Abstract
Aortic dissection is often regarded as a catastrophic aortic syndrome with high rates of mortality. The sensitivity and specificity of transthoracic echocardiography when diagnosing acute type A aortic dissection has been reported as high as 97% and 100%, respectively, in patients with optimal imaging quality when compared to computed tomography. In this article, we discuss the benefit of transthoracic echocardiography in a patient with type A aortic dissection extending from ascending aorta to iliac arteries.
Keywords: Critical care/emergency medicine, echocardiography, aortic dissection, aortic regurgitation, point-of-care ultrasound
Introduction
Aortic dissection (AoD) is often regarded as a rare but catastrophic aortic syndrome associated with high 30-day mortality rates of up to 37%.1 Different schemes have been proposed to describe the presentation of AoD with the Stanford Classification System being one set of criteria used to describe whether there is ascending aortic involvement (type A) or if the dissection is localised to the descending aorta alone (type B). The DeBakey criteria is another descriptive scale that indicates whether the dissection originates at the ascending and involves the whole of the aorta (type I), just the ascending aorta (type II), or solely the descending aorta (type III). The management of AoD is dictated by location with type A dissections frequently requiring surgical intervention while a more conservative approach is adopted for type B dissections. Several risk factors that can increase the predisposition of an AoD have been identified, such as age, hypertension, smoking, chronic cocaine abuse and aortopathy-related genetic conditions such as Marfan syndrome.2 Symptoms experienced by AoD patients include severe chest and back pain often described as a tearing or ripping sensation, syncope, pre-syncope, nausea, vomiting, diaphoresis and dyspnoea.3 Patients with a type A dissection can present with symptoms suggestive of a stroke or myocardial infarction including aphasia and hemiplegia caused by malperfusion and obstruction of the carotid, coronary and/or vertebral arteries. If the subclavian arteries and distal aorta have also been affected, then patients may experience loss or reduced peripheral pulse and abdominal pain secondary to malperfusion-related renal dysfunction, limb and acute bowel ischaemia.4 If left unchecked an AoD can cause an often fatal aortic rupture leading to massive internal haemorrhage, malperfusion syndromes and cardiac tamponade. Computed tomography (CT) of the aorta is the gold standard imaging technique used to identify and confirm the presence and extent of an AoD. However, CT is often oversubscribed, expensive, requires radiation exposure and cannot be done at the bedside often meaning that these limitations can delay the diagnosis and subsequent treatment of AoD. Transthoracic echocardiography (TTE) can support the diagnosis of a type A AoD resulting in a patient being sent for immediate surgery instead of a referral for an emergency CT scan of the aorta. The case review will discuss the use of TTE as an alternative imaging modality for the diagnosis of AoD in a patient presenting with initial signs of acute coronary syndrome.
Case report
A 63-year-old Caucasian male, presented with diaphoresis, retrosternal and intrascapular pain radiating to the mandible. He described symptoms of mild dyspnoea, nausea and anxiety accompanying the chest discomfort that aroused him from sleep, after which he immediately called the emergency services. A 12-lead electrocardiogram showed 2 mm inferior ST elevation with reciprocal ST depression in the lateral leads prompting a call to the local tertiary centre for primary percutaneous coronary intervention (PPCI) to the right coronary artery (RCA). Upon arrival for PPCI, the patient was loaded with 300 mg of aspirin and 60 mg of prasugrel. The patient experienced a cardiac arrest during the procedure requiring one 150-J cardioversion for ventricular fibrillation, which was attributed to myocardial ischaemia. The angiogram prior to PPCI showed an occlusion of the RCA and a 3.5 × 38 mm stent was deployed to the proximal RCA with an excellent angiographic result. Suspicion was raised as to the concomitant diagnosis of type A AoD following cardiac auscultation on the coronary care unit due to ongoing chest pain post PCI, where a diastolic flow murmur was heard.
A standardised TTE was performed by a Highly Specialised Cardiac Physiologist accredited by the British Society of Echocardiography (BSE). The TTE showed a dilated ascending aorta measuring 4.9 cm with a dissection flap best visualised within the modified parasternal long-axis (PLAX) view of the ascending aorta (Supplemental Video 1). The dissection flap was also visualised in the five-chamber (Supplemental Video 2), three-chamber (Supplemental Video 3) and suprasternal views (Supplemental Video 4). Colour Doppler focusing on the aortic valve and left ventricular outflow tract in the PLAX (Supplemental Video 5), apical five (Supplemental Video 6) and three chamber views (Supplemental Video 7) suggested the presence of moderate-to-severe aortic regurgitation. Mild left ventricular systolic impairment with inferior wall akinesia was seen with an ejection fraction of 50%. There was also a suspicion of a dissection flap within the thoracic aorta in the subcostal views. The results of the TTE provided sufficient evidence of a type I AoD, and the patient was recommended immediate surgery to replace the aortic valve and ascending aorta. The patient initially withdrew consent for surgery requesting more a detailed explanation of the condition and time to discuss treatment options with family members. In the interim analgesics, beta-blockers and glyceryl trinitrate were prescribed to prevent further pain and reduce the risk of aortic rupture. This short period of time allowed the opportunity for additional CT imaging to be performed, which would not have been requested had initial consent been obtained. The CT confirmed a large 5-cm aortic root with a type A intimomedial aortic dissection (AoD) flap originating at the ascending aorta extending (Figure 1) along the entire length of the thoracoabdominal aorta terminating at the common iliac arteries bilaterally. The AoD also extended into the brachiocephalic, right common carotid, subclavian arteries and into the left carotid artery while the left subclavian artery arose from the true lumen. The right renal artery connected to the true lumen while the left renal artery arose from a false lumen with a relative reduction in kidney contrast enhancement (Figure 2). The RCA ostia were likely attached to the false lumen obstructing coronary flow and causing the initial presentation of the acute inferior myocardial infarction and subsequent cardiac arrest, which was treated by PPCI.
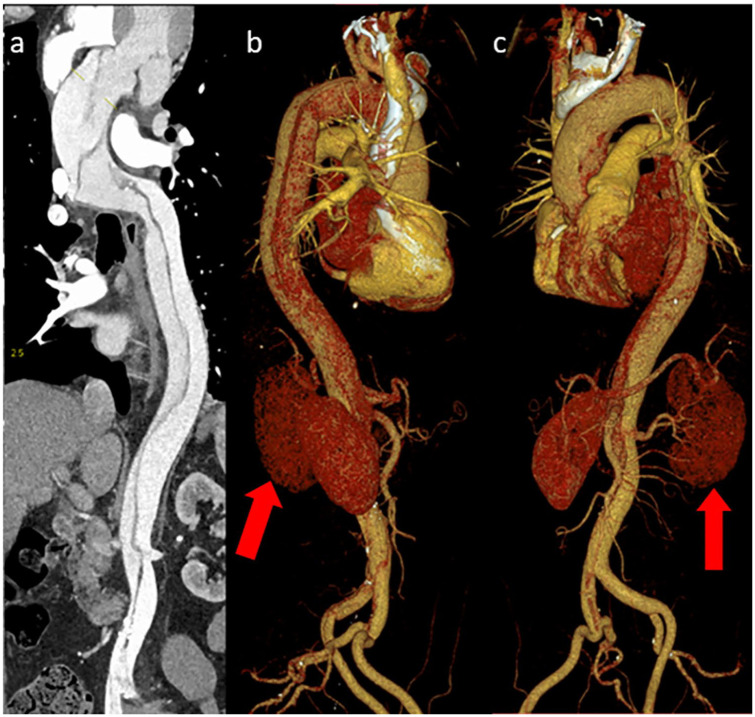
Figure 1.
CT scan (a) showing an unfolded aorta demonstrating the aortic dissection from the ascending aorta to the iliac arteries. Three-dimensional CT renderings of the aorta show the aortic dissection in different views around the aortic arch and down the descending aorta (b and c), thoracic aorta towards the iliac arteries. Note the small reduction in contrast uptake by the left kidney which is attached to the false lumen (red arrow).
Figure 2.
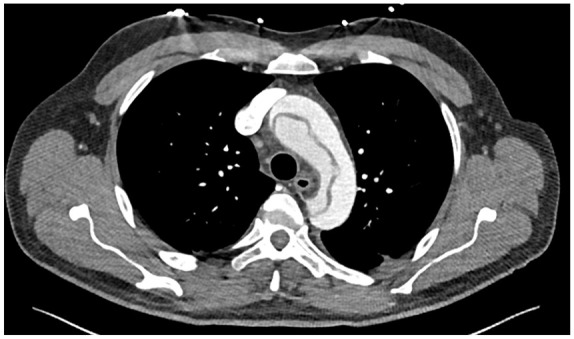
CT of the aorta cross sectional view of the aortic arch showing the aortic dissection.
Following evidence from the TTE and CT scans, surgery to replace the aortic root and valve was discussed with the patient. Following informed consent, a composite ascending aortic graft with left coronary artery reattachment, prosthetic mechanical aortic valve replacement (Carbomedics 23 mm) and saphenous vein graft to the RCA were performed giving an excellent result. Intraoperative transoesophageal echocardiography (TOE) was performed to identify the region of dissection (Supplemental Video 8) and visualise the severity of aortic regurgitation (Supplemental Video 9). The patient was closely monitored on cardiac intensive care before being discharged from hospital 3 weeks following the surgical intervention. On the patient’s most recent clinical follow-up visit, he remains well and is awaiting a repeat CT scan of the aorta.
Discussion
TTE is renowned for being a non-invasive, safe and accurate imaging modality used to evaluate cardiac structure and function. Improvements to imaging technology have increased the sensitivity and specificity of TTE for identifying the presence of an AoD.5 It is feasible that TTE could be the initial imaging modality if an AoD is suspected as non-standardised views used in addition to the standard British Society of Echocardiography TTE template of the ascending and thoracic aorta and aortic valve can provide further evidence of AoD.6 Aortic regurgitation is a frequent finding in patients with type A AoD occurring in 40%–76% of patients which offers the surgeon insight when determining whether the aortic valve should be replaced along with the ascending aorta.7 An AoD can compromise the integrity and geometry of the aortic annulus, sino-tubular junction or ascending aorta causing significant aortic regurgitation secondary to insufficient aortic valve leaflet coaptation. For this reason, cardiac auscultation at the point of admission is currently recommended practice prior to PPCI as an audible murmur around the region of the aortic valve with symptoms of chest and coexisting back pain should arouse suspicion of an AoD. While an important part of any clinical examination, cardiac auscultation has been recognised as a declining skill with modest accuracy when excluding valve disease such as aortic regurgitation.8 Point-of-care ultrasound (POCUS) is a relatively new concept that provides the ability to visualise and assess any aortic regurgitation, while providing accurate, immediate and comparable measurements of the ascending aorta when compared to CT, delivered at the patient’s bedside. In addition, POCUS can also offer immediate evidence of aortic valve morphology and highlight complications which can accompany an AoD such as cardiac tamponade, severe aortic dilation, regional wall motion abnormalities and severe left ventricular systolic dysfunction9 that might influence surgical management. Sobczyk and Nycz9 highlighted that those with aortic valve morphological abnormalities including a bicuspid aortic valve, aortic regurgitation and calcification were 77% more likely to be offered an aortic valve replacement compared to those with normal aortic valvular function. There is an argument that POCUS should be adopted in all PPCI centres which would enable clinicians to effectively triage and identify the presence of an AoD in patients suspected of having an acute myocardial infarction, which may minimise any delay in treatment.10 However, with POCUS being an operator dependent imaging modality this technique relies heavily on sonographer skill and experience. While this is an important consideration to raise, this should not negate its use as operators with in-depth training, ultrasound experience and support from their local echocardiography department will be best prepared to detect the features of AoD.
The European Society of Cardiology guidelines11 recommend the use of TTE in patients who are clinically suspected of an AoD due to its immediate availability (class I, level of evidence C). The superior axial image resolution offered by parasternal long-axis views, mobile dissection membranes within the ascending aorta can be seen in AoD patients with optimal windows.7 The parasternal short-axis view is beneficial to evaluate the morphology of the aortic valve and location of any aortic regurgitation. Due to adequate colour-flow and Doppler alignment, the apical five- and three-chamber views are the best views to quantify the degree of aortic regurgitation. Diastolic flow reversal in the descending aorta and aortic arch and a dilated left ventricular diastolic dimension are often indicators of severe aortic regurgitation. In patients with suitable modified subcostal views, the thoracic aorta may be visualised including a mobile dissection flap and false lumen suggesting the AoD is more extensive and involving the descending and thoracic aorta (type I). Although sub-optimal image quality can be a limitation in some patients, an AoD cannot always be completely ruled out by TTE alone, and further imaging such as TOE or CT of the aorta should be considered if clinical suspicion remains high.12 However, TTE provides the capability for patients with clear evidence of an AoD to be immediately transferred to the operating theatre which has been shown to improve preoperative mortality.13 The advantage of TOE over non-invasive TTE is the ability to provide high-resolution imaging of the entre aortic root with the exception of the distal ascending and proximal aortic arch. The ascending aorta can often be visualised at the mid-oesophageal long and short-axis views within 120° to 150° range provides the ability to visualise atheromatous aortic plaques, intramural hematoma, aneurysmal segments and dissection.14 A mobile dissection flap which expands during systole and recoils in diastole with spontaneous contrast representing low-velocity blood flow seen within the larger false lumen is indicative of an AoD during TOE (Supplemental Video 8). Applying colour flow Doppler at the region of the dissection flap can provide the operator with location of the intimal tear and therefore quantify the type of dissection and likelihood of success of an AoD repair. The benefit of an intraoperative TOE is the ability to check the patency of the aortic valve and if a prosthetic aortic valve is required, assess for paravalvular regurgitation and prosthetic leaflet or disc mobility once attached. While TOE is a superior imaging modality compared to TTE, TOE is an invasive test which requires sedition and is not completely risk free. A theoretical risk of aortic rupture secondary to transient increases in resting systolic pressure due to gagging and retching when introducing the TOE probe has been described.14 However, it is feasible to acknowledge that this is a low-risk phenomenon which could potentially happen at the time of other imaging modalities.
Conclusion
AoD is a rare and often fatal aortic syndrome with patients presenting with acute symptoms such as chest and back pain, which is often mistaken for an acute myocardial infarction. The benefits of TTE provide the ability to visualise the aortic valve and ascending aortic structure in real time at the patient’s bedside to exclude the presence of an AoD. PPCI centres should consider the introduction of POCUS in all suspected acute myocardial infarction patients prior to coronary revascularisation to minimise the avoidable delay in treatment for those experiencing an AoD.
Supplemental Material
Supplemental material, sj-pdf-1-sco-10.1177_2050313X20973086 for Type A aortic dissection: Why there is still a role for echocardiography when every second counts by Peter Luke, Karen Booth, Ali Kindawi and Christopher Eggett in SAGE Open Medical Case Reports
Supplemental material, sj-xps-1-sco-10.1177_2050313X20973086 for Type A aortic dissection: Why there is still a role for echocardiography when every second counts by Peter Luke, Karen Booth, Ali Kindawi and Christopher Eggett in SAGE Open Medical Case Reports
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient for their anonymised information to be published in this article.
ORCID iD: Peter Luke  https://orcid.org/0000-0002-5756-5243
https://orcid.org/0000-0002-5756-5243
Supplemental material: Supplemental material for this article is available online.
References
- 1. Collins JS, Evangelista A, Nienaber CA, et al. Differences in clinical presentation, management, and outcomes of acute type A aortic dissection in patients with and without previous cardiac surgery. Circulation 2004; 110(11Suppl. 1): 237–242. [DOI] [PubMed] [Google Scholar]
- 2. Gawinecka J, Schoenrath F, Von Eckardstein A. Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss Med Wkly 2017; 147: w14489. [DOI] [PubMed] [Google Scholar]
- 3. Conzelmann LO, Weigang E, Mehlhorn U, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016; 49(2): e44–e52. [DOI] [PubMed] [Google Scholar]
- 4. Tran TP, Khoynezhad A. Current management of type B aortic dissection. Vasc Health Risk Manag 2009; 5: 53–63. [PMC free article] [PubMed] [Google Scholar]
- 5. Cecconi M, Chirillo F, Costantini C, et al. The role of transthoracic echocardiography in the diagnosis and management of acute type A aortic syndrome. Am Heart J 2012; 163(1): 112–118. [DOI] [PubMed] [Google Scholar]
- 6. Wharton G, Steeds R, Allen J, et al. A minimum dataset for a standard adult transthoracic echocardiogram: a guideline protocol from the British Society of Echocardiography. Echo Res Pract 2015; 2(1): G9–G24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evangelista A, Flachskampf FA, Erbel R, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr 2010; 11(8): 645–658. [DOI] [PubMed] [Google Scholar]
- 8. Gardezi SKM, Myerson B, Prendergast A, et al. Cardiac auscultation in diagnosing valvular heart disease: a comparison between general practitioners and cardiologists. Eur Heart J 2017; 38(Suppl. 1): P5437. [Google Scholar]
- 9. Sobczyk D, Nycz K. Feasibility and accuracy of bedside transthoracic echocardiography in diagnosis of acute proximal aortic dissection. Cardiovasc Ultrasound 2015; 13: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nishigami K. Update on cardiovascular echo in aortic aneurysm and dissection. Ann Vasc Dis 2018; 11(4): 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erbel R, Aboyans V, Bileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014; 35(41): 2873–2926. [DOI] [PubMed] [Google Scholar]
- 12. Baliga RR, Nienaber CA, Bossone E, et al. The role of imaging in aortic dissection and related syndromes. JACC Cardiovasc Imaging 2014; 7(4): 406–424. [DOI] [PubMed] [Google Scholar]
- 13. Meredith EL, Masani ND. Echocardiography in the emergency assessment of acute aortic syndromes. Eur J Echocardiogr 2009; 10(1): i31–i39. [DOI] [PubMed] [Google Scholar]
- 14. Ramanath VS, Oh JK, Sundt TM, III, et al. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clin Proc 2009; 84(5): 465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-sco-10.1177_2050313X20973086 for Type A aortic dissection: Why there is still a role for echocardiography when every second counts by Peter Luke, Karen Booth, Ali Kindawi and Christopher Eggett in SAGE Open Medical Case Reports
Supplemental material, sj-xps-1-sco-10.1177_2050313X20973086 for Type A aortic dissection: Why there is still a role for echocardiography when every second counts by Peter Luke, Karen Booth, Ali Kindawi and Christopher Eggett in SAGE Open Medical Case Reports