Abstract
Gut microbiota is generally recognized to play a crucial role in maintaining host health and metabolism. The correlation among gut microbiota, glycolipid metabolism, and metabolic diseases has been well reviewed in humans. However, the interplay between gut microbiota and host metabolism in swine remains incompletely understood. Given the limitation in conducting human experiments and the high similarity between swine and humans in terms of anatomy, physiology, polyphagy, habits, and metabolism and in terms of the composition of gut microbiota, there is a pressing need to summarize the knowledge gained regarding swine gut microbiota, its interplay with host metabolism, and the underlying mechanisms. This review aimed to outline the bidirectional regulation between gut microbiota and nutrient metabolism in swine and to emphasize the action mechanisms underlying the complex microbiome–host crosstalk via the gut microbiota–gut–brain axis. Moreover, it highlights the new advances in knowledge of the diurnal rhythmicity of gut microbiota. A better understanding of these aspects can not only shed light on healthy and efficient pork production but also promote our knowledge on the associations between gut microbiota and the microbiome–host crosstalk mechanism. More importantly, knowledge on microbiota, host health and metabolism facilitates the development of a precise intervention therapy targeting the gut microbiota.
Keywords: Gut microbiota, Host nutrient metabolism, Microbiota–gut–brain axis, Microbiota diurnal rhythmicity
1. Introduction
The mammalian gastrointestinal tract hosts trillions of microorganisms. These microorganisms together with their genes are known as the microbiome, which encodes 3.3 million genes and carries genetic messages that are 140 times greater in number than those carried by the human genome (Qin et al., 2010). Accumulating evidence had revealed that gut microbiota plays a pivotal role in maintaining the host physiological homeostasis, in promoting immune system development, and in regulating host metabolism (Kim et al., 2012; Maynard et al., 2012; Sommer and Bäckhed, 2013; Tremaroli and Backhed, 2012). In recent decades, the maturity of high-throughput sequencing and multi-omics techniques has greatly expanded our knowledge of microbial communities in a complex environment and has made it possible to clarify the interactions between gut microbiota and host metabolism homeostasis. The gut microbiota regulates the metabolism of glucolipids (Yin et al., 2018), amino acids (Kawase et al., 2017; Zhang et al., 2020), vitamins (Caesar, 2019), bile acids (Ramírez-Pérez et al., 2018), and other nutrients (Pathak et al., 2020) through the microbiota–gut–brain axis (Carabotti et al., 2015). Conversely, the dysbiosis of gut microbiota leads to metabolic diseases, such as obesity, cardiovascular disease (hyperlipidemia, atherosclerosis and hypertension), insulin resistance, type 2 diabetes, gout, and hyperuricemia (Fan et al., 2018; Liu et al., 2020; Mandal and Mount, 2015; Mendes-Soares et al., 2019; Novoseletskyi, 2013). Fortunately, the homeostasis of gut microbiota can be manipulated by diet, faecal microbiota transplantation, and other approaches (Fuentes et al., 2017; Kelly et al., 2014; Liu et al., 2017; Lee et al., 2019; Thomas and Versalovic, 2010). Further, a well-designed gut microbiota community can be used to improve the human health condition and to promote production efficiency in animal husbandry. Several studies have reviewed the interaction between gut microbiota and human metabolic ability (Cani, 2019b; Karlsson et al., 2013; Rusch and Dave, 2018; Salvatore et al., 2017; Sohail et al., 2017; Vajro et al., 2013; Yolanda et al., 2010). However, most of these works focused on nutrient metabolism and host metabolic diseases in the context of maintaining health. Moreover, most of the trials were carried out on mice or rats. Given the high similarity between swine and humans in terms of anatomy, physiology, polyphagy, habits, and metabolism, swine is possibly one of the most appropriate models that can be used to study nutrient metabolism and health in humans (Olayanju et al., 2019; Walters and Prather, 2013). Research had revealed that the interspecies transplantation of gut microbiota to pig gut produced a donor-like stable microbial community and the microbial ecosystem's succession (Pang et al., 2007). Besides, given that swine is one of the most important food animals worldwide, studies on swine intestinal microbiota and host metabolism will greatly promote the development of swine production.
Although mounting evidence indicated that multilateral crosstalks occur via the gut microbiota–gut–brain axis (Collins et al., 2014; De Vadder et al., 2018; Frost et al., 2014; Mayer et al., 2014), the mechanisms underlying the functions of the gut microbiota on host metabolism remain incompletely understood. Of note, new evidence has indicated that the composition and function of gut microbiota display diurnal rhythmicity in a given day (Leone et al., 2015; Liang et al., 2015; Thaiss et al., 2014, 2016; Zarrinpar et al., 2014), implying that time factor should be taken into consideration in studying the gut microbiota. Thus, this review aimed to elucidate the interplay between gut microbiota and host nutrient metabolism in swine. Additionally, the underlying action mechanisms of gut microbiota via the microbiota–gut–brain axis were outlined. Finally, we systematically reviewed studies on microbial diurnal rhythmicity. A deeper understanding of the interplay between gut microbiota and host metabolism can facilitate progress in the accurate regulation of gut microbiota in order to promote human health and production efficiency in animal husbandry.
2. Swine gut microbiota and their influencing factors
Despite the similarity between humans and swine in terms of anatomy and physiology, considerable interspecies variations in the composition and functions of their gut microbiota exist. Xiao et al. had profiled the gut microbiota of 287 swine fecal samples from France, Denmark, and China. According to the research, a total of 7.7 million non-redundant genes representing 719 metagenomic species were identified. Ninety-six percent of the functional pathways detected in humans are present in the catalogue of pig gut microbiota community, implying the possibility that the human gut microbiota may be investigated using pig models. However, only 78% of the pathways identified in the pig gut metagenome are present in humans, which suggested a more powerful and specific functionality of pig gut microbiota. Of note, the meta-analysis conducted by Holman et al. (2017) revealed that the core genera Prevotella, Clostridium, Alloprevotella, Ruminococcus, and the RC9 were detected in 99% of the faecal samples obtained from commercial swine worldwide. The symbiosis of these core microbiome plays an important role in regulating nutrient metabolism and immunity of the host, ultimately contributing to the health and production performance of pigs (Geng et al., 2018; Kang et al., 2017). To date, a myriad of studies have reported that gut microbiota has a high correlation with the immunity, intestinal development, and production performance of swine (Arnal et al., 2014; McCormack et al., 2017; Saavedra and Dattilo, 2012; Wan et al., 2019; Yang et al., 2018a, 2018b). Specifically, Mach et al. (2015) found that Prevotella was positively correlated with the luminal secretory immunoglobulin A concentrations. Moreover, the gut microbiota of newborn piglets with intrauterine growth restriction demonstrated a lower diversity and a different taxonomic profile; consequently, the piglets displayed a limited production performance (Zhang et al., 2019). Pigs with an extreme feed conversion ratio differed both in terms of composition and abundance of gut microbiota. These differential microbes were mainly enriched in the metabolic pathways of dietary polysaccharides and proteins (Quan et al., 2018; Tan et al., 2018). Also, even when exposed to the same condition, littermates with different birth weights have distinct microbiota communities (Lee et al., 2019).
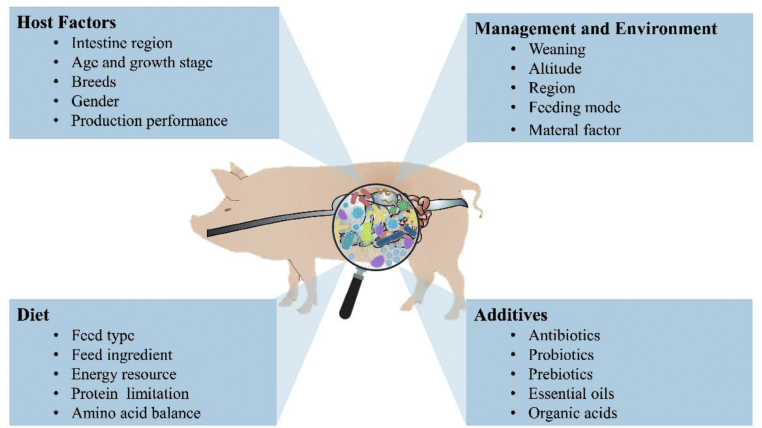
Interestingly, the distribution and composition of gut microbiota might co-evolve with the host and may be subtly influenced by various factors (Fig. 1). It has been well established that the intestinal tract of pigs is characterized by distinct compartmentalization due to differences in anatomical structures, environments, physiological functions, and gut microbiota communities. For instance, the microorganisms in the small intestine (duodenum, jejunum, and ileum) are mainly involved in the digestion and absorption of nutrients (e.g. most of the proteins, lipids, amino acids, monosaccharides, and some oligosaccharides). By contrast, the microorganisms in the large intestine (colon and cecum) are mainly involved in the degradation of nutrients that are indigestible in the small intestine, for instance insoluble cellulose (resistant starch and lignin). The number of microorganisms expands geometrically from the small intestine to the large intestine, and the microbial composition of the ileum significantly differs from that of the cecum and colon. Specifically, the genera Escherichia-Shigella (23.1%), Terrisporobacter (17.9%), Romboutsia (13.7%) and Clostridium sensustricto 1 (12.9%) are more abundant in the ileum than in the cecum and colon. Alloprevotella (7.2%), Lactobacillus (5.0%), and the Prevotellaceae NK3B31 group (4.4%) are the 3 most prevalent genera in the cecum. Streptococcus (10.4%), Lactobacillus (8.8%), and Clostridium (8.0%) are the 3 most prevalent genera in the colon (Quan et al., 2018).
Fig. 1.
The gut microbiota communities of swine are subtle to several endogenous and exogenous factors: host factors, diet, feeding management and environment, and additives.
Age is one of the determinant factors affecting the succession of gut microbiota in neonatal piglets (Bian et al., 2016). For instance, bacterial abundance and diversity increase with age (Wang et al., 2019b). Although controversial, the gut of neonate piglets before birth is generally believed to be sterile and then it becomes immediately occupied by the microbiota encountered in the environments (Funkhouser and Bordenstein, 2013). Moreover, the faecal bacterial composition of swine varies significantly in different growth stages (Kim et al., 2015b). Being one of the important landmarks of the production stage, the weaning transition was found to significantly alter the swine gut microbiota composition and the pathways concerning nutrient metabolism (Meng et al., 2020).
In addition, pigs of different breeds had a distinct gut microbiota composition (Cheng et al., 2017). Guevarra et al. (2015) found that white-coloured breeds, such as Landrace, displayed a higher abundance of cellulolytic bacteria, which possibly indicates a higher fibre digestion efficiency. More specifically, a distinct difference in the composition of gut microbiota was observed amongst Rongchang pig, Tibetan pigs, and Large White pigs (Diao et al., 2016). Further, Crespo-Piazuelo et al. (2019) had revealed that the relative abundance of Akkermansia, CF231, Phascolarctobacterium, Prevotella, SMB53 and Streptococcus was associated with several single-nucleotide polymorphisms.
Gender is also one of the determining factors that shape the gut microbiota composition. Veillonellaceae, Roseburia, Bulleidia and Escherichia had a higher abundance in boars, and Treponema and Bacteroides were over-represented in gilts, indicating that castration significantly shifted the faecal microbiota composition of the boars towards that of gilts (He et al., 2019). Moreover, the biogeographic and geographic distributions of intestinal microbiota vary. For instance, differences in the composition and abundance of gut microorganisms attached to the digesta or intestinal mucosa were observed even when the investigated hosts were found in the same biogeographical area (Adhikari et al., 2019). The gut microbiota of swine was also significantly affected by the geographical differences in the distribution of the phyla Actinobacteria, Verrucomicrobia, Firmicutes, and Fibrobacteres (Kim et al., 2015a).
Diet is one of the most important determinants that shape the profile of gut microbiota communities. Dietary changes including changes in diet composition (Liu et al., 2018; Yu et al., 2019; Zhou et al., 2016), nutritional levels (Lin et al., 2019), energy resources (Papadomichelakis et al., 2012), and diet types (Huang et al., 2020) had been widely proven to shape the gut microbiota communities. Antibiotics (Schweer et al., 2017), prebiotics (Tian et al., 2019; Zhu et al., 2016), probiotics (Cao et al., 2016; Lv et al., 2015), organic acids (Wang et al., 2019a; Yu et al., 2017), essential oils (Li et al., 2018), lactoferrin (Hu et al., 2020), and milk-replacer may also regulate the gut microbiota communities and thus exert beneficial effects on piglets. In addition, other intervention approaches (e.g. faecal microbial transplantation and early feeding) affect the composition and function of gut microbiota (Cheng et al., 2018; Lin et al., 2018; Shi et al., 2018; Wan et al., 2019; Xu et al., 2020).
3. Bidirectional regulation between gut microbiota and swine nutrient metabolism
3.1. Carbohydrate metabolism
Carbohydrates, which are mainly contained in cereals (e.g. corn, wheat and oat), are one of the most important nutrients in feed formulations for swine, accounting for 60% to 70% of the total components of feed formulations. The metabolic pathways of carbohydrates differ depending on their physicochemical characteristics, such as fermentability and water solubility. Water-soluble carbohydrates can be hydrolysed by digestive enzymes, absorbed in the small intestine, and finally metabolized in the entire body. By contrast, insoluble carbohydrates (e.g. non-starch polysaccharides, fibres, and resistant starch), which cannot be digested in the small intestine, pass through the large intestine and are fermented by the gut microbiota. Short-chain fatty acids (SCFA) are microbial metabolites that can be absorbed by the colonic epithelial, hepatic, fat or muscle cells and can further systematically exert physiological effects. Among the SCFA, butyrate is reported to accommodate 10% to 15% energy required by the host and can repair damaged intestinal mucosa, whereas acetate and propionate are involved in liver energy metabolism (Duncan et al., 2004; Tremaroli and Backhed, 2012).
Accumulating evidence had revealed that the gut microbiota plays a key role in fibre metabolism (Yang et al., 2016; Fu et al., 2020). However, studies focused on the relationship between microbiota and fibre fermentation mainly in ruminants (Belanche et al., 2012; Patel et al., 2014). Millions of fibre-degrading microbiota thrive in the large intestine of swine as well as in the rumen of cattle (Metzler and Mosenthin (2008)). Interest on fibre fermentation in the distal gut of swine has increased as dietary fibres play a crucial role in energy homeostasis, in gut development, and in health (Ferrandis Vila et al., 2018; Soto et al., 2019). Metagenomics analysis results showed that the microbiome in the cecum of growing Laiwu pigs had a strong ability to degrade xylan, pectin, and cellulose (Yang et al., 2016). Moreover, Chen et al. (2017) reported that faecal inocula dominated by different fibre-utilizing bacteria belonging to the genera Prevotella or Bacteroides showed differential abilities to ferment fibres with different chemical structures and thus producing different metabolites. Meanwhile, an in vitro study had shown that dietary fibres could specifically and selectively promote the growth of bacteria in the intestines (Yang et al., 2013). For instance, Bifidobacterium was preferably increased by pectin, and Bifidobacterium adolescentis type-2 was increased by resistant starch, and a negative correlation was detected between inulin utilization and Subdoligranulum (Yang et al., 2013). These findings not only implied the direct effects of the gut microbiota on fibre fermentation, but also indirectly provided a theoretical basis for the precise regulation of intestinal microbes to promote homeostasis and health of the gut.
Numerous studies had reported that gut microbes can be domesticated with different carbohydrates, which ultimately influence the feed intake, digestion, absorption, metabolism of nutrients, and physiology of the host (Moran et al., 2016; C Wang et al., 2018a). The results of our previous study suggested that raw potato starch significantly changed the profile of gut microbiota in both colon and cecum by increasing the abundance of Coprococcus, Ruminococcus, and Turicibacter, and by decreasing the abundance of Sarcina and Clostridium (Sun et al., 2016a). We also found that long-term addition of raw potato starch had a profound effect on the composition of colonic microbiota as well as on the metabolome and transcriptome of the liver in pigs (Sun et al., 2015, 2016b). Specifically, the relative abundance of Clostridium, Treponema, Oscillospira, Phascolarctobacterium, RC9, and S24-7 was decreased, whereas the relative abundance of Turicibacter, Blautia, Ruminococcus, Coprococcus, Marvinbryantia, and Ruminococcus bromii was increased (Sun et al., 2015). Fu et al. (2020) had also shown that guar gum and its derivatives could enhance the abundance of Clostridium sensu stricto 1 and Bifidobacterium in vitro. Pectin is also believed to promote the growth and activity of Prevotella, Lactobacillus, and Faecalibacterium, which were found to promote gut health (Chung et al., 2017; Tian et al., 2017). The appropriate percentage of fibre in a diet could stimulate the flourishing of several genera of fibre-degrading bacteria, resulting in increased production of SCFA (e.g. acetic, propionate, and butyrate) that could afford more energy to the host metabolisms in growing Suhuai pigs (Pu et al., 2020). Given its high insoluble dietary fibre content, alfalfa had exerted remarkable impacts on cecal microbiota composition and butyrate concentration (J Wang et al., 2018b). It has been reported that the fibre digestibility of pigs increases with age, and this trend is probably correlated with the abundance of Proteobacteria, Tenericutes and TM7 (Niu et al., 2015). Additionally, Loh et al. (2006) found that inulin alters the colonic microbiota composition no matter the basal diet. By contrast, other researchers had found that the addition of inulin does not affect the gut microbiota composition (Eberhard et al., 2007; Xu et al., 2019). The difference is mainly because of the ages of investigated pigs and the degree of inulin degradation in the porcine upper small intestine (Böhmer et al., 2005). Besides, studies hadshown that addition of 5% dietary corn bran or wheat bran could improve the growth performance of weaned piglets by changing the gut microbiota profile and by improving butyrate production in suckling and weaned piglets (Mu et al., 2017; Zhao et al., 2018).
3.2. Amino acid metabolism
Both the host and the symbiotic bacteria in the gastrointestinal tract require nitrogen sources for metabolism. The major nitrogen sources of the gut microbiota are dietary proteins, amino acids, peptides, endogenous secreted protein, and recycled urea. However, the composition of a nitrogen source might affect the net utilization of amino acids.
Research has revealed that remarkable quantities of protein-fermenting bacteria and amino acid-fermenting bacteria, mainly including Clostridium bifermentans, Peptostreptococcus spp., Fusobacterium spp., Bacteroides spp., Veillonella spp., Megasphaera elsdenii, and Selenomonas ruminantium, are found in the large intestine (Smith and Macfarlane, 1998). The small intestine also hosts different species of amino acid-fermenting bacteria, mainly Escherichia coli, Klebsiella sp., Streptococcus sp., M. elsdenii, and Acidaminococcus fermentans (Dai et al., 2010). Such microorganisms simultaneously synthesize amino acids and microbial proteins, which could feed the host in return (Wang et al., 2011). Using isotope labelling technique, Dai et al. (2012) found that both pure cultures of E. coli, Klebsiella sp., and mixed bacterial cultures isolated from porcine small intestine could rapidly utilize glutamine, lysine, arginine, and threonine. Over 10% of these amino acids are used for protein synthesis; however, the percentage is dependent on the species of the amino acid-fermenting bacterium and on the gut compartment. These findings may provide new insights into the first pass effects reported previously (Stoll et al., 1998). Further, over 90% of the valine needed for bacterial protein synthesis in the small intestine is derived from the diet and from endogenous sources rather than through de novo synthesis (Libao-Mercado et al., 2009). Also, the availability of amino acids not only affects host amino acid metabolism, but also significantly affects the utilization of amino acids by gut microbiota (Libao-Mercado et al., 2009).
The addition of amino acids and their derivatives reshapes gut microbiota communities. Studies on mice and rabbits had found that the addition of some functional amino acids affected the intestinal microbiota composition and ultimately improved gut health and function (Ren et al., 2014; Chamorro et al., 2010; Wada et al., 2013). Xu et al. (2014) had also found that supplementation with N-acetyl cysteine increased the Lactobacillus and Bifidobacterium counts and decreased the E. coli counts in the gut. Moreover, dietary supplementation of L-glutamine alleviated the constipation in sows during late gestation by balancing the compositional structure of the gut microbiota communities (Zhang et al., 2017). Interestingly, Yin et al. (2020a) had found that the balance in branched-chain amino acids (especially leucine and valine) significantly alleviates the detrimental effects on the production performance induced by the protein-restricted diet, and these effects can be modulated by altering the composition of gut microbiota communities, especially the abundance of Spirochaetales, Gammaproteobacteria, Lactobacillales, and Aeromonadales. The conformation of amino acids also affects the gut microbiota communities (Li et al., 2019). Meanwhile, a series of in vitro studies in pigs had shown that the gut microbiota in the small intestine (duodenum, jejunum, and ileum) participated in essential amino acid metabolism (Dai et al., 2010, 2012). Puiman et al. (2013) revealed an increase in plasma threonine concentrations, turnover, and whole-body protein synthesis and proteolysis after an antibiotic intervention to suppress bacterial activity, suggesting that intestinal microbiota can participate in host amino acid metabolism in vivo. Of note, Saraf et al. (2017) found that diet-induced compositional changes in colonic gut microbiota in neonatal piglets mediated the increase of tryptophan metabolism from serotonin to tryptamine. Together, the abovementioned findings have indicated the bidirectional regulatory relationship between the host and the gut microbiota communities.
3.3. Lipid metabolism
Abnormal fat metabolism in humans leads to a series of nutritional metabolic diseases, including hyperlipidemia and hypertension (Ferrario et al., 2002). To date, findings seem to suggest that the gut microbiota is highly correlated with host lipid metabolism (Velagapudi et al., 2010; Caesar et al., 2010; Yang et al., 2018a, Yang et al., 2018b). However, only a few studies had investigated the effects of intestinal microorganisms on fat metabolism in pigs. Given the market's preference for lean pork and the development of various high-quality pork products, it is of great practical significance to investigate the relationship between intestinal microorganisms and fat metabolism in pigs. Comparative analysis had shown that low-fat pigs had a higher abundance of Bacteroidetes and a lower abundance of Firmicutes. Meanwhile, differential functions rich in adipocytokine signalling pathway were detected in the cecum (Yang et al., 2016). Nuclear magnetic resonance based metabonomic results showed that the metabolic pathways involved in lipogenesis, lipid oxidation, energy utilization and partition, protein and amino acid metabolism, and fermentation of gastrointestinal microbes in obese piglets were distinct from those in lean pigs (He et al., 2012). He et al. (2016) suggested that the gut microbiome was an important target in modulating fatness in pigs. Additionally, Guo et al. (2008) had suggested that fat storage could affect the relative abundance of Bacteroidetes in the gut. Interestingly, different breeds of pigs with different body masses showed distinct diversity and numbers of faecal methanogens as was observed in the lean breed Landrace pigs and in the obese breed Erhualian pigs (Luo et al., 2012). The fatty acid composition in the adipose tissue of pigs, however, could be regulated by the oral administration of Bifidobacterium breve NCIMB 702258 (Wall et al., 2009). The interplay between gut microbiota and swine lipid metabolism warrants further investigation.
Bile acid, which is derived mainly from cholesterol metabolism, plays a crucial role in fat metabolism and in maintaining metabolic homeostasis in host. Mainly composed of bile acids, the bile juice is stored temporarily in the gallbladder and is secreted into the gut that hosts trillions of microorganisms. In the gut, most of the bile acid is reabsorbed in the distal part of ileum and is finally recycled by the liver, completing the hepato-intestinal circulation. Part of the remaining bile acids undergoes a series of biotransformation, including deconjugation to liberate free bile acids, reversible epimerization between the α and β orientations, and the oxidation of the 3-, 7-, and 12-hydroxy groups by the gut microbiota (Gerard, 2014; Midtvedt, 1974). The transformation occurs after the deconjugation from a ligand (e.g. glycine and taurine) facilitated by the bile salt hydrolases produced by the microbiota, such as Bacteroides, Clostridium, Lactobacillus, Bifidobacterium, and Listeria (Ridlon et al., 2006). Li et al. (2020) found that, compared with the germ-free piglets, the faecal transplanting group displayed an increased secretion of bile acid and its biotransformation, resulting in a distinctive bile acid profile. Bile acid has been reported to inhibit bacterial growth. Meanwhile, the bacteriostatic effects of deoxycholic acid derived from the bacterial transformation of bile acid were 10-fold higher than those of bile acid, indicating that the profile of bile acid can exert survival pressure on some particular microbes (Kurdi et al., 2006). In relation to this finding, Kusumotoa et al. (2017) had reported that the addition of bile acid-binding resin increased the abundance of Firmicutes and decreased that of Bacteroides. The probable reason as to why high-fat diets impair the abundance and richness of gut microbiota is the increased excretion of bile acids (Yokota et al., 2012).
4. Microbial–host crosstalks through the microbiota–gut–brain axis
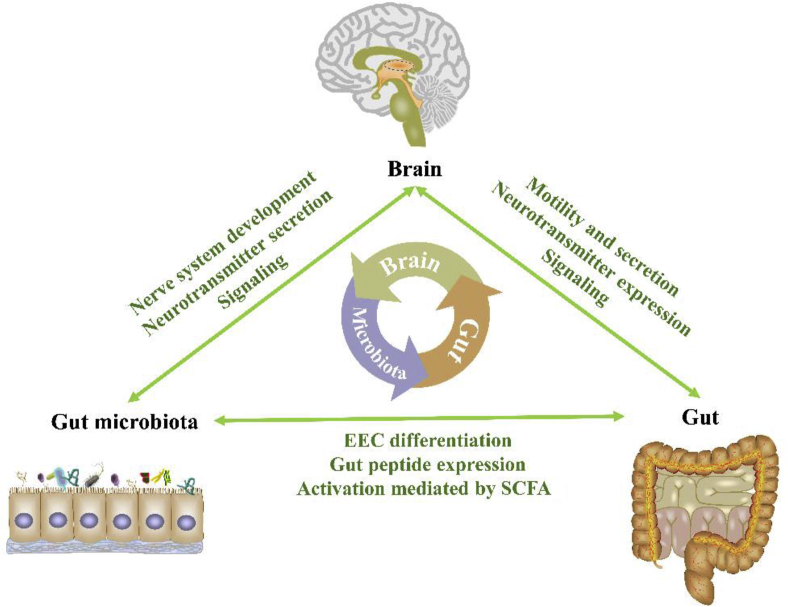
The brain, gut, and the microbiota that had colonized the gut had coevolved into an interactive organic system, and the homeostasis and crosstalks in this system play a vital role in host health, immunity, and nutrient metabolism (Cani et al., 2019a; Cani, 2019b; Krautkramer et al., 2017). It has been suggested that the microbiota functions through the microbiota–gut–brain axis, and dysbiosis in the composition and functions of the gut microbiota have been implicated in various immunologic, neurologic, metabolic, and psychiatric conditions (El Aidy et al., 2016; Turroni et al., 2018). This multi-lateral network of communication between the gut microbiota and the central nervous system (CNS, representing the headquarters of the body) is mediated by the autonomic nervous system (ANS), enteric nervous system, immune system and bacterial metabolites (Junges et al., 2018). The complex microbiome–host crosstalk via the microbiota–gut–brain axis is shown in Fig. 2.
Fig. 2.
The microbiome-host crosstalk via microbiota–gut–brain axis. The mammalian gastrointestinal tract hosts trillions of microorganisms. The symbiotic gut microbiota could promote host nervous system development and neurotransmitter secretion. Besides, the bacterial metabolites might cross the blood–brain barrier and directly modulate signaling in the brain. Meanwhile, the central nervous system in the brain adjust host behavior (e.g. appetite) to change the composition and function of the gut microbiota in turn. The gut affords the nutrients for the microbiota and thus shaping the scenery of its habitat, while the microbiota promotes the differentiation of intestinal epithelial cell, stimulate intestinal peptide secretion, and regulate gut signaling in return. The central nervous system controls the motility and secretion of the gut, while the gut affects the signaling and neurotransmitter expression in the brain. EEC = Enteroendocrine cells; SCFA = short-chain fatty acids.
4.1. Signalling from the gut microbiota to the brain
4.1.1. Gut microbiota affects the host nervous system development and neurotransmitter secretion
Most of the neurons and different brain structures and cell nuclei in the CNS are well formed at the end of the perinatal period (Van Dyck and Morrow, 2017; Watson et al., 2006). However, several studies had indicated that the microbiota can modulate the development, maturation, and function of the CNS (Collins et al., 2014; De Vadder et al., 2018). A decrease in nerve density and in the number of neurons per ganglion together with an increase in the proportion of myenteric nitrergic neurons were found in the jejunum and ileum of germ-free mice. These results suggested that early exposure to intestinal bacteria in the mid to distal small intestine is essential for the postnatal development of the enteric nervous system (ENS) (Collins et al., 2014). Moreover, transplantation of normal mouse gut microbiota into germ-free mice significantly modified the neuroanatomy of the ENS and increased the intestinal transit rates, and these results might have been mediated by the increased production of neuronal and mucosal 5-hydroxytryptamine (5-HT) and by the proliferation of enteric neuronal progenitors in the adult intestine (De Vadder et al., 2018). Another study demonstrated that early-life gut microbiota regulates the hippocampal serotonergic system (Clarke et al., 2013). The results of the use of broad-spectrum antibiotics to deplete the gut microbiota had shown that specific microbiota possibly influences the physiology and neurochemistry of the CNS (Smith, 2015). Further studies found that the lack of specific microbiota causes neurological deficiencies in learning, memory, recognition, and emotional behaviours (Gareau, 2011) as well as causes abnormal variations in neurotransmitters (Foster et al., 2017). However, infusion of antibiotics into the distal-ileal T cannula decreased the concentrations of 5-HT and dopamine in the hypothalamus and upregulated the gene expression levels of neurotransmitter transporters and synthetases accompanied by remarkable changes in the composition of large intestinal microbiota communities (Gao et al., 2018).
4.1.2. Short-chain fatty acids play a key role in signalling from gut microbiota
As signalling molecules, SCFA are considered to play a key role in microbiota–gut–brain crosstalk; SCFA are the main metabolites produced during bacterial fermentation of dietary fibres in the gastrointestinal tract. Mounting evidence had shown that SCFA can cross the blood–brain barrier, although the SCFA uptake is quite minimal (Frost et al., 2014; Kekuda et al., 2013; Mitchell et al., 2011). Accordingly, free fatty acid receptor 2 (FFAR2) and FFAR3, the main SCFA receptors, are expressed in the CNS and in the peripheral nervous system. FFAR3 is highly expressed in rat brain tissue and in sympathetic ganglia (specifically in the superior cervical ganglion) in adult mice, and it has been reported to control the sympathetic nerve activity (Kimura et al., 2011). For instance, reduced nerve activities were found in Ffar3−/− mice (Kimura et al., 2011). FFAR3 is also expressed in the superior cervical ganglion and in the sympathetic ganglia of the thoracic and lumbar sympathetic trunk in mice (Nøhr et al., 2015). Results of extensive studies indicated that SCFA regulate gene expression and chromatin transcription by inhibiting histone deacetylases (HDAC) (Lin et al., 2015; Schilderink et al., 2013). HDAC are a series of key enzymes that have high correlations with the development of the brain and of several neuropsychiatric diseases, such as depression, schizophrenia, and Alzheimer's disease (Volmar and Wahlestedt (2015)). The activity of HDAC had been reported to be inhibited by intracellular butyrate, propionate, and acetate (Soliman and Rosenberger, 2011; Waldecker et al., 2008). Injection of sodium butyrate caused histone hyperacetylation in the hippocampus and frontal cortex, whereas intraperitoneal injection of sodium butyrate caused chronic inhibition of HDAC, improving learning and memory in wild-type mice and mice with brain atrophy (Fischer et al., 2007). Furthermore, acting as endogenous ligands for orphan G protein-coupled receptors, SCFA might affect inflammation and hormonal regulation and thus interact with vagal afferents (Kimura et al., 2011). Additionally, intestinal bacteria can synthesize neurotransmitters (5-HT and dopamine) and neural regulators (d-lactic acid and ammonia) that play a key role in complex communication networks, and the secretion of gut hormones that could directly participate in the gut–brain axis is also affected by the SCFA.
4.1.3. Tryptophan metabolites mediate the signalling from the gut microbiota to brain
Being one of the essential amino acids in animals, tryptophan plays a key role in maintaining development, growth, health, and immunity (Kim et al., 2010; Wen et al., 2014). Results of extensive studies indicated that the gut microbiota exerts profound effects on the metabolism of aromatic amino acids (Gao et al., 2018, 2019). Importantly, being the sole precursor of the neurotransmitter 5-HT, tryptophan and its metabolites play a crucial role in the modulation of neuronal differentiation and migration, axonal outgrowth, myelination, and synapse formation (Gaspar et al., 2003; Homberg et al., 2013; O'Mahony et al., 2015). Although most of the tryptophan derived from the diet is absorbed in the small intestine, the unabsorbed residues enter the large intestine and are then metabolized by the gut microbiota (Agus et al., 2018; Kaluzna-Czaplinska et al., 2019). Specifically, germ-free mice lacking gut microbiota expressed lower tryptamine levels in the gut and higher serumal tryptophan levels in blood circulation compared with normal mice (Marcobal et al., 2013; Clarke et al., 2013). Also, our previous results suggested that increasing hindgut carbohydrate availability to the gut microbiota promotes the synthesis of hypothalamic neurotransmitters; our results had further indicated that aromatic amino acids are possibly the mediator between the gut microbiota and brain (Gao et al., 2019). These findings suggested that the metabolism of aromatic amino acids mediates the crosstalk between the microbiota in the distal intestine and the host brain.
4.2. Signalling from the brain to the gut microbiota
The brain has been shown to directly and indirectly control the gut microbiota (Cerdó et al., 2019). A growing body of evidence had indicated that the composition of the gut microbiota is drastically changed under stress (Aguilera et al., 2013; Tannock and Savage, 1974). Specifically, the composition of the gut microbiota shifts within only 2 h of stress (Galley et al., 2014). These effects might have been mediated by the host neuroendocrine system mainly under the control of the CNS (Santos et al., 1998), which could communicate with the gut microbiota more directly through the intraluminal release of host neurotransmitters and neural regulators, mainly including catecholamines, 5-HT, dynorphin, cytokines, from neurons, and immune cells (Lyte, 1993; Mayer et al., 2014). Knecht et al. (2016) have found that increased serotonin could reinforce quorum sensing in Pseudomonas aeruginosa in both in vitro and in vivo experiments. Further, the increasing serotonin levels could rescue the ability of the avirulent quorum-sensing P. aeruginosa mutant to cause intestinal infection in mice (Knecht et al., 2016).
Mayer (2011) had well established that both branches of the ANS could regulate gut functions and gut physiology (mainly gut motility; secretion of gastric acid, bicarbonate, gut peptides, and antimicrobial peptides; intestinal permeability; and mucosal immune response), thereby significantly influencing the microbial habitat and thus modulating the microbiota composition and activity. Interestingly, a plethora of studies revealed that the composition and functions of the gut microbiota are highly related to intestine motility (Roager et al., 2016; Saad et al., 2010; Vandeputte et al., 2016). Also, it has been reported that the secretion of mucus by intestinal goblet cells is under the modulation of the ANS. By contrast, in a brain injury mouse model, the increased sympathetic nervous system signalling altered the mucoprotein production and goblet cell population size and thus changed the microbiota composition (Houlden et al., 2016; Kim and Ho, 2010).
5. Diurnal rhythmicity of gut microbiota and host metabolism
The homeostasis of intestinal microbiota communities over a given period and the homeostasis is susceptible to diets, to feeding mode, and to the environment is considered to be an important indicator of host health (Liu et al., 2017; Lee et al., 2019). However, growing shreds of evidence had indicated that bacterial biogeography and the composition and function of the gut microbiota undergo diurnal fluctuation in a given day (Leone et al., 2015; Liang et al., 2015; Thaiss et al., 2014, 2016; Zarrinpar et al., 2014). In mice, the relative abundance of over 15% of the detected operational taxonomic units (OTU) was indicated to be involved in rhythmic oscillations under ad libitum feeding with normal chow, whereas a high-fat diet would weaken the proportion of the cycling OTU (Thaiss et al., 2016; Zarrinpar et al., 2014). Besides, the abundance of gut microbiota that adhered to the epithelial layer in the dark phase was up to 10 times higher than that in the light phase in mice subjected to ad libitum feeding (Thaiss et al., 2016). By contrast, the relative abundance of symbiotic lactobacillus in the gut of humans and mice increases in the resting phase (the light phase of mice) and decreases in the active phase (Thaiss et al., 2014). Parts of nutritional substrates and metabolites of gut microbiota also undergo diurnal rhythmic changes (Leone et al., 2015; Thaiss et al., 2016). With the fluctuation in the abundance of gut microbiota, the functions of gut microbiota shift diurnally; this shift coincides with detoxification and chemotaxis, which mainly occur during the resting phase, and with energy collection, DNA repair, and cell growth, which mainly occur during the active phase (Thaiss et al., 2014). Therefore, the gut microbiota itself and its diurnal rhythmicity play a pivotal role in maintaining the normal rhythmicity of physiology, rest–activity cycle, and host metabolism. Thaiss et al. (2016) have indicated that the host acquired compensatory oscillatory in metabolic pathways, such as pyruvate metabolism, glutathione metabolism, and the tricarboxylic acid cycle, upon microbiota depletion, implying the potential effects of the oscillation of gut microbiota towards the host metabolism. Besides, the diurnal fluctuation of gut microbiota could influence the circadian transcriptions of chromatin in the liver, which is essential to the host metabolism. Conversely, the dysbiosis of microbiota rhythmicity reprograms the normal rhythmic transcription of chromatin, particularly including intriguing the genome-wide de novo oscillations in periapical tissue, thereby affecting the normal biorhythms of host physiology and metabolism that lead to obesity and other metabolic syndromes (Thaiss et al., 2016; Voigt et al., 2016). Also, Gong et al. (2018) have indicated that the diurnal rhythmicity of gut microbiota is considered to mediate the diurnal variation of acute liver injury caused by acetaminophen overdose (this drug was taken at night, causing more severe liver damage).
Expectedly, the diurnal rhythmicity of gut microbiota highly depends on host circadian rhythms. In Per1/2−/− mice, where the core molecular clock gene was deleted, bacterial oscillations were remarkably lost (Thaiss et al., 2014). However, a regular feeding pattern could rescue the rhythmic oscillations of gut microbiota (Leone et al., 2015). Both studies indicated that, although affected by host circadian rhythms, the gut microbiota spontaneously oscillated within a day depending on the host feeding time. Feeding time leads to rhythmic changes in the enteric environment, including the richness of nutrient (metabolites), concentrations of digestive enzymes, and pH of the intestinal contents. Besides, the type of diet as a modulator of microbiota composition plays a critical role in shaping the intestinal microbial ecology. Of note, the rhythmicity of bacterial metabolites could in turn drill the oscillating transcription of peripheral clocks, suggesting the mutual relationship between the host and the gut microbiota. However, little is known about the diurnal rhythmicity of gut microbiota in pigs.
Our on-going research has indicated that the relative abundance of gut microbiota in the colonic digesta of growing pigs also showed rhythmic fluctuations (unpublished data). We further found that over 8% of the detected OTU underwent diurnal fluctuation at the phylum level, particularly Firmicutes, Bacteroidetes, Proteobacteria, and Cyanobacteria (unpublished data). Interestingly, the proportion of OTU that demonstrate rhythmicity was quite lower than that reported in mice, suggesting that species specificity exists between mice and pigs concerning this value. However, more work should be done to shed light on the patterns of microbiota diurnal rhythmicity and its effects on a host.
A high-fat diet is one of the most typical characteristics of modern western lifestyle, causing not only a series of metabolic diseases, such as hypertension and hyperlipidemia, but also dampening the cyclical fluctuation of the gut microbiome (Zarrinpar et al., 2014). However, knowledge on the mechanism of the diurnal rhythmicity of gut microbiota and its effects on host metabolism remains incomplete. Li et al. (2019) have found that Lactobacillus reuteri could partly alleviate the metabolic disorder caused by high-fat diets and could modulate the high-fat diet-induced α and β diversity of colonic microbiota in a time-of-day-dependent manner. Moreover, Yin et al. (2020b) have found that exogenous melatonin administration could improve the diurnal rhythms of the gut microbiota in mice fed a high-fat diet. These studies have shed light on new therapies that target the diurnal rhythmicity of gut microbiota. Also, jet lag and shift work had been found to cause a phase shift in the rhythmic fluctuation of the gut microbiota as well as to cause gastrointestinal disturbance, insomnia, hormone secretion disorder, and even cognitive impairments (Waterhouse et al., 2016; Hulsegge et al., 2019), suggesting that the microbiota diurnal rhythmicity is intertwined with the gut microbiota–brain axis.
6. Conclusions and perspectives
Our knowledge concerning swine gut microbiota and its interactions with host metabolism has been greatly expanded over the past several years. The swine gut harbours a powerful gut microbiota that is more similar to that of humans than to that of mice. The gut microbiota plays a crucial role in host nutrient metabolism, including carbohydrate, amino acid, and lipid metabolisms. Further, the microbiota in different part of the intestine demonstrates different ability in the metabolism of various nutrients. For example, the microbiota in the proximal intestine is mainly involved in amino acid metabolism, whereas that in distal intestine mainly participates in dietary fibre fermentation. Interestingly, the gut microbiota may in turn be domesticated by these nutrients from the host. The complex microbiome–host crosstalk is accomplished via the gut microbiota–gut–brain axis. As key messengers, microbial metabolites play crucial roles in the complex communication network. Although numerous studies have focused on the gut microbiota of swine, the action mechanism of the gut microbiota remains incompletely understood. The species-specific response of the gut microbiota to various diets remains unknown. Of note, the existence of the microbiota diurnal rhythmicity has been fully proven in mice; however, the mechanism of diurnal rhythmicity of gut microbiota and its effects on host metabolism remain incompletely understood. Anyhow, these results may imply that the time factor should be taken into consideration during sampling in studies on gut microbiota. Moreover, the diurnal rhythmicity of the intestinal microbiota may be intertwined with the microbiota–gut–brain axis. Thus, studying the interplay between microbiota diurnal rhythmicity and microbiota–gut–brain axis is likely to lead to a better understanding of the mechanism of the microbial–host crosstalk and its effects on host health and metabolism as well as to the development of precise intervention targeting the gut microbiota of pigs.
Author contributions
Yong Su and Hongyu Wang initiated the idea and the outline of this review paper. Hongyu Wang and Yong Su wrote the manuscript. Rongying Xu, He Zhang, Weiyun Zhu provided intellectual oversight, suggestions, and revisions. All authors read and approved the final manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (3187130113) and the National Key R&D Program of China (2018YFD0500404).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Adhikari B., Kim S.W., Kwon Y.M. Characterization of microbiota associated with digesta and mucosa in different regions of gastrointestinal tract of nursery pigs. Int J Mol Sci. 2019;20:1630. doi: 10.3390/ijms20071630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera M., Vergara P., Martinez V. Stress and antibiotics alter luminal and wall-adhered microbiota and enhance the local expression of visceral sensory-related systems in mice. Neuro Gastroenterol Motil. 2013;25:515–529. doi: 10.1111/nmo.12154. [DOI] [PubMed] [Google Scholar]
- Agus A., Planchais J., Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Arnal M.E., Zhang J., Messori S., Bosi P., Smidt H., Lallès J.P. Early changes in microbial colonization selectively modulate intestinal enzymes, but not inducible heat shock proteins in young adult swine. PloS One. 2014;9 doi: 10.1371/journal.pone.0087967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanche A., Doreau M., Edwards J.E., Moorby J.M., Pinloche E., Newbold C.J. Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. J Nutr. 2012;142:1684–1692. doi: 10.3945/jn.112.159574. [DOI] [PubMed] [Google Scholar]
- Bian G., Ma S., Zhu Z., Su Y., Zoetendal E.G., Mackie R. Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environ Microbiol. 2016;18:1566–1577. doi: 10.1111/1462-2920.13272. [DOI] [PubMed] [Google Scholar]
- Böhmer B.M., Branner G.R., Roth-Maier D.A. Precaecal and faecal digestibility of inulin (DP 10 – 12) or an inulin/Enterococcus faecium mix and effects on nutrient digestibility and microbial gut flora. J Anim Physiol Anim Nutr. 2005;89:388–396. doi: 10.1111/j.1439-0396.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- Caesar R., Fåk F., Bäckhed F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J Int Med. 2010;268:320–328. doi: 10.1111/j.1365-2796.2010.02270.x. [DOI] [PubMed] [Google Scholar]
- Caesar R. Pharmacologic and nonpharmacologic therapies for the gut microbiota in type 2 diabetes. Can J Diabetes. 2019;43:224–231. doi: 10.1016/j.jcjd.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Van Hul M., Lefort C., Depommier C., Rastelli M., Everard A. Microbial regulation of organismal energy homeostasis. Nat Metabol. 2019;1:34–46. doi: 10.1038/s42255-018-0017-4. [DOI] [PubMed] [Google Scholar]
- Cani P.D. Microbiota and metabolites in metabolic diseases. Nat Rev Endocrinol. 2019;15:69–70. doi: 10.1038/s41574-018-0143-9. [DOI] [PubMed] [Google Scholar]
- Cao S., Wang L., Jiao L., Lin F., Xiao K., Hu C. Effects of diosmectite-Lactobacillus acidophilus on growth performance, intestine microbiota, mucosal architecture of weaned pigs. Anim Feed Sci Technol. 2016;220:180–186. [Google Scholar]
- Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- Cerdó T., Diéguez E., Campoy C. Early nutrition and gut microbiome: interrelationship between bacterial metabolism, immune system, brain structure, and neurodevelopment. Am J Physiol Endocrinol Metabol. 2019;317:617–630. doi: 10.1152/ajpendo.00188.2019. [DOI] [PubMed] [Google Scholar]
- Chamorro S., De Blas C., Grant G., Badiola I., Menoyo D., Carabaño R. Effect of dietary supplementation with glutamine and a combination of glutamine-arginine on intestinal health in twenty-five-day-old weaned rabbits. J Anim Sci. 2010;88:170–180. doi: 10.2527/jas.2008-1698. [DOI] [PubMed] [Google Scholar]
- Chen T., Long W., Zhang C., Liu S., Zhao L., Hamaker B.R. Fiber-utilizing capacity varies in Prevotella-versus Bacteroides-dominated gut microbiota. Sci Rep. 2017;7:1–7. doi: 10.1038/s41598-017-02995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.S., Wei H.K., Wang P., Yu H.C., Zhang X.M., Jiang S.W. Early intervention with faecal microbiota transplantation: an effective means to improve growth performance and the intestinal development of suckling piglets. Animal. 2018;13:533–541. doi: 10.1017/S1751731118001611. [DOI] [PubMed] [Google Scholar]
- Cheng P.H., Liang J.B., Wu Y.B., Wang Y., Tufarelli V., Laudadio V. In vitro fermentative capacity of swine large intestine: comparison between native Lantang and commercial Duroc breeds. Anim Sci J. 2017;88:1141–1148. doi: 10.1111/asj.12723. [DOI] [PubMed] [Google Scholar]
- Chung W.S.F., Meijerink M., Zeuner B., Holck J., Louis P., Meyer A.S. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol Ecol. 2017;93:127. doi: 10.1093/femsec/fix127. [DOI] [PubMed] [Google Scholar]
- Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatr. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Collins J., Borojevic R., Verdu E.F., Huizinga J.D., Ratcliffe E.M. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neuro Gastroenterol Motil. 2014;26:98–107. doi: 10.1111/nmo.12236. [DOI] [PubMed] [Google Scholar]
- Crespo-Piazuelo D., Migura-Garcia L., Estellé J., Criado-Mesas L., Revilla M., Castelló A. Association between the pig genome and its gut microbiota composition. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-45066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z.L., Li X.L., Xi P.B., Zhang J., Wu G., Zhu W.Y. Metabolism of select amino acids in bacteria from the pig small intestine. Amino Acids. 2012;42:1597–1608. doi: 10.1007/s00726-011-0846-x. [DOI] [PubMed] [Google Scholar]
- Dai Z.L., Zhang J., Wu G., Zhu W.Y. Utilization of amino acids by bacteria from the pig small intestine. Amino Acids. 2010;39:1201–1215. doi: 10.1007/s00726-010-0556-9. [DOI] [PubMed] [Google Scholar]
- De Vadder F., Grasset E., Holm L.M., Karsenty G., Macpherson A.J., Olofsson L.E. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci. 2018;115:6458–6463. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H., Yan H.L., Xiao Y., Yu B., Yu J., He J. Intestinal microbiota could transfer host Gut characteristics from pigs to mice. BMC Microbiol. 2016;16:238. doi: 10.1186/s12866-016-0851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S.H., Holtrop G., Lobley G.E., Calder A.G., Stewart C.S., Flint H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr. 2004;91:915–923. doi: 10.1079/BJN20041150. [DOI] [PubMed] [Google Scholar]
- Eberhard M., Hennig U., Kuhla S., Brunner R.M., Kleessen B., Metges C.C. Effect of inulin supplementation on selected gastric, duodenal, and caecal microbiota and short chain fatty acid pattern in growing piglets. Arch Anim Nutr. 2007;61:235–246. doi: 10.1080/17450390701431631. [DOI] [PubMed] [Google Scholar]
- El Aidy S., Stilling R., Dinan T.G., Cryan J.F. Microbial endocrinology: interkingdom signaling in infectious disease and health. Springer; Cham: 2016. Microbiome to brain: unravelling the multidirectional axes of communication; pp. 301–336. [DOI] [PubMed] [Google Scholar]
- Fan R., Cui J., Ren F., Wang Q., Huang Y., Zhao B. Overexpression of NRK1 ameliorates diet- and age-induced hepatic steatosis and insulin resistance. Biochem Biophys Res Co. 2018;500:476–483. doi: 10.1016/j.bbrc.2018.04.107. [DOI] [PubMed] [Google Scholar]
- Ferrandis Vila M., Trudeau M.P., Hung Y.T., Zeng Z., Urriola P.E., Shurson G.C., Saqui-Salces M. Dietary fiber sources and non-starch polysaccharide-degrading enzymes modify mucin expression and the immune profile of the swine ileum. PloS One. 2018;13 doi: 10.1371/journal.pone.0207196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M., Smith R., Levy P., Strawn W. The hypertension-lipid connection: insights into the relation between angiotensin II and cholesterol in atherogenesis. Am J Med Sci. 2002;323:17–24. doi: 10.1097/00000441-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Fischer A., Sananbenesi F., Wang X., Dobbin M., Tsai L.H. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Foster J.A., Rinaman L., Cryan J.F. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G., Sleeth M.L., Sahuri-Arisoylu M., Lizarbe B., Cerdan S., Brody L. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Wei X., Xiao M., Han Z., Secundo F., Mou H. Properties of hydrolyzed guar gum fermented in vitro with pig fecal inocula and its favorable impacts on microbiota. Carbohydr Polym. 2020:237. doi: 10.1016/j.carbpol.2020.116116. [DOI] [PubMed] [Google Scholar]
- Fuentes S., Rossen N.G., van der Spek M.J., Hartman J.H., Huuskonen L., Korpela K. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 2017;11:1877–1889. doi: 10.1038/ismej.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkhouser L.J., Bordenstein S.R. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;1:1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley J.D., Nelson M.C., Yu Z.T., Dowd S.E., Walter J., Kumar P.S. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. doi: 10.1186/1471-2180-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K., Pi Y., Mu C.L. Increasing carbohydrate availability in the hindgut promotes hypothalamic neurotransmitter synthesis: aromatic amino acids linking the microbiota–brain axis. J Neurochem. 2019;149:641–659. doi: 10.1111/jnc.14709. [DOI] [PubMed] [Google Scholar]
- Gao K., Pi Y., Mu C.L., Peng Y., Huang Z., Zhu W.Y. Antibiotics-induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets. J Neurochem. 2018;146:219–234. doi: 10.1111/jnc.14333. [DOI] [PubMed] [Google Scholar]
- Gaspar P., Cases O., Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Geng S., Cheng S., Li Y., Wen Z., Ma X., Jiang X. Faecal microbiota transplantation reduces susceptibility to epithelial injury and modulates tryptophan metabolism of the microbial community in a piglet model. J Crohns Colitis. 2018;12:1359–1374. doi: 10.1093/ecco-jcc/jjy103. [DOI] [PubMed] [Google Scholar]
- Gerard P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens. 2014;3:14–24. doi: 10.3390/pathogens3010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S., Lan T., Zeng L., Luo H., Yang X., Li N. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J Hepatol. 2018;69:51–59. doi: 10.1016/j.jhep.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevarra R.B., Kim J., Nguyen S.G., Unno T. Comparison of fecal microbial communities between white and black pigs. J Appl Biol Chem. 2015;58:369–375. [Google Scholar]
- Guo X., Xia X., Tang R., Zhou J., Zhao H., Wang K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol. 2008;47:367–373. doi: 10.1111/j.1472-765X.2008.02408.x. [DOI] [PubMed] [Google Scholar]
- He M., Fang S., Huang X., Zhao Y., Ke S., Yang H. Evaluating the contribution of gut microbiota to the variation of porcine fatness with the cecum and fecal samples. Front Microbiol. 2016;7:2108. doi: 10.3389/fmicb.2016.02108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Gao J., Wu J., Zhou Y., Fu H., Ke S. Host gender and androgen levels regulate gut bacterial taxa in pigs leading to sex-biased serum metabolite profiles. Front Microbiol. 2019;10:1359. doi: 10.3389/fmicb.2019.01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Ren P., Kong X., Wu Y., Wu G., Li P. Comparison of serum metabolite compositions between obese and lean growing pigs using an NMR-based metabonomic approach. J Nutr Biochem. 2012;23:133–139. doi: 10.1016/j.jnutbio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Holman D.B., Brunelle B.W., Trachsel J., Allen H.K. Meta-analysis to define a core microbiota in the swine gut. mSystems. 2017;2:e00004–e17. doi: 10.1128/mSystems.00004-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg J.R., Kolk S.M., Schubert D. Editorial perspective of the Research Topic Deciphering serotonin's role in neurodevelopment. Front Cell Neurosci. 2013;7:212. doi: 10.3389/fncel.2013.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden A., Goldrick M., Brough D., Vizi E.S., Lenart N., Martinecz B. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. 2016;57:10–20. doi: 10.1016/j.bbi.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Zhao F., Wang J., Zhu W. Early-life lactoferrin intervention modulates the colonic microbiota, colonic microbial metabolites and intestinal function in suckling piglets. Appl Microbiol Biotechnol. 2020;104:6185–6197. doi: 10.1007/s00253-020-10675-z. [DOI] [PubMed] [Google Scholar]
- Huang L., Ren P., Ouyang Z., Wei T., Kong X., Li T. Effect of fermented feed on growth performance, holistic metabolism and fecal microbiota in weanling piglets. Anim Feed Sci Technol. 2020:114505. [Google Scholar]
- Hulsegge G., Loef B., van Kerkhof L.W., Roenneberg T., van der Beek A.J., Proper K.I. Shift work, sleep disturbances and social jetlag in healthcare workers. J Sleep Res. 2019;28:12802. doi: 10.1111/jsr.12802. [DOI] [PubMed] [Google Scholar]
- Junges V.M., Closs V.E., Nogueira G.M., Gottlieb M.G. Crosstalk between gut microbiota and central nervous system: a focus on Alzheimer's disease. Curr Alzheimer Res. 2018;15:1179–1190. doi: 10.2174/1567205015666180904155908. [DOI] [PubMed] [Google Scholar]
- Kaluzna-Czaplinska J., Gatarek P., Chirumbolo S., Chartrand M.S., Bjorklund G. How important is tryptophan in human health? Crit Rev Food Sci Nutr. 2019;59:72–88. doi: 10.1080/10408398.2017.1357534. [DOI] [PubMed] [Google Scholar]
- Kang C., Wang B., Kaliannan K., Wang X., Lang H., Hui S. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. mBio. 2017;8 doi: 10.1128/mBio.00470-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson F., Tremaroli V., Nielsen J., Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62:3341–3349. doi: 10.2337/db13-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase T., Nagasawa M., Ikeda H., Yasuo S., Koga Y., Furuse M. Gut microbiota of mice putatively modifies amino acid metabolism in the host brain. Br J Nutr. 2017;117:775–783. doi: 10.1017/S0007114517000678. [DOI] [PubMed] [Google Scholar]
- Kekuda R., Manoharan P., Baseler W., Sundaram U. Monocarboxylate 4 mediated butyrate transport in a rat intestinal epithelial cell line. Dig Dis Sci. 2013;58:660–667. doi: 10.1007/s10620-012-2407-x. [DOI] [PubMed] [Google Scholar]
- Kelly C.R., Ihunnah C., Fischer M., Khoruts A., Surawicz C., Afzali A. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.J., Kovacs-Nolan J.A., Yang C., Archbold T., Fan M.Z. Mine Y. L-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Nutr Biochem. 2010;21:468–475. doi: 10.1016/j.jnutbio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Kim H.B., Borewicz K., White B.A., Singer R.S., Sreevatsan S., Tu Z.J. Microbial shifts in the swine distal gut in response to the treatment with antimicrobial growth promoter tylosin. Proc Natl Acad Sci. 2012;109:15484–15490. doi: 10.1073/pnas.1205147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Guevarra R.B., Nguyen S.G., Unno T. Differences in swine gut microbiota in southern region of Republic of Korea. Korean J Microbiol. 2015;51:81–85. [Google Scholar]
- Kim J., Nguyen S.G., Guevarra R.B., Lee I., Unno T. Analysis of swine fecal microbiota at various growth stages. Arch Microbiol. 2015;197:753–759. doi: 10.1007/s00203-015-1108-1. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Ho S.B. Intestinal goblet cells and mucins in health and disease: recent insights and rogress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I., Inoue D., Maeda T., Hara T., Ichimura A., Miyauchi S. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht L., O'Connor G., Mittal R., Liu X.Z., daftarian P., Deo S.K. Serotonin activates bacterial quorum sensing and enhances the virulence of Pseudomonas aeruginosa in the host. EBio Med. 2016;9:161–169. doi: 10.1016/j.ebiom.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautkramer K.A., Dhillon R.S., Denu J.M., Carey H.V. Metabolic programming of the epigenome: host and gut microbial metabolite interactions with host chromatin. Transl Res. 2017;189:30–50. doi: 10.1016/j.trsl.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdi P., Kawanishi K., Mizutani K., Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. 2006;188:1979–1986. doi: 10.1128/JB.188.5.1979-1986.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumotoa Y., Irie J., Iwabu K., Tagawa H., Itoh A., Kato M. Bile acid binding resin prevents fat accumulation through intestinal microbiota in high-fat diet-induced obesity in mice. Metabolism. 2017;71:1–6. doi: 10.1016/j.metabol.2017.02.011. [DOI] [PubMed] [Google Scholar]
- Lee P., Yacyshyn B.R., Yacyshyn M.B. Gut microbiota and obesity: an opportunity to alter obesity through faecal microbiota transplant (FMT) Diabetes Obes Metabol. 2019;21:479–490. doi: 10.1111/dom.13561. [DOI] [PubMed] [Google Scholar]
- Leone V., Gibbons S.M., Martinez K., Hutchison A.L., Huang E.Y., Cham C.M. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17:681–689. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zhou H., Ding Y., Liu Z., Sun J., Li Z. Effects of gut microbiota on bile acid profile and bile acid metabolism in piglets. Biotechnol Bull. 2020;26 [Google Scholar]
- Li N., Huang S., Jiang L., Dai Z., Li T., Han D. Characterization of the early life microbiota development and predominant lactobacillus species at distinct gut segments of low- and normal-birth-weight piglets. Front Microbiol. 2019;10:797. doi: 10.3389/fmicb.2019.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fu X., Ma X., Geng S., Jiang X., Huang Q. Intestinal microbiome -Metabolome responses to essential oils in piglets. Front Microbiol. 2018;9:1988. doi: 10.3389/fmicb.2018.01988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Bushman F.D., FitzGerald G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci. 2015;112:10479–10484. doi: 10.1073/pnas.1501305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libao-Mercado A.J.O., Zhu C.L., Cant J.P., Lapierre H., Thibault J.N., Se`ve B. Dietary and endogenous amino acids are the main contributors to microbial protein in the upper gut of normally nourished pigs. J Nutr. 2009;139:1088–1094. doi: 10.3945/jn.108.103267. [DOI] [PubMed] [Google Scholar]
- Lin C., Wan J., Su Y., Zhu W. Effects of early intervention with maternal fecal microbiota and antibiotics on the gut microbiota and metabolite profiles of piglets. Metabolites. 2018;8:89. doi: 10.3390/metabo8040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M.Y., de Zoete M.R., van Putten J.P.M., Strijbis K. Redirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylases. Front Immunol. 2015;6:554. doi: 10.3389/fimmu.2015.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Yang X., Yuan P., Yang J., Wang P., Zhong H. Undernutrition shapes the gut microbiota and bile acid profile in association with altered gut-liver FXR signaling in weaning pigs. J Agric Food Chem. 2019;67:3691–3701. doi: 10.1021/acs.jafc.9b01332. [DOI] [PubMed] [Google Scholar]
- Liu P., Zhao J., Wang W., Guo P., Lu W., Wang C. Dietary corn bran altered the diversity of microbial communities and cytokine production in weaned pigs. Front Microbiol. 2018;9:2090. doi: 10.3389/fmicb.2018.02090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.X., Li Y., Dai W.K., Li X.S., Qiu C.Z., Ruan M.L. Fecal microbiota transplantation induces remission of infantile allergic colitis through gut microbiota re-establishment. World J Gastroenterol. 2017;23:8570. doi: 10.3748/wjg.v23.i48.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lv Q.L., Ren H.Y., Gao L., Zhao P., Yang X.M. The altered gut microbiota of high-purine-induced hyperuricemia rats and its correlation with hyperuricemia. Peer J. 2020;8 doi: 10.7717/peerj.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.H., Su Y., Wright A.D.G., Zhang L.L., Smidt H., Zhu W.Y. Lean breed Landrace pigs harbor fecal methanogens at higher diversity and density than obese breed Erhualian pigs. Archaea. 2012;2012 doi: 10.1155/2012/605289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv C.H., Wang T., Regmi N., Chen X., Huang K., Liao S.F. Effects of dietary supplementation of selenium-enriched probiotics on production performance and intestinal microbiota of weanling piglets raised under high ambient temperature. J Anim Physiol An N. 2015;99:1161–1171. doi: 10.1111/jpn.12326. [DOI] [PubMed] [Google Scholar]
- Lyte M. The role of microbial endocrinology in infectious disease. J Endocrinol. 1993;137:343–345. doi: 10.1677/joe.0.1370343. [DOI] [PubMed] [Google Scholar]
- Mach N., Berri M., Estellé J., Levenez F., Lemonnier G., Denis C. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environ Microbiol Rep. 2015;7:554–569. doi: 10.1111/1758-2229.12285. [DOI] [PubMed] [Google Scholar]
- Mandal A.K., Mount D.B. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. 2015;77:323–345. doi: 10.1146/annurev-physiol-021113-170343. [DOI] [PubMed] [Google Scholar]
- Marcobal A., Kashyap P.C., Nelson T.A., Aronov P.A., Donia M.S., Spormann A. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. 2013;7:1933–1943. doi: 10.1038/ismej.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A., Savidge T., Shulman R.J. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500–1512. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard C.L., Elson C.O., Hatton R.D., Weaver C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack U.M., Curião T., Buzoianu S.G., Prieto M.L., Ryan T., Varley P. Exploring a possible link between the intestinal microbiota and feed efficiency in pigs. Appl Environ Microbiol. 2017;83 doi: 10.1128/AEM.00380-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Soares H., Raveh-Sadka T., Azulay S., Ben-Shlomo Y., Cohen Y., Ofek T. Model of personalized postprandial glycemic response to food developed for an Israeli cohort predicts responses in Midwestern American individuals. Am J Clin Nutr. 2019;110:63–75. doi: 10.1093/ajcn/nqz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q., Luo Z., Cao C., Sun S., Ma Q., Li Z. Weaning alters intestinal gene expression involved in nutrient metabolism by shaping gut microbiota in pigs. Front Microbiol. 2020;11:694. doi: 10.3389/fmicb.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler B.U., Mosenthin R. A review of interactions between dietary fiber and the gastrointestinal microbiota and their consequences on intestinal phosphorus metabolism in growing pigs. Asian-Australas J Anim Sci. 2008;21:603–615. [Google Scholar]
- Midtvedt T. Microbial bile acid transformation. Am J Clin Nutr. 1974;27:1341–1347. doi: 10.1093/ajcn/27.11.1341. [DOI] [PubMed] [Google Scholar]
- Mitchell R.W., On N.H., Del Bigio M.R., Miller D.W., Hatch G.M. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J Neurochem. 2011;117:735–746. doi: 10.1111/j.1471-4159.2011.07245.x. [DOI] [PubMed] [Google Scholar]
- Moran K., de Lange C.F.M., Ferket P., Fellner V., Wilcock P., van Heugten E. Enzyme supplementation to improve the nutritional value of fibrous feed ingredients in swine diets fed in dry or liquid form. J Anim Sci. 2016;94:1031–1040. doi: 10.2527/jas.2015-9855. [DOI] [PubMed] [Google Scholar]
- Mu C., Zhang L., He X., Smidt H., Zhu W. Dietary fibres modulate the composition and activity of butyrate-producing bacteria in the large intestine of suckling piglets. Anton Leeuw Int JG. 2017;110:687–696. doi: 10.1007/s10482-017-0836-4. [DOI] [PubMed] [Google Scholar]
- Niu Q., Li P., Hao S., Zhang Y., Kim S.W., Li H. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci Rep. 2015;5:9938. doi: 10.1038/srep09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nøhr M.K., Egerod K.L., Christiansen S.H., Gille A., Offermanns S., Schwartz T.W., Møller M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015;290:126–137. doi: 10.1016/j.neuroscience.2015.01.040. [DOI] [PubMed] [Google Scholar]
- Novoseletskyi V. Effectiveness of the dosed individual isometric physical exercise in patients with knee osteoarthritis. Eur J Appl Physiol. 2013;87:108–111. [Google Scholar]
- O'Mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Olayanju A., Jones L., Greco K., Goldring C.E., Ansari T. Application of porcine gastrointestinal organoid units as a potential in vitro tool for drug discovery and development. J Appl Toxicol. 2019;39:4–15. doi: 10.1002/jat.3641. [DOI] [PubMed] [Google Scholar]
- Pang X., Hua X., Yang Q., Ding D., Che C., Cui L. Inter-species transplantation of gut microbiota from human to pigs. ISME J. 2007;1:156–162. doi: 10.1038/ismej.2007.23. [DOI] [PubMed] [Google Scholar]
- Papadomichelakis G., Zoidis E., Mountzouris K.C., Lippas T., Fegeros K. Glycerine kinase gene expression, nutrient digestibility and gut microbiota composition in post-weaned pigs fed diets with increasing crude glycerine levels. Anim Feed Sci Technol. 2012;177:247–252. [Google Scholar]
- Patel V., Patel A.K., Parmar N.R., Patel A.B., Reddy B., Joshi C.G. Characterization of the rumen microbiome of Indian Kankrej cattle (Bos indicus) adapted to different forage diet. Appl Microbiol Biotechnol. 2014;98:9749–9761. doi: 10.1007/s00253-014-6153-1. [DOI] [PubMed] [Google Scholar]
- Pathak S.K., Xiang Y., Huang M., Huang T., Cao X., Liu H. Fused tetracyclic tris [1,2,4] triazolo [1,3,5] triazine as a novel rigid electron acceptor for efficient thermally activated delayed fluorescence emitters. RSC Adv. 2020;10:15529. doi: 10.1039/d0ra01925a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu G., Li P., Du T., Niu Q., Fan L., Wang H. Adding appropriate fiber in diet increases diversity and metabolic capacity of distal gut microbiota without altering fiber digestibility and growth rate of finishing pig. Front Microbiol. 2020;11:533. doi: 10.3389/fmicb.2020.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puiman P., Stoll B., Mølbak L., de Bruijn A., Schierbeek H., Boye M. Modulation of the gut microbiota with antibiotic treatment suppresses whole body urea production in neonatal pigs. AM J Physiol-Gastr L. 2013;304:300–310. doi: 10.1152/ajpgi.00229.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan J., Cai G., Ye J., Yang M., Ding R., Wang X. A global comparison of the microbiome compositions of three gut locations in commercial pigs with extreme feed conversion ratios. Sci Rep. 2018;8:1–10. doi: 10.1038/s41598-018-22692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Pérez O., Cruz-Ramón V., Chinchilla-López P., Méndez-Sánchez N. The role of the gut microbiota in bile acid metabolism. Ann Hepatol. 2018;16:21–26. doi: 10.5604/01.3001.0010.5494. [DOI] [PubMed] [Google Scholar]
- Ren W., Chen S., Yin J., Duan J., Li T., Liu G. Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J Nutr. 2014;144:988–995. doi: 10.3945/jn.114.192120. [DOI] [PubMed] [Google Scholar]
- Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- Roager H.M., Hansen L.B., Bahl M.I., Frandsen H.L., Carvalho V., Gobel R.J. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol. 2016;1:16093. doi: 10.1038/nmicrobiol.2016.93. [DOI] [PubMed] [Google Scholar]
- Rusch J.A., Dave J.A. The gut microbiota and metabolic disease: the new frontier? S Afr J Diabetes. 2018;11:4–9. [Google Scholar]
- Saad R.J., Rao S.S., Koch K.L., Kuo B., Parkman H.P., McCallum R.W. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010;105:403–411. doi: 10.1038/ajg.2009.612. [DOI] [PubMed] [Google Scholar]
- Saavedra J.M., Dattilo A.M. Early development of intestinal microbiota: implications for future health. Gastroenterol Clin. 2012;41:717–731. doi: 10.1016/j.gtc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Salvatore F., Suárez-Zamorano N., Mirko T. Host–microbiota mutualism in metabolic diseases. Front Endocrinol. 2017;8:267. doi: 10.3389/fendo.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J., Saperas E., Nogueiras C., Mourelle M., Antolin M., Cadahia A. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998;114:640–648. doi: 10.1016/s0016-5085(98)70577-3. [DOI] [PubMed] [Google Scholar]
- Saraf M.K., Piccolo B.D., Bowlin A.K., Mercer K.E., LeRoith T., Chintapalli S.V. Formula diet driven microbiota shifts tryptophan metabolism from serotonin to tryptamine in neonatal porcine colon. Microbiome. 2017;5:1–13. doi: 10.1186/s40168-017-0297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilderink R., Verseijden C., de Jonge W.J. Dietary inhibitors of histone deacetylases in intestinal immunity and homeostasis. Front Immunol. 2013;4:226. doi: 10.3389/fimmu.2013.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweer W.P., Patience J.F., Schwartz K., Linhares D., Rademacher C., Allen H.K. A review and evaluation of antibiotic alternatives in the literature. J Anim Sci. 2017;95:148. [Google Scholar]
- Shi C., Zhu Y., Niu Q., Wang J., Wang J., Zhu W. The changes of colonic bacterial composition and bacterial metabolism induced by an early food introduction in a neonatal porcine model. Curr Microbiol. 2018;75:745–751. doi: 10.1007/s00284-018-1442-z. [DOI] [PubMed] [Google Scholar]
- Smith E.A., Macfarlane G.T. Enumeration of amino acid fermenting bacteria in the human large intestine: effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol Ecol. 1998;25:355–368. [Google Scholar]
- Smith P.A. The tantalizing links between gut microbes and the brain. Nature. 2015;526:312–314. doi: 10.1038/526312a. [DOI] [PubMed] [Google Scholar]
- Sohail M.U., Asmaa A., Haseeb A., Rizzi R., Marei H.E. Role of the gastrointestinal tract microbiome in the pathophysiology of diabetes mellitus. J Diabetes Res. 2017;2017:1–9. doi: 10.1155/2017/9631435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman M.L., Rosenberger T.A. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol Cell Biochem. 2011;352:173–180. doi: 10.1007/s11010-011-0751-3. [DOI] [PubMed] [Google Scholar]
- Sommer F., Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Soto J.A., Tokach M.D., Dritz S.S., Gonçalves M.A., Woodworth J.C., DeRouchey J.M. Regression analysis to predict the impact of dietary neutral detergent fiber on carcass yield in swine. Translational Anim Sci. 2019;3:1270–1274. doi: 10.1093/tas/txz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll B., Henry J., Reeds P.J., Yu H., Jahoor F., Burrin D.G. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J Nutr. 1998;128:606–614. doi: 10.1093/jn/128.3.606. [DOI] [PubMed] [Google Scholar]
- Sun Y., Su Y., Zhu W. Microbiome-metabolome responses in the cecum and colon of pig to a high resistant starch diet. Front Microbiol. 2016;7:779. doi: 10.3389/fmicb.2016.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Yu K., Zhou L., Su Y., Zhu W. Metabolomic and transcriptomic responses in the liver of pigs induced by long-term intake of raw potato starch. J Anim Sci. 2016;94:1083–1094. doi: 10.2527/jas.2015-9715. [DOI] [PubMed] [Google Scholar]
- Sun Y., Zhou L., Fang L., Su Y., Zhu W. Responses in colonic microbial community and gene expression of pigs to a long-term high resistant starch diet. Front Microbiol. 2015;6:877. doi: 10.3389/fmicb.2015.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z., Wang Y., Yang T., Chen S., Xing K., Wang C. Differences in gut microbiota composition in finishing Landrace pigs with low and high feed conversion ratios. Antonie Leeuwenhoek. 2018;111:1673–1685. doi: 10.1007/s10482-018-1057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G.W., Savage D.C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun. 1974;9:591–598. doi: 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss C.A., Levy M., Korem T., Dohnalová L., Shapiro H., Jaitin D.A. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 2016;167:1495–1510. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Thaiss C.A., Zeevi D., Levy M., Zilberman-Schapira G., Suez J., Tengeler A.C. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- Thomas C.M., Versalovic J. Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microb. 2010;1:148. doi: 10.4161/gmic.1.3.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Bruggeman G., van den Berg M., Borewicz K., Scheurink A.J., Bruininx E. Effects of pectin on fermentation characteristics, carbohydrate utilization, and microbial community composition in the gastrointestinal tract of weaning pigs. Mol Nutr Food Res. 2017;61:1600186. doi: 10.1002/mnfr.201600186. [DOI] [PubMed] [Google Scholar]
- Tian S., Wang J., Yu H., Zhu W. Changes in ileal microbial composition and microbial metabolism by an early-life galacto-oligosaccharides intervention in a neonatal porcine model. Nutrients. 2019;11:1753. doi: 10.3390/nu11081753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaroli V., Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Turroni S., Brigidi P., Cavalli A., Candela M. Microbiota–host transgenomic metabolism, bioactive molecules from the inside: Miniperspective. J Med Chem. 2018;61:47–61. doi: 10.1021/acs.jmedchem.7b00244. [DOI] [PubMed] [Google Scholar]
- Vajro P., Paolella G., Fasano A. Microbiota and gut-liver axis: a mini-review on their influences on obesity and obesity related liver disease. J Pediatr Gastroenterol Nutr. 2013;56:461. doi: 10.1097/MPG.0b013e318284abb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyck L.I., Morrow E.M. Genetic control of postnatal human brain growth. Curr Opin Neurol. 2017;30:114–124. doi: 10.1097/WCO.0000000000000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte D., Falony G., Vieira-Silva S., Tito R.Y., Joossens M., Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65:57–62. doi: 10.1136/gutjnl-2015-309618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velagapudi V.R., Hezaveh R., Reigstad C.S., Gopalacharyulu P., Yetukuri L., Islam S. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51:1101–1112. doi: 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt R.M., Summa K.C., Forsyth C.B., Green S.J., Engen P., Naqib A. The circadian clock mutation promotes intestinal dysbiosis. Alcohol Clin Exp Res. 2016;40:335–347. doi: 10.1111/acer.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volmar C.H., Wahlestedt C. Histone deacetylases (HDACs) and brain function. Neuroepigenetics. 2015;1:20–27. [Google Scholar]
- Wada S., Sato K., Ohta R., Wada E., Bou Y., Fujiwara M. Ingestion of low dose pyroglutamyl leucine improves dextran sulfate sodium-induced colitis and intestinal microbiota in mice. J Agric Food Chem. 2013;61:8807–8813. doi: 10.1021/jf402515a. [DOI] [PubMed] [Google Scholar]
- Waldecker M., Kautenburger T., Daumann H., Busch C., Schrenk D. Inhibition of histonedeacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. 2008;19:587–593. doi: 10.1016/j.jnutbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Wall R., Ross R.P., Shanahan F. Metabolic activity of the enteric microbiota influences the fatty acid composition of murine and porcine liver and adipose tissues. Am J Clin Nutr. 2009;89:1393–1401. doi: 10.3945/ajcn.2008.27023. [DOI] [PubMed] [Google Scholar]
- Walters E.M., Prather R.S. Advancing swine models for human health and diseases. Mo Med. 2013;110:212–215. [PMC free article] [PubMed] [Google Scholar]
- Wan J.J., Lin C.H., Ren E.D., Su Y., Zhu W.Y. Effects of early intervention with maternal fecal bacteria and antibiotics on liver metabolome and transcription in neonatal pigs. Front Physiol. 2019;10:171. doi: 10.3389/fphys.2019.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Shen Z., Cao S., Zhang Q., Peng Y., Hong Q. Effects of tributyrin on growth performance, intestinal microflora and barrier function of weaned pigs. Anim Feed Sci Technol. 2019;258:114311. [Google Scholar]
- Wang C., Shi C., Zhang Y., Song D., Lu Z., Wang Y. Microbiota in fermented feed and swine gut. Appl Microbiol Biotechnol. 2018;102:2941–2948. doi: 10.1007/s00253-018-8829-4. [DOI] [PubMed] [Google Scholar]
- Wang J., Qin C., He T., Qiu K., Sun W., Zhang X. Alfalfa-containing diets alter luminal microbiota structure and short chain fatty acid sensing in the caecal mucosa of pigs. J Anim Sci Biotechnol. 2018;9:11. doi: 10.1186/s40104-017-0216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Blachier F., Zhao F., Yin Y. Intestinal microbiota: development, metabolism and functions. J Food Agric Environ. 2011;9:121–129. [Google Scholar]
- Wang X., Tsai T., Deng F., Wei X., Chai J., Knapp J. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome. 2019;7:109. doi: 10.1186/s40168-019-0721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse J., Edwards B., Atkinson G., Reilly T., Spencer M., Elsey A. 1st ed. Hubbard; London: 2016. Occupational factors in pilot mental health: sleep loss, jet lag, and shift work. [Google Scholar]
- Watson R.E., Desesso J.M., Hurtt M.E., Cappon G.D. Postnatal growth and morphological development of the brain: a species comparison. Birth Defects Res B Dev Reprod Toxicol. 2006;77:471–484. doi: 10.1002/bdrb.20090. [DOI] [PubMed] [Google Scholar]
- Wen H., Feng L., Jiang W., Liu Y., Jiang J., Li S. Dietary tryptophan modulates intestinal immune response, barrier function, antioxidant status and gene expression of TOR and Nrf2 in young grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2014;40:275–287. doi: 10.1016/j.fsi.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Xu C.C., Yang S.F., Zhu L.H., Cai X., Sheng Y.S., Zhu S.W. Regulation of N-acetyl cysteine on gut redox status and major microbiota in weaned piglets. J Anim Sci. 2014;92:1504–1511. doi: 10.2527/jas.2013-6755. [DOI] [PubMed] [Google Scholar]
- Xu R., Lu Y., Wang J., Liu J., Su Y., Zhu W. Effects of the different dietary fibers on luminal microbiota composition and mucosal gene expression in pig colons. J Funct Foods. 2019;59:71–79. [Google Scholar]
- Xu R., Wan J., Lin C., Su Y. Effects of early intervention with antibiotics and maternal fecal microbiota on transcriptomic profiling ileal mucusa in neonatal pigs. Antibiotics. 2020;9:35. doi: 10.3390/antibiotics9010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Huang X., Fang S., Xin W., Huang L., Chen C. Uncovering the composition of microbial community structure and metagenomics among three gut locations in pigs with distinct fatness. Sci Rep. 2016;6:27427. doi: 10.1038/srep27427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Xiang Y., Robinson K., Wang J., Zhang G., Zhao J. Gut microbiota is a major contributor to adiposity in pigs. Front Microbiol. 2018;9:3045. doi: 10.3389/fmicb.2018.03045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Yang M., Fang S., Huang X., He M., Ke S. Evaluating the profound effect of gut microbiome on host appetite in pigs. BMC Microbiol. 2018;18:215. doi: 10.1186/s12866-018-1364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Martínez I., Walter J., Keshavarzian A., Rose D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe. 2013;23:74–81. doi: 10.1016/j.anaerobe.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Yin J., Li Y., Han H., Chen S., Gao J., Liu G. Melatonin reprogramming of guicrobiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res. 2018;65:e12524. doi: 10.1111/jpi.12524. [DOI] [PubMed] [Google Scholar]
- Yin J., Ma J., Li Y., Ma X., Chen J., Zhang H. Branched-chain amino acids, especially of leucine and valine, mediate the protein restricted response in a piglet model. Food Funct. 2020;11:1304–1311. doi: 10.1039/c9fo01757g. [DOI] [PubMed] [Google Scholar]
- Yin J., Li Y., Han H., Ma J., Liu G., Wu X., Zhu G. Administration of exogenous melatonin improves the diurnal rhythms of the gut microbiota in mice fed a high-fat diet. mSystems. 2020;5:e00002–e20. doi: 10.1128/mSystems.00002-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota A., Fukiya S., Islam K.B., Ooka T., Ogura Y., Hayashi T. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microb. 2012;3:455–459. doi: 10.4161/gmic.21216. [DOI] [PubMed] [Google Scholar]
- Yolanda S., Arlette S., Paola G. Gut microbiota in obesity and metabolic disorders. Proc Nutr Soc. 2010;69:434–441. doi: 10.1017/S0029665110001813. [DOI] [PubMed] [Google Scholar]
- Yu D., Zhu W., Hang S. Effects of long-term dietary protein restriction on intestinal morphology, digestive enzymes, gut hormones, and colonic microbiota in pigs. Animals. 2019;9:180. doi: 10.3390/ani9040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Ren E., Xu J., Su Y., Zhu W. Effects of early intervention with sodium butyrate on lipid metabolism-related gene expression and liver metabolite profiles in neonatal piglets. Livest Sci. 2017;195:80–86. [Google Scholar]
- Zarrinpar A., Chaix A., Yooseph S., Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell metabol. 2014;20:1006–1017. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yue Y., Shi M., Tian M., Ji J., Liao X. Dietary Luffa cylindrica (L.) Roem promotes branched-chain amino acid catabolism in the circulation system via gut microbiota in diet-induced obese mice. Food Chem. 2020:126648. doi: 10.1016/j.foodchem.2020.126648. [DOI] [PubMed] [Google Scholar]
- Zhang W., Ma C., Xie P., Zhu Q., Wang X., Yin Y. Gut microbiota of newborn piglets with intrauterine growth restriction have lower diversity and different taxonomic abundances. J Appl Microbiol. 2019;127 doi: 10.1111/jam.14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lu T., Han L., Zhao L., Niu Y., Chen H. L-glutamine supplementation alleviates constipation during late gestation of mini sows by modifying the microbiota composition in feces. BioMed Res Int. 2017;2017 doi: 10.1155/2017/4862861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Liu P., Wu Y., Guo P., Liu L., Ma N. Dietary fiber increases butyrate-producing bacteria and improves the growth performance of weaned piglets. J Agric Food Chem. 2018;66:7995–8004. doi: 10.1021/acs.jafc.8b02545. [DOI] [PubMed] [Google Scholar]
- Zhou L., Fang L., Sun Y., Su Y., Zhu W. Effects of the dietary protein level on the microbial composition and metabolomic profile in the hindgut of the pig. Anaerobe. 2016;38:61–69. doi: 10.1016/j.anaerobe.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Zhu W.H., Li D.F., Wu H., Li J.T., Chen Y.Q., Guan H.S. Effects of purified polymannuronate on the performance, immune status, antioxidant capacity, intestinal microbial populations and volatile fatty acid concentrations of weaned piglets. Anim Feed Sci Technol. 2016;216:161–168. [Google Scholar]