Abstract
Polyphenols are a class of non-essential phytonutrients, which are abundant in fruits and vegetables. Dietary polyphenols or foods rich in polyphenols are widely recommended for metabolic health. Indeed, polyphenols (i.e., catechins, resveratrol, and curcumin) are increasingly recognized as a regulator of lipid metabolism in host. The mechanisms, at least in part, may be highly associated with gut microbiome. This review mainly discussed the beneficial effects of dietary polyphenols on lipid metabolism. The potential mechanisms of gut microbiome are focused on the effect of dietary polyphenols on gut microbiota compositions and how gut microbiota affect polyphenol metabolism. Together, dietary polyphenols may be a useful nutritional strategy for manipulation of lipid metabolism or obesity care.
Keywords: Polyphenols, Lipid metabolism, Obesity, Gut microbiota
1. Introduction
With the development of society and economy, obesity has become an epidemic health problem worldwide (Morgen and Sørensen, 2014; Duan et al., 2019). Obesity is a result of imbalanced energy intake, energy expenditure, and host lipid metabolism (Adriouch et al., 2017). Various factors could alter lipid metabolism, such as eating habits, exercise, and gut microbiota (Amiot et al., 2016; Yin et al., 2018, 2020; Cao et al., 2019; Song et al., 2019; Li et al., 2020). The direct evidence between gut microbiota and lipid metabolism was demonstrated in germ-free mice, which kept lean even when fed with a high-fat diet (Lewis et al., 2020). Fecal microbial transplantation further confirms the role of gut microbiota in obesity development evidenced by that the colonization of germ-free mice with an ‘obese microbiota’ accumulated more body fat than the colonization with a ‘lean microbiota’ (Zhang et al., 2019b). Accordingly, the manipulation of gut microbiota (dietary polyphenols, probiotics, fiber-rich diet, and other active compounds) may be a potential novel target to influence host lipid metabolism or treat obesity (Azad et al., 2018; Guan et al., 2019; Wang et al., 2019; Guo et al., 2020).
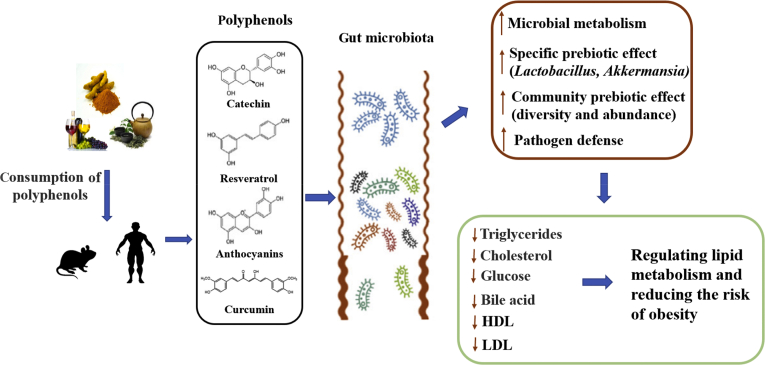
Polyphenols are a large family of bioactive substances from tea, fruits, vegetables, roots, seeds, cocoa, and wine. According to the non-absorbing characteristic in the small intestine, 90% to 95% dietary polyphenols reach the colon and then transform into bioactive products by gut microbiota (Ozdal et al., 2016; Ding et al., 2019). Accumulating evidence suggested that dietary polyphenols and the microbiota-generated metabolites show positive effects on human health, including lipid metabolism (Das et al., 2016; Joseph et al., 2016). Firstly, polyphenols influence gut microbiota compositions in obese subjects, which further affect the host lipid metabolism (Anhê et al., 2019). In turn, gut microbiota metabolize polyphenols into bioactive molecules to improve the lipid regulatory bioavailability (Fig. 1). In this review, we will clarify the relationship between dietary polyphenols and lipid metabolism and discuss the potential role of gut microbiota.
Fig. 1.
Gut microbiota mediates the lipid metabolic benefits of dietary polyphenol. Dietary polyphenols are metabolized by microorganisms to produce small active molecules, which further influence gut microbes to regulate host lipid metabolism. HDL = high-density lipoprotein; LDL = low-density lipoprotein.
2. Polyphenols and lipid metabolism
Polyphenols were first discovered by Nobel Prize laureate Dr. Albert Szent-Györgyi in 1937 and named vitamin P later by other researchers (Grzybowski and Pietrzak, 2013). Vitamin P is a nonessential phytonutrient (mostly flavanones) and these compounds are now classified as polyphenols, including flavonoids, stilbenes, phenolic acids, and lignans. Flavonoids contains various bioactive substances, i.e., anthocyanins (ACN), flavanols, flavones, flavanols, flavanones, and isoflavone. Stilbenes, phenolic acids, and lignans are collectively considered as non-flavonoids polyphenols (Rasines-Perea and Teissedre, 2017; Fraga et al., 2019).
Dietary polyphenols are generally recommended for clinical benefits (Macready et al., 2014), such as metabolic disorders. Growing evidence indicated that polyphenols are involved in lipid metabolism and may prevent obesity development (Adriouch et al., 2017). For example, administration with polyphenol-rich plant extract improved plasma lipid levels and endotoxaemia, macrophage recruitment infiltration to adipose tissues, and adipose accumulation of cholesterol and cholesterol oxides in obese animals (Aires et al., 2019). In clinic trails, significant inverse correlations were observed between dietary polyphenols and body weight, body mass index, waist circumference, and waist-to-height ratio by a 5-year follow-up (Guo et al., 2017). In another randomized, double-blind, placebo-controlled clinical trial, dietary polyphenols might reduce cardiovascular disease risk and atherosclerosis via lowering triglyceride levels (Ishida et al., 2018). To conclude, a polyphenol-rich intake may lead to a heathy life through regulating lipid metabolism and reducing obesity risk.
2.1. Flavonoids and lipid metabolism
Flavonoids are a class of more than 6,000 phenolic compounds widely found in fruits, vegetables, nuts, tea, grains, cocoa, and other plants. Recently, numerous animal and human clinical trials have confirmed the lipid regulatory role of flavonoids and their preventive effects in metabolic diseases (Mulvihill et al., 2016; Rupasinghe et al., 2016; Casanova et al., 2019; Zhang et al., 2019a). In the mice model, dietary baicalin, a major flavonoid in Scutellaria baicalensis, accelerated lipid oxidation and prevented diet-induced obesity (Dai et al., 2018). The intraperitoneal injection of licochalcone A also showed an improvement of lipid metabolism (triglycerides, low-density lipoprotein, and free fatty acids) in high-fat diet-induced obesity and the mechanism might be associated with NAD-dependent deacetylase sirtuin-1 (SIRT1)/5′ AMP-activated protein kinase (AMPK) pathway in obese mice (Liou et al., 2018, 2019). Oxidative stress and inflammation have been widely identified in obese subjects, and antioxidant or anti-inflammatory therapeutic strategies of flavonoids are suggested to treat obesity. For example, dietary flavonoids increased the activity of serum antioxidant enzymes and downregulated pro-inflammatory indexes, which might further improve host lipid metabolism (Wu et al., 2018).
2.2. Stilbenes and lipid metabolism
Resveratrol (RSV) is a major kind of stilbene and has been extensively studied for its anti-obesity, metabolic, and glucose homeostasis effects (León et al., 2017; Ardid-Ruiz et al., 2018; Sun et al., 2018; Andrade et al., 2019; Gimeno-Mallench et al., 2019; Muhammadi and Shafiq, 2019; Springer and Moco, 2019; Yue et al., 2019). Furthermore, the interaction between gut microbiota and resveratrol has recently attracted much interest in the research community because of its potential role in preventing obesity (Chaplin et al., 2018; Zhou et al., 2019a). Dietary resveratrol modified gut microbiota compositions (resveratrol-microorganisms) and reversed the abundances of Lactococcus, Clostridium XI, Oscillibacter, and Hydrogenoanaerobacterium in obese mice (Jung et al., 2016). In addition, resveratrol reduced the level of gut microbiota-derived metabolite trimethylamine-N-oxide (TMAO, an early biomarker of adipose dysfunction) by remodeling the intestinal microbiota (Chen et al., 2016; Barrea et al., 2018). Accordingly, the transplantation of resveratrol-microorganisms into high-fat diet-fed mice promoted the development of beige adipocytes in white adipose tissue and improved lipid metabolism (Wang et al., 2020).
2.3. Phenolic acids and lipid metabolism
Phenolic acids, mainly found in grains, wine, some berries, and nuts, also play a beneficial role in regulating lipid metabolism (Tajik et al., 2017; Naveed et al., 2018). Oral phenolic acid reversed the increase in body weight and improved lipid metabolism (i.e., triglycerides, cholesterol, high density lipoprotein, low density lipoprotein, and very low-density lipoprotein) in mice fed a high-fructose diet and the mechanism might be associated with hormones (i.e., insulin, leptin, and adiponectin) (Ibitoye and Ajiboye, 2018). Previous studies have shown that danshenolic acid regulated obesity and related metabolic diseases by reducing the expression of liver genes (i.e., sterol regulatory element binding protein 1 (SREBP1), fatty acid synthase (FAS), stearyl coenzyme A desaturase 1 (SCD1). Additionally, ferulic acid also improved the glucose and lipid homeostasis in high-fat diet-induced obese mice by inhibiting the protein expression of liver gluconeogenesis, such as phosphoenolpyruvate carboxylase (PEPCK) and glucose-6-phosphatase (G6Pase) (Naowaboot et al., 2016).
2.4. Lignans and lipid metabolism
Lignans mainly include secoisolariciresinol, lariciresinol, matairesinol, pinoresinol, medioresinol, and syringaresinol, and with the clarification of the new lignan structure, the property spectrum has also been broadened (Durazzo et al., 2018). The regulatory role of lignans in lipid metabolism and obesity has been widely reported (Zhang et al., 2015; Scharinger et al., 2016; Jahagirdar et al., 2018). For example, 7-hydroxymatairesinol (7-HMR), a plant-based lignan, limited the weight and fat gain and lowered serum lipids, cholesterols, and triglycerides of mice induced by a high-fructose diet (Biasiotto et al., 2018). The mechanism might be related to increasing the level of peroxisome proliferator–activated receptor a (PPARα) and carnitine palmitoyl transferases 1c and 2 (Cpt1c and Cpt2) (key genes involved in fatty acid oxidation) (Chan et al., 2018). More importantly, further research confirmed that the anti-hyperlipidemic effect of lignan is achieved by down-regulating the liver X receptor α (LXRα)/SREBP1c/FAS/acetyl-CoA carboxylase (ACC) and SREBP2/3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) signaling pathways (Sun et al., 2017).
3. Host–microbe interplay in the lipid metabolic benefits of dietary polyphenols
Although abundant studies reported that polyphenols have antioxidant (Das et al., 2016), anti-inflammatory (Joseph et al., 2016), anti-obesity (Xu et al., 2015), antibacterial (Lu et al., 2018), and anti-cancer (Gorlach et al., 2015) benefits, because of the low bioavailability of polyphenols, a novel view points out that polyphenols interact with intestinal microbes to affect host lipid metabolism (Anhê et al., 2019). On the one hand, polyphenols affect the structure of gut microbiota, such as Firmicutes, Bacteroidetes, and Actinobacteria (Chambers et al., 2019); on the other hand, gut microbiota further improves the bioavailability of polyphenols and promotes the production of polyphenol metabolites including bile acids and short-chain fatty acids (Fernandes et al., 2014), which regulate lipid homeostasis (Fig. 1) (Castro-Barquero et al., 2018).
3.1. Impact of dietary polyphenols on microbial ecology in obese subject
The role of polyphenols in anti-obesity has been widely reviewed and the main mechanisms include causing satiety, promoting energy expenditure by stimulating brown fat-producing heat, regulating fat cells, and inhibiting lipid breakdown (Meydani and Hasan, 2010; Wang et al., 2014; Rupasinghe et al., 2016). However, recent studies also confirm the key role of polyphenols in host lipid metabolism and obesity development through regulating gut microbiota. For example, grape seed extract affected the compositions of microbiota by increasing the relative abundances of Lachnospiraceae, unclassified Clostridales, Lactobacillus, and Ruminococcacceae (Rasines-Perea and Teissedre, 2017). Resveratrol modified intestinal microbes in obese mice by reducing the relative abundances of Turicibacteraceae, Moryella, Lachnospiraceae, and Akkermansia and by increasing the relative abundances of Bacteroides and Parabacteroides (Sung et al., 2017). Meanwhile, in vitro experiments have also shown that polyphenols act as prebiotics by promoting the growth of beneficial bacteria such as Lactobacillus spp. and Bifidobacterium spp., and plum polyphenols have been reported to reduce body weight in obese rats by altering gut microbial structure by increasing Faecalibacterium spp., Lactobacillus spp., and Bacteroides spp. (Noratto et al., 2014). Together, dietary polyphenol intervention can regulate gut microbial ecology, making it more conducive to health by increasing the abundances of probiotics (Marchesi et al., 2016).
3.2. Microbial metabolites of dietary polyphenols
Recent studies have demonstrated the role of dietary polyphenols in the prevention of obesity and obesity-related diseases (Wang et al., 2014). However, most polyphenolic compounds escape the digestion and absorption of the small intestine, and are metabolized by microorganisms in the colon (Ozdal et al., 2016). Polyphenols are mainly hydrolyzed by the microbial enzymes, responsible for the hydrolysis release of o-glucosides and the cleavage of carbon-carbons, resulting in smaller molecules that are more active than natural compounds. Therefore, gut microbiota is highly linked to the bioavailability of polyphenols. In this review, catechins, resveratrol, curcumin, and anthocyanins will be fully introduced.
3.2.1. Catechins
Catechins are the major polyphenolic compounds in green tea, including epigallocatechin-3-gallate (EGCG), epigallocatechin (EGC), epicatechin-3-gallate, epicatechin, gallocatechins, and gallocatechin gallate (Khan and Mukhtar, 2018). EGCG is hydrolyzed to EGC and gallic acid in the first stage of metabolism and then EGC is converted to 1-(3′,4′,5′-trihydroxyphenyl)-3-(2″,4″,6″-trihydroxyphenyl) propan-2-ol, which is further transformed to 1-(3′-dihydroxyphenyl)-3-(2″,4″,6″-trihydroxyphenyl) propan-2-ol. In addition, a part of 1-(3′-dihydroxyphenyl)-3-(2″,4″,6″-trihydroxyphenyl) propan-2-ol is converted to 5-(3′,5′-dihydroxyphenyl)-pentanoic acid and a small portion is converted to 3,5-dihydroxyphenylpropionic acid (3,5-DHPPA) (Mitchell et al., 2019). Furthermore, metabolomics analysis also revealed that EGC produces 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone and then forms 5-(3′,5′-trihydroxyphenyl)-γ-valerolactone in tea polyphenols, and epicatechin is biotransformed into 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone. The γ-valerolactone ring of 5-(3′,5′-trihydroxyphenyl)-γ-valerolactone and 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone could be opened through hydrolysis, yielding 4-hydroxy-5-(dihydroxyphenyl)-valeric acid. Microbial dehydroxylation reactions could further convert 4-hydroxy-5-(dihydroxyphenyl)-valeric acid to 5-(dihydroxyphenyl)-valeric acid and then to 3-hydroxyphenyl-valeric acid (Zhou et al., 2019b). Some microorganisms have been shown to participate in EGCG biotransformation, including Enterobacter aerogenes, Raoultella planticola, Klebsiella pneumoniae susp., Pneumoniae, Bifidobacterium longum subsp. infantis, Clostridium orbiscindens, and Eubacterium ramulus (Takagaki and Nanjo, 2010; Kutschera et al., 2011).
3.2.2. Resveratrol
Resveratrol is metabolized by the gut microbes in the large intestine to generate dihydroresveratrol, lunularin, and 3,4′-dihydroxy-trans-stibene (Muhammadi and Shafiq, 2019). The analysis of 16S rRNA sequences indicated that lunularin is positively associated with higher abundances of Bacteroidetes, Actinobacteria, Verrucomicrobia, Cyanobacteria, and a lower abundance of Firmicutes (Bode et al., 2013). Importantly, new bacterial strains Slackia equolifaciens and Adlercreutzia equolifaciens are found to convert resveratrol to dihydroresveratrol (Bode et al., 2013). Resveratrol and its metabolites have widely been reported to involve in lipid metabolism and the mechanisms include: (1) inhibiting adipocyte growth by modulating a panoply of protein targets such as nuclear hormone receptor-type, nuclear factor kappa-B (NF-κB), enolases, and sirtuins; (2) increasing thermogenesis by regulating central energy pathway signaling and protein targets (AMPK, mammalian target of rapamycin [mTOR], and protein kinase B [PKB, alos known as Akt]); (3) reducing inflammation by inhibiting cyclooxygenases (COX) and quinone reductase 2 (QR2) enzymes, and activating SIRT1 (Leixuri et al., 2014; Muhammadi and Shafiq, 2019).
3.2.3. Curcumin
Due to its low bioavailability, curcumin is generally metabolized by microbiota in the colon (Di Meo et al., 2019). The curcumin metabolic pathway includes reduction, methylation, demethoxylation, hydroxylation, and acetylation by gut microorganisms, and the main products contain tetrahydrocurcumin (THC), dihydroferulic acid (DFA), and 1-(4-hydroxy-3-methoxyphenyl)-2-propanol (Tsuda, 2018; Zam, 2018). In addition, curcumin can be metabolized by Pichia pastoris into 4 major metabolites, such as 5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-1-(4-hydroxyphenyl) heptan-3-one, 5-hydroxy-1,7-bis (4-hydroxy-3-methoxyphenyl) heptan-3-one,5-hydroxy-1,7-bis (4-hydroxyphenyl) heptane-3-one, and 1,7-bis (4-hydroxy-3methoxyphenyl) heptan-3,5-diol (Zam, 2018). Several microbiota such as Escherichia coli, Blautia sp. (mrg-pmf1), Bifidobacterium, Lactobacillus, Pichia anomala, and Bacillus megaterium dcmb-002 have often been found to be involved in the biotransformation of curcumin (Shen and Ji, 2019). Curcumin and its metabolites involve in lipid metabolism mainly by targeting the following pathways: (1) inhibiting the formation and differentiation of adipocytes by downregulating proliferator-activated receptor γ (PPARγ), CCAAT/enhancer-binding protein α (C/EBPα), extracellular-signal-regulated kinase (ERK), c-Jun N-terminal kinases (JNK), and p38 and activating wnt/β-catenin and SIRT1; (2) reducing inflammation via inhibiting monocyte chemoattractant protein-1 from 3T3-L1 adipocytes and down-regulating the inflammatory transcription factors NF-kB and activator protein-1 (AP-1); (3) promoting antioxidants by activating NFE2-related factor 2 (Nrf2) (Bradford, 2013; Zhao et al., 2017).
3.2.4. Anthocyanins
Most of anthocyanins can reach the colon and undergo various enzymatic process by microbiota such as the cleavage of the sugar moiety and the formation of anthocyanin aglycon (Khan et al., 2020), and anthocyanin aglycon can be biotransformed into simple phenolic acids by 2 bacterial enzymes (α-L-rhamnosidase and β-d-glucosidase) (Esposito et al., 2015). Then phenolic acids are further degraded into protocatechuic acid, syringic acid, vanillic acid, phloroglucinol aldehyde, phloroglucinol acid, and gallic acid (Pojer et al., 2013). The metabolic processes of anthocyanin include the cleavage of glycoside bond, the decomposition of anthocyanin heterocyclic ring, and the demethylation, which is associated with E. ramulus and Clostridium saccbarogumia (Tian et al., 2019). Anthocyanins and metabolites improve obesity primarily by inhibiting lipogenesis (Jamar et al., 2017), reducing inflammation (Lee et al., 2017; Peng et al., 2019), promoting energy homeostasis (Elena et al., 2017), and improving insulin resistance (Tarun et al., 2017).
4. Conclusions
During the past decades, remarkable progress has been made in our understanding of dietary polyphenols and host lipid metabolism. In this review, we summarized the basis for the understanding of lipid metabolic benefits of dietary polyphenols and discussed the interaction between dietary polyphenols and gut microbiota, which further regulates the lipid metabolism. Despite the progress made in the understanding of dietary polyphenols, gut microbiota, and lipid metabolism over the past few years, there are still a number of prominent research avenues to be explored. For example, how dietary polyphenols shape gut microbiota and how microbiota further targets host lipid metabolism? Different polyphenols have different roles in gut microbiota, and the specific mechanisms targeting host lipid metabolism should be investigated. In addition, the effects of polyphenols in the clinic should be fully studied and the dietary guidelines of polyphenols to control the development of obesity will become more prominent. In the future, polyphenol recipe will promote a series of effective methods to guide us in maintaining healthy habits and controlling obesity.
Author contributions
Jie Ma: writing—original draft preparation, revision and investigation. Yongmin Zheng: revision. Wenjie Tang: conceptualization. Wenxin Yan and Houfu Nie: investigation. Jun Fang: supervision. Gang Liu: validation.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was supported by National Natural Science Foundation of China (31772642, 31672457), Hunan Provincial Science and Technology Department (2019TP2004), China Postdoctoral Science Foundation (2019T120705, 2018M632963), and Young Elite Scientists Sponsorship Program by CAST (2019-2021QNRC001).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Adriouch S., Kesse-Guyot E., Feuillet T., Touvier M., Olié V., Andreeva V. Total and specific dietary polyphenol intakes and 6-year anthropometric changes in a middle-aged general population cohort. Int J Obes. 2017;42(3):310–317. doi: 10.1038/ijo.2017.227. [DOI] [PubMed] [Google Scholar]
- Aires V., Labbé J., Deckert V., Barros J.-P.P.d., Boidot R., Haumont M. Healthy adiposity and extended lifespan in obese mice fed a diet supplemented with a polyphenol-rich plant extract. Sci Rep. 2019;9(1):9134. doi: 10.1038/s41598-019-45600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiot M.J., Riva C., Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev. 2016;17(7):573–586. doi: 10.1111/obr.12409. [DOI] [PubMed] [Google Scholar]
- Andrade J.M.O., Barcala-Jorge A.S., Batista-Jorge G.C., Paraiso A.F., Freitas K.M., Lelis D.F. Effect of resveratrol on expression of genes involved thermogenesis in mice and humans. Biomed Pharmacother. 2019;112:108634. doi: 10.1016/j.biopha.2019.108634. [DOI] [PubMed] [Google Scholar]
- Anhê F.F., Choi B.S.Y., Dyck J.R.B., Schertzer J.D., Marette A. Host-microbe interplay in the cardiometabolic benefits of dietary polyphenols. Trends Endocrinol Metabol. 2019;30(6):384–395. doi: 10.1016/j.tem.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Ardid-Ruiz A., Ibars M., Mena P., Del Rio D., Muguerza B., Blade C. Potential involvement of peripheral leptin/STAT3 signaling in the effects of resveratrol and its metabolites on reducing body fat accumulation. Nutrients. 2018;10(11):1757. doi: 10.3390/nu10111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad M.A.K., Sarker M., Li T., Yin J. Probiotic species in the modulation of gut microbiota: an overview. BioMed Res Int. 2018;2018:9478630. doi: 10.1155/2018/9478630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrea L., Annunziata G., Muscogiuri G., Di Somma C., Laudisio D., Maisto M. Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients. 2018;10(12):1971. doi: 10.3390/nu10121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasiotto G., Zanella I., Predolini F., Archetti I., Cadei M., Monti E. 7-Hydroxymatairesinol improves body weight, fat and sugar metabolism in C57BJ/6 mice on a high-fat diet. Br J Nutr. 2018;120(7):751–762. doi: 10.1017/S0007114518001824. [DOI] [PubMed] [Google Scholar]
- Bode L.M., Bunzel D., Huch M., Cho G.-S., Ruhland D., Bunzel M. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutr. 2013;97(2):295–309. doi: 10.3945/ajcn.112.049379. [DOI] [PubMed] [Google Scholar]
- Bradford P.G. Curcumin and obesity. BioFactors. 2013;39(1):78–87. doi: 10.1002/biof.1074. [DOI] [PubMed] [Google Scholar]
- Cao G.T., Dai B., Wang K.L., Yan Y., Xu Y.L., Wang Y.X. Bacillus licheniformis, a potential probiotic, inhibits obesity by modulating colonic microflora in C57BL/6J mice model. J Appl Microbiol. 2019;127(3):880–888. doi: 10.1111/jam.14352. [DOI] [PubMed] [Google Scholar]
- Casanova E., Salvado J., Crescenti A., Gibert-Ramos A. Epigallocatechin gallate modulates muscle homeostasis in type 2 diabetes and obesity by targeting energetic and redox pathways: a narrative review. Int J Mol Sci. 2019;20(3):532. doi: 10.3390/ijms20030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Barquero S., Lamuela-Raventós R.M., Doménech M., Estruch R. Relationship between mediterranean dietary polyphenol intake and obesity. Nutrients. 2018;10(10):E1523. doi: 10.3390/nu10101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers K.F., Day P.E., Aboufarrag H.T., Kroon P.A. Polyphenol effects on cholesterol metabolism via bile acid biosynthesis, CYP7A1: a review. Nutrients. 2019;11(11):E2588. doi: 10.3390/nu11112588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.K.W., Bittner S., Bittner A., Atwal S., Shen W.J., Inayathullah M. Nordihydroguaiaretic acid, a lignan from larrea tridentata (Creosote bush), protects against American lifestyle-induced obesity syndrome diet-induced metabolic dysfunction in mice. J Pharmacol Exp Therapeut. 2018;365(2):281–290. doi: 10.1124/jpet.117.243733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin A., Carpéné C., Mercader J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients. 2018;10(11):E1651. doi: 10.3390/nu10111651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.L., Yi L., Zhang Y., Zhou X., Ran L., Yang J. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-Induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio. 2016;7(2):e02210–e02215. doi: 10.1128/mBio.02210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Liang K., Zhao S., Jia W., Liu Y., Wu H. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc Natl Acad Sci U S A. 2018;115(26):E5896–E5905. doi: 10.1073/pnas.1801745115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J., Ramani R., Suraju M.O. Polyphenol compounds and PKC signaling. Biochim Biophys Acta. 2016;1860(10):2107–2121. doi: 10.1016/j.bbagen.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meo F., Margarucci S., Galderisi U., Crispi S., Peluso G. Curcumin, gut microbiota, and neuroprotection. Nutrients. 2019;11(10):2426. doi: 10.3390/nu11102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Xu S., Ma Y., Liu G., Jang H., Fang J. Modulatory mechanisms of the NLRP3 inflammasomes in diabetes. Biomolecules. 2019;9(12):850. doi: 10.3390/biom9120850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., Zhong Y., Xiao H., Zheng C., Song B., Wang W. Gut microbiota mediates the protective effects of dietary beta-hydroxy-beta-methylbutyrate (HMB) against obesity induced by high-fat diets. Faseb J. 2019;33(9):10019–10033. doi: 10.1096/fj.201900665RR. [DOI] [PubMed] [Google Scholar]
- Durazzo A., Lucarini M., Camilli E., Marconi S., Gabrielli P., Lisciani S. Dietary lignans: definition, description and research trends in databases development. Molecules. 2018;23(12):3251. doi: 10.3390/molecules23123251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elena A., Jasminka G., Luigi R.G. Antiobesity effects of anthocyanins in preclinical and clinical studies. Oxidative Med Cell Longevity. 2017;2017:2740364. doi: 10.1155/2017/2740364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito D., Damsud T., Wilson M., Grace M.H., Strauch R., Li X. Black currant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. J Agric Food Chem. 2015;63(27):6172–6180. doi: 10.1021/acs.jafc.5b00963. [DOI] [PubMed] [Google Scholar]
- Fernandes J., Su W., Rahat-Rozenbloom S., Wolever T.M.S., Comelli E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4(6):e121. doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga C.G., Croft Kevin D., Kennedye D.O., Tomás-Barberán F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019;10:514–528. doi: 10.1039/c8fo01997e. [DOI] [PubMed] [Google Scholar]
- Grzybowski Andrzej, Pietrzak K. Albert Szent-Györgyi (1893-1986): the scientist who discovered vitamin C. Clin Dermatol. 2013;31:327–331. doi: 10.1016/j.clindermatol.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Gimeno-Mallench L., Mas-Bargues C., Ingles M., Olaso G., Borras C., Gambini J. Resveratrol shifts energy metabolism to increase lipid oxidation in healthy old mice. Biomed Pharmacother. 2019;118:109130. doi: 10.1016/j.biopha.2019.109130. [DOI] [PubMed] [Google Scholar]
- Gorlach S., Fichna J., Lewandowska U. Polyphenols as mitochondria-targeted anticancer drugs. Canc Lett. 2015;366(2):141–149. doi: 10.1016/j.canlet.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Guan G., Ding S., Yin Y., Duraipandiyan V., Al-Dhabi N.A., Liu G. Macleaya cordata extract alleviated oxidative stress and altered innate immune response in mice challenged with enterotoxigenic Escherichia coli. Sci China Life Sci. 2019;62(8):1019–1027. doi: 10.1007/s11427-018-9494-6. [DOI] [PubMed] [Google Scholar]
- Guo Q., Li F., Duan Y., Wen C., Wang W., Zhang L. Oxidative stress, nutritional antioxidants and beyond. Sci China Life Sci. 2020;63(6):866–874. doi: 10.1007/s11427-019-9591-5. [DOI] [PubMed] [Google Scholar]
- Guo X., Tresserra-Rimbau A., Estruch R., Martinez-Gonzalez M.A., Medina-Remon A., Fito M. Polyphenol levels are inversely correlated with body weight and obesity in an elderly population after 5 Years of follow up (the randomised PREDIMED study) Nutrients. 2017;9(5):452. doi: 10.3390/nu9050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibitoye O.B., Ajiboye T.O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch Physiol Biochem. 2018;124(5):410–417. doi: 10.1080/13813455.2017.1415938. [DOI] [PubMed] [Google Scholar]
- Ishida N., Iizuka M., Kataoka K., Okazaki M., Shiraishi K., Yagi Y. Improvement of blood lipid profiles by Goishi tea polyphenols in a randomised, double-blind, placebo-controlled clinical study. Int J Food Sci Nutr. 2018;69(5):598–607. doi: 10.1080/09637486.2017.1386629. [DOI] [PubMed] [Google Scholar]
- Jahagirdar A., Usharani D., Srinivasan M., Rajasekharan R. Sesaminol diglucoside, a water-soluble lignan from sesame seeds induces brown fat thermogenesis in mice. Biochem Biophys Res Commun. 2018;507(1–4):155–160. doi: 10.1016/j.bbrc.2018.10.195. [DOI] [PubMed] [Google Scholar]
- Jamar G., Estadella D., Pisani L.P. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions. BioFactors. 2017;43(4):507–516. doi: 10.1002/biof.1365. [DOI] [PubMed] [Google Scholar]
- Joseph S.V., Edirisinghe I., Burton-Freeman B.M. Fruit polyphenols: a review of anti-inflammatory effects in humans. CRC Crit Rev Food Technol. 2016;56(3):419–444. doi: 10.1080/10408398.2013.767221. [DOI] [PubMed] [Google Scholar]
- Jung M.J., Lee J., Shin N.R., Kim M.S., Hyun D.W., Yun J.H. Chronic repression of mTOR complex 2 induces changes in the gut microbiota of diet-induced obese mice. Sci Rep. 2016;6:30887. doi: 10.1038/srep30887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.S., Ikram M., Park J.S., Park T.J., Kim M.O. Gut microbiota, its role in induction of alzheimer's disease pathology, and possible therapeutic interventions: special focus on anthocyanins. Cells. 2020;9(4):853. doi: 10.3390/cells9040853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., Mukhtar H. Tea polyphenols in promotion of human health. Nutrients. 2018;11(1):39. doi: 10.3390/nu11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera M., Engst W., Blaut M., Braune A. Isolation of catechin-converting human intestinal bacteria. J Appl Microbiol. 2011;111(1):165–175. doi: 10.1111/j.1365-2672.2011.05025.x. [DOI] [PubMed] [Google Scholar]
- Lee Y.-M., Yoon Y., Yoon H., Park H.-M., Song S., Yeum K.-J. Dietary anthocyanins against obesity and inflammation. Nutrients. 2017;9(10):E1089. doi: 10.3390/nu9101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leixuri A., Alfredo F.-Q., Noemí A., Maria P. Resveratrol: anti-obesity mechanisms of action. Molecules. 2014;19(11):18632–18655. doi: 10.3390/molecules191118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León D., Uribe E., Zambrano A., Salas M. Implications of resveratrol on glucose uptake and metabolism. Molecules. 2017;22(3):E398. doi: 10.3390/molecules22030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P., Oster H., Korf H.W., Foster R.G., Erren T.C. Food as a circadian time cue - evidence from human studies. Nat Rev Endocrinol. 2020;16:213–223. doi: 10.1038/s41574-020-0318-z. [DOI] [PubMed] [Google Scholar]
- Li Y., Ma J., Yao K., Su W., Tan B., Wu X. Circadian rhythms and obesity: timekeeping governs lipid metabolism. J Pineal Res. 2020 doi: 10.1111/jpi.12682. [DOI] [PubMed] [Google Scholar]
- Liou C.J., Lee Y.K., Ting N.C., Chen Y.L., Shen S.C., Wu S.J. Protective effects of licochalcone A ameliorates obesity and non-alcoholic fatty liver disease via promotion of the sirt-1/AMPK pathway in mice fed a high-fat diet. Cells. 2019;8(5):447. doi: 10.3390/cells8050447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou C.J., Wei C.H., Chen Y.L., Cheng C.Y., Wang C.L., Huang W.C. Fisetin protects against hepatic steatosis through regulation of the Sirt1/AMPK and fatty acid beta-oxidation signaling pathway in high-fat diet-induced obese mice. Cell Physiol Biochem. 2018;49(5):1870–1884. doi: 10.1159/000493650. [DOI] [PubMed] [Google Scholar]
- Lu C., Li C., Chen B., Shen Y. Composition and antioxidant, antibacterial, and anti-HepG2 cell activities of polyphenols from seed coat of Amygdalus pcdunculata Pall. Food Chem. 2018;265:111–119. doi: 10.1016/j.foodchem.2018.05.091. [DOI] [PubMed] [Google Scholar]
- Macready A.L., George T.W., Chong M.F., Alimbetov D.S., Yannan J., Alberto V. Flavonoid-rich fruit and vegetables improve microvascular reactivity and inflammatory status in men at risk of cardiovascular disease-FLAVURS: a randomized controlled trial. Am J Clin Nutr. 2014;99(3):479–489. doi: 10.3945/ajcn.113.074237. [DOI] [PubMed] [Google Scholar]
- Marchesi J.R., Adams D.H., Fava F., Hermes G.D.A., Hirschfield G.M., Hold G. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydani M., Hasan S.T. Dietary polyphenols and obesity. Nutrients. 2010;2(7):737–751. doi: 10.3390/nu2070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.B., Sifuentes L.Y., Wissler A., Abd-Elmaksoud S., Lopez G.U., Gerba C.P. Modelling of ultraviolet light inactivation kinetics of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, Clostridium difficile spores and murine norovirus on fomite surfaces. J Appl Microbiol. 2019;126(1):58–67. doi: 10.1111/jam.14103. [DOI] [PubMed] [Google Scholar]
- Morgen C.S., Sørensen T.I. Obesity: global trends in the prevalence of overweight and obesity. Nat Rev Endocrinol. 2014;10(9):513–514. doi: 10.1038/nrendo.2014.124. [DOI] [PubMed] [Google Scholar]
- Muhammadi, Shafiq S. Genetic, structural and pharmacological characterization of polymannuronate synthesized by algG mutant indigenous soil bacterium Pseudomonas aeruginosa CMG1421. J Appl Microbiol. 2019;126(1):113–126. doi: 10.1111/jam.14098. [DOI] [PubMed] [Google Scholar]
- Mulvihill E.E., Burke A.C., Huff M.W. Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annu Rev Nutr. 2016;36:275–299. doi: 10.1146/annurev-nutr-071715-050718. [DOI] [PubMed] [Google Scholar]
- Naowaboot J., Piyabhan P., Munkong N., Parklak W., Pannangpetch P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin Exp Pharmacol Physiol. 2016;43(2):242–250. doi: 10.1111/1440-1681.12514. [DOI] [PubMed] [Google Scholar]
- Naveed M., Hejazi V., Abbas M., Kamboh A.A., Khan G.J., Shumzaid M. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed Pharmacother. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- Noratto G.D., Garcia-Mazcorro J.F., Markel M., Martino H.S., Minamoto Y., Steiner J.M. Carbohydrate-free peach (Prunus persica) and plum (prunus domestica) juice affects fecal microbial ecology in an obese animal model. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdal T., Sela D.A., Xiao J., Boyacioglu D., Chen F., Capanoglu E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients. 2016;8(2):78. doi: 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Yan Y., Wan P., Chen D., Ding Y., Ran L. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic Biol Med. 2019;136:96–108. doi: 10.1016/j.freeradbiomed.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Pojer E., Mattivi F., Johnson D., Stockley C.S. The case for anthocyanin consumption to promote human health: a review. Compr Rev Food Sci Food Saf. 2013;12(5):483–508. doi: 10.1111/1541-4337.12024. [DOI] [PubMed] [Google Scholar]
- Rasines-Perea Z., Teissedre P.-L. Grape polyphenols' effects in human cardiovascular diseases and diabetes. Molecules. 2017;22(1):E68. doi: 10.3390/molecules22010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupasinghe H.P., Sekhon-Loodu S., Mantso T., Panayiotidis M.I. Phytochemicals in regulating fatty acid beta-oxidation: potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol Ther. 2016;165:153–163. doi: 10.1016/j.pharmthera.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Scharinger B., Messner B., Turkcan A., Schuster D., Vuorinen A., Pitterl F. Leoligin, the major lignan from Edelweiss, inhibits 3-hydroxy-3-methyl-glutaryl-CoA reductase and reduces cholesterol levels in ApoE-/- mice. J Mol Cell Cardiol. 2016;99:35–46. doi: 10.1016/j.yjmcc.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Shen L., Ji H.F. Bidirectional interactions between dietary curcumin and gut microbiota. Crit Rev Food Sci Nutr. 2019;59(18):2896–2902. doi: 10.1080/10408398.2018.1478388. [DOI] [PubMed] [Google Scholar]
- Song B., Zhong Y.Z., Zheng C.B., Li F.N., Duan Y.H., Deng J.P. Propionate alleviates high-fat diet-induced lipid dysmetabolism by modulating gut microbiota in mice. J Appl Microbiol. 2019;127(5):1546–1555. doi: 10.1111/jam.14389. [DOI] [PubMed] [Google Scholar]
- Springer M., Moco S. Resveratrol and its human metabolites-effects on metabolic health and obesity. Nutrients. 2019;11(1):143. doi: 10.3390/nu11010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhang C., Kim M., Su Y., Qin L., Dong J. Early potential effects of resveratrol supplementation on skeletal muscle adaptation involved in exercise-induced weight loss in obese mice. BMB Rep. 2018;51(4):200–205. doi: 10.5483/bmbrep.2018.51.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.H., Liu X., Cong L.X., Li H., Zhang C.Y., Chen J.G. Metabolomics study of the therapeutic mechanism of Schisandra Chinensis lignans in diet-induced hyperlipidemia mice. Lipids Health Dis. 2017;16(1):145. doi: 10.1186/s12944-017-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung M.M., Kim T.T., Denou E., Soltys C.-L.M., Hamza S.M., Byrne N.J. Improved glucose homeostasis in obese mice treated with resveratrol is associated with alterations in the gut microbiome. Diabetes. 2017;66:418–425. doi: 10.2337/db16-0680. [DOI] [PubMed] [Google Scholar]
- Tajik N., Tajik M., Mack I., Enck P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur J Nutr. 2017;56(7):2215–2244. doi: 10.1007/s00394-017-1379-1. [DOI] [PubMed] [Google Scholar]
- Takagaki A., Nanjo F. Metabolism of (-)-Epigallocatechin gallate by rat intestinal flora. J Agric Food Chem. 2010;58(2):1313–1321. doi: 10.1021/jf903375s. [DOI] [PubMed] [Google Scholar]
- Tarun B., Seyed N., Seyed N., Solomon H. Dietary anthocyanins and insulin resistance: when food becomes a medicine. Nutrients. 2017;9(10):1111. doi: 10.3390/nu9101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Tan Y., Chen G., Wang G., Sun J., Ou S. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit Rev Food Sci Nutr. 2019;59(6):982–991. doi: 10.1080/10408398.2018.1533517. [DOI] [PubMed] [Google Scholar]
- Tsuda T. Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018;9(2):705–714. doi: 10.1039/c7fo01242j. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhu F., Yang H., Li J., Li Y., Ding X. Epidermal growth factor improves intestinal morphology by stimulating proliferation and differentiation of enterocytes and mTOR signaling pathway in weaning piglets. Sci China Life Sci. 2019 doi: 10.1007/s11427-018-9519-6. [DOI] [PubMed] [Google Scholar]
- Wang P., Li D., Ke W., Liang D., Hu X., Chen F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int J Obes (Lond) 2020;44(1):213–225. doi: 10.1038/s41366-019-0332-1. [DOI] [PubMed] [Google Scholar]
- Wang S., Moustaid-Moussa N., Chen L., Mo H., Shastri A., Su R. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. 2014;25(1):1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Yang L., Guo X., Zhang M., Liu R., Sui W. Raspberry anthocyanin consumption prevents diet-induced obesity by alleviating oxidative stress and modulating hepatic lipid metabolism. Food Funct. 2018;9(4):2112–2120. doi: 10.1039/c7fo02061a. [DOI] [PubMed] [Google Scholar]
- Xu Y., Zhang M., Wu T., Dai S.D., Xu J., Zhou Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015;6(1):297–304. doi: 10.1039/c4fo00970c. [DOI] [PubMed] [Google Scholar]
- Yin J., Li Y., Han H., Chen S., Gao J., Liu G. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res. 2018;65(4) doi: 10.1111/jpi.12524. [DOI] [PubMed] [Google Scholar]
- Yin J., Li Y., Han H., Ma J., Liu G., Wu X. Administration of exogenous melatonin improves the diurnal rhythms of the gut microbiota in mice fed a high-fat diet. mSystems. 2020;5(3) doi: 10.1128/mSystems.00002-20. e00002-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y., Shen P., Chang A.L., Qi W., Kim K.H., Kim D. trans-Trismethoxy resveratrol decreased fat accumulation dependent on fat-6 and fat-7 in Caenorhabditis elegans. Food Funct. 2019;10(8):4966–4974. doi: 10.1039/c9fo00778d. [DOI] [PubMed] [Google Scholar]
- Zam W. Gut microbiota as a prospective therapeutic target for curcumin: a review of mutual influence. J Nutr Metabol. 2018;2018:1367984. doi: 10.1155/2018/1367984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Li Y., Hu J., Shen W.J., Singh M., Hou X. Effect of Creosote bush-derived NDGA on expression of genes involved in lipid metabolism in liver of high-fructose fed rats: relevance to NDGA amelioration of hypertriglyceridemia and hepatic steatosis. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0138203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Li X., Fang H., Guo F., Li F., Chen A. Flavonoids as inducers of white adipose tissue browning and thermogenesis: signalling pathways and molecular triggers. Nutr Metab (Lond) 2019;16:47. doi: 10.1186/s12986-019-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Mocanu V., Cai C., Dang J., Slater L., Deehan E.C. Impact of fecal microbiota transplantation on obesity and metabolic syndrome—a systematic review. Nutrients. 2019;11(10):2291. doi: 10.3390/nu11102291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Chen B., Shen J., Wan L., Zhu Y., Yi T. The beneficial effects of quercetin, curcumin, and resveratrol in obesity. Oxidative Med Cell Longevity. 2017;2017:1459497. doi: 10.1155/2017/1459497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Xiao X., Zhang Q., Zheng J., Deng M. Deciphering the anti-obesity benefits of resveratrol: the "gut microbiota-adipose tissue" Axis. Front Endocrinol (Lausanne) 2019;10:413. doi: 10.3389/fendo.2019.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhang N., Arikawa A.Y., Chen C. Inhibitory effects of green tea polyphenols on microbial metabolism of aromatic amino acids in humans revealed by metabolomic analysis. Metabolites. 2019;9(5):E96. doi: 10.3390/metabo9050096. [DOI] [PMC free article] [PubMed] [Google Scholar]