Abstract
Background and Aims
An increase in root hair length and density and the development of arbuscular mycorrhiza symbiosis are two alternative strategies of most plants to increase the root–soil surface area under phosphorus (P) deficiency. Across many plant species, root hair length and mycorrhization density are inversely correlated. Root architecture, rooting density and physiology also differ between species. This study aims to understand the relationship among root hairs, arbuscular mycorrhizal fungi (AMF) colonization, plant growth, P acquisition and mycorrhizal-specific Pi transporter gene expression in maize.
Methods
Using nearly isogenic maize lines, the B73 wild type and the rth3 root hairless mutant, we quantified the effect of root hairs and AMF infection in a calcareous soil under P deficiency through a combined analysis of morphological, physiological and molecular factors.
Key Results
Wild-type root hairs extended the rhizosphere for acid phosphatase activity by 0.5 mm compared with the rth3 hairless mutant, as measured by in situ zymography. Total root length of the wild type was longer than that of rth3 under P deficiency. Higher AMF colonization and mycorrhiza-induced phosphate transporter gene expression were identified in the mutant under P deficiency, but plant growth and P acquisition were similar between mutant and the wild type. The mycorrhizal dependency of maize was 33 % higher than the root hair dependency.
Conclusions
The results identified larger mycorrhizal dependency than root hair dependency under P deficiency in maize. Root hairs and AMF inoculation are two alternative ways to increase Pi acquisition under P deficiency, but these two strategies compete with each other.
Keywords: Zea mays, rhizosphere, arbuscular mycorrhiza, phosphate acquisition, roots, exudates
INTRODUCTION
Phosphorus (P), a crucial plant macronutrient (Bieleski, 1973; Raghothama and Karthikeyan, 2005) that participates in various metabolic pathways, is a key component of nucleic acids, ATP and phospholipids (Vance et al., 2003; Abel, 2017). Phosphorus deficiency is a major limiting factor for crop production in many regions of the world (Vitousek et al., 2010; Johnston et al., 2014), because of its low mobility and low availability in most soils (Schachtman et al., 1998). Root morphological adaptations, physiological changes that include organic compound exudation, and microbial cooperation are major strategies used by plants to overcome P limitation and increase P acquisition (Lambers et al., 2006; Smith and Read, 2008). Crop species differ in their relative investment in each of these strategies; maize, with its fibrous root system, invests heavily in root morphological adaptations and efficiently establishes arbuscular mycorrhizal fungal symbioses, but exudes carboxylates poorly (Lyu et al., 2016; Wen et al., 2019).
Long total root length, high specific root length, vigorous root branching and long, dense root hairs (especially in the top soil layer) are typical root morphology adaptations, which greatly increase the root surface area and soil volume exploration (Vance et al., 2003; Lynch, 2005; Péret et al., 2014). Root hairs (the tubular-shaped outgrowths from root epidermal cells) are one of the most important root morphological adaptations (Peterson and Farquhar, 1996); they greatly increase the root surface area and play an essential role in inorganic phosphate (Pi) acquisition (Gilroy and Jones, 2000). When plants were P-deficient, maize root hairs were responsible for a >30 % increase in biomass and shoot P content (but did not affect the shoot P concentration), and were even more important under drought (Klamer et al., 2019). Because of the limited solubility and mobility of Pi in soils, the soil volume next to roots and the overall root surface area are most important for Pi acquisition. The rhizosphere, the soil that is in close contact with the root surface and that is strongly influenced by exudates from the plant, is massively extended in the root hair regions compared with hairless regions (X. Ma et al., 2018). Various microorganisms, such as arbuscular mycorrhiza, ectomycorrhiza and ericoid mycorrhizas, enlarge the exploited soil and exude enzymes to mobilize Pi (Smith and Read, 2008; Collavino et al., 2010; van der Heijden et al., 2015), while beneficial plant growth-promoting bacteria often indirectly stimulate plant growth (Ludewig et al., 2019). The majority (probably 70–80 %) of terrestrial plant species are capable of interacting with arbuscular mycorrhizal fungi (AMF) in nature (Bonfante, 2018; Brundrett and Tedersoo, 2018). AMF connect intimately with the root and transfer nutrients into cortical cell layers and can extend up to several centimetres away from the root and form a dense hyphal network (Miller et al., 1995; Smith et al., 2011). The hyphae greatly increase the surface area and soil volume exploited by the root and play a vital role in nutrient acquisition, especially for the sparingly soluble and poorly mobile Pi (Finlay, 2008). Arbuscular mycorrhizal plants have two pathways for the uptake of Pi from the soil solution: the direct Pi uptake pathway via the root epidermis, including root hairs; and the indirect arbuscular mycorrhizal (AM) pathway, where Pi is initially taken up by external AM hyphae (Grace et al., 2009). These different pathways are associated with distinct molecular Pi uptake transporters of the PHT1 Pi transporter family, which play roles specific to the two pathways (Benedetto et al., 2005; Javot et al., 2007). When mycorrhizal fungi colonize plants, the expression of mycorrhiza-specific Pi transporter genes (ZmPht1;6 in maize and variously named orthologues in other species) was greatly enhanced compared with non-colonized roots (Nagy et al., 2006; Sawers et al., 2017).
Both root morphological adaptation and microbial cooperation require plants to allocate photosynthetic carbon below ground to competing sinks, either to promote cellular hair growth or for transfer to the symbiotic partner (Lynch, 2015). The formation and maintenance of root hairs appear to be relatively cheap processes with respect to carbon and energy demand (Jungk, 2001; Bailey et al., 2002; Brown et al., 2013b), while AM colonization is associated with a costly delivery of up to 15–20 % photosynthetic carbon to fungi in exchange for nutrients (Jakobsen and Rosendahl, 1990; Wright et al., 1998; Jakobsen et al., 2005; Ryan et al., 2012). ‘Mycorrhizal dependency’ refers to the difference in plant growth when mycorrhizal and non-mycorrhizal plants are compared (van der Heijden, 2003; Tawaraya, 2003). This dependence differs among plant species and cultivars; for example, mycorrhizal dependency in barley was much lower than in maize (Kaeppler et al., 2000; Tawaraya, 2003). There is compelling evidence of a general trade-off between root hairs and mycorrhizal symbiosis: plant species and genotypes with long and dense root hairs rely less on mycorrhizal fungi for P acquisition (Chen et al., 2005; Brown et al., 2012, 2013a). Comparison of various plant species with different root hair length and mycorrhizal dependency demonstrated that root hairs and mycorrhiza are typically inversely correlated. These alternative, but functionally similar strategies are used by plants to increase phosphate acquisition by increasing the root surface area and broaden the depletion zone around the root (Schweiger et al., 1995).
Maize (Zea mays) is one of the most widely cultivated crops for production of both food and fodder in tropical and temperate soils worldwide (Gore et al., 2009). Two maize genotypes with contrasting root hair morphology, the root hairless mutant 3 (rth3) (Wen and Schnable, 1994) and its corresponding wild type (B73), were used in this study. The wild-type and rth3 roots had similar diameter but contrasting root hair morphology: the wild type’s roots were covered by dense root hairs around 1 mm long, while the root epidermis of rth3 had only very short bumps in the root hair zone and appeared completely bald (Fig. 1). Previous studies showed that there was no growth difference between the wild type and rth3 in terms of shoot biomass, root biomass, mycorrhizal colonization and shoot P content under P-sufficient conditions (Kumar et al., 2019), and rth3 can grow vigorously under standard conditions with sufficient nutrition (Wen and Schnable, 1994; Hochholdinger et al., 2008). In this study we aimed to understand the effects of the presence of root hairs, AM colonization and its interaction with root hairs for P acquisition and plant growth under P deficiency. We hypothesized that (1) the wild type is more adaptive to P deficiency than rth3, because of the presence of root hairs; (2) the contribution of AMF to plant growth and Pi acquisition is generally larger (especially large in rth3) and more critical than that of root hairs, and cannot be compensated by root hairs.
Fig. 1.
Microscopic images of maize root hairless mutant rth3 (left) and wild type (WT, right) grown in soil in rhizoboxes.
MATERIALS AND METHODS
Plant material and cultivation
Maize seed inbred line B73 (wild type) and root hairless mutant rth3 were surface-sterilized by rinsing them in 10 % (v/v) H2O2 solution for 20 min and stored in 10 mm CaSO4 overnight. Seeds were placed for 3 d between filter papers soaked in a 4 mm CaSO4 solution for germination.
The nutrient-poor subsoil used in the study was stored before use for >12 years. Mycorrhization of this soil was checked with classical trypan blue staining methods and was confirmed to be essentially absent (Neumann, 2007; Klamer et al., 2019). The soil had the following properties: pH 7.6, Corg <0.3 %, CaCO3 30 %, mineral concentrations (mg kg−1) 7.9 CAL-P, 59.9 Ca, 11.3 Mg, 15 Mn, 7.8 Fe, 0.6 Zn, 0.2 B and 0.7 Cu. Thirty percentage (w/w) of quartz sand was mixed with the soil for the optimization of soil structure. After mixing, dissolved fertilizers were added (mg kg−1 soil): N (200) as NH4NO3, K (200) as K2SO4, P (32) as Ca(H2PO4)2·H2O, Mg (100) as MgSO4·7H2O, Fe (2) as EDTA iron (III) sodium salt, Zn (2.6) as ZnSO4·7H2O and Cu (1) as CuSO4·5H2O. The P concentration in the final substrate was measured, which was 9.8 mg kg−1 CAL-P and 6.6 mg kg−1 Olsen-P. PVC cylinders (height 48 cm; diameter 10 cm) were longitudinally cut into two halves. Every half-cylinder is referred as a rhizobox. Each rhizobox was filled with mixed soil to reach a final density of 1.3 g cm−3. On the sowing date, six maize seedlings (three wild type, three mutant) were inoculated with Rhizophagus irregularis MUCL41833 (produced by Université Catholique de Louvain Croix du Sud, Louvain-la-Neuve, Belgium) with 8 g of beads per kilogram of soil (Loján et al., 2017). Non-inoculated pots served as the control. The germinated seedling was planted at a depth of 2 cm in each rhizobox. After transplanting, the open part of the half-tube was covered with a transparent plexiglass observation window and secured with tape. Each treatment had three replicates, and a total of 12 rhizoboxes were prepared in the experiment (3 replicates × 2 treatments × 2 genotypes). The experiment used a randomized design and was conducted in the greenhouse (48°42′41.04″ N, 9°12′34.20″ E) in the summer of 2019. During the growth period, the boxes were kept inclined at an angle of 60° so that the roots grew along the lower side of the plexiglass cover. The rhizoboxes were weighed and irrigated from the top with distilled water every 2 d to maintain moisture at 60 % of water-holding capacity. Plants were harvested at 40 d after sowing.
Root hair length and organic acids in the root exudates
At harvest, pictures from the root hair zone were taken with a Stemi 2000-C video microscope equipped with Axio Vision 3.1 software (Zeiss, Oberkochen, Germany). From these pictures, root hair length was determined by taking the average length of ten root hairs per plant (Weber et al., 2018). Afterwards, according to the method described by Neumann et al. (2014), root exudates were collected from a 1-cm subapical root area, visible on the observation window, by applying special sorption filter papers (MN156050, Macherey-Nagel, Munich, Germany). Sampling was conducted with five replicates for each minirhizotron, and after 2 h of collection the sorption filter papers were collected. The collected samples were re-extracted with 1 mL of 80 % (v/v) methanol and centrifuged at 18 000 g for 5 min. The supernatants (around 900 µL) were evaporated using a SpeedVac Concentrator (Savant, Farmington, USA) until dryness at 30 °C and re-dissolved in HPLC elution buffer (18 mm KH2PO4, pH 2.2 adjusted with H3PO4). The organic acids were determined by reversed-phase HPLC in the ion suppression mode with direct UV detection at 210 nm according to the method described by Haase et al. (2007). Isocratic elution was performed with 18 mm KH2PO4, pH 2.2, on a reversed-phase C-18 column (290 × 4.6 mm, GROM-SIL 120 ODS ST, 5 µm particle size), which was equipped with a 20 × 4.6 mm guard column with the same stationary phase (Dr Maisch, Ammerbuch, Germany). Identification and quantitative determination were conducted by comparison with known standards.
Rhizosphere acid phosphatase activities determined by zymography and image analysis
To visualize the acid phosphatase activity in the rhizosphere, direct zymography was used after the collection of the root exudates (Sanaullah et al., 2016). Methylumbelliferyl (MUF) phosphate substrate was dissolved in MES buffer (pH 6.5) to obtain a working solution with a final concentration of 1 mm. Thin polyamide membrane filters (Tao Yuan, China) with a pore size of 0.45 mm were saturated with substrate by soaking in the working solution. The membrane saturated with the substrate was applied directly to the rooted soil surface and incubated for 1 h. Fluorescent substrates in the membranes diffused into the soil and were hydrolysed by enzymes to release fluorescent MUF, which diffused back and was visualized under UV light (Spohn and Kuzyakov, 2014). After incubation the membranes were carefully lifted off the soil surface and all attached soil particles were gently removed using a soft brush. The membranes were placed under UV light (excitation, 365 nm) in a Geneflash Documentation System (Syngene, Cambridge, UK) in a dark room and photographs of the membranes were taken. The imaging equipment, the camera (DSLR-A300, Sony, Japan) settings, exposure time (5 s) and positions of the camera and the membrane were fixed and constant for all samples. A calibration line was set up using membrane squares (4 cm2) that were soaked in solutions of increasing MUF concentration (0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6 and 0.7 mm). The amount of MUF per unit area was calculated by dividing the volume of the solution absorbed by the membrane by the size of the membrane (Ma et al., 2019). The membranes used for the calibration line were imaged in the same way as the samples.
The open-source software ImageJ was used for image processing and analysis. Images of zymograms were converted to 8-bit grey values. Linear regression was used to test the correlation between the grey values of the calibration membranes and the MUF concentrations, and consequently, the correlation with enzyme activities. The calibration point where the MUF concentration was zero was taken as the background signal and subtracted from all images. Five randomly drawn 50-pixel-wide lines across the root on each plant were used to calculate the rhizosphere extent of acid phosphatase activity. The rhizosphere extent of enzyme activity was defined as the distance from the root centre to the position with activity at least 20 % higher than that of bulk soil (Ma et al., 2019).
Shoot dry weight and P analysis
The maize shoots were oven-dried at 60 °C for 3 d to determine shoot dry weight. Dried shoot material was ground to a fine powder. Two hundred and fifty milligrams of shoot dry matter was incubated with 65 % HNO3, 10 mm H2O2 and distilled water in a microwave (MLS Maxi 44, Germany) at a maximum of 210 °C and 1400 W for 65 min. This solution was adjusted to 20 mL and filtered over activated charcoal and through 90-μm mesh filter paper. Phosphorus was measured spectrophotometrically by orthophosphate determination after the addition of molybdate-vanadate reagent (Gericke and Kurmies, 1952) using a spectrophotometer (U-3300, Hitachi, Tokyo, Japan).
Root analyses and mycorrhizal colonization
After shoot excision, roots were vigorously washed with sterilized deionized water in order to remove all soil from the root surface. Root samples were gently dried with clean soft tissue; 0.3 g of fresh root was immediately frozen in liquid nitrogen and stored at −30 °C for RNA extraction. The remaining root was used to calculate the total root length and was determined by flat-bed scanning (Epson Expression 1000 XL, Tokyo, Japan) and analysed with WinRHIZO (Regent Instruments, Canada) software. Root dry biomass was determined and specific root length was calculated as total root length divided by dry mass.
The roots were stained according to Brundrett et al. (1994) with slight modifications. Briefly, the roots were cut into segments 1 cm long, cleared with 10 % KOH at 90 °C for 1 h, rinsed three times with tap water, acidified in 2 m HCl for 2 min and stained with 5 % ink–acetic acid for 30 min at 60 °C. Root pieces were then soaked in tap water acidified with drops of acetic acid overnight to get rid of excess ink (Koske and Gemma, 1989; Vierheilig et al., 1998). Finally, 30 randomly selected root pieces (1 cm long) from each replicate were placed on microscope slides (ten per slide) and observed for AMF colonization under a bright-field light microscope (Axioskop 2, Zeiss, Germany). As in the method described by Trouvelot et al. (1986), the mycorrhizal intensity of colonization (MIC) was determined using colonization classes, which were estimated for each root piece depending on the percentage of the root length colonized by AMF (#0 = 0 %, #1 ≤ 1 %, #2 = 2–10 %, #3 = 11–50 %, #4 = 51–90 %, #5 > 90 %). The MIC was calculated as an average for 30 root pieces using the formula MIC = (95n5 + 70n4 + 30n3 + 5n2 + n1)/N × 100 %, where n5, n4, n3, n2, n1 are the numbers of root pieces scaled as 5, 4, 3, 2 and 1, respectively, and N is the total number of fragments observed. The total colonized root length was calculated as total root length multiplied by MIC.
Mycorrhizal and root hair dependency
‘Mycorrhizal dependency’ refers to the difference in plant growth between mycorrhizal and non-mycorrhizal treatment. It was calculated by the following formula (van der Heijden, 2003; Tawaraya, 2003):
where a is plant dry mass of the inoculated genotype, n is the replicate number inoculated with AMF and b is the mean plant dry mass of the non-inoculated genotype.
‘Root hair dependency’ refers to the difference in plant growth between the hairless mutant and the wild type. It was calculated by the following equation:
where A is plant dry mass of the non-inoculated wild type, N is the number of replicates and B is the mean plant dry mass of non-inoculated rth3.
Gene expression analysis
Total RNA was extracted from ground root tissues with an innuPREP Plant RNA Kit (Analytik Jena, Jena, Germany) following the manufacturer’s instructions. The RNA concentration was determined with a Nanodrop 2000c Spectrophotometer (Thermo Fisher Scientific, USA). RNA (0.5 g) was reverse-transcribed into cDNA using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany).
The AM-induced Pi transporter genes ZmPt1;6 and ZmPt1;11 and the AM-specific marker gene ZmAm3 were quantified by relative real-time PCR on a CFX384 (Bio-Rad, Hercules, CA, USA). qPCRs were carried out in a 20-μL volume with 10 μL of Green Master Mix (2×) without ROX passive reference dye (Genaxxon Bioscience, Ulm, Germany), 1 μL of each primer (10 mmol L−1) and 15 ng of template DNA. The GAPDH and β-actin genes were used as reference genes to normalize the expression data (Gutjahr et al., 2008). All primers are listed in Supplementary Data Table S1. Thermal cycling included an initial denaturation step at 95 °C for 20 min, followed by 50 cycles of denaturation at 95 °C for 15 s and another 20 s at 60 °C. A final melting curve was recorded from 65 to 95 °C with increments of 0.5 °C. Each biological sample was analysed as three technical replicates. For each replication, autoclaved water was used as a negative control. Gene expression was calculated using the Bio-Rad CFX Manager 3.1 software.
Statistical analyses
Normality and homogeneity of the variance for shoot dry weight, root dry weight, total root length, specific root length, P concentration and P content were assessed using the Shapiro–Wilk test and Levene’s test. One-way ANOVA followed by the Duncan test was used to test the significance of differences of these variables in response to AM inoculation, root hairs and total colonized root length. The effect of mycorrhizal inoculation, genotypes and their interactions on shoot dry weight, root dry weight, P content, P concentration, specific root length, total root length and mycorrhizal colonization were tested by two-way ANOVA. Pearson correlation coefficients were checked to determine relationships among AMF colonization, Pi transporter expression parameters, maize growth variables and P acquisition. All statistical analyses were done using the software SPSS v23.0.
RESULTS
Rhizosphere extent, plant growth, P i acquisition and release of organic acid anions of wild type and rth3
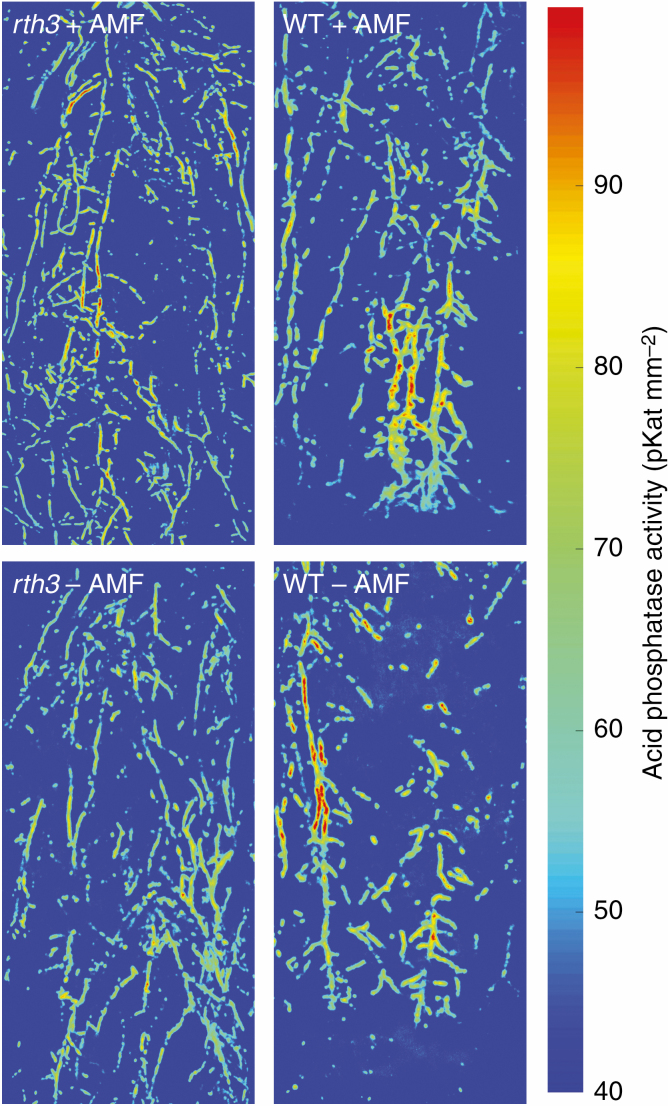
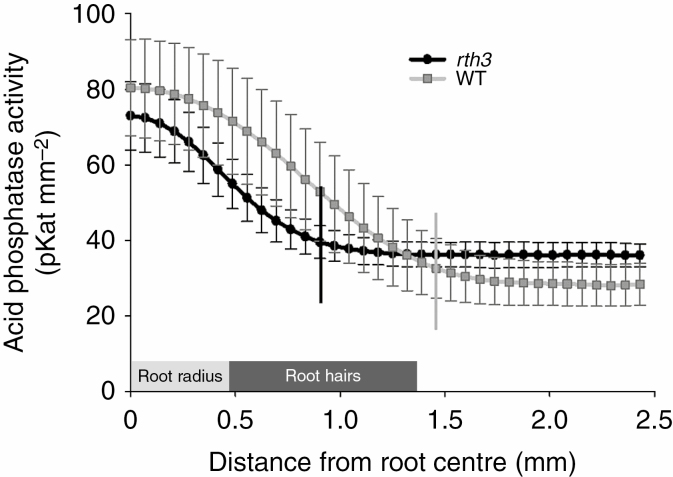
The root hair length was on average 0.9 mm for the wild type while rth3 essentially lacked root hairs completely and was totally bald (Fig. 1). We localized and quantified acid phosphatase activity in both genotypes by in situ zymography (Fig. 2). Visual differences were immediately apparent between rth3 and wild type regardless of AMF inoculation: root-associated acid phosphatase activities were higher and in thicker stretches along the roots, suggesting that the root hairs extended the activity zone of acid phosphatases, and these patterns were not affected by AMF inoculation (Fig. 2). The radial rhizosphere extension of acid phosphatase activity by root hairs was ~0.5 mm, which doubled the radial rhizosphere area for this enzymatic activity (Fig. 3). Malate, citrate, succinate and trans-aconitate were detected in root exudates, malate being the dominant organic acid anion. However, there was no difference between rth3 and the wild type in terms of organic acid anion release from the roots close to the root tips (Supplementary Data Table S2).
Fig. 2.
Spatial distribution of acid phosphatase activity in the rhizosphere of maize root hairless mutant rth3 (left) and wild type (WT, right). Representative examples are shown with AMF (top panels) and without inoculation (lower panels). The colour scale on the right shows linear enzyme activities (pKat mm−2). Top panels show rth3 (left) and wild type (right) with inoculated arbuscular mycorrhizal fungi. Bottom panels show rth3 (left), wild type (right) without inoculation of arbuscular mycorrhizal fungi.
Fig. 3.
Profiles of acid phosphatase activity as a function of distance from the maize root centre in the root-hairless mutant rth3 and the wild type (WT). The root radius (0.45 mm) is shown as a light grey rectangle. The root hair length of wild type (0.9 mm) is shown as a dark grey rectangle. Vertical lines on the curves indicate the distance to which the rhizosphere acid phosphatase activity extended for the root-hairless mutant rth3 and the wild type.
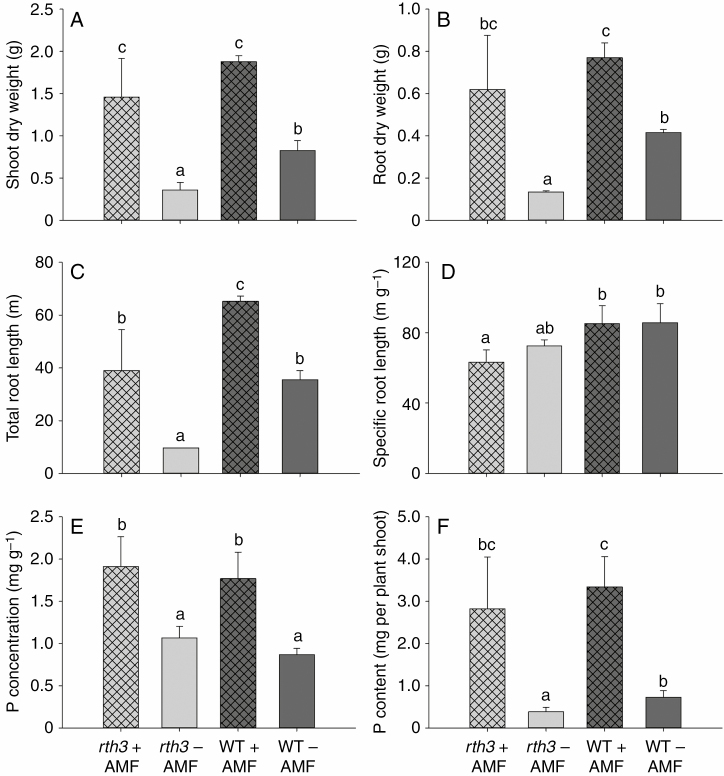
Shoot and root dry weight, total root length and specific root length differed between the two genotypes when grown under limited Pi supply (Fig. 4). The nutritional P status (measured as shoot P concentration, which was around 0.1 %) indicated severely P-deficient conditions (Fig. 1). The non-inoculated rth3 had the lowest shoot and root biomass, shortest total root length and lowest P content. Values for these traits in the wild type were 1.9- to 3.6-fold of those of the root hairless mutant rth3 (Fig. 4A–C, F and Supplementary Data Fig. S1). The specific root length of the wild type was ~25 % higher than that of rth3 (Fig. 4D). The P concentration in the shoot was very low, at around 0.1 %, indicating severe P deficiency, but it was not different between the two genotypes (Fig. 4E and Supplementary Data Fig. S1).
Fig. 4.
The growth defect of the root hairless mutant rth3 on P-deficient soil is compensated by AMF inoculation. Shoot dry weight (A), root dry weight (B), total root length (C), specific root length (D), P concentration (E) and P content (F) of maize rth3 and wild type (WT) inoculated with AMF (+AMF) or not inoculated with AMF (-AMF). Different letters indicate significant differences (P < 0.05, Duncan’s test) between treatments and genotypes.
Effects of AMF inoculation on plant growth, P i acquisition, mycorrhizal colonization, release of organic acid anions and related P i transporter gene expression of wild type and rth3
In AMF-inoculated plants, fine extraradical hyphae were visible across the entire root, but these were absent in non-inoculated conditions (Supplementary Data Fig. S2). Inoculation with AMF significantly improved plant growth and Pi assimilation, and the enhancement was greater than that of root hairs (Table 1, Fig. 4A–C, E, F). The shoot and root biomass of AMF inoculated plants, as well as P content, were 4.0- to 7.4-fold greater those of non-inoculated plants in rth3, while only 1.8- to 4.6-fold greater in wild type (Fig. 4A–C, F). Stimulation of plant growth and Pi acquisition in rth3 by AMF was 1.5- to 3.9-fold greater than in wild type (Fig. 4A–C, E, F), but both AMF-colonized genotypes ultimately had almost the same biomass. The total root length of AMF-inoculated wild-type plants was double that of non-inoculated wild-type plants and was 5-fold increased in rth3, while specific root length was almost not affected (Fig. 4D, Table 1). AMF colonization strongly improved the nutritional status of the plants and increased the internal P concentration ~2-fold to ~0.2 %, but these plants still experienced P deficiency, as the critical sufficiency level for maize is commonly around 0.3 %. Inoculation of AMF in the presence and absence of root hairs did not affect organic acid anion release from roots (Supplementary Data Table S2).
Table 1.
Results of two-way ANOVA showing the statistical significance of the effects of mycorrhizal inoculation, genotype and their interaction on shoot dry weight, root dry weight, P content, P concentration, specific root length, total root length and mycorrhizal colonization
| Effect | Shoot dry weight | Root dry weight | Total root length | Specific root length | Shoot P content | Shoot P concentration | Mycorrhizal colonization | Exudation of organic acids |
|---|---|---|---|---|---|---|---|---|
| Genotype (G) | 0.014* | 0.023* | 0.001** | 0.006** | 0.328 | 0.265 | 0.003** | 0.179 |
| Inoculation (I) | 0.000** | 0.001** | 0.000** | 0.344 | 0.000** | 0.000** | 0.000** | 0.396 |
| G × I interaction | 0.882 | 0.422 | 0.952 | 0.389 | 0.832 | 0.848 | 0.002** | 0.111 |
*P < 0.05; **P < 0.01.
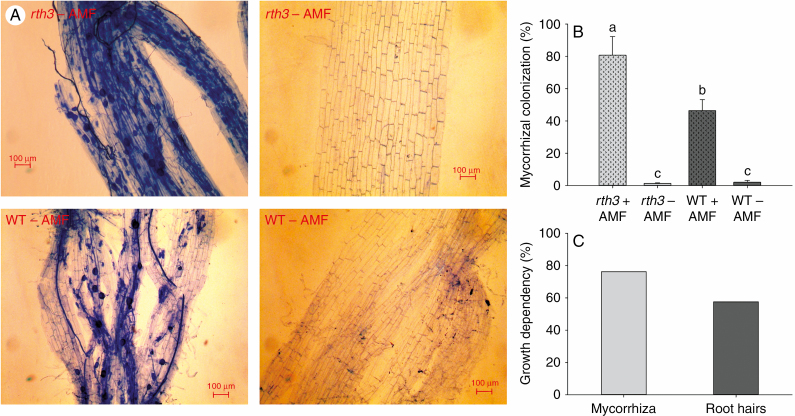
There was significant interaction between mycorrhizal colonization and the presence of root hairs (P = 0.002) (Table 1). The average mycorrhizal colonization of non-inoculated roots was <2 %, but AMF inoculation increased colonization to 46 % for the wild type and to 80 % for rth3 (Fig. 5A, B). However, the total colonized root length was similar in the two genotypes when inoculated (Supplementary Data Fig. S3). Mycorrhizal growth dependency (76 %) was higher than root hair dependency (57 %) (Fig. 5C).
Fig. 5.
Root hairs impair AMF infection. (A) Visualization of AMF colonization in maize roots of rth3 and wild type (WT) inoculated with AMF (left panels) or not inoculated with AMF (right panels). (B) Mycorrhizal colonization and (C) growth dependence on mycorrhiza and root hairs.
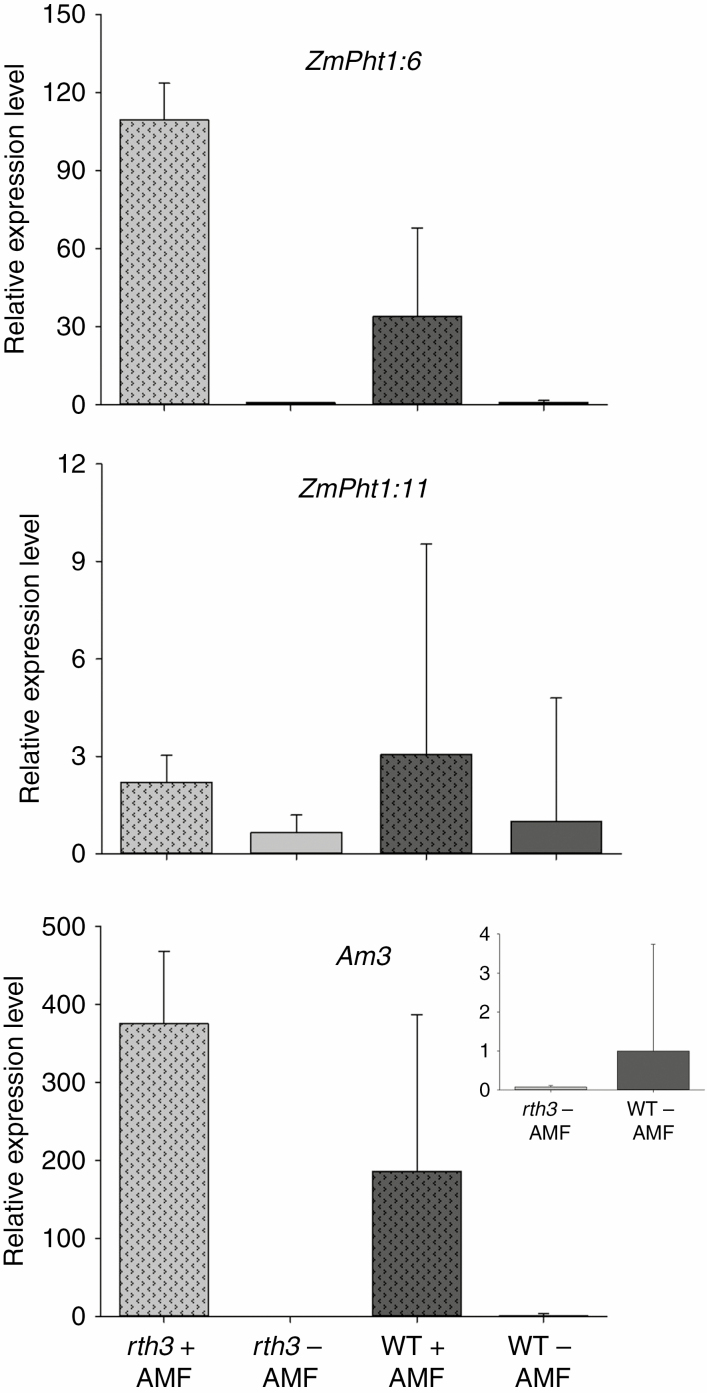
ZmPht1;6, the primary mycorrhiza-specific Pi transporter gene, is exclusively found in AMF-colonized roots and is expressed at a high level. In agreement with the greater infection of rth3 roots, ZmPht1;6 gene expression was higher in the hairless roots. The less expressed, but also mycorrhiza-induced Pi transporter gene ZmPht1;11 and the AMF colonization marker gene Am3 were also induced by mycorrhization, both in AMF-colonized roots of rth3 and in the wild type (Fig. 6).
Fig. 6.
Induction of mycorrhiza-induced Pht genes for phosphate transfer to the plant. Relative expression levels of ZmPht1;6, ZmPht1;11 and Am3 genes in rth3 and wild-type (WT) maize root inoculated or not inoculated with AMF under P deficiency. Data points are means ± s.e.m. (n = 3 biological replicates of three plants each).
Relationships among AM colonization, maize growth, P acquisition and Pi transporter gene expression
Mycorrhizal colonization was positively correlated with ZmPht1:6 and Am3 gene expression, shoot and root biomass, as well as with P concentration and P content (Table 2). The expression of ZmPht1:6 was positively correlated with shoot dry weight, P concentration and P content, indicating the importance of this gene in Pi uptake in maize (Table 2). Both shoot P concentration and shoot P content were also positively correlated with total root length, root and shoot biomass, as well as with each other.
Table 2.
Pearson correlations among mycorrhizal colonization, ZmPht1;6, ZmPht1;11 and Am3 expression, shoot dry weight, root dry weight total root length, P concentration and P content
| Mycorrhizal colonization | ZmPht1;6 | ZmPht1;11 | Am3 | Shoot dry weight | Root dry weight | Total root length | Specific root length | Shoot P concentration | |
|---|---|---|---|---|---|---|---|---|---|
| ZmPht1;6 | 0.945** | ||||||||
| ZmPht1;11 | 0.326 | 0.117 | |||||||
| Am3 | 0.887** | 0.911** | 0.065 | ||||||
| Shoot dry weight | 0.732* | 0.590* | 0.564 | 0.682* | |||||
| Root dry weight | 0.677* | 0.530 | 0.637* | 0.567 | 0.969** | ||||
| Total root length | 0.520 | 0.352 | 0.547 | 0.511 | 0.941** | 0.943** | |||
| Specific root length | −0.375 | −0.464 | −0.158 | −0.154 | 0.089 | 0.052 | 0.366 | ||
| Shoot P concentration | 0.822** | 0.692* | 0.412 | 0.786* | 0.955** | 0.893** | 0.851** | 0.015 | |
| Shoot P content | 0.917** | 0.824** | 0.241 | 0.889** | 0.766** | 0.666* | 0.591* | −0.166 | 0.903** |
*P < 0.05; **P < 0.01.
DISCUSSION
Maize root hairs improved growth and Pi acquisition
As root hairs play an important role in Pi acquisition (Jungk, 2001; Lynch, 2005; Lambers et al., 2006), it was expected that the hairless mutant would perform much worse than the wild type under P-deficient conditions. The primary role of root hairs is to extend the root surface area and to increase the radial rhizosphere diameter to explore a larger soil volume (Ma et al., 2001; Pang et al., 2018). This was confirmed by up to 0.5 mm broader rhizosphere extent of acid phosphatase activity in the wild type compared with the hairless mutant (Figs 2 and 3), irrespective of AMF inoculation. This is in line with previous studies; root hairs greatly improved and extended rhizosphere acid phosphatase activity (Giles et al., 2018) and may contribute 70–90 % of the total surface area relevant for nutrient absorption (Bates and Lynch, 1996; López-Arredondo et al., 2014). Our study further verified that the root hair dependency of maize growth was 57 %. By contrast, the root hair dependency of barley was, according to results shown in Chen et al. (2005) and Jakobsen et al. (2005), around 75 %, which is notably higher than our results in maize, indicating that root hairs play more important roles in barley than in maize. Furthermore, the root hairs enhanced the P content by 44 %, but this increase was not significant and the P concentration was similar or tended to be even slightly decreased in plants with root hairs (Fig. 4E, F). This is in line with previous research (Weber et al., 2018; Klamer et al., 2019; Ludewig et al., 2019). However, root-hairless plants were severely depressed in biomass formation compared with the wild type, meaning that any additionally acquired Pi was immediately invested in producing more shoot and root biomass, leading to bigger shoots and longer roots (Fig. 4). Specific root length is also an important root morphology measure that can affect nutrient acquisition (Lambers et al., 2006). Maize wild type had a higher specific root length than rth3, which indicated that the wild type has longer root length per unit invested in dry mass. This further allowed a greater soil volume to be explored per unit C invested and thus enhanced P uptake efficiency (Laliberté et al., 2015). Therefore, maize root hairs not only improved P acquisition efficiency but also indirectly improved root morphology, with longer total root length and higher specific root length that further improved plant growth compared with the rth3 hairless mutant.
Inoculation of AMF improved plant growth and P i acquisition more effectively in rth3 than in wild type
Inoculation of AMF increased plant growth and Pi acquisition of both the wild type and the rth3 root hairless mutant 1.8- to 7.4-fold (Fig. 4). The P nutritional status, i.e. the shoot P concentration, was massively improved by AMF, facilitating maize growth and further Pi acquisition by AMF and by roots. Phosphorus uptake by the root and root hairs generates a radial depletion zone of up to 2 mm around the roots (Joner et al., 1995) due to the low mobility of Pi (Fontes and Weed, 1996). The hyphal network of AM fungi, by contrast, can extend up to 25 cm from the root, which is far beyond the depletion zone (Li et al., 1991; Jansa et al., 2003). Furthermore, root Pi assimilation is mainly in the root hair zone (Smith et al., 2011), while the AMF also colonizes more mature regions of the root and supplies these regions with assimilated Pi (Richardson et al., 2011). Therefore, hyphae can greatly increase exploration of the soil volume for phosphate. Moreover, the diameter of extracellular hyphae is about one order of magnitude smaller than that of fine roots and often even thinner than that of root hairs; thus, hyphae can enter small soil pores that are inaccessible to thick roots (Drew et al., 2003; Smith and Read, 2008). Even when roots and hyphae have a similar volume, the surface area of hyphae in contact with soil is higher than that of the root due to its small diameter, which can greatly increase Pi assimilation (Jakobsen et al., 2005). All these factors probably contribute to the much larger biomass and greater Pi uptake of AMF-inoculated rth3 compared with wild type under the non-inoculated condition (Fig. 4).
The root-hairless mutant rth3 particularly profited more from the AMF inoculation than the wild type, which is in line with previous studies where root hair length negatively correlated with mycorrhizal dependency of various species, because root hair length contributed to plant Pi uptake (Tawaraya, 2003). Thus, root hairs and AMF provide alternative, inversely correlated pathways for Pi foraging. Although root hairs and AMF provide alternative mechanisms to increase contact with the soil, the 33 % higher mycorrhizal growth dependency than root hair dependency strongly argues that AMF provide a more efficient way to acquire Pi even in young maize (Fig. 5C). Furthermore, the total colonized root length was similar in the two genotypes under the AMF inoculation condition and was accompanied by almost the same final biomass, indicating that AMF played a more important role than root hairs for Pi acquisition in maize. This is in line with previous studies showing that AMF play a critical role in nutrient acquisition, while root hairs are dispensable in maize (Wen and Schnable, 1994; Cozzolino et al., 2013). However, similar studies in barley showed that the root hairs play more important roles than AMF (Jakobsen et al., 2005; Chen et al., 2005; Li et al., 2014). This is probably due to the fact that the root hair length of barley wild type was almost doubled under the P-deficient condition compared with that under the higher P condition (Brown et al., 2012), while the root hair length in maize B73 wild type was not significantly different between P-deficient (this study, 0.90 mm) and P-sufficient conditions (0.83 mm) (Weber et al., 2018). This explanation is supported by observations in Plantago lanceolata, whose root hair length was not responsive to P availability but was highly dependent on AMF for Pi acquisition under P deficiency (Brown et al., 2013a). Other root morphological traits also influence plant dependency on AMF symbiosis; for example, species with thin roots rely less on AMF symbiosis for nutrient uptake than species with thick roots (Kong et al., 2014; Z. Ma et al., 2018). Similar phenomena exist among different root architectures, where the mycorrhizal colonization was lower in the thin first-order root than in the thick second-order roots (Eissenstat et al., 2015). This further indicated that root morphological properties (e.g. root hairs and root diameter) and mycorrhizal colonization act as alternative strategies in Pi uptake. It is also noteworthy that root hairs are already endogenously found in young seedlings from the first days of growth, while AMF establishment requires several weeks to be functionally established (Smith and Read, 2008). Ultimately, AMF could compensate the loss of root hairs and even play a more critical role than root hairs for Pi acquisition under P deficiency even for juvenile maize.
Mycorrhizal colonization and mycorrhiza-specific Pi transporter ZmPht1:6 expression were positively correlated with each other (P < 0.01) and both also positively correlated with plant growth, P status and P acquisition (Table 2). This indicated that the colonization rate and transporter gene expression are positively correlated with symbiotic function and activity. Mycorrhizal colonization promoted maize growth, P status, Pi acquisition and related Pi transporter gene expression in a plant genotype-dependent manner, in which the rth3 hairless mutant benefited more than the wild type. As a consequence, plant biomass and Pi uptake were induced 1.6- to 2.5-fold by AMF colonization in rth3 compared with the wild type (Fig. 4A, B, F). The mycorrhizal colonization of rth3 was 1.7-fold that of the wild type (Fig. 5A, B), leading to higher AM-induced Pi transporter ZmPht1;6 expression in rth3 compared with the wild type (Fig. 6A). The following two mechanisms can explain the lower dependency of the wild type than the hairless mutant on AMF. Morphologically, the wild type, with long and dense root hairs, has a larger root surface area than the root-hairless mutant; thus, dependency on mycorrhiza for Pi acquisition was decreased. Physiologically, root hair growth and AM hypha establishment are both carbon- and energy-consuming and compete for resources (Kuzyakov and Domanski, 2000; Ryan et al., 2012). Hence, the abundance of AM colonization was not only induced by limited soil-available P, but also controlled by phytohormones and molecular mechanisms (Akiyama et al., 2005; Kobae et al., 2018). The phytohormone strigolactone plays an important role in the interaction of root establishment with AM symbiosis interaction (Akiyama et al., 2005; Besserer et al., 2006; Chagas et al., 2018). Colonization by AMF depends on the release of such signalling compounds into the rhizosphere to germinate spores and attract hyphae for root contact; a radially extended rhizosphere was expected to be beneficial for the number of AMF–root contact sites. However, root hairs expand the soil volume into which strigolactone is released, but had only a minor role in maize, as AMF colonization was higher in the hairless mutant than in the wild type (Fig. 5). Molecular signals such as CLE (CLAVATA3/Embryo Surrounding Region-Related) peptides also play a key role in AMF colonization of various plants (Handa et al., 2015; Karlo et al., 2020). Future investigations of genetic and molecular pathways such as the relationship between the release of strigolactones and CLE peptides with AMF colonization are needed to reveal the contrast in mycorrhizal colonization between wild type and rth3 in maize.
Conclusions
Our combined analysis of morphological, physiological and molecular factors and plant growth dependence on root hairs and AMF under P deficiency identified a larger mycorrhizal than root hair dependency. While both root hairs and arbuscular mycorrhizal inoculation can increase Pi uptake under P deficiency, the hairless mutant was more reliant on AMF inoculation than the wild type. Our data are consistent with the general assumption that root hairs and AMF inoculation are two alternative ways to increase Pi acquisition under P deficiency, but these two strategies compete with each other and root hairs cannot compensate for lack of AMF in maize.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: examples of young rth3 and wild-type maize plants inoculated or not inoculated with AMF and grown in rhizoboxes. Figure S2: examples of root-soil areas from maize rth3 and wild type inoculated or not inoculated with AMF and grown in rhizoboxes. Figure S3: total colonized root length calculated as total root length multiplied by MIC. Table S1: primers used for qRT–PCR. Table S2: organic acids captured from rth3 and wild-type maize roots inoculated or not inoculated with AMF.
ACKNOWLEDGEMENTS
We thank H. Ochott and M. Mang for help with nutrient analyses, Professor Dr F. Hochholdinger for providing maize seeds, and Dr M. Weinmann for providing Rhizophagus irregularis MUCL41833 beads and comments. X.M. conceived the research. X.M., X.L. and U.L. designed the research. X.M. and X.L. conducted the experiments and X.M. analysed the data. X.M. and X.L. wrote and U.L. edited the manuscript. All authors read and approved the manuscript. The authors declare that they have no conflict of interest.
FUNDING
We gratefully acknowledge the China Scholarship Council (201506300073) and (201606350143) for supporting X.M. and X.L., respectively. This study was supported by the German Research Foundation (DFG) – 328017493/GRK 2366 (International Research Training Group ‘Adaptation of maize-based food-feed-energy systems to limited phosphate resources’).
LITERATURE CITED
- Abel S. 2017. Phosphate scouting by root tips. Current Opinion in Plant Biology 39: 168–177. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827. [DOI] [PubMed] [Google Scholar]
- Bailey PHJ, Currey JD, Fitter AH. 2002. The role of root system architecture and root hairs in promoting anchorage against uprooting forces in Allium cepa and root mutants of Arabidopsis thaliana. Journal of Experimental Botany 53: 333–340. [DOI] [PubMed] [Google Scholar]
- Bates TR, Lynch JP. 1996. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant, Cell and Environment 19: 529–538. [Google Scholar]
- Benedetto A, Magurno F, Bonfante P, Lanfranco L. 2005. Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza 15: 620–627. [DOI] [PubMed] [Google Scholar]
- Besserer A, Puech-Pagès V, Kiefer P, et al. 2006. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biology 4: 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski RL. 1973. Phosphate pools, phosphate transport, and phosphate availability. Annual Review of Plant Physiology 24: 225–252. [Google Scholar]
- Bonfante P. 2018. The future has roots in the past: the ideas and scientists that shaped mycorrhizal research. New Phytologist 220: 982–995. [DOI] [PubMed] [Google Scholar]
- Brown LK, George TS, Thompson JA, et al. 2012. What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Annals of Botany 110: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LK, George TS, Barrett GE, Hubbard SF, White PJ. 2013a Interactions between root hair length and arbuscular mycorrhizal colonisation in phosphorus deficient barley (Hordeum vulgare). Plant and Soil 372: 195–205. [Google Scholar]
- Brown LK, George TS, Dupuy LX, White PJ. 2013b A conceptual model of root hair ideotypes for future agricultural environments: what combination of traits should be targeted to cope with limited P availability? Annals of Botany 112: 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundrett M, Melville L, Peterson L. 1994. Practical methods in mycorrhiza research. Sidney, Canada: Mycologue Publications. [Google Scholar]
- Brundrett MC, Tedersoo L. 2018. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytologist 220: 1108–1115. [DOI] [PubMed] [Google Scholar]
- Chagas FO, Pessotti RdC, Caraballo-Rodríguez AM, Pupo MT. 2018. Chemical signaling involved in plant-microbe interactions. Chemical Society Reviews 47: 1652–1704. [DOI] [PubMed] [Google Scholar]
- Chen B, Roos P, Borggaard OK, Zhu Y-G, Jakobsen I. 2005. Mycorrhiza and root hairs in barley enhance acquisition of phosphorus and uranium from phosphate rock but mycorrhiza decreases root to shoot uranium transfer. New Phytologist 165: 591–598. [DOI] [PubMed] [Google Scholar]
- Collavino MM, Sansberro PA, Mroginski LA, Aguilar OM. 2010. Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biology and Fertility of Soils 46: 727–738. [Google Scholar]
- Cozzolino V, Di Meo V, Piccolo A. 2013. Impact of arbuscular mycorrhizal fungi applications on maize production and soil phosphorus availability. Journal of Geochemical Exploration 129: 40–44. [Google Scholar]
- Drew EA, Murray RS, Smith SE, Jakobsen I. 2003. Beyond the rhizosphere: growth and function of arbuscular mycorrhizal external hyphae in sands of varying pore sizes. Plant and Soil 251: 105–114. [Google Scholar]
- Eissenstat DM, Kucharski JM, Zadworny M, Adams TS, Koide RT. 2015. Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytologist 208: 114–124. [DOI] [PubMed] [Google Scholar]
- Finlay RD. 2008. Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. Journal of Experimental Botany 59: 1115–1126. [DOI] [PubMed] [Google Scholar]
- Fontes MPF, Weed SB. 1996. Phosphate adsorption by clays from Brazilian oxisols: relationships with specific surface area and mineralogy. Geoderma 72: 37–51. [Google Scholar]
- Gericke S, Kurmies B. 1952. Die kolorimetrische Phosphorsäurebestimmung mit Ammonium-Vanadat-Molybdat und ihre Anwendung in der Pflanzenanalyse. Zeitschrift für Pflanzenernährung, Düngung, Bodenkunde 59: 235–247. [Google Scholar]
- Giles CD, Dupuy L, Boitt G, et al. 2018. Root development impacts on the distribution of phosphatase activity: improvements in quantification using soil zymography. Soil Biology and Biochemistry 116: 158–166. [Google Scholar]
- Gilroy S, Jones DL. 2000. Through form to function: root hair development and nutrient uptake. Trends in Plant Science 5: 56–60. [DOI] [PubMed] [Google Scholar]
- Gore MA, Chia J-M, Elshire RJ, et al. 2009. A first-generation haplotype map of maize. Science 326: 1115–1117. [DOI] [PubMed] [Google Scholar]
- Grace EJ, Cotsaftis O, Tester M, Smith FA, Smith SE. 2009. Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytologist 181: 938–949. [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Banba M, Croset V, et al. 2008. Arbuscular mycorrhiza-specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell 20: 2989–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase S, Neumann G, Kania A, Kuzyakov Y, Römheld V, Kandeler E. 2007. Elevation of atmospheric CO2 and N-nutritional status modify nodulation, nodule-carbon supply, and root exudation of Phaseolus vulgaris L. Soil Biology and Biochemistry 39: 2208–2221. [Google Scholar]
- Handa Y, Nishide H, Takeda N, Suzuki Y, Kawaguchi M, Saito K. 2015. RNA-seq transcriptional profiling of an arbuscular mycorrhiza provides insights into regulated and coordinated gene expression in Lotus japonicus and Rhizophagus irregularis. Plant & Cell Physiology 56: 1490–1511. [DOI] [PubMed] [Google Scholar]
- van der Heijden MGA. 2003. Arbuscular mycorrhizal fungi as a determinant of plant diversity: in search of underlying mechanisms and general principles. In: van der Heijden MGA, Sanders IR, eds. Mycorrhizal ecology. Berlin: Springer, 243–265. [Google Scholar]
- van der Heijden MGA, Martin FM, Selosse M-A, Sanders IR. 2015. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist 205: 1406–1423. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Wen T-J, Zimmermann R, et al. 2008. The maize (Zea mays L.) roothairless3 gene encodes a putative GPI-anchored, monocot-specific, COBRA-like protein that significantly affects grain yield. Plant Journal 54: 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen I, Rosendahl L. 1990. Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytologist 115: 77–83. [Google Scholar]
- Jakobsen I, Chen B, Munkvold L, Lundsgaard T, Zhu Y-G. 2005. Contrasting phosphate acquisition of mycorrhizal fungi with that of root hairs using the root hairless barley mutant. Plant, Cell and Environment 28: 928–938. [Google Scholar]
- Jansa J, Mozafar A, Frossard E. 2003. Long-distance transport of P and Zn through the hyphae of an arbuscular mycorrhizal fungus in symbiosis with maize. Agronomie 23: 481–488. [Google Scholar]
- Javot H, Pumplin N, Harrison MJ. 2007. Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant, Cell and Environment 30: 310–322. [DOI] [PubMed] [Google Scholar]
- Johnston AE, Poulton PR, Fixen PE, Curtin D. 2014. Phosphorus: its efficient use in agriculture. In: Sparks DL, ed. Advances in agronomy. San Diego: Academic Press, 177–228. [Google Scholar]
- Joner EJ, Magid J, Gahoonia TS, Jakobsen I. 1995. P depletion and activity of phosphatases in the rhizosphere of mycorrhizal and non-mycorrhizal cucumber (Cucumis sativus L.). Soil Biology and Biochemistry 27: 1145–1151. [Google Scholar]
- Jungk A. 2001. Root hairs and the acquisition of plant nutrients from soil. Journal of Plant Nutrition and Soil Science 164: 121–129. [Google Scholar]
- Kaeppler SM, Parke JL, Muelle SM, Senior L, Stuber C, Tracy WF. 2000. Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Science 40: 358–364. [Google Scholar]
- Karlo M, Boschiero C, Landerslev KG, et al. 2020. The CLE53-SUNN genetic pathway negatively regulates arbuscular mycorrhiza root colonization in Medicago truncatula. Journal of Experimental Botany 71: 4972–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamer F, Vogel F, Li X, et al. 2019. Estimating the importance of maize root hairs in low phosphorus conditions and under drought. Annals of Botany 124: 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobae Y, Kameoka H, Sugimura Y, et al. 2018. Strigolactone biosynthesis genes of rice are required for the punctual entry of arbuscular mycorrhizal fungi into the roots. Plant and Cell Physiology 59: 544–553. [DOI] [PubMed] [Google Scholar]
- Kong D, Ma C, Zhang Q, et al. 2014. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytologist 203: 863–872. [DOI] [PubMed] [Google Scholar]
- Koske RE, Gemma JN. 1989. A modified procedure for staining roots to detect VA mycorrhizas. Mycological Research 92: 486–488. [Google Scholar]
- Kumar A, Shahbaz M, Koirala M, et al. 2019. Root trait plasticity and plant nutrient acquisition in phosphorus limited soil. Journal of Plant Nutrition and Soil Science 182: 945–952. [Google Scholar]
- Kuzyakov Y, Domanski G. 2000. Carbon input by plants into the soil. Review. Journal of Plant Nutrition and Soil Science 163: 421–431. [Google Scholar]
- Laliberté E, Lambers H, Burgess TI, Wright SJ. 2015. Phosphorus limitation, soil-borne pathogens and the coexistence of plant species in hyperdiverse forests and shrublands. New Phytologist 206: 507–521. [DOI] [PubMed] [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. 2006. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98: 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lin G, Zhang X, Chen Y, Zhang S, Chen B. 2014. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 24: 595–602. [DOI] [PubMed] [Google Scholar]
- Li X-L, George E, Marschner H. 1991. Extension of the phosphorus depletion zone in VA-mycorrhizal white clover in a calcareous soil. Plant and Soil 136: 41–48. [Google Scholar]
- Loján P, Demortier M, Velivelli SLS, et al. 2017. Impact of plant growth-promoting rhizobacteria on root colonization potential and life cycle of Rhizophagus irregularis following co-entrapment into alginate beads. Journal of Applied Microbiology 122: 429–440. [DOI] [PubMed] [Google Scholar]
- López-Arredondo DL, Leyva-González MA, González-Morales SI, López-Bucio J, Herrera-Estrella L. 2014. Phosphate nutrition: improving low-phosphate tolerance in crops. Annual Review of Plant Biology 65: 95–123. [DOI] [PubMed] [Google Scholar]
- Ludewig U, Yuan L, Neumann G. 2019. Improving the efficiency and effectiveness of global phosphorus use: focus on root and rhizosphere levels in the agronomic system. Frontiers of Agricultural Science and Engineering 6: 357–365. [Google Scholar]
- Lynch JP. 2005. Root architecture and nutrient acquisition. In: BassiriRad H, ed. Nutrient acquisition by plants: an ecological perspective. Berlin: Springer, 147–183. [Google Scholar]
- Lynch JP. 2015. Root phenes that reduce the metabolic costs of soil exploration: opportunities for 21st century agriculture. Plant, Cell and Environment 38: 1775–1784. [DOI] [PubMed] [Google Scholar]
- Lyu Y, Tang H, Li H, et al. 2016. Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Frontiers in Plant Science 7: 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Goto S, Tamai K, Ichii M. 2001. Role of root hairs and lateral roots in silicon uptake by rice. Plant Physiology 127: 1773–1780. [PMC free article] [PubMed] [Google Scholar]
- Ma X, Zarebanadkouki M, Kuzyakov Y, Blagodatskaya E, Pausch J, Razavi BS. 2018. Spatial patterns of enzyme activities in the rhizosphere: effects of root hairs and root radius. Soil Biology and Biochemistry 118: 69–78. [Google Scholar]
- Ma X, Mason-Jones K, Liu Y, et al. 2019. Coupling zymography with pH mapping reveals a shift in lupine phosphorus acquisition strategy driven by cluster roots. Soil Biology and Biochemistry 135: 420–428. [Google Scholar]
- Ma Z, Guo D, Xu X, et al. 2018. Evolutionary history resolves global organization of root functional traits. Nature 555: 94–97. [DOI] [PubMed] [Google Scholar]
- Miller RM, Jastrow JD, Reinhardt DR. 1995. External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia 103: 17–23. [DOI] [PubMed] [Google Scholar]
- Nagy R, Vasconcelos MJV, Zhao S, et al. 2006. Differential regulation of five Pht1 phosphate transporters from maize (Zea mays L.). Plant Biology 8: 186–197. [DOI] [PubMed] [Google Scholar]
- Neumann E. 2007. Mycorrhiza technology for sustainable agriculture – results and ideas. Berlin: Mensch & Buch. [Google Scholar]
- Neumann G, Bott S, Ohler MA, et al. 2014. Root exudation and root development of lettuce (Lactuca sativa L. cv. Tizian) as affected by different soils. Frontiers in Microbiology 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J, Bansal R, Zhao H, et al. 2018. The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytologist 219: 518–529. [DOI] [PubMed] [Google Scholar]
- Péret B, Desnos T, Jost R, Kanno S, Berkowitz O, Nussaume L. 2014. Root architecture responses: in search of phosphate. Plant Physiology 166: 1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RL, Farquhar ML. 1996. Root hairs: specialized tubular cells extending root surfaces. Botanical Review 62: 1–40. [Google Scholar]
- Raghothama KG, Karthikeyan AS. 2005. Phosphate acquisition. Plant and Soil 274: 37–49. [Google Scholar]
- Richardson AE, Lynch JP, Ryan PR, et al. 2011. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant and Soil 349: 121–156. [Google Scholar]
- Ryan MH, Tibbett M, Edmonds-Tibbett T, et al. 2012. Carbon trading for phosphorus gain: the balance between rhizosphere carboxylates and arbuscular mycorrhizal symbiosis in plant phosphorus acquisition. Plant, Cell and Environment 35: 2170–2180. [DOI] [PubMed] [Google Scholar]
- Sanaullah M, Razavi BS, Blagodatskaya E, Kuzyakov Y. 2016. Spatial distribution and catalytic mechanisms of β-glucosidase activity at the root-soil interface. Biology and Fertility of Soils 52: 505–514. [Google Scholar]
- Sawers RJH, Svane SF, Quan C, et al. 2017. Phosphorus acquisition efficiency in arbuscular mycorrhizal maize is correlated with the abundance of root-external hyphae and the accumulation of transcripts encoding PHT1 phosphate transporters. New Phytologist 214: 632–643. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Reid RJ, Ayling S. 1998. Phosphorus uptake by plants: from soil to cell. Plant Physiology 116: 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger PF, Robson AD, Barrow NJ. 1995. Root hair length determines beneficial effect of a Glomus species on shoot growth of some pasture species. New Phytologist 131: 247–254. [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis, 3rd edn New York: Academic Press. [Google Scholar]
- Smith SE, Jakobsen I, Gronlund M, Smith FA. 2011. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiology 156: 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spohn M, Kuzyakov Y. 2014. Spatial and temporal dynamics of hotspots of enzyme activity in soil as affected by living and dead roots—a soil zymography analysis. Plant and Soil 379: 67–77. [Google Scholar]
- Tawaraya K. 2003. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Science and Plant Nutrition 49: 655–668. [Google Scholar]
- Trouvelot A, Kough JL, Gianinazzi-Pearson V. 1986. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In: Pearson G, Gianinazzi S, eds. Physiological and genetical aspects of mycorrhizae, Proceedings of the 1st European Symposiurn on mycorrhizae. Paris: Institut National de la Recherche Agronomiqu, 217–221. [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157: 423–447. [DOI] [PubMed] [Google Scholar]
- Vierheilig H, Coughlan AP, Wyss U, Piche Y. 1998. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied and Environmental Microbiology 64: 5004–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek PM, Porder S, Houlton BZ, Chadwick OA. 2010. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecological Applications 20: 5–15. [DOI] [PubMed] [Google Scholar]
- Weber NF, Herrmann I, Hochholdinger F, Ludewig U, Neumann G. 2018. PGPR-induced growth stimulation and nutrient acquisition in maize: do root hairs matter? Scientia Agriculturae Bohemica 49: 164–172. [Google Scholar]
- Wen T-J, Schnable PS. 1994. Analyses of mutants of three genes that influence root hair development in Zea mays (Gramineae) suggest that root hairs are dispensable. American Journal of Botany 81: 833–842. [Google Scholar]
- Wen Z, Li H, Shen Q, et al. 2019. Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytologist 223: 882–895. [DOI] [PubMed] [Google Scholar]
- Wright DP, Read DJ, Scholes JD. 1998. Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant, Cell and Environment 21: 881–891. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.