Abstract
Background:
There is little evidence in the literature on the association between methylphenidate treatment and psychotic symptoms in children and adolescents with attention-deficit/hyperactivity disorder (ADHD).
Objective:
We examine the occurrence of psychotic symptoms during methylphenidate treatment of children and adolescents with ADHD. The data arise from our two Cochrane systematic reviews on methylphenidate, reported elsewhere.
Methods:
Electronic databases were searched up to January 2016 (for observational studies) and March 2017 (for randomized trials). We summarized data as risk ratios and pooled prevalences. Trial Sequential Analysis was used to control for random errors. We assessed the risk of bias and the quality of evidence according to Cochrane guidelines.
Results:
Ten randomized trials (1103 participants), 17 non-randomized studies (76,237 participants) and 12 patient reports or series (18 patients) were identified. In the randomized trials, there was no significant difference in the risk of developing psychotic symptoms [10 of 654 (pooled prevalence, 2.5%) methylphenidate versus 1 of 508 (pooled prevalence, 1.7%) placebo patients; risk ratio, 2.07; 95% confidence interval, 0.58 to 7.35]. Nine of 10 trials had a high risk of bias, and according to the Trial Sequential Analysis, the required information size was not achieved, that is, the meta-analysis was considerably underpowered. There were 873 instances of psychotic symptoms in the non-randomized studies among 55,603 participants (pooled prevalence, 1.2%; 95% confidence interval, 0.7 to 2.4). In the comparative cohort study, methylphenidate significantly increased the risk for any psychotic disorder by 36% (risk ratio, 1.36; 95% confidence interval, 1.17 to 1.57). The overall risk of bias was rated as critical for this study.
Conclusions:
Because of sparse data and low quality of evidence, we cannot confirm or refute whether methylphenidate increases the risk of psychotic symptoms in children and adolescents with ADHD. This possible adverse event may affect 1.1% to 2.5%, and physicians, patients and caregivers should be aware of this to ensure proper treatment in case of occurrence during methylphenidate treatment.
Keywords: adverse events, attention-deficit/hyperactivity disorder, methylphenidate, psychotic symptoms
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder with a worldwide prevalence of around 5.3% among children and adolescents (1). The diagnosis is made by a clinical evaluation of whether a child has presented excessive inattention, hyperactivity and impulsivity. These must be present before six years [International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10)] or 12 years [Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)] and impairing his or her functioning and/or development. ADHD symptoms may persist into adulthood and 15% of the patients continue to fulfil the full criteria for ADHD at the age of 25 (2,3). Psychostimulants, including methylphenidate, are the recommended first-choice drug treatment for ADHD (4-7). Because so many children and adolescents are prescribed methyl-phenidate (8-10), it is important that the risk of adverse events is better understood.
By definition, a substance/medication-induced psychotic disorder must be present during treatment or appear soon after withdrawal (DSM-5), within 2 weeks [ICD-10 (F15.5)] or a month (DSM-IV-TR) depending on the diagnostic criteria used. Psychosis reflects an experience of impaired reality through symptoms such as hallucinations and delusions. However, psychotic symptoms are not always associated with a psychotic disorder and can occur in individuals who have an awareness that their experience does not reflect reality (11).
Psychotic symptoms have been reported in children and adolescents with ADHD prescribed methylphenidate in clinical trials and as patient reports (12). Clinical guidelines recommend a reduction or a withdrawal of methylphenidate if psychotic symptoms occur (13,14) and caution in prescribing methylphenidate in patients with a history of psychotic episodes or a family history of psychotic disorder (15).
To the best of our knowledge, no systematic review of the literature specifically examining psychotic symptoms in relation to methylphenidate use has been performed.
Three publications provide evidence- and expert-based guidance on ADHD medication adverse events, including psychotic symptoms (4,15,16). All three publications are largely based on the same meta-analyses carried out by the Food and Drug Administration (FDA) in 2006 to 2009 (12,17). The FDA examined the occurrence of psychotic symptoms and mania during drug therapy for ADHD (i.e., amphetamine, dextroamphetamine, atomoxetine, modafinil and methylphenidate) by reviewing clinical trial data as well as post-marketing spontaneous reports. Data were provided by manufacturers of ADHD medication and the FDA Adverse Event Reporting System safety database (12). The methodology used resulted in the exclusive inclusion of trials funded by pharmaceutical companies (12). Relevant trials conducted by independent research groups were not sought, and the restricted use of data sources could have influenced the data collection and ultimately the meta-analysis. Study selection of only industry-sponsored trials could have created an additional risk of bias (18). Furthermore, the risk of random errors, risk of bias and trial methodological quality were not assessed systematically. Despite these limitations, the report noted the absence of psychosis and mania events in placebo-treated patients and therefore advised that drugs for ADHD may be associated with such symptoms (17).
We have performed two Cochrane systematic reviews on the efficacy and safety of methylphenidate use in children and adolescents with ADHD, and we sought to avoid any methodological flaws and shortcomings (19-21). In the first review, we included randomized clinical trials (19). In the second review, we included observational studies, the methylphenidate group from randomized clinical trials without placebo or no intervention comparator group, follow-up periods from randomized clinical trials and patient reports (21).
This article highlights one of the many safety outcomes in the two Cochrane publications (19-21) and attempts to establish whether psychotic symptoms occur as an adverse event to methyl-phenidate treatment in randomized clinical trials and observational studies of children and adolescents with ADHD.
Methods
The design and methodology of our study followed the Cochrane Handbook for Systematic Reviews of Interventions (22) and PRISMA guidelines (23,24), which we have described in the protocols (25,26).
Data sources and search criteria
The literature search was carried out and updated for the two Cochrane systematic reviews (19,21) up to February 2015. CINAHL, Cochrane Library, EMBASE, MEDLINE, PsycINFO, ISI CPCI databases, Clinical Trials and ICTRP were searched from origin without restrictions in terms of language, year of publication or type of publication (25,26). Review articles were searched for additional, potentially relevant references. Similarly, the bibliography of a sample of included articles was searched for additional studies. In January 2016, we updated the search for observational studies, and included a “grey literature” search. Unpublished data were also sought from the United States FDA and the European Medicines Agency (21). To collect all new relevant randomized clinical trials, the literature search for randomized clinical trials was updated in March 2017 for the present review.
Where necessary, the authors of the articles included were contacted for additional data. Some authors provided additional, potentially relevant articles. Pharmaceutical companies were contacted for relevant published and unpublished data. Each step of the review was conducted by two review authors, apart from assessment and processing of the final literature search in March 2017, which was conducted by the first author.
Participant inclusion criteria and study selection
After removal of duplicates, authors screened titles and abstracts of the retrieved records. Full-text articles of records judged to be potentially relevant were assessed for eligibility according to our inclusion criteria. Eligibility criteria included children and adolescents with a diagnosis of hyperkinetic disorder or ADHD according to ICD or DSM diagnostic criteria, respectively (ICD-9, DSM-III and newer). At least 75% of the study participants had to be younger than 19 years and the mean age had to be younger than 19 years. We included trials irrespective of comorbidities, but at least 75% of the participants were required to have a normal intellectual capacity (IQ≥70 points). Study designs considered eligible for the assessment of safety were randomized clinical trials on methylphenidate with placebo or no intervention as a comparator, non-randomized studies and patient reports. Furthermore, the methylphenidate group from randomized clinical trials without placebo or no intervention comparator group as well as follow-up periods from randomized clinical trials were included in the observational study category (19,21).
For inclusion in the present review, co-medication was only accepted as over-the-counter drugs or if the co-medication was identical in the intervention and the control group.
Psychotic symptoms
To obtain data on any psychotic phenomena, we included articles reporting assessment and/or occurrence of “psychosis”, “psychotic”, “hallucination” and “delusion”. Only reports of symptoms during methylphenidate treatment or up to a month after withdrawal qualified for inclusion in the present review. Formal assessment of psychotic symptoms was not mandatory, and we accepted both spontaneous reports as well as formal assessment, for example, the Pittsburgh Side Effect Rating Scale, which includes hallucinations as an item (27).
Data extraction and quality assessment
Data were extracted using a template to facilitate a standardized extraction method between researchers. In our analyses, reports of psychotic symptoms leading to withdrawal were not separated from reports of psychotic symptoms in the observed study population. The risk of bias in randomized trials was rated at the study level using the Cochrane Collaboration Handbook for Systematic Reviews (22). We defined low risk of bias trials as trials that had a low risk of bias in all domains. We considered trials with one or more unclear or high risk of bias domains as trials with a high risk of bias (25). This procedure was based on the fact that randomized clinical trials with an unclear or a high risk of bias tend to overestimate benefits and underestimate harms compared with trials with a low risk of bias (28-34). Risk of bias in non-randomized studies, that is, comparative cohort studies and patient–control studies with data, were rated using the ROBINS-I tool (Risk Of Bias In Non-randomized Studies - of Interventions) (35). We assessed and graded the evidence according to Grading of Recommendations Assessment, Development, and Evaluation (GRADE) for a high risk of bias, imprecision, indirectness, heterogeneity and publication bias (36). We report our methodology in greater detail in our Cochrane reviews (19,21).
Data synthesis and statistical analysis
Data from parallel group and cross-over trials were summarized as risk ratios with 95% confidence intervals using the inverse variance method. We combined data from cross-over trials and parallel group trials. Because it might lead to a unit of analysis error, when cross-over trials are analysed as parallel group trials, we carried out a subgroup analysis of study designs. Whenever a cross-over trial reported data on psychotic symptoms in more than one of the groups exposed to methylphenidate, we combined the groups if the patients did not appear in more than one of the groups, otherwise we included data from the group with the highest dose exposure. Furthermore, we carried out a subgroup analysis for dose. For this, we defined low dosage treatment as ≤20 mg/day or ≤0.6 mg/kg/day and moderate/high dosage treatment as >20 mg/day or >0.6 mg/kg/day (19). We also carried out a subgroup analysis on the type of methylphenidate formulation. Here, we compared immediate-release with extended-release inclusive osmotic release oral system and methylphenidate transdermal system. Furthermore, we planned to carry out subgroup analyses on comorbidity and sex. We carried out a sensitivity analysis on age by excluding studies including young adults defined as study participants older than 15 years. We used the random-effect model as the primary method and the fixed-effect model as a sensitivity analysis (37). When carrying out a meta-analysis, a required information size should be estimated to better identify whether an apparent lack of effect may be because of inadequate data just as an a priori sample size calculation is performed for a single randomized clinical trial (38,39). For this, we used Trial Sequential Analysis, which combines the calculation of a required information size with trial sequential monitoring thresholds for benefit, harm or futility (39-41). Furthermore, we planned to assess whether there may be publication bias among randomized clinical trials by testing for funnel plot asymmetry.
Data from comparative cohort studies were summarized as risk ratios with 95% confidence interval using the inverse variance method. The occurrence of psychotic symptoms was calculated as pooled prevalences with 95% confidence intervals in six analyses. We report the pooled prevalence for the methylphenidate and the placebo group in the randomized clinical trials separately, the non-randomized studies and a “grand total” of all non-randomized studies and the methylphenidate group in randomized clinical trials. Furthermore, the pooled prevalence was calculated for two of the study designs included in the non-randomized study category, that is, cohort studies and the methylphenidate group in randomized clinical trials without placebo or no intervention comparator. We also calculated the pooled annual incidence of psychotic symptoms in non-randomized studies.
Data from patient reports were reported qualitatively, that is, number of patients experiencing psychotic symptoms and type of psychotic symptom.
Data were analysed using the program Comprehensive Meta Analysis (42) and the software Review Manager (43).
Results
A total of 14,334 records were retrieved in the literature searches, and 2736 records were considered potentially eligible after screening. After full-text assessments of these 2736 records, 80 articles describing 27 studies (10 randomized trials and 17 non-randomized studies) and 12 patient reports or series were included in the present review (Figure 1). Two studies were included on the basis of their published protocols; however, the results of the studies have still not been published (44,45). No data on psychotic symptoms were obtained from unpublished trials. Four hundred and twelve studies included in the Cochrane reviews on the efficacy and safety of methylphenidate (from the literature searches up to January 2016) did not report assessment or occurrence of psychotic symptoms.
FIGURE 1.
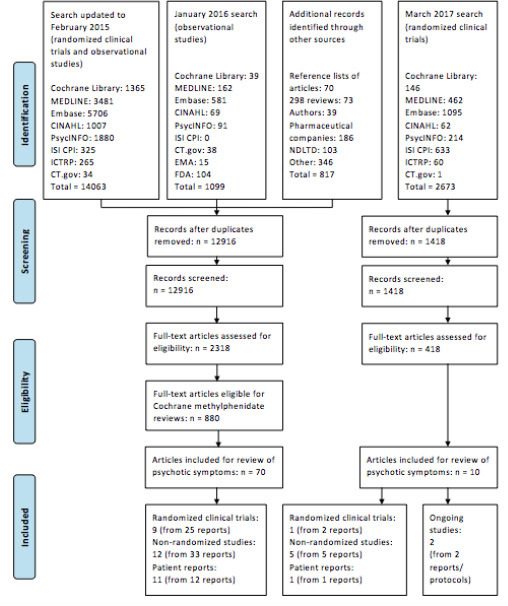
PRISMA flow diagram (24)
Included studies
We included 10 randomized clinical trials [four parallel group trials (46-50) and six cross-over trials (51-57)] totalling 1103 participants, 17 non-randomized studies (49,58-75) totalling 76,237 participants and 12 patient reports or small series describing 18 patients (76-88).
Randomized clinical trials
Table 1 shows the characteristics of the included studies, that is, number, age, sex and methylphenidate-naïvety of the participants; methylphenidate type, dose and dosage regimen; assessment; and type and number of psychotic symptoms. Two trials were designed as summer treatment programs (52,53), one trial used a laboratory classroom design (54) and the remaining were carried out in outpatient settings. Five trials excluded patients with comorbid psychotic disorder, schizophrenia or similar diagnoses (46,47,50,54,55). Two trials only included participants with comorbidity, that is, velocardiofacial syndrome (49) and non-nicotine substance use disorder (50). One trial excluded patients with a history of serious adverse reactions to methylphenidate or lack of response to methylphenidate (54).
TABLE 1.
Characteristics of included randomized clinical trials
| Study ID, country | Study design | N | Age range Mean (SD) (years) | Male [n (%)] | MPH-naïve [n (%)] | MPH type, mean daily dose Dosage regimen | Time of MPH intervention | Mode of assessment | Type and number of psychotic events |
|---|---|---|---|---|---|---|---|---|---|
| Becker 2016/Froehlich 2015, USA | Cross-over | 163 | 7-11 8.41 (1.24) |
117 (72) | 163 (100) | MPH-OROS <25 kg: 18/27/36 mg >25 kg: 18/36/54 mg Once daily |
3 weeks | Pittsburgh Side Effect Rating Scale, Parent rated | Hallucination Placebo: n=0/163 18 mg: n=1/163 27 mg: n=1/31 36 mg: n=4/163 54 mg: n=5/132 |
| Buitelaar 1996, The Netherlands | Cross-over | 52 | 6-13 9.29 (1.63) |
46 (88) | 52 (100) | MPH-IR, 20 mg Twice daily (at breakfast and at noon) |
4 weeks | Modified Stimulant Drug Side Effects Rating scale, Parent rated | Interim analysis Hallucination: n=1 |
| Childress 2009, NCT-00301236, USA | Parallel | 253 | 6-12 8.7 (1.84) |
163 (64) | 175 (69) | MPH-ER, 10/20/30 mg (3 parallel groups) Once daily in the morning |
5 weeks | Regular monitoring of serious adverse events | Tactile hallucination: n=1 (30 mg) |
| Palumbo 2008/Daviss 2008, NCT-00031395, USA | Parallel | 122 | 7-12 9.5 (1.6) |
98 (80) | 57 (47) | MPH-IR, 30.2 mg 1 to 3 times daily (in the morning, noon and at 4 p.m.) | 12 weeks | Pittsburgh Side Effect Rating Scale, Parent and teacher rated. Spontaneous self-reports. | Groups without co-intervention hallucination: n=0/59 |
| Green 2011, NCT-00768820, Israel | Parallel | 34 | 5-20 11.1 (3.7) |
20 (59) | 21 (62) | MPH-IR, 15.7 mg 1 dose | 1 day | Spontaneous reports | Psychotic symptoms: n=0 |
| Pelham 1999, USA, Summer Treatment Program 1988 | Cross-over | 21 | 6-12 10.3 (1.9) |
19 (90) | 7 (33) | MPH-IR, 0.9/0.75/0.3 mg/kg 1 to 3 times daily (morning, noon and afternoon at 3:30 p.m.) | 1day×3 (placebo: 2 daysx3) | Pittsburgh Side Effect Rating Scale. Parent, teacher, and counselor rated | Hallucination: n=1 |
| Pelham 2005, USA, Summer Treatment Program | Cross-over | 36 | 6-13 9.6 (NA) |
33 (92) | 26 (72) | MTS, 0.45/0.9/1.8 mg/h Worn at least 12 hours daily Application time once daily (at 6 or 7 a.m.) |
1 day×2 | Pittsburgh Side Effect Rating Scale. Parent, teacher, and counselor rated | Hallucination: n=0 |
| Riggs 2011, NCT-00264797, USA | Parallel | 303 | 13-18 16.5 (1.3) | 239 (79) | NA | MPH-OROS, 68 mg Once daily in the morning |
16 weeks | Systematic assessment of serious adverse events | Psychotic disorder: n=1 |
| Schachar 2008, USA | Cross-over | 18 | 6-15 11.3 (2.2) |
15* (88) | NA | MPH-IR or MPH-ER, 31.2 mg 1 to 2 times daily (morning- and lunch-time dose) | 1 week | Spontaneous reporting | Psychosis: n=1 |
| Waxmonsky 2008, NCT-00050622, USA, Summer Treatment Program | Cross-over | 101 | 5-12 8.35 (2.05) |
82 (81) | NA | MPH-IR, 15/30/54 mg Thrice daily (7:45 a.m., 11:45 a.m. and 3:45 p.m.) |
1day×3–4×3 | Pittsburgh Side Effect Rating Scale. Staff and parent rated | NA |
ER, extended-release; MPH, methylphenidate; MTS, MPH transdermal system; n, study participants; NA, not available; OROS, osmotic release oral system; IR, immediate-release
The sex of one patient is not stated
The median age for the randomized trials was 9.50 years and the interquartile range was 8.70 to 10.50 years. Across trials, 26% to 100% of the participants were diagnosed with ADHD combined subtype, 20% to 74% with the inattentive subtype, and 0% to 4% with the hyperactive-impulsive subtype. All randomized clinical trials used placebo as a comparator. Three of the six cross-over trials switched interventions daily (52,53,55) and only one cross-over trial included a wash-out period between the interventions (51). Six of the 10 randomized trials used rating scales including an item focusing on psychotic symptoms, that is, visual and auditory hallucinations (47,51-53,55,56), two trials assessed serious adverse effects without further specification (46,50) and two trials recorded spontaneous reports (49,54). Figure 2 presents the forest plot of the meta-analysis of randomized clinical trials. Only one of 10 trials was rated as having a low risk of bias (56). The remaining trials were rated as having a high risk of bias because of vested interest and inadequate information to assess whether the method used could induce bias (see Figure 2). The quality of evidence from the randomized clinical trials assessed according to GRADE guidelines was low owing to a high risk of bias and imprecision.
FIGURE 2.
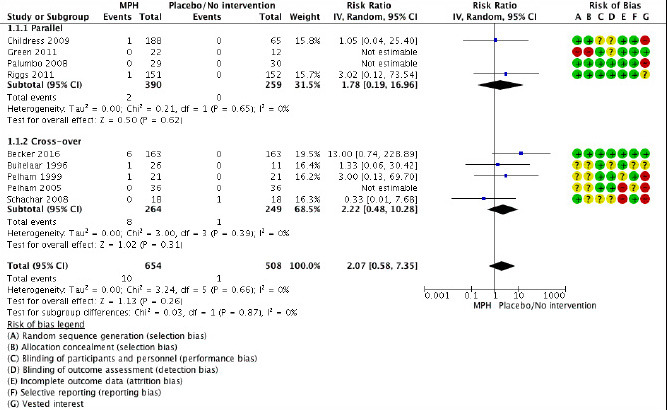
Risk ratio of nine randomized clinical trials comparing methylphenidate versus placebo for patients with ADHD.
The following risk of bias items were rated as low (green), unclear (yellow) or high risk of bias (red): A: Random sequence generation (selection bias). B: Allocation concealment (selection bias). C: Blinding of participants and personnel (performance bias). D: Blinding of outcome assessment (detection bias). E: Incomplete outcome data (attrition bias). F: Selective reporting (reporting bias). G: Vested interest. C and D are for a number of trials assessed as without risk of bias, but because of prevalent and easily recognizable adverse events of methylphenidate, this assessment may well be wrong (19). CI, confidence interval; IV, inverse variance; MPH, methylphenidate; Random, random-effect model.
The meta-analysis yielded 10 of 654 (pooled prevalence, 2.5%; 95% confidence interval, 1.4 to 4.3) methylphenidate patients versus 1 of 508 (pooled prevalence, 1.7%; 95% confidence interval, 0.7 to 4.0) placebo patients with psychotic symptoms (risk ratio, 2.07; 95% confidence interval, 0.58 to 7.35) (see Figure 2). The authors of one trial (55) reported assessment of hallucinations, but data were not available and therefore only nine trials were included in the meta-analysis.
Findings using a fixed-effect model were similar (risk ratio, 2.07; 95% confidence interval, 0.58 to 7.35). The combination of data from parallel group and cross-over trials was tested in a subgroup analysis (see Figure 2), and no significant difference was found between the parallel group trials (risk ratio, 1.78; 95% confidence interval, 0.19 to 16.96) and the cross-over trials (risk ratio, 2.22; 95% confidence interval, 0.48 to 10.28), and the degree of inconsistency across trials in the analysis was 0%. The only reported psychotic episode in the placebo group was in a cross-over trial without any wash-out period between interventions (54). A sensitivity analysis was carried out without this trial (54), but the results were comparable (risk ratio, 2.96; 95% confidence interval, 0.74 to 11.81). Furthermore, we carried out subgroup-analyses for methylphenidate dose and formulation, but there was no statistical difference between low and moderate/high dose of methylphenidate groups: p=0.95 (Figure 3) or between methylphenidate immediate-release and methylphenidate extended-release/osmotic release oral system/transdermal system: p=0.60. The sensitivity analysis of age yielded comparable results with (risk ratio, 2.07; 95% confidence interval, 0.58 to 7.35) and without trials including young adults (49,50) (risk ratio, 1.93; 95% confidence interval, 0.49 to 7.67).
FIGURE 3.
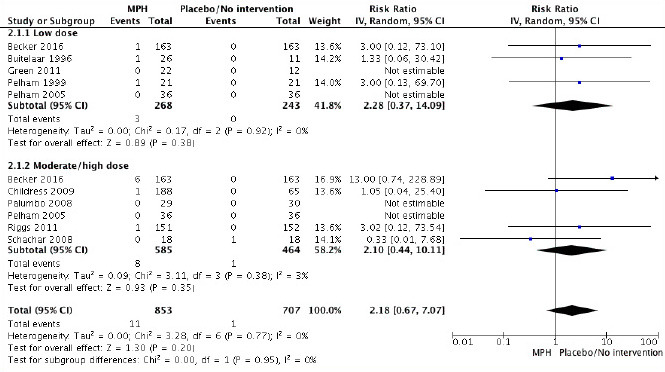
Risk ratio of nine randomized clinical trials comparing methylphenidate versus placebo. Subgroup analysis of dose. Low dose: ≤20 mg/day or ≤0.6 mg/kg/day methylphenidate. Moderate/high dose: >20 mg/day or >0.6 mg/kg/day methylphenidate. CI, confidence interval; IV, inverse variance; MPH, methylphenidate; Random, random-effect model.
We used Trial Sequential Analysis to control the risks of random errors because of sparse data. We calculated the diversity-adjusted required information size by using data from the four parallel group trials that were able to provide data (649 participants), a type 1 error of 5%, a type 2 error of 20%, an assumed control group risk of psychotic symptoms of 2%, a relative risk reduction or increase of 50% and the diversity of the meta-analysis (0%) (Figure 4). With these variables, the diversity-adjusted required information size was 4639 participants, and the meta-analysis is thus considerably underpowered, having only achieved 14.0% (649/4639) of the diversity-adjusted required information size. The unadjusted conventional intervention effect estimate was 1.77 (95% confidence interval, 0.16 to 19.35) using a constant of 0.5 for zero event handling. The Trial Sequential Analysis-adjusted confidence interval, however, ranged from 0.00 to 30,803.
FIGURE 4.
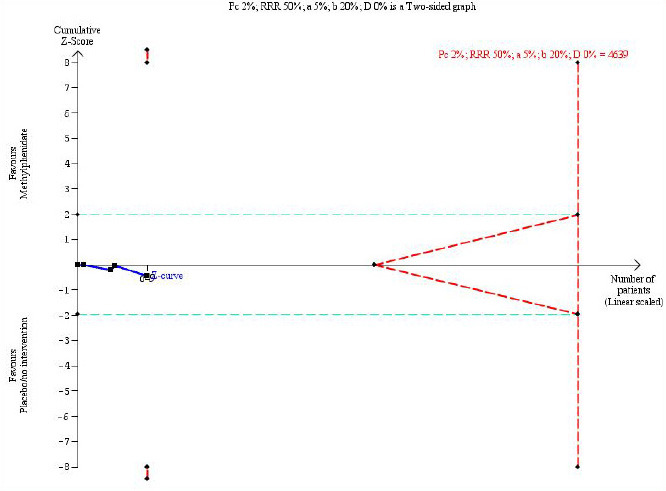
Trial Sequential Analysis of parallel group trials. The diversity-adjusted required information size to demonstrate or reject a relative risk reduction or an increase of 50% with a control group risk of psychotic symptoms of 2%, an alpha of 5%, a beta of 20% and a diversity of 0% is 4639 patients (red vertical dashed line). The red vertical lines to the left represent the trial sequential monitoring boundaries for benefit and harm and the red dashed outward-sloping lines to the right represent the futility boundaries. The horizontal solid blue line is the cumulative Z-curve, showing that only 14.0% (649/4639) of the diversity-adjusted required information size has been accrued. a, alpha; b, beta; D, diversity; DARIS, diversity-adjusted required information size; RRR, relative risk reduction.
Non-randomized studies
Seven of the 17 non-randomized studies were prospective cohort studies (60,63,65-67,71,72,74,75). Three studies were randomized clinical trials without placebo or no-intervention comparators and therefore assessed as prospective cohort studies by only including the methylphenidate groups (58,59,70). One study was a follow-up of a randomized clinical trial where all participants continued methylphenidate treatment, and, therefore, the follow-up period was assessed as a prospective cohort study (49). There were four retrospective cohort studies (61,62,64,73), of which one was a comparative cohort study (73). One retrospective self-controlled patient series study design was included, where psychotic events among patients during periods with no drug exposure were compared with psychotic events during methylphenidate treatment periods (69). One cross-sectional study was included (68).
Study characteristics are presented in Table 2. All studies were carried out in outpatient clinics, except one study, which only included hospitalized patients (64). Comorbid schizophrenia, psychotic disorder or psychiatric disorder were exclusion criteria in two-thirds of the studies (58-60,63,65,66,70,71,73-75). Five studies only included patients with comorbid disorders, including schizophrenia (64), comorbid conduct disorder or oppositional-defiant disorder (59), severe mood dysregulation (60), sleeping difficulties (58) and velocardiofacial syndrome (49). One study only included patients with parents with severe mental illness (68). In the studies, 17.4% to 100% of the patients had the ADHD combined subtype, 11.6% to 70.2% had the inattentive subtype and 0.8% to 55% had the hyperactive-impulsive subtype. The median age for the non-randomized studies was 9.50 years and the interquartile range was 9.20 to 10.49 years.
TABLE 2.
Characteristics of included non-randomised studies
| Study ID, country | N | Age range mean (SD) (years) | Male [n (%)] | MPH-naïve [n (%)] | MPH type, mean daily dose Dosage regimen | Time of intervention | Mode of assessment | Type and number of psychotic events |
|---|---|---|---|---|---|---|---|---|
| Ashkenasi 2011, NCT00989950, USA | 26 | 6-12 9.3 (1.95) |
19 (73) |
NA | MTS, 10 ➔ max 30 mg (optimal dose) Applied once daily in the morning Worn for 9, 10, 11, and 12 h/day in 1 week each |
Titration +4 weeks maintenance | Spontaneous reporting | Withdrawal due to hallucinations n=1 |
| Arnold 2015, TOSCA study, NCT00796302,USA | 168 | 6-12 8.89 (2.01) |
129 (77) |
NA | MPH-OROS, ➔ 44.8 mg Once daily |
3 weeks titration +6 weeks maintenance | NA | Hallucinations n=0/156 in 3 weeks (0/70 in 9 weeks) Delusions n=0/156 in 3 weeks (0/70 in 9 weeks) |
| Baweja 2016,USA | NA | NA NA (NA) |
NA | NA | MPH*, NA NA |
6 weeks | Pittsburgh Side Effects Rating Scale | Hallucinations n=NA |
| Cherland 1999, Canada | 98 | 4-17 NA (NA) |
NA | NA | MPH*, NA Dose range: 5-80 mg NA |
21 months | Spontaneous reporting | Psychotic effects n=7/96 |
| Cortese 2015,Italy | 1426 | 6-18 10.55 (2.75) |
1247 (87) |
NA | MPH-IR, 18.3 mg 2-3 times daily |
up to 5 years | A structured form including any psychiatric symptomatology | Hallucination n=2 |
| Didoni 2011, Italy | 34 | 6-17 10.7 (2.7) |
28 (82) |
34 (100) | MPH-IR, 39.9 mg 2-3 times daily. | > 1 year | Parents were requested in advance to report any adverse events during follow-up visits | Psychotic symptoms n=0 |
| Elman 1998, Israel | 5 | NA NA (NA) | NA | NA | MPH*, NA NA |
NA | NA | n=0 |
| Findling 2009, NCT-00151957, USA | 326 | 6-12 9.2 (1.9) |
212 (65) | ∼ 0 (∼ 0) | MTS, 10➔15➔20➔30 mg Applied once daily (∼7 am) Worn for ∼9 hours (∼4 pm) |
12 months | Systematic assessment | Psychosis/mania n=3 |
| Green 2011, NCT-00768820, Israel | 16 | 5-20 NA (NA) |
NA | 0 (0) | MPH*, NA NA |
6 months | Spontaneous reports | Psychotic symptoms n=0 |
| Lee 2013/NA 2013, Republic of Korea, NCT01060150 | 55 | 12-18 14.33 (1.54) |
43 (78) |
NA | MPH-OROS, 45.78 mg Once daily |
12 weeks | Adverse event checklist and general questioning | Hallucination n=0/55 |
| 121 | 12-18 13.8 (1.49) |
93 (77) |
NA | 54.53 mg Once daily |
12 weeks | Adverse event checklist and general questioning | Withdrawal due to hallucinations n=1/121 | |
| MacKenzie 2016, Canada | 141 +MPH:NA |
6-21 NA (NA) |
67 (48) |
NA | MPH*, NA NA |
>12 months | Schizophrenia proneness instrument-child and youth version and structured interview for prodromal syndrome | Psychotic symptoms: Patients, +mph: n=6/NA Control, -mph: n=4/16 |
| Man 2016, Hong Kong | 76 | 6-19 NA (NA) |
NA | NA | MPH-IR and –ER, NA NA |
Mean: 2.17 years | Psychotic disorder or hallucination diagnostic code in the Clinical Data Analysis and Reporting System | Baseline period (no drug): n=NA Exposed period: n=NA |
| Mohammadi 2004, Iran | 16 | 6-14 8.87 (2.47) |
11 (69) |
16 (100) | MPH*, 1 mg/kg NA |
6 weeks | NA | Hallucination, delusion n=0 |
| Remschmidt 2005/Hoare 2005, UK and Germany | 89 | 6-16 NA (NA) |
NA | 0 (0) | MPH-OROS, 18, 36 or 54 mg Once daily |
1 year | NA | Delusion n=1 (18 mg) |
| Shyu 2015, Taiwan | ADHD: 73,049 | NA 9.4 (3.3) |
58,293 (80) |
NA | MPH*, NA NA |
5 months – 12 years | Schizophrenia spectrum disorders based on insurance status, outpatient and hospitalization claims databases | Psychotic disorder: +MPH: 856/52,646 (1.6%) -MPH: 229/19,125 (1.2%) |
| ADHD+MP H: 53,600 (73%) |
Schizophrenia: +MPH: 452/52,752 (0.9%) -MPH: 120/19,119 (0.6) |
|||||||
| Su 2015, China | 239 | 6-16 9.2 (2.02) |
203 (85) |
NA | MPH-OROS, 18➔ 36 ➔54 mg Once daily |
8-week titration phase | Treatment-emergent adverse events were recorded throughout the study | Hallucination n=1 |
| Wilens 2005, USA | 407 | 6-13 9.2 (1.8) |
338 (83) |
0 (0) | MPH-OROS, 35.2➔44.2 mg Once daily |
21-24 months | Systematic assessment, parent rated | Withdrawal due to hallucinations n=1 |
Note. ➔, titrated to; MPH, methylphenidate; MTS, MPH transdermal system; n, study participants; NA, not available; OROS, osmotic release oral system; IR, immediate-release.
Type of methylphenidate formulation not available
The assessment of psychotic symptoms varied across studies. Rating scales including items focusing on psychotic symptoms were used in two studies (60,68). In one of these studies, structured interviews for prodromal syndrome was also used (68). A structured form including any psychiatric symptomatology was used in one study (62). In two database studies, a diagnostic code of schizophrenia spectrum disorders, psychotic disorders or hallucination was used (69,73). In the rest of the studies, the assessment varied from a systematic assessment of adverse events without further specification to recording of spontaneous reports.
In the non-randomized studies included, 873 instances of psychotic symptoms, including psychotic disorder, hallucinations, delusions and psychosis/mania, were reported to occur during methylphenidate treatment out of a total of 55,603 patients (pooled prevalence, 1.2%; 95% confidence interval, 0.7 to 2.4) (Figure 5). The meta-analysis was based on 14 studies as data from three studies were not available (60,68,69). There was no significant difference in the pooled prevalence in the cohort studies (1.1%; 95% confidence interval, 0.5 to 2.3) (61-67,71-75) compared with the methylphenidate group in the randomized clinical trials without placebo or no intervention comparator (1.9%; 95% confidence interval, 0.4 to 7.8) (58,59,70). The pooled annual incidence in the non-randomized studies was 2.2% (95% confidence interval, 0.2 to 4.1) on the basis of 12 studies with a total of 54,172 patients and 23,375.35 person-years because two studies did not report useable treatment durations (62,64). In the comparative cohort study, methylphenidate was associated with an increased risk for any psychotic disorder of 36% (risk ratio, 1.36; 95% confidence interval, 1.17 to 1.57) (73). The overall risk of bias was rated as critical for this study (Table 3) (73). Risk of bias assessment was only possible for one included study, that is the comparative cohort study (73), since it is a prerequisite for using ROBINS-I that the studies are comparative (35). Non-comparative studies are of critical risk of bias mostly due to confounding factors and therefore we considered all these studies as of critical risk of bias. The quality of evidence assessed according to GRADE guidelines was low owing to study design and a critical risk of bias.
FIGURE 5.
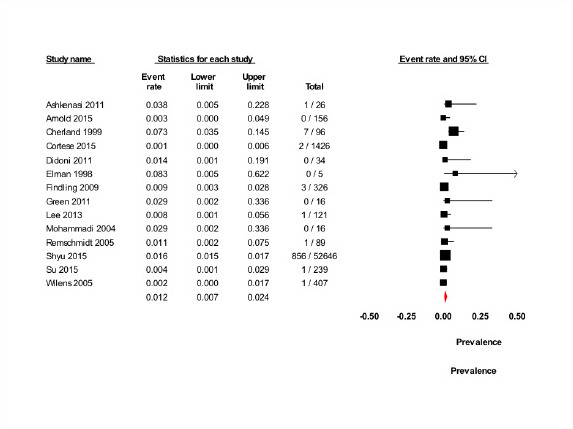
Prevalence of psychotic symptoms in non-randomized studies. CI, confidence interval
TABLE 3.
Risk of bias in non-randomised studies
| Shyu 2015 | |
|---|---|
| Bias due to confounding | Critical |
| Bias in selection of participants into the study | Moderate |
| Bias in classification of interventions | Moderate |
| Bias due to deviations from intended interventions | Critical |
| Bias due to missing data | Critical |
| Bias in measurement of outcomes | Serious |
| Bias in selection of the reported result | No information |
| Risk of bias judgement | Critical |
The “grand total” pooled prevalence summarizing data from the non-randomized study category (58,59,61-67,70-75) and the methylphenidate group in the randomized clinical trials, that is, with placebo as a comparator (46-54,56,57), was 1.8% (95% confidence interval, 1.2 to 2.8).
Patient reports
In the 12 patient reports describing 18 patients (Table 4), 57.9% had ADHD combined subtype, 10.5% had the inattentive subtype and 5.3% had the hyperactive-impulsive subtype.
TABLE 4.
Characteristics of included patient reports
| Study ID, country | N | Age, (years) | Sex | MPH-naïve (n) | MPH type, mean daily dose | Time of intervention | Type and number of psychotic events |
|---|---|---|---|---|---|---|---|
| Aguilera-Albesa 2010, Spain | 2 | 6 | F | NA | 50% MPH-IR/50% MPH-ER, 10➔20 mg Once daily |
4 days | Hallucinations: n=2 |
| 8 | M | NA | MPH-ER, 18 mg Once daily |
2 days | |||
| Coignoux 2009, France | 1 | 14 | M | NA | MPH-ER, 54 mg Once daily in the morning |
6 months | Psychotic symptoms: n=1 |
| Fernández-Fernández 2011, Spain | 1 | 10 | M | 0 | MPH-ER, 1.2 mg/kg Once daily |
1 week | Psychosis: n=1 |
| Goetz 2011, Czech Republic | 1 | 7 | F | 0 | MPH-OROS, 18 mg Once daily |
2.5 months | Hallucination: n=1 |
| Gross-Tsur 2004, Israel | 3 | 7 | M | NA | MPH*, 7.5 mg Once daily |
1 year | Hallucinations: n=3 |
| 12 | M | NA | MPH*, 10 mg Once daily |
Short period | |||
| 7.5 | M | NA | MPH*, 7.5 mg Once daily |
Months | |||
| Halevy 2009, Israel | 1 | 8 | M | 1 | MPH*, 10 mg NA |
Days | Hallucination: n=1 |
| Herguner 2015, Turkey | 1 | 6 | M | NA | MPH-OROS, 18 mg† NA |
2 months | Hallucination: n=1 |
| Irmak 2014, Turkey | 1 | 9 | M | 1 | MPH*, ➔1mg/kg NA |
NA | Hallucination: n=1 |
| Porfirio 2011, Italy | 1 | 11 | M | 1 | MPH-IR, 30 mg Twice daily |
3 years | Hallucination: n=1 |
| Rashid 2007, USA | 1 | 10 | M | 0 | MPH-IR, 20➔30 mg Twice daily |
2 days | Hallucination: n=1 |
| Shibib 2009, UK | 4 | 14 | F | 1 | MPH-ER, 30 mg Once daily |
4 months | Psychosis: n=4 Hallucinations: n=3-4 |
| 8 | M | 1 | MPH-IR, 5➔20 mg Twice daily |
7 days | |||
| 10 | M | 0 | MPH-ER, 36➔54 mg Once daily |
3 weeks | |||
| 14 | M | 0 | MPH-ER, 18 mg NA |
24 hours | |||
| Tomás Vila 2010, Spain | 1 | 10 | M | 0 | 50% MPH-IR/50% MPH-ER, 30 mg NA |
2 weeks | Hallucination: n=1 |
Note. ➔, titrated to; ER, extended-release; F, female; M, male; MPH, methylphenidate; n: study participants; NA, not available; IR: immediate-release; OROS, osmotic release oral system
Type of methylphenidate formulation not available
The hallucinations started when acetaminophen suspension (120 mg/day) was administered in addition to methylphenidate and resolved after withdrawal of acetaminophen
Sixteen patients developed new psychotic symptoms during methylphenidate treatment and two patients experienced exacerbation of pre-existing psychotic symptoms, that is, chronic pattern of partial somatic hallucinations (83) and comorbid schizotypal personality disorder and infantile psychosis (77). The newly developed psychotic symptoms reported were primarily hallucinations, including visual, auditory and tactile hallucinations. The duration of methylphenidate treatment until the development of psychotic symptoms varied from 1 day to 3 years. Time from ingestion of methylphenidate to initiation of psychotic symptoms varied from 1 hour to 1 day. The duration of psychotic symptoms varied from 2 hours to 1 day, except for one patient, who had psychotic symptoms for 1 week coinciding with the co-administration of acetaminophen (88). The psychotic symptoms remitted upon methylphenidate withdrawal in 16 of 16 patients. In two of four patients, a re-challenge with methylphenidate was followed by the recurrence of symptoms (80,81). Furthermore, eight patients were reported to be followed up between 3 months and 3 years after the occurrence of psychotic symptoms (77,79,80,82,83,88), and only one patient continued to have symptoms (77). At follow-up, he was still receiving methylphenidate and was diagnosed with schizophrenia.
The quality of evidence from the patient reports assessed according to GRADE guidelines was very low owing to the study design.
Discussion
The data included here are a subset of data from the most comprehensive systematic reviews of methylphenidate to date (19,21). Despite this, relatively few studies assessed or reported psychotic symptoms.
Only nine of 185 randomized clinical trials and 23 of 259 non-randomized studies and patient reports of methylphenidate in children and adolescents with ADHD reported assessment of psychotic symptoms. One randomized clinical trial and six non-randomized studies and patient reports of psychotic symptoms were identified from the updating search in March 2017. Psychotic symptoms in relation to methylphenidate treatment have occasionally been reported since 1967 (12) and because of the relatively infrequent reports in the literature, it is fair to assume that serious reporting bias exists. We sought to obtain supplemental data from published and unpublished trials through correspondence with pharmaceutical companies, without success. Because of the sparse number of trials included in the meta-analysis, we did not construct a funnel plot. Psychotic symptoms only occurred in six randomized trials (two parallel, four cross-over) and therefore the test power would be too small to distinguish chance from real asymmetry (22).
The evidence that our present results are based on is of low and very low quality according to our assessment following GRADE guidelines and may be prone to bias.
Meta-analysis showed no difference in the risk of psychotic symptoms between the methylphenidate and the placebo groups in the randomized clinical trials (risk ratio, 2.07; 95% confidence interval, 0.58 to 7.35). It is worth noting that the only episode of psychotic symptoms in the placebo group occurred in a cross-over trial without wash-out periods (54), which makes the event dubious. However, the sensitivity analysis without this trial showed comparable results. The combination of data from parallel and cross-over trials was tested in another subgroup analysis and no statistically significant difference was present. A sensitivity analysis on age excluding trials including study participants older than 15 years showed comparable results. A subgroup analysis on dose was carried out showing no statistically significant difference in the occurrence of psychotic symptoms in the low-dose group compared with the moderate/high-dose group. Similarly, a subgroup analysis of methylphenidate formulation showed no statistically significant difference between the methylphenidate immediate-release and the methylphenidate extended-release/osmotic release oral system/transdermal system. It was not possible to carry out subgroup analyses, involving diagnostic criteria, comorbidity or sex because of the limited available data. No data from the first period of cross-over trials were available and thus could not be included in the Trial Sequential Analysis. Our meta-analysis of parallel trials was considerably underpowered according to the Trial Sequential Analysis, having only achieved 14.0% of the diversity-adjusted required information size. The result of the Trial Sequential Analysis highlights that the absence of evidence of an association in our analysis is not evidence suggesting that no association exists. Our results are primarily based on trials at high risk of bias (9/10) and accordingly low-quality evidence. However, the high risk of bias ought not necessarily to be interpreted as adding uncertainty about the occurrence of the reported psychotic symptoms, but rather uncertainty about the low frequency of the occurrence. In fact, the high risk of bias, including vested interest, may be a reason for under-reporting of psychotic symptoms (18,28-34). Furthermore, psychotic symptoms during methylphenidate treatment might not be reported if it is not judged to be an adverse event, but rather a natural occurring phenomenon or symptoms of comorbidity. Reporting bias in the studies included would lead to an underestimation of the prevalence of psychotic symptoms. However, 412 studies included in the two Cochrane systematic reviews (19,21) did not report assessment or occurrence of psychotic symptoms and were excluded from this study. If psychotic symptoms were not reported because they did not occur, the exclusion of the studies might have led to an overestimation of prevalence.
In the hope of finding supplemental important data to the randomized trials, we chose to include non-randomized studies. The risk of bias was critical in the one non-randomized study that could be rated (owing to study designs). This comparative cohort study showed a significantly increased risk of any psychotic disorder with methylphenidate (risk ratio, 1.36; 95% confidence interval, 1.17 to 1.57). As the authors themselves speculate, the patients exposed to methylphenidate might have had a higher symptom severity than the patients not exposed to methylphenidate. An underlining psychotic disorder might develop in time, especially for the patient group with a higher symptom severity, and therefore might not have been caused by methylphenidate treatment, but rather the full development of the disorder itself. Furthermore, because of the study design, the authors could not control for substance use disorders, which could have also confounded the results. Exposure to cannabis, alcohol and other psychoactive drugs is known to be associated with a significantly higher prevalence of subclinical psychosis (11). These limitations are difficult to avoid in non-randomized studies.
The pooled prevalence of psychotic symptoms during methylphenidate treatment in the randomized clinical trials (2.5%; 95% confidence interval, 1.4 to 4.3) was not different from the pooled prevalence in the non-randomized studies (1.2%; 95% confidence interval, 0.7 to 2.4). Estimates from both study designs were smaller than the reported prevalence in the general population [17% for children and 7.5% for adolescents (89)]. However, the same applied for the pooled prevalence in the placebo group in the randomized clinical trials (1.7%; 95% confidence interval, 0.7 to 4.0) and the prevalence in the group without methylphenidate exposure in the comparative cohort study (1.2%, 229/19,125). This might be explained by the way in which psychotic symptoms were evaluated. Nine of 27 studies included in our review used a structured form or rating scales, including items focusing on psychotic symptoms, that is, visual and auditory hallucinations. In the remaining studies, adverse events were assessed by general questioning or recording of spontaneous reports, which are both insufficient and inadequate. Only one study used structured interviews (68). Rating scales and structured interviews are much more sensitive than general questioning about adverse events and recording of spontaneous reports of adverse events, and the proportions reported here might therefore be underestimates of the true value. In the meta-analysis of prevalence in the general population (89), psychotic symptoms were assessed using clinical interviews and questions of whether the child/adolescent ever hears voices or sounds that no one else can hear. A clinical interview is the gold standard for assessing the presence and severity of psychotic symptoms, but is resource-demanding. Screening with self-report questionnaires entails a risk of overestimating the prevalence of psychotic symptoms because of a high rate of false positives (89). However, the above question on auditory hallucinations shows good sensitivity, specificity as well as positive and negative predictive value for psychotic symptoms in general (90), and ought to be included in adverse effect rating scales.
The quality of patient reports is considered very low and by including patient reports in a review a trade-off is made between being all-inclusive and not knowing whether unreliable information is republished (22). Consequently, patient reports can hardly contribute to the causality, but might point towards an association. The percentage of methylphenidate-naïve participants varied considerably (0% to 100%) between studies and the duration of methylphenidate treatment to the occurrence of psychotic symptoms in patient reports varied from 1 day to 3 years. Accordingly, if psychotic symptoms occur as an adverse event, the available data do not suggest whether such symptoms occur in the short or long term.
Genetic studies have shown a possible common heritability of ADHD and schizophrenia, but whether those findings can influence the occurrence of psychotic symptoms in methylphenidate users remain unclear. A Danish study reported an increased relative risk of 4.3 for schizophrenia in adults with ADHD compared with the general population (91). In a nationwide Taiwanese cohort, children with ADHD had an increased risk of developing any psychotic disorder (adjusted hazard ratio, 5.2) or schizophrenia (adjusted hazard ratio, 4.65) compared with non-ADHD controls (73). A small but significant genetic susceptibility was found in rare chromosomal variants (92), but needs to be replicated. Similarly, genetic variations at SNAP25 can be associated differentially with both psychiatric conditions (93).
Approximately two-thirds (16/27) of the included studies, randomized as well as non-randomized, had comorbid psychotic disorders as an exclusion criterion, making the results less generalizable to ADHD patients with known susceptibility to psychotic symptoms. However, in a retrospective cohort study with five patients in a prodromal schizophrenic state, none developed psychotic symptoms in response to methylphenidate (64). In contrast, methylphenidate exacerbated psychotic symptoms in two patient reports (77,83). Methylphenidate mediates its effect through the dopaminergic and noradrenergic neurotransmitter systems, and increases the concentration of dopamine in the synaptic cleft (94). Hyperdopaminergic activity in patients with schizophrenia is believed to cause psychotic symptoms (95). Thus, one might believe that methylphenidate may unmask psychotic symptoms in genetically vulnerable patients through a synergistic mechanism. If this is so, the occurrence of psychotic symptoms during methylphenidate treatment in clinical studies, with the exclusion of these vulnerable patients, would not seem to reflect the occurrence of psychotic symptoms in the population of ADHD patients in a clinical setting.
Results in relation to current knowledge
This systematic review provides an overview of the existing literature and the results are in line with the FDA report (12). Psychotic symptoms occurring during methylphenidate treatment may represent an adverse event. No significant difference in the risk of developing psychotic symptoms was present in the randomized clinical trials, but according to the Trial Sequential Analysis, the meta-analysis was considerably underpowered. In the included comparative cohort study, methylphenidate significantly increased the risk of any psychotic disorder by 36%. The prevalence of psychotic symptoms obtained in our review from the non-randomized studies was much lower than the corresponding numbers for the general population and this was considered to be because of methodological differences, in particular, assessment strategy, but could also be because of bias and low quality of evidence.
Implications for future research
Future high-quality, long-term randomized placebo-controlled trials assessing methylphenidate-induced psychotic symptoms concurrently with beneficial effects are needed and in particular trials with large sample sizes, inclusion of patients vulnerable to psychotic adverse events and assessment of psychotic symptoms by clinical interviews or standardized rating scales. Long duration of placebo administration and inclusion of patients vulnerable to psychotic adverse events might be ethically questionable and therefore also non-randomized studies may be of great importance.
An adverse effect rating scale of methylphenidate should include assessment of psychotic symptoms, for example, a question of whether the child/adolescent ever hears voices or sounds that no one else can hear. Furthermore, the severity and implication for the child of psychotic symptoms ought to be assessed in a clinical interview as psychotic symptoms are not necessarily associated with a psychotic disorder (11).
Conclusions
Because of sparse data and low quality of evidence, we cannot confirm or refute whether methylphenidate increases the occurrence of psychotic symptoms in children and adolescents with ADHD.
It seems that it is not possible to make definitive conclusions on the occurrence of psychotic symptoms in relation to methylphenidate because of methodological issues. A number of limitations are highlighted in the discussion, but the present review does have a number of strengths: it was conducted according to Cochrane guidelines, which involve thoroughness. Protocols were published before the reviews were conducted. The literature search was thorough and systematic, and pharmaceutical companies were contacted to obtain data from unpublished trials. We believe that our approach has led to the best possible gathering of relevant literature on the subject.
The results of the meta-analyses in our main publication (19) suggest that methylphenidate treatment of children and adolescents with ADHD may improve ADHD symptoms, general behaviour and quality of life. However, the magnitude of the beneficial effects cannot be established because of the low quality of the evidence. Within the short study duration typical of the included trials, methylphenidate is associated with an increased risk of non-serious adverse events, such as sleep problems and decreased appetite, but because of sparse data, we could not determine whether methylphenidate increases the risk of serious adverse events (19).
Acknowledgments
The authors thank Susanne Rosendal, PhD, MD, Kirsten Buch Rasmussen, BSc, Trine Lacoppidan Kæstel, BSc, Dorothy Gauci, MSc, Bente Forsboel, MD, Nadia Pedersen, stud.scient.san.publ., Sasja Jul Håkonsen, MScN, PhD student, and Lise Aagaard, PhD (pharm) for participation in the conduction of the Cochrane systematic reviews on Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents and the Cochrane Developmental, Psychosocial and Learning Problems Group for providing help and support.
Footnotes
Clinical significance
Psychotic symptoms may affect 1.1% to 2.5% of children, but there is inadequate evidence to determine that these are caused by methylphenidate treatment. Physicians, patients and caregivers should be aware of this possible adverse event to ensure proper treatment in case of occurrence during methylphenidate treatment. Concerns about this rare possible adverse event should be balanced against the potential beneficial effects of methylphenidate in children and adolescents with ADHD on ADHD symptoms, general behaviour and quality of life (19). Evidence supporting methylphenidate treatment of ADHD patients with a history of psychotic episodes is still awaited.
Funding
The review was funded by the Region Zealand Research Foundation, Denmark; Psychiatric Research Unit, Region Zealand, Slagelse, Denmark; the Copenhagen Trial Unit, the Centre for Clinical Intervention Research, Copenhagen University Hospital, Denmark.
This article is based on two Cochrane Reviews: “Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD)” is published in the Cochrane Database of Systematic Reviews (CDSR) 2015, Issue 11, DOI: 10.1002/14651858.CD009885.pub2 (see www.thecochranelibrary.com for information) and “Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents - assessment of possible adverse events in non-randomised studies” [in press]. Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and the CDSR should be consulted for the most recent version of the review.
Conflicts of interest
Carlos R. Moreira-Maia reports research support from the National Counsel of Technological and Scientific Development (CNPq) and personal fees from Novartis, Libbs, The Health Technology Assessment Institute (IATS), The Federal University of Rio Grande do Sul and the World Federation of ADHD outside the submitted work. Camilla Groth has received funding from the Lundbeck’s Foundation to finish a PhD study – a longitudinal study of children and adolescents with Tourette`s syndrome. Richard Kirubakaran is currently employed by the Cochrane South Asia, salary funded by Effective Healthcare Research Consortium (EHCRC) for the Department for International Development (DFID), UK. The remaining authors have no conflicts of interest to declare.
References
- 1.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA.. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007;164:942–8. [DOI] [PubMed] [Google Scholar]
- 2.Faraone S, Biederman J, Mick E.. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med 2006;36:159–65. [DOI] [PubMed] [Google Scholar]
- 3.Faraone SV., Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers 2015;1:15020. [DOI] [PubMed] [Google Scholar]
- 4.Graham J, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Dittmann RW, et al. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry 2011;20:17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NICE. Attention deficit hyperactivity disorder. Diagnosis and management of ADHD in children, young people and adults National Clinical Practice Guideline Number 72. Leicester (UK): British Psychological Society (UK); 2009. [PubMed] [Google Scholar]
- 6.CADDRA. Canadian ADHD Practice Guidelines (CAP-Guidelines), Third edition Canadian ADHD Resource Alliance; 2011. Available at: www.caddra.ca. [Google Scholar]
- 7.AACAP. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2007;46:894–921. [DOI] [PubMed] [Google Scholar]
- 8.Hodgkins P, Sasané R, Meijer WM.. Pharmacologic treatment of attention-deficit/hyperactivity disorder in children: incidence, prevalence, and treatment patterns in the Netherlands. Clin Ther 2011;33:188–203. [DOI] [PubMed] [Google Scholar]
- 9.Efron D, Davies S, Sciberras E.. Current Australian pediatric practice in the assessment and treatment of ADHD. Acad Pediatr 2013;13:328–33. [DOI] [PubMed] [Google Scholar]
- 10.Renoux C, Shin J-Y, Dell’Aniello S, Fergusson E, Suissa S.. Prescribing trends of attention-deficit hyperactivity disorder (adhd) medications in UK primary care, 1995-2015. Br J Clin Pharmacol 2016;82:858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L.. A systematic review and meta-analysis of the psychosis continuum: Evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med 2009;39:179–95. [DOI] [PubMed] [Google Scholar]
- 12.Mosholder AD, Gelperin K, Hammad TA, Phelan K, Johann-Liang R.. Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children. Pediatrics 2009;123:611–6. [DOI] [PubMed] [Google Scholar]
- 13.NICE. Attention deficit hyperactivity disorder (ADHD): Diagnosis and management of ADHD in children, young people and adults National Clinical Practice Guideline Number 72. NICE; 2014. Available at: https://www.nice.org.uk/guidance/cg72. [Google Scholar]
- 14.FDA. Highlights of prescribing information, Concerta®. 2015. Available at: www.fda.gov; http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021121s015s017lbl.pdf.
- 15.Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry 2013;54:227–46. [DOI] [PubMed] [Google Scholar]
- 16.Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psychiatry 2006;163:1149–52. [DOI] [PubMed] [Google Scholar]
- 17.Mosholder A. Psychiatric adverse events in clinical trials of drugs for ADHD. FDA Report PID D060163 US Food and Drug Administration; 2006. Available at: http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4210b_10_01_Mosholder.pdf. [Google Scholar]
- 18.Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L.. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2017;2:MR000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database Syst Rev 2015;1:CD009885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storebø OJ, Krogh HB, Ramstad E, Moreira-Maia CR, Holmskov M, Skoog M, et al. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ 2015;351:h5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents - assessment of possible adverse events in non-randomised studies. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J, Green S (Eds.). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. Cochrane Collaboration; 2011. Available at: www.cochrane-www.handbook.org. [Google Scholar]
- 23.Liberati A, Altman D, Tetzlaff J, Mulrow C, Gotzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group T. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storebø OJ, Rosendal S, Skoog M, Groth C, Bille T, Buch Rasmussen K, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev 2012;5. [Google Scholar]
- 26.Storebø OJ, Pedersen N, Ramstad E, Krogh HB, Moreira-Maia CR, Magnusson FL, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents -assessment of harmful effects in non-randomised studies. Cochrane Database Syst Rev 2016;2:CD012069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelham WE. Pharmacotherapy of children with attention-deficit hyperactivity disorder. School Psychol Rev 1993;22:199–227. [Google Scholar]
- 28.Gluud L, Thorlund K, Gluud C, Woods L, Harris R, Sterne J.. Correction: reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2008;149:219. [DOI] [PubMed] [Google Scholar]
- 29.Kjaergard LL, Villumsen J, Gluud C.. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982–9. [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609–13. [DOI] [PubMed] [Google Scholar]
- 31.Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med 2012;157:429–38. [DOI] [PubMed] [Google Scholar]
- 32.Savović J, Jones H, Altman D, Harris R, Jűni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technol Assess 2012;16:1–82. [DOI] [PubMed] [Google Scholar]
- 33.Schulz KF, Chalmers I, Hayes RJ, Altman DG.. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408–12. [DOI] [PubMed] [Google Scholar]
- 34.Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterne J, Hernán M, Reeves B, Savović J, Berkman N, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016; 355:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013;66:719–25. [DOI] [PubMed] [Google Scholar]
- 37.Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C.. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brok J, Thorlund K, Gluud C, Wetterslev J.. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 2008;61:763–9. [DOI] [PubMed] [Google Scholar]
- 39.Wetterslev J, Thorlund K, Brok J, Gluud C.. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64–75. [DOI] [PubMed] [Google Scholar]
- 40.Wetterslev J, Jakobsen JC, Gluud C.. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med Res Methodol 2017;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA). Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research; 2011. pp. 1–115. Available at: www.ctu.dk/tsa. [Google Scholar]
- 42.Biostat. Comprehensive meta-analysis [computer program]. Version 2 2008. Available at: https://www.meta-analysis.com/pages/demo.php.
- 43.The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.2 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2012. Available at: http://community.cochrane.org/tools/review-production-tools/revman-5. [Google Scholar]
- 44.Inglis SK, Carucci S, Garas P, Hage A, Banaschewski T, Buitelaar JK, et al. Prospective observational study protocol to investigate long-term adverse effects of methylphenidate in children and adolescents with ADHD: the Attention Deficit Hyperactivity Disorder Drugs Use Chronic Effects (ADDUCE) study. BMJ Open 2016;6:e010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clinicaltrials.gov. NCT02638168, Baweja R. Effects of evening dose of immediate release methylphenidate on sleep in children with ADHD. clinicaltrials.gov. 2015. December 18.
- 46.Childress AC, Spencer T, Lopez F, Gerstner O, Thulasiraman A, Muniz R, et al. Efficacy and safety of dexmethylphenidate extended-release capsules administered once daily to children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2009;19:351–61. [DOI] [PubMed] [Google Scholar]
- 47.Palumbo DR, Sallee FR, Pelham WE, Bukstein OG, Daviss WB, McDermott MP.. Clonidine for attention-deficit/hyperactivity disorder: I. Efficacy and tolerability outcomes. J Ame Acad Child Adolesc Psychiatry 2008;47:180–8. [DOI] [PubMed] [Google Scholar]
- 48.Daviss WB, Patel NC, Robb AS, McDermott MP, Bukstein OG, Pelham J, et al. Clonidine for attention-deficit/hyperactivity disorder: II. ECG changes and adverse events analysis. J Am Acad Child Adolesc Psychiatry 2008;47:189–98. [DOI] [PubMed] [Google Scholar]
- 49.Green T, Weinberger R, Diamond A, Berant M, Hirschfeld L, Frisch A, et al. The effect of methylphenidate on prefrontal cognitive functioning, inattention, and hyperactivity in velocardiofacial syndrome. J Child Adolesc Psychopharmacol 2011;21:589–95. [DOI] [PubMed] [Google Scholar]
- 50.Riggs PD, Winhusen T, Davies RD, Leimberger JD, Mikulich-Gilbertson S, Klein C, et al. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. J Am Acad Child Adolesc Psychiatry 2011;50:903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buitelaar JK, van der Gaag RJ, Swaab-Barneveld H, Kuiper M.. Pindolol and methylphenidate in children with attention-deficit hyperactivity disorder. Clinical efficacy and side-effects. J Child Psychol Psychiatry 1996;37:587–95. [DOI] [PubMed] [Google Scholar]
- 52.Pelham WE, Gnagy EM, Chronis AM, Burrows-MacLean L, Fabiano GA, Onyango AN, et al. A comparison of morning-only and morning/late afternoon adderall to morning-only, twice-daily, and three times-daily methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 1999;104:1300–11. [DOI] [PubMed] [Google Scholar]
- 53.Pelham WE, Manos MJ, Ezzell CE, Tresco KE, Gnagy EM, Hoffman MT, et al. A dose-ranging study of a methylphenidate transdermal system in children with ADHD. J Am Acad Child Adolesc Psychiatry 2005; 44:522–9. [DOI] [PubMed] [Google Scholar]
- 54.Schachar R, Ickowicz A, Crosbie J, Donnelly GAE, Reiz JL, Miceli PC, et al. Cognitive and behavioral effects of multilayer-release methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2008;18:11–24. [DOI] [PubMed] [Google Scholar]
- 55.Waxmonsky J, Pelham WE, Gnagy E, Cummings MR, O’Connor B, Majumdar A, et al. The efficacy and tolerability of methylphenidate and behavior modification in children with attention-deficit/hyperactivity disorder and severe mood dysregulation. J Child Adolesc Psychopharmacol 2008;18:573–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Becker SP, Froehlich TE, Epstein JN.. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Devel Behav Pediatr 2016;37:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Froehlich T, Antonini T, Brinkman W, Langberg J, Simon J, Adams R, et al. Mediators of methylphenidate effects on math performance in children with attention-deficit hyperactivity disorder. J Dev Behav Pediatr 2014;35:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashkenasi A. Effect of transdermal methylphenidate wear times on sleep in children with attention deficit hyperactivity disorder. Pediatr Neurol 2011;45:381–6. [DOI] [PubMed] [Google Scholar]
- 59.Arnold LE, Gadow KD, Farmer CA, Findling RL, Bukstein O, Molina BSG, et al. Comorbid anxiety and social avoidance in treatment of severe childhood aggression: response to adding risperidone to stimulant and parent training; mediation of disruptive symptom response. J Child Adolesc Psychopharmacol 2015;25:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baweja R, Belin PJ, Humphrey HH, Babocsai L, Pariseau ME, Waschbusch DA, et al. The effectiveness and tolerability of central nervous system stimulants in school-age children with attention-deficit/hyperactivity disorder and disruptive mood dysregulation disorder across home and school. J Child Adolesc Psychopharmacol 2016;26:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cherland E, Fitzpatrick R.. Psychotic side effects of psychostimulants: a 5-year review. Can J Psychiatry 1999;44:811–3. [DOI] [PubMed] [Google Scholar]
- 62.Cortese S, Panei P, Arcieri R, Germinario EAP, Capuano A, Margari L, et al. Safety of methylphenidate and atomoxetine in children with attention-deficit/hyperactivity disorder (ADHD): data from the Italian National ADHD Registry. CNS Drugs 2015;29:21. [DOI] [PubMed] [Google Scholar]
- 63.Didoni A, Sequi M, Panei P, Bonati M, Lombardy ADHD Registry Group T. One-year prospective follow-up of pharmacological treatment in children with attention-deficit/hyperactivity disorder. Eur J Clin Pharmacol 2011;67:1061–7. [DOI] [PubMed] [Google Scholar]
- 64.Elman I, Sigler M, Kronenberg J, Lindenmayer JP, Doron A, Mendlovic S, et al. Characteristics of patients with schizophrenia successive to childhood attention deficit hyperactivity disorder (ADHD). Isr J Psychiatry Relat Sci 1998;35:280–6. [PubMed] [Google Scholar]
- 65.Findling RL, Wigal SB, Bukstein OG, Boellner SW, Abikoff HB, Turnbow JM, et al. Long-term tolerability of the methylphenidate transdermal system in pediatric attention-deficit/hyperactivity disorder: A multicenter, prospective, 12-month, open-label, uncontrolled, phase III extension of four clinical trials. Clin Therap 2009;31:1844–55. [DOI] [PubMed] [Google Scholar]
- 66.Lee M-S, Lee SI, Hong SD, Kim J-H, Choi J, Joung Y-S.. Two different solicitation methods for obtaining information on adverse events associated with methylphenidate in adolescents: A 12-week multicenter, open-label study. J Child Adolesc Psychopharmacol 2013;23:22–7. [DOI] [PubMed] [Google Scholar]
- 67.Na K-S, Lee SI, Hong SD, Kim J-H, Shim S-H, Choi J, et al. Effect of osmotic-release oral system methylphenidate on learning skills in adolescents with attention-deficit/hyperactivity disorder: An open-label study. Int Clin Psychopharmacol 2013;28:184–92. [DOI] [PubMed] [Google Scholar]
- 68.MacKenzie LE, Abidi S, Fisher HL, Propper L, Bagnell A, Morash-Conway J, et al. Stimulant medication and psychotic symptoms in offspring of parents with mental illness. Pediatrics 2016;137. [DOI] [PubMed] [Google Scholar]
- 69.Man KKC, Coghill D, Chan EW, Lau WCY, Hollis C, Liddle E, et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl Psychiatry 2016;6:e956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohammadi MR, Kashani L, Akhondzadeh S, Izadian ES, Ohadinia S.. Efficacy of theophylline compared to methylphenidate for the treatment of attention-deficit hyperactivity disorder in children and adolescents: A pilot double-blind randomized trial. J Clin Pharm Ther 2004;29:139–44. [DOI] [PubMed] [Google Scholar]
- 71.Remschmidt H, Hoare P, Ettrich C, Rothenberger A, Santosh P, Schmidt M, et al. Symptom control in children and adolescents with attention-deficit/hyperactivity disorder on switching from immediate-release MPH to OROS MPH Results of a 3-week open-label study. Eur Child Adolesc Psychiatry 2005;14:297–304. [DOI] [PubMed] [Google Scholar]
- 72.Hoare P, Remschmidt H, Medori R, Ettrich C, Rothenberger A, Santosh P, et al. 12-Month efficacy and safety of OROS MPH in children and adolescents with attention-deficit/hyperactivity disorder switched from MPH. Eur Child Adolesc Psychiatry 2005;14:305–9. [DOI] [PubMed] [Google Scholar]
- 73.Shyu Y-C, Yuan S-S, Lee S-Y, Yang C-J, Yang K-C, Lee T-L, et al. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: A nationwide population-based study in Taiwan. Schizophr Res 2015;168:161–7. [DOI] [PubMed] [Google Scholar]
- 74.Su Y, Li H, Chen Y, Fang F, Xu T, Lu H, et al. Remission rate and functional outcomes during a 6-month treatment with osmotic-release oral-system methylphenidate in children with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 2015;35:12. [DOI] [PubMed] [Google Scholar]
- 75.Wilens T, McBurnett K, Stein M, Lerner M, Spencer T, Wolraich M.. ADHD treatment with once-daily OROS methylphenidate: Final results from a long-term open-label study. J Am Acad Child Adolesc Psychiatry 2005;44:1015–23. [DOI] [PubMed] [Google Scholar]
- 76.Aguilera-Albesa S, Yoldi-Petri ME, Molins-Castiella T, Dura-Trave T.. Hallucinations caused by the introduction of methylphenidate at low doses [Spanish]. Rev Neurologia 2010; 51:254–5. [PubMed] [Google Scholar]
- 77.Coignoux Y, Estingoy P, Bastard A.. From hyperactivity to schizophrenia? Clinical, neurobiological and therapeutic discussion of a case. [French]. Ann Médico-Psychologiques (Paris) 2009:57–65. [Google Scholar]
- 78.Fernandez-Fernandez MA, Rufo-Campos M, Mateos-Checa R, Munoz-Cabello B, Madruga-Garrido M, Blanco-Martinez B.. Childhood psychosis secondary to methylphenidate [Spanish]. Rev Neurologia 2011; 52:446–7. [PubMed] [Google Scholar]
- 79.Goetz M, Prihodova I, Hrdlicka M.. Long lasting complex nocturnal hallucinations during Osmotic Release Oral System (OROS) methylphenidate treatment in a 7-year old girl. Neuroendocrinol Letters 2011;32:619–22. [PubMed] [Google Scholar]
- 80.Gross-Tsur V, Joseph A, Shalev RS.. Hallucinations during methylphenidate therapy. Neurology 2004;63:753–4. [DOI] [PubMed] [Google Scholar]
- 81.Halevy A, Shuper A.. Methylphenidate induction of complex visual hallucinations. J Child Neurology 2009; 24:1005–7. [DOI] [PubMed] [Google Scholar]
- 82.Porfirio MC, Giana G, Giovinazzo S, Curatolo P.. Methylphenidate-induced visual hallucinations. Neuropediatrics 2011;42:30–1. [DOI] [PubMed] [Google Scholar]
- 83.Rashid J, Mitelman S.. Methylphenidate and somatic hallucinations. J Am Acad Child Adolesc Psychiatry 2007;46:945–6. [DOI] [PubMed] [Google Scholar]
- 84.Shibib S, Chalhoub N.. Stimulant induced psychosis. Child Adolesc Ment Health 2009;14:20–3. [Google Scholar]
- 85.Tomás Vila M, Izquierdo Quevedo FJ, Cerdán Vera MT, Fernández A, Artés Figueres M, Revert Gomas M.. Visual hallucinations caused by methylphenidate [Spanish]. An Pediatr 2010;72:229–30. [DOI] [PubMed] [Google Scholar]
- 86.Brown University. Somatic hallucinations with methylphenidate in a 10-year-old boy [Case report]. The Brown University Child & Adolesc Psychopharmacol Update 2007;9:11. [Google Scholar]
- 87.Irmak A, Ince-Tasdelen B, Ozmen S, Oztop D.. Treatment choice in association of attention deficit-hyperactivity disorder with Williams and Moebius Syndromes: Case reports. Klinik Psikofarmakoloji Bülteni 2014;24:226. [Google Scholar]
- 88.Herguner S, Ozayhan HY.. Visual hallucinations with methylphenidate and acetaminophen in combination. J Child Adolesc Psychopharmacol 2015;25:598–9. [DOI] [PubMed] [Google Scholar]
- 89.Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M.. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol Med 2012;42:1857–63. [DOI] [PubMed] [Google Scholar]
- 90.Kelleher I, Harley M, Murtagh A, Cannon M.. Are screening instruments valid for psychotic-like experiences? A validation study of screening questions for psychotic-like experiences using in-depth clinical interview. Schizophr Bull 2011;37:362–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dalsgaard S, Mortensen PB, Frydenberg M, Maibing CM, Nordentoft M, Thomsen PH.. Association between attention-deficit hyperactivity disorder in childhood and schizophrenia later in adulthood. Eur Psychiatry 2014;29:259–63. [DOI] [PubMed] [Google Scholar]
- 92.Hamshere ML, Stergiakouli E, Langley K, Martin J, Holmans P, Kent L, et al. Shared polygenic contribution between childhood attention-deficit hyperactivity disorder and adult schizophrenia. Br J Psychiatry 2013;203:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carroll LS, Kendall K, O’Donovan MC, Owen MJ, Williams NM.. Evidence that putative ADHD low risk alleles at SNAP25 may increase the risk of schizophrenia. Am J Med Genet Part B Neuropsychiatr Genet 2009;150B:893–9. [DOI] [PubMed] [Google Scholar]
- 94.Engert V, Pruessner JC.. Dopaminergic and noradrenergic contributions to functionality in ADHD: the role of methylphenidate. Curr Neuropharmacol 2008;6:322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schwartz TL, Sachdeva S, Stahl SM.. Glutamate neurocircuitry: theoretical underpinnings in schizophrenia. Front Pharmacol 2012;3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


