Abstract
Patient acceptance of long-acting injectable antiretroviral (LAI-ARV) HIV-1 regimens will determine uptake. Although previous literature reports high satisfaction, these data stem from clinical trials subject to selection bias. This cross-sectional survey from the HIV practices of an urban academic medical center assessed perceptions and preferences using Likert scales toward overall acceptability, proposed frequencies, injection-site reaction durations, and distribution venue. 59% of surveys were completed resulting 202 respondents. 60% were male, 72% black, and the median age was 49 (IQR 36-58). 93% reported a once daily tablet frequency, 69% reported single tablet regimens, and 59% reported missing zero doses in the prior 30 days. Patients self-categorized as likely (57%) or unlikely (43%) to accept LAI-ARV. Both decreasing frequencies between injections and durations of injection-site reactions resulted higher acceptability scores. 57% of respondents preferred receiving an injectable from their clinician’s office over other potential options. These data demonstrate positive LAI-ARV acceptance potential.
Keywords: HIV, perceptions, injectable, antiretroviral treatment
Background
For many patients, antiretroviral therapy for the treatment of HIV-1 has progressed beyond taking multiple pills multiple times per day to a simplified regimen of 1 tablet taken once daily. Despite minimal pill burden, certain patients struggle with adherence posing a barrier to the 90-90-90 goal from the Joint United Nations Programme on HIV/AIDS.1 Combination antiretroviral therapy (cART), in addition to modern HIV care-management, have increased life expectancies in persons living with HIV (PLWH) and are now similar to overall expectancy rates.2 The increased duration of daily adherence demands have been associated with pill fatigue, spurring research toward alternative mechanisms of medication delivery.3
Alternative delivery mechanisms are not a new concept in the field of HIV-1. Enfuvirtide, a first in class HIV fusion inhibitor approved in 2003, is administered as a twice-daily subcutaneous injection as part of a complete antiretroviral regimen. Enfuvirtide is currently used sparingly in practice due to significant injection-site reactions and alternative medication classes with fewer adverse drug effects.4 Ibalizumab-uiyk, a post attachment inhibitor, is administered as an intravenous infusion once every 2 weeks in addition to an optimized background regimen of oral antiretrovirals.5 Given that neither enfuvirtide nor ibalizumab-uiyk constitute a complete cART regimen, clinical use has remained limited to patients in which resistance patterns preclude the possibility of an all-oral agent regimen.
Many long-acting antiretrovirals administered through alternative mechanisms are under development. An intramuscular cART injectable of cabotegravir (CAB), a novel HIV integrase strand inhibitor, plus the non-nucleoside reverse transcriptase inhibitor rilpivirine (RPV), was approved in Canada in March 2020.6 Examples of other injectable agents under development are lenacapavir (a novel capsid inhibitor) and leronlimab (a novel viral entry inhibitor), both under study as subcutaneous injections. CAB-RPV remains under study with a less frequent dosing interval.7-9 Islatravir, a novel nucleoside reverse transcriptase translocation inhibitor, possesses an intracellular half-life of 100 hours and is under study in combination with other agents for HIV-1 treatment.10
Patient acceptability of and satisfaction with these long-acting alternative modes of medication delivery will be vital to their eventual uptake. Looking to historical precedent, acceptability of twice daily enfuvirtide injections was high in phase III clinical trials indicating relative ease of injection and limited effect on activities of daily living.11 A later enfuvirtide study again demonstrated positive quality of life metrics, however >10% of patients discontinued the medication due to intolerance.12
Most CAB-RPV data exists from patients in clinical trials.13-16 Patients randomized to the injectable-containing arms of the phase 2b CAB-RPV LATTE-2 trial received administrations every 4 or 8 weeks. Study participants overwhelmingly reported injection site pain as an adverse effect, but also reported high rates of satisfaction of both study treatment and treatment continuation.13,14 Patient reported outcome measures at Week 48 of the follow-up phase 3 ATLAS and FLAIR trials again showed high rates of acceptance of injectable therapy, acceptability of injection-site reactions (including pain), and treatment satisfaction.15,16
Data from clinical trials represent, by definition, a select group of participants, and may be subject to selection bias. Selection bias refers to a preferential selection/enrollment of individuals or groups such that there is both a lack of randomization in the studied population and that obtained data may not be truly representative of the population.17,18 Selection bias itself presents a threat to the external validity of findings, and can be minimized through mechanisms such as blinding or randomization. Therefore, study subjects who self-selected to enroll in the described clinical trials involving an injectable medication may not reflect overall acceptability of injectable medications in all populations living with HIV; data outside of the clinical trial environment may be instrumental to understanding real-world acceptability. While minimal data do exist in this area, none represent the full landscape of upcoming treatment options under development.19-22 Our study seeks to further describe patient acceptability of long-acting injectable antiretrovirals through the presentation of multiple formulation and delivery factors.
Methods
Study Design and Population
This anonymous, convenience sampling, cross-sectional study consisted of all PLWH presenting for an appointment at the combined HIV-treating practices of Temple Health (Philadelphia, PA). Patients were offered an English-only survey between March 11 and April 18, 2019, and then either an English or Spanish survey from September 23, 2019 through October 21, 2019. Patients seen by practice providers for reasons other than HIV (e.g., HIV Pre/Post Exposure Prophylaxis or Hepatitis C treatment) were excluded from survey offering.
Survey Instrument and Implementation
Adapting themes from a previously validated medication acceptability survey, this written, self-conducted survey included socio-demographic questions regarding age, gender, race/ethnicity, sexual orientation, endorsement of depressive symptoms, current antiretroviral usage, pill burden/frequency and missed doses.23 Two yes/no questions were asked regarding knowledge of non-oral HIV treatment options both currently available and under development. A list of possible options for the preferred distribution site of a proposed injectable treatment regimen was presented, from which the participant was directed to choose his/her preferred option. A 1 (negative) to 10 (positive) Likert scale was then presented for 13 injectable medication formulation-specific statements regarding overall acceptability, cost, proposed frequency between injections, general comfort with the concept of self- or medical provider-administered injection, comfort with possible sites of injection, and continuation potential based on a proposed injection-site reaction duration. The 1-10 Likert scale was selected based on the proposed statistical analysis plan described below.
A patient care provider or a survey administrator invited patients attending their appointments to participate in survey completion. We obtained verbal consent and assigned each participant a unique study identifier. Although surveys were designed to be self-conducted, a patient could ask for assistance for survey completion from a survey administrator at any time. No personal identifying information was collected during survey administration. Study data were collected, managed, and secured using REDCap electronic data capture tools hosted at Temple University.24
Statistical Analyses
Descriptive statistics summarized patient characteristics and survey responses. Summaries of categorical variables included counts and percentages, while medians and interquartile ranges were used for continuous variables. Survey participants were categorized into 2 groups for analysis using responses to the statement: “I am interested in an injection to treat my HIV.” Participants who responded 1-5 on the Likert scale were categorized as “unlikely acceptors” and 6-10 were categorized as “likely acceptors.” Likely acceptors were compared to unlikely acceptors by Fisher’s exact or Chi-Square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Linear regressions were used to test the trend in acceptability Likert scores of proposed frequency or injection-site reaction duration separately by likely and unlikely acceptors. Values were considered significant if P-values < 0.05. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Ethical Approval and Informed Consent
This study was approved under expedited review by the Institutional Review Board of Temple University (Protocol 25701). Consent was obtained from each patient prior to study initiation.
Results
Fifty-nine percent of offered surveys were completed yielding 202 unique responses. Zero surveys were excluded, therefore 202 completed surveys from PLWH were included to assess perceptions of long-acting injectable antiretrovirals. Table 1 lists demographic data. Participants’ ages ranged from 21 to 73 years with a median of 49 years old. Most respondents self-identified as male, however 38% identified as female and 2% identified as transgender. A large majority of survey respondents reported their race/ethnicity as Black, followed by Hispanic, White and other racial/ethnic identity designations. Approximately one-third of responses indicated that the person identified as Lesbian/Gay/Bisexual/Queer (LGBQ). Most participants endorsed a history of depression or depressive-symptoms.
Table 1.
Demographic Factors of Survey Participants.
| n = 202 | |
| Age, years (IQR) | 49 (36-58) |
| n (%) | |
| Sex/Gender | |
| Male | 122 (60.4) |
| Female | 76 (37.6) |
| Transgender | 4 (2) |
| Race/Ethnicity | |
| Black | 145 (71.8) |
| Hispanic | 36 (17.8) |
| White | 18 (8.9) |
| Other | 3 (1.5) |
| LGBQ Identifying | |
| Yes | 66 (32.7) |
| No | 129 (63.9) |
| Other | 7 (3.5) |
| Depressive symptoms (ever) | |
| Yes | 109 (54) |
| No | 87 (43.1) |
| Other | 6 (3) |
| Antiretroviral pill burden | |
| 1 | 140 (69.3) |
| 2 | 31 (15.3) |
| 3 | 16 (7.9) |
| 4 | 5 (2.5) |
| More than 4 | 7 (3.5) |
| Unknown/Other | 3 (1.5) |
| Antiretroviral pill frequency | |
| Once daily | 188 (93.1) |
| Twice daily | 11 (5.4) |
| Unknown/Other | 3 (1.5) |
| Medication-related Adverse Effects (current) | |
| Yes | 19 (9.4) |
| No | 169 (83.7) |
| Other | 14 (6.9) |
| Missed doses in the past 30 days | |
| Zero | 119 (58.9) |
| 1 | 25 (12.4) |
| 2 | 28 (13.9) |
| Once per week | 12 (5.9) |
| More than once per week | 16 (7.9) |
| Other | 2 (1) |
| Reason for missed doses | |
| I forgot | 52 (25.7) |
| I had trouble getting my medication | 9 (4.5) |
| I had a side effect | 2 (1) |
| Other | 139 (68.8) |
Abbreviation: LGBQ = Lesbian, Gay, Bisexual, or Queer.
Two-hundred participants endorsed currently taking medications for HIV. A majority of regimens consisted of 1 tablet taken once daily and an overall tablet frequency (which may have consisted of multiple tablets) of once daily. A minority of participants endorsed experiencing side effects from their current antiretroviral regimen. While a majority of respondents endorsed missing 0, 1, or 2 doses in the previous 30 days, 12 surveys (6%) indicated that the participant missed 1 dose of medication per week. 16 surveys (8%) indicated that the participant missed more than 1 dose of medication per week. Attributions of missed doses were due to forgetfulness, medication acquisition difficulty, side effects, or other reasons.
Overall responses to the statement “I am interested in an injection to treat my HIV” resulted a median Likert score of 7 (IQR 3-10). One hundred-sixteen respondents (57%) were categorized as “likely acceptors” with a median Likert score of 10 (IQR 8-10), and 86 (43%) were categorized as “unlikely acceptors” with a median Likert score of 1 (IQR 1-5).
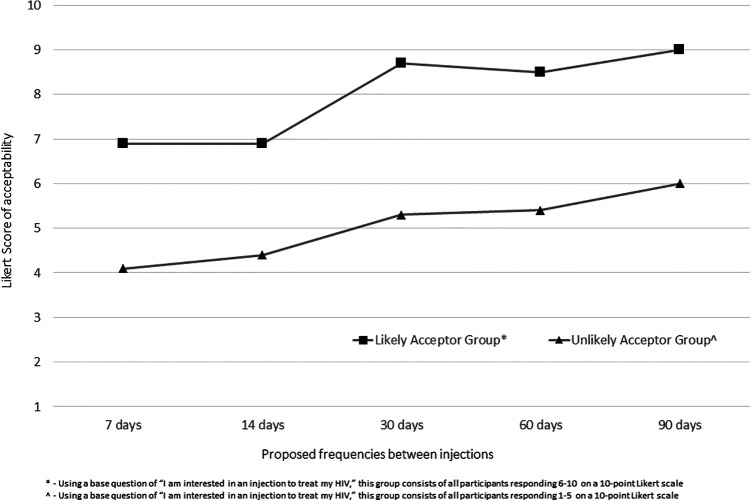
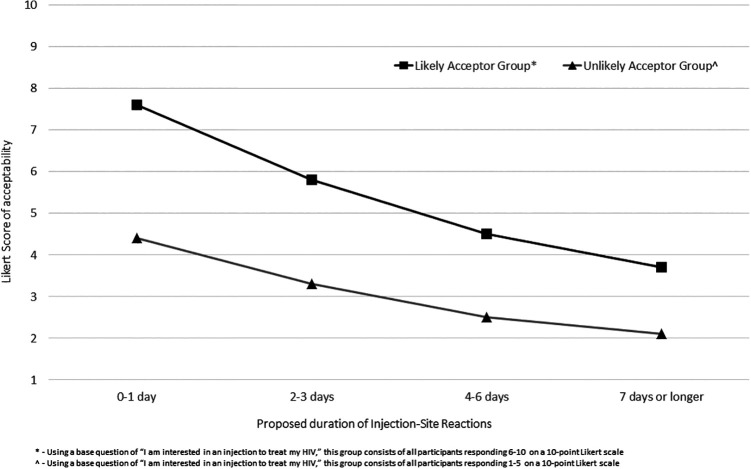
Mean Likert responses for acceptability of a proposed frequency between injections are shown in Figure 1. Scores for likely acceptors were uniformly greater than unlikely acceptors at each given time point with a trend seen toward higher Likert scores of acceptability as frequencies decreased (likely acceptors p = 0.038; unlikely acceptors p = 0.004). Mean Likert responses for acceptability of continuing a medication with a defined proposed injection-site reaction duration are summarized in Figure 2. Likert scores of acceptability for likely acceptors were again uniformly greater than unlikely acceptors at each given time point, and trends were seen toward lower Likert scores as proposed injection-site reaction durations increased (likely acceptors p = 0.014; unlikely acceptors p = 0.02). Associations between acceptor groups and remaining variables were analyzed (sex/gender, race/ethnicity, LGBQ identifying, self-reported depressive symptoms [ever], antiretroviral pill burden, antiretroviral tablet frequency, missed doses in the prior 30 days, and current antiretroviral-related side effect); no variables were statistically significant.
Figure 1.
Likert Scores of long-acting injectable antiretroviral therapy acceptability by proposed frequency between injection administration.
Figure 2.
Likert scores of long-acting injectable antiretroviral therapy acceptability by proposed injection-site reaction duration.
Among a list of presented options for primary preferred distribution venue for an injectable antiretroviral, a clear majority selected the clinician’s office (115, 57%) as their preferred venue. Self-injection (44, 22%), assisted injection at home (18, 9%), pharmacy (7, 4%), and special injection center (3, 2%) comprised the remaining responses (among those who selected a preference).
Participants were given the opportunity to express comfort regarding the idea of self- or medical provider-administered injections on the same Likert scale. Mean Likert scores of acceptability for medical professional-administered injections (likely acceptors 9.2 and unlikely acceptors 7.4) were greater than self-administered injection (likely acceptors 6.9 and unlikely acceptors 4.2).
Discussion
The availability of long-acting cART for the treatment of HIV-1 represents a paradigm shift for patients and providers of HIV care, and careful attention to acceptability, uptake, and implementation should guide future research. We believe that this study represents the most comprehensive data set of patient perceptions outside of a clinical trial to aid in describing attitudes toward injectable antiretroviral medications. We found no statistically significant variables that predicted acceptors versus unlikely acceptors of injectable agents but there were some noteworthy findings, such as the balance between accepting and non-accepting groups. These data differ from existing literature and underscore the importance of comparing real-world opinion to aforementioned acceptability data as part of clinical trials; high published acceptance may reflect selection bias inherent to clinical trials.13-16 These data are in agreement with positive attitudes toward shifting from daily medication taking to injectable options as reported by Khuong-Josses and colleagues.22 When comparing median Likert scores between groups, while the unlikely acceptor IQR spanned the entirety of the score range, the likely acceptor IQR was concentrated among top values. This suggests high likelihood of acceptability among some PLWH after medication approval, and indicates that providers should anticipate adoption of these medications into practice.25
Expected trends among proposed longer intervals between injections and shorter duration of injection-site reactions demonstrate that these patient-friendly characteristics may affect eventual uptake. Of note, these trends were also present among respondents who initially categorized as unlikely acceptors. Although administration comfort with a medical professional was higher than from a self-administered injection, this study did not ask if self-injection would preclude uptake. These data align with previously published concerns toward general hesitation of injections and associated side effects from administration.20
From distribution channel results, although physician’s office and self-injection represent the largest category preferences, participants were only asked to choose 1 option. These data do not purport to compare preferences/attitudes between 2 proposed options, nor do they describe secondary preferences. Pharmacy-delivered injection may or may not be available to patients based on drug formulation, site of injection, and state-by-state regulation.26
Study strengths and limitations
This cross-sectional, convenience-sampling study has multiple strengths, the largest being sample size and patient population. Given general underrepresentation of both minorities and women in HIV clinical trials, data from the population described here can be used to understand and implement these medications into clinical practice upon local approval.27,28 These data represent the most comprehensive known data set on this issue outside of a clinical trial to incorporate a range of possible HIV-1 injectable formulations/characteristics. These data are limited in that they come from a single study site in an urban academic medical center and do not measure some social or psychological determinants of health which may affect results. Notably, Garris and colleagues reported favorable responses that a monthly injection for PLWH would make them feel both less ashamed or stigmatized from HIV. They additionally reported that such an injection may reduce chances that others would discover their diagnosis.21 Further study is needed on these social and environmental factors, which may prove advantageous when attempting to engage patients in challenging or highly-stigmatized locations and settings.
This study only characterizes patient-related attitudes and does not measure real-world barriers such as insurance authorization and procurement. Although we reduced selection bias by conducting this survey outside of a clinical trial, some bias remains due to convenience sampling methods; further implementation study is warranted.
Conclusion and Relevance
Injectable antiretrovirals, used as cART in the treatment of HIV-1 will require new approaches to treatment delivery. In a population enriched with female and minority persons, respondents were closely divided between likely and unlikely accepting groups. Although we found no specific variable predicting groupings, trends in patient-friendly characteristics, namely decreasing injection frequency and decreasing injection-site reaction duration may impact eventual uptake.
Acknowledgments
The Authors would like to thank Milagros Acevedo, the entire staff of the Temple Comprehensive Medical Practice, and the entire Temple Infectious Diseases Section for their assistance with this project.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. D.E.K has served on medical advisory panels for Gilead, Janssen, and Thera, and is an independent consultant for Abbvie and Gilead. The remaining authors have nothing to disclose.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: David E. Koren, PharmD  https://orcid.org/0000-0002-6601-9893
https://orcid.org/0000-0002-6601-9893
Previous Presentations: These data were presented in part at IDWeek 2019 (Abstract 2409) October 2-6, 2019.
References
- 1. The Joint United Nations Programme on HIV/AIDS. 90–90-90 An ambitious treatment target to help end the AIDS epidemic. 2014. Accessed December 6, 2019 https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf
- 2. Antiretroviral Therapy Cohort C. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–e356. doi:10.1016/S2352-3018(17)30066-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Claborn KR, Meier E, Miller MB, Leffingwell TR. A systematic review of treatment fatigue among HIV-infected patients prescribed antiretroviral therapy. Psychol Health Med. 2015;20(3):255–265. doi:10.1080/13548506.2014.945601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oldfield V, Keating GM, Plosker G. Enfuvirtide: a review of its use in the management of HIV infection. Drugs. 2005;65(8):1139–1160. doi:10.2165/00003495-200565080-00007 [DOI] [PubMed] [Google Scholar]
- 5. Bettiker RL, Koren DE, Jacobson JM. Ibalizumab. Curr Opin HIV AIDS. 2018;13(4):354–358. doi:10.1097/COH.0000000000000473 [DOI] [PubMed] [Google Scholar]
- 6. Healthcare V. ViiV healthcare announces first global regulatory approval of CABENUVA; the first complete, long-acting regimen for the treatment of HIV. Accessed June 1, 2020.
- 7. Sager JE, Begley R, Rhee M, et al. Safety and PK of Subcutaneous GS-6207. A novel HIV-1 capsid inhibitor. Presentation at Conference on Retroviruses and Opportunistic Infections, March 7-9, 2019 Seattle WA (Abstract 141). [Google Scholar]
- 8. Dhody K, Kazempour K, Pourhasan N, Maddon PJ. PRO 140 SC: long-acting, single-agent maintenance therapy for HIV-1 infection. Presentation at Conference on Retroviruses and Opportunistic Infections, March 7-9, 2019 Seattle WA (Abstract 486). [Google Scholar]
- 9. ViiV Healthcare. Efficacy, Safety and Tolerability Study of Long-acting Cabotegravir Plus Long-acting Rilpivirine (CAB LA + RPV LA) in human-immunodeficiency virus-1 (HIV-1) infected adults. Accessed December 6, 2019 https://clinicaltrials.gov/ct2/show/NCT03299049.NLMidentifier:NCT03299049
- 10. Markowitz M, Grobler JA. Islatravir for the treatment and prevention of infection with the human immunodeficiency virus type 1. Curr Opin HIV AIDS. 2020;15(1):27–32. doi:10.1097/coh.0000000000000599 [DOI] [PubMed] [Google Scholar]
- 11. Cohen C, Green J, Wintfeld N, Patel K. Fuzeon: Patient acceptance with self-injection of enfuvirtide (Fuzeon) for HIV over 48 of treatment Presentation at 9th European AIDS Conference, October 25-29, 2003 Warsaw, Poland (Abstract 7.1/1). [Google Scholar]
- 12. Pulido F, Del Pozo MA, Fernandez-Guerrero M, et al. Patients’ perception and effectiveness of a treatment containing enfuvirtide when used in HIV-infected patients without very advanced disease. HIV Clin Trials. 2008;9(2):83–90. doi:10.1310/hct0902-83 [DOI] [PubMed] [Google Scholar]
- 13. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510. doi:10.1016/S0140-6736(17)31917-7 [DOI] [PubMed] [Google Scholar]
- 14. Kerrigan D, Mantsios A, Gorgolas M, et al. Experiences with long acting injectable ART: a qualitative study among PLHIV participating in a Phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One. 2018;13(1):e0190487 doi:10.1371/journal.pone.0190487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murray M, Antela A, Mills A, et al. Patient views on long acting HIV treatment: Cabotegravir + rilpivirine as maintenance therapy (ATLAS 48 week results). Presentation at 10th IAS Conference on HIV Science, July 21-24, 2019 Mexico City, Mexico (Abstract MOAB0103). [Google Scholar]
- 16. Murray M, Bernal E, Chounta V, et al. Patient reported outcomes on long-acting Cabotegravir + Rilpivirine as maintenance therapy: FLAIR 48 week results. Presentation at 10th IAS Conference on HIV Science, July 21-24, 2019 Mexico City, Mexico (Abstract MOPEB258). [Google Scholar]
- 17. Mansournia MA, Higgins JP, Sterne JA, Hernan MA. Biases in randomized trials: a conversation between trialists and epidemiologists. Epidemiology. 2017;28(1):54–59. doi:10.1097/EDE.0000000000000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gluud LL. Bias in clinical intervention research. Am J Epidemiol. 2006;163(6):493–501. doi:10.1093/aje/kwj069 [DOI] [PubMed] [Google Scholar]
- 19. Simoni JM, Tapia K, Lee SJ, et al. A conjoint analysis of the acceptability of targeted long-acting injectable antiretroviral therapy among persons living with HIV in the U.S. AIDS Behav. 2019. doi:10.1007/s10461-019-02701-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simoni JM, Beima-Sofie K, Mohamed ZH, et al. Long-acting injectable antiretroviral treatment acceptability and preferences: a qualitative study among us providers, adults living with HIV, and parents of youth living with HIV. AIDS Patient Care STDS. 2019;33(3):104–111. doi:10.1089/apc.2018.0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garris C, Heidenreich S, Arthurs E, et al. Preceptions of and preferences for oral or long-acting injectable antiretroviral treatment regimens in the United States and Canada. Presentation at IDWeek 2019, October 2-6, 2019 Washington, DC (Abstract 2499). [Google Scholar]
- 22. Khuong-Josses MA, Buson M, Charpentier C, Poupard M. Will HIV-infected patients taking oral ARV switch to long-acting injectable ART when it becomes available? Presentation at 17th European AIDS Conference, November 6-9, 2019 Basel, Switzerland (Abstract PE30/3). [Google Scholar]
- 23. Anglia UoE. Medication Acceptability Questionnaire (MAQ). Accessed June 1, 2020 https://www.uea.ac.uk/pharmacy/research/maq
- 24. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi:10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Havlir D, Gandhi M. Implementation challenges for long-acting antivirals as treatment. Curr Opin HIV AIDS. 2015;10(4):282–289. doi:10.1097/COH.0000000000000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oji V, McKoy-Beach Y, Pagan T, Matike B, Akiyode O. Injectable administration privileges among pharmacists in the United States. Am J Health Syst Pharm. 2012;69(22):2002–2005. doi:10.1093/ajhp/69.22.2002 [DOI] [PubMed] [Google Scholar]
- 27. Curno MJ, Rossi S, Hodges-Mameletzis I, Johnston R, Price MA, Heidari S. A systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr. 2016;71(2):181–188. doi:10.1097/QAI.0000000000000842 [DOI] [PubMed] [Google Scholar]
- 28. Castillo-Mancilla JR, Cohn SE, Krishnan S, et al. Minorities remain underrepresented in HIV/AIDS research despite access to clinical trials. HIV Clin Trials. 2014;15(1):14–26. doi:10.1310/hct1501-14 [DOI] [PMC free article] [PubMed] [Google Scholar]