Abstract
Animal protein sources such as fishmeal and plasma powder are excellent and indispensable sources of energy, amino acids, and minerals in animal production. Amino acid imbalance, especially methionine-to-sulfur amino acid (Met:SAA) ratio, caused by an imbalance of animal protein meal leads to growth restriction. This study was conducted to evaluate the effects of imbalanced Met:SAA ratio supplementation of different animal protein source diets on growth performance, plasma amino acid profiles, antioxidant capacity and intestinal morphology in a piglet model. Twenty-four weaned piglets (castrated males; BW = 10.46 ± 0.34 kg), assigned randomly into 3 groups (8 piglets/group), were fed for 28 d. Three experimental diets of equal energy and crude protein levels were as follows: 1) a corn-soybean basal diet with a Met:SAA ratio at 0.51 (BD); 2) a plasma powder diet with a low Met:SAA ratio at 0.41 (L-MR); 3) a fishmeal diet with a high Met:SAA ratio at 0.61 (H-MR). Results revealed that compared to BD, L-MR significantly decreased (P < 0.05) the activities of plasma total antioxidant capacity and glutathione peroxidase, plasma amino acid profiles, and significantly reduced (P < 0.05) villus height and crypt depth in the duodenum and jejunum. Additionally, L-MR significantly reduced (P < 0.05) the mRNA expression level of solute carrier family 7 member 9 (SlC7A9) in the ileum, and significantly increased (P < 0.05) mRNA expression levels of zonula occludens-1 (ZO-1) in the duodenum, and Claudin-1, ZO-1, sodium-coupled neutral amino acid transporters 2 (SNAT2) and SlC7A7 in the jejunum. H-MR significantly increased (P < 0.05) plasma SAA levels, and significantly reduced (P < 0.05) average daily feed intake, villus height, and villus height-to-crypt depth (VH:CD) ratio in the ileum compared to BD. In conclusion, L-MR may result in oxidative stress and villous atrophy but proves beneficial in improving intestinal barrier function and the activity of amino acid transporters for compensatory growth. H-MR may impair intestinal growth and development for weaned piglets. The research provides a guidance on the adequate Met:SAA ratio (0.51) supplementation in diet structure for weaned piglets.
Keywords: Animal protein source, Methionine-to-sulfur amino acid ratio, Antioxidant capacity, Intestinal morphology, Piglet
1. Introduction
Currently, increasing economic attention has been put on exploring feed ingredients for swine with a high growth performance at a lower feeding cost (Owen et al., 1995). With the prohibition in feed antibiotics, greater and more balanced feed ingredients for improving pig performances have become imperative in pig diets. Besides, postnatal growth and development of piglets are sensitive to multiple factors, which can have critical impacts on the growth performance and intestinal health, resulting in great economic losses to the swine industry. Diet has a strong influence on swine growth and intestinal development, especially protein sources (e.g., soybean, fishmeal, plasma powder) (Karasov and Douglas, 2013). Protein supplemented with animal sources is an essential ingredient in pig diets. Compared with plant protein meals, animal protein meals contain relatively high protein and amino acids and no fiber, which may improve feed intake and nutrient digestibility (Gao et al., 2011; Wu et al., 2018). Fishmeal or plasma powder, as an essential and high-quality ingredient of animal protein meals, is commonly used in early weaning diets to improve the growth performance of piglets (Torrallardona et al., 2003, Asghedom et al., 2014). However, little consideration is given to the imbalance of amino acids induced by an inappropriate use of animal protein meal.
Fishmeal, the product from whole fish processing, is rich in all the essential amino acids (EAA), especially Lys and Met that are the most important amino acids for pigs. Plasma powder separated from whole blood has a higher protein content, balanced amino acid, and abundant vitamins and minerals, which could be a functional source of nutrients (Jiang et al., 2000). The immunoglobulins present in plasma powder has been also implicated in improving intestinal health and body immunity (Wu et al., 2018). Although the nutritional composition of fishmeal and plasma powder may be chemically stable, the bioavailability may be variable with the quality of the animal protein. Obviously, once fishmeal or plasma powder is supplied as the entire dietary animal protein, the characteristics of that ingredient will amplify its inherent nutritional excesses or deficiencies. In addition, the uneven distribution of high-quality protein resources is also a constraint when only fishmeal or plasma powder is used as the sole animal protein source. Sulfur amino acid (SAA), containing Cys and Met, is one of the major dietary amino acids in diets (Bauchartthevret et al., 2009). SAA is implicated in animal growth and intestinal health and directly involved in amino acid metabolism (Yan et al., 2018). Adequate Met and Cys are necessary to ensure the body SAA demands. The SAA requirements vary chiefly in response to health status and feedback criteria. Furthermore, dietary SAA can alter the production of mucin to modulate the intestinal barrier (Grimble, 2006). Approximately 30% of dietary SAA is used for mucosal growth and epithelial cell turnover of the intestine. Based on standardized ileal digestion, the National Research Council (NRC, 2012) recommendation of SAA and Met requirements for 7 to 11 kg pigs was 3.5 and 1.8 g/d, respectively, indicating that Met:SAA ratio for 7 to 11 kg healthy pigs was approximately 0.51. The utilization efficiency of SAA in vivo is associated with Met:SAA ratio in food for animals. However, differences in SAA may be caused by feeding different protein sources, which may alter the optimal performance of weaned piglets for the SAA component requirement.
Despite the increased interest in fishmeal and plasma powder as an excellent protein supplement for animal feeding, impact of its imbalanced dietary Met:SAA ratio on nutrition metabolism, antioxidant status, and intestinal development is still unclear. Therefore, this experiment was designed to investigate the effects of imbalanced dietary Met:SAA ratios on growth performance, plasma amino acid profiles, antioxidant capacity and intestinal morphology of weaned piglets. It is also expected to provide theoretical advice on the application of adequate Met:SAA ratio supplementation in diet structure in young pigs.
2. Materials and methods
All procedures were reviewed and approved by the Chinese Academy of Science Institutional Animal Care and Use Committee, Changsha, Hunan, China.
2.1. Experimental design
After an adaptation period of 3 d, 24 castrated male pigs (10.46 ± 0.34 kg BW) were randomly assigned into 3 dietary treatments by body weight (BW). Eight piglets per treatment were housed in individual pens for 28 d. All experimental diets contained the same metabolizable energy, crude protein levels, and other essential nutrients. Experimental diets were: 1) a corn-soybean basal diet with Met:SAA ratio at 0.51 (BD); 2) a plasma powder diet with a low Met:SAA ratio at 0.41 (L-MR); 3) a fishmeal diet with a high Met:SAA ratio at 0.61 (H-MR). Met:SAA ratio in each group diets were provided meeting, lowering, or exceeding the NRC (2012) recommendations for 7- to 11-kg pigs, respectively. The composition and nutrient levels of different experimental diets are shown in Table 1. Dry feed and clean water were freely accessible throughout the experimental period.
Table 1.
Ingredients and chemical composition of experimental piglet diets (as-fed basis).
| Item | Diets1 |
||
|---|---|---|---|
| BD | L-MR | H-MR | |
| Ingredients, g/kg | |||
| Corn | 628.20 | 665.20 | 667.05 |
| Soybean meal | 110.00 | 81.50 | 89.00 |
| Puffed soybean | 156.00 | 75.00 | 73.00 |
| Fishmeal | 11.00 | 0.00 | 78.00 |
| Plasma powder | 13.30 | 70.00 | 0.00 |
| Vitamin and mineral2 | 1.20 | 1.20 | 1.20 |
| Limestone | 12.00 | 14.00 | 8.00 |
| Salt | 2.50 | 2.50 | 2.50 |
| Dicalcium phosphate | 10.00 | 10.00 | 3.50 |
| dl-methionine | 0.60 | 0.20 | 0.90 |
| l-lysine | 5.00 | 4.00 | 5.00 |
| l-threonine | 1.00 | 1.40 | 1.50 |
| l-tryptophan | 0.70 | 0.00 | 0.40 |
| Glucose | 25.00 | 25.00 | 25.00 |
| Whey powder | 12.00 | 37.00 | 31.95 |
| Acidifier | 1.50 | 3.00 | 3.00 |
| Zeolite powder | 10.00 | 10.00 | 10.00 |
| Total | 1,000 | 1,000 | 1,000 |
| Calculated analysis, % | |||
| Digestible energy, kcal/kg | 3.44 | 3.43 | 3.39 |
| Crude protein | 17.62 | 17.61 | 17.61 |
| Calcium | 0.72 | 0.72 | 0.72 |
| Total phosphorus | 0.72 | 0.72 | 0.72 |
| Available phosphorus | 0.57 | 0.57 | 0.58 |
| Lys | 1.36 | 1.36 | 1.36 |
| Met | 0.35 | 0.29 | 0.43 |
| SAA3 | 0.69 | 0.70 | 0.71 |
| Met:SAA ratio3 | 0.51 | 0.41 | 0.61 |
SAA = sulfur amino acid; Met = methionine; Lys = lysine.
BD, a corn-soybean basal diet with a Met:SAA ratio at 0.51; L-MR, a plasma powder diet containing a Met:SAA ratio at 0.41; H-MR, a fishmeal diet with Met:SAA ratio at 0.61.
Provided per kilogram of diet: vitamin A, 1,750 IU; vitamin D3, 200 IU; vitamin E, 11 IU; vitamin K, 0.5 mg; vitamin B1 1.00 mg; vitamin B2, 3.00 mg; vitamin B6, 3.00 mg; biotin, 0.05 mg; folic acid, 0.30 mg; niacin acid, 30.00 mg; pantothenic acid, 300 mg; Cu (CuSO4 5H2O), 5.00 mg; Fe (FeSO4 7H2O), 100.00 mg; Mn (MnSO4 H2O), 3.00 mg; Se, 0.30 mg; I, 0.14 mg; Co, 0.12 mg.
SAA = Met + Cys.
2.2. Growth performance
All piglets were individually weighed at d 1, 14 and at the end of the trial, d 28, and fasting body weight was measured after 10 h. Initial and final body weight, average daily gain (ADG), average daily feed intake (ADFI) and the feed-to-gain (F:G) ratio were recorded and calculated for the whole experimental period (Yin et al., 2001).
2.3. Slaughter procedures and sampling
After the feeding trial, all piglets were fasted for 12 h before slaughter, and then randomly electrocuted and exsanguinated. Intestinal samples for histological analysis were separately collected from the duodenum, jejunum, and ileum by shearing into 2-cm segments. Immediately, each segment was fixed with 4% (wt/vol) phosphate-buffered paraformaldehyde and used for making paraffin-embedded sections (Xiao et al., 2013). Meanwhile, approximately 5 cm of intestinal segments were excised, all the intestinal content was removed with 0.9% saline and then cut into 3 parts and snap-frozen in liquid nitrogen for molecular analysis.
2.4. Plasma amino acid profiles
Before slaughter, blood samples at the end of wk 2 and 4 were harvested from the percaval vein and poured into an ethylene diamine tetraacetic acid (EDTA) vacuum tubes. Then plasma was separated with centrifugation at 3,500 × g for 15 min at 4 °C and stored at −80 °C until analysis for amino acid profiles. Eighteen amino acids, which were l-methionine, l-arginine, l-isoleucine, l-leucine, l-tryptophan, l-phenylalanine, l-lysine, l-histidine, l-valine, l-threonine, l-glutamate, l-serine, l-aspartate, l-alanine, glycine, l-cystine, l-proline and tyrosine, were analyzed according to the stable isotope dilution high performance liquid chromatography–electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS) method (Feng et al., 2014).
2.5. Plasma antioxidant capacity analysis
Total antioxidant capacity (T-AOC), superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px), and glutathione (GSH) content and malondialdehyde (MDA) content, as important antioxidant indexes, in the plasma were determined using commercial spectrophotometric kits in accordance with the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.6. Histomorphological investigations
Histomorphological changes in the small intestines were measured in accordance with the description of Tan et al. (2009). Briefly, samples were removed from fixation fluid, dehydrated, and embedded in paraffin. Cryosections were performed on approximately 5-μm thickness of transverse, stained with hematoxylin and eosin, and imaged with a Leica DMI3000B microscopy. Villus height and crypt depth were measured on 10 microscopic fields with randomly selected 100× magnified images from individual animal. Villus height was considered from the top to the villus–crypt junction, and crypt depth was considered from the villus–crypt junction to the villus base. The villus height-to-crypt depth (VH:CD) ratio was also calculated.
2.7. Real-time quantitative PCR (RT-PCR)
Total RNA from liquid nitrogen frozen duodenum, jejunum, and ileum samples was extracted with Trizol Reagent (Invitrogen, Carlsbad, CA, USA) and dissolved in diethyl pyrocarbonate (DEPC)-treated water (Yin et al., 2018). The RNA concentration and quality of each sample were evaluated by Eppendorf Biophotometer (Eppendorf AG, Hamburg, Germany) and 1% agarose gel, respectively. Further, first-strand cDNA synthesis was performed on 1.0 μg of total RNA incubated with DNase I, and reverse-transcribed using Oligo (dT) primers (Takara, Otsu, Japan). All primers were designed using Primer 6.0 based on the mRNA sequences of Sus scrofa (Table 2). Reactions of quantitative RT-PCR were performed in triplicate and the β-actin housekeeping gene was used to normalize target gene expression. The performing protocol was performed as previously described (Bai et al., 2018). The relative expression of target genes was presented as a ratio to the control gene.
Table 2.
Primers used in this study.
| Gene | Accession No. | Primer (5ʹ–3ʹ) | Size, bp |
|---|---|---|---|
| β-actin | XM_021086047.1 | F: CTGCGGCATCCACGAAACT R: AGGGCCGTGATCTCCTTCTG |
147 |
| Claudin-1 | NM_001244539.1 | F: AAGGACAAAACCGTGTGGGA R: CTCTCCCCACATTCGAGATGATT |
247 |
| Occludin | NM_001163647.2 | F: ACGAGCTGGAGGAAGACTGGATC R: CCCTTAACTTGCTTCAGTCTATTG |
238 |
| ZO-1 | XM_021098896.1 | F: CCTGCTTCTCCAAAAACTCTT R: TTCTATGGAGCTCAACACCC |
252 |
| SNAT2 | NM_001317081.1 | F: CTGAGCAATGCGATTGTGGG R: ACGTGGTCGGCAAGAATCAT |
615 |
| SLC7A7 | NM_001110421.1 | F: CTCGGGCATCTTCGTCT R: CCCAGTTCCGCATAACA |
126 |
| SLC7A6 | XM_021094157.1 | F: TCTGTTGTGGGTGCCCTTTG R: GACGGCTGGATGATGTAGTTGG |
194 |
| SLC7A9 | NM_001110171.1 | F: AGGAACCGCCAGAGTAAC R: CATCAGGAAGAAATAGCCAC |
100 |
ZO-1 = zonula occludens-1; SNAT2 = sodium-coupled neutral amino acid transporters 2; SLC7A7 = solute carrier family 7 member 7.
2.8. Statistical analysis
Results were analyzed using IBM SPSS Statistics 23.0 software (SPSS Inc., Chicago, IL, USA), and one-way ANOVA and Duncan's multiple-range test were utilized for comparing the differences among treatments. All data are presented as means ± the standard errors (SEM), 0.05 < P < 0.10 was defined as a trend, and P < 0.05 a statistically significance.
3. Results
3.1. Growth performance
Compared to BD, L-MR and H-MR significantly reduced the BW on d 14 (P < 0.05, Table 3), L-MR reduced (P < 0.05) the BW on d 28. Dietary supplementation with H-MR increased the BW on d 14 (P < 0.05) compared to the L-MR. From d 1 to 14, piglets on H-MR had a lower ADG (P < 0.05) and ADFI (P < 0.05) compared to those on BD. Compared to the BD, L-MR had significantly reduced ADG (P < 0.05) and increased the F:G ratio (P < 0.05). ADG in the H-MR group was increased (P < 0.05) and F:G ratio in the H-MR group was decreased (P < 0.05), as compared with the L-MR group. From d 14 to 28, pigs fed BD or L-MR had a higher ADFI (P < 0.05) than those in H-MR group. L-MR tended to increase ADG (P = 0.075) compared to BD. Overall (d 1 to 28), compared to BD, L-MR tended to decrease ADG (P = 0.063). ADFI in the H-MR group was significantly reduced (P < 0.05) compared to the BD group. Pigs on L-MR had a higher (P < 0.05) F:G ratio compared to those on BD or H-MR.
Table 3.
Effects of imbalanced dietary Met:SAA ratio on the growth performance in weaned piglets.
| Item | Diets1 |
P-value | ||
|---|---|---|---|---|
| BD | L-MR | H-MR | ||
| BW, kg | ||||
| Day 1 | 10.45 ± 0.12 | 10.45 ± 0.13 | 10.49 ± 0.12 | 0.96 |
| Day 14 | 15.56 ± 0.32c | 13.05 ± 0.34a | 14.38 ± 0.31b | <0.01 |
| Day 28 | 21.52 ± 0.47b | 19.24 ± 0.82a | 20.04 ± 0.40ab | 0.038 |
| Day 1 to 14 | ||||
| ADG, g | 393.75 ± 22c | 223.08 ± 23.31a | 299.04 ± 23.28b | <0.01 |
| ADFI, kg | 786.54 ± 25.75b | 665.38 ± 50.15ab | 639.42 ± 37.65a | 0.021 |
| F:G ratio | 2.02 ± 0.07a | 3.03 ± 0.1b | 2.19 ± 0.15a | <0.01 |
| Day 14 to 28 | ||||
| ADG, g | 425.45 ± 19.5 | 476.79 ± 31.58 | 404.46 ± 11.72 | 0.075 |
| ADFI, kg | 1,159.65 ± 47.03b | 1,195.15 ± 57.47b | 1,013.32 ± 41a | 0.033 |
| F:G ratio | 2.75 ± 0.11 | 2.53 ± 0.06 | 2.5 ± 0.06 | 0.108 |
| Day 1 to 28 | ||||
| ADG, g | 410.19 ± 16.46 | 354.63 ± 26.86 | 353.7 ± 13.71 | 0.063 |
| ADFI, kg | 980 ± 32.92b | 940.08 ± 52.7ab | 834.22 ± 36.6a | 0.038 |
| F:G ratio | 2.4 ± 0.05a | 2.67 ± 0.06b | 2.36 ± 0.08a | 0.008 |
Met = methionine; SAA = sulfur amino acid; BW = body weight; ADG = average daily gain; ADFI = average daily feed intake; F:G ratio = feed-to-gain ratio.
a, b, c Within a row, means sharing different superscript letters differ significantly (P < 0.05).
BD, a corn-soybean basal diet with Met:SAA ratio at 0.51; L-MR, a plasma powder diet with a low Met:SAA ratio at 0.41; H-MR, a fishmeal diet with a high Met:SAA ratio at 0.61.
3.2. Plasma antioxidant capacity analysis
The results of plasma antioxidant indexes are shown in Table 4. On d 14, L-MR significantly (P < 0.05) reduced T-AOC and tended to reduce the activity of SOD (P = 0.077) compared to the BD group. On d 28, compared to the BD group, L-MR reduced the activity of GSH-Px (P < 0.05) in the plasma.
Table 4.
Effects of imbalanced dietary Met:SAA ratio on plasma amino acid profiles in weaned piglets at d 14 and 28.
| Item | Diets1 |
P-value | ||
|---|---|---|---|---|
| BD | L-MR | H-MR | ||
| Day 14 | ||||
| T-AOC, U/mL | 2.07 ± 0.25b | 1.37 ± 0.15a | 1.63 ± 0.12ab | 0.046 |
| SOD, U/mL | 14.32 ± 0.55 | 12.64 ± 0.35 | 14.21 ± 0.7 | 0.077 |
| CAT, U/mL | 23.83 ± 3.79 | 11.43 ± 1.85 | 15.97 ± 8.03 | 0.187 |
| GSH-Px, U/mL | 789.66 ± 42.16 | 764.67 ± 53.81 | 750.61 ± 50.26 | 0.836 |
| GSH, mg/L | 2.08 ± 0.05 | 2.21 ± 0.08 | 2.23 ± 0.13 | 0.484 |
| MDA, nmol/mL | 6.45 ± 0.23 | 5.37 ± 0.47 | 6.56 ± 0.76 | 0.207 |
| Day 28 | ||||
| T-AOC, U/mL | 1.08 ± 0.29 | 1.05 ± 0.21 | 1.27 ± 0.16 | 0.798 |
| SOD, U/mL | 13.05 ± 0.8 | 13.77 ± 0.39 | 13.22 ± 0.4 | 0.650 |
| CAT, U/mL | 18.45 ± 4.27 | 16.68 ± 3.25 | 13.56 ± 3.47 | 0.639 |
| GSH-Px, U/mL | 806.98 ± 79.6b | 563.67 ± 50.41a | 685.32 ± 44.37ab | 0.032 |
| GSH, mg/L | 1.42 ± 0.05 | 1.42 ± 0.05 | 1.44 ± 0.05 | 0.948 |
| MDA, nmol/mL | 6.27 ± 0.57 | 6.45 ± 0.69 | 5.97 ± 0.4 | 0.831 |
Met = methionine; SAA = sulfur amino acid; T-AOC = total antioxidant capacity; SOD = superoxide dismutase; CAT = catalase; GSH-Px = glutathione peroxidase; GSH = glutathione; MDA = malondialdehyde.
a, b Within a row, means sharing different superscript letters differ significantly (P < 0.05).
BD, a corn-soybean basal diet with Met:SAA ratio at 0.51; L-MR, a plasma powder diet with a low Met:SAA ratio at 0.41; H-MR, a fishmeal diet with a high Met:SAA ratio at 0.61.
3.3. Plasma amino acid profiles
The concentrations of SAA, total amino acid (TAA), EAA, nonessential amino acid (NEAA) and the EAA-to-NEAA (EAA:NEAA) ratio were calculated based on the analyzed plasma amino acid. As shown in Table 5, on d 14, compared to BD, pigs on L-MR had significantly decreased concentrations of Met, leucine, tryptophan, and glutamate (P < 0.05), and increased plasma threonine concentration (P < 0.05). Meanwhile, pigs with H-MR had decreased (P < 0.05) concentrations of the branched-chain amino acids (BCAA, including leucine, isoleucine, and valine), histidine and phenylalanine, and increased (P < 0.05) concentrations of threonine, serine, aspartate, and glycine in plasma compared to BD. The concentrations of Met, Glu, Ser, Asp, Gly were lower (P < 0.05), and the histidine and BCAA concentrations were higher (P < 0.05) in plasma of pigs on L-MR compared to those on H-MR. Compared to H-MR, L-MR tended to decrease the concentrations of arginine (P = 0.051) and proline (P = 0.063) in plasma. As shown in Table 6, on d 28, compared to BD, L-MR reduced (P < 0.05) concentrations of Met, arginine, glutamate, aspartate, proline, and tyrosine in plasma. Compared to the BD group, pigs on H-MR had reduced (P < 0.05) concentrations of arginine, histidine, and glutamate. The concentrations of Met and tyrosine were significantly decreased (P < 0.05) in the L-MR group, whereas the concentration of histidine was markedly increased (P < 0.05) compared to the H-MR group. L-MR also tended to reduce the alanine concentration (P = 0.055) in plasma compared to BD.
Table 5.
Effects of imbalanced dietary Met:SAA ratio on plasma amino acid profiles in weaned piglets at d 14 (μg/g).
| Item | Diets1 |
P-value | ||
|---|---|---|---|---|
| BD | L-MR | H-MR | ||
| Essential amino acids | ||||
| l-methionine | 2.55 ± 0.32b | 2 ± 0.11a | 4.04 ± 0.18b | <0.001 |
| l-arginine | 58.87 ± 4.74 | 49.5 ± 2.78 | 62.86 ± 3.31 | 0.051 |
| l-isoleucine | 10.73 ± 1.3b | 8.79 ± 1.01b | 5.69 ± 0.55a | 0.007 |
| l-leucine | 16.69 ± 1.3c | 13.1 ± 1.47b | 9.18 ± 0.58a | 0.001 |
| l-tryptophan | 6.63 ± 0.79b | 4.05 ± 0.63a | 5.09 ± 0.57ab | 0.04 |
| l-phenylalanine | 9.23 ± 0.69b | 7.8 ± 0.42ab | 7.08 ± 0.38a | 0.023 |
| l-lysine | 26.24 ± 2.38 | 25.34 ± 0.96 | 30.1 ± 2.5 | 0.248 |
| l-histidine | 4.16 ± 0.33b | 4.27 ± 0.3b | 2.37 ± 0.25a | <0.001 |
| l-valine | 14.11 ± 2.08b | 13.43 ± 1.75b | 5.73 ± 0.52a | 0.002 |
| l-threonine | 10.92 ± 1.69a | 21.23 ± 2.32b | 23.19 ± 4.1b | 0.015 |
| Non-essential amino acids | ||||
| l-glutamate | 40.87 ± 3.5b | 26.46 ± 1.79a | 43.27 ± 4.19b | 0.003 |
| l-serine | 14.84 ± 0.74a | 13.13 ± 0.69a | 18.2 ± 1.09b | 0.002 |
| l-aspartate | 1.62 ± 0.2a | 1.16 ± 0.13a | 2.73 ± 0.53b | 0.01 |
| l-alanine | 42.97 ± 3.51ab | 33.97 ± 2.25a | 45.78 ± 3.53b | 0.038 |
| Glycine | 76.97 ± 7.42a | 64.01 ± 4.76a | 113.32 ± 8.39b | <0.001 |
| l-cystine | 2.08 ± 0.18 | 2.1 ± 0.24 | 1.77 ± 0.08 | 0.359 |
| l-proline | 21.23 ± 1.52 | 19.11 ± 0.61 | 23.07 ± 1.02 | 0.063 |
| l-tyrosine | 9.23 ± 0.81 | 7.14 ± 0.75 | 8.12 ± 0.41 | 0.119 |
| SAA | 4.63 ± 0.39a | 4.09 ± 0.28a | 5.81 ± 0.23b | 0.003 |
| TAA | 403.41 ± 21.59b | 316.59 ± 12.08a | 411.59 ± 20.73b | 0.003 |
| EAA | 163.65 ± 5.07 | 149.50 ± 7.21 | 155.33 ± 6.74 | 0.312 |
| NEAA | 221.03 ± 11.60b | 167.08 ± 8.66a | 256.26 ± 14.85b | 0.001 |
| EAA:NEAA ratio | 0.72 ± 0.06a | 0.91 ± 0.06b | 0.61 ± 0.02a | 0.002 |
Met = methionine; SAA = sulfur amino acid; TAA = total amino acid; EAA = essential amino acid; NEAA = non-essential amino acid.
a, b, c Within a row, means sharing different superscript letters differ significantly (P < 0.05).
BD, a corn-soybean basal diet with Met:SAA ratio at 0.51; L-MR, a plasma powder diet with a low Met:SAA ratio at 0.41; H-MR, a fishmeal diet with a high Met:SAA ratio at 0.61.
Table 6.
Effects of imbalanced dietary Met:SAA ratios on plasma amino acid profiles in weaned piglets at d 28 (μg/g).
| Item | Diets1 |
P-value | ||
|---|---|---|---|---|
| BD | L-MR | H-MR | ||
| Essential amino acids | ||||
| l-methionine | 3.45 ± 0.3b | 2.61 ± 0.22a | 4.11 ± 0.32b | 0.005 |
| l-arginine | 54.69 ± 1.54b | 45.13 ± 2.79a | 45.19 ± 3.34a | 0.028 |
| l-isoleucine | 9.26 ± 0.61 | 8.6 ± 0.93 | 10.97 ± 1.44 | 0.280 |
| l-leucine | 16.77 ± 1.01 | 14.29 ± 1.73 | 17.31 ± 2.17 | 0.426 |
| l-tryptophan | 8.71 ± 1.25 | 6.46 ± 1.2 | 6.96 ± 0.39 | 0.285 |
| l-phenylalanine | 9.73 ± 0.58 | 8.62 ± 0.77 | 9.63 ± 0.84 | 0.512 |
| l-lysine | 25.85 ± 1.64 | 20.79 ± 1.46 | 25.65 ± 2.4 | 0.122 |
| l-histidine | 6.66 ± 1.01b | 5.14 ± 0.57ab | 3.88 ± 0.49a | 0.042 |
| l-valine | 19.01 ± 2.4 | 15.77 ± 2.56 | 16.24 ± 2.38 | 0.607 |
| l-threonine | 14.97 ± 2.26 | 13.15 ± 1.29 | 10.72 ± 1.12 | 0.206 |
| Non-essential amino acids | ||||
| l-glutamate | 52.37 ± 4.94b | 31.46 ± 2.15a | 40.77 ± 3.95a | 0.004 |
| l-serine | 14.49 ± 1.91 | 11.37 ± 0.79 | 14.4 ± 1.1 | 0.203 |
| l-aspartate | 2.32 ± 0.52b | 1.22 ± 0.16a | 1.93 ± 0.21ab | 0.09 |
| l-alanine | 45.25 ± 3.69 | 34.76 ± 2.99 | 37.75 ± 1.9 | 0.055 |
| Glycine | 69.42 ± 8.49 | 61.72 ± 5.68 | 66.87 ± 5.22 | 0.708 |
| l-cystine | 2.32 ± 0.16 | 2.64 ± 0.32 | 2.6 ± 0.21 | 0.609 |
| l-proline | 24.51 ± 1.38b | 19.64 ± 0.67a | 21.68 ± 1.1ab | 0.016 |
| l-tyrosine | 11.76 ± 0.81b | 8.83 ± 0.95a | 11.47 ± 0.74b | 0.041 |
| SAA | 5.77 ± 0.35ab | 5.25 ± 0.40a | 6.71 ± 0.25b | 0.021 |
| TAA | 391.55 ± 15.64 | 375.89 ± 17.46 | 348.14 ± 18.21 | 0.217 |
| EAA | 169.10 ± 6.46 | 165.64 ± 7.28 | 150.57 ± 12.51 | 0.337 |
| NEAA | 222.45 ± 15.61 | 210.26 ± 15.92 | 197.47 ± 10.06 | 0.470 |
| EAA:NEAA ratio | 0.79 ± 0.07 | 0.82 ± 0.08 | 0.77 ± 0.06 | 0.863 |
Met = methionine; SAA = sulfur amino acid; TAA = total amino acid; EAA = essential amino acid; NEAA = non-essential amino acid.
a, b Within a row, means sharing different superscript letters differ significantly (P < 0.05).
BD, a corn-soybean basal diet with Met:SAA ratio at 0.51; L-MR, a plasma powder diet with a low Met:SAA ratio at 0.41; H-MR, a fishmeal diet with a high Met:SAA ratio at 0.61.
Overall, after the different animal protein diets fed for 28 d, the concentrations of SAA, TAA and NEAA in plasma of pigs on L-MR were significantly decreased (P < 0.05), and the EAA:NEAA ratio was improved (P < 0.05) compared to those on BD and H-MR for 14 d. However, no significant difference was observed on the concentrations of TAA, EAA, NEAA and the EAA:NEAA ratio in plasma of weaned piglets for 28 d among the 3 treatments. Pigs on H-MR had higher (P < 0.05) SAA levels in plasma compared to those on L-MR. In short, the amino acid profiles in plasma showed more variance on d 14 than on d 28, evident by the dietary sulfur amino acid levels.
3.4. Amino acids transporters
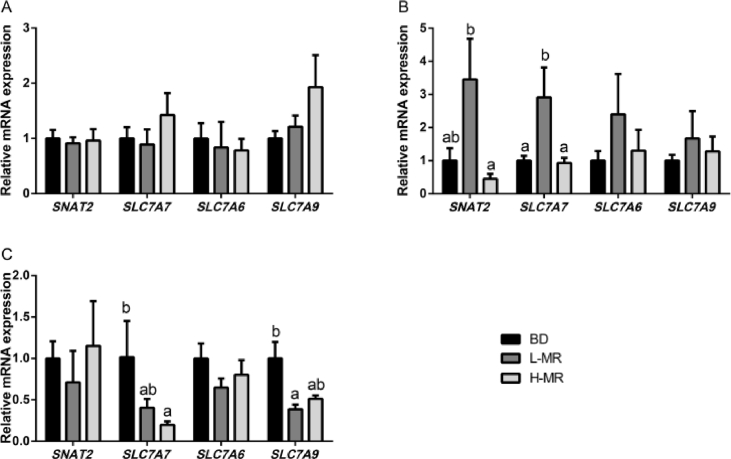
Analyzed expression levels of intestinal amino acid transporters are detailed in Fig. 1. The relative mRNA expression levels of sodium-coupled neutral amino acid transporters 2 (SNAT2) and solute carrier family 7 member 7 (SLC7A7) were up-regulated (P < 0.05) in the jejunum of pigs supplemented with L-MR compared to the BD and H-MR. Compared to the BD group, decreased SLC7A7 mRNA level (P < 0.05) in the ileum was observed in dietary H-MR supplementation. The expression level of SLC7A9 was lower (P < 0.05) in pigs on L-MR than BD. However, no significant difference (P > 0.10) was observed on SNAT2, SLC7A7, SLC7A6 and SLC7A9 expression between groups in the duodenum.
Fig. 1.
Effects of imbalanced dietary Met:SAA ratios on mRNA expression of intestinal amino acids transporters in (A) duodenum, (B) jejunum, and (C) ileum of weaned piglets. BD, a corn-soybean basal diet containing the Met:SAA ratio at 0.51; L-MR, a plasma powder diet containing a low Met:SAA ratio at 0.41; H-MR, a fishmeal diet containing a high Met:SAA ratio at 0.61. The mRNA expression abundances of SNAT2, SLC7A7, SLC7A6, and SLC7A9 were normalized using β-actin as an internal control. Data are expressed as means ± SEM (n = 8). Bar with different superscripts are significantly different (P < 0.05). Met = methionine; SAA = sulfur amino acids; SNAT2 = sodium-coupled neutral amino acid transporters 2; SLC7A7 = solute carrier family 7 member 7.
3.5. Histomorphological investigations
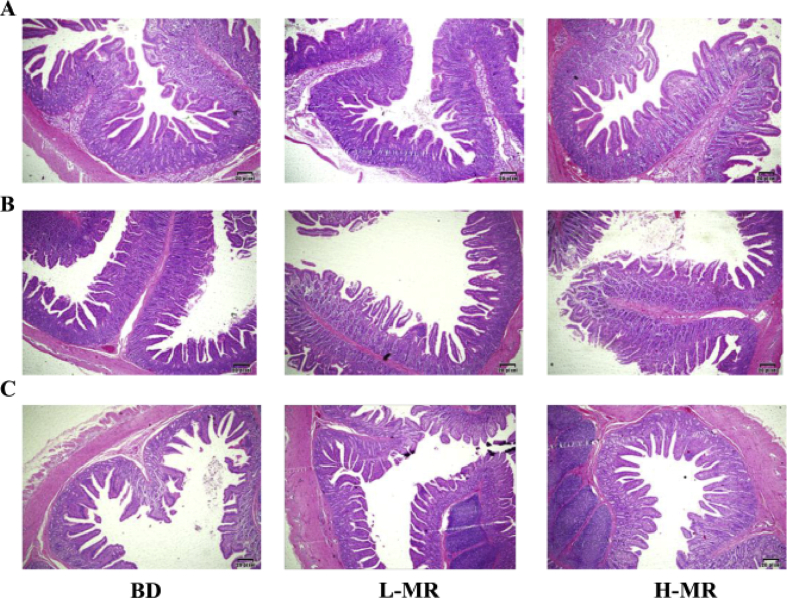
Compared to the L-MR and H-MR, the villi were arranged more closely and tightly in duodenum and jejunum when pigs fed with the BD diet, as shown in Fig. 2. However, pigs on the BD group had shorter villi of the duodenum than the other 2 groups. The villi were shorter and the extent of the injury was higher in the jejunum of pigs on the L-MR as opposed to the BD. With digestion time prolongs, the effect of growth promotion on the small intestine was reduced in the L-MR and H-MR groups.
Fig. 2.
Hematoxylin and eosin (H&E) stained sections of the intestine (200× magnification). (A) Duodenum. (B) Jejunum. (C) Ileum. BD, a corn-soybean basal diet containing the Met:SAA ratio at 0.51; L-MR, a plasma powder diet containing a low Met:SAA ratio at 0.41; H-MR, a fishmeal diet containing a high Met:SAA ratio at 0.61. Met = methionine; SAA = sulfur amino acids.
As shown in Table 7, the villous height, crypt depth, and VH:CD ratio of the duodenum, jejunum and ileum were measured. Compared to the BD and H-MR groups, the L-MR decreased the crypt depth in the duodenum of pigs. Pigs on L-MR had a higher VH:CD ratio of the duodenum compared to those on BD. Compared to the BD and H-MR, L-MR had significantly (P < 0.05) decreased the villous height and crypt depth of the jejunum. The H-MR reduced (P < 0.05) the villous height of the ileum compared to the BD, and decreased the VH:CD ratio of the ileum compared to the BD and L-MR (P < 0.05). In addition, H-MR had a significant trend (P = 0.066) to increase the crypt depth of the ileum compared to BD.
Table 7.
Effects of imbalanced dietary Met:SAA ratio on the morphology of the small intestine in weaned piglets.
| Item | Diets1 |
P-value | ||
|---|---|---|---|---|
| BD | L-MR | H-MR | ||
| Duodenum | ||||
| Villous height, μm | 959.2 ± 29.76 | 986.5 ± 32.88 | 1051.33 ± 26.3 | 0.099 |
| Crypt depth, μm | 869.28 ± 35.29b | 734.09 ± 23.5a | 861.65 ± 32.86b | 0.002 |
| VH:CD ratio | 1.19 ± 0.07a | 1.43 ± 0.07b | 1.29 ± 0.05ab | 0.034 |
| Jejunum | ||||
| Villous height, μm | 865.76 ± 19.42b | 387.4 ± 12.34a | 819.17 ± 54.27b | <0.01 |
| Crypt depth, μm | 622.97 ± 21.55b | 267.82 ± 8.97a | 568.02 ± 34.97b | <0.01 |
| VH:CD ratio | 1.5 ± 0.08 | 1.51 ± 0.06 | 1.45 ± 0.07 | 0.804 |
| Ileum | ||||
| Villous height, μm | 934.99 ± 24.96b | 883.99 ± 19.38ab | 854.63 ± 18.41a | 0.027 |
| Crypt depth, μm | 513.09 ± 19.44 | 505.97 ± 21.53 | 568.87 ± 21.31 | 0.066 |
| VH:CD ratio | 1.94 ± 0.08b | 1.92 ± 0.09b | 1.61 ± 0.07a | 0.006 |
Met = methionine; SAA = sulfur amino acid; VH:CD ratio = villous height to crypt depth ratio.
a, b Within a row, means sharing different superscript letters differ significantly (P < 0.05).
BD, a corn-soybean basal diet with Met:SAA ratio at 0.51; L-MR, a plasma powder diet with a low Met:SAA ratio at 0.41; H-MR, a fishmeal diet with a high Met:SAA ratio at 0.61.
3.6. Intestinal barrier function
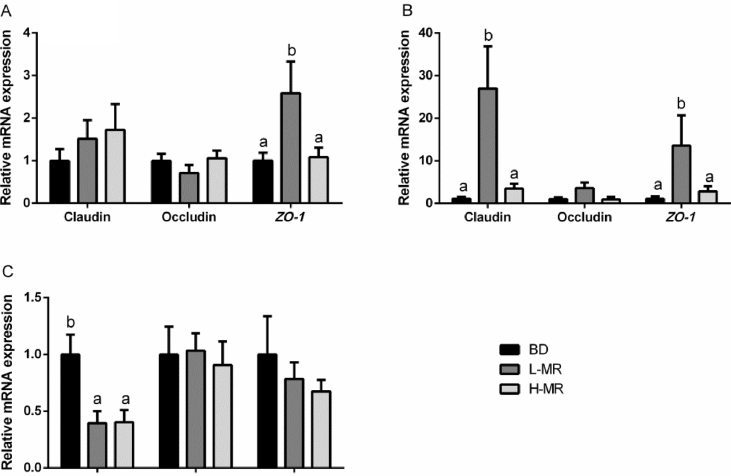
Analyzed the mRNA expression levels of Claudin-1, Occludin and zonula occludens-1 (ZO-1) in the small intestine were presented in Fig. 3. L-MR supplementation remarkably (P < 0.05) up-regulated the expression of ZO-1 in the duodenum and jejunum, and increased (P < 0.05) the jejunal expression of Claudin-1, compared to these in BD and H-MR groups. There was a significantly higher (P < 0.05) expression level of Claudin-1 of the ileum in the BD group compared to both the L-MR or H-MR groups.
Fig. 3.
Effects of imbalanced dietary Met:SAA ratio on mRNA expression of the tight junction of the small intestine in weaned piglets. (A) Duodenum; (B) Jejunum; (C) Ileum. BD, a corn-soybean basal diet containing the Met:SAA ratio at 0.51; L-MR, a plasma powder diet containing a low Met:SAA ratio at 0.41; H-MR, a fishmeal diet containing a high Met:SAA ratio at 0.61. The mRNA expression abundances of Claudin-1, Occludin, and ZO-1 were normalized using β-actin as an internal control. Data are expressed as means ± SEM (n = 8). Bar with different superscripts are significantly different (P < 0.05). Met = methionine; SAA = sulfur amino acids; ZO-1 = zonula occludens-1.
4. Discussion
The present study is the first to explore the potential influence of imbalanced Met:SAA ratio in dietary supplementation from different animal protein sources for weaned pigs. Numerous studies indicate that sulfur amino acid play crucial metabolic and functional roles in animal nutrition. Especially Met, as the second limiting amino acid in traditional diets, is commonly supported to optimize pigs’ growth performance. Generally, the requirement of amino acid for protein deposition would be higher in younger pigs than in older pigs (NRC, 2012). The negative effect on the growth rate of pigs was more serious in early periods than late-finisher periods. In the present study, supplementation with a low Met:SAA ratio diet had a reduced growth performance during the 14 d and the whole experimental period. The reason for the reduced growth performance may be interlinked with a suppressed effect on the intestinal growth of dietary SAA deficiency. Instead, increasing dietary SAA levels could improve the growth performance of piglets, increasing gain-to-feed ratio from d 0 to 14 (Zong et al., 2018). However, from d 14 to 28, as compared to the BD group, the ADG, and ADFI of piglets on L-MR increased by 12.0% and 3.0%, respectively, and the F: G ratio reduced by 8.0%. The results indicate a compensation action of L-MR on growth performance when weaned piglets were fed a diet with a lower Met:SAA ratio.
Meanwhile, an improvement of F:G ratio responded to dietary supplementation with SAA (via Met). Previous studies have demonstrated that dietary SID SAA:Lys ratios are positively correlated with ADG and F:G ratio (Ren et al., 2018). However, results of the present study showed that the high Met:SAA ratio of fishmeal diet (H-MR) had no effect on growth performance, but reduced the ADFI of pigs. The discrepancies may be due to the large proportion of fishmeal in feed ingredients, leading to poor palatability for pigs. Thus, our results suggest that the Met:SAA ratio recommended by the NRC (2012) for pigs would correspond to better growth performance. According to the F:G ratio, H-MR also had a positive effect on pig performance.
Predominantly, as building blocks for polypeptides and protein, plasma amino acids are associated with animal growth performance and health (Jeon et al., 2018, Ren et al., 2014). Amino acid flux in the serum is more susceptible to changes in dietary nutrients during the early periods. In the present study, increased plasma Met level is a direct result of increased Met:SAA ratio diet. In accordance with previous studies, it was not surprising to find that plasma levels of Met and Cys increased with an increase in dietary Met (Bauchartthevret et al., 2009, Kim et al., 2012). Conversely, the BCAA (Ile, Leu, Val) levels in plasma decreased with an increase in dietary Met:SAA ratio. As BCAA is essential for synthesizing circulating glutamine and alanine, it constitutes a major fuel for rapidly proliferating cells (Wu, 2014). Accordingly, large amounts of BCAA were utilized for synthesizing intestinal mucosal protein and thus are expected to increase as the Met:SAA ratio in the diet decreases. Additionally, the ratio of EAA to NEAA is an important index to evaluate the nutritional status of animals. The increased plasma EAA:NEAA ratio suggests an adequate supply of EAA in the serum. This is beneficial to maintain the amino acid balance and improve protein deposition and improve the growth performance of piglets (Lewis, 1992). Recent studies show that plasma EAA:NEAA ratio was higher in the pigs on L-MR than on BD and H-MR at d 14. However, no positive effect on growth performance was found in pigs on L-MR in the early stages of clinical trials. A possible explanation is that the reduced concentrations of plasma SAA, TAA, EAA, and NEAA is a response to lower levels of Met:SAA ratio in the L-MR diet in combination with the limitation of growth performance found in the present study. Numerous studies have also reported that Met may be the first limiting amino acid in diets for piglets when the level of plasma powder was more than 6% (Owen et al., 1995, Wu et al., 2018). Instead, the compensation effect on the growth of piglets may be associated with higher absorption of the small intestine.
The absorption and transportation of amino acids in cells mainly require specific transporters to regulate (Yang et al., 2016). The levels of amino acid transporters, affected by dietary nutrient content, are indicative of the availability of amino acids in the small intestine (Laspiur et al., 2009). The SNAT2, SlC7A7, SLC7A6, and SLC7A9 are Na+-independent neutral amino acid transporters (Verrey et al., 2004). SNAT2 is mainly responsible for transporting Cys, Glu, Gly, and Ala, and other neutral aliphatic amino acids (Hyde et al., 2007). SlC7A7, SLC7A6, and SLC7A9 are mainly involved in the transport of BCAA, Asp, Glu, and some small neutral amino acids (Fuchs et al., 2005). In this study, mRNA expression levels of amino acid transporters (SNAT2 and SlC7A7) were increased in the jejunum of pigs on the L-MR diet. The mRNA expression of SLC7A6, SLC7A7 and SLC7A9 in the ileum was the highest in piglets fed the basal diet. Instead, a high SAA-diet reduced the mRNA expression levels of amino acid transporters in the small intestine. A possible explanation is the effect of Met on intestinal morphology growth in pigs is associated with lower small intestinal digestion and absorption capacities.
Due to the imbalance between the production and limitation of the reactive oxygen species, oxidative stress can impair the cellular antioxidant defense system of the body (Zhang et al., 2015). Oxidative stress or weaning stress is reported to cause villous atrophy, increased intestinal permeability and infection in piglets (Thomson et al., 1998, Spahr et al., 2007). In the present study, L-MR-diet had a decrease in the activity of plasma T-AOC and GSH-Px, indicating that dietary decreasing Met:SAA ratio can reduce the enzymatic antioxidant defense systems for piglets. The highest activities of antioxidant enzymes (including T-AOC and GSH-Px) were found in the plasma of piglets on BD-diet, shows that adequate Met:SAA ratio are necessary to meet the amino acid balance for the enzymatic antioxidant defense systems of the body. In particular, methionine is an indispensable component of glutathione and maintains cellular redox homeostasis.
The intestinal morphology is closely associated with the absorption of nutrients and growth performance in animals (Lee et al., 2013). Villus atrophy and crypt hyperplasia, as significant changes in the intestinal morphology, directly induce the malabsorption, diarrhea, and growth inhibition in pigs (Xiong et al., 2016). Moreover, the VH:CD ratio is an important index for estimating the influence of small intestinal morphology, which can reflect the capacity of nutrient digestion and absorption. Previous studies reported that dietary supplementation of methionine or N-acetyl-cysteine could improve villus development and the epithelial cell metabolism (Hou et al., 2012, Shen et al., 2014). Here, we have found that the decrease in the villus height of the jejunum and ileum was shown in the L-MR and H-MR supplemented diets. Inappropriate Met:SAA ratio diet probably results in the impairment of epithelial proliferation. Besides, the highest crypt depth in the duodenum, and the highest villus height and VH:CD ratio in the ileum and crypt depth in the jejunum, along with the highest villus height and VH:CD ratio in the ileum of the level of Met:SAA ratio at 0.51 of group, which meet the nutritional requirements as recommended by the NRC (2012), indicated that the nutrient could be preferentially digested and absorbed in this group. This indicates that SAA deficiency or excess could suppress and impair intestinal growth and development.
Tight junction proteins are regarded as key markers of barrier functions in the epithelial, endothelial, and intestinal paracellular pathway (Ren et al., 2014, Xiong et al., 2015). Tight junction proteins are composed of integral membrane proteins associated with cytoplasmic plaque proteins. Decreased tight-junction protein expression is associated with sustained impairment in the intestinal barrier (Hu et al., 2013). Similarly, the results showed that the mRNA expression of ZO-1 in duodenum and Claudin-1 and ZO-1 in the jejunum was increased in pigs on diet supplementation with a low Met:SAA ratio level (L-MR), and the mRNA expression of Claudin-1 in the ileum was increased in the BD group compared to other groups. This suggests that the addition of excessive amounts of fish meal to the diet seriously compromised the intestinal barrier integrity in pigs. It may be due to the excess level of Met and Lys in the H-MR diet. A previous study showed that a high Met-diet decreased the mRNA expression of Claudin-1, and compromised the intestinal epithelium integrity of piglets (Grimble, 2006). Dietary adequate Met or SAA levels could improve intestinal integrity of weaning piglets (Chen et al., 2014). Further research is warranted to investigate the signaling pathways related to high Met on intestinal barrier function.
5. Conclusions
In conclusion, supplementation with dietary Met:SAA ratio at 0.41 reduced antioxidant capacity, plasma amino acid level, and mucosal growth, but improved intestinal barrier function and amino acid transporters activity in the duodenum and jejunum for compensatory growth in the later stage of pigs. The diet with the Met:SAA ratio at 0.61 would hamper the ADFI, and suppress intestinal growth and nutrient absorption in weaned piglets. This study contributes to enhancing the understanding of the negative effects of feeding a single animal protein source diet with different Met:SAA ratios on weaned piglets.
Author contributions
Hongnan Liu, Jinping Deng, Ruilin Huang and Yulong Yin designed the research and analysed the data. Miaomiao Bai, Lei Wang and Kang Xu conducted the research. Miaomiao Bai and Lei Wang wrote the article. Hongnan Liu, Jinping Deng, Ruilin Huang and Yulong Yin had primary responsibility for the final content, and all authors read and approved the final manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
We would like to express sincere gratitude to the National Key Research and Development Program of China (2018YFD0501101) and Natural Science Foundation of Hunan Province of China (2018JJ3579). The research was also funded by the research program of the National Natural Science Foundation of China (Grant No. 31872985), Youth Talent Program of Hunan Province (2018RS3110), Youth Innovation Promotion Association, CAS (2019356), Youth Innovation Team Project of ISA, CAS (2017QNCXTD_TBE), Changsha Key Research System (kq1907074) and the China Agricultural Research System (CARS-35).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Hongnan Liu, Email: liuhn@isa.ac.cn.
Jinping Deng, Email: dengjinping@scau.edu.cn.
References
- Asghedom G., Kjos N.P., Austbo D. Effect of fishmeal supplementation on body weight gain of weaned pigs in Eritrea. Tanzania J Agric Sci. 2014;2(7):97–104. [Google Scholar]
- Bai M.M., Liu H.N., Xu K., Zou B.J., Yu R., Liu Y.L. Effects of dietary coated cysteamine hydrochloride on pork color in finishing pigs. J Sci Food Agric. 2018;98(5):1743–1750. doi: 10.1002/jsfa.8647. [DOI] [PubMed] [Google Scholar]
- Bauchartthevret C., Barbara S., Shaji C., Burrin D.G. Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am J Physiol Endocrinol Metab. 2009;296(6):E1239. doi: 10.1152/ajpendo.91021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li D.F., Dai Z.L., Piao X.S., Wu Z.L., Wang B. L-Methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets. Amino Acids. 2014;46(4):1131–1142. doi: 10.1007/s00726-014-1675-5. [DOI] [PubMed] [Google Scholar]
- Feng Z.M., Zhou X.H., Wu F., Yao K., Kong X.F., Li T.J. Both dietary supplementation with monosodium L-glutamate and fat modify circulating and tissue amino acid pools in growing pigs, but with little interactive effect. PloS One. 2014;9(1) doi: 10.1371/journal.pone.0084533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs B.C., Bode B.P. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15(4):254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Gao Y.Y., Jiang Z.Y., Lin Y.C., Zheng C.T., Zhou G.L., Chen F. Effects of spray-dried animal plasma on serous and intestinal redox status and cytokines of neonatal piglets. J Anim Sci. 2011;89(1):150–157. doi: 10.2527/jas.2010-2967. [DOI] [PubMed] [Google Scholar]
- Grimble R.F. The effects of sulfur amino acid intake on immune function in humans. J Nutr. 2006;136(6):1660S–1665S. doi: 10.1093/jn/136.6.1660S. [DOI] [PubMed] [Google Scholar]
- Hou Y.Q., Wang L., Zhang W., Yang Z.G., Ding B.Y., Zhu H.L. Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids. 2012;43(3):1233–1242. doi: 10.1007/s00726-011-1191-9. [DOI] [PubMed] [Google Scholar]
- Hu C.H., Xiao K., Luan Z.S., Song J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci. 2013;91(3):1094–1101. doi: 10.2527/jas.2012-5796. [DOI] [PubMed] [Google Scholar]
- Hyde R., Cwiklinski E.L., MacAulay K., Taylor P.M., Hundal H.S. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability. J Biol Chem. 2007;282(27):19788–19798. doi: 10.1074/jbc.M611520200. [DOI] [PubMed] [Google Scholar]
- Jeon J.S., Oh J.J., Kwak H.C., Yun H.Y., Kim H.C., Kim Y.M. Age-related changes in sulfur amino acid metabolism in male C57BL/6 Mice. Biomol Ther. 2018;26(2):167–174. doi: 10.4062/biomolther.2017.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R.H., Chang X.Y., Stoll B., Fan M.Z., Arthington J., Weaver E. Dietary plasma protein reduces small intestinal growth and lamina propria cell density in early weaned pigs. J Nutr. 2000;130(1):21–26. doi: 10.1038/sj.ijo.0801096. [DOI] [PubMed] [Google Scholar]
- Karasov W.H., Douglas A.E. Comparative digestive physiology. Comp Physiol. 2013;3(2):741–783. doi: 10.1002/cphy.c110054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.C., Mullan B.P., Frey B., Payne H.G., Pluske J.R. Whole body protein deposition and plasma amino acid profiles in growing and/or finishing pigs fed increasing levels of sulfur amino acids with and without Escherichia coli lipopolysaccharide challenge. J Anim Sci. 2012;90(Suppl):362–365. doi: 10.2527/jas.53821. [DOI] [PubMed] [Google Scholar]
- Laspiur J.P., Burton J.L., Weber P.S., Moore J., Kirkwood R.N., Trottier N.L. Dietary protein intake and stage of lactation differentially modulate amino acid transporter mRNA abundance in porcine mammary tissue. J Nutr. 2009;139(9):1677. doi: 10.3945/jn.108.103549. [DOI] [PubMed] [Google Scholar]
- Lee H., Park J.H., Park D.I., Kim H.J., Cho Y.K., Sohn C.I. Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J Neurogastroenterol Motil. 2013;19(2):244–250. doi: 10.5056/jnm.2013.19.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A.J. CHAPTER 3 - Determination of the amino acid requirements of animals. In: Nissen S., editor. Modern methods in protein nutrition and metabolism. Academic Press; 1992. pp. 67–85. [DOI] [Google Scholar]
- National Research Council . 11th rev ed. National Academy Press; Washington (DC): 2012. Nutrient requirements of swine. [Google Scholar]
- Owen K.Q., Nelssen J.L., Goodband R.D., Tokach M.D., Kats L.J., Friesen K.G. Added dietary methionine in starter pig diets containing spray-dried blood products. J Anim Sci. 1995;73(9):2647–2654. doi: 10.2527/1995.7392647x. [DOI] [PubMed] [Google Scholar]
- Ren W.K., Yin J., Wu M.M., Liu G., Yang G., Xion Y. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PloS One. 2014;9(2) doi: 10.1371/journal.pone.0088335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P., Orlando U.A.D., Goncalves M.A.D., Vázquez-Añón M. Effects of increasing ratios of standardized ileal digestible total sulfur amino acid to lysine on growth performance of 7 to 17 kg pigs under antibiotics or antibiotics-free regime. J Anim Sci. 2018;96(Suppl 2) doi: 10.1093/jas/sky073.206. 111-111. [DOI] [Google Scholar]
- Shen Y.B., Weaver A.C., Kim S.W. Effect of feed grade l-methionine on growth performance and gut health in nursery pigs compared with conventional DL-methionine. J Anim Sci. 2014;92:5530–5539. doi: 10.2527/jas.2014-7830. [DOI] [PubMed] [Google Scholar]
- Spahr L., Bresson-Hadni S., Amann P., Kern I., Golaz O., Frossard J.L. Allopurinol, oxidative stress and intestinal permeability in patients with cirrhosis: an open-label pilot study. Liver Int. 2007;27(1):54–60. doi: 10.1111/j.1478-3231.2006.01382.x. [DOI] [PubMed] [Google Scholar]
- Tan B.E., Yin Y.L., Liu Z.Q., Li X.G., Xu H.J., Kong X.F. Dietary l-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids. 2009;37(1):169–175. doi: 10.1007/s00726-008-0148-0. [DOI] [PubMed] [Google Scholar]
- Thomson A., Hemphill D., Jeejeebhoy K.N. Oxidative stress and antioxidants in intestinal disease. Dig Dis. 1998;16(3):152–158. doi: 10.1159/000016859. [DOI] [PubMed] [Google Scholar]
- Torrallardona D., Conde M.R., Badiola I., Polo J., Brufau J. Effect of fishmeal replacement with spray-dried animal plasma and colistin on intestinal structure, intestinal microbiology, and performance of weanling pigs challenged with Escherichia coli K99. J Anim Sci. 2003;81(5):1220–1226. doi: 10.0000/PMID12772849. [DOI] [PubMed] [Google Scholar]
- Verrey F., Closs E.I., Wagner C.A., Palacin M., Endou H., Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflügers Archiv Eur Jo Phy. 2004;447(5):532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- Wu G. Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotechnol. 2014;5(1):34. doi: 10.1186/2049-1891-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Pan L., Tian Q.Y., Piao X.S. Comparative digestibility of energy and ileal amino acids in yeast extract and spray-dried porcine plasma fed to pigs. Arch Anim Nutr. 2018;72(1):76–84. doi: 10.1080/1745039X.2017.1413827. [DOI] [PubMed] [Google Scholar]
- Xiao D.F., Tang Z.R., Yin Y.L., Zhang B., Hu X.G., Feng Z.M. Effects of dietary administering chitosan on growth performance, jejunal morphology, jejunal mucosal sIgA, occluding, claudin-1 and TLR4 expression in weaned piglets challenged by enterotoxigenic Escherichia coli. Int Immunopharm. 2013;17(3):670–676. doi: 10.1016/j.intimp.2013.07.023. [DOI] [PubMed] [Google Scholar]
- Xiong X., Yang H.S., Wang X.C., Hu Q., Liu C.X., Wu X. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J Anim Sci. 2015;93(3):1089–1097. doi: 10.2527/jas.2014-7851. [DOI] [PubMed] [Google Scholar]
- Xiong X., Yang H.S., Hu X.H., Wang X.C., Li B., Long L.N. Differential proteome analysis along jejunal crypt-villus axis in piglets. Front Biosci. 2016;21:343–363. doi: 10.2741/4392. [DOI] [PubMed] [Google Scholar]
- Yan S.L., Long L.N., Zong E.Y., Huang P.F., Yang H.S., Li J.Z. Dietary sulfur amino acids affect jejunal cell proliferation and functions by affecting antioxidant capacity, Wnt/β-Catenin and the mechanistic target of rapamycin signaling pathways in weaning piglets. J Anim Sci. 2018 doi: 10.1093/jas/sky349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.S., Wang X.C., Xiong X., Yin Y.L. Energy metabolism in intestinal epithelial cells during maturation along the crypt-villus axis. Sci Rep. 2016;6:31917. doi: 10.1038/srep31917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y.L., Baidoo S.K., Schulze H., Simmins P.H. Effects of supplementing diets containing hulless barley varieties having different levels of non-starch polysaccharides with β-glucanase and xylanase on the physiological status of the gastrointestinal tract and nutrient digestibility of weaned pigs. Livest Prod Sci. 2001;71(2):97–107. doi: 10.1016/S0301-6226(01)00214-7. [DOI] [Google Scholar]
- Yin J., Li Y.Y., Han H., Liu Z.J., Zeng X.F., Li T.J. Long-term effects of lysine concentration on growth performance, intestinal microbiome, and metabolic profiles in a pig model. Food Func. 2018;9(8):4153–4163. doi: 10.1039/C8FO00973B. [DOI] [PubMed] [Google Scholar]
- Zhang G.J., Thacker P.A., Htoo J.K., Qiao S.Y. Optimum proportion of standardized ileal digestible sulfur amino acid to lysine to maximize the performance of 25-50 kg growing pigs fed reduced crude protein diets fortified with amino acids. Czech J Anim Sci. 2015;60(7):302–310. doi: 10.17221/8276-CJAS. [DOI] [Google Scholar]
- Zong E.Y., Huang P.F., Zhang W., Li J.Z., Li Y.L., Ding X.Q. The effects of dietary sulfur amino acids on growth performance, intestinal morphology, enzyme activity, and nutrient transporters in weaning piglets. J Anim Sci. 2018;96(3):1130–1139. doi: 10.1093/jas/skx003. [DOI] [PMC free article] [PubMed] [Google Scholar]