Abstract
Objectives: To determine the prevalence and factors associated with the use of opioids among patients with chronic non-cancer pain (CNCP).
Methods: A systematic review and meta-analysis. Comprehensive literature searches in Medline-PubMed, Embase and SCOPUS databases. Original studies published between 2009 and 2019 with a cross-sectional design were included. The quality of the studies was assessed with Critical Appraisal Checklist for Studies Reporting Prevalence Data from the Joanna Briggs Institute. Protocol registered in the International Prospective Register of Systematic Reviews with reference number: CRD42019137990.
Results: Out of the 1,310 potential studies found, 25 studies fulfilled the inclusion criteria. Most of the studies were of high quality. High levels of heterogeneity were found in the studies included. In the general population, the prevalence of long-term opioid use was 2.3% (95% CI: 1.5–3.6%), the prevalence of short-term opioid use was 8.1% (95% CI: 5.6–11.6%), and among people with chronic low back pain it was 5.8% (95% CI: 0.5–45.5%). The prevalence of opioid use among patients from the health records or medical surveys was 41% (95% CI: 23.3–61.3%). Finally, in patients with musculoskeletal pain, the prevalence was 20.5% (95% CI: 12.9–30.9%) and in patients with fibromyalgia, 24.5% (95% CI: 22.9–26.2%). A higher prevalence of opioid use was observed among men, younger people, patients receiving prescriptions of different types of drugs, smokers and patients without insurance or with noncommercial insurance. In addition, non-white and Asian patients were less likely to receive opioids than non-Hispanic white patients.
Conclusions: The prevalence of opioid use among patients with CNCP was higher in subjects with short or occasional use compared to those with long-term use. Men, younger people, more chronic pain conditions, and patients without insurance or with noncommercial insurance were most related to opioid use. However, non-white and Asian patients, and those treated by a physician trained in complementary medicine were less likely to use opioids.
Keywords: chronic pain, meta-analysis, opioids, prevalence, systematic review
Introduction
Chronic pain (CP) is a major public health concern (Vos et al., 2012) that is associated with disability, distress, and a decrease in the quality of life of affected individuals (de Sola et al., 2016). The prevalence of moderate to severe CP in the general adult population ranges from 2 to 55% in different countries (Azevedo et al., 2012; Mohamed Zaki and Hairi, 2015; Mills et al., 2019), with an estimated global annual cost of over US$245 billion (GSK, 2017).
The physiopathology of CP has been recognized to involve complex interactions between physical, psychological, and social factors, and that its appropriate management requires a multidisciplinary approach (Broekmans et al., 2010). However, pharmacological therapy remains a mainstay for treating these patients (Timmerman et al., 2016), opioids being one class of pharmacotherapies that is frequently prescribed to modulate pain (Parsells Kelly et al., 2008).
Opioid therapy has attracted growing interest recently related to the increased use observed in CP patients (Cheung et al., 2014; Alam and Juurlink, 2015; Webster et al., 2017). This situation is of particular concern in patients with chronic non-cancer pain (CNCP), where the evidence of opioid therapy benefits may be less robust than that observed in patients with acute or cancer pain (Von Korff et al., 2011; Scholten, 2013; Campbell et al., 2015). The duration of opioid therapy is also important with regard to the benefits for patients, since the prescription of opioids may be appropriate for short-term pain relief, but long-term opioid therapy (LTOT) cannot be associated with improvements in pain or function (Karmali et al., 2020).
Furthermore, despite the lack of information on the efficacy of opioids (Warner, 2012), the introduction of high-dose and extended-release oral tablet formulations of opioids has been shown to increase the total prescriptions among CNCP patients, especially in the last decade (Von Korff et al., 2011; Alam and Juurlink, 2015; Severino et al., 2018). In some European countries, such as Spain, the use of opioids increased by 83.59% from 2008 to 2015 (De Sola et al., 2020). Additionally, in 2016, more than one-third of adults were prescribed opioids in the United States (Walker, 2018), making it an important social problem (Sehgal et al., 2012; Salazar et al., 2019). The differences in opioid prescribing patterns have been related to age, gender, ethnicity, pain diagnosis, number of total medications, payment type, physician specialty, and patient relationship with provider (Rasu and Knell, 2018).
Determining the prevalence of their use and factors underlying its prevalence can advance our understanding of current treatment practice and its impact on public health. Thus, it is necessary to collect updated information about the prevalence of the therapeutic use of opioids for CNCP in different countries, and summarize the information published. Additionally, it is necessary to take into account the length of the treatment and factors associated with it to produce international estimates.
To this end, we carried out a systematic review of the literature to know the prevalence of the therapeutic use of opioids in patients with CNCP and, as a second aim, to analyze the factors associated with their use. We also performed a meta-analysis of the prevalence of the therapeutic use of opioids to summarize the information obtained.
Following the PICOS method, the research question of this systematic review is: What are the prevalence and factors associated with the use of opioids among patients with CNCP?
Methods
Protocol and Registration
The present systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (Shamseer et al., 2015) (Supplementary Material SI). The study protocol was registered in the International Prospective Register of Systematic Reviews with reference number: CRD42019137990.
Design of the Study
Systematic review and meta-analysis.
Search Strategy
A systematic search strategy was built according to PICOS method and performed in the Medline-PubMed, Embase and SCOPUS databases. The terms/keywords of interest were “opioid,” “analgesic,” and “pain.” The terms were combined with the tag for searching in title, abstract and keywords. Search terms and search strategies were adapted to each database (Supplementary Material SII, TI). In light of the differences in the prevalence of opioid use in the last decade, the recent original studies, i.e., published in English or Spanish from January 2009 to December 2019 with a cross-sectional design were included.
Once the search strategies for all the databases were executed, we imported all the references found into the Covidence online tool (Veritas Health Innovation Ltd., 2019). The process of duplicate removal, screening, data extraction and risk of bias analysis were performed by this web-based systematic review tool.
Eligibility Criteria
The population of interest was people (all ages) with chronic non-malignant pain. Those studies related to CNCP located in specific body regions (e.g. musculoskeletal CP) were also included. Thus, the term “CNCP” was not included in the search strategy in order not to limit the searches to studies presenting only data from general CNCP. The criteria to define CNCP and the specific body regions that each study focuses on are specified in Table 2.
TABLE 2.
Characteristics of the studies included in the systematic review.
| First author, Year | Primary aim | Population | Sample source and timeframe | Method for data retrieval | N | Response Rate | Pain definition | Prevalence of CP | Method for obtaining the opioids prevalence | Prevalence of opioid use | FA to opioid use in CP patients |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Miller et al., 2019) | To analyze the prevalence of non-opioid prescribing among commercially- insured patients with CP | The commercially insured population (18–64 years) | MarketScan database. 2014 | Truven health MarketScan Research | 21,745,233 | NA | Patients with at least two outpatient visits in 90 days for CP ICD-9-CM code | 9.5% | Prescription drugs coverage using the National Drug code schema | 28.4% had prescriptions for both opioids and a non-opioid, 15.9% prescription for an opioid | — |
| (Rodondi et al., 2019) | To investigate among PC patients and their physicians in western Switzerland the prevalence of use, perceived usefulness, and communication about treatments for CLBP including complementary medicine | Patients with CLBP recruited during regular medical appointment. (≥18 years) | PC physician in western French-speaking area of Switzerland from November 1, 2015, to May 31, 2016 | Self-reported questionnaire | 499 | 37% | CLBP, defined as pain lasting or recurring for 3 months or more | The whole sample had CLBP (N = 499) | Self-reported use of assessed therapies | 52.5% of the CLBP patients | Current smoking was associated with using opioids (OR = 1.8; 95% CI: 1.1–3.1) - Patients from PC physician who were trained in complementary medicine were significantly less likely to use opioids (OR = 0.5; 95% CI: 0.3–0.9) |
| (Callhoff et al., 2020) | To analyze FA with the burden of OA, taking the pattern of joint involvement into account | Patients with OA of the knee or hip or with POA (30–79 years) | German statutory health insurance database (BARMER). Year 2016 | Survey and claims data | 8,995 | 42% | Persons with ICD- 10- GM (German Modification) diagnoses of OA in 2014 | The whole sample had OA (N = 3,564) - 758 POA - 959 hip OA. - 399 hip and knee OA- 1,448 knee OA | Analgesics were identified using ATC codes, counting patients as users if they had ≥1 prescription of the drug in that year | 14.9% (n = 531: 106 POA+134 hip OA+88 hip and knee+203 knee OA) of the total OA patients - 14% POA. - 14% hip OA. - 22% hip and knee OA- 14% knee OA. | — |
| (Lin et al., 2019) | To examine how prescription drug monitoring programs share data with bordering states and its association with patients being prescribed opioids for non-cancer CP treatment | Adult patients with CNCP (≥18 years) | National Ambulatory Medical Care Survey (NAMCS) 2014 | The NAMCS | Weighted N = 66,198,751; unweighted N = 2,846 | NA | The ICD-9-CM diagnosis codes provided by the NAMCS | The whole sample had CNCP (N = 2,846) | Electronic health records, including information on prescribed medications | 33.1% of the study sample | - Patients aged 25–49 vs. 18–25 years (OR = 2.78; 95% CI: 0.93–8.33) - Patients with Medicare (OR = 1.56; 95% CI: 1.03–2.38) or Medicaid coverage (OR = 2.08; 95% CI: 1.15–3.85) vs. who had private insurance coverage. - Patients being followed by the physician vs. naïve patients (OR = 2.33; 95% CI: 1.49–3.57) |
| (Van Den Driest et al., 2019) | To examine the analgesic used by patients with OA related pain and how the analgesics were used in the preceding month | Patients with rheumatic diseases (age not specified) | The panel of the Dutch Arthritis Foundation | Online questionnaire | 842 | 56% | Generalized OA was defined as self-reported OA in 3 or more groups of joints | The whole sample had OA (N = 842) | Self-reported medications used in the preceding month for OA related pain | 22% (n = 186: 44 use only opioids +142 use opioids combined with others drugs) of the patients with OA-related pain | — |
| (Shmagel et al., 2018) | To examine patterns of drugs prescription among Americans with CLBP in a nationally representative, community-based sample | A representative sample of US adult population (aged 20–69) | The National health and Nutrition Examination Survey. 2009–2010 | Home-based interviews with pill bottle verification to capture prescribed medications for CP. | 5,103 | NR | CLBP was defined as self-reported pain in the area between the lower posterior margin of the ribcage and the horizontal gluteal fold on most days for at least 3 months | 13.7% had CLBP (N = 700) | Self-reported prescription medications used within the past 30 days | 18.8% of working-age Americans with CLBP | - Low levels of education: For less than high school (OR = 3.07; 95% CI: 1.12–8.39) and for high school or associates’ degree (OR = 4.17; 95% CI: 1.73–10.03) compared with college education - <35,000$ of annual household income (OR = 1.92; 95% CI: 1.19–3.11) vs > 65,000 2 or more medical comorbidities (OR = 3.32; 95% CI: 1.74–6.35) vs none or one |
| (Scala et al., 2018) | To evaluate the level of readiness to practice different types of active self-care among CP patients | Patients with CP. (≥18 years) | Patients seeking care at the Pain Center University Hospital, Switzerland between June 2013 and March 2015 | Self- administered questionnaire | 639 | 41.9% | Pain lasting 6 months or more | The whole sample had CP (N = 639). The locations were back (71.4%), lower limb (68.4%), cervical spine (25.8%), an upper limb (25.2%) or a shoulder (23.0%) | Patients were asked whether they used non-opioid painkillers, opioids or dietary supplements ‘against pain” during the last six months | 64.6% of the study sample | — |
| (Sites et al., 2018) | To understand the relationship between prescription opioid use and satisfaction with care among adults with musculoskeletal conditions | Patients with musculoskeletal conditions (≥18 years) | Nationally representative data from the 2008–2014 Medical Expenditure Panel Survey | 5 rounds of telephone interviews over a 30-month period and questionnaire | 19,566 | NA | A combination of (ICD-9-CM) codes and patient self-reported data | The whole sample had musculoskeletal pain (N = 19,566) | Participants were asked to report prescription medication use and pharmacies were contacted to validate these prescriptions | 13.1% opioid users. - 29.2% as low-level users (2–4 opioid prescriptions) - 28.9% as moderate users (5–9) - 41.9% as heavy users. (10 or more) | — |
| (Knoop et al., 2017) | To describe the use of analgesics; and to determine FA with analgesic use in patients with knee and/or hip OA referred to an outpatient center | Patients referred to an outpatient center with knee and/or hip OA diagnosed (age not specified) | Amsterdam Osteoarthritis (AMS-OA) cohort in an outpatient center (reade, center for rehabilitation and rheumatology, the Netherlands) from December 2009 to July 2016 | Questionnaire | 656 | NR | Clinical knee and/or hip OA diagnosed, according to the American college of rheumatology criteria | The whole sample had OA (N = 656) | Patients were asked to list all medication used at that moment | 12% use of opioids 6% Tramadol 3% codeine 1%Prednisone 3% Other | — |
| (Miller et al., 2017) | To estimate the prevalence of CP and analgesia use in the Australian population by: age and sex; the severity of pain in the population with CP by sex; and the distribution of recent pain severity in those using analgesia by age and sex | Representative sample of Australian population. (All ages) | The ABS | Face-to-face interviews conducted by trained ABS interviewers in participants' homes | n = 20,426 participants from 15,565 private residences. 1 adult and 1 child aged 0–17 years (if applicable) in each participating household | 84.8% | Self-reported pain, which persisted over a 6-month period | - 12.7% of all ages (N = 2.8 million) - 15.4% (aged ≥15 years). -14.6% males -16.1% females | Opioid analgesia use included the any type of opioid analgesia over the previous 2 weeks. Participants were asked for the name or brand of all medication and to provide the packages to the interviewer | 12% males vs. 13.4% females (aged ≥15 years) | — |
| (Romanelli et al., 2017) | To evaluate opioid prescribing in an ambulatory setting among patients with CNCP | Adult patients with CP with a medical record in the EHR (≥18 years) | Using Sutter EHR (community-based open-network healthcare system in northern California) | The EHR | 1,784,114 | NA | Patients with 2 records of ICD-9 CM, diagnoses for a CNCP condition (pain lasts longer 3 months) at least 30 days apart | 6.8% (N = 120,481) | The electronic health records, including information on prescribed medications | Patients receiving any opioids among all CP Patients: 58.1% Short-acting (immediate-release) opioids: 57.4%. Long-acting opioid: 7% | CP Conditions per Patient by CP category: Arthritis/joint pain (OR = 1.39; 95% CI: 1.36–1.42) Back/cervical pain (OR = 1.07; 95% CI: 1.05–1.09) Neuropathies/neuralgias (OR = 1.65; 95% CI: 1.61–1.69) Headaches/migraines (OR = 1.51; 95% CI: 1.47–1.56), unclassified pain (OR = 1.48; 95% CI: 1.44–1.53). Patient demographic characteristics Older patients (≥66 years vs 18–45 years) (OR = 0.55; 95% CI: 0.52–0.58) Those with moderate chronic disease burden (CCI score = 2–3 vs 0) (OR = 0.92; 95% CI: 0.88–0.96) asians (vs. Non-Hispanic-White) (OR = 0.37; 95% CI: 0.33–0.40) Patients with higher odds of receiving an opioid were: Men (over women). Patients with non-commercial insurance, especially Medicaid (OR = 2.77; 95% CI: 2.56–3.01) Patients with more CP conditions (OR = 3.27; 95% CI: 3.15–3.40) |
| (Fain et al., 2017) | To quantify prescription analgesic use of elderly nursing home residents with persistent non-cancer pain and to identify individual and facility traits associated with no treatment | Elderly nursing home residents with persistent non-cancer pain. (≥65 years) | Individuals residing in a nursing home in U.S. at any time between December 2007, and November 2008 | The Minimum Data Set; the Online Survey, certification, and Reporting database; and Medicare Part D | 2.99 million individuals | NA | Moderate to severe daily pain lasting at least 3 months | 3.8% (N = 18,526) of eligible nursing home residents had persistent pain | Prescription drug used from Medicare Part D records. An opioid prescription dated within 30 days before or after persistent pain onset. | - 81.2% received an opioid drug (alone or in combination with acetaminophen or prescription NSAID). -16.2% had only opioids prescription | — |
| (Gouveia et al., 2017) | To analyze and characterize the intake profile of pain-relief drugs in a population-based study of adults with CLBP. | Portuguese adult population With self-reported active CLBP (>18 years) | Households selected by random route methodology | Face-to-face interview | 10,661 | NR | LBP lasting at least 90 days | 10.4% (CI 9.56%; 11.9%) (N = 1,487) | Information regarding analgesic and other pain-relief drugs was collected and organized according to the national drug agency classification | 1.6% (95% CI: 0.9–2.2) among population with active CLBP | — |
| (Ahn et al., 2016) | To assess medical care and costs of the 3 highest prevalence lumbar disorders -nLBP, IDD and SS- to provide basic information for standards of appropriate management | Patients included in 2011 Korean health Insurance Review and assessment Service (HIRA) (all ages) | National health Insurance billing data provided by HIRA. Year 2011 | 2011 HIRA National Patient Sample (NPS) | 1,375,842 | NA | Patients with a lumbar disorder coded by the Korean Classification of Diseases, adapted from the ICD-10 | 27% (Patient visiting medical institution with lumbar/spinal diagnostic codes N = 371,858) | Treatment prescriptions classified according to National Evidence-based healthcare collaborating Agency reports | 2.3% (n = 4,300: 761 nLBP +1994 IDD +1545 SS) of the total registers with lumbar disorder included for analyses (N = 188,985: 111,544 nLBP +48,413 IDD +28,842 SS) | — |
| (Birke et al., 2016) | To examine the trends regarding the prevalence of CNCP, dispensed opioids, and concurrent use of BZD/BZD-related drugs in the Danish population | Participants with CP (≥16 years) | The Danish National Cohort Study (DANCOS). Years 2000, 2005, 2010 and 2013 | In 2000 and 2005, face-to-face interviews and self- administered questionnaire. In 2010 and 2013, postal or web questionnaire | 16,684 in 2000 10,916 in 2005 25,000 in 2010 25,000 in 2013 | 63% in 2000 51% in 2005 61% in 2010 57% in 2013 | Pain lasting 6 months or more | -18.9% in 2000 - 20.2% in 2005 - 26.2% in 2010 - 26.8% in 2013 | Dispensed medicines from the Danish National Prescription registry using ATC codes. Long-term, having used at least one prescription/month for 6 months. Short- term having used at least one prescription in the previous year | Opioid users among individuals with CP Long-term - 1.3% in 2000. - 1.3% in 2005 - 1.7% in 2010. - 1.8% in 2013. Short-term - 2.8% in 2000. - 3.1% in 2005 - 3.8% in 2010. - 3.9% in 2013 | — |
| (Wand et al., 2016) | To present the outcomes of a comprehensive evaluation of the psychometric properties of the Fremantle Back Awareness Questionnaire and explore the potential relationships between body perception, nociceptive sensitivity, distress, and beliefs about back pain and the contribution these factors might play in explaining pain and disability | People with axial CLBP (between 18 and 70 years) | From 2 metropolitan hospitals in Western Australia, private metropolitan physiotherapy clinics, pain management and general practice clinics. Also, via multimedia advertisements circulated throughout the general community Western Australia | Self- administered questionnaire and a combination of clinical bedside tests and laboratory tests | 251 | NR | To have experienced LBP for >3 months, scored ≥2 on a numeric rating scale, and ≥5 on the roland Morris Disability Questionnaire | The whole sample had experienced LBP | Self-reported questionnaire about current pain medications | 15.9% of the 251 people with CLB | — |
| (Vincent et al., 2015) | To evaluate the problem of multiple chronic conditions and polypharmacy in patients with fibromyalgia | Patients with fibromyalgia. (≥21 years) | Patients identified via the rochester Epidemiology Project in Olmsted county, Minnesota. Between January 2005 and December 2009 | 1,111 | NR | Patients with a diagnosis of fibromyalgia (HICDA or ICD-9) | The whole sample had fibromyalgia | Using a unit (or dossier) medical record system, whereby data from an individual (demographics, diagnoses and billing records) | 22.4% among the 1,111 patients with fibromyalgia | — | |
| (Larochelle et al., 2015) | To characterize trends in opioid prescribing and co-prescribing of sedative hypnotics at acute and chronic musculoskeletal pain visits from 2001 to 2010 | Patients with musculoskeletal pain. (≥18 years) | Combining the NAMCS and National Hospital Ambulatory Medical Care Survey | Data collection was carried out by physicians, hospital staff, or census field representatives | 35,302 | NA | Pain lasting at least 3 months | 53% of the visits were for CP. | Drugs prescribed using Multum drug classification. The primary outcome was prescription or continuation of an opioid medication during the visit | Combining all years, opioids were prescribed to 20.8% (95% CI 18.9–22.6%) of CP visits. 12.9% (95% CI 9.7–16.0%) in 2001.28.2% (95% CI 21.4–34.9%) in 2007.23.1% (95% CI 18.3–27.9%) in 2010 | Patients aged 35–49 years vs. 50–64 years (OR = 1.32; 95% CI: 1.11–1.56). Hispanic vs. non-Hispanic whites (OR = 0.54; 95% CI: 0.39–0.74). Patients with Medicaid (OR = 1.46; 95% CI: 1.16–1.85), Medicare patients under age 65 years (OR = 2.34; 95% CI: 1.77–3.10), and patients without insurance (OR = 1.54; 95% CI: 1.21–1.96) vs. private insurance. Patients visiting their assigned PC provider (OR = 1.39; 95% CI: 1.15–1.68) and patients previously seen in that office (OR = 1.94; 95% CI: 1.52–2.49) |
| (Marschall et al., 2015) | To determine the prevalence and the demographic and medical predictors of LTOT, of high doses of LTOT and of abuse/addiction of prescribed opioids in a cohort of insureds with CNCP of a large German statutory health insurance | Persons insured by the German statutory medical health insurance. (Age not specified) | From the records of outpatient (Association of Statutory health Insurance Physicians bills) and inpatient care (hospital bills) of persons, insured by the German statutory medical health insurance plan Barmer GEK January 2012 and December 2012 | The Barmer GEK. | 870,000 | NA | According to the ICD-10-GM | The whole sample had CP. | Oral opioid prescriptions of outpatient care were identified by the Anatomical Therapeutic chemical Classification (ATC). The insurance organization LTOT prescriptions: Defined by at least one opioid prescription per quarter for at least three consecutive quarters (one quarter = 3 months) over the last 12 months. High-dose opioid therapy (defined by ≥ 100 mg MEQ/day) | LTOT prescription all insureds with CNCP 1.3% (range 1.2%; 1.4%). High-dose opioid therapy among LTOT patients 15.5% (range 14.2%; 16.5%) | — |
| (Kingsbury et al., 2014) | To examine the impact of peripheral joint OA across five large European countries and how people with OA use pharmacotherapies | The general population using the internet panel maintained by Lightspeed Research. (≥18 years) | Data were derived from the 2011 five European countries (5 EU) National health and Wellness Survey (NHWS) | Respondents were emailed a link to the survey to complete on their own. ≥65-year-old population were recruited by telephone and they had the choice to complete the interview on the phone | 57, 512: France: n = 15,000 Germany:n = 15,001 Italy: n = 7,500 Spain: n = 5,011 UK: n = 15,000 | NR | Respondents who self-reported a physician diagnosis of OA | OA prevalence 6.5% - UK 10.9% - France 6.4% - Germany 3.8% - Spain 6.3% - Italy 3.6% | Respondents were asked whether they currently use prescription to treat their arthritis; if so, they were asked to indicate what they were currently using | 16.7% among respondents with diagnosis of OA (N = 3,750) - 19.3% in the UK (N = 1,635) - 27.7% in France (N = 961) -3.5% in Germany (N = 570) - 6.9% in Spain (N = 316) - 0.7% in Italy (N = 268) | — |
| (Fredheim et al., 2014) | To know the prevalence of persistent opioid use among people in the general population with self-reported CNCP | All inhabitants in the county of Nord-Trondelag in Norway (≥20 years) | Linkage of the National Norwegian prescription database and the Nord-Trøndelag health study 3 2006–2008 | 2 Postal questionnaires and a physical examination | 45,837 | NR | Pain lasting 6 months or more and pain of at least moderate intensity during the last week before participation in HUNT 3 | 31.6% | Prescription drugs dispensed at pharmacies from The National Norwegian Prescription Database. Two different definitions of persistent opioid use included: The wide definition clinically corresponds to using opioids most days of the week (>180 DDD or 4500 OMEQ) -The strict definition to using opioids around the clock all days (>730 DDD or 18,000 OMEQ). Data on dispensed opioid prescriptions during the 6 months immediately before participation in HUNT 3 | Opioid users among individuals with CP Persistent opioid use 2.9% Occasional opioid use 12.3% | - Being younger than 56 years old (OR = 2.22; 95% CI: 1.65; 2.99) - Male (OR = 1.49; 95% CI: 2–1.11) - A current smoker (OR = 2.95; 95% CI: 1.36–2.94) - using more than 100 DDD of benzodiazepines per year (OR = 5.55; 95% CI: 3.74–8.23) - Receiving prescriptions of drugs from several ATC classes (OR = 4.98; 95% CI: 3.31–7.48) |
| (Azevedo et al., 2013) | To describe the prevalence and FA with opioid use in subjects with CP in Portugal and to evaluate satisfaction and self-assessed treatment effectiveness | A representative sample of the adult Portuguese population (≥18 years) | Random digit dialing | A structured questionnaire conducted by CATI. | 5,094 | 76% among responding households and 51% among all identified households | Pain lasting at least 3 months | 35.7% (95% CI: 34.38–37.02) | Respondents were asked if they were using any pain medicine. If so, they were asked for the drugs and the frequency | 4.24% (95% CI: 3.31–5.41) among participants who responded if they were using any pain medicine (N = 1786) | -Pain-related disability PDI (per increase in 10 units) (OR = 1.23; 95% CI: 1.02–1.50) |
| (Henderson et al., 2013) | To determine the prevalence of CP, its causes, severity, management, impact on sleep, mood and activity levels, and GP and patient satisfaction with pain management | Patients attending General Practice. (All ages) | The BEACH (Bettering the Evaluation and Care of health), an Australian General Practice program | Questionnaires were completed by the GP in discussion with the patient, using the combined knowledge of both | 5,793 | 79% | Pain experienced every day for three months in the six months prior to this consultation | 18.8% (95% CI: 17.8–19.8) | Respondents were asked if their pain was being managed and how. If the answer was “with medication”, they were asked to specify which medication | 32.7% (n = 343: 365 took opioids - 22 people with cancer) among respondents with CNCP who responded to the management question (N = 1,049 =1,074 – 25 people with cancer) | — |
| (Häuser et al., 2012) | To conduct the first European FMS consumer reports on the effectiveness and side effects of FMS-therapies in routine clinical care | Members of the self-help organizations with diagnosis of FMS (age not specified) | From the two largest German FMS-self-help organizations and nine clinical institutions. 2010–2011 | Self-reported questionnaires | 1,661 | NR | FMS-diagnosis >1 month’s duration | The whole sample had FMS | Participants were asked to “indicate whether they currently use any interventions for FMS”. The interventions, including drugs, were listed in different sections | - 17.6% Weak opioids - 8.4% Strong opioids | — |
| (Kurita et al., 2012) | To estimate the current prevalence of CP in the Danish population, CP prevalence in immigrants of different origin in Denmark, CP prevalence related to potential FA. | Individuals living in Denmark (≥16 years) | From the Danish health Survey and official Danish health and socioeconomic, individual-based registers. 2010 | A self-reported survey questionnaire and administrative registers | 25,000 | 60.7% | Chronic Pain: Participants who responded they had chronic/long-lasting pain lasting 6 months or more | 24.7% (95% CI: 24.0–25.4) CNCP (N = 14,925) | Dispensed prescription medications obtained from the Danish National Prescription registry and linked on an individual level to the survey data. Use of medication was defined as at least 1 dispensed drug during the 90 days before survey completion | 12.3% (N = 3,305) | — |
EHR, Electronic health Record system; ABS, Australian Bureau of Statistics; NAMCS, National Ambulatory Medical Care Survey; POA, polyarthritis; FMS, fibromyalgia syndrome; LTOT, long-term opioid therapy; CP, chronic pain; PC, primary care, CLBP, chronic low back pain; LBP, low back pain; nLBP, non-specific low back pain; IDD, intervertebral disc disorder; SS, spinal stenosis; CNCP, chronic non-cancer pain; OA, osteoarthritis; BDZ, benzodiazepine; FA, factors associated; GP, general practitioner; CATI, computer-assisted telephone interviews; NA, not applicable; NR, not reported (Mezcla de mayúsculas y minúsculas).
In this review, we exclusively focused on opioid treatment as an intervention for pain. The use of opioid treatment can be defined as self-reported use, self-reported prescription medications and prescription, or dispensed drugs retrieved from electronic health records (see Table 2). A study was selected when its main outcome was the prevalence of the use of opioids in CNCP, as long as these data were shown within the study or it was possible to calculate prevalence from it. Studies analyzing the factors associated with the use of opioids were also included.
Studies reporting the use of opioids in patients with acute, post-operative, palliative, or cancer pain were excluded. Studies focusing on the opinions or attitudes of physicians about opioid prescription or on the disorders derived from their consumption were also excluded (Supplementary Material SII, T2).
Study Selection
Two authors (MD and HS) independently screened the title and abstract of all of studies. Shortlisted studies were then analyzed in depth according to the inclusion criteria, and their reference lists were also revised to identify studies that could be included in the review.
Risk of Bias Assessment
The quality of the studies was assessed following the Critical Appraisal Checklist for Studies Reporting Prevalence Data from the Joanna Briggs Institute (Munn et al., 2015). This checklist consists of nine items regarding sample frame, appropriate recruitment, adequate sample size, appropriate description of the subjects and setting, data analysis, method used, the reliability of condition measures, appropriateness of the statistical analysis and response rate (Table 1). Each item was assessed as “yes,” “no,” “unclear” or “not applicable.” For standardization, “yes” was considered to imply a low risk of bias, and “no” and “unclear” a high risk of bias. Since there is not a standard classification for the “Critical Appraisal Checklist for Studies Reporting Prevalence Data from the Joanna Briggs Institute,” some systematic reviews were consulted to guide our classification (Porto De Toledo et al., 2017; Bett et al., 2019; Peterson et al., 2019). A study was considered to have a low risk of bias (i.e., high-quality study) when it accumulated at least seven items answered as “yes” and a moderate risk of bias when the study reached 4–6 “yes.” Any disagreements regarding the suitability of a study were resolved by a third author (AS).
TABLE 1.
Risk of bias assessment. Checklist for studies reporting prevalence data from the Joanna Briggs Institute (N = 25).
| Author name, year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 |
|---|---|---|---|---|---|---|---|---|---|
| Ahn, 2016 | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | NA |
| Azevedo, 2013 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Birke, 2016 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Callhof, 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Fain, 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA |
| Fredheim, 2014 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Gouveia, 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Häuser, 2012 | Yes | Yes | Unclear | Yes | Yes | Yes | No | Yes | No |
| Henderson, 2013 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Kingsbury, 2014 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Knoop, 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Kurita, 2012 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Larochelle, 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA |
| Lin, 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA |
| Marschall, 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA |
| Miller, 2017 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Miller, 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA |
| Rodondi, 2019 | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Romanelli, 2017 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | NA |
| Scala, 2018 | Yes | Yes | Unclear | Yes | Yes | No | Yes | Yes | No |
| Shmagel, 2018 | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Sites, 2018 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA |
| Van den Driest, 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Vincent, 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Wand, 2016 | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
Q1, Was the sample frame appropriate? Q2, Participants were appropriately recruited? Q3, Sample size was adequate? Q4, Study subjects and setting were described? Q5, Was the data analysis conducted with sufficient coverage of the identified sample? Q6, Were valid methods used for the identification of the condition? Q7, Was the condition measured in a standard, reliable way for all participants? Q8, Was there appropriate statistical analysis? Q9, Was the response rate adequate or managed appropriately? NA, Not applicable.
Data Extraction
Information was extracted about the primary aim of the study, the characteristics of the population, the sample source, sample size, method for data retrieval, response rate, the definition of CNCP considered in each study, the prevalence of CNCP in the population studied, the prevalence of opioid use, the method for obtaining this prevalence data, and the factors associated with opioid use (Table 2).
Statistical Analysis
A descriptive analysis of the characteristics of all the studies included in the systematic review was carried out. A meta-analysis was performed if two or more studies reported the same characteristics in the information provided and the same measure of effect. In order to manage heterogeneity, the studies were first grouped according to the following aspects: the source of the sample (the general population or health registries/medical surveys); the duration of opioid treatment [long-term, commonly defined as more 3 months or short-term (Karmali et al., 2020)] and the type of pain (Table 3).
TABLE 3.
Characteristics of the subgroups and results of the meta-analysis.
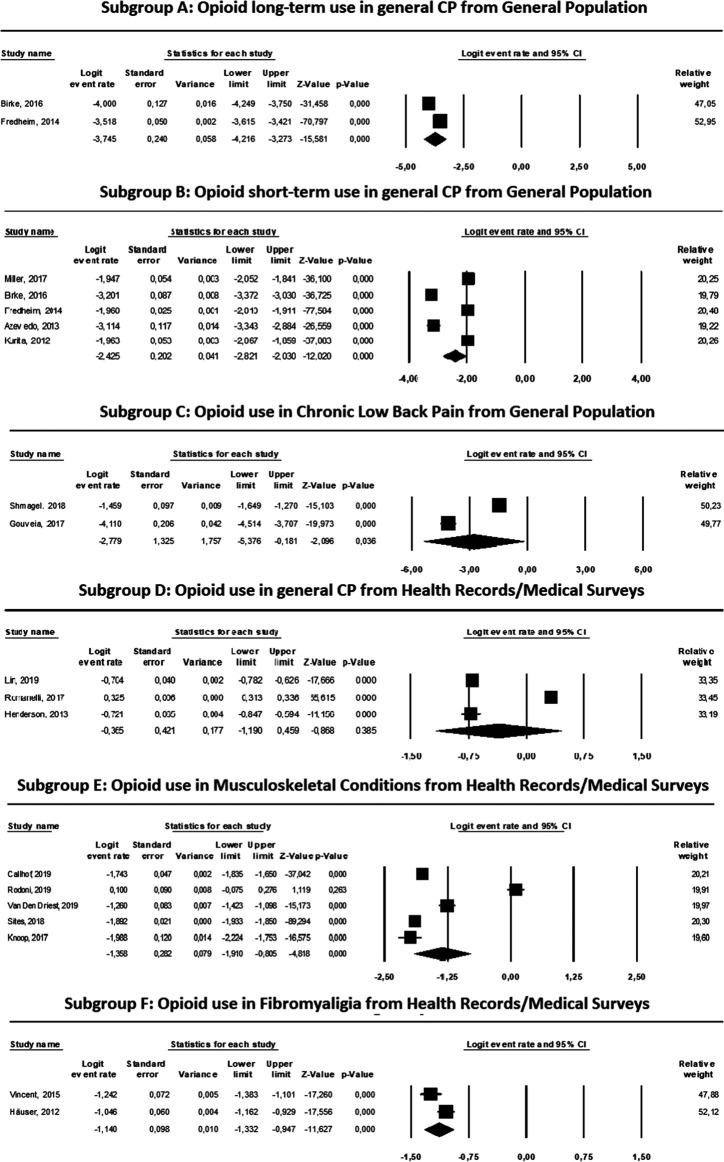
| Subgroup | Source | Type of pain | Treatment duration | Heterogeneity test | Study | Events | Sample Size | Prevalence (CI 95%) | Publication Bias |
|---|---|---|---|---|---|---|---|---|---|
| A | General Population Surveys | General Chronic pain | Long-term use | Q = 12.44; df = 1; p < 0.001 I 2 = 91.96 There is heterogeneity | Birke, 2016 | 63 | 3,501 | 1.8 (1.4–2.3) | — |
| Fredheim, 2014 | 417 | 14,477 | 2.9 (2.6–3.2) | ||||||
| Summary Prevalence | 2.3 (1.5–3.6) | ||||||||
| B | General Population Surveys | General Chronic pain | Short-term use | Q = 275.47; df = 4; p < 0.001 I 2 = 98.55 There is heterogeneity | Miller, 2017 | 393 | 3,146 | 12.5 (11.4–13.7) | Egger’s test: p = 0.1119 Begg’s test: p = 0.2207 |
| Birke, 2016 | 137 | 3,501 | 3.9 (3.3–4.6) | ||||||
| Fredheim, 2014 | 1787 | 14,477 | 12.3 (11.8–12.9) | ||||||
| Azevedo, 2013 | 76 | 1786 | 4.3 (3.4–5.3) | ||||||
| Kurita, 2012 | 407 | 3,305 | 12.3 (11.2–13.5) | ||||||
| Summary Prevalence | 8.1 (5.6–11.6) | ||||||||
| C | General Population Surveys | Chronic Low Back Pain | Unspecified | Q = 135.96; df = 1; p < 0.001 I 2 = 99.26 There is heterogeneity | Shmagel. 2018 | 132 | 700 | 18.9 (16.1–21.9) | — |
| Gouveia, 2017 | 24 | 1,487 | 1.6 (1.1–2.4) | ||||||
| Summary Prevalence | 5.8 (0.5–45.5) | ||||||||
| D | Health records or Medical Surveys | General Chronic pain | Unspecified | Q = 901.59; df = 2; p < 0.001 I 2 = 99.78 There is heterogeneity | Lin, 2019 | 942 | 2,846 | 33.1 (31.4–34.9) | Egger’s test: p = 0.1662. Begg’s test: p = 0.6015 |
| Romanelli, 2017 | 69,935 | 120,481 | 58.0 (57.8–58.3) | ||||||
| Henderson, 2013 | 356 | 1,088 | 32.7 (30.0–35.6) | ||||||
| Summary Prevalence | 41.0 (23.3–61.3) | ||||||||
| E | Health records or Medical Surveys | Musculoskeletal Conditions | Unspecified | Q = 509.24; df = 4; p < 0.001 There is heterogeneity | Callhof, 2019 | 531 | 3,564 | 14.9 (13.8–16.1) | Egger’s test: p = 0.2391 Begg’s test: p = 0.3272 |
| Rodoni, 2019 | 262 | 499 | 52.5 (48.1–56.9) | ||||||
| Van den driest, 2019 | 186 | 842 | 22.1 (19.4–25.0) | ||||||
| Sites, 2018 | 2,564 | 19,566 | 13.1 (12.6–13.6) | ||||||
| Knoop, 2017 | 79 | 656 | 12.0 (9.8–14.8) | ||||||
| Summary Prevalence | 20.5 (12.9–30.9) | ||||||||
| F | Health records or Medical Surveys | Fibromyalgia | Unspecified | Q = 4.412; df = 1; p = 0.036 I 2 = 77.34 There is heterogeneity | Vincent, 2015 | 249 | 1,111 | 22.4 (20.1–25.0) | — |
| Häuser, 2012 | 381 | 1,465 | 26.0 (23.8–28.3) | ||||||
| Summary Prevalence | 24.5 (22.9–26.2) | ||||||||
All the models are random effects models, given the heterogeneity observed in all the subgroups. Q, Cochran’s Q; df, degrees of freedom; p, p-value; I 2, The I 2 statistic (percentage of variation across studies that is due to heterogeneity)
Six subgroups were established. Group A included studies carried out in the general population including people with CNCP, where the duration of the use of opioids was long-term or persistent (Fredheim et al., 2014; Birke et al., 2016). Group B included studies in the general population including people with CNCP, but in which the duration of the use of opioids was short-term (Kurita et al., 2012; Azevedo et al., 2013; Fredheim et al., 2014; Birke et al., 2016; Miller et al., 2017). Group C included studies in the general population which analyze people with chronic low back pain (CLBP) who had been using opioids (Gouveia et al., 2017; Shmagel et al., 2018). Group D consisted of studies that included patients with CNCP from health registries who had been using opioids (Henderson et al., 2013; Romanelli et al., 2017; Lin et al., 2019). Group E included studies with patients from medical surveys with musculoskeletal conditions [comprising musculoskeletal pain, osteoarthritis and CLBP, following The International Classification of Diseases (Ministerio De Sanidad Consumo y Bienestar Social, 2020)] and who had been using opioids at the moment of the study (Knoop et al., 2017; Sites et al., 2018; Rodondi et al., 2019; Van Den Driest et al., 2019; Callhoff et al., 2020). Finally, group F included studies of fibromyalgia patients from medical surveys who had been using opioids (Häuser et al., 2012; Vincent et al., 2015) (Table 3).
Studies carried out in a population of a specific age (Fain et al., 2017), those that could not be compared with any other study, such as those focused on a specific type of pain (Kingsbury et al., 2014; Marschall et al., 2015), those from specific sample sources (Wand et al., 2016; Scala et al., 2018; Miller et al., 2019), and those focused on visits rather than the patients (with the potential overlapping of the records of the patients) (Larochelle et al., 2015; Ahn et al., 2016) were excluded from the meta-analysis.
The measurement of effect for each study and the summary measure (prevalence of opioid use, defined as the number of subjects taking opioids divided by the number of individuals with CNCP) were calculated with 95% CI. Also, the logit transformation with 95% CI and with standard error and variance were obtained to stabilize the variance (Barendregt et al., 2013). Studies were weighted according to the prevalence of the effect size and the inverse of the study variance.
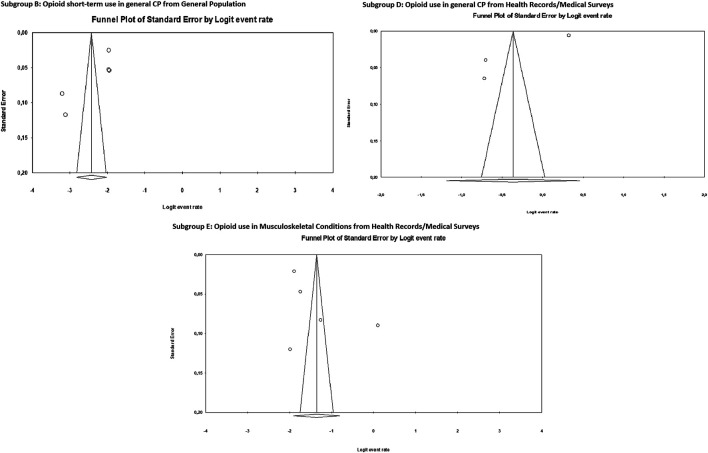
The heterogeneity between the studies was determined by the DerSimonian and Laird method with Cochran’s Q statistic. As heterogeneity was observed in all the study subgroups, random effects models were performed, which considers the variability of the results due to the differences between the studies. The proportion of total variability due to the heterogeneity of the studies was estimated using the I 2 value. The results of the meta-analysis are presented in forest plots. To assess the potential publication bias in groups with three or more studies, a funnel plot, along with Begg’s rank correlation and Egger’s weighted regression methods, were used. Two-tailed p < 0.05 was considered indicative of a statistically significant publication bias.
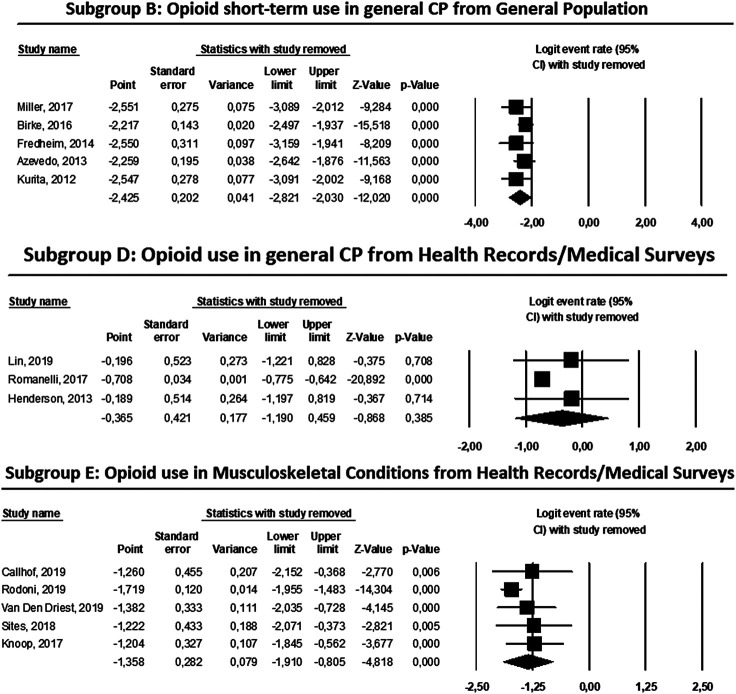
Finally, a sensitivity analysis was carried out in groups with three or more studies to determine the influence of each of the studies on the overall estimate of the effect, and therefore the robustness or stability of the final measurement obtained, through influence graphs.
The data were analyzed using Comprehensive Meta-Analysis Software Version 3.0 (Biostat, Englewood, NJ, USA).
Results
The search identified 1,310 potential studies. After the selection process (Figure 1), 22 suitable studies were identified. Three more studies obtained by the additional search strategies (citation search) were added.
FIGURE 1.
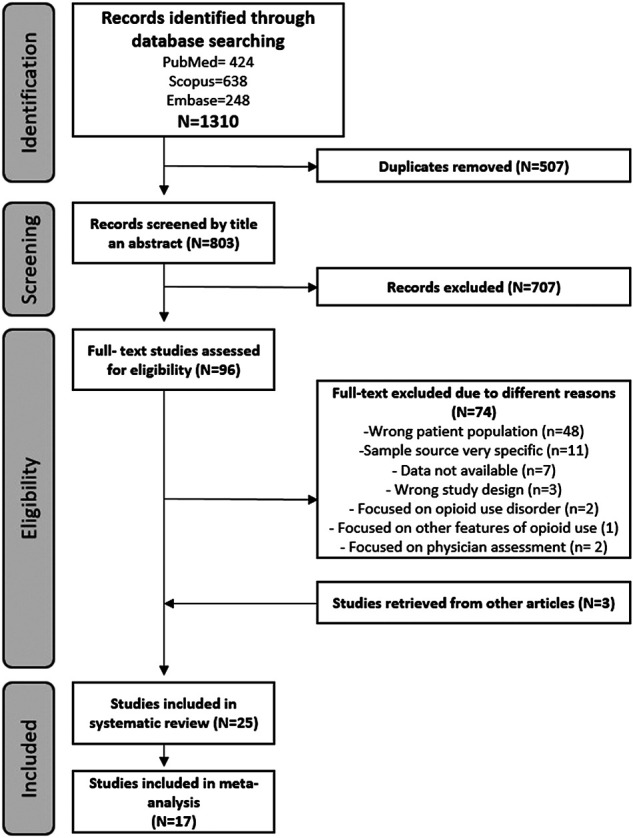
Flowchart.
Risk of Bias Assessment
Twenty-two of the 25 studies that remained fulfilled at least seven items of the checklist, indicating a low risk of bias. The response rate (Q9) was not reported by seven studies and the use of valid methods for the identification of the condition (Q6) was not reported by 4 (Table 1).
Study Characteristics
Out of these 25 studies, nine had been performed in the general population (Kurita et al., 2012; Azevedo et al., 2013; Fredheim et al., 2014; Kingsbury et al., 2014; Ahn et al., 2016; Birke et al., 2016; Gouveia et al., 2017; Miller et al., 2017; Shmagel et al., 2018), and sixteen in patients with CNCP from medical surveys or medical records (Häuser et al., 2012; Henderson et al., 2013; Larochelle et al., 2015; Marschall et al., 2015; Vincent et al., 2015; Wand et al., 2016; Fain et al., 2017; Knoop et al., 2017; Romanelli et al., 2017; Scala et al., 2018; Sites et al., 2018; Lin et al., 2019; Miller et al., 2019; Rodondi et al., 2019; Van Den Driest et al., 2019; Callhoff et al., 2020) (Table 2). The data were gathered from thirteen countries. Most of the studies (n = 15) were restricted to adult populations (18 years or older), whereas two study also included adolescents (≥16 years) (Kurita et al., 2012; Birke et al., 2016), three included children (all ages) (Henderson et al., 2013; Ahn et al., 2016; Miller et al., 2017), and one only included people over 65 (Fain et al., 2017). In four studies, the age was not specified. Thirteen studies were performed in patients suffering from a chronic painful process of specific cause (e.g., musculoskeletal pain) (Häuser et al., 2012; Kingsbury et al., 2014; Larochelle et al., 2015; Vincent et al., 2015; Ahn et al., 2016; Wand et al., 2016; Gouveia et al., 2017; Knoop et al., 2017; Shmagel et al., 2018; Sites et al., 2018; Rodondi et al., 2019; Van Den Driest et al., 2019; Callhoff et al., 2020). The reported participation rates in the studies ranged from 37% (Rodondi et al., 2019) to 84.8% (Miller et al., 2017), but in some instances, the information given by the authors was missing or unclear (Table 2). CP was defined as pain lasting at least 6 months in five of the studies included (Kurita et al., 2012; Fredheim et al., 2014; Birke et al., 2016; Miller et al., 2017; Scala et al., 2018), while in the rest, it was considered as pain lasting longer than 3 months. The prevalence of CNCP in the studies carried out in the general population ranged from 6.8% (Romanelli et al., 2017) to 35.7% (Azevedo et al., 2013) (Table 2).
Prevalence of Opioid use
Out of the nine studies set in the general population, two distinguished between short-term or occasional opioid users and long-term or persistent opioid users (Fredheim et al., 2014; Birke et al., 2016). The prevalence was higher in those in which the use was short or occasional (3.9%–12.3% vs. 1.8%–2.9%) (Kurita et al., 2012; Fredheim et al., 2014; Birke et al., 2016). Three studies (out of nine in the general population) focused on CLBP, and the prevalence ranged from 1.6% (Gouveia et al., 2017) to 18.8% (Shmagel et al., 2018). Another study retrieving data from five countries focused on osteoarthritis, the total prevalence of opioid being use 16.7% (Kingsbury et al., 2014).
In the studies analyzing the population from medical registries or medical surveys, the use of opioids was variable: 32.7% in patients treated in general practices (Henderson et al., 2013), 28.4% among commercially insured patients (Miller et al., 2019) and 64.4% in patients receiving care in a pain center (Scala et al., 2018). In the studies in patients suffering a specific pain condition, the use of opioids ranged from 13.1 to 20.8% in the case of musculoskeletal pain (Larochelle et al., 2015; Sites et al., 2018), from 12% (Knoop et al., 2017) to 22% in osteoarthritis (Callhoff et al., 2020) and from 8.4% (Häuser et al., 2012) to 22.4% in fibromyalgia (Vincent et al., 2015). The highest prevalence of opioid use was 81.2% in a study performed in a nursing home with people ≥65 years (Fain et al., 2017) (Table 2).
Factors Associated with the use of Opioids
Seven of the studies included in the review analyzed the factors associated with the use of opioids, reporting a greater use of these drugs in men (Fredheim et al., 2014; Romanelli et al., 2017), in young people (Fredheim et al., 2014; Larochelle et al., 2015; Romanelli et al., 2017), in patients receiving prescriptions for different kinds of drugs (Fredheim et al., 2014), in people with a lower educational level (Shmagel et al., 2018), in smokers (Rodondi et al., 2019), and in patients without insurance or with noncommercial insurance, especially Medicaid and Medicare, vs. those with private insurance (Larochelle et al., 2015; Romanelli et al., 2017; Lin et al., 2019) (Table 2).
The use of opioids was also related to the physician. Patients followed by a physician had higher odds of being prescribed an opioid than naive patients (Lin et al., 2019). Moreover, if the primary care physician was trained in complementary medicine, he/she was significantly less likely to prescribe opioids (Rodondi et al., 2019).
Additionally, opioid use was greater in patients with a pain-related disability (Azevedo et al., 2013) and in those with more CP conditions (Romanelli et al., 2017; Shmagel et al., 2018). However, patients with a higher score on the Charlson Comorbidity Index (2–3 vs. 0) had lower odds of receiving an opioid (Romanelli et al., 2017) (Table 2).
Race was related to the use of opioids in two studies, which showed that non-white patients (Larochelle et al., 2015) and Asian patients (Romanelli et al., 2017) were less likely to receive opioids than non-Hispanic white patients (Table 2).
Results of the Meta-Analysis
The characteristics and results of the meta-analysis (heterogeneity tests, estimated prevalences with 95% CI, relative weights and tests for publication bias) of the studies included in each of the six subgroups are shown in Table 3, and the results of the logit transformations are shown in Figure 2.
FIGURE 2.
Results and ForestPlots of the meta-analyses for opioid use in different types of pain from diferent sources.
As shown in Table 3, we found heterogeneity within the groups, demonstrating a marked variability among the estimates (I 2 > 77, p < 0.05, in all cases). Therefore, the model used for the estimations of the summarized prevalence and logit transformation was the random effects model.
Among the results obtained, it is noteworthy that in the general population, the prevalence of long-term opioid use among patients with general CNCP was 2.3% (95% CI: 1.5–3.6%), the prevalence of short-term opioid use was 8.1% (95% CI: 5.6–11.6%), and the prevalence in CLBP was 5.8% (95% CI: 0.5–45.5%). The prevalence among patients from health registries or medical surveys was 41% (95% CI: 23.3–61.3%) in patients with general CNCP. The prevalence in patients with musculoskeletal conditions was 20.5% (95% CI: 12.9–30.9%) and 24.5% in patients with fibromyalgia (95% CI: 22.9–26.2%) (Table 3).
Regarding the results obtained with the logit transformations, negative summary measures were obtained in all the groups, ranging from −3.745 in subgroup A, comprising subjects receiving long-term opioid treatment for general CNCP from general population surveys, to −0.365 in subgroup D, patients receiving opioids for general CNCP from health records or medical surveys (Figure 2).
Figure 3 shows the funnel plot for the meta-analysis of subgroups B, D and E, suggesting no evidence of publication bias. Neither Egger’s test nor Begg’s test were statistically significant for the publication bias (Table 3).
FIGURE 3.
Publication bias. Funnel plots of the subgroups B, D and E of the meta-analyses.
Finally, Figure 4 shows the result of the sensitivity analysis for subgroups B, D and E, indicating in the three cases that none of the studies included would substantially change the overall result of the summarized logit transformation if the studies were eliminated from the meta-analysis. This finding indicates that the results are robust, since none of the studies exerted a great influence on the final result.
FIGURE 4.
Influence graphics for sensibility analysis of the subgroups B, D and E of the meta-analyses.
Discussion
This study analyzes the information published about the prevalence of the use of opioids in patients with CNCP and examines the factors associated with their use.
The results reveal that there were differences in the prevalence of the use of these drugs depending on the length of the treatment (2.3% in long duration or 8.1% in occasional use) (Fredheim et al., 2014; Birke et al., 2016). It was also observed that when the information derives from health registries, the prevalence is much higher than in the general population, and more variable depending on the chronic pain condition.
The lower prevalence found in patients with longer treatments seems reasonable if we take into account, on the one hand, the prescribers’ concern about the risk of addiction and the improper use of these drugs by some patients (Allen et al., 2013) and, on the other hand, the treatment dropout, possibly due to the appearance of analgesic tolerance, induced hyperalgesia, side effects frequently associated with these drugs, and insufficient pain relief (Kalso et al., 2004; Noble et al., 2008; Sehgal et al., 2013). In this line, a recent systematic review about opioids for CNCP concludes that the benefits of opioids for pain and functioning are similar to non-opioid alternatives. Opioid use was associated with small improvements in pain and physical functioning, and increased risk of vomiting compared with placebo (Busse et al., 2018).
The higher prevalence in the studies based on health registries could be explained because these patients are usually treated in specialized pain units, especially those patients with complex types of pain conditions who do not respond to conventional treatment and require special drugs, such as opioids (Henderson et al., 2013; Pérez et al., 2013; De Sola et al., 2020). Furthermore, a lack of healthcare providers offering effective treatment alternatives is likely to have an impact on other treatment choices (White et al., 2019). Another possible reason is the difficulty in opioid deprescribing when patients have poor function and unremitting pain or aberrant behavior and misuse, since tapering is a complex process with little current guidance available (White et al., 2019).
Regarding the specific pathologies, the prevalence in patients with musculoskeletal conditions was 20.5% and in patients with fibromyalgia 24.5% (Luo et al., 2004; Romanelli et al., 2017). Musculoskeletal conditions are one of the most common causes of pain (Breivik et al., 2006; Pierce et al., 2019) and the use of weak opioids was recommended to relieve pain and disability in the short-term in these patients (Airaksinen et al., 2006). However, recent studies have found that LTOT is associated with unrelieved pain, greater functional impairment, and lower return to work rates (Turner et al., 2016; Krebs et al., 2018). In line with this, current treatment guidelines do not recommend opioids for fibromyalgia management (Peng et al., 2015), possibly because opioids are unable to target the pathophysiological processes involved in this central sensitization syndrome (Menzies et al., 2017). Our findings suggest that, despite a lack of scientific support of opioid treatment in people with fibromyalgia and for musculoskeletal conditions, clinicians are nevertheless prescribing them for symptom management in these patients.
In the analysis of the factors associated with the use of opioids, younger individuals showed greater use. One explanation could be that opioids are not always recommended for the elderly population due to a higher probability of liver or kidney dysfunction, greater risk of respiratory depression, drug interactions, organ dysfunction, co-morbidity and side effects, such as constipation, drowsiness or sedation, which can have more serious consequences in this population (Romanelli et al., 2017). In addition, it has been shown that medical personnel sometimes underestimate pain in the elderly, which leads to a lower prescription of opioids in these patients (Raymond and John, 2002; Seers et al., 2018).
Regarding race, different studies have shown that the pain experience is different according to the ethnic group. This finding has been attributed to different responses to painful stimuli and the different coping strategies for managing pain between groups (Campbell and Edwards, 2012; Larochelle et al., 2015; Ringwalt et al., 2015; Romanelli et al., 2017). Additionally, according to Anderson et al. (2009), there are other factors that could influence these differences, such as selective care and differences in the process of evaluation and allocation of treatment according to the ethnic group of the patient.
Another factor to consider is the type of care received by the patient. The type of medical insurance can influence the manner of approaching the pain and consequently determine the use of opioids. Patients with private insurance have been reported to obtain better results than patients with public coverage (Meineche-Schmidt et al., 2012) since, in addition to the fact that the care is more immediate, a multidisciplinary approach is more common and produces better results, decreasing the use of analgesic treatment (Becker et al., 2000). In this vein, Rodondi et al. (2019) highlighted that the training of the physician in complementary medicine also results in the prescription of less opioid treatments, since being specialized in integrative and complementary medicine could help physicians to inform and guide patients about the most effective treatment options, their potential interactions with conventional therapies, and their side effects.
Finally, it would be reasonable to think that in those studies where the prevalence of CP is higher, the use of opioids would also be greater (Barnett et al., 2012). However, when we compare the results from different countries, this hypothesis is not confirmed, as the factors that may be important are the method of data collection and the characteristics of the population included in the studies (Miller et al., 2017; Steingrímsdóttir et al., 2017). Likewise, the cultural perceptions of pain could help us to understand the differences in the prevalence of opioid use, the perception of opioid use, the widespread marketing campaign for opioids, and the regulations controlling the prescription of opioids (Severino et al., 2018). Most of the studies included in this review were performed in the US and Western Europe, where there are significant differences in healthcare systems and healthcare regulatory oversight, the financial incentive behind the treatment of pain, and restrictions on the length of validity for these drugs, among others (Meyer et al., 2020).
Some limitations of this review should be noted. It is worth mentioning that three of the subgroups in the meta-analysis included only two studies. The minimum number of studies to include in a meta-analysis has been discussed previously in the literature, without clear agreement (Cooper et al., 2009; Valentine et al., 2010; Pigott, 2012). Some researchers consider that a minimum of five studies are desirable, or even required. Others argue that, as long as the studies meet the quality criteria and statistical requirements, the meta-analysis can be carried out, as it is just a statistical combination of the results. The number of studies in the literature on a topic is out of our control, and the lack of studies on these topics (in our case, studies carried out in the general population focused on the prevalence of the long-term use of opioids; in the general population focused on the prevalence of the use of opioids in CLBP; and from health records or medical surveys focused on the use of opioids in fibromyalgia) is itself a relevant result, and it shows the need for further research on the topics. Of course, the number of studies has a direct impact on the statistical power and precision, but if those few studies are relevant and their quality is high, we believe that it is worth drawing conclusions from them. In this vein, Pigott (2012) argued that the quick answer for the minimum number of studies is two, but recommend computing the statistical power a priori “using assumptions about the size of an important effect in a given context, and the typical sample sizes used in a given field.” Finally, Valentine et al. (2010) state that a meta-analysis is always the best option to synthesize information (even if we have few studies) as other alternatives “are likely to be based on less defensible assumptions and on less transparent processes.” Consequently, we decided to perform these three meta-analyses which, however, need to be interpreted with caution, given the limited statistical power.
In order to strengthen our findings, we attempted to limit the impact of the clinical and methodological heterogeneity, classifying the studies into subgroups with similar characteristics. However, the tests for statistical heterogeneity among the studies included in the meta-analyses still demonstrated substantial variability between them (I 2 > 77, p < 0.05). A stratified analysis grouped by the different nationalities of the patients or by the different definitions of the prevalence of use of opioids in each study could have been another potential way of classification based on the importance of cultural aspects, healthcare systems, and healthcare regulations in opioid use. However, the few studies with this information available, and the marked heterogeneity within the groups made another way to stratify them impossible. Also, the differences in sociodemographic structure, socioeconomic level and the duration of pain could contribute to the differences between the study populations. However, without access to individual patient data, it was not possible to control these factors.
Another limitation of our review was the risk of bias in the studies. Although 22 out of the 25 studies fulfilled at least seven items of the checklist, only 12 fulfilled the nine items of the Critical Appraisal Checklist for Studies Reporting Prevalence Data, and just over half of the 25 studies included were not primarily designed to produce prevalence data (Table 2). This was reflected in the variability of reporting of important variables. For example, population denominators and response rates were not always identifiable and there were occasional numerical discrepancies between the data presented in the study abstract, main text, and results tables. Despite the fact that some of the studies could have a potential bias, the flaws are not sufficient to invalidate the results since they satisfied other criteria in the assessment of the risk of bias and provided important information in line with the objectives of this review.
Despite extensive database searches, it is possible that some references from the gray literature were missed. Furthermore, language is one of the methodological limitations. All the studies were in English, conducted in predominantly Western settings, making generalizability to other parts of the world difficult. As a strength of the study, we would like to highlight its novelty since, to the best of our knowledge, no systematic review and meta-analysis of the prevalence of the therapeutic use of opioids has been published previously.
Conclusion
This study shows that the prevalence of opioid use among patients with CNCP was higher in clinical studies based on health registries and in subjects with short or occasional use compared to those with long term use. Men, younger people, more CP conditions, and patients without insurance or with noncommercial insurance were most related to opioid use. In contrast, non-white and Asian patients, and those treated by a physician trained in complementary medicine were less likely to use opioids.
Author Contributions
I Failde supervised the study. HS and MD led the writing. HS, MD, AS and PJ conducted the search strategy and the analysis. All the authors conceptualized the study, contributed to its writing, and made a critical review of the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.564412/full#supplementary-material
References
- Ahn Y.-J., Shin J.-S., Lee J., Lee Y. J., Kim M.-R., Park K. B., et al. (2016). Evaluation of use and cost of medical care of common lumbar disorders in Korea: cross-sectional study of Korean Health Insurance Review and Assessment Service National Patient Sample data. BMJ Open 6, e012432 10.1136/bmjopen-2016-012432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen O., Brox J. I., Cedraschi C., Hildebrandt J., Klaber-Moffett J., Kovacs F., et al. (2006). Chapter 4: European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 15, 192–300. 10.1007/s00586-006-1072-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A., Juurlink D. N. (2015). The prescription opioid epidemic: an overview for anesthesiologists. Can. J. Anesth. Can. D Anesth. 63, 61–68. 10.1007/s12630-015-0520-y [DOI] [PubMed] [Google Scholar]
- Allen M. J. M., Asbridge M. M., MacDougall P. C., Furlan A. D., Tugalev O. (2013). Self-reported practices in opioid management of chronic noncancer pain: a survey of Canadian family physicians. Pain Res. Manag. 18, 177–184. 10.1155/2013/528645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. O., Green C. R., Payne R. (2009). Racial and ethnic disparities in pain: causes and consequences of unequal care. J. Pain 10, 1187–1204. 10.1016/j.jpain.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Azevedo L. F., Costa-Pereira A., Mendonça L., Dias C. C., Castro-Lopes J. M. (2012). Epidemiology of chronic pain: a population-cased nationwide study on its prevalence, characteristics and associated disability in Portugal. J. Pain 13, 773–783. 10.1016/j.jpain.2012.05.012 [DOI] [PubMed] [Google Scholar]
- Azevedo L. F., Costa-Pereira A., Mendonza L., Dias C. C., Castro-Lopes J. M. (2013). A population-based study on chronic pain and the use of opioids in Portugal. Pain 154, 2844–2852. 10.1016/j.pain.2013.08.022 [DOI] [PubMed] [Google Scholar]
- Barendregt J. J., Doi S. A., Lee Y. Y., Norman R. E., Vos T. (2013). Meta-analysis of prevalence. J. Epidemiol. Comm. Health 67, 974–978. 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- Barnett K., Mercer S. W., Norbury M., Watt G., Wyke S., Guthrie B. (2012). Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380, 37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- Becker N., Sjogren P., Bech P., Olsen A. K., Eriksen J. (2000). Treatment outcome of chronic non-malignant pain patients managed in a Danish multidisciplinary pain centre compared to general practice: a randomised controlled trial. Pain 84, 203–211. 10.1016/s0304-3959(99)00209-2 [DOI] [PubMed] [Google Scholar]
- Bett J. V. S., Batistella E. Â., Melo G., Munhoz E. de. A., Silva C. A. B., Guerra E. N. da. S., et al. (2019). Prevalence of oral mucosal disorders during pregnancy: a systematic review and meta-analysis. J. Oral Pathol. Med. 48, 270–277. 10.1111/jop.12831 [DOI] [PubMed] [Google Scholar]
- Birke H., Kurita G. P., Sjøgren P., Højsted J., Simonsen M. K., Juel K., et al. (2016). Chronic non-cancer pain and the epidemic prescription of opioids in the Danish population: trends from 2000 to 2013. Acta Anaesthesiol. Scand. 60, 623–633. 10.1111/aas.12700 [DOI] [PubMed] [Google Scholar]
- Breivik H., Collett B., Ventafridda V., Cohen R., Gallacher D. (2006). Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur. J. Pain 10, 287–333. 10.1016/j.ejpain.2005.06.009 [DOI] [PubMed] [Google Scholar]
- Broekmans S., Dobbels F., Milisen K. (2010). Pharmacologic pain treatment in a multidisciplinary pain center. Clin. J. Pain 26, 81–86. 10.1097/AJP.0b013e3181b91b22 [DOI] [PubMed] [Google Scholar]
- Busse J. W., Wang L., Kamaleldin M., Craigie S., Riva J. J., Montoya L., et al. (2018). Opioids for chronic noncancer pain. JAMA 320, 2448 10.1001/jama.2018.18472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callhoff J., Albrecht K., Redeker I., Lange T., Goronzy J., Gunther K.-P., et al. (2020). Disease burden of patients with osteoarthritis: results of a cross-sectional survey linked to claims data. Arthritis Care Res. (Hoboken). 72, 193–200. 10.1002/acr.24058 [DOI] [PubMed] [Google Scholar]
- Campbell C. M., Edwards R. R. (2012). Ethnic differences in pain and pain management. Pain Manag. 2, 219–230. 10.2217/pmt.12.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G., Nielsen S., Larance B., Bruno R., Mattick R., Hall W., et al. (2015). Pharmaceutical opioid use and dependence among people living with chronic pain: associations observed within the pain and opioids in treatment (POINT) cohort. Pain Med. 16, 1745–1758. 10.1111/pme.12773 [DOI] [PubMed] [Google Scholar]
- Cheung C. W., Qiu Q., Choi S.-W., Moore B., Goucke R., Irwin M. (2014). Chronic opioid therapy for chronic non-cancer pain: a review and comparison of treatment guidelines. Pain Physician 17, 401–414. [PubMed] [Google Scholar]
- Cooper H., Hedges L., Valentine J. (2009). The handbook of research synthesis and meta-analysis. New York, NY: Russell Sage Foundation. [Google Scholar]
- De Sola H., Maquibar A., Failde I., Salazar A., Goicolea I. (2020). Living with opioids: a qualitative study with patients with chronic low back pain. Health Expect. 00, 1–11. 10.1111/hex.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sola H., Salazar A., Dueñas M., Ojeda B., Failde I. (2016). A nationwide cross-sectional study of the impact of chronic pain on an individual´s employment. Relationship with the family and the social support. BMJ Open 6, e012246 10.1136/bmjopen-2016-012246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain K. M., Alexander G. C., Dore D. D., Segal J. B., Zullo A. R., Castillo-Salgado C. (2017). Frequency and predictors of analgesic Prescribing in U.S. nursing home residents with persistent pain. J. Am. Geriatr. Soc. 65, 286–293. 10.1111/jgs.14512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredheim O. M. S., Mahic M., Skurtveit S., Dale O., Romundstad P. P. P., Borchgrevink P. C. (2014). Chronic pain and use of opioids: a population-based pharmacoepidemiological study from the Norwegian prescription database and the Nord-Trondelag health study. Pain 155, 1213–1221. 10.1016/j.pain.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Gouveia N., Rodrigues A., Ramiro S., Eusebio M., Machado P. M., Canhao H., et al. (2017). The use of analgesic and other pain-relief drugs to manage chronic low back pain: results from a national survey. Pain Pract. 17, 353–365. 10.1111/papr.12455 [DOI] [PubMed] [Google Scholar]
- GSK (2017). Global pain index 2017 research report. London, United Kingdom: Global Report Findings, 1–49. [Google Scholar]
- Häuser W., Jung E., Erbslöh-Möller B., Gesmann M., Kühn-Becker H., Petermann F., et al. (2012). The German fibromyalgia consumer reports - a cross-sectional survey. BMC Muscoskel. Disord. 13, 2–7. 10.1186/1471-2474-13-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J. V., Harrison C. M., Britt H. C., Bayram C. F., Miller G. C. (2013). Prevalence, causes, severity, impact, and management of chronic pain in Australian General Practice Patients. Pain Med. 14, 1346–1361. 10.1111/pme.12195 [DOI] [PubMed] [Google Scholar]
- Kalso E., Edwards J. E., Moore R. A., McQuay H. J. (2004). Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 112, 372–380. 10.1016/j.pain.2004.09.019 [DOI] [PubMed] [Google Scholar]
- Karmali R. N., Bush C., Raman S. R., Campbell C. I., Skinner A. C., Roberts A. W. (2020). Long-term opioid therapy definitions and predictors: a systematic review. Pharmacoepidemiol. Drug Saf. 29, 252–269. 10.1002/pds.4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury S. R., Gross H. J., Isherwood G., Conaghan P. G. (2014). Osteoarthritis in Europe: impact on health status, work productivity and use of pharmacotherapies in five European countries. Rheumatology (Oxford) 53, 937–947. 10.1093/rheumatology/ket463 [DOI] [PubMed] [Google Scholar]
- Knoop J., van Tunen J., van der Esch M., Roorda L. D., Dekker J., van der Leeden M., et al. (2017). Analgesic use in patients with knee and/or hip osteoarthritis referred to an outpatient center: a cross-sectional study within the Amsterdam Osteoarthritis Cohort. Rheumatol. Int. 37, 1747–1755. 10.1007/s00296-017-3785-3 [DOI] [PubMed] [Google Scholar]
- Krebs E. E., Gravely A., Nugent S., Jensen A. C., DeRonne B., Goldsmith E. S., et al. (2018). Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain the SPACE randomized clinical trial. JAMA 319, 872–882. 10.1001/jama.2018.0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita G. P., Sjogren P., Juel K., Hojsted J., Ekholm O. (2012). The burden of chronic pain: a cross-sectional survey focussing on diseases, immigration, and opioid use. Pain 153, 2332–2338. 10.1016/j.pain.2012.07.023 [DOI] [PubMed] [Google Scholar]
- Larochelle M. R., Zhang F., Ross-Degnan D., Wharam J. F. (2015). Trends in opioid prescribing and co-prescribing of sedative hypnotics for acute and chronic musculoskeletal pain: 2001–2010. Pharmacoepidemiol. Drug Saf. 24, 885–892. [DOI] [PubMed] [Google Scholar]
- Lin H.-C., Wang Z., Simoni-Wastila L., Boyd C., Buu A. (2019). Interstate data sharing of prescription drug monitoring programs and associated opioid prescriptions among patients with non-cancer chronic pain. Prev. Med. 118, 59–65. 10.1016/j.ypmed.2018.10.011 [DOI] [PubMed] [Google Scholar]
- Luo X., Pietrobon R., Hey L. (2004). Patterns and trends in opioid use among individuals with back pain in the United States. Spine . 29, 884–890. 10.1097/00007632-200404150-00012 [DOI] [PubMed] [Google Scholar]
- Marschall U., L’hoest H., Radbruch L., Häuser W. (2015). Long-term opioid therapy for chronic non-cancer pain in Germany. Eur. J. Pain 20, 767–776. 10.1002/ejp.802 [DOI] [PubMed] [Google Scholar]
- Meineche-Schmidt V., Jensen N. H., Sjøgren P. (2012). Long-term outcome of multidisciplinary intervention of chronic non-cancer pain patients in a private setting. Scand. J. Pain 3, 99–105. 10.1016/j.sjpain.2011.10.002 [DOI] [PubMed] [Google Scholar]
- Menzies V., Thacker L. R., Mayer S. D., Young A. M., Evans S., Barstow L. (2017). Polypharmacy, opioid use, and fibromyalgia. Biol. Res. Nurs. 19, 97–105. 10.1177/1099800416657636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., LeClair C., McDonald J. V. (2020). Prescription opioid prescribing in western Europe and the United States. R. I. Med. J. (2013) 103, 45–48. [PubMed] [Google Scholar]
- Miller A., Sanderson K., Bruno R., Breslin M., Neil A. L. (2017). The prevalence of pain and analgesia use in the Australian population: findings from the 2011 to 2012 Australian National Health Survey. Pharmacoepidemiol. Drug Saf. 26, 1403–1410. 10.1002/pds.4301 [DOI] [PubMed] [Google Scholar]
- Miller G. F., Guy G. P., Zhang K., Mikosz C. A., Xu L. (2019). Prevalence of nonopioid and opioid prescriptions among commercially insured patients with chronic pain. Pain Med. 20, 1948–1954. 10.1093/pm/pny247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. E. E., Nicolson K. P., Smith B. H. (2019). Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 123, e273–e283. 10.1016/j.bja.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministerio De Sanidad Consumo y Bienestar Social (2020). Clasificación internacional enfermedades (CIE-10-ES). Available at: https://eciemaps.mscbs.gob.es/ecieMaps/browser/metabuscador.html (Accessed January 2, 2020).
- Mohamed Zaki L. R., Hairi N. N. (2015). A systematic review ofthe prevalence and measurement of chronic pain in Asian adults. Pain Manag. Nurs. 16, 440–452. 10.1016/j.pmn.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C. (2015). Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int. J. Evid. Based Health 13, 147–153. 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- Noble M., Tregear S. J., Treadwell J. R., Schoelles K. (2008). Long-term opioid therapy for chronic noncancer pain: a systematic review and meta-analysis of efficacy and safety. J. Pain Symptom Manag. 35, 214–228. 10.1016/j.jpainsymman.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Parsells Kelly J., Cook S. F., Kaufman D. W., Anderson T., Rosenberg L., Mitchell A. A. (2008). Prevalence and characteristics of opioid use in the US adult population. Pain 138, 507–513. 10.1016/j.pain.2008.01.027 [DOI] [PubMed] [Google Scholar]
- Peng X., Robinson R. L., Mease P., Kroenke K., Williams D. A., Chen Y., et al. (2015). Long-term evaluation of opioid treatment in fibromyalgia. Clin. J. Pain 31, 7–13. 10.1097/AJP.0000000000000079 [DOI] [PubMed] [Google Scholar]
- Pérez C., Margarit C., Serrano M. (2013). Survey of European patients assessing their own noncancer chronic pain: results from Spain. Curr. Med. Res. Opin. 29, 643–651. 10.1185/03007995.2013.787978 [DOI] [PubMed] [Google Scholar]
- Peterson M. E., Li Y., Shanks H., Mile R., Nair H., Kyaw M. H. (2019). Serogroup-specific meningococcal carriage by age group: a systematic review and meta-analysis. BMJ Open 9, e024343 10.1136/bmjopen-2018-024343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce D. P. R., Pierce B., Cheng C.-I., Perzhinsky J. (2019). Assessing treatment and monitoring of musculoskeletal conditions using opioid versus nonopioid therapy: a cross-sectional study. Medicine (Baltimore) . 98, e15128 10.1097/MD.0000000000015128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott T. D. (2012). Advances in meta-analysis. New York, NY: Springer US; 10.1007/978-1-4614-2278-5 [DOI] [Google Scholar]
- Porto De Toledo I., Stefani F. M., Porporatti A. L., Mezzomo L. A., Peres M. A., Flores-Mir C., et al. (2017). Prevalence of otologic signs and symptoms in adult patients with temporomandibular disorders: a systematic review and meta-analysis. Clin. Oral Invest. 21, 597–605. 10.1007/s00784-016-1926-9 [DOI] [PubMed] [Google Scholar]
- Rasu R. S., Knell M. E. (2018). Determinants of opioid prescribing for nonmalignant chronic pain in US outpatient settings. Pain Med. (United States) 19, 524–532. 10.1093/pm/pnx025 [DOI] [PubMed] [Google Scholar]
- Raymond C. T., John T. C. (2002). Pain in older subacute care patients: associations with clinical status and treatment. Pain Med. 3, 231–239. 10.1046/j.1526-4637.2002.02031 [DOI] [PubMed] [Google Scholar]
- Ringwalt C., Roberts A. W., Gugelmann H., Skinner A. C. (2015). Racial disparities across provider specialties in opioid prescriptions dispensed to medicaid beneficiaries with chronic noncancer pain. Pain Med. (United States) 16, 633–640. 10.1111/pme.12555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodondi P. Y., Bill A. S., Danon N., Dubois J., Pasquier J., Florence, et al. (2019). Primary care patients’ use of conventional and complementary medicine for chronic low back pain. J. Pain Res. 12, 2101–2112. 10.2147/JPR.S200375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli R. J., Ikeda L. I., Lynch B., Craig T., Cappelleri J. C., Jukes T., et al. (2017). Opioid prescribing for chronic pain in a community- based healthcare system. Am. J. Manag. Care 23, e138–e145. [PubMed] [Google Scholar]
- Salazar A., Moreno S., De Sola H., Moral-Munoz J. A., Dueñas M., Failde I. (2019). The evolution of opioid-related mortality and potential years of life lost in Spain from 2008 to 2017. Differences between Spain and the United States. Curr. Med. Res. Opin. 36, 285–291. 10.1016/j.neulet.2008.12.049 [DOI] [PubMed] [Google Scholar]
- Scala E., Decosterd I., Faouzi M., Burnand B., Rodondi P.-Y. (2018). Level of readiness of chronic pain patients to practise active self-care. Eur. J. Pain 22, 1800–1812. 10.1002/ejp.1276 [DOI] [PubMed] [Google Scholar]
- Scholten W. (2013). Access to opioid analgesics: essential for quality cancer care. Cancer Pain 249–262. 10.1007/978-0-85729-230-19 [DOI] [Google Scholar]
- Seers T., Derry S., Seers K., Moore R. A. (2018). Professionals underestimate patients’ pain. Pain 159, 1 10.1097/j.pain.0000000000001165 [DOI] [PubMed] [Google Scholar]
- Sehgal N., Colson J., Smith H. S. (2013). Chronic pain treatment with opioid analgesics: benefits versus harms of long-term therapy. Expert Rev. Neurother. 13, 1201–1220. 10.1586/14737175.2013.846517 [DOI] [PubMed] [Google Scholar]
- Sehgal N., Manchikanti L., Smith H. S. (2012). Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician 15, ES67-92. [PubMed] [Google Scholar]
- Severino A. L., Shadfar A., Hakimian J. K., Crane O., Singh G., Heinzerling K., et al. (2018). Pain therapy guided by purpose and perspective in light of the opioid epidemic. Front. Psychiatr. 9, 119 10.3389/fpsyt.2018.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349, 1–25. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- Shmagel A., Ngo L., Ensrud K., Foley R. (2018). Prescription medication use among community-based US adults with chronic low back pain: a cross-sectional population based study. J. Pain 19, 1104–1112. 10.1016/j.jpain.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sites B. D., Harrison J., Herrick M. D., Masaracchia M. M., Beach M. L., Davis M. A. (2018). Prescription opioid use and satisfaction with care among adults with musculoskeletal conditions. Ann. Fam. Med. 16, 6–13. 10.1370/afm.2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrímsdóttir Ó. A., Landmark T., Macfarlane G. J., Nielsen C. S. (2017). Defining chronic pain in epidemiological studies – a systematic review and meta-analysis. Pain 158, 2092–2107. 10.1097/j.pain.0000000000001009 [DOI] [PubMed] [Google Scholar]
- Timmerman L., Stronks D. L., Groeneweg J. G., Huygen F. J. (2016). Prevalence and determinants of medication non-adherence in chronic pain patients: a systematic review. Acta Anaesthesiol. Scand. 60, 416–431. 10.1111/aas.12697 [DOI] [PubMed] [Google Scholar]
- Turner J. A., Shortreed S. M., Saunders K. W., LeResche L., Korff M. Von. (2016). Association of levels of opioid use with pain and activity interference among patients initiating chronic opioid therapy: a longitudinal study. Pain 157, 849–857. 10.1016/j.physbeh.2017.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine J. C., Pigott T. D., Rothstein H. R. (2010). How many studies do you need? A primer on statistical power for meta-analysis. J. Educ. Behav. Stat. 35, 215–247. 10.3102/1076998609346961 [DOI] [Google Scholar]
- Van Den Driest J. J., Pijnenburg P., Bindels P. J. E. E., Bierma-Zeinstra S. M. A. A., Schiphof D. (2019). Analgesic use in Dutch patients with osteoarthritis: frequent but low doses. J. Clin. Rheumatol. 25, 297–303. 10.1097/RHU.0000000000000853 [DOI] [PubMed] [Google Scholar]
- Veritas Health Innovation Ltd. (2019). Covidence. Available at: www.covidence.org (Accessed May 1, 2020).
- Vincent A., Whipple M. O., McAllister S. J., Aleman K. M., St Sauver J. L. (2015). A cross-sectional assessment of the prevalence of multiple chronic conditions and medication use in a sample of community-dwelling adults with fibromyalgia in Olmsted County, Minnesota. BMJ Open 5, 1–5. 10.1136/bmjopen-2014-006681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M., Kolodny A., Deyo R. A., Chou R. (2011). Long-term opioid therapy reconsidered. Ann. Emerg. Med. 155, 325–328. 10.1059/0003-4819-155-5-201109060-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T., Flaxman A. D., Naghavi M., Lozano R., Michaud C., Ezzati M., et al. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. (2018). The opioid crisis: a 21st century pain. Drugs Today 54, 283–286. 10.1358/dot.2018.54.4.2812620 [DOI] [PubMed] [Google Scholar]
- Wand B. M., Catley M. J., Rabey M. I., O’Sullivan P. B., O’Connell N. E., Smith A. J. (2016). Disrupted self-perception in people with chronic low back pain. Further evaluation of the fremantle back awareness questionnaire. J. Pain 17, 1001–1012. 10.1016/j.jpain.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Warner E. A. (2012). Opioids for the treatment of chronic noncancer pain. Am. J. Med. 125, 1155–1161. 10.1016/j.amjmed.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Webster F., Bremner S., Oosenbrug E., Durant S., McCartney C. J., Katz J. (2017). From opiophobia to overprescribing: a critical scoping review of medical education training for chronic pain. Pain Med. 18, 1467–1475. 10.1093/pm/pnw352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R., Hayes C., Boyes A. W., Chiu S., Paul C. L. (2019). General practitioners and management of chronic noncancer pain: a cross-sectional survey of influences on opioid deprescribing. J. Pain Res. 12, 467–475. 10.2147/JPR.S168785 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.