Abstract
Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) is a rare peripheral cytotoxic T-cell lymphoma, clinically resembling panniculitis. Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening syndrome of immune overactivation, triggered by underlying conditions. SPTCL presenting with HLH may represent a severe and rapidly progressive disease course. Currently, there is no standardized approach to treatment of HLH secondary to underlying SPTCL. A 34-year-old Asian male presented with a several months history of high fevers, weight loss, and nonpruritic purple discoloration of the skin. He had a skin biopsy showing atypical lymphohistiocytic panniculitis with dermal mucinosis and erythrophagocytosis consistent with SPTCL. The patient was initiated on treatment with dexamethasone and cyclosporine A. Almost immediate improvement of his skin lesions was noted and laboratory abnormalities trended toward baseline within 2 weeks. He noted complete symptom resolution after 3 months on therapy. SPTCL may be treated effectively with cyclosporine A and steroids to achieve rapid clinical and symptom management of this rare malignancy.
Keywords: subcutaneous panniculitis-like T-cell lymphoma, panniculitis, hemophagocytic lymphohistiocytosis, cyclosporine A, corticosteroids
Introduction
Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) is a rare peripheral cytotoxic lymphoma that can clinically resemble panniculitis and is characterized by infiltration of skin tissue by cytotoxic T cells. It has come to recognition over the past 2 decades and accounts for <1% of cutaneous lymphomas.1 This rare malignancy represents a heterogeneous disease, characterized by 2 distinct immune-phenotypical entities: an αβ subtype and a γδ subtype. The αβ subtype is characterized by CD8 positive and CD4 negative neoplastic T cells, while the γδ subtype is characterized by a worse prognosis and T cells negative for both CD4 and CD8. The most recent revision of the World Health Organization classification of lymphoid neoplasms now considers only the αβ subtype as SPTCL and regards the γδ subtype as a distinct entity.2
The clinical course of SPTCL varies but patients often report B symptoms, such as fever, weight loss, and night sweats.1 Hemophagocytic lymphohistiocytosis (HLH) has been reported to be a presenting syndrome in 37% of patients with SPTCL.3 Co-occurrence of HLH and SPTCL was previously thought to signify a progressive disease course with worse prognosis; however, recently published data brings this into dispute. One report states that patients with HLH have worse outcomes despite aggressive chemotherapy, with the median survival being 15 months, while other reports show remission in patients with less toxic immunosuppressive regimens.4,5
While there is no standard treatment for SPTCL, traditionally CHOP-based chemotherapy regimens have been implemented initially.1 There are limited case series and case reports using cyclosporine A as first-line treatment or for refractory disease.1,6,7
In this case, we present a 34-year-old man diagnosed with SPTCL after presenting with HLH, who subsequently responded to treatment with cyclosporine A and dexamethasone.
Case Synopsis
A 34-year-old Asian male with no known past medical history, presented to the emergency department on referral from his primary care physician, for evaluation of fever, weight loss, and skin rash of 2 months duration. The patient initially noticed firm, nontender, nonpruritic purple discoloration of his lower back that progressively extended to cover the entire lower back and bilateral flanks. During this time, he also developed fevers, peaking at a maximum of 102 °F, along with fatigue, chills, night sweats, loss of appetite, and a total weight loss of 16 pounds. He eventually developed similar skin lesions on the right posterior neck and in the right groin. His primary care physician initially suspected an infectious etiology and he was treated with ceftriaxone and cefuroxime. When his fevers persisted, he received a trial of clindamycin and trimethoprim-sulfamethoxazole, without improvement in symptoms. He was not taking any other medications at that time. He had computed tomography (CT) scans of the chest, abdomen, and pelvis that were negative for lymphadenopathy or masses. Social history included a period of incarceration 2 years prior to his symptom onset, but no recent travel outside of the country. Family history was negative for malignancy and autoimmune disease. He was sexually active with one female partner, and had no previous history of sexually transmitted infections. He denied cough, chest pain, shortness of breath, oral lesions, dysuria, hematuria, blood in the stool, and exposure to any sick contacts.
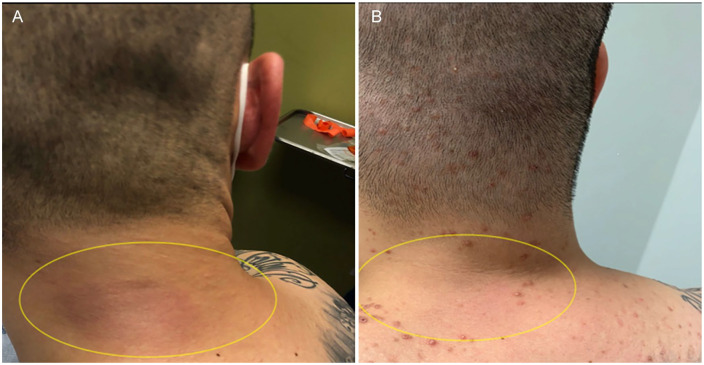
Physical examination showed erythematous-to-violaceous nontender, nonpurulent plaques overlying the right posterior neck (Figure 1A), bilateral lower back and hips, right upper lateral abdomen, and right groin. These areas were warm to the touch and markedly indurated on palpation. He was also noted to have faint erythematous macules on the trunk and bilateral upper extremities.
Figure 1.
(A) Patient’s posterior neck nodule before treatment. (B) Patient’s posterior neck nodule after 3 months of treatment.
Laboratory studies were remarkable for an elevated ferritin, C-reactive protein, and erythrocyte sedimentation rate. Aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase (LDH), activated partial thromboplastin time, serum interleukin (IL)-6, and IL-2 were also found to be elevated. Additionally, C2, C3, C4, and C5 were elevated in conjunction with an elevated total complement activity (Table 1). The patient’s lupus anticoagulant was positive and he had normocytic anemia. Throughout hospitalization serial comprehensive metabolic panels showed consistently elevated transaminases, alkaline phosphatase, γ-glutamyltransferase, and triglyceride levels (Table 1). Urinalysis was remarkable for 0.20 mg/dL of blood. Serum protein electrophoresis was remarkable for mild hypoalbuminemia with an increased alpha-1 globulin fraction (0.6 g/dL). No abnormal bands were seen by immunosubtraction immunotyping. Kappa/lambda free light chains and ratio were within normal limits. Treponema pallidum antibody, HIV, hepatitis panel, severe acute respiratory syndrome coronavirus 2 polymerase chain reaction, blood cultures, fungal blood cultures, and urine cultures were all negative. Tissue culture, including fungal and acid fact bacilli tissue cultures, were all negative. Epstein-Barr virus immunoglobulin (Ig)M was negative, but Epstein-Barr Virus IgG was positive at 373 U/mL. Epstein-Barr virus IgG to early (D) antigen was negative, Epstein-Barr viral polymerase chain reaction was negative. Parvovirus B19 IgM was negative but parvovirus B19 IgG was positive at 1.52 IV. ANA, ANCA, mitochondrial M2 IgG, anti-phospholipid panel, dsDNA antibody, ENA antibody panel, rheumatoid factor, cyclic citrullinated peptide IgG, complement C1q, lactic acid, fibrinogen, prothrombin time international normalized ratio, and thyroid-stimulating hormone were also negative.
Table 1.
Patient’s Laboratory Results Throughout His Clinical Course.
| Variable | Reference range | Value during hospitalization | Value at 3 months of treatment |
|---|---|---|---|
| White blood cell count | 4.5-11 × 103/µL | 5.1 × 103/µL | 6 × 103/µL |
| Hemoglobin | 13.5-17.5 g/dL | 10.6 g/dL | 11.5 g/dL |
| Hematocrit | 40% to 51% | 31.6% | 33% |
| Mean corpuscular volume | 80-100 fL | 81.4 fL | 85.7 fL |
| C-reactive protein | <0.9 mg/dL | 13.2 mg/dL | <0.05 mg/dL |
| Erythrocyte sedimentation rate | 0-15 mm/h | 73 mm/h | 47 mm/h |
| Lactate dehydrogenase | <201 U/L | 708 U/L | 246 U/L |
| Ferritin | 20-300 ng/mL | 10 842 ng/mL | 512.2 ng/mL |
| Activated partial thromboplastin time | 24-37 seconds | 43.6 seconds | 37.5 seconds |
| Serum IL-2 | ≤2.0 pg/mL | 1538.4 pg/mL | — |
| Serum IL-6 | 175.3-858.2 pg/mL | 7.1 pg/mL | — |
| C2 | 1.6-4 mg/dL | 4.9 mg/dL | 2.7 mg/dL |
| C3 | 83-180 mg/dL | 190 mg/dL | 164 mg/dL |
| C4 | 18-55 mg/dL | 67 mg/dL | 51 mg/dL |
| C5 | 7-20 mg/dL | 23 mg/dL | 28 mg/dL |
| Total complement activity | 38.7-89.9 U/mL | >95 U/mL | 80.8 U/mL |
| AST | <45 U/L | 351 U/L | 24 U/L |
| ALT | <46 U/L | 320 U/L | 17 U/L |
| ALP | 20-120 U/L | 137 U/L | 60 U/L |
| GGT | <81 U/L | 131 U/L | 17 U/L |
| Triglycerides | <150 mg/dL | 209 mg/dL | 195 mg/dL |
| Uric acid | 3.5-7.5 mg/dL | 3.5 mg/dL | 7.9 mg/dL |
Abbreviations: IL, interleukin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyltransferase.
The patient’s chest X-ray was unremarkable; however, the CT scan of the chest with contrast showed body wall edema and skin thickening, most pronounced at the right lower hemithorax and presternal soft tissues. There were no pulmonary masses, consolidation, or lymphadenopathy observed on imaging. The CT scan of the abdomen and pelvis with contrast was remarkable for extensive tissue stranding and edema within the subcutaneous soft tissues of the abdomen, flanks, back, and groin, most notably along the right flank, extending inferiorly into the right groin. Nonspecific foci were also identified, with a 1-cm hyperattenuating focus seen in the dome of the liver and a lucency observed within the left iliac bone.
A bone marrow biopsy showed normocellular marrow for age with trilineage hematopoiesis and a full range of maturation. The myeloid:erythroid ratio was within normal limits. An immunohistochemical CD68 stain performed on the core biopsy highlighted rare cells suspicious for hemophagocytes; however, interpretation was limited by the presence of high background staining. Flow cytometry immunophenotying was performed on aspirate material and showed no immunophenotypic evidence of hematopoietic neoplasia.
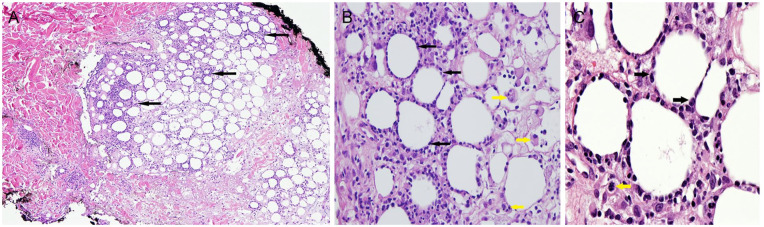
A skin biopsy was performed from the lower back and revealed an edematous dermis with an infiltrate of histiocytes and extravasation of erythrocytes concentrated around eccrine coils. There was a concentration of histiocytic and lymphocytic infiltrate in a lobular pattern in the subcutis. Adipocytes in the subcutis were rimmed by mild to moderately atypical, hyperchromatic lymphoid cells, best highlighted by CD8 (Figure 2A-C). These lymphocytes stained positive for CD3 with diminished staining of CD4. CD5 and CD8 stained greater numbers of cells than CD4 with a reversal in ratio of CD4:CD8. CD30, CD20, CD56, and periodic acid–Schiff staining was negative. CD68 highlighted the histiocytes. The delta TCR chain immunohistochemical stain was negative, while β-F1 with appropriate control demonstrated nuclear staining of the atypical lymphoid cells rimming adipocytes, favoring a diagnosis of αβ subtype SPTCL. Alcian blue demonstrated mucin in the dermis, subcutis, and in the histiocytes within the subcutis. The pattern was that of an atypical lymphohistiocytic panniculitis with dermal mucinosis and erythrophagocytosis. Erythrophagocytosis was easily identified in dermal histiocytes. These findings were consistent with SPTCL.
Figure 2.
(A) Lymphocytic and histiocytic infiltrate (solid black arrows) in a lobular pattern in the subcutis. (B) Adipocytes in subcutis are rimmed by mild to moderately atypical lymphoid cells (solid black arrows). Histiocytes some with phagocytosed hematic cellular fragments (solid yellow arrows). (C) Adipocytes in subcutis are rimmed by mild to moderately atypical, hyperchromatic lymphoid cells (solid black arrows). An occasional mitotic figure is present (solid yellow arrow).
Based on elevated ferritin, fasting triglycerides, elevated soluble IL-2, persistent fevers >38.5 °F, and the hemophagocytosis seen on skin biopsy, he was diagnosed with hemophagocytic lymphohistiocystosis and was treated with dexamethasone 10 m/m2 daily for 2 weeks. He was started on cyclosporine A 4 mg/kg with significant improvement in skin lesions.
Of note, the patient reported severe bilateral knee pain beginning 1 week after the initiation of cyclosporine A. He was treated in the emergency room and pain resolved with ibuprofen administration. He was subsequently weaned off steroids completely and remained on single agent cyclosporine A.
At 3-month follow-up, the patient reported complete resolution of symptoms with cyclosporine A treatment. The patient demonstrated near resolution of posterior neck lesions (Figure 1B) and improvement in the posterior back lesion. Laboratory results also improved, with comprehensive metabolic panel demonstrating normalization of aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase (Table 1). His ferritin remained elevated but was trending downward (Table 1). His activated partial thromboplastin time and complements have normalized. Although his ferritin, triglycerides, and LDH remain elevated, the values are trending down toward normal. His uric acid, however, has increased (Table 1).
Discussion
Patients with SPTCL commonly present with erythematous subcutaneous lesions for which the clinical course is varied. Those who present with HLH, elevated LDH levels, leukopenia, or γδ subtype have been reported to have a poorer prognosis.8,9 The γδ subtype of SPTCL was excluded in our patient by immunohistochemistry with lymphoma cells staining negative for delta TCR chain and positive for β-F1.1 The diagnosis was made via skin biopsy. The biopsy revealed dense subcutaneous infiltrates with a pattern of lobular panniculitis, with atypical lymphocytes rimming individual adipocytes.
The most common bone marrow abnormality in SPTCL is hemophagocytosis, as seen in our patient.1 HLH is a life-threatening syndrome of immune over activation. It is usually triggered by an underlying event, condition, or infection that disrupts normal activity of the immune system. HLH is diagnosed based on clinical presentation and diagnostic criteria from the HLH-2004 trial. Typical clinical features include fever, splenomegaly, bicytopenia (commonly anemia and thrombocytopenia), hypertriglyceridemia or hypofibrinogenemia, hemophagocytosis identified on tissue biopsy, in addition to ferritin levels >500 µg/L, elevated soluble CD25, and low or absent natural killer cell activity. HLH is diagnosed based on meeting at least 5 of these 8 criteria, in the setting of a compatible clinical picture. In our patient, HLH was able to be diagnosed as he met 5 of the 8 criteria with elevated ferritin, persistent fevers, elevated fasting triglycerides, soluble IL-2, and hemophagocytosis visualized on skin biopsy. Furthermore, liver function abnormalities, such as those observed in this patient, are also reported in the majority of HLH patients.10
No standardized approach to the treatment of patients with HLH secondary to underlying SPTCL currently exists. Previously, many patients were treated with cytotoxic chemotherapy regimens (cyclophosphamide, vincristine, doxorubicin, and prednisolone) but several recent cases showed clinical response to treatment with oral steroids alone or a combination of oral steroids with methotrexate or cyclosporine A.11-13 The patient’s case and other recently published reports demonstrate that HLH does not necessarily predict more aggressive disease course and worse prognosis. Interestingly, the treatment regimen utilized in this case showed rapid and profound clinical improvement in our patient with minimal toxicity. The majority of the patient’s laboratory values began trending toward normal within mere days and he noted symptomatic improvement almost immediately. The exception to this trend is the uric acid level, which had increased at his 3-month follow-up appointment. Increasing uric acid is likely secondary to the cyclosporine A, as hyperuricemia is a common side effect of this therapy.14
This highlights the importance of initial treatment with steroids and immune-modulating drugs as patients who respond well to these treatments, such as our patient, may avoid more aggressive therapies. This case demonstrates that a patient with clinical features, previously deemed to carry a poor prognosis, could potentially respond in a quick and durable manner to treatment with cyclosporine A and dexamethasone.
The literature available on optimal treatment options for SPTCL is sparse and limited to case reports and a few case series, due to the rarity of this disease. Commonly, patients who presented with SPTCL-induced HLH were initially treated with an etoposide-based regimen, based on standard of care recommendations; however, relapse was frequently observed in these patients.15 Induction of robust and long-lasting responses to treatment with cyclosporine A, even in patients initially treated with multiagent regimens who relapsed on such treatments, is a consistent finding in the available literature. As both patients initially treated with cyclosporine A and those treated with cyclosporine A as second- or third-line therapy after relapse tend to have a good response, this raises the question: Is SPTCL a unique disease entity distinct from other T-cell lymphomas? The answer to this question will require further research.
Although our patient experienced both an objective and subjective improvement in his condition, this drug regimen was not without adverse effects. After 2 weeks of taking cyclosporine A and dexamethasone, the patient developed severe bilateral knee and muscle pain, thought to be secondary to steroid-induced myopathy. His pain responded to nonsteroidal anti-inflammatory drugs and resolved after being weaned off steroids. Given that the patient has been off steroids for over 2 months, it is unlikely that his response can be solely attributed to the steroids but this is a possibility. Frequent follow-up will be pursued to ensure the patient continues to respond while utilizing a single-agent regimen.
In the event of disease relapse, we plan to utilize methotrexate 20 mg per week administered with folic acid daily (except days on which he takes methotrexate). Third-line therapy would consist of 200 to 300 mg/m2 bexarotene for approximately 2 months followed by de-escalation of treatment. If the patient were to relapse despite multiple lines of therapy, allogenic stem cell transplant would be pursued.
Overall, cyclosporine A and dexamethasone appear to be effective in treatment of our patient; however, studies utilizing a larger patient population are necessary to further elucidate the role of cyclosporine A in treatment of SPTCL.
Conclusion
In summary, our findings suggest that HLH secondary to SPTCL may be treated with cyclosporine A and dexamethasone to achieve rapid symptom and clinical improvement of this rare malignancy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
References
- 1. Willemze R, Jansen PM, Cerroni L, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:838-845. doi: 10.1182/blood-2007-04-087288 [DOI] [PubMed] [Google Scholar]
- 2. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues: WHO Classification of Tumours Revised. 4th ed Vol 2 World Health Organization; 2017. [Google Scholar]
- 3. Hung G, Chen Y, Chen D. Subcutaneous panniculitis-like T-cell lymphoma presenting with hemophagocytic lymphohistiocytosis and skin lesions with characteristic high-resolution ultrasonographic findings. Clin Rheumatol. 2007;26:775-778. doi: 10.1007/s10067-005-0193-y [DOI] [PubMed] [Google Scholar]
- 4. Foss FM, Edelson RL, Wilson LD. Lymphomas: cutaneous T-cell lymphomas. In: VT Jr Devita, TS Lawrence, RA Weinberg, eds. Devita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology. 8th ed. Lippincott Williams & Wilkins; 2005:2143-2158. [Google Scholar]
- 5. López-Lerma I, Peñate Y, Gallardo F, et al. Subcutaneous panniculitis-like T-cell lymphoma: clinical features, therapeutic approach, and outcome in a case series of 16 patients. J Am Acad Dermatol. 2018;79:892-898. doi: 10.1016/J.JAAD.2018.05.1243 [DOI] [PubMed] [Google Scholar]
- 6. Rojnuckarin P, Nakorn TN, Assanasen T, Wannakrairot P, Intragumtornchai T. Cyclosporin in subcutaneous panniculitis-like T-cell lymphoma. Leuk Lymphoma. 2007;48:560-563. doi: 10.1080/10428190601078456 [DOI] [PubMed] [Google Scholar]
- 7. Go SI, Lee WS, Kang MH, Kim IS, Kim DC, Lee JH. Cyclosporine A treatment for relapsed subcutaneous panniculitis-like T-cell lymphoma: a case with long-term follow-up. Korean J Hematol. 2012;47:46-49. doi: 10.5045/kjh.2012.47.2.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404-1413. doi: 10.1002/cncr.20502 [DOI] [PubMed] [Google Scholar]
- 9. Huang JJ, Cai MY, Ye S, Li ZM, Huang HQ, Lin TY. Clinical analysis of 19 cases of subcutaneous panniculitis T-cell lymphoma with literature review [in Chinese]. Chin J Cancer. 2009;28:1093-1099. [DOI] [PubMed] [Google Scholar]
- 10. Emmenegger U, Schach DJ, Larroche C, Neftel KA. Haemophagocytic syndromes in adults: current concepts and challenges ahead. Swiss Med Wkly. 2005;135:299-314. doi: 10.4414/smw.2005.10976 [DOI] [PubMed] [Google Scholar]
- 11. Lee WS, Hwang JH, Kim MJ, et al. Cyclosporine A as a primary treatment for panniculitis-like T cell lymphoma: a case with a long-term remission. Cancer Res Treat. 2014;46:312-316. doi: 10.4143/crt.2014.46.3.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu ZL, Sang H, Deng L, Li ZH. Subcutaneous panniculitis-like T-cell lymphoma in children: a review of the literature. Pediatr Dermatol. 2015;32:526-532. doi: 10.1111/pde.12452 [DOI] [PubMed] [Google Scholar]
- 13. Sirka C, Pradhan S, Patra S, Padhi S, DasMajumdar S, Panda D. Hemophagocytic lymphohistiocytosis: a rare, potentially fatal complication in subcutaneous panniculitis like T-cell lymphoma. Indian J Dermatol Venereol Leprol. 2018;85:481-485. doi: 10.4103/ijdvl.IJDVL_277_17 [DOI] [PubMed] [Google Scholar]
- 14. Lin HY, Rocher LL, McQuillan MA, Schmaltz S, Palella TD, Fox IH. Cyclosporine-induced hyperuricemia and gout. N Engl J Med. 1989;321:287-292. [DOI] [PubMed] [Google Scholar]
- 15. Michonneau D, Petrella T, Ortonne N, et al. Subcutaneous panniculitis-like T-cell lymphoma: Immunosuppressive drugs induce better response than polychemotherapy. Acta Derm Venereol. 2017;97:358-364. doi: 10.2340/00015555-2543 [DOI] [PubMed] [Google Scholar]