Abstract
Background:
This study was designed to examine effects of telmisartan; an angiotensin receptor blocker; self-nanoemulsifying drug delivery system (SNEDDS) in reversing already-established hepatic fibrosis.
Method:
Forty rats were given thioacetamide (200 mg/kg, intraperitoneally) twice/week for 8 weeks then divided into 5 groups (n = 8), PC and 4 treated groups. Treatments were given orally for another 2 months as follows: telmisartan low and high doses (TL and TH: 1.8 and 3.6 mg/kg/day) and telmisartan SNEDDS at the same doses (TLS and THS). At end of treatment, blood was obtained and liver was isolated.
Results:
Rats showed significant elevations of plasma ALT and AST and hepatic IL-6, TNF-α, and MDA, significant reductions of plasma albumin, hepatic GSH, and body weight, and hepatic histopathological damage. All treatments except for TL significantly reversed these thioacetamide-induced changes. THS group showed significant differences from all groups. Regarding ratio of free telmisartan concentration in hepatic homogenate to that of plasma, TH and TLS groups showed non-significant variation between each other while THS group showed significant differences from them. No significant changes were detected in blood pressure, hemoglobin, white blood cells, and platelets.
Conclusion:
Telmisartan SNEDDS, compared with telmisartan, more effectively reversed chronic hepatic fibrosis with good safety profile.
Keywords: telmisartan, liver fibrosis, thioacetamide
Introduction
Chronic hepatic fibrosis is a condition with severe disturbance of hepatic metabolism and functions with no standard therapy till date. Stimulation of the renin angiotensin system (RAS) in liver is related to fibrosis and the angiotensin II (AT II) receptors are expressed in the activated hepatic stellate cells (HSCs) during progression of fibrosis. RAS inhibitor therapy causes a decrease of liver fibrosis in the patients with good safety profile.1 Hence, it is a more reasonable to test “therapeutic” effects of potential anti-fibrotic agents in a model of “established fibrosis.”2 Use of nanoformulation for antifibrotic agents help achieving high hepatic concentrations, targeting receptors expressed by the active HSCs, getting maximum efficacies in treatment of liver fibrosis, and also minimizing associated systemic side effects compared to the standard drugs.3 Telmisartan; a small long-acting non-peptide angiotensin II type-1 receptor (AT1 R) blocker (ARB); is an arterial blood pressure lowering agent with anti-inflammatory effects.4 It shows the most satisfactory effects on fasting blood glucose and lipid profile compared with olmesartan and losartan.5 Its affinity for the AT1 receptor is about 3,000 times much more than that for the AT2 receptor. Telmisartan shows different degrees of insurmountable AT1 receptor antagonism because it forms a loose complex followed by a tight binding complex.6 The members of the ARBs have different degrees of selectivity to the angiotensin receptors, but to detect whether this is clinically useful, large clinical trials are needed, thus it seems safest to take these differences into consideration.7 In a clinical trial, the PK parameters of a single daily administration of oral and IV telmisartan in healthy volunteers and in hypertensive patients were measured. The steady state was observed after 5-7 days and there was no accumulation at 28 days. The prolonged elimination half-life of telmisartan allows its administration once per day.8
Taken together, the present work was designed to examine the hypothesis that preparation of telmisartan in a nanostructure form will improve its delivery and in vivo performance in treating and reversing hepatic fibrosis in thioacetamide-treated rats more effectively than telmisartan in its regular dosage form. This study could help use of telmisatan nanoformulation as a “therapeutic” agent in hepatic fibrosis.
Materials and Methods
In Vitro Study
Formulation of telmisartan SNEDDS
Oleic acid (oil) 10% w/w, Tween 80 and span 85 (1:1 weight ratio) 50% w/w (surfactant), and labrasol 40% w/w (co-surfactant) were utilized as SNEDDS formula components and were prepared as previously described [23]. Briefly, the SNEDDS was prepared by mixing formula. Telmisartan was utilized at a concentration of 10 mg/g SNEDDS formula.
Telmisartan SNEDSS globule size analysis
The prepared telmisartan SNEDDS was assayed for globule size using particle size analyzer (Zetasizer Nano ZSP, Malvern Panalytical Ltd, Malvern, UK). The formulation was diluted with deionized water. The average of globule size was expressed using 3 replicate samples.
Telmisartan SNEDSS transmission electron microscope examination
Telmisartan SNEDDS sample was investigated by transmission electron microscope (TEM) JEOL-JEM-1011 (JEOL-Tokyo, Japan) by staining the sample, loaded on a copper grid, using phosphotungstic acid. After that, excess acid was removed, and sample was dried then investigated by TEM.
In Vivo Study
Induction of hepatic fibrosis and treatment groups
This work was approved by the KAU Research Ethics Committee (Reference number 561-19) and obeyed the international guidelines for the Care and Use of Laboratory Animals. Male adult Sprague–Dawley rats (200-250 g) were acquired from King Fahd Medical Research Center and housed in cages at 20-22°C room temperature in a 12 h light-dark cycle. After 1 week of acclimatization, rats were used in the experiment. Food and water were obtainable ad libitum. All drugs and chemicals were purchased from Sigma Aldrich (St. Louis, US) unless stated otherwise. Telmisartan self-nanoemulsifying drug delivery system (SNEDDS) were prepared as previously described with some modifications.9 Telmisartan standard drug (Sigma-Aldrich, MO, USA) was suspended in distilled water with 0.5% w/v carboxymethyl cellulose.10 Besides the normal control (NC) group receiving the vehicle, 5 groups of rats (n = 8) were given freshly prepared thioacetamide (TAA) (200 mg/kg, intraperitoneally) twice weekly for 8 weeks.11-13 The 5 TAA groups included a positive control group (PC, untreated) and 4 treated groups orally for another 2 months as follows: the first and second groups received telmisartan standard drug low & high doses (TL & TH: 1.8 and 3.6 mg/kg/day) while the third and fourth groups received telmisartan SNEDDS at the same low and high doses (TLS & THS).9,14,15 Then blood samples were obtained and then rats were sacrificed. The liver was isolated 2 h after final administration of telmisartan, a part is used for histopathological study while another part was homogenized. Also concentrations of the telmisartan in plasma and liver homogenate was measured by HPLC/MS.
Measurement of plasma ALT and AST
Blood samples were obtained and plasma was separated and kept at −80°C till assay of ALT and AST by using ELISA commercially available kits (MyBioSource, Inc. CA, USA, Catalog numbers: MBS269614 and MBS264975) following the manufacturer’s protocol.
Measurement of IL-6, TNF-α, MDA, and GSH in liver homogenate
A part of the liver was rapidly homogenized using phosphate-buffered saline (0.01 M PBS, pH 7.4) using beads for sample disruption with the TissueLyser II (Qiagen) at 4°C to produce a 10% homogenate which was centrifuged at 10000 rpm for 15 min at 4°C and the supernatant and kept at −80°C till time of analysis.14 The homogenate levels of TNF-α, IL-6, malondialdehyde (MDA), and reduced glutathione (GSH) were assayed by the ELISA kits (MyBioSource, Inc. CA, USA, Catalog numbers: MBS355371, MBS726707, MBS738685, and MBS265966 respectively). The protein content in the liver homogenate was determined by using a colorimetric kit (Catalog number: MBS355526) based on Bradford method16 and the levels of IL-6, TNF-α, GSH, and MDA were expressed/mg protein.
Histopathological examination
Samples of the hepatic tissue were fixed in 10% phosphate-buffered formalin followed by its embedding in paraffin. Sections (3-5 μm) were cut, stained with H&E, examined for estimation of fibrosis, inflammatory cell infiltration, and hepatocytes degeneration, and the injury was reported as mild, moderate, or severe.17
Measurement of free telmisartan concentration in plasma and hepatic homogenate
Triple quad mass spectrometer (Agilent 6460, Agilent Technologies, USA) coupled with DAD and qQq-MS detector has been utilized in the study. The MassHunter software (version B.03.01, Build 3.1.346.0) controlled the system. Gas temperature, 330°C with gas flow rate, 11 L/min; nebulizer pressure; 35 psi, and capillary voltage, 4300 V. The mode of ionization used was positive mode. Agilent Eclipse Plus C18, 3.5 μm, 4.6 x 100 mm column with a pore size of 100 Angstrom was used for chromatographic separation and was maintained at 25 ± 2°C. The mobile phase was acetonitrile: water having 0.05% w/v formic acid, 70: 30 v/v, and pumped at isocratic flow, 0.5 mL/min. (Table 1). Valsartan was used as internal standard. The sample extraction and analysis were done by applying the procedure previously published for telmisartan analysis in rat plasma18 with certain modifications. Briefly, 200 µl of plasma or homogenate was blended with 100 µl internal standard solution (valsartan, 100 ng/μl) and 700 µl acetonitrile. The mixture was centrifuged, and the supernatant (200 μl) was transferred to a total recovery autosampler vial. After that, a 5 µl sample was injected for LC-MS/MS-DAD analysis. The calibration curve for telmisartan was made. The stock solution of telmisartan and internal standard (1 mg/ml) were prepared in acetonitrile. A series of calibrator working solutions of telmisartan were prepared using acetonitrile as diluting agent. The calibration solutions were prepared with telmisartan solutions to give a concentration from 1.0 to 8000.0 ng/mL and a fixed concentration of internal standard (10 µg/ml). The peak area ratios of telmisartan to valsartan (internal standard) were found linear in range from 1.0 to 8000 ng/ml of telmisartan.
Table 1.
MRM transition of Telmisartan and the Internal Standard.
| Compound Name | Precursor Ion, m/z | Product Ion, m/z | Dwell | Fragmentor, v | Collision Energy, v |
|---|---|---|---|---|---|
| telmisartan | 515.1 | 497.1 | 200 | 150 | 40 |
| Valsartan-internal standard | 436.2 | 306.1 | 200 | 150 | 19 |
Assessment of safety endpoints
The safety endpoints of the new formulation were evaluated at the end of treatment by measuring the body weight, hepatic weight, and hepatic/body weight ratio. Also the plasma albumin was measured using a commercial kit (MyBioSource, Inc. CA, USA, Catalog numbers MBS2540439). Moreover, the systolic blood pressure was measured using the non-invasive indirect rat tail blood pressure system of conscious rats (Harvard Apparatus. Com., USA) as previously described.19 In addition, EDTA blood samples were obtained to measure hemoglobin level, white blood cells count, and platelets count to detect any potential affection.
Statistical Analysis
All the values are presented as means ± SEM. Comparisons between different groups were made by using 1-way analysis of variance (ANOVA) followed by Bonferroni test for multiple comparisons. The SPSS software, version 22 (US) was used to conduct these statistical tests. The difference was considered significant when P < 0.05.
Results
Formulation and Characterization of Telmisartan SNEDDS
Telmisartan SNEDDS formula showed almost clear solution when mixed with water that indicate immediate distribution of SNEDDS globules in the nano-size range within the aqueous dispersion medium. Globule size analysis showed average globule size of the prepared SNEDDS formula of 92.2 ± 13.4 nm as measured by the particle size analyzer. Representative TEM micrograph of telmisartan SNEDDS is shown in Figure 1. Examination of the SNEDDS formulation using TEM showed spherical shape vesicles with comparable average sized vesicles of 97.4 ± 33.2 nm relative to the vesicle size measured by the particle size analyzer.
Figure 1.
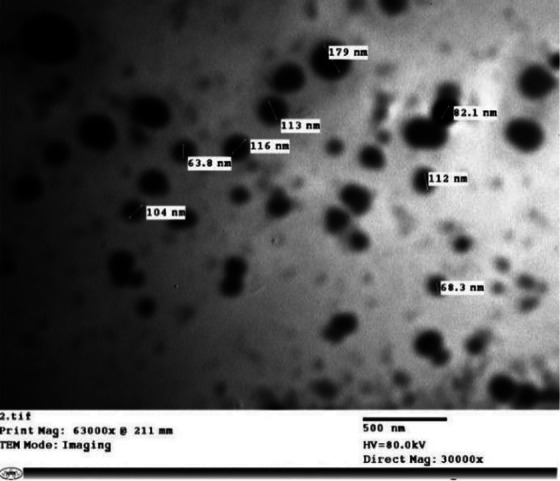
Transmission electron microscope (TEM) of Telmisartan SNEDDS formula (X 30,000 magnification).
Plasma levels of ALT and AST
The hepatic fibrotic rats demonstrated significant elevations of the plasma levels of ALT and AST. All treatments except TL significantly reversed these changes. The TH and TLS groups showed non-significant variation between each other while the THS group showed significant differences from all groups (Figure 2).
Figure 2.

Effects of telmisartan low and high doses as standard drug (TL, TH) and as SNEDDS formula (TLS, THS) on plasma levels of ALT and AST in TAA-induced hepatic fibrosis rats (n = 8). Data are expressed as mean ± SEM. *: P < 0.05 vs. Normal control (NC), #: P < 0.05 vs. Positive control (PC) and TL, ^: P < 0.05 vs. TH & TLS.
Inflammatory and oxidative markers
The TAA-induced fibrotic rats displayed significant elevations of the levels of IL-6, TNF-α, and MDA and a significant decrease of GSH level in the liver homogenate. All treatments except for TL significantly reversed these TAA-induced changes. The TH and TLS groups showed non-significant variation between each other while the THS group showed significant differences from all groups (Table 2).
Table 2.
Effects of Telmisartan Low and High Doses as Standard Drug (TL, TH) and as SNEDDS Formula (TLS, THS) on Levels of IL-6, THF-α, GSH, and MDA in Liver Homogenate in TAA-Induced Hepatic Fibrosis Rats (n = 8).
| NC | PC | TL | TH | TLS | THS | |
|---|---|---|---|---|---|---|
| TNF-α (pg/mg protein) | 112.71 ± 2.67 | 321.05 ± 10.66 | 306.30 ± 9.80 * | 234.23 ± 10.44 *,# | 216.00 ± 10.52 *,# | 156.82 ± 11.12*,#,^ |
| IL-6 (pg/mg protein) | 50.49 ± 3.52 | 216.50 ± 7.66 | 194.51 ± 11.27 * | 143.83 ± 8.67 *,# | 135.32 ± 11.66 *,# | 89.16 ± 3.26 *,#,^ |
| GSH (ng/mg protein) | 146.38 ± 6.75 | 57.90 ± 3.17 | 63.38 ± 3.42 * | 87.22 ± 4.04 *,# | 92.18 ± 4.67 *,# | 113.47 ± 4.18 *,#,^ |
| MDA (nmol/mg protein) | 4.03 ± 0.12 | 35.55 ± 2.01 | 34.72 ± 2.13 * | 24.14 ± 2.54 *,# | 26.35 ± 1.60 *,# | 15.12 ± 1.49 *,#,^ |
Data are expressed as mean ± SEM. *: P < 0.05 vs. Normal control (NC), #: P < 0.05 vs. Positive control (PC) and TL, ^: P < 0.05 vs. TH & TLS.
Histopathological examination
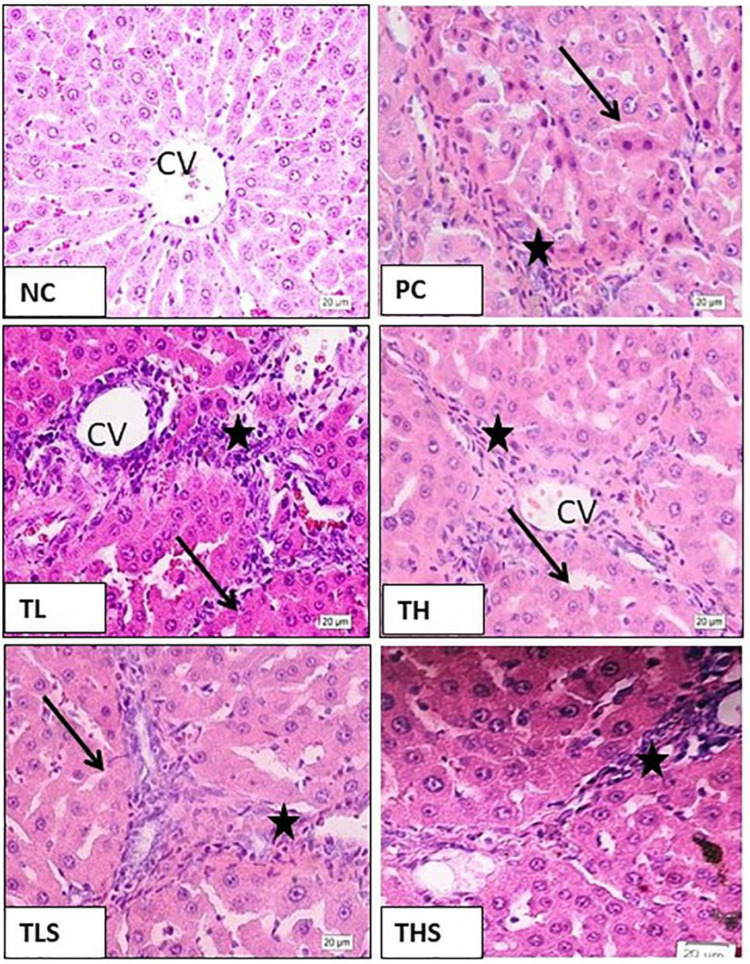
The NC rats showed normal hepatic architecture with normal appearance of hepatocytes. The TAA-induced fibrotic rats showed definitive hepatic lobulation due to proliferation of fibroblasts, marked inflammatory infiltration, and degeneration of hepatocytes. All treatments except for TL reversed these TAA-induced changes with varying degrees. The TH and TLS groups showed mild improvement while the THS group showed moderate protection but still different from normal rats (Figure 3).
Figure 3.
Microphotographs of liver sections of rats of: (NC) normal control group showing normal hepatic structure and hepatocytes around the central vein (CV). (PC) thioacetamide group showing definitive hepatic lobulation (arrows), marked inflammatory infiltration (stars), and degenerative hepatocytes. (TL) telmisartan low dose group showing no improvement. (TH) telmisartan high dose group and (TLS) telmisartan low dose-SNEDDS showing mild improvement with moderate hepatic lobulation (arrows), inflammatory infiltrates (stars), and hepatocyte degeneration. (THS) telmisartan high-SNEDDS group showing moderate protection with mild inflammatory infiltrate (stars) and degenerative appearance of the hepatocytes (H&E, High power (X20)).
Free Telmisartan Measurement in Plasma and Hepatic Homogenate
Method validation
The Agilent QQQ-MS is characterized by a wide linear dynamic range. Moreover, the calibration data are enough linear over the studied range. The calibration curve for telmisartan was assessed using free-drug-plasma and free drug homogenate as a calibration matrix. The stock solution of telmisartan and valsartan (internal standard) were prepared separately by dissolving 10 mg of each in acetonitrile to obtain a concentration of 1 mg/ml. A series of calibrator working solutions of telmisartan were prepared from its stock solutions applying serial dilution technique using acetonitrile as a diluting solvent The calibration solutions were prepared by spiking separately the plasma free drug and free drug homogenate with telmisartan solutions to give a concentration spanning the range of 1.0 to 8000.0 ng/ml of telmisartan and a fixed concentration of internal standard 10 µg/ml. The calibrated solutions were extracted, and analyzed by the developed method. The peak area ratios of telmisartan to internal standard were found linear in the concentration range 1.0 to 8000 ng/ml of telmisartan. The control matrix samples were spiked with 4 nominated concentrations including (LLOQ) and the calibration curve was constructed according to the experimental procedure as described before. The recovery was studied by matching the results obtained from the injected standard solutions versus the same concentrations after spiking in the corresponding matrix. Related to validation of the developed method, in regarding to recovery from matrix and matrix effect, 3 concentrations ranges including 1,50,8000 ng/ml were spiked into control matrix and analyzed, the recoveries were NLT 95.20 ± 2.12 (n = 6) for the 3 levels determined. These results deemed acceptable selectivity with minimal matrix effect due to co-extracted matrix biogenic materials. The RSD values of the extracted analytes from matrix were calculated to estimator intraday and inter-day precision, the results obtained for both types of precisions expressed as RSD value were NMT 2.2%. The obtained data indicate that the analytical method used is precise, selective and accurate because all validation parameters are within the allowed limits.
Free Telmisartan Concentration in Plasma and Hepatic Homogenate
Representative MRM transition chromatograms of telmisartan in plasma and hepatic homogenate are shown in Figure 4. Regarding the ratio of the free telmisartan concentration in hepatic homogenate to that of plasma (H/P ratio), the TH and TLS groups showed non-significant variation between each other while the THS group showed significant differences from them (Figure 5).
Figure 4.
Representative MRM transition chromatograms of telmisartan in: A. Plasma and B. Liver homogenate.
Figure 5.

The ratio of the free telmisartan concentration in hepatic homogenate to that of plasma (H/P ratio) with telmisartan low and high doses as raw drug (TL, TH) and as SNEDDS formula (TLS, THS) in TAA-induced hepatic fibrosis rats (n = 8). Data are expressed as mean ± SEM. *: P < 0.05 vs. TL, #: P < 0.05 vs. TH, TLS.
Safety Endpoints
The hepatic fibrotic rats showed significant reductions in body weight and significant increases in hepatic weight and hepatic/body weight ratio. Also they showed a significant reduction in plasma albumin level. All treatments except the TL significantly reversed these changes. The TH and TLS groups showed non-significant variations between each other while the THS group showed significant differences from all groups. In addition, no significant changes were detected in the systolic blood pressure level, hemoglobin level, white blood cells count, and platelets count among the groups (Table 3).
Table 3.
Effects of Telmisartan Low and High Doses as Standard Drug (TL, TH) and as SNEDDS Formula (TLS, THS) on the Safety Endpoints Including Body Weight, Hepatic Weight, and Hepatic/Body Weight Ratio, Plasma Albumin Level, Systolic Blood Pressure Level, Hemoglobin Level, White Blood Cells Count, and Platelets Count in TAA-Induced Hepatic Fibrosis Rats (n = 8).
| NC | PC | TL | TH | TLS | THS | |
|---|---|---|---|---|---|---|
| Body weight (g) | 239.80 ± 5.25 | 159.25 ± 1.58 | 162.50 ± 2.30 * | 181.25 ± 2.73 *,# | 180.43 ± 6.38 *,# | 203.25 ± 1.93 *,#,^ |
| Hepatic weight (g) | 4.86 ± 0.10 | 9.93 ± 0.52 | 9.89 ± 0.50 * | 8.24 ± 0.26 *,# | 8.19 ± 0.28 *,# | 6.47 ± 0.17 *,#,^ |
| Hepatic /Body weight ratio | 2.04 ± 0.07 | 6.22 ± 0.29 | 6.13 ± 0.33 * | 4.55 ± 0.16 *,# | 4.59 ± 0.27 *,# | 3.18 ± 0.09 *,#,^ |
| Plasma albumin (g%) | 4.98 ± 0.08 | 1.92 ± 0.14 | 1.74 ± 0.12 * | 2.82 ± 0.16 *,# | 2.80 ± 0.19 *,# | 3.94 ± 0.25 *,#,^ |
| SBP (mmHg) | 114.63 ± 1.93 | 116.25 ± 2.43 | 120.75 ± 3.11 | 118.38 ± 3.17 | 122.75 ± 1.25 | 115.38 ± 2.63 |
| Hemoglobin (g%) | 14.09 ± 0.29 | 13.47 ± 0.50 | 13.24 ± 0.42 | 14.76 ± 0.44 | 13.22 ± 0.38 | 13.65 ± 0.52 |
| WBCs (×103/cmm) | 8.24 ± 0.26 | 7.95 ± 0.23 | 8.19 ± 0.28 | 7.64 ± 0.25 | 7.56 ± 0.09 | 8.19 ± 0.28 |
| Platelets (×105/cmm) | 5.04 ± 0.20 | 5.83 ± 0.36 | 6.05 ± 0.37 | 5.65 ± 0.17 | 6.10 ± 0.30 | 5.60 ± 0.31 |
Data are expressed as mean ± SEM. *: P < 0.05 vs. Normal control (NC), #: P < 0.05 vs. Positive control (PC) and TL, ^: P < 0.05 vs. TH & TLS.
Discussion
In the current study, the doses used (3.6 and 1.8 mg/kg) are equivalent to the human doses used clinically (usually 40 mg/day, but sometimes only 20 mg/day could be useful). In patients with mild to moderate hepatic impairment, the dose of telmisartan should not exceed 40 mg once daily. The SNEDDS formulation was used to further enhance telmisartan bioavailability aiming to show that using SNEDDS formula of the smaller dose (1.8 mg/kg) succeeded to improve hepatic fibrosis while the same dose of the standard drug failed to produce any effect. Using the higher dose (3.6 mg/kg) was to show the dose-related effects of the formulation. Although this higher dose did not deteriorate the conditions of rats, but it must be used carefully. In a clinical trial, single oral doses of telmisartan 20 mg and 120 mg were well tolerated in both hepatically-impaired and healthy subjects. The most common side effect potentially related to telmisartan was headache. Changes in the vital signs and laboratory measurements were transient and of no clinical importance. Hepatic impairment resulted in an apparent increase in the absolute bioavailability of telmisartan, and a significant reduction of its total clearance compared with healthy volunteers. Plasma protein binding of telmisartan was equal to or more than 99.5% in both hepatically impaired and in healthy subjects. This shows that in hepatic patients use of small doses of telmisartan to control hypertension is effective and safe with fewer side effects. The increased bioavailability of telmisartan in patients with hepatic impairment suggests that lower doses should be considered.20
The selection of the components of the SNEDDS formula was based on preliminary data that showed improved solubility of telmisartan in the selected oil, surfactant, and co-surfactant. The effect of similar surfactants on the organic anion transporting polypeptide (OATP) drug uptake transporters and the efflux P-glycoprotein transporters was discussed in previous studies. It was reported that the SNEDDS formulation containing telmisartan (20 mg), Tween, Carbitol, and Acrysol EL 135 showed quicker release with rapid emulsification. It showed significant rises in the dissolution rate, oral absorption, and bioavailability compared to the aqueous drug suspension. Thus, SNEDDS can be considered as a new and commercially achievable alternative to the standard telmisartan formula.21 The doses used (3.6 and 1.8 mg/kg) are equivalent to the human doses used clinically (usually 40 mg/day, but sometimes only 20 mg/day could be useful). The hepatic organic anion transporting polypeptide (OATP) drug uptake transporters (OATP1B1 and OATP1B3 in human) impact liver uptake of many drugs. Telmisartan is a substrate for OATP and it inhibits human OATP-mediated hepatic uptake at concentrations higher than those used clinically indicating low risk of drug interactions.22 Moreover, the predominant contribution of OATP1B3 in hepatic uptake of telmisartan in rats has been demonstrated by using the positron emission tomography methodology. It was shown that the hepatic uptake of radiolabeled telmisartan mainly consists of a saturable process mediated by OATP where it was taken up into the liver as rapidly as the hepatic blood flow rate and the radio-metabolite was subsequently excreted into the bile. When rifampicin, the prototypic OATP inhibitor, was co-administered with radiolabeled telmisartan, the hepatic uptake clearance of radiolabeled telmisartan decreased while its biliary efflux clearance was not changed.23 Being a substrate for the hepatic OATP1B3, radioactive telmisartan was rapidly taken up by the liver in both rats and human.24,25 Using the oral administration of 14C-telmisartan, a single dose (1 mg/kg) given to fasted rats or repeated doses (1 mg/kg/day for 7 days) given to fed rats, showed elevations in levels of radioactivity in the liver and gastrointestinal tract. The concentrations of radioactivity in all tissues were lower than that in plasma except in the gastrointestinal, liver, kidney, and lung. Telmisartan was mainly distributed in liver with a hepatic concentration of 39-46 times higher than that of plasma. The concentrations of radioactivity in tissues and plasma decreased with time indicating no accumulation of telmisartan in any tissue. Fed rats showed lower plasma levels than fasted rats due to effect of food intake.26 Telmisartan exhibits high partition coefficient and extensive tissue distribution due to high lipophilicity. Using the cynomolgus monkey as a model for prediction of hepatic clearance made through the OATP transporters in human, telmisartan; among other OATP substrates; was investigated in plated cynomolgus monkey and human hepatocytes. The partition coefficient (Kp, uu); ratio of unbound drug level in tissue to that in plasma; of telmisartan was more than 2.27 In another study using the beagle dog as a preclinical model, telmisartan Kpuu less than 5.28
Telmisartan improved non-alcoholic fatty liver disease and also fibrosis in non-alcoholic steatohepatitis cases with few adverse events.29 In thioacetamide-induced liver fibrosis rats, daily intraperitoneal injection of telmisartan significantly decreased the levels of ALP, TNF-α, TGF-β-1, hepatic inflammation, and fibrosis while increased the hepatic levels of GSH. Also it significantly decreased plasma level of IL-6 and increased paraoxonase 1 anti-oxidant enzyme activity. This indicates that telmisartan has anti-inflammatory and anti-oxidative effects in the thioacetamide model of liver fibrosis.30 Telmisartan shows anti-fibrotic activity, but its clinical use is restricted due to systemic hypotension. To overcome this, telmisartan macromolecular prodrugs were formulated to optimize its special release in diseased liver tissue. The release of active telmisartan in the fibrotic liver was optimized to be depot-like concomitant with a low release of the drug systemically. This improved antifibrotic efficacy and decreased the dose-limiting systemic hypotension in mouse models of hepatic fibrosis. In rats and dogs, the prodrugs were hold long time in liver enabling infrequent dosing in treatment of liver fibrosis and they were well-tolerated.31 Being hydrophobic, nanosuspension is a suitable method for preparing nanoformulas of solid oral dosage forms of telmisartan.32 A self-nanoemulsifying Drug Delivery System (SNES) was developed of telmisatan. The PK parameters after oral administration of SNES were compared with similar doses of the oral tablet and pure drug aqueous suspension (1.8 mg/kg). The results demonstrated a 4.3-fold elevation in oral bioavailability of drug compared with the tablet while that of the tablet was only a 1.8-fold higher than that compared with the pure drug aqueous suspension. The plasma concentration after half an hour of administration of SENS formulation was 10 times higher than the tablet, while that of the tablet was 2 times higher than the pure drug suspension.9
The steady state of telmisartan is reached within 3-5 days of treatment. In rats, telmisartan after IV or oral administration showed the highest levels in the liver, kidney, blood, and lung respectively. Very low levels remained, 24 hours post-dosing except in the liver. It is to be noticed that the plasma protein binding of telmisartan is high, nearly 98%, but it is not restrictive as shown by the large volume of distribution and rapid plasma clearance. The absolute bioavailability of telmisartan in humans is 40-60% depending on dose and it increases in subjects with hepatic impairment to about 100% which could be explained by presence of the extrahepatic shunts which allow it to escape the first-pass hepatic metabolism. Thus in hepatic impairment, drug elimination of telmisartan was not affected and the elimination half-life continued to be unchanged allowing telmisartan to be used once-daily.33,34
At the doses used in the current study, there were no observed adverse effects. This agrees with a previous study in rats which reported that no adverse effects were observed at a low dose of telmisartan (12 mg/kg orally for 28 days) and even at a high dose (48 mg/kg orally for 28 days), although there were some significant hematological and biochemical changes, but they were transient and reversible.35 In rats, TAA administration resulted in significant reductions in body weight, and significant increases in hepatic weight and hepatic/body weight ratio in addition to significant reduction in plasma albumin level.36 The reduction of body weight is due to the TAA toxic effects and it is considered the most reliable symptom of toxicity in experimental animals.37 The reduced level of albumin usually shows the severity of the liver dysfunction and it is a reliable index of prognosis.38 In the current study, telmisartan low dose failed to reverse these changes, but telmisartan high dose and the SNEDDS formulations of both low and high doses reversed them with varying degrees. The systolic blood pressure was measured as a functional safety endpoint and no significant changes were detected among groups. This finding is in agreement with a previous study in normotensive rats which showed that telmisartan low and high doses (0.1 and 10 mg/kg/day) produced a 15-20% drop in the systolic blood pressure, but all rats remained normotensive.39 Moreover, in a clinical trial in normotensive Japanese patients with type 2 DM, telmisartan decreased microalbuminuria without significant changes in blood pressure. It was safe and well tolerated.40 Also the potential neuroprotective effect of telmisartan was tested in acute cerebral ischemia in normotensive rats. The mean arterial blood pressure remained unchanged compared to the vehicle-treated group.41 In addition, no significant changes were detected in the hemoglobin level, white blood cells count, and platelets count between groups. Consequently, comparing the benefits versus risks for the doses used seems satisfactory, however it should be assessed following long-term toxicity studies in order to rule out long-term adverse effect of the formulations used. In addition, it would be better to test them in a combined model of hepatic fibrosis and hypertension.
Conclusion
Telmisartan nano-formula (self-nanoemulsifying drug delivery system, SNEDDS), compared with the same identical doses of the standard telmisartan, produced a higher hepatic free drug concentration and more effectively reversed hepatic fibrosis with a good safety profile in a model of already-established chronic hepatic fibrosis. Thus it is suggested to use the SNEDDS formula of a smaller dose in hepatic patients rather than using a higher dose of the standard form. This will help clarify the therapeutic role of telmisartan nanostructures in treatment of hepatic fibrosis.
Acknowledgments
The participation of the medical students Muhammed Fhaid Alamri, Khalild Faisal Alzibali, Ahmad Samir Momina, Abdulrhman Qathal Al-sulme, Abdullah Faisal Attar, and Ziad Tariq Al-Malki is gratefully acknowledged.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the Deanship of Scientific Research, (DSR) at King Abdulaziz University (KAU), Jeddah under grant number (G: 64-828-1440). The authors, therefore, acknowledge with thanks DSR for technical and financial support.
ORCID iD: Hussam Murad  https://orcid.org/0000-0002-8406-4946
https://orcid.org/0000-0002-8406-4946
Osama Ahmed  https://orcid.org/0000-0002-3204-381X
https://orcid.org/0000-0002-3204-381X
References
- 1. Zhu Q, Li N, Li F, et al. Therapeutic effect of renin angiotensin system inhibitors on liver fibrosis. J Renin-Angio-Aldo S. 2016;17(1):1470320316628717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weiskirchen R. Hepatoprotective and anti-fibrotic agents: it’s time to take the next step. Front Pharmacol. 2016;6:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giannitrapani L, Soresi M, Bondì ML, Montalto G, Cervello M. Nanotechnology applications for the therapy of liver fibrosis. World J Gastroenterol. 2014;20(23):7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakayama S, Watada H, Mita T, et al. Comparison of effects of olmesartan and telmisartan on blood pressure and metabolic parameters in Japanese early-stage type-2 diabetics with hypertension. Hypertens Res. 2008;31(1):7. [DOI] [PubMed] [Google Scholar]
- 5. Kalikar M, Nivangune KS, Dakhale GN, et al. Efficacy and tolerability of olmesartan, telmisartan, and losartan in patients of stage I hypertension: a randomized, open-label study. J Pharmacol Pharmacother. 2017;8(3):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Le M, Pugsley M, Vauquelin G, Van Liefde I. Molecular characterisation of the interactions between olmesartan and telmisartan and the human angiotensin II AT1 receptor. Brit J Pharmacol. 2007;151(7):952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siragy HM. Angiotensin Receptor Blockers: How Important Is Selectivity? Oxford University Press; 2002. [DOI] [PubMed] [Google Scholar]
- 8. Stangier J, Su C, Roth W. Pharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients. J Int Med Res. 2000;28(4):149–167. [DOI] [PubMed] [Google Scholar]
- 9. Ahmad J, Kohli K, Mir SR, Amin S. Formulation of self-nanoemulsifying drug delivery system for telmisartan with improved dissolution and oral bioavailability. J Dispers Sci Technol. 2011;32(7):958–968. [Google Scholar]
- 10. Kishi T, Hirooka Y, Sunagawa K. Sympathoinhibition caused by orally administered telmisartan through inhibition of the AT 1 receptor in the rostral ventrolateral medulla of hypertensive rats. Hypertens Res. 2012;35(9):940–946. [DOI] [PubMed] [Google Scholar]
- 11. Eraky SM, El-Mesery M, El-Karef A, Eissa LA, El-Gayar AM. Silymarin and caffeine combination ameliorates experimentally-induced hepatic fibrosis through down-regulation of LPAR1 expression. Biomed Pharmacother. 2018;101:49–57. [DOI] [PubMed] [Google Scholar]
- 12. Nakajima M, Iwata K, Yamamoto T, Funae Y, Yoshida T, Kuroiwa Y. Nicotine metabolism in liver microsomes from rats with acute hepatitis or cirrhosis. Drug Metabo Dispos. 1998;26(1):36–41. [PubMed] [Google Scholar]
- 13. El Awdan SA, Rahman RFA, Ibrahim HM, et al. Regression of fibrosis by cilostazol in a rat model of thioacetamide-induced liver fibrosis: Up regulation of hepatic cAMP, and modulation of inflammatory, oxidative stress and apoptotic biomarkers. PloS One. 2019;14(5):e0216301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nie J, Zhao Q, Huang J, Xiang B, Feng YQ. Determination of telmisartan in rat tissues by in-tube solid-phase microextraction coupled to high performance liquid chromatography. J Sep Sci. 2006;29(5):650–655. [DOI] [PubMed] [Google Scholar]
- 15. Patel JM, Dhingani AP, Garala KC, Raval MK, Sheth NR. Development and validation of bioanalytical HPLC method for estimation of telmisartan in rat plasma: application to pharmacokinetic studies. Dhaka Univ J Pharm Sci. 2012;11(2):121–127. [Google Scholar]
- 16. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. [DOI] [PubMed] [Google Scholar]
- 17. Ramadan A, Afifi N, Yassin NZ, Abdel-Rahman RF, El-Rahman SSA, Fayed HM. Mesalazine, an osteopontin inhibitor: the potential prophylactic and remedial roles in induced liver fibrosis in rats. Chem Bio Interact. 2018;289:109–118. [DOI] [PubMed] [Google Scholar]
- 18. Sengupta P, Chatterjee B, Mandal UK, Gorain B, Pal TK. Development and validation of a high throughput LC–MS/MS method for simultaneous quantitation of pioglitazone and telmisartan in rat plasma and its application to a pharmacokinetic study. J Pharm Anal. 2017;7(6):381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Vliet BN, Chafe LL, Antic V, Schnyder-Candrian S, Montani J-P. Direct and indirect methods used to study arterial blood pressure. J Pharmacol Toxicol Method. 2000;44(2):361–373. [DOI] [PubMed] [Google Scholar]
- 20. Stangier J, Su CAP, Schöndorfer G, Roth W. Pharmacokinetics and safety of intravenous and oral telmisartan 20 mg and 120 mg in subjects with hepatic impairment compared with healthy volunteers. J Clin Pharmacol. 2000;40(12):1355–1364. [PubMed] [Google Scholar]
- 21. Patel J, Kevin G, Patel A, Raval M, Sheth N. Design and development of a self-nanoemulsifying drug delivery system for telmisartan for oral drug delivery. Int J Pharm Investig. 2011;1(2):112–118. doi:10.4103/2230-973x.82431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Durmus S, Lozano-Mena G, van Esch A, Wagenaar E, van Tellingen O, Schinkel AH. Preclinical mouse models to study human OATP1B1- and OATP1B3-mediated drug-drug interactions in vivo. Mol Pharm. 2015;12(12):4259–6429. doi:10.1021/acs.molpharmaceut.5b00453 [DOI] [PubMed] [Google Scholar]
- 23. Takashima T, Hashizume Y, Katayama Y, et al. The involvement of organic anion transporting polypeptide in the hepatic uptake of telmisartan in rats: PET studies with [11C]telmisartan. Mol Pharm. 2011;8(5):1789–1798. doi:10.1021/mp200160t [DOI] [PubMed] [Google Scholar]
- 24. Shimizu K, Takashima T, Yamane T, et al. Whole-body distribution and radiation dosimetry of [11C] telmisartan as a biomarker for hepatic organic anion transporting polypeptide (OATP) 1B3. Nucl Med Biol. 2012;39(6):847–853. [DOI] [PubMed] [Google Scholar]
- 25. Li R, Barton HA, Varma MV. Prediction of pharmacokinetics and drug–drug interactions when hepatic transporters are involved. Clin Pharmacokinet. 2014;53(8):659–678. [DOI] [PubMed] [Google Scholar]
- 26. Shimasaki M, Yamashita K, Imanishi R, et al. Pharmacokinetics of 14C-telmisartan (1): absorption, distribution and protein binding of 14C-telmisartan after a single oral administration to rats. Drug Metabol Pharmacokinet. 1999;14(6):425–431. [Google Scholar]
- 27. De Bruyn T, Ufuk A, Cantrill C, et al. Predicting human clearance of OATP substrates using cynomolgus monkey: in vitro-in vivo scaling of hepatic uptake clearance. Drug Metabol Dispos. 2018; 46(7):989–1000. [DOI] [PubMed] [Google Scholar]
- 28. Matsunaga N, Ufuk A, Morse BL, et al. Hepatic organic anion transporting polypeptide–mediated clearance in the beagle dog: assessing in vitro–in vivo relationships and applying cross-species empirical scaling factors to improve prediction of human clearance. Drug Metabol Disp. 2019;47(3):215–226. [DOI] [PubMed] [Google Scholar]
- 29. Alam S, Kabir J, Mustafa G, Gupta U, Hasan S, Alam A. Effect of telmisartan on histological activity and fibrosis of non-alcoholic steatohepatitis: a 1-year randomized control trial. Saudi J Gastroenterol. 2016;22(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Czechowska G, Celinski K, Korolczuk A, et al. The effect of the angiotensin II receptor, type 1 receptor antagonists, losartan and telmisartan, on thioacetamide-induced liver fibrosis in rats. J Physiol Pharmacol. 2016;67(4):575–586. [PubMed] [Google Scholar]
- 31. Golder MR, Liu J, Andersen JN, et al. Reduction of liver fibrosis by rationally designed macromolecular telmisartan prodrugs. Nat Biomed Eng. 2018;2(11):822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel J, Dhingani A, Garala K, Raval M, Sheth N. Design and development of solid nanoparticulate dosage forms of telmisartan for bioavailability enhancement by integration of experimental design and principal component analysis. Powder Technol. 2014;258:331–343. [Google Scholar]
- 33. Wienen W, Entzeroth M, van Meel JC, et al. A review on telmisartan: a novel, long-acting angiotensin II-receptor antagonist. Cardiovasc Drug Rev. 2000;18(2):127–154. [Google Scholar]
- 34. Li R, Ghosh A, Maurer TS, Kimoto E, Barton HA. Physiologically based pharmacokinetic prediction of telmisartan in human. Drug Metabol Disp. 2014;42(10):1646–1655. [DOI] [PubMed] [Google Scholar]
- 35. Nandi U, Karmakar S, Das AK, et al. Pharmacokinetics, pharmacodynamics and toxicity of a combination of metoprolol succinate and telmisartan in Wistar albino rats: safety profiling. Regul Toxicol Pharmacol. 2013;65(1):68–78. [DOI] [PubMed] [Google Scholar]
- 36. Ra S-H, Shin R-H, Ri H-C, Ri J-H, Ri H-C, Ri A-J. Effect of lesimarin against thioacetamide-induced liver cirrhosis in rat. Braz J Pharm Sci. 2019;55. [Google Scholar]
- 37. Ljubuncic P, Song H, Cogan U, Azaizeh H, Bomzon A. The effects of aqueous extracts prepared from the leaves of Pistacia lentiscus in experimental liver disease. J Ethnopharmacol. 2005;100(1-2):198–204. [DOI] [PubMed] [Google Scholar]
- 38. Thapa B, Walia A. Liver function tests and their interpretation. Indian J Pediatr. 2007;74(7):663–671. [DOI] [PubMed] [Google Scholar]
- 39. Villa L, Boor P, Konieczny A, et al. Effects and mechanisms of angiotensin II receptor blockade with telmisartan in a normotensive model of mesangioproliferative nephritis. Nephrol Dial Transplant. 2011;26(10):3131–3143. [DOI] [PubMed] [Google Scholar]
- 40. Makino H, Haneda M, Babazono T, et al. Microalbuminuria reduction with telmisartan in normotensive and hypertensive Japanese patients with type 2 diabetes: a post-hoc analysis of the Incipient to Overt: Angiotensin II Blocker, Telmisartan, Investigation on Type 2 Diabetic Nephropathy (INNOVATION) study. Hypertens Res. 2008;31(4):657–664. [DOI] [PubMed] [Google Scholar]
- 41. Thoene-Reineke C, Rumschüssel K, Schmerbach K, et al. Prevention and intervention studies with telmisartan, ramipril and their combination in different rat stroke models. PLoS One. 2011;6(8):e23646. [DOI] [PMC free article] [PubMed] [Google Scholar]