter fluency: r(151)=-0.016, p = 0.422].
Fig. 4.
The correlation between Rapid LN and brain activation in the anterior vOT for rhyme processing (left) and onset processing (right). Rapid LN refers to rapid letter naming.
To ascertain whether letter fluency was specifically related to the anterior but not posterior vOT for rhyme but not onset, we compared the correlation of rhyme in the anterior vOT [r(151)=-0.214, p = 0.004] with the correlation in the posterior vOT [r(151)=-0.016, p = 0.422], and compared the correlation in the anterior vOT for rhyme [r(151)=-0.214, p = 0.004] with the correlation for onset [r(151)=-0.103, p = 0.103]. We found that the correlation of letter fluency to brain activation for rhyme in the anterior vOT was significantly greater than in the posterior vOT (Fisher z = -2.665, p = 0.004). There was also a trend that the correlation of letter fluency to anterior vOT’s activation for rhyme was greater than that for onset (Fisher z =-1.637, p = 0.051). All together, these analyses showed that, in 7−8-year-old children, letter fluency was only correlated with brain activation in the anterior vOT for rhyme but not onset.1
After the analyses of vOT, we conducted a voxel-wise regression analysis at whole brain level outside the vOT masks for onset and rhyme processing. However, we did not find any significant clusters correlated with either word ID or rapid LN.
Because we only showed a brain-behavioral correlation for rhyme but not onset, we conducted follow-up analyses to examine if onset processing relied on refined phonological representations stored in STG. We analyzed the correlations between phonological awareness (PA) skill and brain activation for onset and rhyme in the left anterior STG and in the mid STG. In the anterior STG, we found that PA was significantly correlated with brain activation for onset [r(150) = 0.186, p = 0.011]. However, we did not find a significant correlation for rhyme [r(150) = 0.151, p = 0.031] after the Bonferroni correction (p = 0.05/2 = 0.025) for multiple comparisons. Fig. 5 shows the scatterplot of the correlation between PA and brain activation in the anterior STG for rhyme processing (left) and for onset processing (right). In contrast to the anterior STG, we found no significant correlations of PA to brain activation in the mid STG for either onset [r(150) = 0.070, p = 0.196] or rhyme [r(150) = 0.039, p = 0.317]. Together, we only observed a significant correlation between phonological skill and brain activation in the anterior STG for onset.
Fig. 5.
The correlation between Phonological Awareness skill and brain activation in the anterior STG for rhyme processing (left) and onset processing (right).
Similar to our analyses of vOT, we examined the specificity of the correlation effect. We compared the correlation of PA to the anterior STG’s activation for onset [r(150) = 0.186, p = 0.011] versus for rhyme [r(150) = 0.151, p = 0.031], and compared the correlation of PA to onset in the anterior STG [r(150) = 0.186, p = 0.011] versus mid STG [r(150) = 0.070, p = 0.196]. We found that there was no significant difference between onset and rhyme in the anterior STG (Fisher z = 0.664, p = 0.253). However, a trend of a greater correlation of PA to onset in the anterior STG than in the mid STG was indicated (Fisher z = 1.527, p = 0.063).
4. Discussion
Previous studies have shown that the posterior vOT, a region involved in representing letters, is often automatically activated during auditory phonological tasks in young preschool and kindergarten children, whereas the anterior vOT, a region involved in processing larger orthographic grain sizes such as rimes, is often found in older elementary and middle school children. In the current study, we aimed to address the age gap in previous studies and examine if this automatic activation of spelling representations in the anterior vOT emerges in younger elementary children. To answer this question, we recruited 7- to 8-year-old children, which filled the age gap in previous studies on younger (i.e. age 3–6) and older children (i.e. age 8–15). We measured children’s brain activity during an auditory phonological task that included both small (i.e. onset) and large (i.e. rhyme) grain size conditions. We found that in 7- to 8-year-old children, reading-related skill, as measured by letter fluency was related to brain activation in the anterior vOT for the rhyme condition. Letter fluency was not related to activation in posterior vOT for the rhyme condition, or during onset judgments in either region of interest. Moreover, reading accuracy was not correlated with activation in any comparisons. These findings suggest that 7- to 8-year-old children have already begun to automatically activate spelling representations at the large grain size in the anterior vOT for spoken words, similar to older elementary children and adults. The findings also suggest that this automatic engagement of orthography when listening to words was driven by children’s automaticity in naming familiar visual symbols but not the ability to accurately decode visual words, as the effects only emerged with letter fluency.
Our main finding was that reading-related skill only correlated with brain activation in the anterior vOT for rhyme processing, suggesting that 7- to 8-year-old children automatically activate large grain orthographic representations during auditory phonological tasks. This result is consistent with previous studies on older elementary children aged 8–15 (e.g. Cone et al., 2008; Desroches et al., 2010; Dębska et al., 2019) which found that reading skills were associated with activation in the anterior but not posterior vOT during rhyme and pseudoword matching tasks. In contrast to studies on older children, previous studies on children aged 3–6 (e.g. Raschle et al., 2012; Hutton et al., 2015; Powers et al., 2016; Wang et al., 2018) showed that reading was associated with brain activation only in the posterior but not anterior vOT, suggesting that younger children automatically activate their small grain orthographic representations during auditory phonological tasks. This transition from posterior to anterior vOT during auditory phonological tasks is likely a result of reading acquisition. According to Frith (1985), children’s reading develops from the alphabetic stage, using small grain letter-to-phoneme mapping, to the orthographic stage, employing large grain orthography-to-phonology mapping at the word level. Our study showed evidence that automatic activation in the anterior vOT during spoken language processing occurs as young as 7–8 years old, similar to older children and adults, suggesting that early elementary children have likely transitioned to the orthographic stage of reading, employing large grain mapping.
We only found that letter fluency, but not reading accuracy, was significantly related to activation in the anterior vOT, suggesting that letter fluency plays a more important role than reading accuracy in the automatic activation of vOT. Based on literature showing that typical children learn to decode accurately first, and then become fluent with lots of practice (Norton and Wolf, 2012), we expected letter fluency to play a more important role than reading accuracy in the automatic activation in vOT in older compared to younger children. Previous studies partially support this idea by showing that reading accuracy plays a role in the automatic activation of vOT in younger children or those with lower reading skills. In 5- to 6-year-olds, Wang et al. (2018) found that reading accuracy was related to activation in posterior vOT. In 9- to 15-year-olds, however, Desroches et al. (2010) found no correlation between reading accuracy and brain activation in vOT in typically developing children. Instead, they found a brain-behavior correlation only in children with dyslexia. In contrast to other studies, which only used reading accuracy (e.g. Wang et al., 2018; Desroches et al., 2010) or used a combination of reading accuracy and fluency (e.g. Dębska et al., 2019), we separately examined the effects of reading accuracy and letter fluency. Together with our previous study on 5- to 6-year-old children (e.g. Wang et al., 2018), our current findings with 7- to 8-year-old children suggests a progression from the role of early reading accuracy to later letter fluency in the determinants of orthographic involvement in spoken language processing.
Even though we found a reliable correlation between letter fluency and brain activation in the anterior vOT for rhyme, the correlational coefficient was relatively low (r=-0.214), probably because factors other than letter fluency also affect the activation in vOT during spoken language processing. For example, studies have found that task demand modulated the activation in vOT at a similar location to our current study, with greater activation in vOT during more lexically demanding tasks (e.g. Planton et al., 2019). The vOT also appears to contain some spoken language selective neurons, the activation of which is independent of written language skills (e.g. Pattamadilok et al., 2019). Children could choose to rely on either orthographic or phonological strategies to complete an auditory phonological awareness task (Stuart, 1990). All of these factors may contribute to the low correlation coefficient in our study. However, the fact that we found a significant correlation between letter fluency and brain activation in vOT suggests that children automatically converted spoken language to spelling patterns during spoken language tasks.
A limitation of the current study is that letter fluency, as measured by the rapid letter naming task, could be considered a measurement of small grain size. Measuring reading fluency using words or pseudowords allows a direct examination of the automaticity of mapping between visual letters and sound at a larger grain size, which future studies should investigate. However, rapid letter naming may provide a better measure of mapping automaticity between visual symbols to sound, with less impact from individual differences in lexical skills. Previous research has shown that rapid naming correlates with word reading fluency across languages (e.g. see meta-analyses in Scarborough, 1998; Swanson et al., 2003; Araújo et al., 2015; Song et al., 2016). Onochie-Quintanilla et al. (2019) showed that rapid naming made comparable contributions to word/nonword reading speed with both high and low syllable frequencies, suggesting that rapid naming operates at all grain size levels from the grapho-phonemic to the grapho-syllabic to the whole-word levels. Therefore, even though on the face of it rapid letter naming appears to tap into small grain size, it has been shown to reflect the automaticity of naming familiar prints at different grain size levels.
We did not find any correlations between reading-related skills and brain activation in vOT for small grain sizes as measured by our onset condition. This is probably because reading has transitioned to the orthographic stage in our 7- to 8-year-old children. Instead of automatically activating orthographic letter representations in the posterior vOT like younger beginning readers (e.g. Raschle et al., 2012; Hutton et al., 2015; Powers et al., 2016; Wang et al., 2018), 7-to 8-year-old children may rely more on their refined phonological skills to perform tasks requiring phonemic judgments at the level of the onset. Previous behavioral research has shown that children aged 3–7 become more refined in phonological output from the syllabic to phonemic level (Nittrouer et al., 1989). The left superior temporal gyrus is a region that is reliably engaged during auditory phonological awareness tasks (e.g. Boets et al., 2013; Leonard and Chang, 2014; Weiss et al., 2018; Vandermosten et al., 2020; Wang et al., 2020). Therefore, it is possible that 7- to 8-year-old children rely more on their refined phonological representations in the left superior temporal gyrus to solve small grain size problems.
Our additional analysis on the relation between phonological awareness skills (PA) and brain activation in the anterior and mid STG for either onset or rhyme supports this idea. We found that PA was only correlated with brain activation in the anterior STG for onset. This finding is consistent with a previous study (Blau et al., 2009) on adults with dyslexia where phonological skills were positively related to brain activation in STG during a passive listening task on phonemes. The lack of a significant correlation between phonological skills and rhyme processing in our study is also consistent with a previous study on children aged 8–13 years old (McNorgan et al., 2013), which found no relation between phonemic awareness skill and brain activation during an auditory rhyming task. According to the developmental theory of phonological awareness, rhyme awareness matures earlier than phonemic awareness (Anthony and Francis, 2005). It is likely that phonemic judgment relies more on phonological representation in STG with skill improvement. Although a direct comparison of the correlations between onset and rhyme in our study did not reveal a significant difference, our finding of a significant correlation between PA and onset suggests that 7−8-year-old children rely on phonological representations in STG for small grain phonemic awareness judgment.
One interesting finding of this additional analysis is that the correlation between phonological skill and brain activation for onset was only found in the anterior STG but not the mid STG. This is unexpected because according to the meta-analysis by Dewitt et al. (2012), the mid STG should be sensitive to single phonemes whereas the anterior STG should be sensitive to combinations of phonemes at the word level. So, we should expect to find a correlation for the small grain onset condition in the mid STG. A previous study by Blau et al. (2009) also showed that reading skill was correlated with brain activation in the mid STG during a passive listening task on phonemes. The fact that we found a correlation in the anterior but not mid STG is probably because we used auditory words as stimuli, unlike Blau et al. (2009) which used single phonemes as stimuli.
Overall, our current findings were based on English-speaking children and we showed that 7- to 8-year olds who have likely transitioned to the orthographic stage of reading relied on automatic activation of orthographic representations in vOT for larger grain rhyme processing. They also relied on phonological representations in STG for smaller grain onset processing. However, English, as an opaque language, has less consistent mapping between letters and phonemes, but more consistent mapping from spelling to pronunciation at the rhyme level (Treiman et al., 1995). Unlike opaque languages, both rhyme and phoneme could be processed similarly at a small grain size phonemic level in transparent languages. Whether children speaking transparent langauges will rely on automatic orthographic activation for larger grain phonological processing remains to be seen. A previous literature review (Richlan, 2014) showed that there is greater activation in the superior temporal gyrus (STG) for transparent than opaque languages in skilled readers. Therefore, we expect that as children transition to the orthographic stage of reading, those speaking more transparent languages will not rely on orthographic representations in vOT for rhyme but will rely on their phonological representations in STG for both phonemic and rhyme processing. However, this hypothesis remains to be examined in future studies.
In conclusion, the current study recruited 7- to 8-year-old children to examine the automatic activation of vOT during spoken language processing. We found that reading-related skill was only correlated with brain activation in the anterior vOT for the rhyme condition, suggesting that these early elementary school children have already engaged larger grain orthographic representation for spoken words, similar to older children and adults. In addition, we showed that letter fluency, but not reading accuracy, was associated with the automatic activation of anterior vOT, suggesting that the automaticity of mapping visual letters to sounds is a critical determinant in the engagement of orthographic representations for spoken words.
Data statement
We are in the process of making the data from the entire project available on openNeuro, and will have it posted at the latest by September 2021. We will also supply the data to individual researchers before that time upon request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by National Institute of Health (R01 DC013274) to James R. Booth.
Footnotes
We not only used 6mm smoothing kernel but also applied 4mm smoothing kernel to re-analyze our data using top 100 voxels. Our results remained the same. In addition, we used the top 50 and top 150 to examine the stability of our results at different threshold of ROI selections. We again found the same effects.
References
- Anthony J.L., Francis D.J. Development of phonological awareness. Curr. Dir. Psychol. Sci. 2005;14(5):255–259. [Google Scholar]
- Araújo S., Reis A., Petersson K.M., Faísca L. Rapid automatized naming and reading performance: a meta-analysis. J. Educ. Psychol. 2015;107(3):868. [Google Scholar]
- Balota D.A., Yap M.J., Cortese M.J., Hutchison K.A., Kessler B., Loftis B., Neely J.H., Nelson D.L., Simpson G.B., Treiman R. The english lexicon project. Behav. Res. Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Blau V., van Atteveldt N., Ekkebus M., Goebel R., Blomert L. Reduced neural integration of letters and speech sounds links phonological and reading deficits in adult dyslexia. Curr. Biol. 2009;19(6):503–508. doi: 10.1016/j.cub.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Boets B., de Beeck H.P.O., Vandermosten M., Scott S.K., Gillebert C.R., Mantini D., Bulthe J., Sunaert S., Wouters J., Ghesquière P. Intact but less accessible phonetic representations in adults with dyslexia. Science. 2013;342(6163):1251–1254. doi: 10.1126/science.1244333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger D.J., Hornickel J., Cone N.E., Burman D.D., Booth J.R. Neural correlates of orthographic and phonological consistency effects in children. Hum. Brain Mapp. 2008;29(12):1416–1429. doi: 10.1002/hbm.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S., Halder P., Bucher K., Summers P., Martin E., Brandeis D. Tuning of the visual word processing system: distinct developmental ERP and fMRI effects. Hum. Brain Mapp. 2009;30(6):1833–1844. doi: 10.1002/hbm.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S., Bach S., Kucian K., Kujala J.V., Guttorm T.K., Martin E., Lyytinen H., Brandeis D., Richardson U. Brain sensitivity to print emerges when children learn letter–speech sound correspondences. Proc. Natl. Acad. Sci. 2010;107(17):7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chall J.S. 2nd ed. Harcourt-Brace; Fort Worth, TX: 1996. Stages of Reading Development. [Google Scholar]
- Cone N.E., Burman D.D., Bitan T., Bolger D.J., Booth J.R. Developmental changes in brain regions involved in phonological and orthographic processing during spoken language processing. NeuroImage. 2008;41(2):623–635. doi: 10.1016/j.neuroimage.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W., Reynolds R.C., Taylor P.A. AFNI and clustering: false positive rates redux. BioRxiv. 2016 doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dębska A., Chyl K., Dzięgiel G., Kacprzak A., Łuniewska M., Plewko J., Marchewka A., Grabowska A., Jednoróg K. Reading and spelling skills are differentially related to phonological processing: behavioral and fMRI study. Dev. Cogn. Neurosci. 2019;39 doi: 10.1016/j.dcn.2019.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S., Jobert A., Naccache L., Ciuciu P., Poline J.B., Le Bihan D., Cohen L. Letter binding and invariant recognition of masked words: behavioral and neuroimaging evidence. Psychol. Sci. 2004;15(5):307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L., Sigman M., Vinckier F. The neural code for written words: a proposal. Trends Cogn. Sci. (Regul. Ed.) 2005;9(7):335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Pegado F., Braga L.W., Ventura P., Nunes Filho G., Jobert A., Dehaene-Lambertz G., Kolinsky R., Morais J., Cohen L. How learning to read changes the cortical networks for vision and language. Science. 2010;330(6009):1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- Desroches A.S., Cone N.E., Bolger D.J., Bitan T., Burman D.D., Booth J.R. Children with reading difficulties show differences in brain regions associated with orthographic processing during spoken language processing. Brain Res. 2010;1356:73–84. doi: 10.1016/j.brainres.2010.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt I., Rauschecker J.P. Phoneme and word recognition in the auditory ventral stream. Proc. Natl. Acad. Sci. 2012;109(8):E505–E514. doi: 10.1073/pnas.1113427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Hsieh P.J., Nieto-Castañón A., Whitfield-Gabrieli S., Kanwisher N. New method for fMRI investigations of language: defining ROIs functionally in individual subjects. J. Neurophysiol. 2010;104(2):1177–1194. doi: 10.1152/jn.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U. Beneath the surface of developmental dyslexia. Surface dyslexia. 1985;32:301–330. [Google Scholar]
- Hutton J.S., Horowitz-Kraus T., Mendelsohn A.L., DeWitt T., Holland S.K., C-Mind Authorship Consortium Home reading environment and brain activation in preschool children listening to stories. Pediatrics. 2015;136(3):466–478. doi: 10.1542/peds.2015-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A.S., Kaufman N.L. second edition. Pearson; Bloomington, MN: 2004. Kaufman Brief Intelligence Test. [Google Scholar]
- Leonard M.K., Chang E.F. Dynamic speech representations in the human temporal lobe. Trends Cogn. Sci. (Regul. Ed.) 2014;18(9):472–479. doi: 10.1016/j.tics.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Schurz M., Kronbichler M., Richlan F. Reading in the brain of children and adults: a meta‐analysis of 40 functional magnetic resonance imaging studies. Hum. Brain Mapp. 2015;36(5):1963–1981. doi: 10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika P., Hoeft F., Glover G.H., Reiss A.L. Methods and software for fMRI analysis for clinical subjects. Human Brain Mapping Conference. 2009 [Google Scholar]
- McCandliss B.D., Cohen L., Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McNorgan C., Randazzo-Wagner M., Booth J.R. Cross-modal integration in the brain is related to phonological awareness only in typical readers, not in those with reading difficulty. Front. Hum. Neurosci. 2013;7:388. doi: 10.3389/fnhum.2013.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.L., McEvoy C.L., Schreiber T.A. 1998. The University of South Florida word Association, Rhyme, and Word Fragment Norms.http://www.usf.edu/FreeAssociation/ [DOI] [PubMed] [Google Scholar]
- Nittrouer S., Studdert-Kennedy M., McGowan R.S. The emergence of phonetic segments: evidence from the spectral structure of fricative-vowel syllables spoken by children and adults. J. Speech Lang. Hear. Res. 1989;32(1):120–132. [PubMed] [Google Scholar]
- Norton E.S., Wolf M. Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilities. Annu. Rev. Psychol. 2012;63:427–452. doi: 10.1146/annurev-psych-120710-100431. [DOI] [PubMed] [Google Scholar]
- Onochie-Quintanilla E., Defior S.A., Simpson I.C. RAN and orthographic processing: what can syllable frequency tell us about this relationship? J. Exp. Child Psychol. 2019;182:1–17. doi: 10.1016/j.jecp.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Pattamadilok C., Planton S., Bonnard M. Spoken language coding neurons in the visual word form area: evidence from a TMS adaptation paradigm. NeuroImage. 2019;186:278–285. doi: 10.1016/j.neuroimage.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Planton S., Chanoine V., Sein J., Anton J.L., Nazarian B., Pallier C., Pattamadilok C. Top-down activation of the visuo-orthographic system during spoken sentence processing. NeuroImage. 2019;202 doi: 10.1016/j.neuroimage.2019.116135. [DOI] [PubMed] [Google Scholar]
- Powers S.J., Wang Y., Beach S.D., Sideridis G.D., Gaab N. Examining the relationship between home literacy environment and neural correlates of phonological processing in beginning readers with and without a familial risk for dyslexia: an fMRI study. Ann. Dyslexia. 2016;66(3):337–360. doi: 10.1007/s11881-016-0134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N.M., Zuk J., Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc. Natl. Acad. Sci. 2012;109(6):2156–2161. doi: 10.1073/pnas.1107721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F. Functional neuroanatomy of developmental dyslexia: the role of orthographic depth. Front. Hum. Neurosci. 2014;8:347. doi: 10.3389/fnhum.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough H.S. Predicting the future achievement of second graders with reading disabilities: contributions of phonemic awareness, verbal memory, rapid naming, and IQ. Ann. Dyslexia. 1998;48(1):115–136. [Google Scholar]
- Seymour H.N., Roeper T., De Villiers J.G. DELV: diagnostic evaluation of language variation: screening test. PsychCorp. 2003 [Google Scholar]
- Song S., Georgiou G.K., Su M., Hua S. How well do phonological awareness and rapid automatized naming correlate with Chinese reading accuracy and fluency? a meta-analysis. Sci. Stud. Read. 2016;20(2):99–123. [Google Scholar]
- Stuart M. Processing strategies in a phoneme deletion task. Q. J. Exp. Psychol. 1990;42(2):305–327. doi: 10.1080/14640749008401224. [DOI] [PubMed] [Google Scholar]
- Suárez‐Pellicioni M., Booth J.R. Fluency in symbolic arithmetic refines the approximate number system in parietal cortex. Hum. Brain Mapp. 2018;39(10):3956–3971. doi: 10.1002/hbm.24223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson H.L., Trainin G., Necoechea D.M., Hammill D.D. Rapid naming, phonological awareness, and reading: a meta-analysis of the correlation evidence. Rev. Educ. Res. 2003;73(4):407–440. [Google Scholar]
- Taylor J.S.H., Rastle K., Davis M.H. Can cognitive models explain brain activation during word and pseudoword reading? a meta-analysis of 36 neuroimaging studies. Psychol. Bull. 2013;139(4):766. doi: 10.1037/a0030266. [DOI] [PubMed] [Google Scholar]
- Tong Y., Chen Q., Nichols T.E., Rasetti R., Callicott J.H., Berman K.F., Weinberger D.R., Mattay V.S. Seeking optimal region-of-interest (ROI) single-value summary measures for fMRI studies in imaging genetics. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0151391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiman R., Mullennix J., Bijeljac-Babic R., Richmond-Welty E.D. The special role of rimes in the description, use, and acquisition of English orthography. J. Exp. Psychol. Gen. 1995;124(2):107–136. doi: 10.1037//0096-3445.124.2.107. [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Correia J., Vanderauwera J., Wouters J., Ghesquière P., Bonte M. Brain activity patterns of phonemic representations are atypical in beginning readers with family risk for dyslexia. Dev. Sci. 2020;23(1) doi: 10.1111/desc.12857. [DOI] [PubMed] [Google Scholar]
- Vitevitch M.S., Luce P.A. A web-based interface to calculate phonotactic probability for words and nonwords in English. Behav. Res. Methods Instrum. Comput. 2004;36(3):481–487. doi: 10.3758/bf03195594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R., Torgesen J., Rashotte C., Pearson N.A. Pro-ed; Austin, TX: 1999. CTOPP-2: Comprehensive Test of Phonological Processing–Second Edition. [Google Scholar]
- Wang J., Joanisse M.F., Booth J.R. Reading skill related to left ventral occipitotemporal cortex during a phonological awareness task in 5–6-year old children. Dev. Cogn. Neurosci. 2018;30:116–122. doi: 10.1016/j.dcn.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Joanisse M.F., Booth J.R. Neural representations of phonology in temporal cortex scaffold longitudinal reading gains in 5-to 7-year-old children. NeuroImage. 2020;207 doi: 10.1016/j.neuroimage.2019.116359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss Y., Cweigenberg H.G., Booth J.R. Neural specialization of phonological and semantic processing in young children. Hum. Brain Mapp. 2018;39(11):4334–4348. doi: 10.1002/hbm.24274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig E.H., Semel E., Secord W.A. 5th ed.) Pearson; San Antonio: 2013. Clinical Evaluation Language Fundamentals (CELF-5) [Google Scholar]
- Wilke M., Altaye M., Holland S.K., CMIND Authorship Consortium CerebroMatic: a versatile toolbox for spline-based MRI template creation. Front. Comput. Neurosci. 2017;11:5. doi: 10.3389/fncom.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock R.W., McGrew K.S., Mather N. Riverside Publishing; Itasca, IL: 2001. Woodcock-Johnson III. [Google Scholar]
- Yoncheva Y.N., Zevin J.D., Maurer U., McCandliss B.D. Auditory selective attention to speech modulates activity in the visual word form area. Cereb. Cortex. 2009;20(3):622–632. doi: 10.1093/cercor/bhp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger J.W., Lee K.W., Demir‐Lira O.E., Booth J.R. Brain lateralization of phonological awareness varies by maternal education. Dev. Sci. 2019;22(6) doi: 10.1111/desc.12807. [DOI] [PubMed] [Google Scholar]