Abstract
Cytokines are proteins secreted in the central nervous system by neurons, microglia, astrocytes and infiltrating peripheral immune cells under physiological and pathological conditions. Over the last 20 years, a growing number of reports have investigated the effects of these molecules on brain plasticity. In this review, we describe how the key cytokines interleukin 1β, interleukin 6 and tumour necrosis factor α were found to support long-term plasticity and learning and memory processes in physiological conditions. In contrast, during inflammation where cytokines levels are elevated such as in models of brain injury or infection, depression or neurodegeneration, the effects of cytokines are mostly detrimental to memory mechanisms, associated behaviours and homeostatic plasticity.
Keywords: Cytokines, plasticity, inflammation, IL-6, IL-1β, TNF-α
Introduction
Brain plasticity is the ability of the brain to change its activity and modify its connections throughout life in response to extrinsic or intrinsic stimuli. Indeed, synaptic plasticity supports learning and memory processes (Konorski, 1948) during which the strength and efficacy of the synaptic transmission change between neurons, accompanied by structural modifications of spines, dendrites and axons. We will describe the role of cytokines in plasticity such as long-term potentiation (LTP), a key process for memory formation involving N-methyl-d-aspartate receptors (NMDAR) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR; Bear, 1994), and synaptic scaling, an operation by which a neuron adjusts the postsynaptic strength of all its synapses in order to maintain normal neuronal network functions (Turrigiano, 2008). Neurons were, for a long time, thought to be the sole actors involved in plasticity but over the last decades, the roles of glial cells have switched from passive homeostatic elements to active modulators of synaptic plasticity and information processing. Among these glial cells are astrocytes that engulf synapses and surround blood vessels. Their many functions involve, among others, uptake and clearance of neurotransmitters such as glutamate and gamma aminobutyric acid (GABA; Sattler and Rothstein, 2006; Schousboe and Waagepetersen, 2005) and regulation of synaptic functions (Halassa and Haydon, 2010). Microglial cells also play a key role in modulating neuronal plasticity. They are a specialised population of tissue-resident macrophages broadly distributed in the brain parenchyma. Resting microglial cells are constantly scanning their environment by targeting synapses to monitor and regulate neuronal activity (Dissing-Olesen et al., 2014; Li et al., 2012). In response to infections, stress, degenerative diseases or any changes in the nervous system both activated astrocytes and microglia produce cytokines that modulate a large variety of physiological and pathological processes (Hanisch, 2002; Hanisch and Kettenmann, 2007; Sofroniew, 2013).
Cytokines are small pleiotropic signalling proteins classically secreted in response to pathogens or injury by cells of the immune system, including monocytes, macrophages, lymphocytes and vascular endothelial cells. This group of proteins comprises interleukins, chemokines, tumour necrosis factors, interferons, growth and cell stimulating factors and neurotrophins. In the brain, cytokines are constitutively expressed in various brain regions by activated glial and neuronal cells and are involved in several normal and pathological processes including sleep regulation (Krueger, 2008), neuronal development (Deverman and Patterson, 2009), alteration of the blood–brain barrier (Yarlagadda et al., 2009), modulation of neurotransmitter metabolism and synaptic plasticity (Miller et al., 2009; Raison et al., 2006). There is an increased interest for the role of cytokines in the brain due to the presence of inflammation in the brain in a wide range of diseases such as Alzheimer’s disease (AD), major disorder depression, epilepsy, stroke, amyotrophic lateral sclerosis and arthritis. In this review, we will discuss how cytokines modulate learning and memory and plasticity in the normal central nervous system (CNS) function and under pathological inflammatory conditions. Learning and memory experiments provide a significant amount of data as they are an effective way of integrating molecular and cellular plasticity mechanisms with systems-level and behavioural changes. We will focus mainly on interleukin 1β (IL-1β), interleukin 6 (IL-6) and tumour necrosis factor α (TNF-α) as they are the most studied cytokines in the brain so far.
The role of cytokines (IL-1β, IL-6, TNF-α) on learning, memory and plasticity in physiological conditions
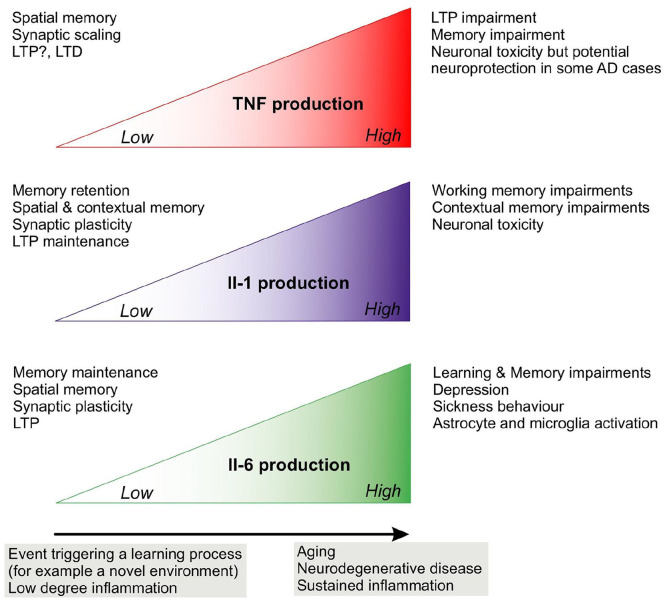
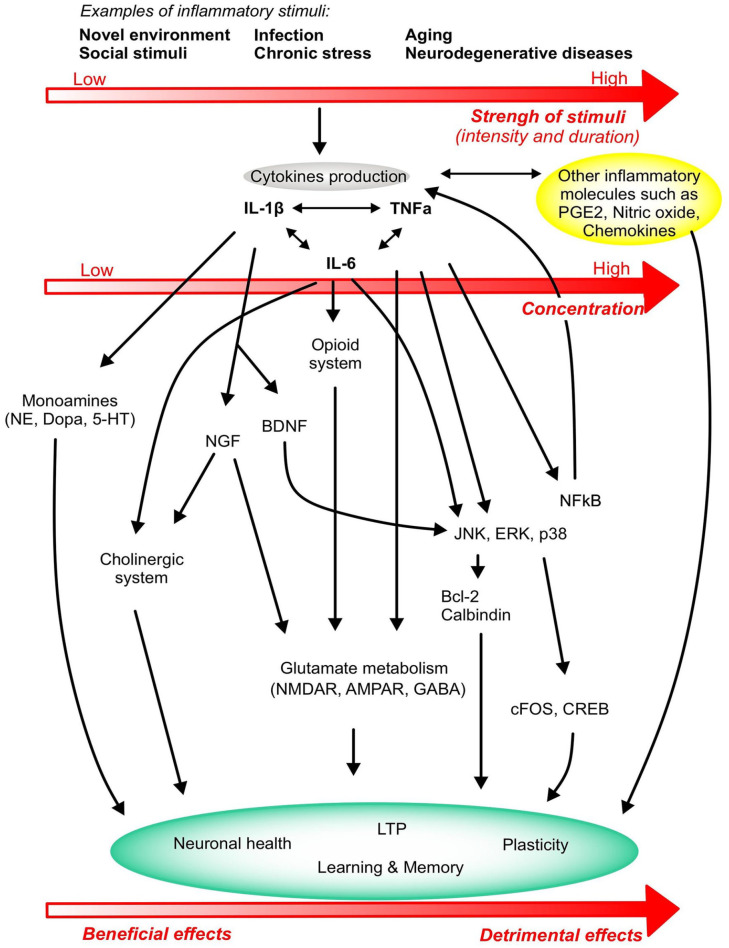
Cytokines and their receptors are expressed in the brain by neurons, microglia and astrocytes and their involvement in structural changes at the synaptic level was reported over 20 years ago (for review, Boulanger et al., 2001; Tonelli and Postolache, 2005). Periodic changes in synaptic transmission that underlie modification of behavioural states are often associated with adjustments in neurotransmitters at the synapse and those are influenced by cytokines (Miller et al., 2013). An overview of the effects of each cytokine on plasticity and learning and memory are summarised in Figure 1 and the mechanisms of action are presented in Figure 2.
Figure 1.
Summary of the effects of cytokines on learning, memory and plasticity.
Figure 2.
Overview of the major mechanisms of action of cytokines on plasticity and learning and memory. Stimuli of different intensity and duration activate the production of cytokines IL-1β, TNF-α and IL-6 that in turn modulate several metabolic and molecular pathways, ultimately affecting neurocircuits that regulate learning and memory function. The strength and duration of the stimulus determine the concentration and production levels of cytokines, leading the cytokine response to generate either beneficial effects on learning and memory or detrimental effects that ultimately progress towards neuronal death and cognitive deficits. Cytokine production also activates other inflammatory systems like PGE2, nitric oxide and other chemokines that will impact on the inflammatory status of the brain and the learning, memory and plasticity responses.
IL-1β
Under normal resting conditions, IL-1β levels are usually below the level of detection and a stimulus is necessary to augment IL-1β expression. Many reports support the role of IL-1β in spatial recognition and contextual learning. IL-1β messenger RNA (mRNA) expression was found to be induced following fear conditioning and during spatial memory tasks (Depino et al., 2004; Goshen et al., 2007; Labrousse et al., 2009). No impairment was detected in IL-1 receptor (IL-1r) knockout (KO) mice for auditory cued memory nor visual memory (Avital et al., 2003) and intrahippocampal injection of IL-1β after training impaired the consolidation of contextual but not auditory fear conditioning memory (Gonzalez et al., 2009). However, mice depleted of any endogenous IL-1 receptor displayed hippocampus-dependent learning deficits (Avital et al., 2003), which highlight the complexity of IL-1 function in the brain (Table 1) and point to a possible role of IL-1β and IL-1α in normal memory function. A few studies have also shown that IL-1β is induced during short- and long-term plasticity and is necessary for LTP maintenance (Avital et al., 2003; del Rey et al., 2013). It is important to stress that IL-1 effects on memory are dose- and age-dependent (see Table 1): at low concentrations, injections of IL-1β appeared to promote synaptic plasticity and improved performance in passive avoidance conditioning whereas injections of higher IL-1β doses following a learning task resulted in impaired memory (Brennan et al., 2003; Goshen et al., 2007; Yirmiya et al., 2002). Most reports use ‘adult’ mice, usually between 2- and 4-month-old; however, an interesting study points out that the role of IL-1 might change when animals get older as only young (3 months) but not older mice (6 months) display deficits in spatial recognition (Takemiya et al., 2017). The authors suggest that synaptic sensitivity to IL-1β and that IL-1β function might evolve with age and with the appearance of age-related brain inflammation and elevation of proinflammatory molecules.
Table 1.
Dose- and age-dependent effects of IL-1 on learning and memory.
| References | Brain region | Animal model and compound concentrations | Age | Task | Effects |
|---|---|---|---|---|---|
| Goshen (2007) | Hippocampus | IL-1ra overexpression; IL-1ra 100 μg/mouse IL-1β low 1 ng or high 10 ng/mouse | Mice 2–4 months old | Contextual fear conditioning; water maze | Deficit |
| Brennan (2003); Yirmiya (2002) | Low-dose IL-1β 1–3 μg/kg; IL-1β 10 ng/rat | Brennan: rats 2-month-old Yirmiya: rats 6–8 months old | Passive avoidance | Improvement | |
| Goshen (2007); Yirmiya (2002) | High-dose IL-1β 6–1000 μg/kg; IL-1ra 100 μg/rat | Yirmiya: rats 6–8 months old | Fear conditioning; passive avoidance | Deficit | |
| Takemiya (2017) | IL-1ra knockout; IL-1β knockout; | Mice 3 and 6 months old | Water maze | Deficit for young mice | |
| Avital (2003); Matousek (2010); Moore (2009) | IL-1 receptor type 1 knockout or IL-1β overexpression | Avita: mice 2–4 months old; others: adult mice | Contextual fear conditioning; water maze (spatial version) | Deficit | |
| Avita (2003); Goshen (2007); Gonzalez (2009) | Amygdala; non- hippocampal-dependent tests | IL-1ra knockout; IL-1ra overexpression; IL-1ra 100 μg/mouse IL-1β low 1 ng or high 10 ng/mouse; IL-1β 5 ng/hippocampus | Mice 2–4 months old | Auditory cued fear conditioning; water maze (visual version) | No effect |
IL: interleukin.
Peripheral IL-1β injection stimulates the release of monoamines in the brain (Song et al., 1999; Zalcman et al., 1994), which are known to modulate learning and memory (for review, Myhrer, 2003); for example, passive avoidance and acquisition of the water maze task were impaired in rats treated with a norepinephrine agonist (Sirviö et al., 1992); spatial memory performance was impaired in rats treated with 5-HT receptor agonist (Carli et al., 1992) and in rats having depleted dopamine (Whishaw and Dunnett, 1985). IL-1β can also modulate plasticity and memory by promoting the production of neurotrophic factors: nerve growth factor (NGF) stimulates acetylcholinesterase activity and facilitates LTP induction (Conner et al., 2009); brain-derived neurotrophic factor (BDNF) activates multiple signalling pathways involved in synaptic plasticity and memory formation such as TrkB–ERK pathway that leads to glutamate and GABA release, potentiation of NMDAR and upregulation of membrane AMPAR (reviewed in the study by Cunha et al., 2010).
IL-6
Rodent studies have shed light on the role of IL-6 in learning and memory (Figure 1) in different brain regions. IL-6 KO mice showed deficits in spatial learning tasks (Braida et al., 2004) and when IL-6 was blocked via the injection of anti-IL-6 antibodies in orbitofrontal cortex (OFC), rats had deficits in a reversal learning task (Donegan et al., 2014). IL-6 role in the striatum was investigated by Brennan et al; they showed that an intraperitoneal (IP) injection of IL-6 prior to an escape/avoidance task did not have any effect on the performance of the animal (Brennan et al., 2004). This task involves the dorsal striatal learning system and it is possible that IL-6 might not be required in that instance, or that the cytokine concentration was too low to have an effect. Several publications have also described a role of IL-6 in the hippocampus. Hippocampal-dependent avoidance learning performance was impaired when IL-6 was injected in the hippocampus (Ma and Zhu, 2000). Young and adult IL-6 KO mice perform better in a radial maze task compared to their wild-type (WT) counterparts (Braida et al., 2004); this difference was particularly noticeable in young mice, which suggest that the chronic lack of IL-6 facilitates learning and memory processes at a juvenile stage. IL-6 mRNA levels were upregulated after LTP induction in vitro and in freely moving rats, and blocking IL-6 led to prolonged LTP at the perforant path and improved memory in a Y-maze task (Balschun et al., 2004). Overall, these results suggest that IL-6 production and function in memory and plasticity might vary with age and brain region (Table 2).
Table 2.
Effects of IL-6 on learning and memory.
| References | Brain region | Mouse model | Task | Effects |
|---|---|---|---|---|
| Braida (2004) | Hippocampus | IL-6 knockout | Radial maze | Facilitatory effect |
| Heyser (1997) | IL-6 overexpressed (GFAP-IL-6 transgene) | Discriminated avoidance task | Deficit (increases with age) | |
| Ma and Zhu (2000) | IL-6 infusion | Passive avoidance task | Deficit | |
| Wu and Lin (2008) | IL-6 infusion | Forced swim task | Increased immobility | |
| IL-6 inhibitor | Forced swim task | Reduced immobility | ||
| Amygdala | IL-6 infusion | Forced swim task | Increased immobility | |
| IL-6 inhibitor | Forced swim task | Reduced immobility | ||
| Donegan (2014) | Orbitofrontal cortex | Anti-IL-6 antibodies | Reversal learning (attentional set shifting test) after chronic intermittent cold (CIC) stress | Deficit |
| Donegan (2014) | IL-6 overexpression (AAV vector) | Reversal learning (attentional set shifting test) after chronic intermittent cold (CIC) stress | Deficit attenuated | |
| Wu and Lin (2008) | Frontal cortex | IL-6 infusion or IL- 6 inhibitor | Forced swim task | No effect |
| Brennan (2004) | Dorsal striatum | IP injection of IL-6 | Leverpress escape/avoidance task | No effect |
| Donegan (2014) | Anti-IL-6 antibodies | Reversal learning (attentional set shifting test) after chronic intermittent cold (CIC) stress | No effect | |
| Roberts (2019) | Limbic and hypothalamic regions | IL-6 overexpression (GFAP-IL-6 transgene) | Open field, light/dark transfer, digging tests | Deficit |
| Tail suspension test | No effect | |||
| Forced swim task | Deficit |
IL: interleukin; GFAP: glial fibrillary acidic protein; AAV: adeno-associated virus; IP: intraperitoneal.
The mechanisms by which IL-6 modulates cognitive function are far from being elucidated (Figure 2). Decreased ERK1/2 activation after IL-6 application correlates with impairments of LTP (Tancredi et al., 2000). A hypothesis is that the memory facilitation observed in mice lacking IL-6 could be due to the absence of inhibition from the endogenous opioid system as µ-opioid receptors were downregulated in IL-6 KO mice (Bianchi et al., 1999) and therefore unable to dampen learning and memory (Ukai et al., 2000; Ukai and Lin, 2002). IL-6 is also known to interact with the cholinergic system and peripheral injection of this cytokine reduced scopolamine-induced impairment of a passive avoidance task (Bertolucci et al., 1997). Moreover, intracellular calcium response to NMDA or glutamate was enhanced following chronic treatment of cultured cerebellar-granule neurons with IL-6 (Holliday et al., 1995; Qiu et al., 1995) meaning that IL-6 can also potentiate neurotransmitter responses and thus any dependent behaviour.
TNF-α
Very few studies have investigated TNF-α role under physiological conditions. Mice lacking TNF-α performed poorly in a novel object recognition task and in the Barnes maze test that evaluates spatial memory and learning effectiveness (Baune et al., 2008). A positive effect of TNF-α on learning and memory was also described in an escape/avoidance task where rats injected with TNF-α (6 μg/kg, intraperitoneal) made more avoidance responses and fewer escape responses compared to controls (Brennan et al., 2004).
TNF-α role in synaptic activity has been more extensively studied. TNF-α regulates synaptic scaling by promoting the insertion of AMPAR lacking GluR2 subunit at the plasma membrane of synapses, hereby promoting excitatory signalling over inhibitory signalling. TNF-α also modulates gliotransmission by elevating astrocytic calcium levels which in turn increase by twofold the number of glutamatergic exocytic vesicles. Released glutamate activates neuronal NMDAR at granule cells synapses of the dentate gyrus, hereby increasing excitatory synaptic activity and potentiation of GC synapses (Santello et al., 2011).
Beattie et al. showed, using neuronal cultures and acute hippocampal slices, that TNF-α was required for preservation of synaptic strength and increasing the number of AMPAR at the membrane, the number of synapses and the frequency of miniature excitatory post-synaptic currents (Beattie et al., 2002). Concomitant to these changes, TNF-α was found responsible for an increase in spine size of dendritic branches with recent spine loss, which constitute another synaptic scaling feature (Barnes et al., 2017). Other studies have confirmed that constitutive TNF-α is necessary for maintaining AMPAR at the synapse, and described how TNF-α also triggered GABAA receptors internalisation, highlighting the unique ability of this cytokine to modulate both excitatory and inhibitory plasticity (Stellwagen et al., 2005; Stück et al., 2012). Even though TNF-α can be produced by neurons, the above effects were generated by glial TNF-α in response to extracellular glutamate (Stellwagen and Malenka, 2006).
The role of TNF-α in other forms of plasticity is unclear. Some studies reported that LTP induction or maintenance did not require TNF-α as TNF-α KO mice displayed normal LTP in the CA1 pyramidal layer (Albensi and Mattson, 2000; Stellwagen and Malenka, 2006). However, a recent report highlighted a significant TNF-α-dependent inhibition of LTP in the stratum radiatum but not the stratum oriens of rats after either high-frequency priming stimulation or treatment with TNF-α (1.8 nM) via the phosphorylation of p38-MAPK, ERK and JNK (Singh et al., 2019), pointing to a differential regulation of LTP in the CA1 basal and apical dendrites. Findings regarding TNF-α and LTD are also conflicted. Although Albensi et al. showed that TNF-α is a key factor for the induction of hippocampal LTD via nuclear factor kappa B (NFκB) activation (Albensi and Mattson, 2000), that was not the case in other laboratories (Stellwagen and Malenka, 2006). Overall, these varying results could be explained by different experimental conditions such as theta burst or high-frequency stimulation, the area in which the stimulation and recordings are made, the use of different TNF-α concentration, time of incubation, cell culture or brain slices.
The role of cytokines in plasticity, learning and memory under inflammatory conditions
Central and peripheral inflammation in response to pathogens, injury or disease is a defence mechanism by which microglia and astrocytes become activated and potentially produce chemoattractant proteins that promote extravasation of monocytes from the periphery into the brain (Ransohoff et al., 2003). Therefore, in the inflamed brain, cytokines are produced by microglia, astrocytes, neurons, peripheral inflammatory cells, endothelial cells, pericytes and choroid plexus; and cytokine receptors are present on neurons, astrocytes, microglia and vascular endothelial and perivascular cells (Kennedy and Silver, 2016; Konsman et al., 2006; Verma et al., 2006). TNF-α, IL-1β and IL-6 have the ability to stimulate each other’s production and can act synergistically (Donzis and Tronson, 2014) to modulate neuronal responses, plasticity and learning and memory (Figure 2), often translated by an elevated excitability of the brain and learning and memory changes that we describe below.
IL-1β
A detrimental role of IL-1β on learning and memory was described in an inflammatory environment (Figure 1) created by generating chronic elevated IL-1 levels in the brain. Spatial memory deficits were reported after chronic injection of IL-1β into the lateral ventricles of rats in an eight-arm radial maze (Taepavarapruk and Song, 2010). Mouse models of inflammation using chronic hippocampal overexpression of IL-1β for 2 weeks led to the appearance of inflammatory markers such as activated glia, elevated prostaglandin E2 (PGE2), increased hippocampal proinflammatory cytokine and chemokine mRNAs and lower levels of the plasticity-related gene Arc (Hein et al., 2010; Moore et al., 2009). These transgenic mice displayed delayed acquisition and decreased retention in the spatial water maze task, and impaired long-term contextual fear memory, while hippocampal-independent and short-term memory remained intact (Hein et al., 2010; Moore et al., 2009).
Models of peripheral infections have also been used to study cytokines in the brain. Peripheral infection can cause significant impairment of cognitive functions in individuals already suffering from neurodegenerative disease (Table 3; Perry et al., 2003) and to a lesser extent in healthy individuals (Dantzer et al., 2008). When injected intraperitoneally with Escherichia coli, IL-1β is upregulated in the aged rat hippocampus (Barrientos et al., 2009). The involvement of IL-1β in learning and memory was also studied in mice injected with Legionella pneumophila before subjecting them to the Morris water maze (MWM). Sick mice displayed impaired learning; their task performance was, however, restored to control levels if they were treated with an anti-IL-1β antibody (Gibertini et al., 1995). It is worth noticing that the effects of IL-1β depend (1) on the intensity of the protocol, as a high frequency of training sessions were more likely to highlight an IL-1β–related memory impairment in rodents than when spaced protocols were used (Gibertini, 1998) and (2) on the concentration of IL-1β that is injected in the animal, with low dose (100 ng/mouse) leading to learning impairment and high dose of IL-1β (1000 ng/mouse) facilitating spatial navigation learning (Gibertini, 1998). Lipopolysaccharide (LPS) is another compound regularly used to study the effects of cytokines during an infection and it classically generates sickness behaviour-related symptoms (Dantzer, 2001; Goshen et al., 2008; Kent et al., 1992; Larson and Dunn, 2001; Maier and Watkins, 1998). Following LPS injection in mice lacking caspase-1, the enzyme that cleaves the inactive pro-IL-1 into its active form, mice displayed reduced sickness behaviours and decreased levels of proinflammatory markers such as PGE2 (Mastronardi et al., 2007). IP injection of LPS increased IL-1β protein levels in the cortex, hippocampus and hypothalamus of rat (Nguyen et al., 1998) and impaired contextual but not auditory fear conditioning (Nguyen et al., 1998; Pugh et al., 1998), an effect that was abolished by subcutaneous injection of the endogenous antagonist IL-1ra (Pugh et al., 1998). A mouse model of septic encephalopathy with blood–brain barrier disruption was characterised by astrogliosis and upregulation of hippocampal IL-1β and its receptor IL-1R1, and altered LTP was recovered when hippocampal slices were pre-incubated with an IL-1R1 antagonist (Imamura et al., 2011). IL-1β impairment of LTP was recorded in the Schaffer collateral–CA1 synapses and in the associational/commissural fibre–CA3 synapses; LTP deficit was found to be dependent on the activation of mitogen-activated protein kinases (MAPKs) and the presence of NMDAR (Hoshino et al., 2017; Kaminska et al., 2009). Interestingly, LTP was not impaired at mossy fibre–CA3 synapses, possibly because these synapses are NMDAR-independent (Nicoll and Schmitz, 2005).
Table 3.
Effects of cytokines on patient’s cognition in different conditions.
| References | Condition | Cytokine | Effects |
|---|---|---|---|
| Perry et al., (2003) | Peripheral infection added to neurodegenerative disease | IL-1β | Cognitive impairment |
| Dantzer (2008) | Peripheral infection in healthy individuals | IL-1β | Cognitive impairment |
| Weaver (2002), Economos (2013), Elderkin-Thompson et al. (2012), Foster (2002), Palotás (2002) | Chronic inflammatory diseases; ageing | IL-6 | Sickness and depressive behaviours; Cognitive decline |
| Cohen (2003) | LPS injection | IL-6 | Declarative and working memory impairments |
| Mao (2016), Mutso (2012) | Chronic pain | TNF-α | Memory deficits, reduced hippocampal volume |
IL: interleukin; LPS: lipopolysaccharide; TNF: tumour necrosis factor.
Stressors like inescapable shocks also trigger an increase of IL-1β protein levels in the brain, although this increase can be difficult to highlight as it is likely to be masked by an inhibitory action of glucocorticoids (Nguyen et al., 1998). Chronic mild stress triggered an increase of hippocampal IL-1β protein levels and depression-like symptoms in rodents, but mice lacking the IL-1 receptor or with an astrocytic overexpression of an IL-1 antagonist did not display stress-induced behavioural nor neuroendocrine changes (Goshen et al., 2008). Social isolation also elevated Il-1β protein levels in the hippocampus and the cerebral cortex and consequently led to contextual fear-conditioning impairment (Pugh et al., 1999). Similar to isolation studies, rats infused with IL-1β in the hippocampus displayed contextual but not auditory deficits after fear conditioning, and this effect was prevented by intracerebral injection of IL-1ra (Pugh et al., 1999). IL-1 was also found to impair working memory using a three-panel runway task (Matsumoto et al., 2001, 2004). In a rodent model of seizure where the mouse cecum is ligated then punctured, IL-1β mRNA expression in the hippocampus was upregulated, escape latency in the MWM was impaired and LTP decreased; the use of an IL-1 antagonist rescued LTP and learning and memory abilities (Han et al., 2016). High hippocampal IL-1β levels found in aged or stressed rats was also associated with impaired LTP (Murray and Lynch, 1998). Under stress conditions, it is likely that microglial IL-1β production increase is linked to the activation of the purinergic receptor P2R×7 (Aricioglu et al., 2019), which is a key protein that supports stress and depression mechanisms (Ribeiro et al., 2019).
In a model of Parkinson’s disease, elevated IL-1β production in the brain worsened neurodegeneration course by supporting neuronal death, either directly or via nitric oxide production (Pott Godoy et al., 2008). IL-1β levels are elevated in AD brain as IL-1β was produced in response to Aβ exposure in cell culture (Meda et al., 1999) and was shown to contribute to cognitive deficits, tau phosphorylation and Aβ pathology in aged 3× Tg-AD mice (Kitazawa et al., 2011). Aβ-induced astrocytic IL-1β is also suspected of supporting the overexpression and activity of acetylcholinesterase hence exacerbating the cholinergic dysfunction linked to cognitive impairment observed in AD (Li et al., 2000).
Some mechanisms by which IL-1β modulates memory function have been described. It has been reported that the synaptic sensitivity to IL-1β increases with age due to a ratio change of the IL-1-receptor type 1 subunits AcP (proinflammatory) and AcPb (prosurvival) that facilitates memory impairment (Prieto et al., 2015). There is evidence also for the arachidonic cascade and the glutamatergic system to be influenced by IL-1β. Bilateral injection of IL-1β in the hippocampus of rats increased cyclooxygenase-2 (COX2) protein levels and activated the arachidonic cascade and the release of PGE2 in the hippocampus (Matsumoto et al., 2004; Molina-Holgado et al., 2000). Hippocampal PGE2 injection led to more working memory errors than in control group (Matsumoto et al., 2004). Similarly, selective and competitive NMDAR antagonists caused deficits in working memory performance in the three-panel runway task (Ohno et al., 1992). An NMDAR agonist concurrently administered with IL-1β into the hippocampus reversed the working memory impairment induced by the cytokine. IL-1β also modulates calcium influx through NMDARs (Viviani et al., 2003). Moreover, elevated IL-1β protein levels in the brain could inhibit LTP by interfering with BDNF signal transduction and dendritic spine morphology (Tong et al., 2012).
IL-6
In illnesses such as depression, arthritis and others where chronic inflammation is settled, patients report sickness and depressive behaviours and cognitive decline, often accompanied by elevated levels of IL-6 (Weaver et al., 2002). Individuals given a dose of LPS did not report feeling sick but performed poorly during declarative memory tests and better in working memory tasks (Cohen et al., 2003). A lot of rodent studies have confirmed these detrimental effects of IL-6 on cognition and behaviour. Mice lacking IL-6 displayed reduced sickness behaviour and depressive-like social behaviour induced by systemic and central injections of IL-1β and LPS was lower in IL-6−/− mice than in their WT counterparts (Bluthé et al., 2000). Similarly, turpentine-induced abscess or influenza infection did not cause any behavioural deficits in IL-6−/− mice (Kozak et al., 1997). Sparkman et al. evaluated the ability of these animals to integrate new information after the acquisition period of an MWM task by injecting LPS. Contrary to WT mice, IL-6 KO animals failed to display any working memory impairment and lacked the expected LPS-induced increase in TNF-α and IL-1β mRNA in hippocampal neurons but not in the periphery (Sparkman et al., 2006). However, LPS injection still activated a marker of neuronal activity c-Fos in neurons of the nucleus tractus solitarius that receive peripheral information via the vagal nerve (Sparkman et al., 2006; Vallières and Rivest, 1999). These data support the fact that IL-6 is not fundamental for periphery-to-brain communication but is required for LPS-induced production of TNF-α and IL-1β in the brain and for the expression of some behavioural impairments. Mice lacking or expressing IL-6 have also been used to highlight the role of IL-6 in the limbic system in relation to anxiety-like and depressive-like behaviours. IL-6 KO mice showed alterations in such behaviours (Chourbaji et al., 2006; Wu and Lin, 2008). Decreased exploratory behaviour in a light–dark transfer test and digging behaviours were reported in mice overexpressing IL-6 together with a longer time spent immobile in a forced-swim test (Roberts et al., 2019; Table 2) as a readout of the ability of the animal to adapt upon exposure to an inescapable stressor (Molendijk and de Kloet, 2019).
IL-6 plays a key role in activating astrocytes and microglia during inflammatory conditions. IL-6 KO mice displayed reduced reactive astrogliosis and microgliosis after IP injection of the toxic glutamate receptor agonist kainic acid (model of seizure; Penkowa et al., 2001) or subcutaneous injection of the neurotoxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (model of selective dopaminergic cell injury, which occurs in Parkinson’s disease; Cardenas and Bolin, 2003). Indeed, IL-6 KO mice had a reduced astrocytic population and a decreased ability to activate microglia (Klein et al., 1997); and in a murine model of experimental autoimmune encephalomyelitis, IL-6 blocked the astrocytic response to interferon-γ and the release of microglial inhibitory molecules such as galectin-1 and HO-1 (Savarin et al., 2015). This glial cell activation by IL-6 is likely to happen in ageing and neurodegenerative diseases. Studies have described the elevation of IL-6 in the plasma of elderly individuals and its correlation with cognitive decline, with females being more sensitive to higher IL-6 levels (Table 3; Economos et al., 2013; Elderkin-Thompson et al., 2012; Weaver et al., 2002). These results were confirmed in a mouse model of accelerated ageing in which IL-6 protein levels were increased by 50% and 30% in the hippocampus and cortex of aged mice, respectively, compared to controls (Tha et al., 2000). Similarly, IL-6 gene and protein expression in glial cells were found to be upregulated in the cerebellum, cortex and hippocampus of mice as they age naturally (Ye and Johnson, 1999). IL-6 detrimental effects on cognition could be linked to neurodegeneration and calcium homeostasis which is perturbed in the brain of Alzheimer’s patients and aged individuals (Foster and Kumar, 2002; Palotás et al., 2002), with the expression of a key calcium-binding and buffering protein calbindin being reduced in rodent models of dementia (Heyser et al., 1997; Palop et al., 2003; Figure 2). Mice chronically expressing astrocytic IL-6 were found to express a progressive age-related decline in avoidance learning performance that correlated with pre-synaptic loss, typically associated with behavioural deficits (Cunningham et al., 2003) and a decrease in cortical and hippocampal neuronal calbindin that plays a protective role in AD (Kook et al., 2014). However, in the early phase of AD, IL-6 participates in a reduction in Aβ deposition as IL-6 overexpression in a mouse model of AD led to enhanced microglia activation and phagocytosis activity (Chakrabarty et al., 2010).
TNF-α
The role of TNF-α in learning and memory under inflammatory conditions is likely to be age dependent. Indeed, studies using 30-day-old mice overexpressing TNF-α did not find any learning and memory impairment in the water maze test (Aloe et al., 1999; Fiore et al., 1996), whereas older mice showed impaired memory in a passive avoidance task (Fiore et al., 1996), in the water maze (Bjugstad et al., 1998) and in a three-panel runway task (Matsumoto et al., 2002). Adult rats chronically overexpressing five times the normal levels of murine neuronal TNF-α also display spatial memory impairments in a water maze paradigm (Pettigrew et al., 2008, 2016). In these animals, hippocampal synaptic plasticity measured by LTP and paired-pulse facilitation are comparable to those measured in the immature hippocampus of neonates, which support a role of TNF-α in maturation of the synaptic network (Pettigrew et al., 2008, 2016). Another report points to a beneficial role of TNF-α in memory recovery as mice lacking TNF-α that have recovered from pneumococcal meningitis displayed impaired water maze performance compared to WT controls (Gerber et al., 2004).
Chronic pain is often accompanied by memory deficits and a reduced hippocampal volume (Table 3; Mao et al., 2016; Mutso et al., 2012). In a model of chronic pain, a pared sciatic nerve leads to microglia activation, increases TNF-α levels and impairs LTP in the hippocampus, reduces the density of presynaptic boutons, as well as the functional synaptic connectivity and BDNF expression in CA1 neurons (Liu et al., 2017; Ren et al., 2011). These synaptic changes are TNF-α-dependent as similar results were obtained when TNF-α was injected in the brain, but not when the experiment was repeated with mice lacking the TNF-α receptor TNFR1 (Liu et al., 2017; Ren et al., 2011).
Astrocytic and microglial TNF-α release under inflammatory conditions is a feature of neurodegenerative diseases (Chung and Benveniste, 1990; Rojo et al., 2008; Yin et al., 2017) and is associated with increased levels of Aβ, tau and neuronal cell death (Janelsins et al., 2008; McAlpine et al., 2009). CSF levels of TNF-α, produced by brain cells, are increased in some AD patients (Tarkowski et al., 1999); TNF-α mediates LTP inhibition as well as memory deficits caused by Aβ (Rowan et al., 2007; Tobinick, 2009; Wang et al., 2005). In aged APP/PS1 mice, a model of AD in which TNF-α levels are twofold higher than in their WT counterparts, there is a marked TNF-α-dependent inhibition of LTP possibly due to elevated TNF-α production by Aβ-dependent glial cell activation (Singh et al., 2019). Several other studies have reported that in mice and hippocampal slices, TNF-α abolished LTP and had deleterious effects on working memory (Cunningham et al., 1996; Curran and O’Connor, 2003; Pickering and O’Connor, 2007; Ren et al., 2011; Tancredi et al., 1992; Wall et al., 2015), while cognitive deficit is reduced by pharmacological inhibition of TNF-α in mice (Shin et al., 2014). Interestingly, earlier in vitro studies have reported a protective role of TNF-α in AD, with human TNF-α levels inversely correlated with levels of a marker of neuronal degradation tau and in vitro data linking TNF-α incubation of neuronal cells to an increase in bcl-2, which can downregulate apoptosis (Tarkowski et al., 1999). TNF-α has also been shown to protect against glutamate, free radical and Aβ toxicity in enriched cultures of primary neurons (Barger et al., 1995).
In an inflammatory context, TNF-α levels can rise from picomolar to millimolar levels; phosphorylation of MAPKs (Figure 2) have been described during TNF-α inhibition of LTP in the molecular layer of the dentate gyrus and hippocampal apical dendrites (Butler et al., 2004; Singh et al., 2019). Elevated TNF-α concentration can also activate NFκB and potentiate NMDAR and AMPAR via TNFR1 activation, blocking glutamate transporter activity and promoting glutamate neurotoxicity (Bernardino et al., 2005; Zou and Crews, 2005), leading eventually to neuronal and oligodendrocyte cell death (Akassoglou et al., 1998; Li et al., 2004). Moreover, TNF-α favours synaptic transfer of calcium-permeable AMPAR lacking GluR2 subunit, hence potentially increasing the risk of neuronal toxicity (Stellwagen et al., 2005).
Surprisingly, the effects described above do not occur in the dorsolateral striatum of rodents. In this region rich in inhibitory GABAergic neurons, TNF-α promotes the internalisation of AMPAR permeable to calcium thereby reducing synaptic strength, which could be an adaptative mechanism of the brain to delay motor symptoms in diseases characterised by movement disorders and elevated TNF-α levels (Lewitus et al., 2014). Overall TNF-α roles on plasticity depend on the anatomical location of TNF-α action and on the level of expression of TNF-α (Butler et al., 2004; Cunningham et al., 1996; Tancredi et al., 1992; Wall et al., 2015).
Conclusion
Cytokines are much more than basic signalling inflammatory molecules as they have key roles in modulating cognitive functions in physiological conditions. We propose that the length, strength and location in a particular area of the brain of cytokine activity will dictate the activation of specific signalling pathways together with the stimulation of other inflammatory systems that will contribute to the complexity of the response. This renders the distinction between mechanisms that would be solely engaged either in physiological or in inflammatory conditions very difficult. We suggest that the homeostasis equilibrium shifts when pathological, chronic expression of cytokines sustains in time; then the cytokine actions often become detrimental and lead to neuronal death and learning and memory impairments. In that view, a better understanding of the interaction between the whole immune system and synaptic plasticity would certainly be key to develop treatments for neuronal, cognitive and mood disorders that present deficits in these mechanisms.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Julie-Myrtille Bourgognon  https://orcid.org/0000-0001-6772-3965
https://orcid.org/0000-0001-6772-3965
References
- Akassoglou K, Bauer J, Kassiotis G, et al. (1998) Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: Models for multiple sclerosis with primary Oligodendrogliopathy. The American Journal of Pathology 153(3): 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albensi BC, Mattson MP. (2000) Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse 35(2): 151–159. [DOI] [PubMed] [Google Scholar]
- Aloe L, Properzi F, Probert L, et al. (1999) Learning abilities, NGF and BDNF brain levels in two lines of TNF-α transgenic mice, one characterized by neurological disorders, the other phenotypically normal. Brain Research 840(1): 125–137. [DOI] [PubMed] [Google Scholar]
- Aricioglu F, Ozkartal CS, Bastaskin T, et al. (2019) Antidepressant-like effects induced by chronic blockade of the purinergic 2X7 receptor through inhibition of non-like receptor protein 1 inflammasome in chronic unpredictable mild stress model of depression in rats. Clinical Psychopharmacology and Neuroscience 17(2): 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital A, Goshen I, Kamsler A, et al. (2003) Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus 13(7): 826–834. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, Del Rey A, et al. (2004) Interleukin-6: A cytokine to forget. The FASEB Journal 18(14): 1788–1790. [DOI] [PubMed] [Google Scholar]
- Barger SW, Hörster D, Furukawa K, et al. (1995) Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: Evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proceedings of the National Academy of Sciences of the United States of America 92(20): 9328–9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SJ, Franzoni E, Jacobsen RI, et al. (2017) Deprivation-induced homeostatic spine scaling in vivo is localized to dendritic branches that have undergone recent spine loss. Neuron 96(4): e.8718825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, et al. (2009) Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain, Behavior, and Immunity 23(1): 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Wiede F, Braun A, et al. (2008) Cognitive dysfunction in mice deficient for TNF- and its receptors. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 147B(7): 1056–1064. [DOI] [PubMed] [Google Scholar]
- Bear MFRCM. (1994) Synaptic plasticity: LTP and LTD. Current Opinion in Neurobiology 4(3): 389–399. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, et al. (2002) Control of synaptic strength by glial TNFα. Science 295(5563): 2282–2285. [DOI] [PubMed] [Google Scholar]
- Bernardino L, Xapelli S, Silva AP, et al. (2005) Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. The Journal of Neuroscience 25(29): 6734–6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolucci M, Perego C, De Simoni MG. (1997) Interleukin-6 is differently modulated by central opioid receptor subtypes. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 273(3): R956–R959. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Maggi R, Pimpinelli F, et al. (1999) Presence of a reduced opioid response in interleukin-6 knock out mice. European Journal of Neuroscience 11(5): 1501–1507. [DOI] [PubMed] [Google Scholar]
- Bjugstad KB, Flitter WD, Garland WA, et al. (1998) Preventive actions of a synthetic antioxidant in a novel animal model of AIDS Dementia. Brain Research 795(1): 349–357. [DOI] [PubMed] [Google Scholar]
- Bluthé R-M, Michaud B, Poli V, et al. (2000) Role of IL-6 in cytokine-induced sickness behavior: A study with IL-6 deficient mice. Physiology & Behavior 70(3): 367–373. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Huh GS, Shatz CJ. (2001) Neuronal plasticity and cellular immunity: Shared molecular mechanisms. Current Opinion in Neurobiology 11(5): 568–578. [DOI] [PubMed] [Google Scholar]
- Braida D, Sacerdote P, Panerai AE, et al. (2004) Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behavioural Brain Research 153(2): 423–429. [DOI] [PubMed] [Google Scholar]
- Brennan FX, Beck KD, Servatius RJ. (2003) Low doses of interleukin-1β improve the leverpress avoidance performance of Sprague–Dawley rats. Neurobiology of Learning and Memory 80(2): 168–171. [DOI] [PubMed] [Google Scholar]
- Brennan FX, Beck KD, Servatius RJ. (2004) Proinflammatory cytokines differentially affect leverpress avoidance acquisition in rats. Behavioural Brain Research 153(2): 351–355. [DOI] [PubMed] [Google Scholar]
- Butler MP, O’Connor JJ, Moynagh PN. (2004) Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience 124(2): 319–326. [DOI] [PubMed] [Google Scholar]
- Cardenas H, Bolin LM. (2003) Compromised reactive microgliosis in MPTP-lesioned IL-6 KO mice. Brain Research 985(1): 89–97. [DOI] [PubMed] [Google Scholar]
- Carli M, Lazarova M, Tatarczynska E, et al. (1992) Stimulation of 5-HT1A receptors in the dorsal hippocampus impairs acquisition and performance of a spatial task in a water maze. Brain Research 595(1): 50–56. [DOI] [PubMed] [Google Scholar]
- Chakrabarty P, Jansen-West K, Beccard A, et al. (2010) Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: Evidence against inflammation as a driving force for amyloid deposition. FASEB Journal 24(2): 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S, Urani A, Inta I, et al. (2006) IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiology of Disease 23(3): 587–594. [DOI] [PubMed] [Google Scholar]
- Chung IY, Benveniste EN. (1990) Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. The Journal of Immunology 144(8): 2999–3007. [PubMed] [Google Scholar]
- Cohen O, Reichenberg A, Perry C, et al. (2003) Endotoxin-induced changes in human working and declarative memory associate with cleavage of plasma ‘readthrough’ acetylcholinesterase. Journal of Molecular Neuroscience 21(3): 199–212. [DOI] [PubMed] [Google Scholar]
- Conner JM, Franks KM, Titterness AK, et al. (2009) NGF is essential for hippocampal plasticity and learning. The Journal of Neuroscience 29(35): 10883–10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL. (2010) A simple role for BDNF in learning and memory? Frontiers in Molecular Neuroscience 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AJ, Murray CA, O’Neill LAJ, et al. (1996) Interleukin-1β (IL-1β) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neuroscience Letters 203(1): 17–20. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Deacon R, Wells H, et al. (2003) Synaptic changes characterize early behavioural signs in the ME7 model of murine prion disease. The European Journal of Neuroscience 17(10): 2147–2155. [DOI] [PubMed] [Google Scholar]
- Curran BP, O’Connor JJ. (2003) The inhibition of long-term potentiation in the rat dentate gyrus by pro-inflammatory cytokines is attenuated in the presence of nicotine. Neuroscience Letters 344(2): 103–106. [DOI] [PubMed] [Google Scholar]
- Dantzer R. (2001) Cytokine-induced sickness behavior: Where do we stand? Brain, Behavior, and Immunity 15(1): 7–24. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, et al. (2008) From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews. Neuroscience 9(1): 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey A, Balschun D, Wetzel W, et al. (2013) A cytokine network involving brain-borne IL-1β, IL-1ra, IL-18, IL-6, and TNFα operates during long-term potentiation and learning. Brain, Behavior, and Immunity 33: 15–23. [DOI] [PubMed] [Google Scholar]
- Depino AM, Alonso M, Ferrari C, et al. (2004) Learning modulation by endogenous hippocampal IL-1: Blockade of endogenous IL-1 facilitates memory formation. Hippocampus 14(4): 526–535. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. (2009) Cytokines and CNS development. Neuron 64(1): 61–78. [DOI] [PubMed] [Google Scholar]
- Dissing-Olesen L, LeDue JM, Rungta RL, et al. (2014) Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth. The Journal of Neuroscience 34(32): 10511–10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan JJ, Girotti M, Weinberg MS, et al. (2014) A novel role for brain interleukin-6: Facilitation of cognitive flexibility in rat orbitofrontal cortex. The Journal of Neuroscience 34(3): 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzis EJ, Tronson NC. (2014) Modulation of learning and memory by cytokines: Signaling mechanisms and long term consequences. Neurobiology of Learning and Memory 115: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economos A, Wright CB, Moon YP, et al. (2013) Interleukin 6 plasma concentration associates with cognitive decline: The northern Manhattan study. Neuroepidemiology 40(4): 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Irwin MR, Hellemann G, et al. (2012) Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. The American Journal of Geriatric Psychiatry 20(9): 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Probert L, Kollias G, et al. (1996) Neurobehavioral alterations in developing transgenic mice expressing TNF-α in the brain. Brain, Behavior, and Immunity 10(2): 126–138. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. (2002) Calcium dysregulation in the aging brain. The Neuroscientist 8(4): 297–301. [DOI] [PubMed] [Google Scholar]
- Gerber J, Böttcher T, Hahn M, et al. (2004) Increased mortality and spatial memory deficits in TNF-α-deficient mice in ceftriaxone-treated experimental pneumococcal meningitis. Neurobiology of Disease 16(1): 133–138. [DOI] [PubMed] [Google Scholar]
- Gibertini M. (1998) Cytokines and cognitive behavior. Neuroimmunomodulation 5(3–4): 160–165. [DOI] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, et al. (1995) Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-β. Brain, Behavior, and Immunity 9(2): 113–128. [DOI] [PubMed] [Google Scholar]
- Gonzalez PV, Schiöth HB, Lasaga M, et al. (2009) Memory impairment induced by IL-1β is reversed by α-MSH through central melanocortin-4 receptors. Brain, Behavior, and Immunity 23(6): 817–822. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, et al. (2008) Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Molecular Psychiatry 13(7): 717–728. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, et al. (2007) A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 32(8): 1106–1115. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. (2010) Integrated brain circuits: Astrocytic networks modulate neuronal activity and behavior. Annual Review of Physiology 72(1): 335–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T, Qin Y, Mou C, et al. (2016) Seizure induced synaptic plasticity alteration in hippocampus is mediated by IL-1β receptor through PI3K/Akt pathway. American Journal of Translational Research 8(10): 4499–4509. [PMC free article] [PubMed] [Google Scholar]
- Hanisch U-K. (2002) Microglia as a source and target of cytokines. Glia 40(2): 140–155. [DOI] [PubMed] [Google Scholar]
- Hanisch U-K, Kettenmann H. (2007) Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience 10(11): 1387–1394. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, et al. (2010) Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain, Behavior, and Immunity 24(2): 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, Samimi A, et al. (1997) Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proceedings of the National Academy of Sciences of the United States of America 94(4): 1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday J, Parson K, Curry J, et al. (1995) Cerebellar granule neurons develop elevated calcium responses when treated with interleukin-6 in culture. Brain Research 673(1): 141–148. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Hasegawa K, Kamiya H, et al. (2017) Synapse-specific effects of IL-1β on long-term potentiation in the mouse hippocampus. Biomedical Research (Tokyo, Japan) 38(3): 183–188. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Wang H, Matsumoto N, et al. (2011) Interleukin-1β causes long-term potentiation deficiency in a mouse model of septic encephalopathy. Neuroscience 187: 63–69. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Mastrangelo MA, Park KM, et al. (2008) Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. The American Journal of Pathology 173(6): 1768–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska B, Gozdz A, Zawadzka M, et al. (2009) MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. The Anatomical Record 292(12): 1902–1913. [DOI] [PubMed] [Google Scholar]
- Kennedy RH, Silver R. (2016) Neuroimmune signaling: Cytokines and the central nervous system BT. In: Pfaff DW, Volkow ND. (eds) Neuroscience in the 21st Century: From Basic to Clinical. New York: Springer, pp. 601–641. [Google Scholar]
- Kent S, Bluthé R-M, Kelley KW, et al. (1992) Sickness behavior as a new target for drug development. Trends in Pharmacological Sciences 13(1): 24–28. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Cheng D, Tsukamoto MR, et al. (2011) Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer’s disease model. Journal of Immunology 187(12): 6539–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MA, Möller JC, Jones LL, et al. (1997) Impaired neuroglial activation in interleukin-6 deficient mice. Glia 19(3): 227–233. [DOI] [PubMed] [Google Scholar]
- Konorski J. (1948) Conditioned Reflexes and Neuron Organization. Conditioned Reflexes and Neuron Organization. New York: Cambridge University Press. [Google Scholar]
- Konsman JP, Drukarch B, Van Dam A-M. (2006) (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clinical Science 112(1): 1–25. [DOI] [PubMed] [Google Scholar]
- Kook S-Y, Jeong H, Kang MJ, et al. (2014) Crucial role of calbindin-D28k in the pathogenesis of Alzheimer’s disease mouse model. Cell Death and Differentiation 21(10): 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak W, Poli V, Soszynski D, et al. (1997) Sickness behavior in mice deficient in interleukin-6 during turpentine abscess and influenza pneumonitis. American Journal of Physiology 272(2): R621–R630. [DOI] [PubMed] [Google Scholar]
- Krueger JM. (2008) The role of cytokines in sleep regulation. Current Pharmaceutical Design 14(32): 3408–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse VF, Costes L, Aubert A, et al. (2009) Impaired interleukin-1β and c-Fos expression in the hippocampus is associated with a spatial memory deficit in P2X7 receptor-deficient mice. PLoS One 4(6): e6006pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson SJ, Dunn AJ. (2001) Behavioral effects of cytokines. Brain, Behavior, and Immunity 15(4): 371–387. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Pribiag H, Duseja R, et al. (2014) An adaptive role of TNFα in the regulation of striatal synapses. The Journal of Neuroscience 34(18): 6146–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Yang L, Lindholm K, et al. (2004) Tumor necrosis factor death receptor signaling cascade is required for amyloid-beta protein-induced neuron death. The Journal of Neuroscience 24(7): 1760–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Du X, Liu C, et al. (2012) Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Developmental Cell 23(6): 1189–1202. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu L, Kang J, et al. (2000) Neuronal-glial interactions mediated by interleukin-1 enhance neuronal acetylcholinesterase activity and mRNA expression. The Journal of Neuroscience 20(1): 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhou L-J, Wang J, et al. (2017) TNF-α differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia-dependent mechanisms after peripheral nerve injury. The Journal of Neuroscience 37(4): 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TC, Zhu XZ. (2000) Effects of intrahippocampal infusion of interleukin-6 on passive avoidance and nitrite and prostaglandin levels in the hippocampus in rats. Arzneimittel-Forschung 50(3): 227–231. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. (1998) Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review 105(1): 83–107. [DOI] [PubMed] [Google Scholar]
- Mao CP, Bai ZL, Zhang XN, et al. (2016) Abnormal subcortical brain morphology in patients with knee osteoarthritis: A cross-sectional study. Frontiers in Aging Neuroscience 8: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronardi C, Whelan F, Yildiz OA, et al. (2007) Caspase 1 deficiency reduces inflammation-induced brain transcription. Proceedings of the National Academy of Sciences 104(17): 7205–7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Watanabe S, Suh Y-H, et al. (2002) Effects of intrahippocampal CT105, a carboxyl terminal fragment of β-amyloid precursor protein, alone/with inflammatory cytokines on working memory in rats. Journal of Neurochemistry 82(2): 234–239. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Yamaguchi T, Watanabe S, et al. (2004) Involvement of arachidonic acid cascade in working memory impairment induced by interleukin-1 beta. Neuropharmacology 46(8): 1195–1200. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Yoshida M, Watanabe S, et al. (2001) Involvement of cholinergic and glutamatergic functions in working memory impairment induced by interleukin-1β in rats. European Journal of Pharmacology 430(2): 283–288. [DOI] [PubMed] [Google Scholar]
- McAlpine FE, Lee J-K, Harms AS, et al. (2009) Inhibition of soluble TNF signaling in a mouse model of Alzheimer’s disease prevents pre-plaque amyloid-associated neuropathology. Neurobiology of Disease 34(1): 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda L, Baron P, Prat E, et al. (1999) Proinflammatory profile of cytokine production by human monocytes and murine microglia stimulated with beta-amyloid. Journal of Neuroimmunology 93(1–2): 45–52. [DOI] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, et al. (2013) Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depression and Anxiety 30(4): 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. (2009) Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry 65(9): 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk ML, de Kloet ER. (2019) Coping with the forced swim stressor: Current state-of-the-art. Behavioural Brain Research 364: 1–10. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Ortiz S, Molina-Holgado F, et al. (2000) Induction of COX-2 and PGE(2) biosynthesis by IL-1beta is mediated by PKC and mitogen-activated protein kinases in murine astrocytes. British Journal of Pharmacology 131(1): 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AH, Wu M, Shaftel SS, et al. (2009) Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience 164(4): 1484–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. (1998) Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 18(8): 2974–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutso AA, Radzicki D, Baliki MN, et al. (2012) Abnormalities in hippocampal functioning with persistent pain. The Journal of Neuroscience 32(17): 5747–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhrer T. (2003) Neurotransmitter systems involved in learning and memory in the rat: A meta-analysis based on studies of four behavioral tasks. Brain Research Reviews 41(2): 268–287. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, et al. (1998) Exposure to acute stress induces brain interleukin-1beta protein in the rat. The Journal of Neuroscience 18(6): 2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. (2005) Synaptic plasticity at hippocampal mossy fibre synapses. Nature Reviews Neuroscience 6(11): 863–876. [DOI] [PubMed] [Google Scholar]
- Ohno M, Yamamoto T, Watanabe S. (1992) Effects of intrahippocampal injections of N-methyl-D-aspartate receptor antagonists and scopolamine on working and reference memory assessed in rats by a three-panel runway task. Journal of Pharmacology and Experimental Therapeutics 263(3): 943–950. [PubMed] [Google Scholar]
- Palop JJ, Jones B, Kekonius L, et al. (2003) Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proceedings of the National Academy of Sciences 100(16): 9572–9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palotás A, Kálmán J, Palotás M, et al. (2002) Fibroblasts and lymphocytes from Alzheimer patients are resistant to β-amyloid-induced increase in the intracellular calcium concentration. Progress in Neuro-Psychopharmacology and Biological Psychiatry 26(5): 971–974. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Molinero A, Carrasco J, et al. (2001) Interleukin-6 deficiency reduces the brain inflammatory response and increases oxidative stress and neurodegeneration after kainic acid-induced seizures. Neuroscience 102(4): 805–818. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. (2003) The impact of systemic infection on the progression of neurodegenerative disease. Nature Reviews Neuroscience 4(2): 103–112. [DOI] [PubMed] [Google Scholar]
- Pettigrew LC, Kindy MS, Scheff S, et al. (2008) Focal cerebral ischemia in the TNFalpha-transgenic rat. Journal of Neuroinflammation 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew LC, Kryscio RJ, Norris CM. (2016) The TNFα-transgenic rat: Hippocampal synaptic integrity, cognition, function, and post-ischemic cell loss. PLoS One 11(5): e0154721–e0154721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering M, O’Connor JJ. (2007) Pro-inflammatory cytokines and their effects in the dentate gyrus. In: Scharfman H. (ed.) The Dentate Gyrus: A Comprehensive Guide to Structure, Function, and Clinical Implications. Amsterdam, The Netherlands: Elsevier, pp. 339–354. [DOI] [PubMed] [Google Scholar]
- Pott Godoy MC, Tarelli R, Ferrari CC, et al. (2008) Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain 131(pt 7): 1880–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto GA, Snigdha S, Baglietto-Vargas D, et al. (2015) Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1β in the aged hippocampus . Proceedings of the National Academy of Sciences 112(36): E5078–E5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, et al. (1998) Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain, Behavior, and Immunity 12(3): 212–229. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Nguyen KT, Gonyea JL, et al. (1999) Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behavioural Brain Research 106(1): 109–118. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Parsons KL, Gruol DL. (1995) Interleukin-6 selectively enhances the intracellular calcium response to NMDA in developing CNS neurons. The Journal of Neuroscience 15(10): 6688–6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. (2006) Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology 27(1): 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Kivisäkk P, Kidd G. (2003) Three or more routes for leukocyte migration into the central nervous system. Nature Reviews Immunology 3(7): 569–581. [DOI] [PubMed] [Google Scholar]
- Ren W-J, Liu Y, Zhou L-J, et al. (2011) Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology 36(5): 979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro DE, Roncalho AL, Glaser T, et al. (2019) P2X7 receptor signaling in stress and depression. International Journal of Molecular Sciences 20(11): 2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Khom S, Bajo M, et al. (2019) Increased IL-6 expression in astrocytes is associated with emotionality, alterations in central amygdala GABAergic transmission, and excitability during alcohol withdrawal. Brain, Behavior, and Immunity 82: 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo L, Fernández J, Maccioni A, et al. (2008) Neuroinflammation: Implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Archives of Medical Research 39(1): 1–16. [DOI] [PubMed] [Google Scholar]
- Rowan MJ, Klyubin I, Wang Q, et al. (2007) Synaptic memory mechanisms: Alzheimer’s disease amyloid beta-peptide-induced dysfunction. Biochemical Society Transactions 35(pt 5): 1219–1223. [DOI] [PubMed] [Google Scholar]
- Santello M, Bezzi P, Volterra A. (2011) TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69(5): 988–1001. [DOI] [PubMed] [Google Scholar]
- Sattler R, Rothstein JD. (2006) Regulation and dysregulation of glutamate transporters BT – Neurotransmitter transporters. In: Sitte HH, Freissmuth M. (eds) Handbook of Experimental Pharmacology. Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 277–303. [DOI] [PubMed] [Google Scholar]
- Savarin C, Hinton DR, Valentin-Torres A, et al. (2015) Astrocyte response to IFN-γ limits IL-6-mediated microglia activation and progressive autoimmune encephalomyelitis. Journal of Neuroinflammation 12(1): 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A, Waagepetersen HS. (2005) Role of astrocytes in glutamate homeostasis: Implications for excitotoxicity. Neurotoxicity Research 8(3): 221–225. [DOI] [PubMed] [Google Scholar]
- Shin J-W, Cheong Y-J, Koo Y-M, et al. (2014) α-asarone ameliorates memory deficit in lipopolysaccharide-treated mice via suppression of pro-inflammatory cytokines and microglial activation. Biomolecules & Therapeutics 22(1): 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Jones OD, Mockett BG, et al. (2019) Tumor necrosis factor-α-mediated metaplastic inhibition of LTP is constitutively engaged in an Alzheimer’s disease model. The Journal of Neuroscience 39(46): 9083–9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirviö J, Riekkinen P, Ekonsalo T, et al. (1992) The effects of dexmedetomidine, an alpha2 agonist, on learning and memory, assessed using passive avoidance and water maze tasks in rats. Neuropharmacology 31(2): 163–168. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. (2013) Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. The Neuroscientist 20(2): 160–172. [DOI] [PubMed] [Google Scholar]
- Song C, Merali Z, Anisman H. (1999) Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience 88(3): 823–836. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JRR, et al. (2006) Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. The Journal of Neuroscience 26(42): 10709–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. (2006) Synaptic scaling mediated by glial TNF-α. Nature 440(7087): 1054–1059. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, et al. (2005) Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. The Journal of Neuroscience 25(12): 3219–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stück ED, Christensen RN, Huie JR, et al. (2012) Tumor necrosis factor alpha mediates GABA(A) receptor trafficking to the plasma membrane of spinal cord neurons in vivo. Neural Plasticity 2012: 261345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taepavarapruk P, Song C. (2010) Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1β administrations: Effects of omega-3 fatty acid EPA treatment. Journal of Neurochemistry 112(4): 1054–1064. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Fumizawa K, Yamagata K, et al. (2017) Brain interleukin-1 facilitates learning of a water maze spatial memory task in young mice. Frontiers in Behavioral Neuroscience 11: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancredi V, D’Antuono M, Cafè C, et al. (2000) The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. Journal of Neurochemistry 75(2): 634–643. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D’Arcangelo G, Grassi F, et al. (1992) Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neuroscience Letters 146(2): 176–178. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Blennow K, Wallin A, et al. (1999) Intracerebral production of tumor necrosis factor-alpha, a local neuroprotective agent, in Alzheimer disease and vascular dementia. Journal of Clinical Immunology 19(4): 223–230. [DOI] [PubMed] [Google Scholar]
- Tha KK, Okuma Y, Miyazaki H, et al. (2000) Changes in expressions of proinflammatory cytokines IL-1β, TNF-α and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Research 885(1): 25–31. [DOI] [PubMed] [Google Scholar]
- Tobinick E. (2009) Tumour necrosis factor modulation for treatment of Alzheimer’s disease. CNS Drugs 23(9): 713–725. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Postolache TT. (2005) Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurological Research 27(7): 679–684. [DOI] [PubMed] [Google Scholar]
- Tong L, Prieto GA, Kramár EA, et al. (2012) Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1β via p38 mitogen-activated protein kinase. The Journal of Neuroscience 32(49): 17714–17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. (2008) The self-tuning neuron: Synaptic scaling of excitatory synapses. Cell 135(3): 422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukai M, Lin HP. (2002) Involvement of μ₁-opioid receptors and cholinergic neurotransmission in the endomorphins-induced impairment of passive avoidance learning in mice. Behavioural Brain Research 129(1–2): 197–201. [DOI] [PubMed] [Google Scholar]
- Ukai M, Watanabe Y, Kameyama T. (2000) Effects of endomorphins-1 and -2, endogenous μ-opioid receptor agonists, on spontaneous alternation performance in mice. European Journal of Pharmacology 395(3): 211–215. [DOI] [PubMed] [Google Scholar]
- Vallières L, Rivest S. (1999) Interleukin-6 is a needed proinflammatory cytokine in the prolonged neural activity and transcriptional activation of corticotropin-releasing factor during endotoxemia. Endocrinology 140(9): 3890–3903. [DOI] [PubMed] [Google Scholar]
- Verma S, Nakaoke R, Dohgu S, et al. (2006) Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain, Behavior, and Immunity 20(5): 449–455. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, et al. (2003) Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. The Journal of Neuroscience 23(25): 8692–8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H, Siegmund D. (2019) TNFR1 and TNFR2 in the control of the life and death balance of macrophages. Frontiers in Cell and Developmental Biology 7: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall AM, Mukandala G, Greig NH, et al. (2015) Tumor necrosis factor-α potentiates long-term potentiation in the rat dentate gyrus after acute hypoxia. Journal of Neuroscience Research 93(5): 815–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wu J, Rowan MJ, et al. (2005) Beta-amyloid inhibition of long-term potentiation is mediated via tumor necrosis factor. The European Journal of Neuroscience 22(11): 2827–2832. [DOI] [PubMed] [Google Scholar]
- Weaver JD, Huang M-H, Albert M, et al. (2002) Interleukin-6 and risk of cognitive decline. Neurology 59(3): 371–378. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Dunnett SB. (1985). Dopamine depletion, stimulation or blockade in the rat disrupts spatial navigation and locomotion dependent upon beacon or distal cues. Behavioural Brain Research 18(1): 11–29. [DOI] [PubMed] [Google Scholar]
- Wu T-H, Lin C-H. (2008) IL-6 mediated alterations on immobile behavior of rats in the forced swim test via ERK1/2 activation in specific brain regions. Behavioural Brain Research 193(2): 183–191. [DOI] [PubMed] [Google Scholar]
- Yarlagadda A, Alfson E, Clayton AH. (2009) The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry 6(11): 18–22. [PMC free article] [PubMed] [Google Scholar]
- Ye S-M, Johnson RW. (1999) Increased interleukin-6 expression by microglia from brain of aged mice. Journal of Neuroimmunology 93(1): 139–148. [DOI] [PubMed] [Google Scholar]
- Yin Z, Raj D, Saiepour N, et al. (2017) Immune hyperreactivity of Aβ plaque-associated microglia in Alzheimer’s disease. Neurobiology of Aging 55: 115–122. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Winocur G, Goshen I. (2002) Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiology of Learning and Memory 78(2): 379–389. [DOI] [PubMed] [Google Scholar]
- Zalcman S, Green-Johnson JM, Murray L, et al. (1994) Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain Research 643(1–2): 40–49. [DOI] [PubMed] [Google Scholar]
- Zou JY, Crews FT. (2005) TNFα potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: Neuroprotection by NFκB inhibition. Brain Research 1034(1): 11–24. [DOI] [PubMed] [Google Scholar]