Abstract
Background and Aims
Crassulacean acid metabolism (CAM) is an adaptation to increase water use efficiency in dry environments. Similar biochemical patterns occur in the aquatic lycophyte genus Isoëtes. It has long been assumed and accepted that CAM-like behaviour in these aquatic plants is an adaptation to low daytime carbon levels in aquatic ecosystems, but this has never been directly tested.
Methods
To test this hypothesis, populations of Isoëtes engelmannii and I. tuckermanii were grown in climate-controlled chambers and starved of atmospheric CO2 during the day while pH was measured for 24 h.
Key Results
We demonstrate that terrestrial plants exposed to low atmospheric CO2 display diel acidity cycles similar to those in both xerophytic CAM plants and submerged Isoëtes.
Conclusions
Daytime CO2 starvation induces CAM-like nocturnal acid accumulation in terrestrial Isoëtes, substantiating the hypothesis that carbon starvation is a selective pressure for this physiological behaviour.
Keywords: CAM, Isoetaceae, CO2 manipulation, Isoëtes engelmannii, Isoëtes tuckermanii, quillwort, isoetid physiology, aquatic CAM
INTRODUCTION
Metabolic shifts play an essential role in the survival of plants in extreme habitats. Many desert-adapted species, for example, minimize water loss by temporally segregating the light and dark reactions of photosynthesis. By closing their stomata during the day and only opening them at night, these plants can limit their daytime water loss, and incorporate CO2 into four-carbon acids in the evening (Kluge and Ting, 1978; Sipes and Ting, 1985; Lüttge, 2004). This physiological behaviour is known as Crassulacean acid metabolism (CAM) and leads to noticeable diel (24-h) cycles in acidity of photosynthetic organs. This metabolism is critical for the success of many xerophytic plants and is regarded as one of the most important photosynthetic adaptations to dry environments (Kluge and Ting, 1978). Although CAM in xerophytes allows for high water use efficiency (WUE), the pathway behind this metabolism (C4, Hatch/Slack/Korshak pathway) is fundamentally a carbon-concentrating mechanism which increases the selectivity of Rubisco by altering the CO2 : O2 ratio within the chloroplast (Keeley and Rundel, 2003; Edwards, 2019).
In 1981, diel acidity cycles like those observed in CAM plants were discovered in the aquatic lycophyte genus Isoëtes (Keeley, 1981). This behaviour was called ‘aquatic CAM’ to highlight its similarity to acidity cycles in xerophytic plants. However, unlike xerophytes, Isoëtes are not water-limited in any sense, and generally grow aquatically or seasonally emergent in eutrophic or oligotrophic lakes. In the habitat of Isoëtes, available aquatic carbon levels are known to be low – either diurnally in eutrophic lakes, or perpetually in oligotrophic lakes (Keeley et al., 1983b; Keeley and Busch, 1984). Furthermore, investigations of CAM-like behaviour in Isoëtes have demonstrated a high degree of plasticity, which seems to correspond to habitat (submerged or terrestrial). For example, while growing terrestrially and exposed to atmospheric CO2 levels, Isoëtes howellii does not accumulate acid nocturnally. In contrast, I. karstenii and I. palmeri do accumulate acid nocturnally regardless of their environment, demonstrating that this behaviour can be facultative or constitutive (Keeley, 1998; Keeley and Rundel, 2003). Together, these observations led to the assumption that CAM-like nocturnal acidification in Isoëtes is a response to low carbon availability in aquatic ecosystems. While this has become the accepted hypothesis for explaining this behaviour, it has hitherto remained largely hypothetical and correlative (Keeley, 1982, 1998; Keeley and Bowes, 1982; Keeley and Rundel, 2003). To test the hypothesis that nocturnal acidification in Isoëtes is a direct response to carbon starvation, we grew terrestrial plants of two species in environmentally controlled growth chambers, starved them of atmospheric CO2 during the day, and sampled leaf pH from multiple individuals for 24 h. We show that diurnal atmospheric hypocarbia does induce CAM-like diel acidity cycles in terrestrial Isoëtes engelmannii, like those observed in xerophytic CAM plants and submerged Isoëtes species. This provides direct evidence suggesting that facultative nocturnal carbon accumulation in extant Isoëtes species can be induced by low CO2 availability, which adds substantial support to the hypothesis that CO2 limitation is the selection pressure that led to the evolution of this photosynthetic behaviour in Isoëtes (Keeley, 1998).
The aim of this study was to empirically test the long-held hypothesis explaining why species of aquatic lycophytes – which are not water-limited in any sense – accumulate acid nocturnally like xerophytic CAM plants. This study is the first to provide direct evidence that CO2 starvation induces CAM-like nocturnal acidification and carbon accumulation in this genus or in any other facultative CAM plant. Knowledge of carbon uptake and photosynthetic strategies in evolutionarily distinct lineages such as the Isoetaceae represents an important step forward in understanding the evolution of CAM and other carbon-concentrating mechanisms. Moreover, the induction of this behaviour in terrestrial Isoëtes by atmospheric CO2 starvation raises interesting questions about the evolution of carbon-concentrating mechanisms in Isoetalean ancestors as well as the environments and selection pressures to which these extinct plants were exposed.
MATERIALS AND METHODS
Field experiments
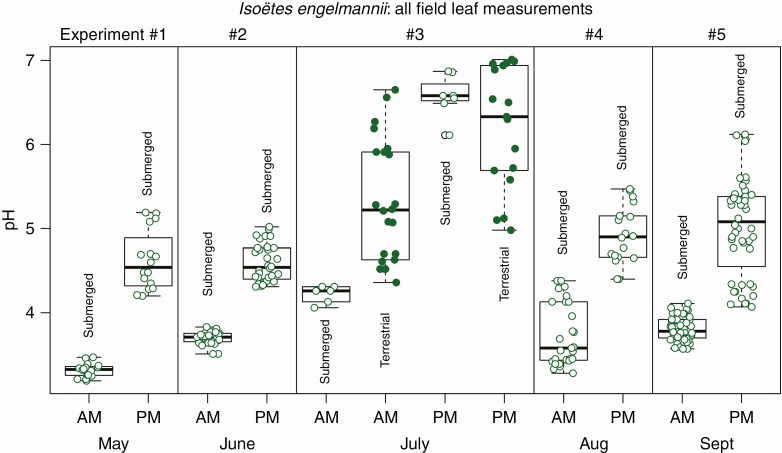
The Edmund Niles Huyck Preserve is located in Rensselaerville, NY, USA, at 425–525 m elevation. Plants of Isoëtes engelmannii were studied from May to September in a population on the north shore of Lake Myosotis (42°31′26.8″N, 74°9′7.2″W; Fig. 1A, B) (Russell, 1958). Three to eight samples each composed of leaves, corms or roots, from five or six plants were collected five times during the summer of 2018 from May to September (Fig. 2). During May, June and July, samples were collected only in the morning at 0600 and in the evening at 1800; in August, samples were collected every 3 h from 0600 to 1800, and in September collections were made every 3 h for a full 24 h (1800–1800). During all experiments plants were cleaned of algae and soil in deionized water (DI) in the lab, which was located 1 km from the study site.
Fig. 1.
(A) Lake Myosotis study site during average water levels for most of the year. (B) Lake Myosotis in July when water levels drop. (C) Exposed plants of I. tuckermanii in lab conditions. (D) Exposed plants of I. engelmannii in situ.
Fig. 2.
Field experiments. Diel pH measurements of leaves from field-grown plants throughout the summer months. In July some plants were growing terrestrially and did not show significant differences in pH. Each point represents a single pH measurement. A total of 0.2–0.5 g of leaf material was pooled from five or six individuals and separated into three to eight distinct samples. During September more than two time points were measured throughout the day (see Fig. 3).
In the lab, leaves, corms and roots were separated using scalpels and forceps and organs were washed and blotted dry. At each time point, leaves from five or six individuals of I. engelmannii were randomized and separated into three to eight distinct samples, depending on the amount of material available. Each of the individual samples was composed of 0.2–0.5 g of leaf, corm or root tissue. Tissue from each of the three to eight samples was macerated using a plastic dowel in a 1.7-mL Eppendorf tube, and 0.5 mL of DI H2O was added to each sample. Samples were then resuspended by mixing using a vortex mixer at maximum intensity for 10 s, followed by centrifugation at 10 000 r.p.m. for 10 s. The supernatant was carefully extracted using a pipette, and placed on the Horiba LAQUAtwin pH-22 meter, for pH readings. The pH was measured twice from each sample to ensure accuracy of the reading (Fig. 3). The pH meter was cleaned with DI water and dried between each sample. The pH meter was recalibrated between each time point using pH 4 and 7 standardized buffers. These measurements were made instead of the historically used acid titration method because of the ease of use of handheld pH meters, the small amount of material needed and the accuracy of measurements. The main difference between measuring acid levels using pH meters compared to acid titration is that pH measured from two solutions with the same amount of acid can be different if they have different buffering capacities (Sadler and Murphy, 2010). Even though there are difficulties with comparing pH and titrant concentration with weak acids in solutions of different concentrations, one can still relate the two by using the Henderson–Hasselbalch equation (Sadler and Murphy, 2010). We make the assumption that the buffering capacity of leaves of Isoëtes does not substantially change over the course of a 24-h period, which justifies the use of a pH meter to quantify change during the diel cycle. If leaves from different species had been compared, buffering capacity could potentially be different and pH measurements potentially harder to compare between samples without quantitative titration.
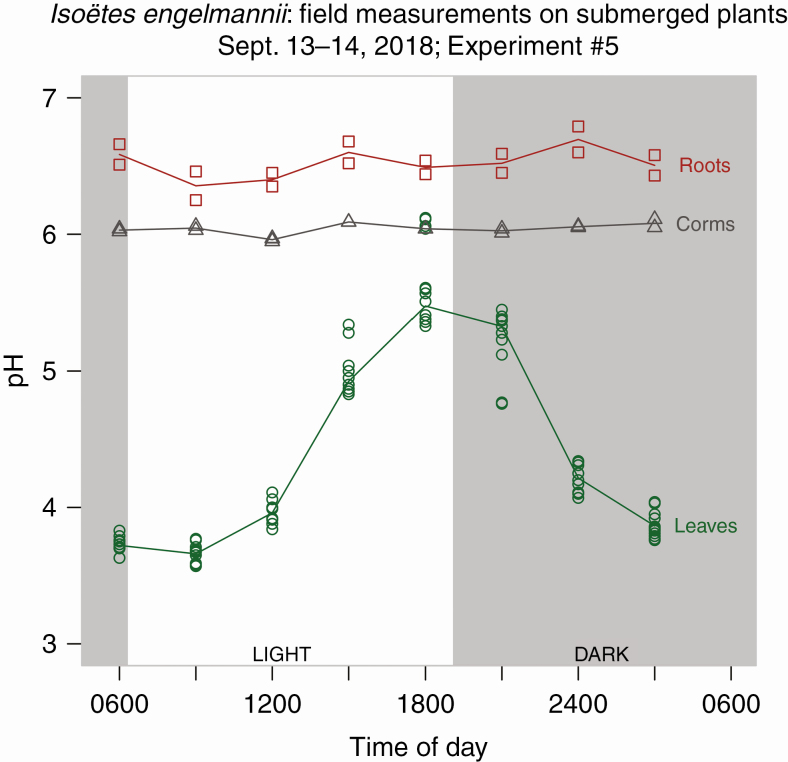
Fig. 3.
Field experiments. pH measurements of leaves, corms and roots, for 24 h in September. A clear cycle in pH is demonstrated in leaves while corms and roots show no diel change. Each point represents a single pH measurement of three to eight samples consisting of 0.2–0.5 g of pooled leaves from five or six individuals; pH was measured twice on each sample.
Laboratory experiments
Isoëtes engelmannii specimens collected in the field throughout summer 2018 in Lake Myosotis were cultivated in growth chambers in the Weld Hill Research Building of the Arnold Arboretum of Harvard University. In addition, populations of I. tuckermanii already growing in the glasshouse (collected from the NW shore of Lake Mattawa, in Orange, MA, USA, 42°34′11.9″N, 72°19′34.1″W), were used in the growth chamber experiments. For 2 months, plants were grown fully submerged at ambient CO2 levels (~400 ppm), at 20 °C, with a 12-h photoperiod, 150 µmol m−2 s−1 photosynthetically active radiation. Submerged pH was measured on a diel cycle following the field protocol (see above) (Fig. 4A, D). Containers were then drained of water (Fig. 1C), and the plants were allowed to acclimate for 1–3 d. While plants were terrestrial under ambient CO2 levels, pH was measured every 3–5 h for 24 h following the aforementioned protocol (Fig. 4B, E). Plants were then moved to a growth chamber set to a diel cycle of atmospheric CO2: 100 ppm of CO2 during the 12-h photoperiod (the minimum obtainable in the chamber) and 400 ppm (equivalent to ambient atmospheric levels) during the dark period. Temperature and light intensity were not changed from the ambient CO2 conditions. Starting at 0700, plants were harvested every 3–5 h for 24 h. At each time point leaves from two to four individuals were randomized and separated into two or three distinct samples consisting of 0.2–0.5 g of leaf tissue and pH was measured following the field protocol (Fig. 4C, F).
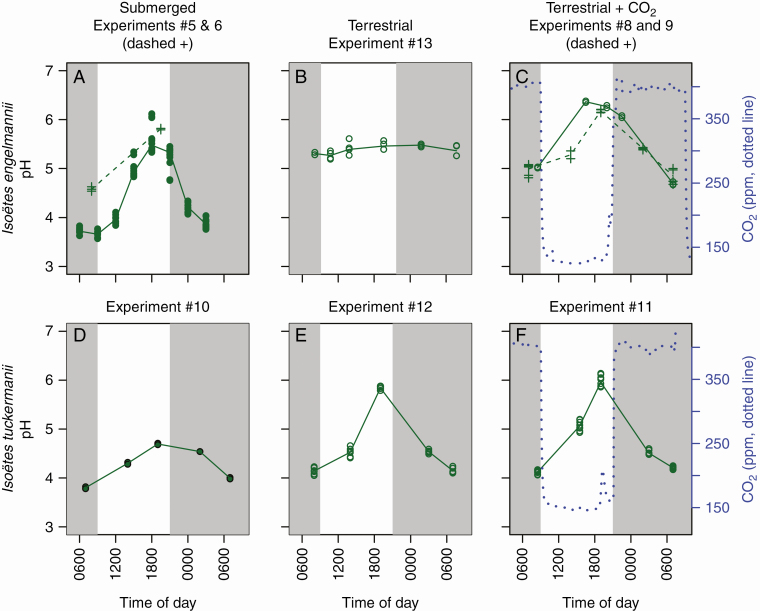
Fig. 4.
Laboratory experiments. Leaf pH measurements of I. engelmannii and I. tuckermanii under (A, D) submerged ambient CO2 levels; (B, E) terrestrial ambient atmospheric CO2 levels; and (C, F) terrestrial CO2 manipulation. A clear diel change in pH was not observed in I. engelmannii when emergent but was induced upon CO2 starvation. Solid circles represent submerged plants and open circles represent terrestrial plants. Each point represents a single pH measurement of two or three samples consisting of 0.2–0.5 g of pooled leaves from two to four individuals; pH was measured twice on each sample. *Circles in A are measurements from the field, while crosses represent lab experiments. In C, two separate 24-h experiments were conducted (crosses and circles).
Statistics
To quantify the similarity in the diel cycle of acidity between two experiments, we employed two statistical methodologies. First, we applied the Brown–Forsythe test for the equality of variance (Brown and Forsythe, 1974). Second, we calculated the overlap in tail probabilities of the probability distribution of the maximum value unbiased estimator (MVUE) for the maximum diel range. Neither of these approaches is optimal, but each provides some indication of the likelihood that the observed diel cycles are significantly different at the indicated probability level. In the case of the Brown–Forsythe test, this is a conventional P-value; in the alternative MVUE approach, it is a (one-tailed) probability that the larger diel range would be observed, given the distribution observed in the sample with a smaller diel range. All manipulations and the pairwise comparisons between all experiments are provided and fully documented in the R code, which can be accessed upon request from the authors. In the text, the results of a statistical comparison between two experiments are shown in the format x:y where the comparison is between experiment x and y, prefixed by the type of test (Brown–Forsythe or MVUE). So ‘Brown–Forsythe (5:6), P = 0.03’ means that the Brown–Forsythe test rejected the null hypothesis that experiments 5 and 6 had equal variances at the 5% level (but not at the 1% level). Experiment numbers referred to in the text correspond to those shown in the figures (Supplementary Data File S1).
RESULTS
Field experiments
In our first series of field measurements from May to September, we measured morning and evening pH in Isoëtes engelmannii leaves, roots and corms, monthly (Fig. 2). We found that in the field, submerged plants of I. engelmannii accumulated carbon on a diel cycle (Figs 2 and 3). The mean morning pH of the leaves throughout the summer was 3.71 and the mean evening pH was 4.90 (Fig. 2). The pH of non-photosynthetic organs (roots and corms) did not change on a diel cycle, showing that the nocturnal acidification was restricted to the leaves (Fig. 3); corms: mean morning pH 5.94, mean evening pH 5.97; roots: mean morning pH 6.32, mean evening pH 6.36 (Fig. 3). Upon recession of the shoreline in July many of the plants were left growing terrestrially, exposed to atmospheric conditions (Fig. 1D). In the plants growing terrestrially in July, pH variability was higher in both the morning and the evening (Fig. 2), and the variance of terrestrial measurements was significantly higher than submerged values throughout the summer (P < 0.001 based on a Brown–Forsythe test for equality of variance and tail probabilities of a minimum variance unbiased estimator of maximum diel range (MVUE; 3:1–5). Prior observations of I. engelmannii additionally also suggest that this species accumulates carbon nocturnally only when submerged.
Lab experiments
For laboratory manipulations, we collected I. engelmannii from the same population measured in the field as wells as specimens of I. tuckermanii, a related species that generally grows completely submerged. All plants collected were brought into the lab and cultivated in climate-controlled growth chambers. Under ambient CO2 levels the diel pH variation of submerged individuals of I. engelmannii was similar to submerged individuals in the field (Fig. 4A); MVUE [Exp. 5:6] P = 0.01; Brown–Forsythe [Exp. 5:6] P = 0.03; mean leaf morning pH was 4.58, and mean evening pH was 5.81. Submerged plants of I. tuckermanii had a qualitatively lower diel shift in pH, compared to I. engelmannii; MVUE [Exp. 5:10] P < 0.001, [Exp. 6:10] P = 0.04; Brown–Forsythe [Exp. 5:10] P = 0.28, [Exp. 6:10] P = 0.01, but still experienced a diel fluctuation (Fig. 4A, D); mean morning pH was 3.89, and mean evening pH was 4.70. When emergent, as expected from the field experiments, individuals of I. engelmannii no longer displayed a diel shift in pH (Fig. 4B); MVUE [Exp. 5:13] P < 0.001, [Exp. 6:13] P < 0.001; Brown–Forsythe [Exp. 5:13] P < 0.001, [Exp. 6:13] P = 0.53; mean morning pH was 5.31, and mean evening pH was 5.46. Experiment 6 only comprised two time points, and thus statistical support for determining differences is lower when compared to other experiments (Supplementary Data File S1). Isoëtes tuckermanii, however, showed a larger diel change in pH, when emergent compared to when it was submerged; MVUE [Exp. 10:12] P = 0.001; Brown–Forsythe [Exp. 10:1] P = 0.02 (Fig. 4E); mean morning pH was 4.15, and mean evening pH was 5.84. These results demonstrate different behaviour of nocturnal acid accumulation in these two species. Isoetes engelmannii induces nocturnal acidification when submerged but not when emergent, while I. tuckermanii demonstrated constitutive nocturnal acid accumulation, with similar diel variation in pH irrespective of water depth, and only a slight increase in acid accumulation when emergent.
We next grew the plants in growth chambers with diurnal CO2 starvation and nocturnal enrichment to mimic the CO2 conditions in a eutrophic lake. Individuals of I. engelmannii grown under these conditions demonstrated a diel cycle in pH similar to that observed in the submerged specimens, but different from emergent plants in the field and lab [MVUE (5:8) P = 0.01, (6:8): P = 0.17, (5:9) P < 0.001, (6:9) P = 0.34); Brown–Forsythe (5:8) P < 0.001, (6:8) P = 0.29, (5:9) P < 0.001, (6:9) P = 0.72] (Fig. 4C). In two independent experiments with multiple replicates per time point, we measured pH for 24 h. For Exp. 8, mean morning pH was 4.69 and mean evening pH was 6.37. For Exp. 9, mean morning pH was 4.85 and mean evening pH was 6.18 (Fig. 4C). The magnitude of pH change in the terrestrial plants grown under diurnal CO2 starvation was similar to that in submerged plants in the field experiments, although slightly dampened (Figs 3 and 4C). This may be due to the fact that predawn CO2 concentrations in the field in eutrophic lakes and ponds can exceed 2500 ppm (Keeley and Bowes, 1982), and the maximum CO2 enrichment during our experiment approximated ambient atmospheric concentrations of 400 ppm (Fig. 4C).
When grown under these manipulated CO2 conditions, I. tuckermanii continued to show nocturnal acid accumulation (Fig. 3F) with a mean morning pH of 4.12, and a mean evening pH of 5.98. The pH fluctuation of I. tuckermanii during diurnal hypocarbia did not differ from that of the terrestrially growing plants in ambient CO2 [MVUE (11:12) P = 0.02; Brown–Forsythe (11:12) P = 0.38] (Fig. 4E), suggesting that this species is obligate in its nocturnal acidification. Unlike I. engelmannii, the individuals of I. tuckermanii we examined had no stomata, a possible explanation of this constitutive behaviour. These results provide a possible explanation for the plasticity of nocturnal acidification in the genus Isoëtes observed in the past (Keeley, 1998). Moreover, we show that laboratory-based experimental modification of natural conditions can replicate field conditions and that carbon starvation imposed on terrestrial plants in the lab produces a diel change in pH comparable to the change observed in submerged aquatic plants.
DISCUSSION
Since the first documentation of nocturnal acidification in Isoëtes (Keeley, 1981), it has been suggested that aquatic CO2 starvation – either diurnally in eutrophic lakes, or perpetually in oligotrophic lakes – was the selection pressure driving the evolution of this behaviour in the genus (Keeley, 1981; Keeley et al., 1983b). While these hypotheses are commonly accepted and widely taught, they have remained largely speculative, and untested, over the last 40 years. In this study we use field- and lab-based experiments, in conjunction with growth chamber CO2 manipulations, to confirm that nocturnal acidification, like that in xerophytic CAM plants, is induced in Isoëtes by limiting day-time CO2 levels (Fig. 4C). These findings demonstrate that carbon limitation directly induces CAM-like behaviour in Isoëtes and provide new and substantial evidence to support the hypothesis that CO2 limitation may be the selection pressure that led to the evolution of this behaviour in Isoëtes.
We find that low atmospheric CO2 levels have the same effect of inducing nocturnal acidification as suggested for low aquatic CO2 levels (Fig. 4A, C). We are not aware of any study that demonstrates this behaviour in facultative xerophytic CAM plants under well-watered conditions. However, some studies have demonstrated that there are other environmental stressors involved in the inducibility of facultative CAM, including salinity (Winter and von Willert, 1972), high light levels (Brulfert et al., 1988), nutrient deficiencies (Ota, 1988; Paul and Cockburn, 1990) and of course drought (Borland and Griffiths, 1990). The inducibility of CAM by these stressors in facultative xerophytic CAM plants is usually rapid and reversable. Similarly, we note that even within 24 h plants can change their photosynthetic behaviour from CAM-like to C3 or vice versa (Supplementary Data File S1). Because CAM-like nocturnal acid accumulation (facultative or constitutive) is first and foremost a carbon-concentrating mechanism, daily CO2 starvation experiments should lead to the induction of CAM in well-watered facultative xerophytic CAM species, comparable to what we have observed in Isoëtes (Fig. 4C).
While almost ubiquitous across the genus, there is a high level of behavioural plasticity of nocturnal acidification among individuals and between species of Isoëtes (Keeley, 1982). Almost all aquatic Isoëtes species have been shown to be constitutive; most seasonally emergent taxa are facultative; and most terrestrial Isoëtes species have not been observed to accumulate acid nocturnally (Keeley, 1998). Based on our results, the seasonally emergent taxon I. engelmannii facultatively accumulates acid nocturnally (Fig. 4A–C). In contrast, I. tuckermanii is also seasonally emergent, but obligately accumulates acid nocturnally, regardless of its habitat (Fig. 4D–F). This contradicts most observations that emergent taxa are facultative (Keeley et al., 1983a). Interestingly, in I. tuckermanii, it seems that upon emergence the magnitude of diel acidity cycles is greater compared with when it is submerged (Fig. 4E; Supplementary Data File S1). This has been demonstrated before in certain species such as I. palmeri (Keeley, 1998). This behaviour of increased diel acidity cycling upon emergence may be the result of increased nocturnal acidification or increased daytime carbon fixation while emergent compared to submerged. That is, higher concentrations of atmospheric CO2, compared to aquatic levels, may lead to increased nocturnal acid storage and lower nocturnal pH. Additionally, lower light levels under water may lead to decreased fixation of stored carbon during the day. In I. tuckermanii we do not see a decrease in nocturnal pH between submerged and emergent individuals (Fig. 4D, E), but rather an increase in daytime pH, suggesting that the amount of acid stored nocturnally between emergent and submerged individuals does not change, but that daytime use of stored acid differs. This may be due to increased available sunlight upon emergence, as documented in natural populations during overcast days (Keeley, 1983; Keeley et al., 1983b).
While important in understanding the photosynthetic behaviours of Isoëtes, stomata of species in this genus have seldom been investigated. Descriptions have mostly been qualitative: some species seem to lack them completely, while others have various stomatal densities (Keeley, 1982). Interestingly, stomata on submerged taxa have been hypothesized to be non-functional (Sculthorpe, 1967), but this is highly speculative. There are potential implications for lacking, or having non-functional, stomata in nocturnal carbon uptake in Isoëtes with CAM-like behaviour. For instance, if plants completely lack stomata, while submerged or emergent, they are entirely dependent on diffusion of CO2 through their cuticle and would not be able to actively regulate carbon uptake, as a plant with stomata can. Because gases diffuse 10 000 times slower in water compared to air, emergent plants would be able to fix much more carbon, leading to an increased nocturnal storage. Furthermore, it has been demonstrated in the fully terrestrial Isoëtes andicola (and other aquatic macrophytes) that roots have the potential for carbon uptake (Søndergaard and Sand-Jensen, 1979;Keeley et al., 1984; Richardson et al., 1984), which undoubtedly has further implications for aquatic vs. terrestrial carbon uptake dynamics. Additionally, CO2 converted from malate reserves may be transported through the lacuna in the leaves and respired CO2 may also be recycled in a similar manner, which could occur in the absence of functional stomata (Madsen, 1987). Further investigations of the distribution and functionality of Isoëtes stomata are warranted to tease apart these dynamics.
While the evidence provided here determines that extant aquatic Isoëtes employ CAM-like nocturnal acidification and carbon accumulation in response to low CO2 levels, we cannot conclusively identify the source of the selection pressure of low CO2 that led to the evolution of this behaviour. Previous discussions of the adaptive significance of CAM-like photosynthesis in Isoëtes have focused on CO2 limitation in the aquatic ecosystem as a selective force (Keeley, 1998). However, our experiments show that atmospheric carbon limitation on terrestrially growing Isoëtes can have the same effect. It may be that the low atmospheric CO2 levels employed in our experiments mimic the aquatic environment of extant Isoëtes in eutrophic or oligotrophic lakes. If this is the case then it can be interpreted that the selection pressure on the evolution of this behaviour was due to low CO2 levels in the aquatic ecosystem inhabited by Isoëtes since the Jurassic (Keeley and Rundel, 2003). However, now extinct Isoetalean relatives were common during the Carboniferous, and these taxa were not exclusively aquatic. Furthermore, low atmospheric CO2 was a notable feature of this time period (Beerling, 2002; Van Der Meer et al., 2014). In the Carboniferous atmosphere, a carbon-concentrating mechanism that increased carbon gain and minimized the photorespiratory loss of energy in a high-oxygen/low-CO2 atmosphere would have been advantageous. For these reasons, it is possible that nocturnal acidification and carbon accumulation may have evolved in Isoetalean lycopsids during the low atmospheric CO2 conditions of the Carboniferous. Similarities between leaf and root anatomy of extant Isoëtes species and extinct terrestrial carboniferous Isoetalean lycopsids suggest that they may have also had a related physiology (Green, 2010). From the results presented here, we can only conclude that terrestrial CO2 starvation induces CAM-like nocturnal acidification and carbon accumulation in at least one species of Isoëtes – mimicking the behaviour observed in aquatic populations in the wild. This adds substantial evidence supporting the hypothesis that CO2 limitation is the selection pressure on the evolution of this behaviour in the genus. In addition, the fact that we can induce this behaviour combined with the prevalence and plasticity of similar behaviours throughout the genus suggests a deep evolutionary history worthy of future examination.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. File S1: Field- and lab-based measurements of diel pH; results of the Brown–Forsythe statistical comparisons between experiments; and results of the MVUE statistical comparisons between experiments.
ACKNOWLEDGEMENTS
We thank Katharine E. Black, Sylvia P. Kinosian and Weston L. Testo for their helpful edits of the manuscript. We thank the Edmund Niles Huyck Preserve for funding, access to plant materials and lodging during the field experiment, as well as the growth facilities staff at Weld Hill Research Building of the Arnold Arboretum of Harvard University for assisting in plant cultivation and growth chamber accommodations.
FUNDING
This work was supported by The Edmund Niles Huyck Preserve Graduate Research Grant awarded to J.S.S. in 2018. J.S.S. is funded in part through the Department of Organismic and Evolutionary Biology graduate student research fellowships as well as the Arnold Arboretum fellowship programme.
LITERATURE CITED
- Beerling DJ. 2002. Low atmospheric CO2 levels during the Permo-Carboniferous glaciation inferred from fossil lycopsids. Proceedings of the National Academy of Sciences of the United States of America 99: 12567–12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland AM, Griffiths H. 1990. The regulation of CAM and respiratory recycling by water supply and light regime in the C3-CAM intermediate Sedum telephium. Functional Ecology 4: 33–39. [Google Scholar]
- Brown MB, Forsythe AB. 1974. Robust tests for the equality of variances. Journal of the American Statistical Association 69: 364–367. [Google Scholar]
- Brulfert J, Kluge M, Güclü S, Queiroz O. 1988. Interaction of photoperiod and drought as CAM inducing factors in Kalanchoe blossfeldiana Poelln., cv. Tom thumb. Journal of Plant Physiology 133: 222–227. [Google Scholar]
- Edwards EJ. 2019. Evolutionary trajectories, accessibility and other metaphors: the case of C4 and CAM photosynthesis. The New Phytologist 223: 1742–1755. [DOI] [PubMed] [Google Scholar]
- Green WA. 2010. The function of the aerenchyma in arborescent lycopsids: evidence of an unfamiliar metabolic strategy. Proceedings of the Royal Society B: Biological Sciences 277: 2257–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley JE. 1981. Isoetes howellii: a submerged aquatic CAM plant? American Journal of Botany 68: 420–424. [Google Scholar]
- Keeley JE. 1982. Distribution of diurnal acid metabolism in the genus Isoetes. American Journal of Botany 69: 254–257. [Google Scholar]
- Keeley JE. 1983. Crassulacean acid metabolism in the seasonally submerged aquatic Isoetes howellii. Oecologia 58: 57–62. [DOI] [PubMed] [Google Scholar]
- Keeley JE. 1998. CAM photosynthesis in submerged aquatic plants. The Botanical Review; Interpreting Botanical Progress 64: 121–175. [Google Scholar]
- Keeley JE, Bowes G. 1982. Gas exchange characteristics of the submerged aquatic crassulacean acid metabolism plant, Isoetes howellii. Plant Physiology 70: 1455–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley JE, Busch G. 1984. Carbon assimilation characteristics of the aquatic CAM plant, Isoetes howellii. Plant Physiology 76: 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley JE, Mathews RP, Walker CM. 1983a Diurnal acid metabolism in Isoetes howellii from a temporary pool and permanent lake. American Journal of Botany 70: 854–857. [Google Scholar]
- Keeley JE, Osmond CB, Raven JA. 1984. Stylites, a vascular land plant without stomata absorbs CO2 via its roots. Nature 310: 694–695. [Google Scholar]
- Keeley JE, Rundel PW. 2003. Evolution of CAM and C4 carbon-concentrating mechanisms. International Journal of Plant Sciences 164: S55–S77. [Google Scholar]
- Keeley JE, Walker CM, Mathews RP. 1983b Crassulacean acid metabolism in Isoetes bolanderi in high elevation oligotrophic lakes. Oecologia 58: 63–69. [DOI] [PubMed] [Google Scholar]
- Kluge M, Ting IP. 1978. Crassulacean acid metabolism: an ecological analysis. Ecological Studies Series 30: 209. [Google Scholar]
- Lüttge U. 2004. Ecophysiology of crassulacean acid metabolism (CAM). Annals of Botany 93: 629–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen TV. 1987. Interactions between internal and external CO2 pools in the photosynthesis of the aquatic CAM plants Littorella uniflora (L.) Aschers and Isoetes lacustris L. The New Phytologist 106: 35–50. [Google Scholar]
- Ota K. 1988. Stimulation of CAM photosynthesis in Kalanchoë blossfeldiana by transferring to nitrogen-deficient conditions. Plant Physiology 87: 454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Cockburn W. 1990. The stimulation of CAM activity in Mesembryanthemum crystallinum in nitrate and phosphate-deficient conditions. The New Phytologist 114: 391–398. [DOI] [PubMed] [Google Scholar]
- Richardson K, Griffiths H, Reed ML, Raven JA, Griffiths NM. 1984. Inorganic carbon assimilation in the Isoetids, Isoetes lacustris L. and Lobelia dortmanna L. Oecologia 61: 115–121. [DOI] [PubMed] [Google Scholar]
- Russell NH. 1958. The vascular flora of the Edmund Niles Huyck Preserve, New York. The American Midland Naturalist 59: 138–145. [Google Scholar]
- Sadler GD, Murphy PA. 2010. pH and titratable acidity. In: Nielsen SS, ed. Food analysis. Boston: Springer US, 219–238. [Google Scholar]
- Sculthorpe CD. 1967. Biology of aquatic vascular plants. New York: St. Martin’s Press. [Google Scholar]
- Sipes DL, Ting IP. 1985. Crassulacean acid metabolism and crassulacean acid metabolism modifications in Peperomia camptotricha. Plant Physiology 77: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søndergaard M, Sand-Jensen K. 1979. Carbon uptake by leaves and roots of Littorella uniflora (L.) Aschers. Aquatic Botany 6: 1–12. [Google Scholar]
- Van Der Meer DG, Zeebe RE, van Hinsbergen DJ, Sluijs A, Spakman W, Torsvik TH. 2014. Plate tectonic controls on atmospheric CO2 levels since the Triassic. Proceedings of the National Academy of Sciences of the United States of America 111: 4380–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, von Willert DJ. 1972. NaCl-induzierter crassulaceensäurestoffwechsel bei Mesembryanthemum crystallinum. Zeitschrift für Pflanzenphysiologie 67: 166–170. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.