Abstract
Background
Few data exist concerning conversion to secondary progressive MS in patients treated with disease-modifying therapies.
Objective
Determine the proportion of alemtuzumab-treated patients converting from relapsing-remitting to secondary progressive MS during the CARE-MS core and extension studies.
Methods
Patients (N = 811) were analyzed post hoc for secondary progressive MS conversion. Optimal conversion definition: Expanded Disability Status Scale (EDSS) score ≥4, pyramidal functional system score ≥2, and confirmed progression over ≥3 months including confirmation within the functional system leading to progression, independent of relapse.
Results
Over 6.2 years median follow-up, 20 alemtuzumab-treated patients converted (Kaplan-Meier estimate, 2.7%; 95% confidence interval, 1.8%–4.2%). Sensitivity analysis accounting for dropouts showed similar results (3%), as did analyses using alternative definitions with different EDSS thresholds and/or confirmation periods, and analysis of core study subcutaneous interferon beta-1a-treated patients who received alemtuzumab in the extension. Patients converting to secondary progressive MS were older, and had higher EDSS scores and greater brain lesion volumes at baseline, but did not need additional alemtuzumab or other therapies.
Conclusions
The 6-year conversion rate to secondary progressive MS was low for alemtuzumab-treated patients, supporting further study of the role alemtuzumab may play in reducing risk of secondary progression.
ClinicalTrials.gov identifiers: NCT00530348, NCT00548405, NCT00930553.
Keywords: Relapsing-remitting multiple sclerosis, secondary progressive multiple sclerosis, alemtuzumab, disease progression
Introduction
Conversion to secondary progressive MS is an important predictor of long-term prognosis in patients with relapsing-remitting MS.1 Following conversion, disability accumulates independently of relapses2 and burden of illness is higher than in patients with relapsing-remitting MS.3 Over half of untreated relapsing-remitting MS patients progress to secondary progressive MS within 10 years of diagnosis.4 Delaying progression from relapsing-remitting to secondary progressive MS is an important treatment goal; however, little information exists concerning how current treatments may delay conversion.1,5
Lack of a consensus definition for secondary progressive MS presents a major challenge. In 2016, Lorscheider et al. developed an objective definition for secondary progressive MS using data from a large registry of MS patients (MSBase): Expanded Disability Status Scale (EDSS) score ≥4, pyramidal score ≥2, and disability progression by 1 EDSS point (if EDSS score ≤5.5) or 0.5 EDSS points (if EDSS score ≥6) without relapse, with confirmed progression over ≥3 months including confirmation within the functional system leading to the progression event.6 Using this definition, approximately 18% of registry patients converted to secondary progressive MS over a median 5.8 years (range, 3.4–9.6).6
Alemtuzumab (LEMTRADA®; Sanofi Genzyme, Cambridge, MA), a humanized monoclonal antibody approved for relapsing-remitting MS, significantly improved clinical and MRI outcomes over 2 years in patients with active relapsing-remitting MS versus subcutaneous interferon beta-1a (SC IFNB-1a; Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis [CARE-MS] I [NCT00530348]7 and II [NCT00548405]8). An extension study demonstrated continued efficacy for 4 additional years.9,10 Adverse events associated with alemtuzumab treatment in clinical trials and postmarketing experience include infusion-associated reactions, increased frequency of infection and the potential for opportunistic infections, secondary autoimmunity (thyroid disorders, immune thrombocytopenia, nephropathies, autoimmune cytopenias, autoimmune hepatitis, and other less common autoimmune events), acute acalculous cholecystitis, and cardiovascular and pulmonary events possibly related to infusion.7–15
Here we describe a post hoc analysis of CARE-MS patients to determine the proportion converting from relapsing-remitting to secondary progressive MS through 6 years using the definition developed by Lorscheider et al.6 Additional sensitivity analyses are also presented.
Methods
Design of CARE-MS and extension studies
The CARE-MS core and extension study designs were described previously.7–10 Briefly, the 2-year, phase 3 CARE-MS studies compared alemtuzumab with SC IFNB-1a in patients with active relapsing-remitting MS who were either treatment-naive (CARE-MS I; aged 18–50 years)7 or had inadequate response to prior therapy (CARE-MS II; aged 18–55 years).8 Patients received alemtuzumab 12 mg/day intravenously on 5 consecutive days at baseline and 3 consecutive days 12 months later. Patients completing the phase 3 studies could enter the 4-year CARE-MS extension (NCT00930553), wherein they could receive additional alemtuzumab courses (12 mg/day on 3 consecutive days ≥12 months after most recent course) as needed for relapse or MRI activity, or other licensed disease-modifying therapy (DMT) at the investigator’s discretion; core study SC IFNB-1a-treated patients who entered the extension switched to 2 alemtuzumab courses (extension baseline and 12 months later), followed by as-needed additional alemtuzumab, or other DMT at investigator’s discretion.9,10 Local institutional ethics review boards of participating sites approved all procedures. Patients provided written informed consent.
Primary definition of secondary progressive MS conversion
The primary definition of secondary progressive MS in our analysis was based on the optimal definition from Lorscheider et al.6 (following personal communication with Dr. Lorscheider to clarify algorithmic derivation of conversion, to ensure appropriate application to the CARE-MS data): disability progression by 1 EDSS point if baseline EDSS score ≤5.5 or by 0.5 EDSS points if baseline EDSS score ≥6, both without relapse (i.e. progression start date is ≥180 days after prior relapse); minimum EDSS score of 4 and minimum pyramidal functional system score of 2; confirmed progression over ≥3 months, including confirmation within the functional system leading to the progression event. EDSS was assessed quarterly by raters blinded to core study treatment assignment and treatment history.7–10
Statistical analyses
Statistical analyses included patients who received ≥2 courses of alemtuzumab 12 mg in the CARE-MS studies (‘alemtuzumab-only’) and core study SC IFNB-1a-treated patients who received ≥2 courses of alemtuzumab 12 mg in the extension (‘IFN-alemtuzumab’); analyses were carried out for the pooled CARE-MS studies (and for the separate CARE-MS studies in alemtuzumab-only patients); data cut-off was September 15, 2015.
The percentage of patients with secondary progressive MS6 was derived using the Kaplan-Meier method, which accounted for patients who discontinued prematurely or did not enter the extension. Cumulative estimates and 95% confidence intervals (CIs) are reported. Baseline characteristics were assessed descriptively for patients who did or did not convert to secondary progressive MS and for alemtuzumab-only patients who discontinued or did not enter the extension. In patients who converted to secondary progressive MS, further descriptive analyses in alemtuzumab-only patients included percentage of patients who received additional alemtuzumab and/or treatment with another DMT, reasons for additional alemtuzumab, temporal relationship between additional alemtuzumab courses and conversion to secondary progressive MS, efficacy endpoints (annualized relapse rate [ARR], mean EDSS score, and number of new gadolinium [Gd]-enhancing and new/enlarging T2 hyperintense lesions [also assessed for IFN-alemtuzumab]) by year over 6 years, and quality-of-life (QoL) assessments (Functional Assessment of Multiple Sclerosis [FAMS; version 4],16 Short-Form-36 [version 2] physical component summary and mental component summary [SF-36 PCS and MCS],17 and European Quality of Life-5 Dimensions Visual Analog Scale [EQ-VAS])18 from baseline to Year 6 (higher scores indicate better QoL on all instruments assessed; p values were based on Wilcoxon signed-rank test).19
Sensitivity analyses of the optimal definition from Lorscheider et al.6 in alemtuzumab-only patients included varying confirmation periods (6, 12, or 24 months) and an alternative threshold using a minimum EDSS score of 3 with confirmation periods of 3, 6, 12, or 24 months. Definitions using EDSS score ≥3 were found by Lorscheider et al. to have lower specificity and lower overall accuracy than definitions using EDSS score ≥4 (particularly when using 6-month confirmation).6 In addition, sensitivity analysis investigating the number of patients who may have converted to secondary progressive MS was carried out on those from the primary analysis lacking the full 6-year data. These patients were used to determine the impact on the primary estimate of conversion to secondary progressive MS using the optimal definition from Lorscheider et al.
All statistical analyses used SAS (version 9.4, The SAS Institute, Cary, NC).
Results
Patients
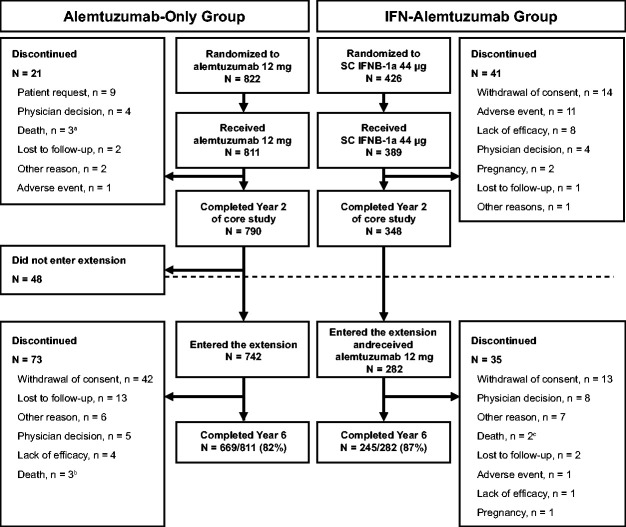
Of the 811 CARE-MS alemtuzumab-only patients (mean age, 34.0 years; standard deviation [SD], 8.24), 669 (82%) completed Year 6. Twenty-one patients discontinued the core studies, 48 did not enroll in the extension, and 73 discontinued from the extension (due to lack of efficacy [extension; n = 4]; adverse event [core studies; n = 1]; Figure 1). Through 6 years, 56% of alemtuzumab-only patients received neither additional alemtuzumab nor another DMT (no additional alemtuzumab: 59%; no other DMT: 93%). Total follow-up time in the pooled CARE-MS alemtuzumab-only population was 4740 patient-years; median follow-up time was 6.2 years. Among 282 IFN-alemtuzumab patients (mean age, 34.5 years; SD, 8.81), 245 (87%) completed Year 6 (follow-up: 1169 patient-years; median, 4.2 years).
Figure 1.
Patient disposition in the CARE-MS core and extension studies. Abbreviations: CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis. aDeemed unrelated to alemtuzumab treatment (3 deaths). bDeemed unrelated (1 death) and possibly related (2 deaths) to alemtuzumab treatment. cDeemed unrelated to alemtuzumab treatment (1 death) and relationship to alemtuzumab treatment unable to be determined (1 death).
Secondary progressive MS conversion
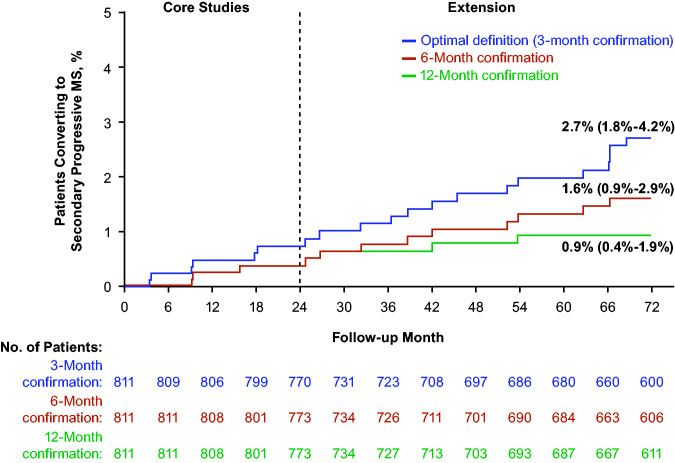
In the pooled alemtuzumab-only population, 20 patients over 6 years (Kaplan-Meier estimate, 2.7%; 95% CI, 1.8%–4.2%; CARE-MS I, n = 4; CARE-MS II, n = 16) converted to secondary progressive MS according to the Lorscheider et al. optimal definition (median time to meet definition criteria for conversion, 37.5 months; range, 3.2–68.6).6 Conversion was less frequent when longer confirmation periods (6 and 12 months) were used (Figure 2, Table 1). No patients converted to secondary progressive MS when a 24-month confirmation period was used. Estimates of secondary progressive MS conversion in the individual CARE-MS studies were similar to the pooled population (Table 1). The most common reason for patients meeting the secondary progressive MS definition at 3 months but not at 24 months was failure to achieve the required EDSS increase (n = 11, 55%). Other reasons were: insufficient time available for the confirmation period to reach the required duration (n = 5, 25%), pyramidal functional system score <2 (n = 2, 10%), patient decision to discontinue the study early (n = 1, 5%; reason for discontinuation unknown), and EDSS score decrease to <4 (n = 1, 5%; this patient received 1 additional alemtuzumab course between the start and end of secondary progression). Ten IFN-alemtuzumab patients (3.6%; 95% CI, 2.0%–6.7%; median time to conversion, 41.8 months; range, 23.7–60.0) converted to secondary progressive MS (optimal definition).
Figure 2.
Kaplan-Meier estimate of secondary progressive MS conversion in the pooled CARE-MS alemtuzumab-only population (EDSS threshold ≥4; different confirmation periods). Abbreviations: CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; EDSS, Expanded Disability Status Scale. No patients converted to secondary progressive MS when a confirmation period of 24 months was used.
Table 1.
Kaplan-Meier estimates of proportions of alemtuzumab-only patients converting to secondary progressive MS.a
| Kaplan-Meier estimate, % (95% CI) |
|||
|---|---|---|---|
| EDSS threshold | CARE-MS I | CARE-MS II | Pooled CARE-MS I and II |
| EDSS ≥4 (optimal definition) | |||
| 3-mo. confirmation | 1.2 (0.4–3.0) | 4.2 (2.6–6.7) | 2.7 (1.8–4.2) |
| 6-mo. confirmation | 0.9 (0.3–2.7) | 2.3 (1.2–4.4) | 1.6 (0.9–2.9) |
| 12-mo. confirmation | 0.3 (0.0–2.1) | 1.5 (0.7–3.2) | 0.9 (0.4–1.9) |
| 24-mo. confirmation | 0 | 0 | 0 |
| EDSS ≥3 | |||
| 3-mo. confirmation | 3.4 (1.9–5.9) | 12.2 (9.3–15.8) | 8.0 (6.3–10.2) |
| 6-mo. confirmation | 2.6 (1.3–4.9) | 7.0 (4.9–10.0) | 4.9 (3.6–6.7) |
| 12-mo. confirmation | 1.4 (0.6–3.4) | 3.9 (2.4–6.3) | 2.8 (1.8–4.2) |
| 24-mo. confirmation | 0 | 0.7 (0.2–2.3) | 0.4 (0.1–1.2) |
Abbreviations: CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; CI, confidence interval; EDSS, Expanded Disability Status Scale; mo., month.
aProgression start date ≥180 days after prior relapse.
Baseline characteristics of patients who converted to secondary progressive MS or discontinued
Because alemtuzumab-only patients who discontinued or did not enter the extension might have had a higher likelihood of converting to secondary progressive MS had they remained on study, we compared baseline characteristics of that subgroup with those of patients who did or did not convert to secondary progressive MS over 6 years of follow-up. At baseline, patients who converted to secondary progressive MS were older and had higher EDSS scores, numbers of Gd-enhancing lesions, and T1 and T2 lesion volumes compared with those who did not convert (Table 2). CARE-MS II patients who converted also had longer disease duration than those who did not convert (eTable). The baseline characteristics of patients who discontinued or did not enter the extension (n = 138) were similar to those of patients who did not convert to secondary progressive MS over 6 years (Table 2), except for T2 lesion volume in CARE-MS I, which showed more similarity between patients who discontinued and those who converted to secondary progressive MS (eTable).
Table 2.
Baseline characteristics of alemtuzumab-only patients who did and did not convert to secondary progressive MSa (optimal definition), and who discontinued or did not enter the extension: pooled CARE-MS I and II studies.
| Characteristicb | Patients who converted to secondary progressive MS (n = 20) | Patients who did not convert to secondary progressive MS (n = 791) | Patients who discontinued or did not enter the extension (n = 138)c |
|---|---|---|---|
| Age, years | 36.6 (6.7) | 33.9 (8.3) | 33.3 (8.4) |
| EDSS score | 3.4 (1.2) | 2.4 (1.1) | 2.6 (1.2) |
| MS disease duration, years | 4.6 (2.8) | 3.3 (2.4) | 3.5 (2.4) |
| No. of relapses in prior year | 1.6 (0.7) | 1.7 (0.9) | 1.7 (0.9) |
| No. of relapses in prior 2 years | 2.7 (0.7) | 2.7 (1.1) | 2.8 (1.2) |
| No. of Gd-enhancing lesions | 6.1 (7.3) | 2.2 (5.5) | 1.6 (3.4) |
| Proportion with Gd-enhancing lesions, n/N (%) | 16/19 (84) | 336/781 (43) | 51/137 (37) |
| T2 hyperintense lesion volume, cm3 | 12.5 (10.3) | 8.7 (10.9) | 8.3 (9.5) |
| T1 hypointense lesion volume, cm3 | 3.0 (4.7) | 1.6 (3.2) | 1.2 (2.2) |
| Brain parenchymal fraction | 0.82 (0.03) | 0.82 (0.02) | 0.82 (0.02) |
Abbreviations: CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; EDSS, Expanded Disability Status Scale; Gd, gadolinium; SD, standard deviation.
aProgression start date ≥180 days after prior relapse.
bValues represent mean (SD) unless indicated otherwise.
cOf 142 patients who discontinued or did not enroll in the extension, 4 converted to secondary progression prior to discontinuation and are omitted here.
At baseline in the IFN-alemtuzumab group, patients who converted to secondary progressive MS were older and had higher EDSS scores, lower numbers of Gd-enhancing lesions, and lower T1 and T2 lesion volumes compared with those who did not convert (Table 3).
Table 3.
Baseline characteristics of IFN-alemtuzumab patients who did and did not convert to secondary progressive MSa (optimal definition).
| Characteristicb | Patients who converted to secondary progressive MS (n = 10) | Patients who did not convert to secondary progressive MS (n = 272) | |
|---|---|---|---|
| Age, years | 38.1 (7.2) | 34.3 (8.9) | |
| EDSS score | 3.2 (1.2) | 2.3 (1.0) | |
| MS disease duration, years | 4.9 (3.6) | 3.4 (2.6) | |
| No. of relapses in prior year | 1.2 (0.4) | 1.7 (0.8) | |
| No. of relapses in prior 2 years | 2.3 (0.5) | 2.6 (0.9) | |
| No. of Gd-enhancing lesions | 0.7 (1.9) | 2.0 (4.2) | |
| Proportion with Gd-enhancing lesions, n/N (%) | 2/10 (20) | 132/269 (49) | |
| T2 hyperintense lesion volume, cm3 | 5.6 (4.1) | 8.0 (10.0) | |
| T1 hypointense lesion volume, cm3 | 1.2 (1.3) | 1.4 (2.3) | |
| Brain parenchymal fraction | 0.82 (0.03) | 0.82 (0.02) |
Abbreviations: CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; EDSS, Expanded Disability Status Scale; Gd, gadolinium; SD, standard deviation.
aProgression start date ≥180 days after prior relapse.
bValues represent mean (SD) unless indicated otherwise.
Efficacy and QoL over 6 years
Over 6 years, clinical and MRI efficacy were similar in patients who did or did not convert to secondary progressive MS, except for increased and less stable EDSS scores in patients who converted (eFigures 1 and 2). Among alemtuzumab-only patients who converted, QoL scores worsened over 6 years on the FAMS (mean [SD] baseline score, 113.8 [24.0]; Year 6, 95.3 [40.0]; p = 0.02), SF-36 PCS (39.7 [9.6]; 35.1 [8.2]; p = 0.08), SF-36 MCS (43.0 [13.9]; 40.8 [14.4]; p = 0.20), and EQ-VAS (63.5 [19.7]; 62.0 [21.1]; p = 0.33).
Percentage of alemtuzumab-only patients who received additional treatment and temporal relationship between additional alemtuzumab courses and conversion to secondary progressive MS
Of 20 alemtuzumab-only patients who converted to secondary progressive MS, 11 received neither additional alemtuzumab nor another DMT, 6 received additional alemtuzumab only (due to relapse and/or MRI activity), 2 received both additional alemtuzumab and another DMT, and 1 received another DMT only. Of 8 who received any additional alemtuzumab, 4 received 1 additional course (i.e. Course 3) and 4 received 2 additional courses (Courses 3 and 4). Additional courses were received before conversion to secondary progressive MS in 4 of 8 patients and after conversion in 3 of 8 patients; in the remaining patient, conversion occurred between Courses 3 and 4.
Sensitivity analyses
An EDSS score threshold ≥3 indicated generally higher percentages of alemtuzumab-only patients converting to secondary progressive MS; however, conversion was low (≤8%) overall (Table 1).
Among 811 alemtuzumab-only CARE-MS patients, 195 did not have full 6-year data. We further investigated the number of potential secondary progressive MS converters in this subgroup. Fifty of these 195 patients had at least 5.75 years of follow-up and did not convert to secondary progressive MS, so they could not have converted over 6 years; these 50 patients were thus excluded from this sensitivity analysis. A further 4 patients were identified as converting to secondary progressive MS before leaving the study and were therefore also excluded from this sensitivity analysis, since they had already been counted among the 20 patients who converted to secondary progressive MS in the main analysis. Of the remaining 141 patients, we assumed a conversion rate of 2.7% (i.e. the proportion converting in the main analysis), yielding a further 4 potential patients who could have converted, and thus an overall total of 24 patients, i.e. 3.0% of the 811 patients in the main analysis population, meeting the secondary progressive MS definition through 6 years.
Discussion
This exploratory analysis demonstrated that 2.7% of CARE-MS alemtuzumab-only patients converted to secondary progressive MS over 6 years of follow-up, according to criteria developed by Lorscheider et al.6 The 3-month confirmation had the highest sensitivity and reduced diagnostic uncertainty, with minimal compromise on specificity (86% compared with physicians’ diagnosis).6 Although it could be proposed that the patients who discontinued our study may have had a higher likelihood of developing secondary progressive MS, leading to underestimation of the percentage of patients who converted in our analysis, our evidence argues against this. The baseline characteristics of the patients who discontinued were similar to those of patients who did not convert to secondary progressive MS, and were not consistent with those of patients who did convert. Nonetheless, we carried out an additional sensitivity analysis to address the possibility that some of the patients for whom full 6-year data were not available could have converted to secondary progressive MS. This conservative analysis, in which we assumed that the proportion converting to secondary progressive MS among the patients who discontinued would have been the same as for the entire study population (2.7%), still yielded an overall proportion of only 3% converting to secondary progressive MS. Results from other sensitivity analyses in the whole study population using longer confirmation periods and a lower EDSS threshold, or including the 4 patients previously determined to have converted to secondary progressive MS while on study, also showed low proportions converting to secondary progressive MS (≤8% in the pooled population). In addition, no patients met the 24-month confirmation period for conversion to secondary progressive MS, further underscoring the low rate of conversion in alemtuzumab-treated patients. These findings also align with the significant improvements in disability often observed with alemtuzumab compared with SC IFNB-1a,8 and the subsequent longevity of those effects through 8 years.7–10 Importantly, no relationship was evident between conversion to secondary progressive MS and administration of additional alemtuzumab courses or other DMTs.
A low rate of conversion to secondary progressive MS in alemtuzumab-treated patients has important clinical implications. Compared with relapsing-remitting MS, patients with secondary progressive MS experience more frequent neurologic symptoms, increased disability, and further decreases in physical functioning, negatively affecting overall QoL including ability to engage in work and activities of daily living.3 The onset of secondary progressive MS is also associated with increased rates of hospitalization and increased economic costs.3 Treatments that delay disease progression to secondary progressive MS, therefore, may increase QoL and decrease overall costs associated with the disease.
Comparison of our data with other studies is confounded by patient heterogeneity across data sets, including disease duration, methods to evaluate disability and secondary progressive MS conversion, and variable DMT use.6 However, the 2.7% conversion rate in CARE-MS patients is consistent with other estimates. For example, a review of other retrospective analyses of patients treated with IFNB over a similar timeframe indicated a 9.2% conversion rate over 5.7 years,20 while an observational cohort study of patients with active relapsing-remitting MS showed that 5% of patients treated with alemtuzumab experienced secondary progression (4 of 87 patients) over a median of 7 years, using an operational definition of 2 consecutive discrete confirmed disability worsening events, independent of relapses.21 In another observational study involving a population mostly derived from the MSBase cohort, lower proportions converting from relapsing-remitting to secondary progressive MS were reported with alemtuzumab, fingolimod, or natalizumab compared with IFNB or glatiramer acetate (hazard ratio, 0.66; 95% CI, 0.44–0.99; p = 0.046; N = 615; median follow-up, 5.8 years).22 Follow-up of IFNB-treated clinical trial patients over a time frame similar to our study (7–8 years after study baseline) revealed 19.7% of patients converting to secondary progressive MS.23 In patients treated with DMTs after secondary progressive MS conversion, some benefits in reducing relapses and slowing disability worsening have been reported with siponimod,24 teriflunomide,25 and alemtuzumab26,27; only recently have DMTs started to receive approval for use in patients with secondary progressive MS.28–30
Older age and early disability accumulation are well established as predictors of a secondary progressive course.1,31–33 While statistical comparisons for baseline characteristics in the present study were not performed due to the small numbers of patients converting to secondary progressive MS, patients who converted were older and had higher EDSS scores at baseline. The baseline MRI lesion burden in the patients who converted was greater in the alemtuzumab-only group but lower in the IFN-alemtuzumab group. In a recent study of the MSBase cohort, no correlation between MRI activity and clinical course was established.34 Further research, using more reliable and standardized MRI assessments, may help to resolve this “clinicoradiological paradox.”35 As expected, over 6 years among patients who converted to secondary progressive MS, EDSS scores steadily increased whereas relapse rates and MRI lesion counts did not worsen. Worsened QoL in these patients was in contrast to the improved or stable QoL recently reported in the overall CARE-MS II population.19
Results of this post hoc analysis must be interpreted with caution. Currently, no consensus diagnostic criteria exist for secondary progressive MS. We used an objective definition developed by an independent group, using functional system scores to increase diagnostic accuracy and a short confirmation period to reduce the period of diagnostic uncertainty while maintaining specificity.6 We also acknowledge the lack of a comparator for rates of secondary progressive MS conversion, although we did assess the group who switched to alemtuzumab in the extension after core study SC IFNB-1a, and found that the proportion converting to secondary progressive MS over 6 years was similar to, albeit slightly higher than, that of the alemtuzumab-only group. Finally, the 6-year follow-up time for which functional systems scores were available limited our ability to detect conversion to secondary progression. Follow-up over a longer term presumably would have increased the number of patients converting.
Conclusions
Our data suggest alemtuzumab may affect the natural history of MS by slowing progression to more severe stages of the disease. Over 80% of patients remained in the extension study at Year 6, supporting the robustness of these data. Further confirmation of these results over longer time periods and in real-world cohorts will be important.
Supplemental Material
Supplemental material, sj-pdf-1-mso-10.1177_2055217320972137 for Proportion of alemtuzumab-treated patients converting from relapsing-remitting multiple sclerosis to secondary progressive multiple sclerosis over 6 years by Dana Horáková, Aaron Boster, Antonio Bertolotto, Mark S Freedman, Isabel Firmino*, Steven J Cavalier, Alan K Jacobs*, Karthinathan Thangavelu*, Nadia Daizadeh, Elizabeth M Poole*, Darren P Baker, David H Margolin*, Tjalf Ziemssen and on behalf of the CARE-MS I, CARE-MS II, and CAMMS03409 Investigators in Multiple Sclerosis Journal—Experimental, Translational and Clinical
Acknowledgements
The authors and Sanofi thank the patients for their participation in the CARE-MS I, CARE-MS II, and CAMMS03409 studies, as well as the Steering Committee and the Investigators. Critical review of the manuscript for scientific accuracy was provided by Ericka M Bueno, PhD, and Colin P Mitchell, PhD, of Sanofi. Editorial support was provided by Richard J Hogan, PhD, and Linda V Wychowski, PhD, of Eloquent Scientific Solutions, and was funded by Sanofi.
Footnotes
Conflict of Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DH reported receiving consulting fees/speaker honoraria from Biogen, Merck, Novartis, Roche, Sanofi, and Teva; and receiving support from the Czech Ministry of Education project Progres Q27/LF1. ABo reported receiving consulting fees and/or fees for non-CME services from Biogen, Mallinckrodt, Medtronic, Novartis, Sanofi, and Teva. ABe reported serving on advisory boards for and/or receiving speaker honoraria from Almirall, Bayer, Biogen, Novartis, Sanofi, and Teva; and receiving grant support from Almirall, Associazione San Luigi Gonzaga ONLUS, Bayer, Biogen, Fondazione per la Ricerca Biomedica ONLUS, Merck, Novartis, Sanofi, Teva, and the Italian Multiple Sclerosis Society. MSF reported receiving honoraria/consulting fees from Actelion, Bayer, Biogen, Canada Innovation, Chugai, EMD Canada, Merck Serono, Novartis, Roche, Sanofi, and Teva; serving as a member of an advisory board, board of directors, or other similar group for Actelion, Bayer, Biogen, Merck Serono, Novartis, Opexa, Roche, and Sanofi; and participation in speakers bureau for Sanofi. SJC, ND, and DPB reported receiving personal compensation as employees of Sanofi. IF, AKJ, KT, EMP, and DHM reported receiving personal compensation as employees of Sanofi during study conduct and analysis. TZ reported receiving consulting and/or speaking fees from Almirall, Bayer, Biogen, Merck, Novartis, Roche, Sanofi, and Teva; and grant/research support from Biogen, Novartis, Sanofi, and Teva.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The CARE-MS I, CARE-MS II, and CAMMS03409 studies were funded by Sanofi and Bayer HealthCare Pharmaceuticals. Sanofi contributed to the conduct of the studies; collection, management, analysis, and interpretation of the data; and review of the manuscript. Apart from the Sanofi employees listed as authors, the funders had no role in preparation, approval, and decision to submit the manuscript for publication.
ORCID iDs: Dana Horáková https://orcid.org/0000-0003-1915-0036
Aaron Boster https://orcid.org/0000-0003-3056-9065
Antonio Bertolotto https://orcid.org/0000-0002-7052-1907
Tjalf Ziemssen https://orcid.org/0000-0001-8799-8202
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Dana Horáková, Department of Neurology and Center of Clinical Neuroscience, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic.
Aaron Boster, The Boster Center for Multiple Sclerosis, Columbus, USA.
Antonio Bertolotto, AOU San Luigi, Orbassano, Torino, Italy.
Mark S Freedman, University of Ottawa and the Ottawa Hospital Research Institute, Ottawa, Canada.
David H Margolin*, Sanofi, Cambridge, USA.
Tjalf Ziemssen, Center of Clinical Neuroscience, Carl Gustav Carus University Hospital, Dresden, Germany *Employees of Sanofi during study conduct and analysis..
References
- 1.Scalfari A, Neuhaus A, Daumer M, et al. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J Neurol Neurosurg Psychiatry 2014; 85: 67–75. [DOI] [PubMed] [Google Scholar]
- 2.Frohman EM, Filippi M, Stüve O, et al. Characterizing the mechanisms of progression in multiple sclerosis: evidence and new hypotheses for future directions. Arch Neurol 2005; 62: 1345–1356. [DOI] [PubMed] [Google Scholar]
- 3.Gross HJ, Watson C. Characteristics, burden of illness, and physical functioning of patients with relapsing-remitting and secondary progressive multiple sclerosis: a cross-sectional US survey. Neuropsychiatr Dis Treat 2017; 13: 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markowitz CE. Multiple sclerosis update. Am J Manag Care 2013; 19: 294–300. [PubMed] [Google Scholar]
- 5.Fox RJ, Thompson A, Baker D, et al. Setting a research agenda for progressive multiple sclerosis: the International Collaborative on Progressive MS. Mult Scler 2012; 18: 1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorscheider J, Buzzard K, Jokubaitis V, et al. Defining secondary progressive multiple sclerosis. Brain 2016; 139: 2395–2405. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012; 380: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 8.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012; 380: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 9.Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 2017; 89: 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology 2017; 89: 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanofi Belgium. LEMTRADA summary of product characteristics, www.ema.europa.eu/en/documents/product-information/lemtrada-epar-product-information_en.pdf (2020, accessed 27 August 2020).
- 12.Azevedo CJ, Kutz C, Dix A, et al. Intracerebral haemorrhage during alemtuzumab administration. Lancet Neurol 2019; 18: 329–331. [DOI] [PubMed] [Google Scholar]
- 13.Cuker A, Bass AD, Nadj C, et al. Immune thrombocytopenia in alemtuzumab-treated MS patients: incidence, detection, and management. Mult Scler 2020; 26: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phelps R, Winston JA, Wynn D, et al. Incidence, management, and outcomes of autoimmune nephropathies following alemtuzumab treatment in patients with multiple sclerosis. Mult Scler 2019; 25: 1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wray S, Havrdova E, Snydman DR, et al. Infection risk with alemtuzumab decreases over time: pooled analysis of 6-year data from the CAMMS223, CARE-MS I, and CARE-MS II studies and the CAMMS03409 extension study. Mult Scler 2019; 25: 1605–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cella DF, Dineen K, Arnason B, et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology 1996; 47: 129–139. [DOI] [PubMed] [Google Scholar]
- 17.Ware J, Kosinski M, Bjorner J, et al. Determining important differences in scores. In: User’s manual for the SF-36v2® health survey. Lincoln, RI: QualityMetric Inc., 2007, pp.125–133.
- 18.Van Reenen M, Oppe M. EQ-5D-3L user guide Rotterdam, Netherlands: EuroQol Research Foundation, 2015.
- 19.Arroyo R, Bury DP, Guo JD, et al. Impact of alemtuzumab on health-related quality of life over 6 years in CARE-MS II trial extension patients with relapsing-remitting multiple sclerosis. Mult Scler 2020; 26: 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T, Shirani A, Zhao Y, BC MS Clinic Neurologists et al. Beta-interferon exposure and onset of secondary progressive multiple sclerosis. Eur J Neurol 2015; 22: 990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuohy O, Costelloe L, Hill-Cawthorne G, et al. Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry 2015; 86: 208–215. [DOI] [PubMed] [Google Scholar]
- 22.Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 2019; 321: 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kappos L, Traboulsee A, Constantinescu C, et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology 2006; 67: 944–953. [DOI] [PubMed] [Google Scholar]
- 24.Kappos L, Bar-Or A, Cree B, et al. Efficacy and safety of siponimod in secondary progressive multiple sclerosis – results of the placebo controlled, double-blind, phase III EXPAND study. Mult Scler 2016; 22 (S3): 828–883. [Google Scholar]
- 25.Miller AE, O'Connor P, Wolinsky JS, et al. Pre-specified subgroup analyses of a placebo-controlled phase III trial (TEMSO) of oral teriflunomide in relapsing multiple sclerosis. Mult Scler 2012; 18: 1625–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkovich R. Effects of alemtuzumab on disability and cognition in patients with secondary progressive multiple sclerosis (SPMS). Neurology 2017; 88 (16 Supplement): P5.356. [Google Scholar]
- 27.Coles AJ, Wing MG, Molyneux P, et al. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol 1999; 46: 296–304. [DOI] [PubMed] [Google Scholar]
- 28.Biogen. BG00012 and disability progression in secondary progressive multiple sclerosis (SPMS) (INSPIRE), https://clinicaltrials.gov/ct2/show/NCT02430532 (2017, accessed 15 November 2017).
- 29.US Food and Drug Administration. FDA approves new oral treatment for multiple sclerosis, www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm634837.htm (2019, accessed 15 April 2019).
- 30.Novartis. Novartis receives FDA approval for Mayzent® (siponimod), the first oral drug to treat secondary progressive MS with active disease, www.novartis.com/news/media-releases/novartis-receives-fda-approval-mayzent-siponimod-first-oral-drug-treat-secondary-progressive-ms-active-disease (2019, accessed 3 June 2019).
- 31.Bergamaschi R, Berzuini C, Romani A, et al. Predicting secondary progression in relapsing-remitting multiple sclerosis: a Bayesian analysis. J Neurol Sci 2001; 189: 13–21. [DOI] [PubMed] [Google Scholar]
- 32.Riise T, Gronning M, Fernandez O, et al. Early prognostic factors for disability in multiple sclerosis, a European multicenter study. Acta Neurol Scand 1992; 85: 212–218. [DOI] [PubMed] [Google Scholar]
- 33.Trojano M, Avolio C, Manzari C, et al. Multivariate analysis of predictive factors of multiple sclerosis course with a validated method to assess clinical events. J Neurol Neurosurg Psychiatry 1995; 58: 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fambiatos A, Jokubaitis V, Horakova D, et al. Risk of secondary progressive multiple sclerosis: a longitudinal study. Mult Scler 2020; 26: 79–90. [DOI] [PubMed] [Google Scholar]
- 35.Lövblad KO, Anzalone N, Dörfler A, et al. MR imaging in multiple sclerosis: review and recommendations for current practice. Am J Neuroradiol 2010; 31: 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mso-10.1177_2055217320972137 for Proportion of alemtuzumab-treated patients converting from relapsing-remitting multiple sclerosis to secondary progressive multiple sclerosis over 6 years by Dana Horáková, Aaron Boster, Antonio Bertolotto, Mark S Freedman, Isabel Firmino*, Steven J Cavalier, Alan K Jacobs*, Karthinathan Thangavelu*, Nadia Daizadeh, Elizabeth M Poole*, Darren P Baker, David H Margolin*, Tjalf Ziemssen and on behalf of the CARE-MS I, CARE-MS II, and CAMMS03409 Investigators in Multiple Sclerosis Journal—Experimental, Translational and Clinical