Abstract
This study was to evaluate the effect of xylanase supplementation and the addition of live yeast, Saccharomyces cerevisiae, on growth performance and intestinal microbiota in piglets. One hundred and eighty commercial crossbred 23-d-old piglets (PIC 417) were sorted by initial BW and allocated to 3 treatments: control (CTR) diet, CTR diet supplemented with xylanase at 16,000 birch xylan units/kg (XYL) and XYL diet supplemented with live yeast (2 × 1010 CFU/g) at 1 kg/t (XYL + LY). Each treatment had 10 replicates, with 6 animals each. A sorghum-based diet and water were available ad libitum for 42 d of the study. Average daily gain (ADG) and average daily feed intake (ADFI) were measured from 0 to 42 d (23- to 65-d-old) and feed conversion ratio (FCR) calculated. At the end of the study, bacterial identification through 16S rRNA (V3 to V4) sequencing of the ileal and caecal digesta from one piglet per replicate was performed. No treatment effects were observed on ADFI. Pigs offered the live yeast in addition to the xylanase had increased ADG compared with those supplemented with xylanase alone (XYL + LY vs. XYL; P = 0.655). FCR was improved with XYL and XYL + LY compared with CTR (P = 0.018). Clostridiaceae counts in the ileum tended to reduce by 10% with XYL and 14% with XYL + LY compared to CTR (P = 0.07). XYL and XYL + LY increased the counts of Lactobacillaceae in the caecum compared with CTR (P < 0.0001). Dietary supplementation of live yeast combined with xylanase improved growth performance and microbial balance of piglets during the nursery phase.
Keywords: Sorghum, Nursery piglet, Xylanase, Live yeast, Growth performance, Microbiota
1. Introduction
Prior to 2006 the common practise worldwide in animal production was to include antibiotics in feedstuffs at sub-therapeutic levels as growth promoters. As of Jan. 1, 2006, the European Union (EU) was the first trading block to ban the use of antibiotic growth promoters, due to the potential link of antimicrobial resistance transference from animals to humans (Smith et al., 2002). Since that ban, there is a focus on looking for nutritional solutions to mitigating certain common illnesses that increased in the absence of antibiotic use as well as the use of different feed ingredients.
Enzymes and probiotics have been proposed as antibiotic alternatives in livestock (Gaggia et al., 2010; Thacker, 2013). Enzyme supplementation aims to decrease the resistant of feed components to endogenous enzymes and improve nutrient digestibility and animal performance. There are several exogenous enzyme types available in the market, but with arabinoxylan making up the highest percentage of non-starch polysaccharide (NSP) in a typical pig or poultry diet (Masey-O'Neill et al., 2014). Xylanase is the most important NSP degrading enzyme. The hydrolysis of soluble arabinoxylan to reduce digesta viscosity (Choct et al., 2004) coupled with abrasion of feedstuff cell walls (Bedford, 2018) and the subsequent release of nutrients entrapped (Bedford, 2002) are the main suggested mechanisms of xylanase activity. Moreover, the release of xylo-oligosaccharides (XOS) as a result of xylan degradation in the distal sections of the gastrointestinal tract (GIT) may additionally play an important role, especially in non-viscous diets providing a substrate for fermentation by the intestinal microbiota (De Maesschalck et al., 2015).
Probiotics are live cultures that, when administered to humans or animals in adequate amounts, equilibrate the intestinal microbiota and benefit the host (Fuller, 1989). The most widely used probiotics are lactobacilli and bifidobacteria, but live yeast Saccharomyces cerevisiae var. boulardii has long been known effective for treating gastroenteritis (Hatoum et al., 2012). The modification of the intestinal environment is the result of several mechanisms such as the synthesis of inhibitor compounds, increase of mucins secretion, adherence-site competition against pathogens and for nutritional sources, bacterial toxin inhibition and improvement of nutrients absorption (Strompfova et al., 2004). All these mechanisms and others such as oxygen scavenging (Julien et al., 2015) and pathogenic binding (Jiang et al., 2015; Kiros et al., 2018) are important and potentially play a role in live yeast functionality.
The hypothesis of the study was that the positive effects of xylanase on piglet performance through the in situ production of oligosaccharides in the intestine can be further improved with live yeast supplementation in low viscosity diets through modification of the GIT environment and therefore the microbiota composition. The objective of the study was to evaluate the effect of xylanase supplementation and the addition of live yeast (S. cerevisiae, strain 1242) on the performance and intestinal microbiota in piglets fed a sorghum-based diet.
2. Materials and methods
The study was carried out following the recommendations from the Guide for the Care and Use of Laboratory Animals of the National Animal Experimentation Control Council of Brazil (CEUA). The trial was approved by the Ethics Committee of Animal Experiments of Akei Animal Research (protocol number: 001.2017).
2.1. Animals and housing
A total of 180 commercial crossbred 23-d-old piglets (PIC 417), 50% males and 50% females, were purchased from a local farm (Taguaí, São Paulo, Brazil). Upon arrival, piglets (weaned at 23 d of age; BW was 6.84 ± 0.195 kg) were placed immediately in 30 pens in an environmentally controlled room, with 6 piglets per pen. Animals were distributed into 5 blocks by initial BW. Within each block, pigs were distributed in pens for a balanced BW distribution. Each pen was 1.7 m length and 1.5 m width (total area 2.55 m2) and was equipped with a nipple water drinker, a lidded 20-cm hopper per piglet. Test diets and water were provided ad libitum throughout the trial.
2.2. Experimental diets
Sorghum, soybean meal, and soya oil were used as primary ingredients to formulate the experimental diets that met or exceeded nutrient recommendations for weaned piglets fed in 2 phases: pre-starter, from 23 to 44 d of age; and starter, from 44 to 65 d of age (NRC, 2012). The composition of the experimental diets is shown in Table 1. For each phase, one basal diet was made, then split equally into 3 batches, each of which were supplemented with or without different experimental products: 1) control (CTR) diet, without any added supplement; 2) xylanase diet, with xylanase (Econase XT 25P, AB Vista, Marlborough, UK; 160,000 birch xylan unit/g) at 100 g/t (XYL); and 3) xylanase diet supplemented additionally with live yeast (S. cerevisiae strain 1242; Vistacell, AB Vista, Marlborough, UK; 2 × 1010 CFU/g) at 1 kg/t (XYL + LY) resulting in 3 experimental treatments, each having 10 replicates and 6 pigs per pen. Feed additives were added on top. All diets were supplemented with phytase (Quantum Blue, AB Vista, Marlborough, UK; 10,000 phytase units [FTU]/g) at 500 FTU/kg. Experimental feeds were presented in a mash form and did not contain any antibiotic. ZnO and CuSO4 were included at therapeutic doses (Zn at 1,500 mg/kg and Cu at 150 mg/kg) in the pre-starter diet only.
Table 1.
Ingredients and calculated composition of the experimental diets (as-fed, %).
| Item | Pre-Starter | Starter |
|---|---|---|
| Ingredients | ||
| Sorghum | 52.51 | 67.76 |
| Soybean meal | 22.11 | 26.14 |
| Soya oil | 3.26 | 2.95 |
| Fish meal | 5.00 | 0.00 |
| Whey (5.5% DM) | 15.00 | 0.00 |
| Salt | 0.05 | 0.50 |
| Limestone | 0.27 | 0.39 |
| Dicalcium phosphate (18% P) | 0.54 | 0.86 |
| l-Try | 0.01 | 0.01 |
| Lys·HCl | 0.41 | 0.52 |
| Met | 0.16 | 0.16 |
| Thr | 0.17 | 0.21 |
| Vitamin and mineral premix1 | 0.50 | 0.50 |
| Phytase2 | 0.005 | 0.005 |
| Calculated nutrient composition | ||
| CP | 21.72 | 20.12 |
| DM | 87.57 | 87.50 |
| Ca | 0.80 | 0.70 |
| P | 0.74 | 0.68 |
| Fat | 5.64 | 5.38 |
| Fibre | 1.90 | 2.34 |
| Dig. Met + Cys | 0.75 | 0.68 |
| Dig. Lys | 1.36 | 1.24 |
| Dig. His | 0.48 | 0.46 |
| Dig. Try | 0.24 | 0.22 |
| Dig. Thr | 0.88 | 0.81 |
| Dig. Arg | 1.13 | 1.09 |
| Dig. Iso | 0.81 | 0.73 |
| Dig. Leu | 1.72 | 1.67 |
| Dig. Val | 0.88 | 0.80 |
| Dig. Gly | 0.76 | 0.65 |
| Dig. Ser | 0.84 | 0.80 |
| Dig. Phe + Tyr | 1.53 | 1.48 |
| Available P | 0.45 | 0.37 |
| Metabolizable energy, MJ/kg | 14.23 | 14.02 |
Dig. = digestible.
One kilogram of feed contains: 30,000 IU vitamin A; 7,500 IU vitamin D3; 75 mg vitamin E; 7.50 mg vitamin K3; 6.75 mg vitamin B1; 20 mg vitamin B2; 10 mg vitamin B6; 100 mg vitamin B12; 0.10 mg niacin; 47 mg pantothenic acid; 3 mg folic acid; 0.40 biotin; 1.50 mg selenium; 0.50 mg iron; 0.05 mg copper; 0.20 g manganese; 5 mg cobalt.
Quantum Blue 10G, AB Vista, Marlborough, UK; 10,000 phytase units (FTU)/g.
2.3. Experimental procedures
Piglets were weighted individually on d 0, 21, and 42 of the trial (23, 44, and 65 d of age respectively), to measure mean BW and calculate average daily gain (ADG) for each period and cumulatively. Feed consumption was determined by pen, and the mortality was checked twice daily, and the weights of dead piglets were used to adjust the feed conversion ratio (FCR). On 65 d of age, 30 piglets (one per pen) were randomly selected and desensitised by using 2 electrodes at 350 V and 1.3 A during approximate 3 s (Petrovina Ò IS 2000), then the piglets were exsanguinated by severing the jugular vein in the neck. The total GIT was removed immediately from the abdominal cavity. The digesta content from the ileum and caecum was immediately collected on an individual basis by gently squeezing each section into a tube, rapidly frozen in dry ice and then stored at −80 °C for subsequent analysis of microbiota profile. The faecal quality was evaluated twice a day. The absence of diarrhoea was considered by normal, and diarrhoea occurrence was characterized by liquid or loose faecal appearance (Giang et al., 2012).
2.4. Sample analyses
Diets were analysed for xylanase activity by enzyme linked immunosorbent assay (ELISA) method using Quantiplate Kits for Econase XT (Enzyme Services & Consultancy, Innovation & Technology Centre, Ystrad Mynach, UK) and phytase activity by an ELISA method, using Quantiplate Kits for Quantum Blue supplied by Envirologix (Enzyme Services & Consultancy, Innovation & Technology Centre, Ystrad Mynach, UK). Live yeast recovery in feeds was analysed by viable yeast count using the pour plate method using malt extract agar and CFU reported per gram of feed (Enzyme Services & Consultancy, Innovation & Technology Centre, Ystrad Mynach, UK).
Ileal and caecal samples from piglets were collected and bacterial DNA was extracted using AMPureXP beads (Beckman Couleter, Brea, CA) after thermal lysis process of 96 °C for 10 min. Amplicon sequencing library preparation was performed for bacteria using the V3 to V4 16S rRNA gene primers 341F (5′-CCTACGGGRSGCAGCAG-3′, Wang and Qian (2009)) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′, Caporaso et al. (2012)), with the following conditions: the first PCR primers contain the Ilumina sequences based on TruSeq structure adapter (Ilumin, San Diego, CA), allowing the second PCR with indexing sequences. The PCR were always carried out in triplicate using Platinum Taq (Invitrogen, USA) with the following conditions: PCR1, 95 °C for 5 min, 25 cycles of 95 °C for 45 s, 55 °C for 30 s and 72 °C for 45 s and a final extension of 72 °C for 2 min for; PCR2, 95 °C for 5 min, 10 cycles of 95 °C for 45 s, 66 °C for 30 s and 72 °C for 45 s and a final extension of 72 °C for 2 min; PCR3, the reaction was cleaned up using AMPureXP beads (Beckman Coulter, Brea, CA) and samples were pooled in the sequencing libraries for quantification. The DNA concentration of the pool amplicon was estimated with Picogreen drDNA assays (Invitrogen, USA), and then the pooled libraries were diluted for accurate quantitative PCR quantification using KAPA Library Quantification Kit for Ilumina Platforms (KAPA Byosystems, Woburn, MA). The library pool was adjusted to a final concentration of 11.5 pmol/L and sequenced in a MiSeq system by using the standard Ilumina primers provided in the kit. Single-end sequencing (300 nt) was performed using a V2 × 300 sequencing kit. After sequencing, the bioinformatics pipeline performs sequence demultiplexing, adaptor and primer trimming. The reads were size normalized to 283 bp. Read quality filter (E) was performed converting each nucleotide Q score in error probability (ei), that was summed and divided by read length (L). If Q was minor or equal to 20 (1%), the read was considered in downstream analysis. To increase the reliability of the read, excluding possible diversity generated by chimeric amplicons or erroneous nucleotide incorporated in PCR, 100% identical reads were clustered (Vieira et al., 2019). If any cluster was represented by fewer than 5 reads, it was not considered in further analysis. In the pipeline, each cluster gets a unique identification stablished as the operational taxonomic unit (OTU). Clustered sequences, i.e., OTU, were then subjected to taxonomic classification and compared using a 16S rRNA database (NeoRefdb, Neoprospecta Microbiome Technologies, Brazil). Sequences with at least 99% of identity in the reference database were taxonomically assigned and the samples were then evaluated based on their microbiome composition, focussing on bacterial profiles and their quantity amounts.
2.5. Statistical analyses
Animal performance were subjected to a one-way analysis of variance using JMP Pro 12 (SAS). Pen was used as an experimental unit for all performance measurements, and the piglet was considered an experimental unit when the microbial populations from the ileum and caecum were analysed. The initial BW was included as a covariate. Means were separated only when the treatment P-value was at a significant level and then compared by using a protected least significant difference (LSD) test (Snedecor and Cochran, 1967). Chi-square comparisons were used to evaluate the diarrhoea frequency. Biostatistical analysis for microbiota were performed in an open source software R-Studio v.3.6.1. Diversity was analysed at OTU level using the vegan package (Oksanen et al., 2019). Richness and alpha diversity were calculated with raw counts based on Simpson, Shannon and Inverse-Simpson estimators. Beta diversity was evaluated by multivariate ANOVA based on dissimilarities through envfit and adonis function. Finally, differential abundance analysis was performed with taxa relative abundances under a zero-inflated log normal mixture model, and P-values were corrected by false-discovery rate (FDR) with the metagenomeseq package (Paulson et al., 2019). Statements of significance were based on P-value of equal to or less than 0.05, and a P-value between 0.05 and 0.10 was considered as a trend. The correlation plot was created using R package “corrplot” (Wei and Simko, 2017).
3. Results
Analysed enzyme activities and live yeast recoveries in feed samples were all close to our expected values (Table 2).
Table 2.
Analysed enzyme activities and live yeast recoveries in feed samples.1
| Item | Pre-Starter |
Starter |
||||
|---|---|---|---|---|---|---|
| CTR | XYL | XYL + LY | CTR | XYL | XYL + LY | |
| Phytase2, FTU/kg | 764 | 753 | 622 | 374 | 403 | 513 |
| Xylanase3, BXU/kg | <2,000 | 24,700 | 24,000 | <2,000 | 13,900 | 16,000 |
| Live yeast, CFU/g | <1 × 104 | <1 × 104 | 2.13 × 107 | <1 × 104 | <1 × 104 | 2.90 × 107 |
FTU = phytase unit; BXU = birch xylan unit.
CTR, control diet; XYL, CTR supplemented with xylanase at 16,000 BXU/kg; XYL + LY, XYL supplemented with live yeast (2 × 1010 CFU/g) at 1 kg/t.
One FTU is defined as the amount of enzyme required to release 1 μmol of inorganic P/min from sodium phytate at 37 °C and pH 5.5.
One BXU is defined as the amount of enzyme that produces 1 nmol of reducing sugars from birchwood xylan in 1 s at 50 °C and pH 5.3.
3.1. Growth performance and diarrhoea
Overall mortality was 0.8% and no differences were observed between the experimental treatments (P > 0.05) (data not shown). The effects of experimental treatments on growth performance from d 23 to 44, from d 44 to 65, and from d 23 to 65 are shown in Table 3. No differences were observed on BW at 44 d of age (P = 0.415) between experimental treatments, but a significant trend was observed on BW at 65 d of age (P = 0.100). Piglets in XYL + LY treatment were 0.9 kg heavier compared to CTR (24.6 vs. 23.7 kg; P = 0.043). No differences were observed for average daily feed intake (ADFI) in any of the measured periods or the overall length of the study. ADG or FCR were not improved by XYL or XYL + LY treatments during the pre-starter phase (P > 0.05). During the starter phase, a significant trend in experimental treatments was observed on ADG (P = 0.098); piglets in XYL + LY treatment gained 37 g/d more than those in CTR treatment (P = 0.040); FCR during this period tended to be improved with XYL (P = 0.080) and improved with XYL + LY (P = 0.002) compared to CTR treatment. When the overall period was considered, pigs in XYL + LY treatment had increased ADG compared with those in XYL treatment (XYL + LY vs. XYL, P = 0.655). Effect of treatment on FCR was significant from 23 to 65 d (P = 0.018). FCR tended to be improved with XYL by 0.08 (P = 0.058) and XYL + LY by 0.12 (P = 0.010) compared to CTR treatment.
Table 3.
Effects of xylanase and live yeast supplementation on growth performance of piglets fed a sorghum-based diet1.
| Item | CTR | XYL | XYL + LY | Pooled SD |
P-values |
|
|---|---|---|---|---|---|---|
| Treatment | Initial BW | |||||
| BW, kg | ||||||
| 44 d of age | 13.4 | 13.3 | 13.6 | 0.513 | 0.415 | <0.001 |
| 65 d of age | 23.7 | 23.9 | 24.6 | 0.905 | 0.100 | <0.001 |
| 23 to 44 d of age | ||||||
| ADG, g/d | 301 | 305 | 320 | 28 | 0.255 | <0.001 |
| ADFI, g/d | 477 | 472 | 456 | 29 | 0.264 | <0.001 |
| FCR, g/g | 1.576 | 1.501 | 1.487 | 0.114 | 0.195 | 0.801 |
| 44 to 65 d of age | ||||||
| ADG, g/d | 488 | 496 | 525 | 38 | 0.098 | <0.001 |
| ADFI, g/d | 989 | 957 | 955 | 72 | 0.503 | <0.001 |
| FCR, g/g | 2.036a | 1.924ab | 1.829b | 0.137 | 0.009 | 0.610 |
| 23 to 65 d of age | ||||||
| ADG, g/d | 402 | 406 | 422 | 21 | 0.102 | <0.001 |
| ADFI, g/d | 730 | 706 | 716 | 47 | 0.516 | <0.001 |
| FCR, g/g | 1.819a | 1.740ab | 1.698b | 0.089 | 0.018 | 0.867 |
SD = standard deviation; ADG = average daily gain; ADFI = average daily feed intake; FCR = feed conversion ratio.
a, bMeans in the same row with different superscripts differ at P < 0.05.
Mean values for 10 replicate pens with 6 piglets per replicate pen. CTR, control diet; XYL, CTR supplemented with xylanase at 16,000 birch xylan units/kg; XYL + LY, XYL supplemented with live yeast (2 × 1010 CFU/g) at 1 kg/t.
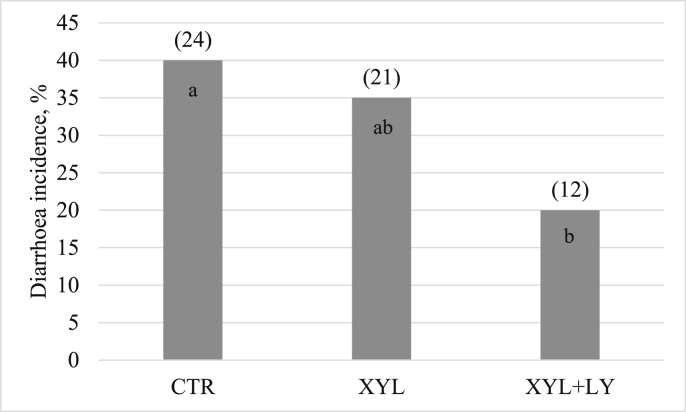
Experimental treatments influenced the incidence of diarrhoea (P < 0.05) (Fig. 1). Diarrhoea occurred in 40% of the piglets in non-supplemented CTR treatment, 35% of the pigs in XYL treatment, and only 20% of the pigs in XYL + LY treatment.
Fig. 1.
Effects of xylanase and live yeast supplementation on diarrhoea incidence of piglets fed a sorghum-based diet. CTR, control diet; XYL, CTR supplemented with xylanase at 16,000 birch xylan units/kg; XYL + LY, XYL supplemented with live yeast (2 × 1010 colony forming units/g) at 1 kg/t. Values in parentheses represent the number of animals with diarrhoea. Data columns with different letters differ at P < 0.05.
3.2. Microbial profile
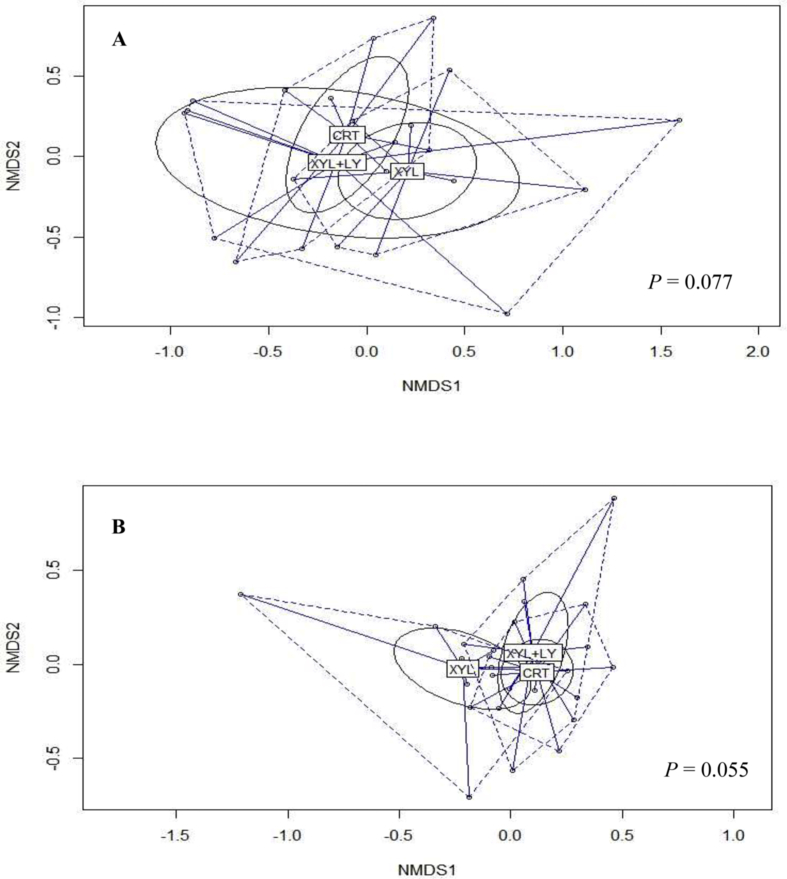
Three alpha-diversity estimators (Simpson, Shannon and inverse Simpson) were performed in the ileal and caecal digesta of piglets at 65 d of age (Table 4). No differences were observed in any of the estimators in the caecum (P > 0.05). Shannon and inverse Simpson estimators in the ileum diversity were not influenced by the experimental treatments, but XYL + LY treatment reduced Simpson estimator when compared to CTR and XYL treatments (P < 0.05). XYL and XYL + LY treatments tended to influence beta diversity in the ileum (Fig. 2A) and the caecal digesta (Fig. 2B) (P < 0.10).
Table 4.
Effects of xylanase and live yeast supplementation on alpha diversity in the ileal and caecal microbiota of piglets fed a sorghum-based diet1.
| Item | CTR | XYL | XYL + LY | Pooled SD | P-value |
|---|---|---|---|---|---|
| Ileum | |||||
| Shannon | 1.09 | 1.14 | 0.73 | 0.501 | 0.237 |
| Simpson | 0.60a | 0.55a | 0.32b | 0.214 | 0.044 |
| Inverse Simpson | 2.56 | 2.55 | 2.31 | 1.349 | 0.925 |
| Cecum | |||||
| Shannon | 1.89 | 1.60 | 1.66 | 0.354 | 0.184 |
| Simpson | 0.77 | 0.66 | 0.69 | 0.124 | 0.134 |
| Inverse Simpson | 4.82 | 3.48 | 3.81 | 1.444 | 0.114 |
SD = standard deviation.
a, bMeans in the same row with different superscripts differ at P < 0.05.
Mean values from 10 piglets per treatment. CTR, control diet; XYL, CTR supplemented with xylanase at 16,000 birch xylan units/kg; XYL + LY, XYL supplemented with live yeast (2 × 1010 CFU/g) at 1 kg/t.
Fig. 2.
Comparison of beta-diversity of ileum (A) and caecum (B) microbiota between CTR, XYL and XYL + LY treatments. CTR, control diet; XYL, CTR supplemented with xylanase at 16,000 birch xylan units/kg; XYL + LY, XYL supplemented with live yeast (2 × 1010 colony forming units/g) at 1 kg/t. The nonmetric-multidimensional scaling (NMDS) plots was generated using Bray–Curtis distances. P-value was obtained from analysis of similarities (ANOSIM).
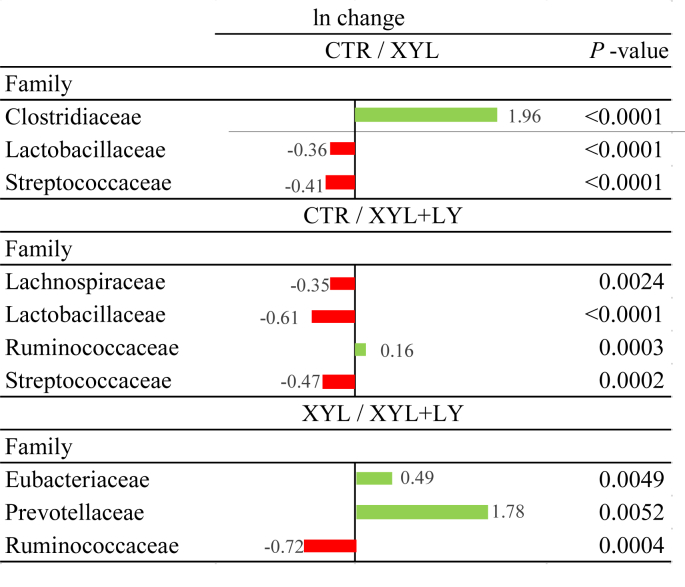
In both intestinal regions, 5 phyla were detected being Firmicutes the predominant but some family members in the ileal and caecal digesta were influenced by XYL and XYL + LY treatments (Table 5 and Fig. 3). The relative proportion of Clostridiaceae was reduced by 9.8% in the ileum with XYL and 14.2% with XYL + LY compared to CTR (P = 0.068). The relative proportions of Bifidobacteriaceae, Clostridiaceae and Enterobacteriaceae in the caecal digesta were also influenced with the experimental treatments (P = 0.011, P = 0.076 and P = 0.021, respectively). No differences were observed in the natural logarithm (ln) values for the ileal microbiota (data not shown). In contrast, the ln changes promoted by treatments at family level in the caecal microbiota are presented in Fig. 3. The count of Clostridiaceae in CTR treatment was 196% higher than that in XYL treatment (P < 0.0001), but lower counts of Lactobacillaceae (P < 0.0001) and Streptococcaceae (P < 0.0001) were observed in CTR treatment. Ruminococcaceae ln value in CTR treatment was 16% higher than that in XYL + LY treatment (P = 0.0003), but the ln values of Lachnospiraceae (P = 0.0024), Lactobacillaceae (P < 0.0001) and Streptococcaceae (P = 0.0002) in XYL + LY treatment were higher than those in CTR treatment. Finally, differences were also observed due to live yeast supplementation. The ln values of Eubacteriaceae and Prevotellaceae in XYL treatment were 49% and 178% higher than those in XYL + LY treatment (P = 0.0049 and P = 0.0052, respectively), and the ln value of Ruminococcaceae in XYL + LY treatment was 72% lower than that in XYL treatment (P = 0.0004).
Table 5.
Relative proportion of microbial populations in the ileal and caecal digesta based on the operational taxonomic units1.
| Item | Ileum |
Cecum |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTR | XYL | XYL + LY | Pooled SD | P-value 2 | Adjusted P-value 3 |
CTR | XYL | XYL + LY | Pooled SD | P-value2 | Adjusted P-value 3 |
|
| Bacteroidaceae | 0.0 | 0.0 | 0.2 | 0.22 | 1.000 | 1.000 | n.d | n.d | n.d | |||
| Bifidobacteriaceae | 0.0 | 0.0 | 0.2 | 0.24 | 1.000 | 1.000 | 0.5 | 0.3 | 0.1 | 0.58 | 0.011 | 0.115 |
| Clostridiaceae | 20.0 | 10.2 | 5.8 | 11.92 | 0.068 | 0.163 | 4.5 | 1.6 | 4.5 | 5.75 | 0.076 | 0.241 |
| Enterobacteriaceae | 7.8 | 3.2 | 0.7 | 9.54 | 0.129 | 0.257 | 2.2 | 0.0 | 3.4 | 6.89 | 0.021 | 0.115 |
| Enterococcaceae | n.d | n.d | n.d | 0.0 | 0.0 | 0.1 | 0.22 | 1.000 | 1.000 | |||
| Eubacteriaceae | 0.0 | 0.2 | 1.6 | 2.24 | 1.000 | 1.000 | 8.8 | 7.9 | 3.6 | 5.18 | 0.218 | 0.399 |
| Lachnospiraceae | 0.0 | 0.7 | 0.6 | 1.52 | 1.000 | 1.000 | 5.6 | 3.6 | 5.4 | 3.29 | 0.128 | 0.282 |
| Lactobacillaceae | 53.9 | 64.3 | 78.7 | 20.44 | 0.468 | 0.702 | 41.2 | 56.4 | 49.2 | 14.70 | 0.654 | 0.720 |
| Mycoplasmataceae | 0.0 | 0.2 | 0.0 | 0.41 | 1.000 | 1.000 | n.d | n.d | n.d | |||
| Prevotellaceae | 0.0 | 0.0 | 0.9 | 1.29 | 1.000 | 1.000 | 1.6 | 1.5 | 0.3 | 1.91 | 0.420 | 0.513 |
| Ruminococcaceae | 0.0 | 3.6 | 2.9 | 7.82 | 1.000 | 1.000 | 17.5 | 13.7 | 14.0 | 8.15 | 0.088 | 0.241 |
| Streptococcaceae | 18.4 | 17.7 | 7.4 | 15.38 | 0.318 | 0.545 | 5.3 | 6.5 | 6.0 | 4.26 | 0.358 | 0.492 |
| Veillonellaceae | 0.0 | 0.0 | 1.0 | 1.37 | 1.000 | 1.000 | 12.9 | 8.6 | 13.3 | 8.76 | 0.281 | 0.442 |
SD = standard deviation; n.d = non-determined.
Mean values from 10 piglets per treatment. CTR, control diet; XYL, CTR supplemented with xylanase at 16,000 birch xylan units/kg; XYL + LY, XYL supplemented with live yeast (2 × 1010 CFU/g) at 1 kg/t.
Obtained using a zero-inflated Gaussian mixture model.
P-values corrected by false-discovery rate.
Fig. 3.
Logarithm (ln) value changes promoted in treatment contrasts (adjusted P < 0.05) in family taxa in the caecal microbiota. CTR, control diet; XYL, CTR supplemented with xylanase at 16,000 birch xylan units/kg; XYL + LY, XYL supplemented with live yeast (2 × 1010 CFU/g) at 1 kg/t. Positive values and negative values indicate greater and lower abundance based on the absolute operational taxonomic units, respectively. Differences presented are based on all taxa detected in samples per treatment.
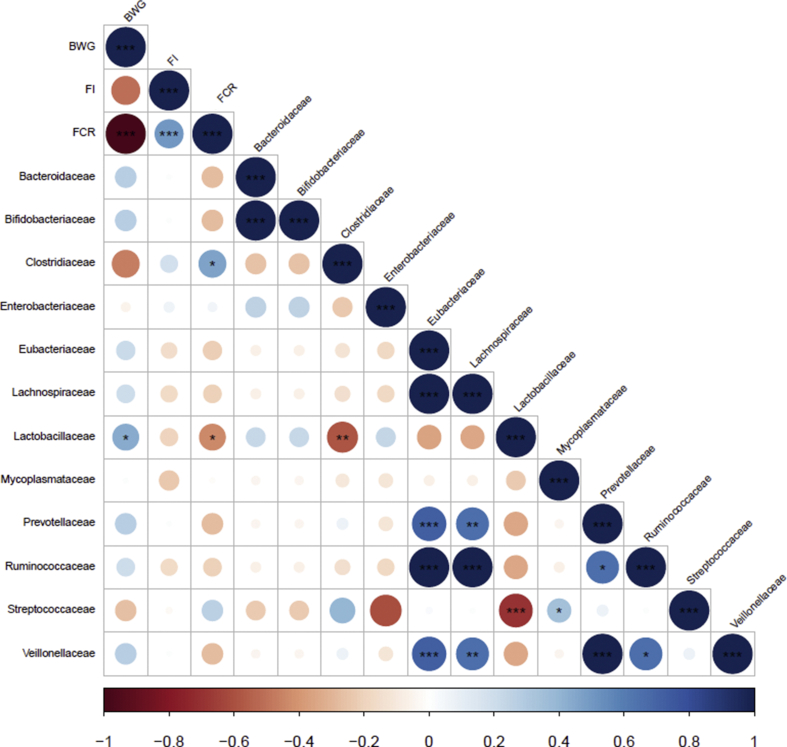
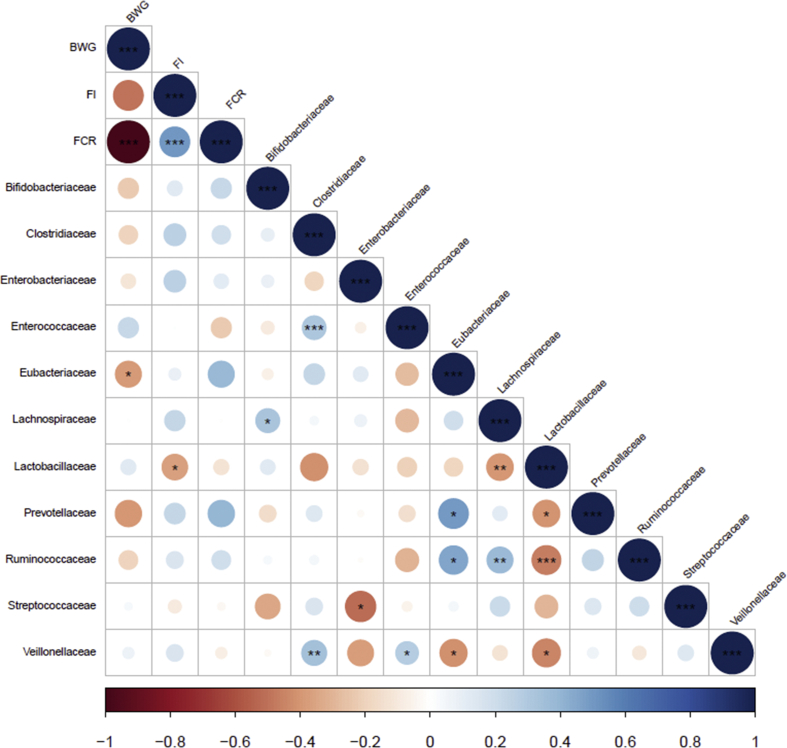
The correlations between animal performance and the relative abundance of bacterial families in the ileum and the caecal digesta are presented in Fig. 4, Fig. 5, respectively. A positive and significant correlation between BW gain and the relative abundance of Lactobacillaceae in the ileum was observed. Looking at the implications of microbiota on FCR, the relative abundance of Lactobacillaceae was negatively correlated with FCR whereas the relative abundance of Clostridiaceae was positively correlated with FCR. A negative and significant correlation between BW gain and the relative abundance of Eubacteriaceae in the caecal content was observed. The relative abundance of Lactobacillaceae in the caecal content was negatively correlated with feed intake.
Fig. 4.
Correlations between performance parameters and relative abundances of bacterial family taxa in the ileum content. BWG = BW gain; FI = feed intake; FCR = feed conversion ratio. The ∗(P < 0.05), ∗∗(P < 0.01) and ∗∗∗(P < 0.001) symbols indicates a statistically significant correlation. The scale colours (Spearman's ρ from −1 to +1) indicate whether the correlation is positive (blue coloured circles) or negative (red coloured circles) between factors.
Fig. 5.
Correlations between performance parameters and relative abundances of bacterial family taxa in the caecal contents. BWG = BW gain; FI = feed intake; FCR = feed conversion ratio. The ∗(P < 0.05), ∗∗(P < 0.01) and ∗∗∗(P < 0.001) symbols indicates a statistically significant correlation. The scale colours (Spearman's ρ from −1 to +1) indicate whether the correlation is positive (blue coloured circles) or negative (red coloured circles) between factors.
4. Discussion
The literature for the use of sorghum as the sole cereal source in nursery diet of piglets is limited and therefore the effects of xylanase and live yeast on piglets fed sorghum-based diets are scarce. The effects of xylanase supplemented in sorghum-based diets have attracted less attention because sorghum has less soluble NSP compared to wheat and corn, and the viscosity is likely irrelevant in this grain (Bach Knudsen, 2014). Nevertheless, arabinoxylan is the main cell wall component in cereals, and in the present study, xylanase supplementation over a 42-d period improved animal performance in sorghum-based diets justifying that sorghum has sufficient arabinoxylan content to elicit a response to xylanase. Different mechanism of action through xylanase supplementation could explain the positive results observed in the present study: 1) viscosity reduction, although it is unlikely to be the main reason as the soluble NSP content is very low (Choct et al., 2004); 2) abrasion of feedstuff cell walls and the subsequent release of nutrients entrapped (Bedford, 2002) and 3) arabinoxylo-oligosaccharides (AXOS) production in the GIT not only from the endogenous enzyme action by the microbial populations but also from the action of exogenous xylanase (Morgan et al., 2017, 2019). Therefore, these AXOS could be acting as prebiotic or signalling compounds in the GIT to stimulate fibre degrading microbiota as reviewed by G. González-Ortiz et al. (2019). The physicochemical properties of digesta, nutrient or energy digestibility have not been evaluated in the present study, but if the previous hypotheses are right, the fibre-degrading enzymes produced in situ by the microbial communities in the GIT due to the stimulation of the xylo-oligomers produced by the xylanase would potentially increase further the breakdown of cell walls with greater efficiency than supplemented enzyme. However, the efficiency of arabinoxylan breakdown by endogenous or exogenous xylanase has not been studied and is a potential area of future research. In the case of sorghum, the release of entrapping nutrients is of great interest as the protein structure in the grain and its distribution in the endosperm is proportionally greater and has a denser surface and higher water penetration resistance compared to corn and other grains (Selle et al., 2018). Starch granules are surrounded by kafirin protein bodies and cell wall fibre in the sorghum endosperm. This complex matrix can be cross-linked, forming structures that limit enzymatic action, especially compromising amino acid and starch digestibility (Liu et al., 2016; Pan et al., 2017). Therefore, xylanase supplementation in sorghum-fed pigs may be a feasible strategy to improve nutrient and energy profitability and the release of AXOS to have a better animal performance.
Further to the evaluation of xylanase, it was considered to study if the supplementation of live yeast in addition to the xylanase provided any extra benefit. Piglets in XYL + LY treatment were 0.7 kg heavier at 65 d of age compared to those supplemented with xylanase. Overall, live yeast supplementation combined with xylanase during the nursery phase increased ADG and improved FCR with no effects on ADFI. The enhancement of FCR of piglets observed in the present study was in agreement with previously published reports on live yeast (Jiang et al., 2015). During the weaning phase, piglets have to undergo many stressors (environmental, social and dietary) with consequences in animal performance. Together, with the immaturity of their intestinal and immune systems, it may lead the animals to develop anorexia, intestinal stasis, a low rate of feed digestion and the risk of diarrhoea (Lallès et al., 2007). This post-weaning lag may increase mortality, reduce growth in animals from wean to finish and increase heterogeneity of weight lots. Nutritional strategies such as yeast supplementation are used for improving intestinal development and immune function in pigs. It has been reported that some strains of live yeast and components of yeasts can improve growth performance, the small intestine architecture and immune function in weaned piglets (Bontempo et al., 2006; Jiang et al., 2015; Li et al., 2006; Trevisi et al., 2015, 2017). A variety of components make up yeasts such as β-glucans, mannans, nucleotides and peptides which have been related with the positive effects observed when live yeast, cell wall extracts or dead yeasts have been supplemented (Kollar et al., 1997). However, the main differential feature of live yeast compared to other types of yeast by-products is the oxygen scavenging capacity. A fully functional hindgut has to offer a strictly anaerobically environment to allow fermentation of the undigested nutrients, mainly NSP, coming from the small intestine (Rinttilä and Apajalahti, 2013). Presence of oxygen in samples obtained from the hindgut of humans and rats clearly demonstrated that changes in bacterial composition reduce the presence of fibre-degrading members (Albenberg et al., 2014). Ruminal digesta from animals receiving live yeast had lower prevailing concentrations of oxygen, but improved fibre digestion due to the protection of highly oxygen-sensitive fibre-degrading bacteria like Fibrobacter succinogenes (Chaucheyras-Durand and Fonty, 2001). If the same findings can be found in monogastric animals, live yeast supplemented to piglets may also reduce the presence of oxygen in the hindgut, and increase the survival and presence of fibrolytic bacteria. As Jha and Berrocoso (2016) also discussed, the reduction on dissolved oxygen stimulated fermentation and production of short-chain fatty acids (SCFA). Moreover, increasing fermentation and production of SCFA may reduce the opportunities for pathogens to colonize the intestinal environment. Taken together with the increased quantities of acid glycoconjugates in the mucous cells of live yeast-supplemented piglets (Bontempo et al., 2006), the new environment may confer a greater resistance to bacterial infection and consequently less diarrhoea episodes. In the present study, diarrhoea indices were high in the CTR treatment, suggesting some kind of postweaning disturbance. Xylanase supplementation marginally reduced the incidence, but it is noteworthy the further reduction of the diarrhoea occurrence in piglets supplemented with xylanase in combination with live yeast. Many studies have demonstrated that supplementation with live yeast or polysaccharides from its cell walls may improve disease resistance and enhance performance through immunostimulation and maintenance of a favourable intestinal environment of animals (Badia et al., 2012b). Yeast cells are natural mannose-rich products that can be used as a substrate for adhesion of Gram-negative bacteria (Gedek, 1999). Administration of live yeast helped reduce postweaning diarrhoea after enterotoxigenic Escherichia coli (ETEC) infection (Che et al., 2017; Trckova et al., 2014; Trevisi et al., 2015), possibly through the interaction of live yeast with ETEC binding to the intestinal receptors (Badia et al., 2012a, 2012b). Animals from the present study were not diagnosed for the aetiology agent causing the faecal consistency lost, but based in other studies, it can be speculated that live yeast had an effect on the piglet immune response, fermentation activity, epithelial integrity and possibly also impairing the attachment of pathogenic bacteria to the intestinal receptors, improving disease resistance, nutrient digestibility, animal performance and the faecal consistency (Badia et al., 2012b; Trevisi et al., 2015; Zanello et al., 2009).
The ileal and caecal microbiota diversity was analysed as both xylanase and live yeast supplementation are related with microbial shifts in the GIT (Bedford and Cowieson, 2012; Gemma González-Ortiz et al., 2017; Le Bon et al., 2010). Clostridiaceae from the ileum and caecum were reduced with the experimental treatments, and in the caecum, XYL and XYL + LY treatments increased the presence of Lactobacillaceae, Streptococcaceae and Lachnospiraceae. Some members belonging to the Clostridiaceae family can be pathogenic. For example, Clostridium perfringens, Clostridium botulinum, Clostridium tetani, Clostridium septicum and Clostridium difficile are some of the most known species threatening human and animal health (Gibbs, 2009), but others like Faecalibacterium prausnitzi have been identified as butyrate producers (Rios-Covian et al., 2015). The identification of the microbiota at the genus and specie levels in the Clostridiaceae family did not reveal any influence of the experimental treatments (data not shown), but it cannot be discarded an inhibitory effect of xylanase and the combination with live yeast in other members of the family not detected in the current study. In contrast, the presence of Lactobacillaceae tended to increase with xylanase and even further when xylanase was combined with live yeast. Lactobacillus amylovorus was the most influenced strain by the experimental treatments. L. amylovorus has demonstrated to be an anti-inflammatory strain inhibiting Toll-like receptors when exposed to ETEC (Finamore et al., 2014). Although there was no difference in the relative proportion of Enterobacteriace levels between treatments, it is interesting to highlight the numerically improvement on Lactobacillaceae to Enterobacteriaceae ratio in XYL and XYL + LY treatments compared to CTR (20 and 112 vs. 7, respectively, in the ileum, and 35 and 14 vs. 9, respectively, in the caecum). This ratio is generally considered an indicator of gut health (Demecková et al., 2002). It can be hypothesised that the Lactobacillaceae to Enterobacteriaceae ratio may have partially contributed for the improved FCR observed in the present study as observed in the multivariate analysis. In the cecum, Lactobacillaceae count was influenced by the experimental treatments as well as the presence of Eubacteriaceae. XYL and XYL + LY treatments increased Lactobacillaceae count compared to CTR pigs, and Eubacteriaceae count was higher in XYL compared to XYL + LY. The shifts in microbial diversity observed in the present study may be the consequence of several factors. On the one hand, xylanase, through XOS production, may stimulate lactate and butyrate producing bacteria, and xylanase often has cross-feeding interactions with butyrate producing bacteria (De Maesschalck et al., 2015); on the other hand, the resistance to pathogen adhesion displayed by live yeast (Posadas et al., 2017) may be an additional reason of the beneficial effects observed in performance and microbial diversity in the present study. However, it cannot be discarded the synergistic effect that both additives may have on the stimulation of fibrolytic bacteria and as a consequence better fibre digestibility.
5. Conclusion
Xylanase application during the nursery phase improved animal performance and the microbial balance by increasing Lactobacillaceae and reducing Clostridiaceae in piglets fed sorghum-based diets. Additional live yeast supplementation further improved these parameters. These findings suggest that xylanase and live yeast can stimulate the growth of lactic acid bacteria and the development of a fibrolytic fermentation in the GIT, thus improving nutrient digestibility and animal performance.
Author contritbutions
Gemma González-Ortiz: conceptualization writing - original draft, writing - review & editing.
Marco A. Callegari: investigation.
Pete Wilcock: conceptualization.
Diego Melo-Duran: formal analysis.
Michael R. Bedford: supervision, conceptualization.
Hilário R. V. Oliveira: investigation.
Marcos A. A. da Silva: investigation.
Carlos R. Pierozan: investigation.
Caio A. da Silva: supervision.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
The authors acknowledge the help of Paloma Menezes Rubin from Neoprospecta, Brazil, for the microbial analyses.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Albenberg L., Esipova T.V., Judge C.P., Bittinger K., Chen J., Laughlin A. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147(5):1055–1063. doi: 10.1053/j.gastro.2014.07.020. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach Knudsen K.E. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poultry Sci. 2014;93(9):2380–2393. doi: 10.3382/ps.2014-03902. [DOI] [PubMed] [Google Scholar]
- Badia R., Lizardo R., Martinez P., Badiola I., Brufau J. The influence of dietary locust bean gum and live yeast on some digestive immunological parameters of piglets experimentally challenged with Escherichia coli. J Anim Sci. 2012;90(Suppl 4):260–262. doi: 10.2527/jas.53894. [DOI] [PubMed] [Google Scholar]
- Badia R., Zanello G., Chevaleyre C., Lizardo R., Meurens F., Martinez P. Effect of Saccharomyces cerevisiae var. Boulardii and beta-galactomannan oligosaccharide on porcine intestinal epithelial and dendritic cells challenged in vitro with Escherichia coli F4 (K88) Vet Res. 2012;43:4. doi: 10.1186/1297-9716-43-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford MRs. McNab JM and Boorman KN. Poultry feedstuffs: supply, composition and nutritive value. CABI Publishing; Oxon, United Kingdom: 2002. The role of carbohydrases in feedstuff digestion; pp. 319–336. [Google Scholar]
- Bedford M.R. The evolution and application of enzymes in the animal feed industry: the role of data interpretation. Br Poultry Sci. 2018;59(5):486–493. doi: 10.1080/00071668.2018.1484074. [DOI] [PubMed] [Google Scholar]
- Bedford M.R., Cowieson A.J. Exogenous enzymes and their effects on intestinal microbiology. Anim Feed Sci Technol. 2012;173(1):76–85. [Google Scholar]
- Bontempo V., Di Giancamillo A., Savoini G., Dell' Orto V., Domeneghini C. Live yeast dietary supplementation acts upon intestinal morpho-functional aspects and growth in weanling piglets. Anim Feed Sci Technol. 2006;129:224–236. [Google Scholar]
- Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Huntley J., Fierer N. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaucheyras-Durand F., Fonty G. Establishment of cellulolytic bacteria and development of fermentative activities in the rumen of gnotobiotically-reared lambs receiving the microbial additive Saccharomyces cerevisiae CNCM I-1077. Reprod Nutr Dev. 2001;41(1):57–68. doi: 10.1051/rnd:2001112. [DOI] [PubMed] [Google Scholar]
- Che L., Xu Q., Wu C., Luo Y., Huang X., Zhang B. Effects of dietary live yeast supplementation on growth performance, diarrhoea severity, intestinal permeability and immunological parameters of weaned piglets challenged with enterotoxigenic Escherichia coli K88. Br J Nutr. 2017;118(11):949–958. doi: 10.1017/S0007114517003051. [DOI] [PubMed] [Google Scholar]
- Choct M., Kocher A., Waters D.L., Pettersson D., Ross G. A comparison of three xylanases on the nutritive value of two wheats for broiler chickens. Br J Nutr. 2004;92(1):53–61. doi: 10.1079/BJN20041166. [DOI] [PubMed] [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl Environ Microbiol. 2015;81(17):5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demecková V., Kelly D., Coutts A.G.P., Brooks P.H., Campbell A. The effect of fermented liquid feeding on the faecal microbiology and calostrum quality of farrowing sows. Int J Food Microbiol. 2002;79(1–2):85–97. doi: 10.1016/s0168-1605(02)00182-4. [DOI] [PubMed] [Google Scholar]
- Finamore A., Roselli M., Imbinto A., Seeboth J., Oswald I.P., Mengheri E. Lactobacillus amylovorus inhibits the TLR4 inflammatory signaling triggered by enterotoxigenic Escherichia coli via modulation of the negative regulators and involvement of TLR2 in intestinal Caco-2 cells and pig explants. PloS One. 2014;9(4):e94891. doi: 10.1371/journal.pone.0094891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- Gaggia F., Mattarelli P., Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol. 2010;141(Suppl 1):S15–S28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Gedek B.R. Adherence of Escherichia coli serogroup O 157 and the Salmonella typhimurium mutant DT 104 to the surface of Saccharomyces boulardii. Mycoses. 1999;42(4):261–264. doi: 10.1046/j.1439-0507.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- Giang H.H., Viet T.Q., Ogle B., Lindberg J.E. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with a complex of lactic acid bacteria alone or in combination with Bacillus subtilis and Saccharomyces boulardii. Livest Sci. 2012;143(2):132–141. [Google Scholar]
- Gibbs PAs. 2nd ed. Woodhead Publishing; 2009. 23-Pathogenic Clostridium species. In: blackburn CdW and McClure PJ. Foodborne pathogens; pp. 820–843. [Google Scholar]
- González-Ortiz G., Gomes G.A., Dos Santos T.T., MRs Bedford. New strategies influencing gut functionality and animal performance. In: González-Ortiz G., Bedford M.R., Bach Knudsen K.E., Courtin C.M., Classen H.L., editors. The value of fibre. Engaging the second brain for animal nutrition Wageningen. Wageningen Academic Publishers; The Netherlands: 2019. pp. 233–254. [Google Scholar]
- González-Ortiz G., Kozłowski K., Drażbo A., Bedford M.R. Response of turkeys fed wheat-barley-rye based diets to xylanase supplementation. Anim Feed Sci Technol. 2017;229:117–123. [Google Scholar]
- Hatoum R., Labrie S., Fliss I. Antimicrobial and probiotic properties of yeasts: from fundamental to novel applications. Front Microbiol. 2012;3(421):1–12. doi: 10.3389/fmicb.2012.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Berrocoso J.F.D. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim Feed Sci Technol. 2016;212:18–26. [Google Scholar]
- Jiang Z., Wei S., Wang Z., Zhu C., Hu S., Zheng C. Effects of different forms of yeast Saccharomyces cerevisiae on growth performance, intestinal development, and systemic immunity in early-weaned piglets. J Anim Sci Biotechnol. 2015;6:47. doi: 10.1186/s40104-015-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C., Marden J.P., Auclair E., Moncoulon R., Cauquil L., Peyraud J.L. Interaction between live yeast and dietary rumen degradable protein level: effects on diet utilization in early-lactating dairy cows. Agric Sci. 2015;6:1–13. [Google Scholar]
- Kiros T.G., Derakhshani H., Pinloche E., D'Inca R., Marshall J., Auclair E. Effect of live yeast Saccharomyces cerevisiae (Actisaf Sc 47) supplementation on the performance and hindgut microbiota composition of weanling pigs. Sci Rep-UK. 2018;8(1):5315. doi: 10.1038/s41598-018-23373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollar R., Reinhold B.B., Petrakova E., Yeh H.J., Ashwell G., Drgonova J. Architecture of the yeast cell wall. Beta(1→6)-glucan interconnects mannoprotein, beta(1)→3-glucan, and chitin. J Biol Chem. 1997;272(28):17762–17775. doi: 10.1074/jbc.272.28.17762. [DOI] [PubMed] [Google Scholar]
- Lallès J.-P., Bosi P., Smidt H., Stokes C.R. Weaning — a challenge to gut physiologists. Livest Sci. 2007;108(1):82–93. [Google Scholar]
- Le Bon M., Davies H.E., Glynn C., Thompson C., Madden M., Wiseman J. Influence of probiotics on gut health in the weaned pig. Livest Sci. 2010;133(1):179–181. [Google Scholar]
- Li J., Li D., Gong L., Ma Y., He Y., Zhai H. Effects of live yeast on the performance, nutrient digestibility, gastrointestinal microbiota and concentration of volatile fatty acids in weanling pigs. Arch Anim Nutr. 2006;60(4):277–288. doi: 10.1080/17450390600785343. [DOI] [PubMed] [Google Scholar]
- Liu S.Y., Truong H.H., Khoddami A., Moss A.F., Thomson P.C., Roberts T.H. Comparative performance of broiler chickens offered ten equivalent diets based on three grain sorghum varieties as determined by response surface mixture design. Anim Feed Sci Technol. 2016;218:70–83. [Google Scholar]
- Masey-O'Neill H.V., Smith J.A., Bedford M.R. Multicarbohydrase enzymes for non-ruminants. Asian-Australas J Anim Sci. 2014;27(2):290–301. doi: 10.5713/ajas.2013.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N.K., Choct M., Wallace A., Hawkins K.L., Wu S.B., Bedford M.R. Book in vitro evaluation of xylo-oligosaccharide production from different batches of wheat with and without xylanase. University of Syndey; Syndey, Australia: 2019. In vitro evaluation of xylo-oligosaccharide production from different batches of wheat with and without xylanase; pp. 59–60. [Google Scholar]
- Morgan N.K., Wallace A., Bedford M.R., Choct M. Efficiency of xylanases from families 10 and 11 in production of xylo-oligosaccharides from wheat arabinoxylans. Carbohydr Polym. 2017;167:290–296. doi: 10.1016/j.carbpol.2017.03.063. [DOI] [PubMed] [Google Scholar]
- NRC . The National Academies Press; Washington, DC: 2012. Nutrient requirements of swine. Eleventh revised edition. [Google Scholar]
- Oksanen J., Guillaume Blanchet R.K.F., Legendre P., Minchin P.R., O'Hara R.B., Simpson G.L. 2019. Package 'vegan. R packag. version 3.4.0. [Google Scholar]
- Pan L., Shang Q.H., Wu Y., Ma X.K., Long S.F., Liu L. Concentration of digestible and metabolizable energy, standardized ileal digestibility, and growth performance of pigs fed diets containing sorghum produced in the United States or corn produced in China. J Anim Sci. 2017;95(11):4880–4892. doi: 10.2527/jas2017.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson N.J., Olson N.D., Braccia D.J., Wagner J., Talukder H., Pop M. 2019. Package 'metagenomeSeq'. [Google Scholar]
- Posadas G.A., Broadway P.R., Thornton J.A., Carroll J.A., Lawrence A., Corley J.R. Yeast pro- and paraprobiotics have the capability to bind pathogenic bacteria associated with animal disease. Transl Anim Sci. 2017;1:60–68. doi: 10.2527/tas2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinttilä T., Apajalahti J. Intestinal microbiota and metabolites—implications for broiler chicken health and performance. J Appl Poultry Res. 2013;22(3):647–658. [Google Scholar]
- Rios-Covian D., Gueimonde M., Duncan S.H., Flint H.J., de los Reyes-Gavilan C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol Lett. 2015;362(21) doi: 10.1093/femsle/fnv176. [DOI] [PubMed] [Google Scholar]
- Selle P.H., Moss A.F., Truong H.H., Khoddami A., Cadogan D.J., Godwin I.D. Outlook: sorghum as a feed grain for Australian chicken-meat production. Animal Nutr. 2018;4(1):17–30. doi: 10.1016/j.aninu.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.L., Harris A.D., Johnson J.A., Silbergeld E.K., Morris J.G., Jr. Animal antibiotic use has an early but important impact on the emergence of antibiotic resistance in human commensal bacteria. Proc Natl Acad Sci USA. 2002;99(9):6434–6439. doi: 10.1073/pnas.082188899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor G., Cochran W. 6th ed. the Iowa State University Press; Ames: 1967. Statistical methods. [Google Scholar]
- Strompfova V., Laukova A., Ouwehand A.C. Selection of enterococci for potential canine probiotic additives. Vet Microbiol. 2004;100(1–2):107–114. doi: 10.1016/j.vetmic.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Thacker P.A. Alternatives to antibiotics as growth promoters for use in swine production: a review. J Anim Sci Biotechnol. 2013;4(35):1–12. doi: 10.1186/2049-1891-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trckova M., Faldyna M., Alexa P., Sramkova Zajacova Z., Gopfert E., Kumprechtova D. The effects of live yeast Saccharomyces cerevisiae on postweaning diarrhea, immune response, and growth performance in weaned piglets. J Anim Sci. 2014;92(2):767–774. doi: 10.2527/jas.2013-6793. [DOI] [PubMed] [Google Scholar]
- Trevisi P., Colombo M., Priori D., Fontanesi L., Galimberti G., Calo G. Comparison of three patterns of feed supplementation with live Saccharomyces cerevisiae yeast on postweaning diarrhea, health status, and blood metabolic profile of susceptible weaning pigs orally challenged with Escherichia coli F4ac. J Anim Sci. 2015;93(5):2225–2233. doi: 10.2527/jas.2014-8539. [DOI] [PubMed] [Google Scholar]
- Trevisi P., Latorre R., Priori D., Luise D., Archetti I., Mazzoni M. Effect of feed supplementation with live yeast on the intestinal transcriptome profile of weaning pigs orally challenged with Escherichia coli F4. Animal. 2017;11(1):33–44. doi: 10.1017/S1751731116001178. [DOI] [PubMed] [Google Scholar]
- Vieira D.A.P., Cabral L., Noronha M.F., Júnior G.V.L., Sant'Ana A.S. Microbiota of eggs revealed by 16S rRNA-based sequencing: from raw materials produced by different suppliers to chilled pasteurized liquid products. Food Contr. 2019;96:194–204. [Google Scholar]
- Wang Y., Qian P.Y. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PloS One. 2009;4(10):e7401. doi: 10.1371/journal.pone.0007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T., Simko V.R. 2017. Package "corrplot": visualization of a correlation matrix (version 0.84) [Google Scholar]
- Zanello G., Meurens F., Berri M., Salmon H. Saccharomyces boulardii effects on gastrointestinal diseases. Curr Issues Mol Biol. 2009;11(1):47–58. [PubMed] [Google Scholar]