Abstract
Dietary fiber is a critical nutrient in sow diet and has attracted interest of animal nutritionists for many years. In addition to increase sows’ satiety, dietary fiber has been found to involve in the regulation of multiple biological functions in the sow production. The interaction of dietary fiber and gut microbes can produce bioactive metabolites, which are of great significance to sows' metabolism and reproductive performance. This article reviewed the interaction between dietary fiber and gut microbes in regulating sows' gut microbial diversity, intestinal immune system, lactation, and production performance, with the aim to provide a new strategy for the use of dietary fiber in sow diets.
Keywords: Dietary fiber, Gut microbe, Reproductive performance, Sow
1. Introduction
During gestation, sows are restrictively fed to maintain normal reproductive performance. However, excessive feeding restrictions makes it difficult for sows to produce satiety and sows show abnormal behaviors such as vacuum chewing, frequent rising and lying during pregnancy, which leads to a negative impact on their reproductive performance (Sekiguchi and Koketsu, 2004). In addition, the feed intake of sows during pregnancy is closely related to their voluntary feed intake during lactation. Excessive feeding restriction during pregnancy will result in a decrease in sows' voluntary feed intake during lactation (Matte et al., 1994; Sun et al., 2014, 2015). Dietary fiber supplementation is an efficient way to maintain proper satiety for sows. Dietary fiber is not easily digested by digestive enzymes in the gastrointestinal tract, thus avoiding the sow from taking in excessive energy (Huang et al., 2020).
Interestingly, dietary fiber not only increases the satiety, but also improves reproductive performance and lactation performance of sows. Previous studies have found that adding dietary fiber to sow diets during pregnancy increased the total litter birth, weaning litter weights, and milk fat content in colostrum (Che et al., 2011; Loisel et al., 2013; Matte et al., 1994; Veum et al., 2009). Moreover, sows fed with dietary fiber during multiple reproductive cycles have better reproductive and lactation performances (Che et al., 2011; Matte et al., 1994; Veum et al., 2009). The physiological metabolism of sows undergoes complex changes during pregnancy. As a result, the stability of the intestinal flora structure is fragile and easily broken (Newbern and Freemark, 2011). The destruction of intestinal flora stability results in intestinal epithelium damage and intestinal inflammation. The gut microbiota is sensitive to the physical and chemical properties of specific dietary fibers. Many recent studies have reported that the addition of dietary fibers to diets during pregnancy effectively alters sow gut microbiota composition and diversity, and their interaction affects the sow's nutritional, physiological, and immune processes in a variety of ways (Cheng et al., 2018a; Jarrett and Ashworth, 2018; Wu et al., 2020; Xu et al., 2020a; Zhuo et al., 2020). The present review summarized the effects of dietary fiber on the composition and diversity of sows' gut microbes, as well as their interactions on the regulation of sows' gut health, and reproductive and lactation performances.
2. Changes of gut microbiota composition during different stages of pregnancy
During the reproductive cycle, the physiology and metabolism of the sow undergo complex changes. Gut microbes play a vital role in the function and health of their host (such as, nutrient absorption, metabolism, immune system, and resistance to pathogens) and are also affected by changes in the physiological state of their host. Therefore, the relationship between pregnancy and gut microbes is of particular concern. With the increase of gestational age, the alpha diversity of gut microbiota is also increased. Kong et al. (2016) and Ji et al. (2017) analyzed the ileal and colonic luminal microbiota composition of Huanjiang mini-pigs during pregnancy. From the first to third trimester of pregnancy, the dominant bacteria in the colon of Huanjiang mini-pigs are Firmicutes and Bacteroides, and the dominant genera include Lactobacillus, Treponema, Ruminococcus, Clostridium, and Prevotella (Kong et al., 2016). And, Firmicutes (account for 69.99% to 85.44% of the total reads) and Proteobacteria (5.82% to 15.17%) are dominant in the ileum (Ji et al., 2017). From the 45th day to the 75th day of pregnancy, the number of Firmicutes and Lactobacillus bacteria in the ileum is decreased, which is contrast to the number of Proteobacteria, Enterobacteriaceae, and Bacteroides. The significant increase of Proteobacteria (mainly pathogenic bacteria) is an indicator of ileal inflammation (Flint et al., 2008; Koren et al., 2012; Wu et al., 2011).
These changes of intestinal microbes could further regulate the metabolism of the host. Recently, Huang et al. (2019) analyzed the gut microbe characteristics of sows from late pregnancy (5 d before delivery) to postpartum (within 6 h after delivery). The results showed that the ratio of Bacteroidetes to Firmicutes and the relative abundance of Prevotella were significantly reduced, and the dominant genus Lactobacillus was significantly increased. Consistent with these changes, the predicted functional capacities of the gut microbe associated with glycan biosynthesis, amino acid metabolism, and the metabolism of cofactors and vitamins were significantly decreased. However, the abundance of the functional capacities related to carbohydrate and lipid metabolism were increased. Intriguingly, it has also been reported that the progression of pregnancy is related to several carbohydrate-degrading bacteria (Prevotella, Succinivibrio, Bacteroides, and Parabacteroides) (Ji et al., 2019). In addition, from pregnancy to parturition, changes in the composition of gut microbes also modified the intestinal levels of pro-inflammatory IL-6 and anti-inflammatory IL-10 (Cheng et al., 2018b). Thus, the interaction of gut microbes at different stages of pregnancy affects the metabolism of the host, which may increase nutrient metabolism and regulate sows’ lactation performance.
3. Dietary fiber regulates the abundance and diversity of sow gut microbes
Dietary fiber has an intimate relationship with the intestinal microbes (Makki et al., 2018). Generally, fiber is divided into soluble fiber and insoluble fiber. The amount of soluble and insoluble fiber varies in different feed ingredients (Jarrett and Ashworth, 2018). Soluble fiber (such as inulin) is hardly digested in the mouth, stomach, and small intestine. This fiber is transported to the hindgut as intact molecules and broken down by the coliform, which produce a small amount of energy and various bioactive substances (Makki et al., 2018). Insoluble fiber (such as cellulose) is difficult to be fermented by intestinal microbes, which could enhance intestinal health by promoting intestinal peristalsis (Capuano, 2017). Firmicutes and Actinobacteria are dominant responders in the gut to break down dietary fiber (Deehan et al., 2017). The digestibility for fiber could be different in the different breeds of sows. For example, Rongchang sows are more capable of digesting high-fiber feed compared with Landrace sows, which is related to the abundant Ruminococcaceae in the gut of Rongchang sows (Liu et al., 2019).
Difference in dietary fiber sources and gut microbe sensitivity partly determine the complex microbial system of the gut. In sows, Bacteroidetes and Firmicutes are the most dominant phyla in the intestine, accounting for about 73% of the total sequence of microorganisms (Niu et al., 2019). Moreover, Lactobacillus, Oscillibacter, and Treponema accounted for more than 49% of the total sequence (Niu et al., 2019). There is a close relationship between the gut microbiota composition and the apparent digestibility of crude fiber. For example, the abundance of some microorganisms in sow fecal samples was positively correlated with the apparent digestibility of crude fiber (such as Anaerofustis and Robinsoniella), neutral detergent fiber (such as Collinsella and Sutterella), and acid detergent fiber (such as Clostridium, Collinsella, Robinsoniella, and Turicibacter). Dietary fiber is the main source of energy for gut microbiota, which means that adding an appropriate amount of dietary fiber can increase the abundance of specific microorganisms (Sappok et al., 2015). In addition, dietary fiber also increases the proportion of beneficial bacteria and reduces the proportion of pathogenic bacteria in the gut (Guan et al., 2019; Jiang et al., 2019; Wu et al., 2020; Xu et al., 2020a).
Therefore, dietary fiber can improve the utilization of protein and fat by regulating the abundance of specific gut microbes. The strategy for dietary fiber to regulate protein fermentation in the intestine has been summarized before (Jha and Berrocoso, 2016). Briefly, dietary fiber effectively relieves the adverse effects of certain amino acid fermentation products (for example, ammonia, histamine) and decreases the harmful strains (such as, enterotoxigenic strains of Escherichia coli) on the intestinal tract. Furthermore, dietary fiber reduced the sow weight gain and fat accumulation that are caused by adding fat in diets (Zhou et al., 2017), which might be caused by the modification of gut microbial structure. These results indicate that dietary fiber can regulate the utilization of protein and fat by intestinal microorganisms, which further promotes sows’ intestinal health.
In summary, the addition of different levels and types of dietary fiber are related to the gut microbiota abundance and diversity. However, for the level of dietary fiber added to the diet, there is currently insufficient data to form a reference standard. Moreover, the research on the effect of dietary fiber types on the gut microbes of sows is still in the preliminary exploratory stage and a large amount of data is still needed to enrich this aspect.
4. Biological effects of dietary fiber fermentation by-products in the gut
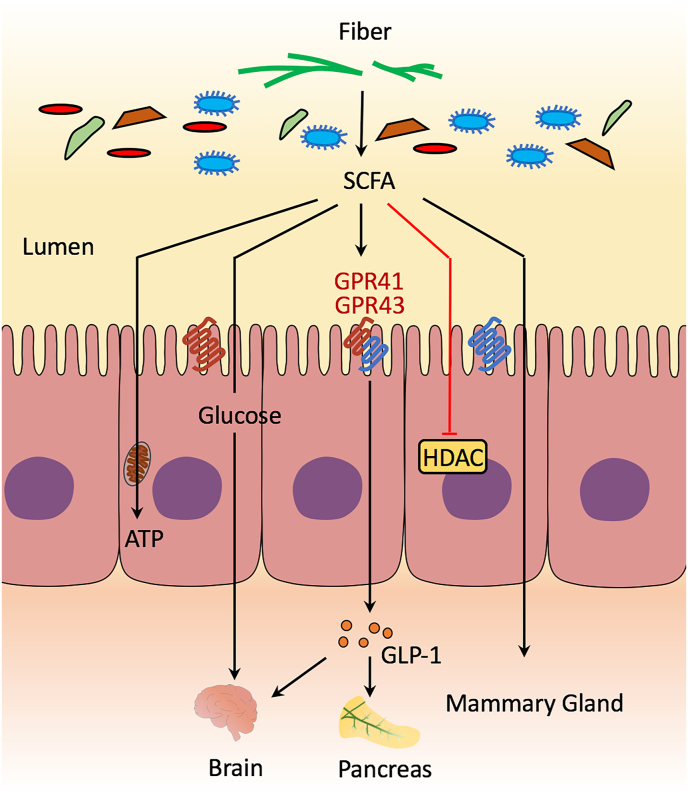
Dietary fiber is a critical energy source for cecal and colonic epithelial cells (Fig. 1). Under specific intestinal environmental conditions, dietary fiber is fermented and utilized by anaerobic bacteria and produces a variety of metabolites, such as short-chain fatty acids (SCFA), especially acetate, propionate and butyrate (Jarrett and Ashworth, 2018; Patil et al., 2020). They are an important substrate of gluconeogenesis and participate in the regulation of central metabolism. Short-chain fatty acids trigger host signals by inhibiting histone deacetylase (HDAC) or activating G protein-coupled receptors (such as GPR41 and GPR43), which are activated to regulate the release of glucagon-like peptide 1 (GLP-1) and neuropeptide Y which lead to satiety.
Fig. 1.
Mechanisms of signaling from the gut microbiota to the sow. SCFA = short-chain fatty acids; GPR = G protein-coupled receptors; HDAC = histone deacetylase; ATP = adenosine triphosphate; GLP-1 = glucagon-like peptide 1.
Short-chain fatty acids involved in the regulation of sow metabolism, immunity, and cell proliferation (Li et al., 2019; Wu et al., 2020; Xu et al., 2020a). Dietary supplementation of 3% purified fiber-mixture significantly increase the concentrations of butyrate and propionate in the feces of sows, which was related to the increase in the abundance of Eubacterium-hallii-group and Bacteroides in the gut (Wu et al., 2020). Different types of dietary fiber contribute to the variation of SCFA (Tokach et al., 2019). Short-chain fatty acids are transported to the peripheral circulation through the portal vein, thereby acting on the liver and peripheral tissues. They have been proposed to act as signal molecules to regulate different physiological activities of the host (Koh et al., 2016), such as regulating immunity and the expression of antioxidant enzymes, inflammatory and pro-inflammatory factors (Li et al., 2019; Shang et al., 2019; Guo et al., 2020; Xu et al., 2020a).
5. Embryo development and survival
To date, it has been widely accepted that dietary fiber supplementation improves the survival rate of sow embryos during pregnancy. Oocytes are very sensitive to maternal nutrient levels. Dietary nutrients intake changes the physiological levels of circulating hormones and metabolites, which ultimately affects the function of the ovaries (Ashworth et al., 2009). Administration of fiber promotes the maturation of follicles and oocytes in the sow ovary (Ferguson et al., 2007; Renteria-Flores et al., 2008; Weaver et al., 2013). The proportions of oocytes reaching Metaphase II were higher in the diet fiber group than in the control group (Weaver et al., 2013).
The modification of ovary function may be due to the dietary fiber fermentation products in the gut. Short-chain fatty acids could bind with G protein-coupled receptors GPR41 and GPR43 and activate their downstream target proteins in the gut. Adenosine 5′-monophosphate-activated protein kinase (AMPK)/mammalian target of rapamycin complex 1 (mTORC1) is a classic signaling pathway which largely regulates cellular biology and metabolism. This pathway also has an important significance for the activation and survival of primordial follicles (Zhuo et al., 2019). Short-chain fatty acids mediates multiple beneficial effects on the host metabolism through the activation of AMPK (Hu et al., 2010). AMPK is a negative regulator of mTORC1. Consistently, the phosphorylation level of mTORC1 and its downstream target S6 in the ovary decrease linearly with the increase of dietary fiber (Cao et al., 2019). In addition, the extracellular signal-regulated protein kinases 1/2 (ERK1/2) signal is also suppressed as the level of dietary fiber increases (Cao et al., 2019). Inhibition of ERK1/2 decrease the mTORC1 signaling, which participates in the activation of primordial follicles. These results indicate that increasing the level of dietary fiber intake can preserve a greater reserve of primordial follicles. Interestingly, these results show that the mTOR signaling pathway is inhibited. Whether this result is really beneficial to the reserve of primordial follicles still needs more evidence.
The other potential pathway for dietary fiber to regulate follicular maturation is through neuropeptide Y. This may be the result of the fermentation of dietary fiber by microorganisms in the intestine. However, to date, there is no direct evidence that microorganisms play a key role in this process. Neuropeptide Y has been shown to be involved not only in regulating food intake, but also related to the secretion of gonadotropin releasing hormone. A high-fiber diet changes the circulating concentrations of estradiol and luteinizing hormone, which are important for oocyte maturation (Ferguson et al., 2007). These results also explain why long-term feeding of dietary fiber increases the total number of births of sows, whereas short-term feeding does not. Because sows fed dietary fiber for a long time not only retain a larger reserve of primordial follicles, but also create a better physiological environment for follicle maturation than short-term feeding sows and gilts.
6. Milk composition
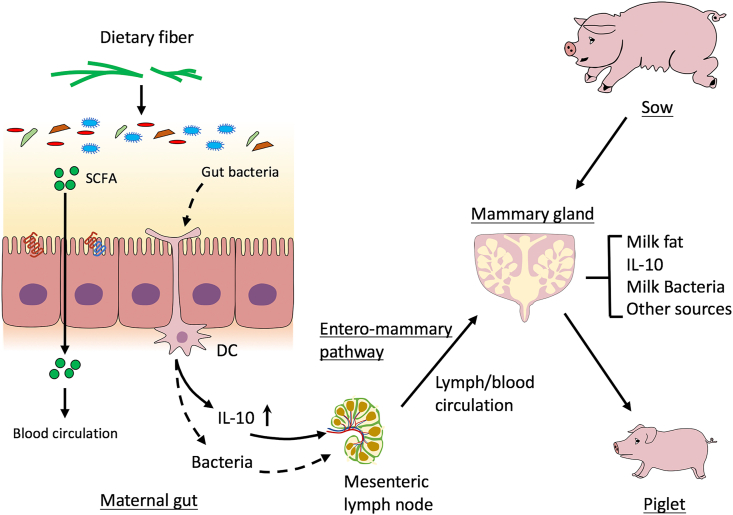
Milk is a unique nutrition source for newborns. During pregnancy and lactation, the mammary gland is one of the most active tissues in sows. In order to provide enough nutrients for newborns, a large amount of nutrients absorbed by the gut are transported to the mammary glands through the blood circulation to synthesize milk. Dietary nutrient level is an important factor affecting the synthesis and composition of sow milk. Although dietary fiber cannot be used directly by sows, its fermentation product is an important source of nutrients for sow milk synthesis. As we mentioned before, dietary fiber could be fermented by gut microorganisms to produce SCFA (Fig. 2). Mammary glands use SCFA as precursors for de novo synthesis of milk fat. Compared with the low-fiber group, the high-fiber group had increased milk fat content in the colostrum (Loisel et al., 2013; Feyera et al., 2019).
Fig. 2.
Dietary fiber and gut microbe interaction changes the composition of milk. Dietary fiber and gut microbe interaction produces a variety of fermentation products, including short-chain fatty acids (SCFA). Parts of the SCFA are transferred to the mammary gland through the blood circulation, then used as substrates for de novo synthesis of milk fat. Also, some immune factors (e.g. IL-10) from gut are transported to the mammary gland through the intestinal-lymphatic circulation system. Interestingly, there is a possibility that gut microbe can enter the lymph nodes through dendritic cells (DC) and then reach the mammary gland through the intestinal-lymphatic circulation system. These factors result in the changes of sow milk composition. Ultimately, the beneficial effects of the interaction between dietary fiber and gut microbes are passed from mother to offspring through lactation.
The effects of dietary fiber on milk immunoglobin (Ig) secretion is inconsistent. Loisel et al. (2013) and Feyera et al. (2019) found the concentrations of IgA and IgG in colostrum of sows in the high-fiber group was reduced. Whereas, Shang et al. (2019) reported the concentration of IgA in sow colostrum is significantly increased when sows were fed the high-fiber diet. These inconsistent results may be due to the different types of dietary fibers (Loisel et al., 2013; Shang et al., 2019). Recent studies also reported that dietary fiber increases the IL-10 concentration of sow colostrum and normal milk, which is critical to promote piglet intestinal health (Lv et al., 2018; Shang et al., 2019). There is no doubt that the utilization of dietary fiber by sows requires intestinal microbial fermentation, which leads to the changes of related physiological indicators. Unfortunately, these studies did not explore the role of gut microbes in this process.
In addition to nutrients, milk also contains a lot of microorganisms. It is proposed that a portion of the milk microorganisms may derive from the gut, but there is yet a definite conclusion (Moossavi and Azad, 2019; Rodríguez, 2014; Zhang et al., 2018). During the third trimester of pregnancy and lactation, there is an important outflow of intestinal immune cells to the mammary glands (Macpherson and Uhr, 2004; Newburg, 2005; Rescigno et al., 2001; Vazquez-Torres et al., 1999). Gut microbes might transfer into the lymph nodes with the help of dendritic cells and then reach the mammary gland through the intestinal-lymphatic circulation system (Fernández et al., 2013; Rodríguez, 2014). Certain bacteria in the gut have been found to co-exist in maternal peripheral blood and lymphocytes isolated from milk (Avershina et al., 2018; Perez et al., 2007). The main dominant bacteria in sow milk are Ruminococcaceae, Streptococcus, Lactobacillus, and Clostridiales, which largely exist in the gut of animals (Chen et al., 2018). Intriguingly, Ruminococcaceae and Lactobacillus are important bacterial genera for the dietary fiber fermentation in the gut (Cheng et al., 2018a). These studies provide some primary evidence that dietary fiber composition may change the microbial composition of milk.
7. Weaning-to-estrus interval
The weaning-to-estrus interval (WEI) is a crucial indicator to evaluate the reproductive performance of sows. During the reproductive cycle, a relatively fixed WEI (approximately 5 to 7 d) is essential for sows to ovulate efficiently and maintain the embryo survival rate during pregnancy. Estrus is a complex physiological activity and regulated by a variety of reproductive hormones, such as follicle stimulating hormone, luteinizing hormone, and estradiol. The bidirectional interaction between gut microbes and the secretion and metabolism of host sex hormones has been demonstrated (Markle et al., 2013; Melvin, 2016). In rats, transplanting Lactobacillus or faecal microbes from healthy rats into polycystic ovary syndrome rats increases the abundance of Lactobacillus in the gut and significantly increases the plasma concentrations of estradiol and estrone (Guo et al., 2016). In sows, the plasma reproductive hormone levels (oestradiol, follicle-stimulating hormone and luteinizing hormone) in sows with a short WEI (not more than 7 d) are significantly higher than those with a long WEI (>14 d) sows (Xu et al., 2020b). Importantly, in the gut of sows with short WEI, Prevotella and Bacteroides are lower at the genus level, whereas Firmicutes and Lentisphaerae are higher at the phylum level (Xu et al., 2020b). Prevotella and Bacteroides are important genera that degrade carbohydrates in the gut, which indicates dietary fibers might regulate WEI of sows. To our surprise, most studies have shown that increasing dietary fiber has no significant effect on the WEI of sows. Thus, the minor effects of microorganisms on the secretion of reproductive hormones might not efficiently modify the WEI of sows.
8. Effects on offspring piglets
The beneficial effects of the interaction between dietary fiber and gut microbes can be passed from the mother to the offspring. Dietary fiber is reported to enhance the reproduction performance of sows. Wu et al. (2020) fed gestational sows with a diet supplemented with 3% purified fiber-mixture, which significantly increased the number of live-born piglets. Veum et al. (2009) reported feeding sows a diet supplemented with wheat straw for a long period significantly increased the number of weaned piglets per litter, the weight of total litter births, and weaning litter. Furthermore, a short-term addition of dietary fiber in the late pregnancy also reduced the proportion of stillborn piglets and the total mortality of piglets during lactation (Feyera et al., 2017). The effects of dietary fiber on reproduction performance of sows might be partially dependent on the physical condition of sows and dietary fiber level. Che et al. (2011) fed sows with 3 fiber levels (10.8%, 15.8% and 20.8% neutral detergent fiber) for 2 reproductive cycles. In the first parity, the total litter births, litter births alive, and litter weight in the low-fiber group were significantly higher than those in other groups; likewise, the low-fiber group and medium-fiber group had greater litter weights at parturition and weaning. However, in the second parity, the total number of births, the total litter births and litter births alive, and litter weight in the medium-fiber group were significantly higher than those in other groups. Interestingly, the high-fiber group had the highest survival rate and heavier weight of piglets at weaning (after 22 d of lactation) compared with other groups. These results indicate that sows fed dietary fiber-added diets for a long time is more conducive to improving its production performance, and the addition level of fiber in the diet can be appropriately increased with the extension of feeding time.
Some recent studies reported that the changes of sow production performance caused by dietary fiber is related to the modification of gut microbes. Intestinal microbes may lead to the changes of intestinal antioxidant capacity (Zhou et al., 2017). Adding inulin to high-fat diets enhanced the activities of superoxide dismutase (T-SOD) and glutathione peroxidase (GSH-Px) and reduced the malondialdehyde (MDA) concentrations in the serum of sows (Wang et al., 2016). The production performance and antioxidant capacity of sows are positively correlated with Bacteroides of Bacteroidaceae, but negatively correlated with Phascolarctobacterium and Streptococcus (Wang et al., 2019a,b; Wang et al., 2018). It worth noting that T-SOD, GSH-Px, and MDA could be transferred from sows to piglets through milk (Chen et al., 2019a; Chen et al., 2019b; Chen et al., 2020; Wang et al., 2019a,b). Thus, there is a close relationship between dietary fiber, gut microbes, antioxidant abilities, and sow production performance.
9. Conclusion
The application value of dietary fiber in improving the satiety of sows has long been recognized. However, some recent reports suggested that dietary fiber has many other benefits in production of sow. With the reduction in the use of antibacterial drugs on a global scale, the application value of dietary fiber is particularly valued. Dietary supplementation of fiber can change the abundance and diversity of gut microbiota and thereby promote gut health and reduce the need for antibiotics. In addition, the application of dietary fiber to sow diets provides opportunities to improve sow production and reproduction in several aspects. Dietary fiber and gut microbe interaction affects the diversity and abundance of gut microbes, immune system, milk composition, embryonic development, and the production performance of sows. However, there are still many problems that need to be resolved: 1) The source of dietary fiber is rich and the composition is complex, which makes it difficult to determine a reference standard in production; 2) People's understanding of gut microbes mostly stays at the level of phylum and genus, which makes it difficult to isolate the key bacteria related to dietary fiber fermentation; 3) A large number of obligate anaerobic bacteria are present in the milk and passed to the piglets. Do these bacteria come from the gut of the sow? Therefore, although dietary fiber and gut microbes play an important role in improving sow performance, a lot of data are still needed to explain the regulation mechanism in the future.
Author contributions
Min Tian and Shihai Zhang initiated the idea, scope, and outline of this review paper. Min Tian, Shihai Zhang, Jiaming Chen, Jiaxin Liu, and Fang Chen studied and analyzed all of the publications cited in this paper and prepared the initial manuscript. Shihai Zhang and Wutai Guan made the final revision. All authors read and approved the final manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 31802067 and 31872364) and the Natural Science Foundation of Guangdong Province (No. 2018A030310201).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Wutai Guan, Email: wutaiguan1963@163.com.
Shihai Zhang, Email: zhangshihai@scau.edu.cn.
References
- Ashworth C.J., Toma L.M., Hunter M.G. Nutritional effects on oocyte and embryo development in mammals: implications for reproductive efficiency and environmental sustainability. Philos Trans R Soc Lond B Biol Sci. 2009;364:3351–3361. doi: 10.1098/rstb.2009.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avershina E., Angell I.L., Simpson M., Storrø O., Øien T., Johnsen R., Rudi K. Low maternal microbiota sharing across gut, breast milk and vagina, as revealed by 16S rRNA gene and reduced metagenomic sequencing. Genes. 2018;9:231. doi: 10.3390/genes9050231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M., Zhuo Y., Gong L.H., Tang L.C., Li Z.P., Li Y., Yang M., Xu S.Y., Li J., Che L.Q., Lin Y., Feng B., Fang Z.F., Wu D. Optimal dietary fiber intake to retain a greater ovarian follicle reserve for gilts. Animals. 2019;9:881. doi: 10.3390/ani9110881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit Rev Food Sci Nutr. 2017;57:3543–3564. doi: 10.1080/10408398.2016.1180501. 2017. [DOI] [PubMed] [Google Scholar]
- Che L., Feng D., Wu D., Fang Z., Lin Y., Yan T. Effect of dietary fibre on reproductive performance of sows during the first two parities. Reprod Domest Anim. 2011;46:1061–1066. doi: 10.1111/j.1439-0531.2011.01787.x. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhang F., Guan W., Song H., Tian M., Cheng L., Shi K., Song J., Chen F., Zhang S., Yang F., Ren C., Zhang Y. Increasing selenium supply for heat-stressed or actively cooled sows improves piglet preweaning survival, colostrum and milk composition, as well as maternal selenium, antioxidant status and immunoglobulin transfer. J Trace Elem Med Biol. 2019;52:89–99. doi: 10.1016/j.jtemb.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Chen J., Tian M., Guan W., Wen T., Yang F., Chen F., Zhang S., Song J., Ren C., Zhang Y., Song H. Increasing selenium supplementation to a moderately-reduced energy and protein diet improves antioxidant status and meat quality without affecting growth performance in finishing pigs. J Trace Elem Med Biol. 2019;56:38–45. doi: 10.1016/j.jtemb.2019.07.004. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhang Y., You J., Song H., Zhang Y., Lv Y., Qiao H., Tian M., Chen F., Zhang S., Guan W. The effects of dietary supplementation of Saccharomyces cerevisiae fermentation product during late pregnancy and lactation on sow productivity, colostrum and milk composition, and antioxidant status of sows in a subtropical climate. Front Vet Sci. 2020;7:71. doi: 10.3389/fvets.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Mi J., Lv N., Gao J., Cheng J., Wu R., Ma J., Lan T., Liao X. Lactation stage-dependency of the sow milk microbiota. Front Microbiol. 2018;9:945. doi: 10.3389/fmicb.2018.00945. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Wei H., Xu C., Xie X., Jiang S., Peng J. Maternal soluble fiber diet during pregnancy changes the intestinal microbiota, improves growth performance, and reduces intestinal permeability in piglets. Appl Environ Microbiol. 2018;84 doi: 10.1128/AEM.01047-18. e01047-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Wei H., Yu H., Xu C., Jiang S., Peng J. Metabolic syndrome during perinatal period in sows and the link with gut microbiota and metabolites. Front Microbiol. 2018;9:1989. doi: 10.3389/fmicb.2018.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan E.C., Duar R.M., Armet A.M., Perez-Muñoz M.E., Jin M., Walter J. Modulation of the gastrointestinal microbiome with nondigestible fermentable carbohydrates to improve human health. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.bad-0019-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E.M., Slevin J., Hunter M.G., Edwards S.A., Ashworth C.J. Beneficial effects of a high fibre diet on oocyte maturity and embryo survival in gilts. Reproduction. 2007;133:433–439. doi: 10.1530/REP-06-0018. [DOI] [PubMed] [Google Scholar]
- Fernández L., Langa S., Martín V., Maldonado A., Jiménez E., Martín R., Rodríguez J.M. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res. 2013;69:1–10. doi: 10.1016/j.phrs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Feyera T., Højgaard C.K., Vinther J., Bruun T.S., Theil P.K. Dietary supplement rich in fiber fed to late gestating sows during transition reduces rate of stillborn piglets. J Anim Sci. 2017;95:5430–5438. doi: 10.2527/jas2017.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyera T., Zhou P., Nuntapaitoon M., Sørensen K.U., Krogh U., Bruun T.S., Purup S., Jørgensen H., Poulsen H.D., Theil P.K. Mammary metabolism and colostrogenesis in sows during late gestation and the colostral period. J Anim Sci. 2019;97:231–245. doi: 10.1093/jas/sky395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint H.J., Bayer E.A., Rincon M.T., Lamed R., White B.A. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- Guan G., Ding S., Yin Y., Duraipandiyan V., Al-Dhabi N.A., Liu G. Macleaya cordata extract alleviated oxidative stress and altered innate immune response in mice challenged with enterotoxigenic Escherichia coli. Sci China Life Sci. 2019;62:1019–1027. doi: 10.1007/s11427-018-9494-6. [DOI] [PubMed] [Google Scholar]
- Guo Q., Li F., Duan Y., Wen C., Wang W., Zhang L., Huang R., Yin Y. Oxidative stress, nutritional antioxidants and beyond. Sci China Life Sci. 2020;63:866–874. doi: 10.1007/s11427-019-9591-5. [DOI] [PubMed] [Google Scholar]
- Guo Y., Qi Y., Yang X., Zhao L., Wen S., Liu Y., Tang L. Association between polycystic ovary syndrome and gut microbiota. PloS One. 2016;11 doi: 10.1371/journal.pone.0153196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., Chen G., Xu H., Ge R., Lin J. Activation of the AMP activated protein kinase by short-chain fatty acids is the main mechanism underlying the beneficial effect of a high fiber diet on the metabolic syndrome. Med Hypotheses. 2010;74:123–126. doi: 10.1016/j.mehy.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Huang S., Wei J., Yu H., Hao X., Zuo J., Tan C., Deng J. Effects of dietary fiber sources during gestation on stress status, abnormal behaviors and reproductive performance of sows. Animals. 2020;10:141. doi: 10.3390/ani10010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Gao J., Zhao Y., He M., Ke S., Wu J., Zhou Y., Fu H., Yang H., Chen C., Huang L. Dramatic remodeling of the gut microbiome around parturition and its relationship with host serum metabolic changes in sows. Front Microbiol. 2019;10:2123. doi: 10.3389/fmicb.2019.02123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett S., Ashworth C.J. The role of dietary fibre in pig production, with a particular emphasis on reproduction. J Anim Sci Biotechnol. 2018;9:59. doi: 10.1186/s40104-018-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Berrocoso J.F.D. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim Feed Sci Technol. 2016;212:18–26. [Google Scholar]
- Ji Y., Kong X., Li H., Zhu Q., Guo Q., Yin Y. Effects of dietary nutrient levels on microbial community composition and diversity in the ileal contents of pregnant Huanjiang mini-pigs. PloS One. 2017;12 doi: 10.1371/journal.pone.0172086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y., Li H., Xie P., Li Z., Li H., Yin Y., Blachier F., Kong X. Stages of pregnancy and weaning influence the gut microbiota diversity and function in sows. J Appl Microbiol. 2019;127:867–879. doi: 10.1111/jam.14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Lu N., Xue Y., Liu S., Lei H., Tu W., Lu Y., Xia D. Crude fiber modulates the fecal microbiome and steroid hormones in pregnant Meishan sows. Gen Comp Endocrinol. 2019;277:141–147. doi: 10.1016/j.ygcen.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Kong X., Ji Y., Li H., Zhu Q., Blachier F., Geng M., Chen W., Yin Y. Colonic luminal microbiota and bacterial metabolite composition in pregnant Huanjiang mini-pigs: effects of food composition at different times of pregnancy. Sci Rep. 2016;6:37224. doi: 10.1038/srep37224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O., Goodrich J.K., Cullender T.C., Spor A., Laitinen K., Bäckhed H.K., Gonzalez A., Werner J.J., Angenent L.T., Knight R., Backhed F., Isolauriet E., Salminen S., Ley R.E. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–480. doi: 10.1016/j.cell.2012.07.008. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu H., Zhang L., Yang Y., Lin Y., Zhuo Y., Fang Z., Che L., Feng B., Xu S., Li J., Wu D. Maternal dietary fiber composition during gestation induces changes in offspring antioxidative capacity, inflammatory response, and gut microbiota in a sow model. Int J Mol Sci. 2019;21:31. doi: 10.3390/ijms21010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Hou C., Li N., Zhang X., Zhang G., Yang F., Zeng X., Liu Z., Qiao S. Microbial and metabolic alterations in gut microbiota of sows during pregnancy and lactation. Faseb J. 2019;33:4490–4501. doi: 10.1096/fj.201801221RR. [DOI] [PubMed] [Google Scholar]
- Loisel F., Farmer C., Ramaekers P., Quesnel H. Effects of high fiber intake during late pregnancy on sow physiology, colostrum production, and piglet performance. J Anim Sci. 2013;91:5269–5279. doi: 10.2527/jas.2013-6526. [DOI] [PubMed] [Google Scholar]
- Lv D., Xiong X., Yang H., Wang M., He Y., Liu Y., Yin Y. Effect of dietary soy oil, glucose, and glutamine on growth performance, amino acid profile, blood profile, immunity, and antioxidant capacity in weaned piglets. Sci China Life Sci. 2018;61:1233–1242. doi: 10.1007/s11427-018-9301-y. [DOI] [PubMed] [Google Scholar]
- Macpherson A.J., Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- Makki K., Deehan E.C., Walter J., Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Markle J.G., Frank D.N., Mortin-Toth S., Robertson C.E., Feazel L.M., Rolle-Kampczyk U., von Bergen M., McCoy K.D., Macpherson A.J., Danska J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- Matte J.J., Robert S., Girard C.L., Farmer C., Martineau G.P. Effect of bulky diets based on wheat bran or oat hulls on reproductive performance of sows during their first two parities. J Anim Sci. 1994;72:1754–1760. doi: 10.2527/1994.7271754x. [DOI] [PubMed] [Google Scholar]
- Melvin R. 2016. The effects of exercise and estrogen on gut microbiota in female mice. [DOI] [Google Scholar]
- Moossavi S., Azad M.B. Origins of human milk microbiota: new evidence and arising questions. Gut Microb. 2019;12:1667722. doi: 10.1080/19490976.2019.1667722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern D., Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes. 2011;18:409–416. doi: 10.1097/MED.0b013e32834c800d. [DOI] [PubMed] [Google Scholar]
- Newburg D.S. Innate immunity and human milk. J Nutr. 2005;135:1308–1312. doi: 10.1093/jn/135.5.1308. [DOI] [PubMed] [Google Scholar]
- Niu Q., Li P., Hao S., Kim S.W., Du T., Hua J., Huang R. Characteristics of gut microbiota in sows and their relationship with apparent nutrient digestibility. Int J Mol Sci. 2019;20:870. doi: 10.3390/ijms20040870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil Y., Gooneratne R., Ju X. Interactions between host and gut microbiota in domestic pigs: a review. Gut Microb. 2020;11:310–334. doi: 10.1080/19490976.2019.1690363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez P.F., Doré J., Leclerc M., Levenez F., Benyacoub J., Serrant P., Segura-Roggero I., Schiffrin E.J., Donnet-Hughes A. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007;119:e724–e732. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- Renteria-Flores J.A., Johnston L.J., Shurson G.C., Moser R.L., Webel S.K. Effect of soluble and insoluble dietary fiber on embryo survival and sow performance. J Anim Sci. 2008;86:2576–2584. doi: 10.2527/jas.2007-0376. [DOI] [PubMed] [Google Scholar]
- Rescigno M., Urbano M., Valzasina B., Francolini M., Rotta G., Bonasio R., Granucci F., Kraehenbuhl J.P., Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Rodríguez J.M. The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv Nutr. 2014;5:779–784. doi: 10.3945/an.114.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappok M.A., Peréz Gutiérrez O., Smidt H., Pellikaan W.F., Verstegen M.W., Bosch G., Hendriks W.H. Adaptation of faecal microbiota in sows after diet changes and consequences for in vitro fermentation capacity. Animal. 2015;9:1453–1464. doi: 10.1017/S1751731115000865. [DOI] [PubMed] [Google Scholar]
- Sekiguchi T., Koketsu Y. Behavior and reproductive performance by stalled breeding females on a commercial swine farm. J Anim Sci. 2004;82:1482–1487. doi: 10.2527/2004.8251482x. [DOI] [PubMed] [Google Scholar]
- Shang Q., Liu H., Liu S., He T., Piao X. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets1. J Anim Sci. 2019;97:4922–4933. doi: 10.1093/jas/skz278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Tan C., Wei H., Zou Y., Long G., Ao J., Xue H., Jiang S., Peng J. Effects of different amounts of konjac flour inclusion in gestation diets on physio-chemical properties of diets, postprandial satiety in pregnant sows, lactation feed intake of sows and piglet performance. Anim Reprod Sci. 2015;152:55–64. doi: 10.1016/j.anireprosci.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Sun H., Zhou Y., Tan C., Zheng L., Peng J., Jiang S. Effects of konjac flour inclusion in gestation diets on the nutrient digestibility, lactation feed intake and reproductive performance of sows. Animal. 2014;8:1089–1094. doi: 10.1017/S175173111400113X. [DOI] [PubMed] [Google Scholar]
- Tokach M.D., Menegat M.B., Gourley K.M., Goodband R.D. Review: nutrient requirements of the modern high-producing lactating sow, with an emphasis on amino acid requirements. Animal. 2019;13:2967–2977. doi: 10.1017/S1751731119001253. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A., Jones-Carson J., Bäumler A.J., Falkow S., Valdivia R., Brown W., Le M., Berggren R., Parks W.T., Fang F.C. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–808. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- Veum T.L., Crenshaw J.D., Crenshaw T.D., Cromwell G.L., Easter R.A., Ewan R.C., Nelssen J.L., Miller E.R., Pettigrew J.E., Ellersieck M.R. The addition of ground wheat straw as a fiber source in the gestation diet of sows and the effect on sow and litter performance for three successive parities. J Anim Sci. 2009;87:1003–1012. doi: 10.2527/jas.2008-1119. [DOI] [PubMed] [Google Scholar]
- Wang H., Hu C., Cheng C., Cui J., Ji Y., Hao X., Li Q., Ren W., Deng B., Yin Y., Deng J., Tan C. Unraveling the association of fecal microbiota and oxidative stress with stillbirth rate of sows. Theriogenology. 2019;136:131–137. doi: 10.1016/j.theriogenology.2019.06.028. [DOI] [PubMed] [Google Scholar]
- Wang H., Ji Y., Yin C., Deng M., Tang T., Deng B., Ren W., Deng J., Yin Y., Tan C. Differential analysis of gut microbiota correlated with oxidative stress in sows with high or low litter performance during lactation. Front Microbiol. 2018;9:1665. doi: 10.3389/fmicb.2018.01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhu F., Yang H., Li J., Li Y., Ding X., Xiong X., Ji F., Zhou H., Yin Y. Epidermal growth factor improves intestinal morphology by stimulating proliferation and differentiation of enterocytes and mTOR signaling pathway in weaning piglets. Sci China Life Sci. 2019 doi: 10.1007/s11427-018-9519-6. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhou P., Liu H., Li S., Zhao Y., Deng K., Cao D., Che L., Fang Z., Xu S., Li Y., Feng B., Li J., Wu D. Effects of inulin supplementation in low- or high-fat diets on reproductive performance of sows and antioxidant defence capacity in sows and offspring. Reprod Domest Anim. 2016;51:492–500. doi: 10.1111/rda.12707. [DOI] [PubMed] [Google Scholar]
- Weaver A.C., Kelly J.M., Kind K.L., Gatford K.L., Kennaway D.J., Herde P.J., van Wettere W.H. Oocyte maturation and embryo survival in nulliparous female pigs (gilts) is improved by feeding a lupin-based high-fibre diet. Reprod Fertil Dev. 2013;25:1216–1223. doi: 10.1071/RD12329. [DOI] [PubMed] [Google Scholar]
- Wu G., Chen J., Hoffmann C., Bittinger K., Chen Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R., Walters W.A., Knight R., Sinha R., Gilroy E., Gupta K., Baldassano R., Nessel L., Li H., Bushman F.D., Lewis J.D. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Xiong Y., Zhong M., Li Y., Wan H., Wu D., Liu Q. Effects of purified fibre-mixture supplementation of gestation diet on gut microbiota, immunity and reproductive performance of sows. J Anim Physiol Anim Nutr. 2020;104:1144–1154. doi: 10.1111/jpn.13287. [DOI] [PubMed] [Google Scholar]
- Xu C., Cheng C., Zhang X., Peng J. Inclusion of soluble fiber in the gestation diet changes the gut microbiota, affects plasma propionate and odd-chain fatty acids levels, and improves insulin sensitivity in sows. Int J Mol Sci. 2020;21:635. doi: 10.3390/ijms21020635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Bai M., Liu H., Duan Y., Zhou X., Wu X., Liao P., Li T., Yin Y. Gut microbiota and blood metabolomics in weaning multiparous sows: associations with oestrous. J Anim Physiol Anim Nutr. 2020;104:1155–1168. doi: 10.1111/jpn.13296. [DOI] [PubMed] [Google Scholar]
- Zhang S., Chen F., Zhang Y., Lv Y., Heng J., Min T., Li L., Guan W. Recent progress of porcine milk components and mammary gland function. J Anim Sci Biotechnol. 2018;9:77. doi: 10.1186/s40104-018-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Zhao Y., Zhang P., Li Y., Gui T., Wang J., Jin C., Che L., Li J., Lin Y., Xu S., Feng B., Fang Z., Wu D. Microbial mechanistic insight into the role of inulin in improving maternal health in a pregnant sow model. Front Microbiol. 2017;8:2242. doi: 10.3389/fmicb.2017.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo Y., Feng B., Xuan Y., Che L., Fang Z., Lin Y., Xu S., Li J., Feng B., Wu D. Inclusion of purified dietary fiber during gestation improved the reproductive performance of sows. J Anim Sci Biotechnol. 2020;11:47. doi: 10.1186/s40104-020-00450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo Y., Hua L., Feng B., Jiang X., Li J., Jiang D., Huang X., Zhu Y., Li Z., Yan L., Jin C., Che L., Fang Z., Lin Y., Xu S., Li J., Wu D. Fibroblast growth factor 21 coordinates adiponectin to mediate the beneficial effects of low-protein diet on primordial follicle reserve. EBioMedicine. 2019;41:623–635. doi: 10.1016/j.ebiom.2019.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]