Abstract
The purpose of this study was to investigate the potential benefits of yeast cell wall (YCW) on the gastrointestinal development of weaned calves. Twenty healthy Holstein male calves (BW = 92 ± 8.29 kg and 60 ± 5 d of age) were randomly allocated into 2 groups: CON with no YCW, and YCW (accounted for 0.16% of the basal diet). The dietary concentrate-to-roughage ratio was 40:60. All the calves were fed regularly twice a day at 09:00 and 16:00 and had free access to water. The experiment lasted for 60 d. The results showed that calves fed YCW showed higher (P < 0.05) length, width, and surface area of papillae in the ventral sac of the rumen as compared to CON. For the dorsal sac of the rumen, the muscularis thickness was thicker (P < 0.05) in the YCW group when compared with CON group. The villus height of YCW calves was higher (P < 0.05) than that of CON in the ileum. Calves supplemented with YCW also showed a higher (P < 0.05) villus height-to-crypt depth ratio in the ileum. The YCW calves exhibited a greater (P < 0.05) thickness of the wall in the duodenum and jejunum. Calves supplemented with YCW improved (P < 0.05) the claudin 1 mRNA expression in the ileum and occludin mRNA expression in the jejunum and ileum. The YCW increased (P < 0.05) the contents of secretory immunoglobulin A in the jejunum and ileum of calves. In conclusion, dietary supplementation with YCW could improve the gastrointestinal development of weaned calves.
Keywords: Weaned calf, Yeast cell wall, Ruminal histology, Intestinal histology, Intestinal barrier function, Gastrointestinal development
1. Introduction
The calf stage is an important period in the life of dairy cows. Early weaning is a common way in rearing calves, because early weaning can reduce production cost and speed up ruminal development (Rezazadeh et al., 2019). However, calves have an obvious stress response to weaning because of the changes in feeding methods and feedstuffs. Besides, the digestive organs of weaned calves are in the developmental phase and have less resistance to the external environment (Hulbert et al., 2011). Because of this, the weaned calves are vulnerable to pathogenic bacteria, which can cause gastrointestinal dysfunction (Terré et al., 2007). Therefore, under the modern large-scale and intensive dairy farming development processes, mitigating the weaning stress of calves is an urgent issue to be addressed.
Yeast cell wall (YCW) is commonly used as a nutritional supplement in animals' diet, and it contains 2 main polysaccharides including mannan-oligosaccharide and β-glucan. These polysaccharides of YCW can absorb pathogens and enhance the release of cytokines, which then lead to improve the immunity (Liu et al., 2018). Previous studies reported that YCW or its ingredients have positive effects on animals (Salinas-Chavira et al., 2015; Xue et al., 2017). Besides, YCW can improve the intestinal histology of non-ruminant animals (Han et al., 2017). In ruminants, some studies have shown that dietary supplementation with YCW is beneficial for milk production (Nocek et al., 2011), growth performance (Terré et al., 2007), ruminal development (Lesmeister et al., 2004a), and intestinal development (Shen et al., 2009). Moreover, another study found that supplementation of yeast products in neonatal calves' diet could enhance the papilla length and width of ruminal epithelium, and also increase the villus height-to-crypt depth ratio of the small intestine (Xiao et al., 2016). Although yeast products can enhance the gastrointestinal development of animals, the research about the role of YCW as a feed additive in weaned calves is limited. Our previous study found that YCW could improve the growth performance of weaned calves, and the optimum addition level was 0.16% of the basal diet. Based on previous studies, we hypothesized that YCW may be an effective feed additive to improve the gastrointestinal development of weaned calves. Therefore, the current study was conducted to evaluate the effects of YCW on ruminal histology, intestinal histology, and intestinal barrier function of weaned calves.
2. Materials and methods
2.1. Animal ethics statement
The animal experiment was conducted according to the Regulation of the Administration of Laboratory Animals (2017 Revision) promulgated by Decree No. 676 of the State Council. All procedures involving animal care and management were approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University (Chengdu, Sichuan, China).
2.2. Experimental design and diets
This study was conducted at the experimental site of the Animal Nutrition Institute, Sichuan Agricultural University (Ya'an, Sichuan, China). Twenty Holstein male calves were used in this experiment. The selected calves (BW = 92 ± 8.29 kg and 60 ± 5 d of age) were marked with ear tags and randomly allocated into 2 dietary treatment groups: CON with no YCW (Angel Yeast Co., Ltd., Yichang, Hubei, China; main ingredients: β-glucan ≥ 30%, mannan-oligosaccharide ≥ 35%), and YCW (YCW accounted for 0.16% of the basal diet, based on our previous study). Calves in the 2 groups were housed in 20 pens, with 1 calf in each pen. All the calves were regularly provided rations twice a day at 09:00 and 16:00. Calves had free access to water during the experiment. A 7-d adjustment period is followed by 60 d of experimental period.
All calves were fed the total mixed ration, and the YCW was supplemented to the basal diet at the rate of 0.16% and mixed with other ingredients. The basal diet was formulated according to NRC (2001), and the nutritional requirements of calves were based on the BW (125 kg) and average daily gain (0.8 kg). The ratio of concentrate to roughage was adjusted to 40:60. The roughages were rice straw, alfalfa pellet, and vinasse, which accounted for 27%, 18%, and 15% of the total mixed ration on dry matter basis respectively. The concentrates mainly included corn, rapeseed cake, wheat bran, soybean meal, premix, and salt. The feed ingredients and nutrient levels of basal diet are shown in Table 1.
Table 1.
Ingredients and nutrient levels of the experimental diets (dry matter basis)1.
| Item | CON | YCW |
|---|---|---|
| Ingredients, % | ||
| Rice straw | 27.00 | 27.00 |
| Alfalfa pellet | 18.00 | 18.00 |
| Vinasse | 15.00 | 15.00 |
| Corn | 20.04 | 20.04 |
| Soybean meal | 6.63 | 6.63 |
| Rapeseed cake | 3.21 | 3.21 |
| Wheat bran | 7.23 | 7.07 |
| CaHPO4 | 0.67 | 0.67 |
| CaCO3 | 0.44 | 0.44 |
| NaHCO3 | 0.67 | 0.67 |
| NaCl | 0.67 | 0.67 |
| Yeast cell wall | 0.00 | 0.16 |
| Premix2 | 0.44 | 0.44 |
| Total | 100.00 | 100.00 |
| Nutrient levels, % | ||
| NEg3, MJ/kg | 4.06 | 4.06 |
| Crude protein | 13.87 | 13.89 |
| Ether extract | 3.36 | 3.36 |
| Acid detergent fiber | 24.88 | 24.81 |
| Neutral detergent fiber | 36.62 | 36.58 |
| Ca | 0.64 | 0.64 |
| P | 0.41 | 0.41 |
YCW = yeast cell wall; NEg = net energy for gain.
CON, the control with no YCW (Angel Yeast Co., Ltd., Yichang, Hubei, China); YCW, YCW accounted for 0.16% of the basal diet.
The premix provided the following per kilogram of the diet: vitamin A 8,000 IU, vitamin D 1,200 IU, vitamin E 50 IU, Cu (as copper sulfate) 10 mg, Fe (as ferrous sulfate) 100 mg, Mn (as manganese sulfate) 40 mg, Zn (as zinc sulfate) 60 mg, I (as potassium iodide) 0.50 mg, Se (as sodium selenite) 0.3 mg, Co (as cobalt chloride) 0.1 mg.
NEg was calculated according to the Nutrient Requirements of Dairy Cattle, NRC (2001).
2.3. Sample collection and analytical determination
2.3.1. Chemical analysis
Samples of fresh basal diet were collected and analyzed for nutrient levels. Acid detergent fiber and neutral detergent fiber were determined in an ANKOM fiber analyzer (ANKOM A200i, USA) (Van Soest et al., 1991). In addition, the samples were analyzed for crude protein (No. 984.13), ether extract (No. 954.02), calcium (No. 977.29), and phosphorus (No. 995.11) according to the AOAC international methods (AOAC, 2000).
2.3.2. Slaughter
On d 60 of the experiment, 6 calves in each group, which were close to the group average BW, were slaughtered after fasted 12 h by captive bolt stunning and exsanguinated humanely. The slaughter process was according to the conventional procedures of Sichuan Agricultural University. After the slaughtering, the abdominal cavity was immediately opened and the gastrointestinal tract was removed, afterwards each tissue of the digestive tract (rumen, duodenum, jejunum, and ileum) was isolated and tied off. The weights of rumen, reticulum, omasum, and abomasum were recorded. The samples were collected according to the following methods.
2.3.3. Ruminal and intestinal tissues
The tissues of rumen, duodenum, jejunum, and ileum were separated. The digesta was removed and tissues were rinsed with ice-cold and sterile phosphate buffer. Ruminal epithelia in the ventral and dorsal sac were cut (2 cm × 2 cm) and placed into 4% paraformaldehyde overnight. Subsequently, tissues were dehydrated and embedded in paraffin, and then cut into 3 sections with a thickness of 5 μm, and then stained with Hematoxylin and Eosin for histological study under a light microscope. The histological characteristics, including papilla length (PL), papilla width (PW), papilla surface area (PSA), and muscularis thickness, of 5 to 8 slides for each sample were recorded by an optical binocular microscope (Olymplus BX 61; Olympus, Warsaw, Poland) fitted with a digital camera, which was equipped with the Image-Pro Plus 6.0 software (Media Cybernetics Inc., Bethesda, MD, USA). For each analyzed parameter, 30 measurements were taken. The muscularis thickness of ruminal tissue was determined using a vernier caliper (0 to 150 mm, Chengdu Shuangtuo Testing Instrument Co., Ltd., Chengdu, China) and an analytical balance (Chengdu Shuangtuo Testing Instrument Co., Ltd., Chengdu, China). The PL and PW were determined by a paraffin slicing machine (Shenzhen Huiwo Technology Co., Ltd., Shenzhen, China) and a scanning electron microscope (Carl Zeiss Management Co., Ltd., Shanghai, China). The cross-sectional PSA was calculated by multiplying PL by PW (Xiao et al., 2016). For the small intestine, a 4-cm long looped section (mid-duodenum, mid-jejunum, and mid-ileum) was placed into 4% paraformaldehyde. The samples were separately cut into 2 cross sections with 1 mm thick, washed, and then embedded in paraffin blocks. For each block, 5 cuts of 3 to 4 μm thick sections were obtained, leading to 10 areas of per site for histological analysis. The section images were taken and quantitative morphometric analysis was carried out to determine the villus height (VH), villus width (VW), villus surface area (VSA), crypt depth, villus height-to-crypt depth ratio (VCR), and thickness of the wall by the measurement methods as described above. The cross-sectional VSA was calculated by multiplying VH by VW, whereas VCR was calculated by dividing VH by crypt depth. In addition, the mucosa of jejunum and ileum (mid-portion) of calves were scratched carefully using a sterile glass microscope slide, and stored at −80 °C for determination of mRNA expression of tight junction proteins (the detection method is described below). The content of secretory immunoglobulin A (sIgA) of small intestinal mucosa was analyzed by a ELISA kit according to the manufacturer instructions (Camex biotechnology Co., Ltd., Shanghai, China) (Zbrun et al., 2012).
2.3.4. Tight junction protein gene expressions detected by real-time PCR
The mRNA expression of zonula occludens 1 (ZO-1), claudin 1, and occludin in the jejunal and ileal mucosa were detected by real-time PCR. The amplification primers of these genes were designed using primer 5.0 software, and all the primers were commercially synthesized by Sangon Biotechnology Co., Ltd., (Shanghai, China). The information of primers is presented in Table 2. Total RNA was extracted from the intestinal epithelial mucosa, and then cDNA was reversely transcribed from the extracted RNA using the cDNA Synthesis Kit (Takara, Shanghai, China) according to the instructions. The real-time PCR was conducted by the SYBR Green Kit (Takara, Shanghai, China) and CFX96 Touch Real-Time PCR Detection System (Bio-Rad Inc, Hercules, CA, USA). All samples had 3 replicates. Beta-actin was used as the housekeeping gene, and the relative expression of genes was calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 2.
Sequences of primer used to analyze gene expression in the intestinal mucosa of calves by quantitative PCR.
| Gene | Primer | Sequence (5′-3′) | Accession number | Annealing temperature, °C | Product size, bp |
|---|---|---|---|---|---|
| Claudin 1 | F | TTCGACTCCTTGCTGAATCTGA | NM001001854.2 | 59 | 159 |
| R | AGCCATCCGCATCTTCTGTG | ||||
| Occludin | F | CCAGAGCGGCAAAGGGATT | XM005221440.3 | 63.3 | 147 |
| R | CCAGGACGGCGGTCACTATTA | ||||
| ZO-1 | F | CAACCACAATCTCTTGCCGAAT | XM010817146.1 | 61.4 | 120 |
| R | TCCACGCCACTGTCAAACTCA | ||||
| β-actin | F | CTAGGCACCAGGGCGTAATG | NM173979.3 | 59.5 | 177 |
| R | CCACACGGAGCTCGTTGTAG |
F = forward; R = reverse; ZO-1 = zonula occludens 1.
2.4. Statistical analysis
Data were based on pen as the experimental unit, and the homogeneity of variances and normality of data were tested first. Then, all the data were analyzed using a completely randomized design (single factor) with independent samples t-test of the SPSS statistical software (Ver. 20.0 for Windows; SPSS, Chicago, IL, USA). Data were shown as means and SEM. A significance level was indicated at P < 0.05, and a trend was declared at 0.05 ≤ P < 0.10.
3. Results
3.1. The weight of stomach
Effects of YCW on the weight of the stomach in weaned calves are shown in Table 3. No significant difference in the weight of rumen, reticulum, omasum, and abomasum between CON and YCW groups was observed. However, compared with CON, the weight of rumen in YCW was higher (P > 0.05).
Table 3.
Effects of YCW on the stomach weight of weaned calves (g)1.
| Item | Treatments |
SEM | P-value | |
|---|---|---|---|---|
| CON | YCW | |||
| Rumen | 3,588.43 | 3,803.37 | 91.05 | 0.256 |
| Reticulum | 503.31 | 498.28 | 25.27 | 0.927 |
| Reti-rumen2 | 4,091.74 | 4,301.65 | 84.78 | 0.232 |
| Omasum | 870.03 | 801.70 | 41.24 | 0.434 |
| Abomasum | 891.31 | 965.07 | 63.33 | 0.579 |
| Total3 | 5,853.08 | 6,068.42 | 132.67 | 0.440 |
YCW = yeast cell wall.
CON, control with no YCW (Angel Yeast Co., Ltd., Yichang, Hubei, China); YCW, YCW accounted for 0.16% of the basal diet.
Reti-rumen = rumen + reticulum.
Total = rumen + reticulum + omasum + abomasum.
3.2. Ruminal histology
Table 4 shows the effects of YCW on PL, PW, PSA, and muscularis thickness in the ventral and dorsal sac of the rumen. Notably, calves fed YCW exhibited greater PL (P = 0.041), PW (P = 0.035), and PSA (P = 0.027) in the ventral sac of the rumen, but no significant difference in muscularis thickness was found between CON and YCW groups. Interestingly, the results in the dorsal sac of the rumen were different from those from the ventral sac. Evaluation of parameters (PL, PW, and PSA) indicated no noticeable difference between CON and YCW groups. However, calves supplemented with YCW showed a higher (P = 0.043) muscularis thickness in the dorsal sac of the rumen.
Table 4.
Effects of YCW on the ruminal histology of weaned calves1.
| Item | Treatments |
SEM | P-value | |
|---|---|---|---|---|
| CON | YCW | |||
| Ventral sac of rumen | ||||
| PL, μm | 2,004.52 | 2,187.33 | 46.19 | 0.041 |
| PW, μm | 501.74 | 543.71 | 10.34 | 0.035 |
| PSA, μm2 | 1,007,540.64 | 1,191,951.48 | 43,845.02 | 0.027 |
| Muscularis thickness, μm | 3,515.06 | 3,520.28 | 55.55 | 0.965 |
| Dorsal sac of rumen | ||||
| PL, μm | 607.73 | 606.03 | 12.65 | 0.950 |
| PW, μm | 352.79 | 360.53 | 8.00 | 0.651 |
| PSA, μm2 | 215,220.77 | 219,743.76 | 9,180.87 | 0.819 |
| Muscularis thickness, μm | 3,631.61 | 3,879.9 | 63.31 | 0.043 |
YCW = yeast cell wall; PL = papilla length; PW = papilla width; PSA = papilla surface area.
CON, control with no YCW (Angel Yeast Co., Ltd., Yichang, Hubei, China); YCW, YCW accounted for 0.16% of the basal diet.
3.3. Intestinal histology
Effects of YCW on the VH, VW, VSA, crypt depth, VCR, and thickness of the wall in the small intestine are presented in Table 5. Except for higher (P < 0.05) VH of the ileum in the YCW group, numerical differences (P > 0.05) were detected between CON and YCW groups for VH in the duodenum and jejunum. No difference of VW, VSA, and crypt depth in any segments of the small intestine was observed between 2 groups. Calves supplemented with YCW showed a greater (P = 0.030) VCR in the ileum. No difference in VCR of duodenum and jejunum was found between CON and YCW groups. However, calves fed YCW significantly increased (P < 0.05) the thickness of the wall of duodenum and jejunum.
Table 5.
Effects of YCW on the small intestinal histology of weaned calves1.
| Item | Treatments |
SEM | P-value | |
|---|---|---|---|---|
| CON | YCW | |||
| Duodenum | ||||
| VH, μm | 861.01 | 875.89 | 14.53 | 0.632 |
| VW, μm | 162.55 | 161.42 | 6.57 | 0.936 |
| VSA, μm2 | 139,549.89 | 141,282.11 | 5,535.59 | 0.884 |
| Crypt depth, μm | 216.93 | 217.52 | 5.26 | 0.958 |
| VCR | 3.99 | 4.06 | 0.11 | 0.763 |
| Thickness of wall, μm | 1,291.67 | 1,410.12 | 30.71 | 0.047 |
| Jejunum | ||||
| VH, μm | 894.02 | 908.17 | 16.84 | 0.695 |
| VW, μm | 166.47 | 163.06 | 5.78 | 0.783 |
| VSA, μm2 | 149,625.68 | 148,171.01 | 6,737.95 | 0.920 |
| Crypt depth, μm | 203.25 | 201.94 | 4.97 | 0.902 |
| VCR | 4.42 | 4.54 | 0.15 | 0.694 |
| Thickness of wall, μm | 1,318.77 | 1,426.6 | 27.67 | 0.045 |
| Ileum | ||||
| VH, μm | 845.11 | 905.79 | 15.78 | 0.048 |
| VW, μm | 163.61 | 165.72 | 6.34 | 0.877 |
| VSA, μm2 | 138,113.33 | 150,147.47 | 6,117.96 | 0.349 |
| Crypt depth, μm | 200.90 | 191.67 | 5.19 | 0.400 |
| VCR | 4.22 | 4.76 | 0.13 | 0.030 |
| Thickness of wall, μm | 1,322.99 | 1,339.31 | 20.23 | 0.706 |
YCW = yeast cell wall; VH = villus height; VW = villus width; VSA = villus surface area; VCR = villus height-to-crypt depth ratio.
CON, control with no YCW (Angel Yeast Co., Ltd., Yichang, Hubei, China); YCW, YCW accounted for 0.16% of the basal diet.
3.4. Intestinal barrier function
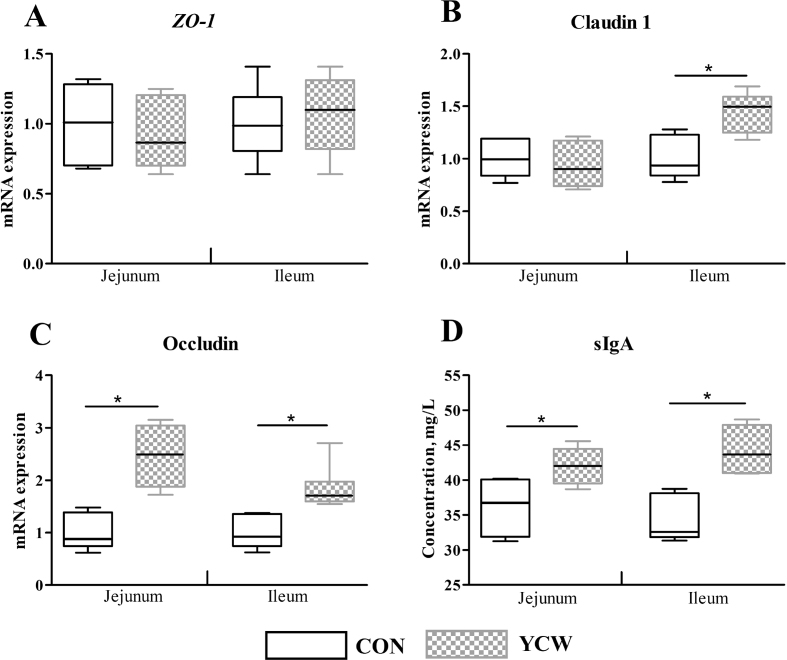
Fig. 1 shows the effects of YCW on the mRNA expression of ZO-1, claudin 1, occludin, and the content of sIgA in the jejunum and ileum. No significant difference in ZO-1 mRNA expression of jejunum or ileum was observed between CON and YCW groups (Fig. 1A, P > 0.05). The mRNA expression of claudin 1 in the jejunum was similar. However, calves supplemented with YCW significantly increased the mRNA expression of claudin 1 in the ileum (Fig. 1B, P < 0.05). Calves fed YCW exhibited a higher mRNA expression of occludin both in the jejunum and ileum (Fig. 1C, P < 0.05). Additionally, the mRNA expressions of occludin in the jejunum and ileum of the YCW group were 146.33% and 83.33% higher than those of the CON group. As shown in Fig. 1D, the contents of sIgA of calves supplemented with YCW also significantly increased (P < 0.05) in the jejunum and ileum.
Fig. 1.
Effects of YCW on the intestinal barrier function of weaned calves. (A) mRNA expression of ZO-1. (B) mRNA expression of claudin 1. (C) mRNA expression of occludin. (D) The concentration of sIgA. CON = control with no YCW (Angel Yeast Co., Ltd., Yichang, Hubei, China); YCW, yeast cell wall accounted for 0.16% of the basal diet. ZO-1 = zonula occludens 1; sIgA = secretory immunoglobulin A. The asterisk indicates a significant difference between 2 groups (P < 0.05).
4. Discussion
4.1. The weight of stomach
Rumen is an important organ of ruminants for nutrient digestion and absorption. The healthy development of rumen at young age is closely related to production performance in the future (Cristobal-Carballo et al., 2019). The present study did not find a statistical difference in stomach weight between CON and YCW groups. Only numerical improvements were found in the weights of rumen and abomasum when YCW was added in the diet. These findings were consistent with the results from Xiao et al. (2016), who found that yeast fermentation products had no obvious effects on the weight of the stomach.
4.2. Ruminal histology
Ruminal epithelium plays an essential role in the absorption of volatile fatty acids (Penner et al., 2009), the regulation of homeostasis to prevent severe cytosolic acidosis (Gäbel et al., 2002), the nitrogen transportation, and urea recycling (Abdoun et al., 2006). Ruminal papilla length and width are the most critical factors to evaluate the relative development of ruminal epithelium, followed by the thickness of the gastric wall (Lesmeister et al., 2004b). Normal development of rumen is crucial to the utilization of nutrients and directly affects the amount of volatile fatty acids absorption in the rumen (Graham and Simmons, 2005). A previous finding has shown that the gastrointestinal histology is sensitive to physical or chemical changes of the ration (Lesmeister and Heinrichs, 2004). Effects of yeast products on the ruminal histology have been studied previously. The yeast fermentation products improve PL and PW of the rumen (Brewer et al., 2014; Xiao et al., 2016). Similar positive effects of ruminal papilla were found in the present research. Besides, this study also found that calves fed YCW significantly increased the PSA in the ventral sac of the rumen. These results indicated that the rumen had a larger absorption area for volatile fatty acids and other nutrients. Effects of YCW on the dorsal sac of the rumen mainly reflected in the muscularis thickness. The results from our study showed that YCW could effectively improve nutrient absorption area and development of weaned calves' rumen. According to our data (data not published), calves supplemented with YCW could increase the content of propionate in the rumen (CON vs. YCW: 13.42 vs. 15.04 mmol/L, P = 0.011). Propionate is used as an energy source by the ruminal epithelium and can promote the development of ruminal epithelium (Liang et al., 2017). Therefore, the increased content of propionate might have positive effects on the ruminal development. In short, YCW could promote the development of weaned calves' rumen.
4.3. Intestinal histology
The higher VH of the small intestine can result in the larger epithelial surface area, and the larger area means a stronger ability to digest and absorb nutrients to avoid nutritional diarrhea caused by the excessive fermentation of posterior intestine (Graham et al., 1984). A previous study has demonstrated that villus shortening occurs via the increase in the rate of cell loss, resulting in increased crypt cell production, and finally, increased crypt depth (Pluske et al., 1997). Deeper crypt depth coinciding with lower VCR value may cause nutrient malabsorption and diarrhea (Pearson et al., 1978). In the present study, the results of YCW calves exhibited an increase for VH and a decrease for crypt depth of ileum, which resulted in improved VCR of ileum. In addition, the thickness of the wall in duodenum and jejunum were significantly increased. Similar results were observed by Zhao et al. (2012), who reported that dietary supplementation of mannan-oligosaccharide resulted in greater intestinal VH and VCR. Another study also reported that broilers fed yeast products could improve intestinal histology (Gao et al., 2008). Although YCW had limited effects on the VW and VSA, YCW showed an ability to increase the VH and decrease the crypt depth, which had positive effects for improving VCR. Another study found that both mannan-oligosaccharide and β-glucan in YCW could protect the intestinal epithelium by preventing the colonization of pathogenic bacteria (Bode, 2012). However, the potential mechanism of YCW on the intestinal structural development of calves needs further elucidation.
4.4. Intestinal barrier function
The tight junction is a zonal junction structure between intestinal epithelial cells. It is located at the top of the epithelial cells and plays a critical role in sealing the cell space. It is an important structure to prevent harmful pathogenic bacteria and toxic substances from entering the submucosa through the intestinal epithelial space. Besides, the tight junction is useful to regulate the permeability of the epithelium and to selectively transport nutrients (Han et al., 2015). The tight junctions consist of various tight junction proteins, mainly including zonula occludens, claudin, and occludin proteins, and these proteins are widely distributed among the epithelial cells of the digestive tract. The mRNA expression levels of tight junction proteins are positively correlated with the epithelial barrier function. Moreover, the occurrence of various intestinal diseases are correlated with the abnormal expression of tight junction proteins (Musch et al., 2006). Effects of YCW on the tight junction protein mRNA expression in the small intestine of calves have not been studied. Previously, a study reported that dietary supplementation with β-glucan could up-regulate the mRNA expressions of claudin 1 and occludin in the small intestinal mucosa of salmonella-infected broilers (Shao et al., 2013). Our research found that calves fed YCW significantly increased the mRNA expression of occludin in the mucosa of jejunum and ileum, and the difference in claudin 1 mRNA expression occurred in the ileum. The above results indicated that adding YCW in the diet could protect the intestinal health of weaned calves. The possible reason is that YCW can adhere to the surface of pathogenic bacteria to prevent their colonization and invasion in the intestinal tract. Thus, YCW could protect the integrity of the intestinal epithelial structure and stimulate the secretion of cytokines in the mucosa, which affect the expression of tight junction proteins. The findings were consistent with the results of relatively complete intestinal development of YCW calves.
The sIgA is the first line of defense against enterotoxins and pathogenic microorganisms in the intestinal epithelium, and also plays a vital role in maintaining the homeostasis of intestinal mucosa (Mantis et al., 2011). A previous study has shown that feeding β-glucan could increase the contents of small intestinal sIgA in salmonella-infected broilers (Shao et al., 2013). In the current study, the mucosal sIgA contents of the small intestine were significantly higher in the YCW calves than in the CON calves. Furthermore, according to our data (not yet published), calves supplemented with YCW could increase the average daily gain (CON vs. YCW: 0.93 vs. 1.17 kg/d, P = 0.025) and neutral detergent fiber digestibility (CON vs. YCW: 69.79% vs. 74.05%, P = 0.030). Based on all these results, we can speculate that weaning stress could cause gastrointestinal dysplasia of calves. Whereas, YCW could improve the gastrointestinal development of weaned calves and subsequently increase the nutrient digestibility, which finally promotes the growth performance of weaned calves. In the future, more studies should focus on the effects of YCW on absorptive and metabolic mechanisms in calves' gastrointestinal development.
5. Conclusions
The weaned calves receiving YCW supplementation had better PL, PW, and PSA in the ventral sac of rumen. Moreover, calves fed YCW exhibited increased VH and VCR in the ileum. On the other hand, dietary supplementation with YCW could improve the barrier function of the small intestine of weaned calves. Therefore, YCW can be used as an effective feed additive to improve the gastrointestinal development of weaned calves.
Author contributions
Jian Ma and Zhisheng Wang: conceptualization; Jian Ma and Zhisheng Wang: methodology; Zhisheng Wang and Kun Kang: funding acquisition; Jian Ma and Yaqun Shao: investigation; Jian Ma and Ali Mujtaba Shah: formal analysis; Zhisheng Wang and Huawei Zou: supervision; Jian Ma: writing—original draft; Jian Ma, Ali Mujtaba Shah, Zhisheng Wang, Huawei Zou and Kun Kang: writing—review & editing.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the National Key Research and Development Program (no. 2017YFD0502005) and China Agriculture (Beef Cattle/Yak) Research System (CARS-37). We would like to thank the experimental site of Animal Nutrition Institute, Sichuan Agricultural University (Ya'an, Sichuan, China) for providing the facilities. We also greatly acknowledge the Angel Yeast Co., Ltd., for supplying the YCW for the experiment. Moreover, gratitude is expressed to Dr. Sajid Hameed for linguistic assistance.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abdoun K., Stumpff F., Martens H. Ammonia and urea transport across the rumen epithelium: a review. Anim Health Res Rev. 2006;7:43–59. doi: 10.1017/S1466252307001156. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis of the AOAC. 17th Edition. Association of Official Analytical Chemists; Virginia, USA: 2000. [Google Scholar]
- Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22:1147–1162. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer M.T., Anderson K.L., Yoon I., Scott M.F., Carlson S.A. Amelioration of salmonellosis in pre-weaned dairy calves fed Saccharomyces cerevisiae fermentation products in feed and milk replacer. Vet Microbiol. 2014;172:248–255. doi: 10.1016/j.vetmic.2014.05.026. [DOI] [PubMed] [Google Scholar]
- Cristobal-Carballo O., Khan M.A., Knol F.W., Lewis S.J., Stevens D.R., Laven R.A. Impact of weaning age on rumen development in artificially-reared lambs. J Anim Sci. 2019;97:3498–3510. doi: 10.1093/jas/skz148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gäbel G., Aschenbach J.R., Müller F. Transfer of energy substrates across the ruminal epithelium: implications and limitations. Anim Health Res Rev. 2002;3:15–30. doi: 10.1079/ahrr200237. [DOI] [PubMed] [Google Scholar]
- Gao J., Zhang H.J., Yu S.H., Wu S.G., Yoon I., Quigley J. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poultry Sci. 2008;87:1377–1384. doi: 10.3382/ps.2007-00418. [DOI] [PubMed] [Google Scholar]
- Graham C., Simmons N.L. Functional organization of the bovine rumen epithelium. Am J Physiol Regul Integr Comp Physiol. 2005;288:R173–R181. doi: 10.1152/ajpregu.00425.2004. [DOI] [PubMed] [Google Scholar]
- Graham D.Y., Sackman J.W., Estes M.K. Pathogenesis of rotavirus-induced diarrhea. Digest Dis Sci. 1984;29:1028–1035. doi: 10.1007/BF01311255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F.F., Zhang H.W., Xia X., Xiong H.T., Song D.G., Zong X. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J Immunol. 2015;194:1882–1893. doi: 10.4049/jimmunol.1402300. [DOI] [PubMed] [Google Scholar]
- Han F.F., Fan H.X., Yao M., Yang S.S., Han J.Z. Oral administration of yeast β-glucan ameliorates inflammation and intestinal barrier in dextran sodium sulfate-induced acute colitis. J Funct Foods. 2017;35:115–126. [Google Scholar]
- Hulbert L.E., Cobb C.J., Carroll J.A., Ballou M.A. The effects of early weaning on innate immune responses of Holstein calves. J Dairy Sci. 2011;94:2545–2556. doi: 10.3168/jds.2010-3983. [DOI] [PubMed] [Google Scholar]
- Lesmeister K.E., Heinrichs A.J. Effects of corn processing on growth characteristics, rumen development, and rumen parameters in neonatal dairy calves. J Dairy Sci. 2004;87:3439–3450. doi: 10.3168/jds.S0022-0302(04)73479-7. [DOI] [PubMed] [Google Scholar]
- Lesmeister K.E., Heinrichs A.J., Gabler M.T. Effects of supplemental yeast (Saccharomyces cerevisiae) culture on rumen development, growth characteristics, and blood parameters in neonatal dairy calves. J Dairy Sci. 2004;87:1832–1839. doi: 10.3168/jds.S0022-0302(04)73340-8. [DOI] [PubMed] [Google Scholar]
- Lesmeister K.E., Tozer P.R., Heinrichs A.J. Development and analysis of a rumen tissue sampling procedure. J Dairy Sci. 2004;87:1336–1344. doi: 10.3168/jds.S0022-0302(04)73283-X. [DOI] [PubMed] [Google Scholar]
- Liang Y.S., Li G.Z., Li X.Y., Lü J.Y., Li F.D., Tang D.F. Growth performance, rumen fermentation, bacteria composition, and gene expressions involved in intracellular pH regulation of rumen epithelium in finishing Hu lambs differing in residual feed intake phenotype1. J Anim Sci. 2017;95:1727–1738. doi: 10.2527/jas.2016.1134. [DOI] [PubMed] [Google Scholar]
- Liu N., Wang J.Q., Liu Z.Y., Wang Y.C., Wang J.P. Effect of supplemental yeast cell walls on growth performance, gut mucosal glutathione pathway, proteolytic enzymes and transporters in growing broiler chickens. J Anim Sci. 2018;96:1330–1337. doi: 10.1093/jas/sky046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔ CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mantis N.J., Rol N., Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch M.W., Walsh-Reitz M.M., Chang E.B. Roles of ZO-1, occludin, and actin in oxidant-induced barrier disruption. Am J Physiol Gastrointest Liver Physiol. 2006;290:G222–G231. doi: 10.1152/ajpgi.00301.2005. [DOI] [PubMed] [Google Scholar]
- Nocek J.E., Holt M.G., Oppy J. Effects of supplementation with yeast culture and enzymatically hydrolyzed yeast on performance of early lactation dairy cattle. J Dairy Sci. 2011;94:4046–4056. doi: 10.3168/jds.2011-4277. [DOI] [PubMed] [Google Scholar]
- NRC . 7th rev. ed. Natl. Acad. Sci.; Washington, DC: 2001. Nutrient requirements of dairy Cattle. [Google Scholar]
- Pearson G.R., McNulty M.S., Logan E.F. Pathological changes in the small intestine of neonatal calves naturally infected with reo-like virus (rotavirus) Vet Rec. 1978;102:454–458. doi: 10.1136/vr.102.21.454. [DOI] [PubMed] [Google Scholar]
- Penner G.B., Aschenbach J.R., Gäbel G., Rackwitz R., Oba M. Epithelial capacity for apical uptake of short chain fatty acids is a key determinant for intraruminal pH and the susceptibility to subacute ruminal acidosis in sheep. J Nutr. 2009;139:1714–1720. doi: 10.3945/jn.109.108506. [DOI] [PubMed] [Google Scholar]
- Pluske J.R., Hampson D.J., Williams I.H. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci. 1997;51:215–236. [Google Scholar]
- Rezazadeh F., Kowsar R., Rafiee H., Riasi A. Fermentation of soybean meal improves growth performance and immune response of abruptly weaned Holstein calves during cold weather. Anim Feed Sci Technol. 2019;254:114206. [Google Scholar]
- Salinas-Chavira J., Arzola C., González-Vizcarra V., Manríquez-Núñez O.M., Montaño-Gómez M.F., Navarrete-Reyes J.D. Influence of feeding enzymatically hydrolyzed yeast cell wall on growth performance and digestive function of feedlot cattle during periods of elevated ambient temperature. Asian-Australas J Anim Sci. 2015;28:1288–1295. doi: 10.5713/ajas.15.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y.J., Guo Y.M., Wang Z. β-1, 3/1, 6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poultry Sci. 2013;92:1764–1773. doi: 10.3382/ps.2013-03029. [DOI] [PubMed] [Google Scholar]
- Shen Y.B., Piao X.S., Kim S.W., Wang L., Liu P., Yoon I. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J Anim Sci. 2009;87:2614–2624. doi: 10.2527/jas.2008-1512. [DOI] [PubMed] [Google Scholar]
- Terré M., Calvo M.A., Adelantado C., Kocher A., Bach A. Effects of mannan oligosaccharides on performance and microorganism fecal counts of calves following an enhanced-growth feeding program. Anim Feed Sci Technol. 2007;137:115–125. [Google Scholar]
- Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Xiao J.X., Alugongo G.M., Chung R., Dong S.Z., Li S.L., Yoon I. Effects of Saccharomyces cerevisiae fermentation products on dairy calves: ruminal fermentation, gastrointestinal morphology, and microbial community. J Dairy Sci. 2016;99:5401–5412. doi: 10.3168/jds.2015-10563. [DOI] [PubMed] [Google Scholar]
- Xue G.D., Wu S.B., Choct M., Swick R.A. Effects of yeast cell walls on growth performance, immune responses and intestinal short chain fatty acid concentrations of broiler in an experimental necrotic enteritis model. Anim Nutr. 2017;3:399–405. doi: 10.1016/j.aninu.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbrun M.V., Zielinski G.C., Piscitelli H.C., Descarga C., Urbani L.A., Defain Tesoriero M.V. Evaluation of anti-Moraxella bovis pili immunoglobulin-A in tears following intranasal vaccination of cattle. Res Vet Sci. 2012;93:183–189. doi: 10.1016/j.rvsc.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Zhao P.Y., Jung J.H., Kim I.H. Effect of mannan oligosaccharides and fructan on growth performance, nutrient digestibility, blood profile, and diarrhea score in weanling pigs. J Anim Sci. 2012;90:833–839. doi: 10.2527/jas.2011-3921. [DOI] [PubMed] [Google Scholar]