Abstract
Objective
Near-infrared fluorescence cholangiography (NIRF-C) can help to identify the bile duct during laparoscopic cholecystectomy. This retrospective study was performed to investigate the effect of NIRF-C in laparoscopic cholecystectomy.
Methods
Consecutive patients who underwent NIRF-C-assisted laparoscopic cholecystectomy (n = 34) or conventional laparoscopic cholecystectomy (n = 36) were enrolled in this study. Identification of biliary structures, the operation time, intraoperative blood loss, and postoperative complications were analyzed.
Results
Laparoscopic cholecystectomy was completed in all patients without conversion to laparotomy. The median operation time and intraoperative blood loss were not significantly different between the two groups. No intraoperative injuries or postoperative complications occurred in either group. In the NIRF-C group, the visualization rate of the cystic duct, common bile duct, and common hepatic duct prior to dissection was 91%, 79%, and 53%, respectively. The success rate of cholangiography was 100% in the NIRF-C group. NIRF-C was more effective for visualizing biliary structures in patients with a BMI of <25 than >25 kg/m2.
Conclusions
NIRF-C is a safe and effective technique that enables real-time identification of the biliary anatomy during laparoscopic cholecystectomy. NIRF-C helps to improve the efficiency of dissection.
Keywords: Near-infrared imaging, fluorescence cholangiography, indocyanine green, laparoscopic cholecystectomy, biliary anatomy, intraoperative visualization
Introduction
Laparoscopic cholecystectomy (LC) has become the gold standard for the treatment of benign lesions of the gallbladder.1 It has the advantages of effectiveness, minimal invasion, and rapid recovery; however, it is also associated with surgical complications, the most serious of which is bile duct injury (BDI). The incidence of BDI has remained steady at 0.4% to 0.7%.2 BDI is a rare but serious complication that may lead to prolonged hospitalization, increased health costs, and decreased quality of life. Among the many causes of intraoperative BDI, unclear identification of the bile duct during the procedure accounts for 71% to 97% of cases.3 Bile duct identification is especially problematic in patients with inflammatory edema, obesity, and biliary tract variation.
Intraoperative cholangiography can help surgeons to correctly identify the anatomy of the bile duct to avoid BDI.4 Intraoperative cholangiography is currently the accepted gold standard method for intraoperative identification of the biliary anatomy.5 However, intraoperative cholangiography has some drawbacks, such as being time-consuming, requiring additional equipment and technicians, risking radiation exposure among staff and patients,6–8 and requiring the injection of contrast material into the bile duct, which may increase the risk of BDI.
Near-infrared fluorescence cholangiography (NIRF-C) is a new technique for bile duct visualization during LC procedures. It requires preoperative intravenous injection of indocyanine green (ICG), a water-soluble dye. After entering the human blood circulation, ICG binds to plasma protein and rapidly enters hepatocytes; it is finally excreted through the intestine with the bile. Because ICG has no chemical reaction in vivo, it is excreted in its original form only through the bile. However, ICG can emit fluorescence when combined with protein in bile. When illuminated with near-infrared light, protein-bound ICG shows fluorescence at a wavelength of around 840 nm.9 Because of the weak penetration of 840-nm light, it is rarely absorbed by water or protein; this allows the protein-bound ICG to be captured by the infrared light camera for imaging.10 This property has led to the increasing use of ICG in clinical medicine.11–14 In 2009, Ishizawa et al.14 first reported the application of ICG fluorescence imaging technology in LC. In 2010, the same authors published the largest series of patients (52 patients) who underwent LC with ICG NIRF-C and concluded that fluorescent cholangiography can be used to identify the biliary anatomy in real time during dissection of Calot’s triangle.15
NIRF-C is noninvasive, efficient, and safe.2,3,16,17 In this study, we investigated the feasibility and safety of NIRF-C in detecting the anatomy of the biliary tree during LC.
Materials and methods
Patients
Patients who met the following criteria were included in this retrospective clinical observational study: age of ≥18 years, benign gallbladder lesions, ability to tolerate laparoscopic surgery, and complete clinical records. Patients were excluded if they had one of the following conditions: acute cholecystitis, cholangitis, pregnancy, cirrhosis, organ dysfunction, conversion to an open procedure, previous abdominal surgery, or known allergy to ICG.
Consecutive patients who underwent LC from January 2018 to January 2020 were enrolled. The patients were divided into two groups: the NIRF-C group, in which NIRF-C was used in LC, and the conventional group, in which conventional LC was performed.
This study was approved by the medical ethics committee of the Third Affiliated Hospital of Sun Yat-sen University (Guangzhou, China) in January 2020, and all patients provided written informed consent. We have de-identified all patient details. The study complied with the Equator Network guidelines (https://www.equator-network.org/).
Procedures
The patients in the conventional group were treated as follows. Following anesthesia, a 1.5-cm incision was made under the umbilicus, an air needle was inserted to establish pneumoperitoneum, and the intra-abdominal air pressure was maintained at 1.3 kPa. A 10-mm trocar was placed in the subumbilical incision and then inserted into a conventional laparoscope. A trocar was also placed under the xiphoid process and on the right abdominal wall, respectively, and an ultrasonic knife and grasping forceps were placed through the trocars. Calot’s triangle was dissected, and the cystic duct and cystic artery were exposed and disconnected. The seromuscular layer of the gallbladder was cut at about 0.5 cm from the liver margin, and the mesangium of the gallbladder was cut off with an ultrasonic knife or electrocoagulation hook. The gallbladder was removed.
The patients in the NIRF-C group were treated as follows. First, all patients received an intradermal sensitivity test for ICG. Patients who were not allergic to ICG were injected with 1 mL of ICG (2.5 mg/mL) into the elbow vein approximately 30 minutes prior to the laparoscopic procedure. The cholecystectomy techniques were the same as those in the conventional group except that near-infrared fluorescence was used to observe the structure of the bile duct before, during, and after dissection of Calot’s triangle.
NIRF-C was performed using the PINPOINT system (NOVADAQ, Mississauga, Ontario, Canada). The core components of the PINPOINT system include laparoscopic lighting and video processing, a high-definition laparoscopic camera, and a set of high-definition laparoscopes. The system can collect and display high-definition white light and near-infrared fluorescence images in real time.
Statistical analysis
All statistical analyses were performed with SAS software version 22.0 (SAS Institute Inc., Cary, NC, USA). Normally distributed data are presented as mean ± standard deviation, and differences between groups were assessed with a t test. Non-normally distributed data are presented as median (lower quartile, upper quartile), and differences between groups were evaluated with Wilcoxon’s test. Enumeration data are presented as proportions. Comparisons between groups were performed using the chi-squared test or Fisher’s exact test. A p value of <0.05 was considered statistically significant.
Results
Seventy patients were included in this study. The NIRF-C group comprised 34 patients (18 women, 16 men; mean age, 50.0 ± 12.7 years; mean body mass index (BMI), 21.5 ± 2.5 kg/m2), and the conventional group comprised 36 patients (22 women, 14 men; mean age, 45.7 ± 13.9 years; mean BMI, 22.6 ± 3.2 kg/m2). There were no significant differences in the patients’ characteristics between the two groups (Table 1).
Table 1.
Patients’ demographics.
| Factors | Conventional group (n = 36) | NIRF-C group (n = 34) | p value |
|---|---|---|---|
| Sex | 0.490 | ||
| Male | 14 (39) | 16 (47) | |
| Female | 22 (61) | 18 (53) | |
| BMI, kg/m2 | 22.6 ± 3.2 | 21.5 ± 2.5 | 0.531 |
| Age, years | 45.7 ± 13.9 | 50.0 ± 12.7 | 0.183 |
| Diagnosis | a0.611 | ||
| Cholecystolithiasis | 23 (64) | 25 (75) | |
| Gallbladder polyps | 4 (11) | 3 (9) | |
| Cholecystic adenomyosis | 7 (19) | 3 (9) | |
| Cholecystic adenoma | 2 (56) | 3 (9) |
Data are presented as n (%) or mean ± standard deviation.
aFisher’s exact test.
NIRF-C, near-infrared fluorescence cholangiography; BMI, body mass index.
Patients in both groups completed LC without conversion to laparotomy, and no procedure-related injury to the common bile duct (CBD), common hepatic duct (CHD), or cystic duct occurred. The operation time was shorter in the NIRF-C group than in the conventional group, but the difference was not statistically significant. There was no significant difference in the intraoperative bleeding volume between the two groups (Table 2).
Table 2.
Surgical outcomes.
| Factors | Conventional group (n = 36) | NIRF-C group (n = 34) | p value |
|---|---|---|---|
| Operation time, minutes | 71 (58, 93) | 67 (57, 84) | 0.522 |
| Intraoperative bleeding volume, mL | 5 (5, 10) | 5 (2, 10) | 0.802 |
Data are presented as median (lower quartile, upper quartile).
NIRF-C, near-infrared fluorescence cholangiography.
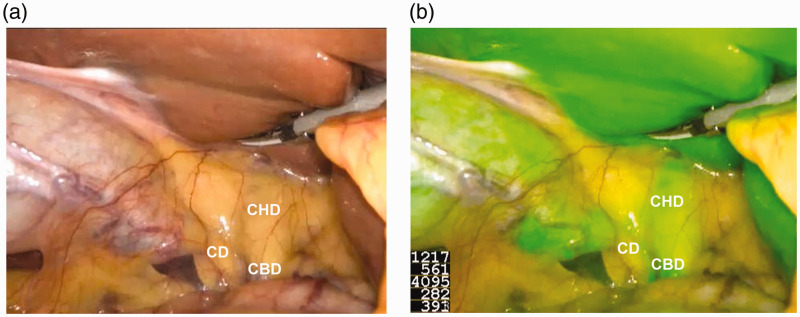
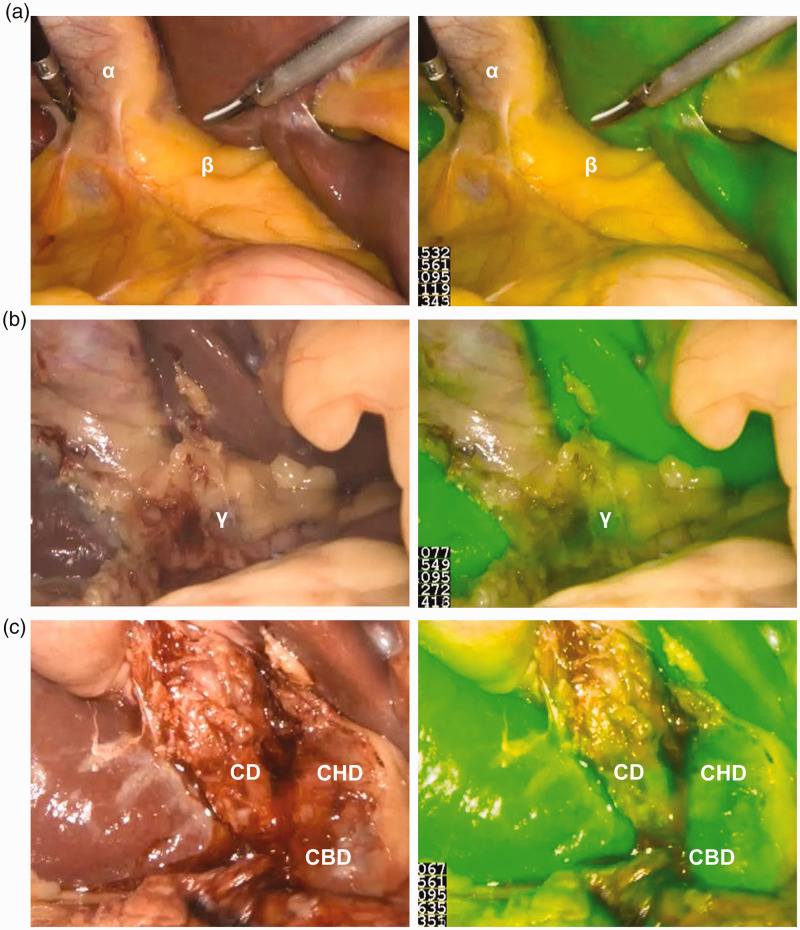
Prior to dissection, we were able to delineate at least one biliary structure in all patients in the NIRF-C group with a 100% success rate of cholangiography. The use of ICG provided stable and clear images for the operator (Figure 1). The visualization rates of the cystic duct, CHD, and CBD were higher in the NIRF-C group than in the conventional group (91% vs. 74%, 53% vs. 21%, and 79% vs. 47%, respectively), although the difference was significant only for the visualization rates of the CHD and CBD (p < 0.05) (Table 3). We also found that NIRF-C provided better visualization of biliary structures in patients with a BMI of <25 than >25 kg/m2 (Table 4), although only the difference in CBD visualization was statistically significant (p < 0.05). In obese patients, however, NIRF-C also helped to identify the extrahepatic biliary structures by repeated intraoperative fluorescence imaging (Figure 2). No drug allergy or other drug-related complications occurred during the use of ICG. No postoperative complications such as abdominal bleeding, cholecystic fistula, abdominal infection, or wound infection occurred in either group.
Figure 1.
Intraoperative identification of extrahepatic bile ducts with (a) conventional visual mode and (b) near-infrared fluorescence cholangiography mode.
CD, cystic duct; CHD, common hepatic duct; CBD, common bile duct.
Table 3.
Identification rates for extrahepatic bile ducts in CV mode and NIRF-C mode.
| CV group (n = 34) | NIRF-C group (n = 34) | p value | |
|---|---|---|---|
| CD | 25 (74) | 31 (91) | 0.056 |
| CHD | 7 (21) | 18 (53) | 0.001 |
| CBD | 16 (47) | 27 (79) | 0.006 |
Data are presented as n (%).
CV, conventional visual; NIRF-C, near-infrared fluorescence cholangiography; CD, cystic duct; CHD, common hepatic duct; CBD, common bile duct.
Table 4.
Identification rates for extrahepatic bile ducts with near-infrared fluorescence cholangiography in obese and non-obese patients.
| BMI of <25 kg/m2 (n = 22) | BMI of >25 kg/m2 (n = 12) | p value | |
|---|---|---|---|
| CD | 21 (95) | 10 (83) | 0.234 |
| CHD | 16 (73) | 2 (17) | 0.002 |
| CBD | 18 (82) | 9 (75) | 0.677a |
Data are presented as n (%).
aFisher’s exact test.
BMI, body mass index.
Figure 2.
Intraoperative identification of extrahepatic bile ducts with conventional visual mode and near-infrared fluorescence cholangiography (NIRF-C) mode in obese patients. (a) Before dissection, Calot’s triangle was covered with thick fat (β), and NIRF-C failed to visualize the extrahepatic bile ducts. α: Gallbladder. Left panel: conventional visual mode; right panel: NIRF-C mode. (b) After partial dissection, the extrahepatic bile ducts began to be visualized (γ). Left panel: conventional visual mode; right panel: NIRF-C mode. (c) After complete dissection, the extrahepatic bile ducts were completely visualized.
CD: cystic duct; CHD: common hepatic duct; CBD: common bile duct. Left panel: conventional visual mode; right panel: NIRF-C mode.
Discussion
This study was performed to determine the feasibility and safety of NIRF-C in the identification of extrahepatic biliary structures in LC. Early recognition of iatrogenic BDI is of paramount importance.18 Our results show that NIRF-C is able to identify at least one extrahepatic bile duct structure in all cases and is completely safe. The visualization rates of extrahepatic biliary structures, especially the CHD and CBD, clearly increased in NIRF-C mode. No drug allergies were evident during the use of ICG, and no postoperative complications occurred.
The key step in LC is the dissection of Calot’s triangle. This is also the most time-consuming step, especially in patients with acute cholecystitis, acute biliary pancreatitis, a fibrotic atrophic gallbladder, gallstones stuck in Hartmann’s pouch, Mirizzi syndrome, or an abnormal biliary anatomy. NIRF-C provides the surgeon with a road map of the biliary tree in real time and at any time during dissection of Calot’s triangle,19 making it possible to perform rapid dissection of Calot’s triangle without causing BDI. Dip et al.20 recently conducted a single-blind, randomized, two-arm trial comparing the efficacy of NIRF-C (n = 321) versus white light alone (n = 318) during LC. The authors concluded that NIRF-C was statistically superior to white light alone in visualizing extrahepatic biliary structures during LC. NIRF-C is also easy to perform, only requiring preoperative ICG injection without the need for additional equipment or technicians. All of these advantages of NIRF-C help to shorten the operation time. Our study showed a shorter operative time in the NIRF-C group than in the conventional group.
The surgeon’s experience contributes to the incidence of BDI during LC21 because inexperienced surgeons often lack a thorough understanding of anatomical relationships, particularly the anatomical variations under laparoscopy. NIRF-C is a popular application of fluorescence imaging-guided surgery (FIGS). The European registry on FIGS (www.euro-figs.eu) aims to obtain a snapshot of the current practice of FIGS and is a valuable tool with which to promote and monitor FIGS-related educational and consensus activities in Europe.22 NIRF-C allows for visualization of the bile duct and thus helps young surgeons to identify the anatomical structure of the bile duct, reducing the incidence of BDI. NIRF-C is therefore a very useful tool for the teaching of LC.
ICG is a nearly non-toxic fluorescent dye and the only agent approved by the Food and Drug Administration for use in human subjects.23 The injected ICG is rapidly cleared by hepatic metabolism, leaving no metabolites in the body. The risk of anaphylaxis is approximately 0.003% at doses of >0.5 mg/kg.24 The ICG is rapidly excreted via the biliary tract after peripheral intravenous injection, and bile containing ICG begins to be excreted in a matter of minutes and lasts about 6 hours.22 Hence, ICG has very high safety.3,25 Lehrskov et al.26 recently conducted a non-inferiority blinded randomized controlled trial of patients who underwent either intraoperative fluorescence cholangiography (n = 60) or X-ray cholangiography (n = 60) during elective LC. The authors concluded that fluorescence cholangiography was non-inferior to X-ray cholangiography in visualizing the critical junction during LC. In the present study, intravenous injection of ICG 30 minutes before the operation enabled the surgeon to obtain clear and stable images of the biliary anatomy in the NIRF-C group. In addition, there was no need to perform bile duct puncture during the whole procedure of NIRF-C, substantially reducing the risk of BDI. Moreover, NIRF-C requires no X-ray machine, thus avoiding the risk of radiation injury. Together, these advantages allow NIRF-C to improve the level of safety in LC.
A disadvantage of NIRF-C is that its tissue penetration ability is limited to 5 to 10 mm. In obese patients, Calot’s triangle is often covered with a thick layer of fat, limiting the effectiveness of NIRF-C. In this study, we investigated the relationship between visualization of the extrahepatic bile duct and the BMI and found that visualization of biliary structures by NIRF-C was better in patients with a BMI of <25 than >25 kg/m2, especially for visualization of the CHD. Importantly, repeated images of the bile duct structure during dissection of Calot’s triangle can be obtained during NIRF-C-assisted procedures. Hence, NIRF-C is a highly suitable technique for obese patients.
The association between the timing and dose of ICG and optimal visualization of the bile ducts when performing NIRF-C remains unknown. In most recent studies, the timing of ICG injection varied from 30 to 60 minutes prior to the start of surgery.3,23,27 Longer times of 2.5 to 24 hours have also been reported.2 Finally, adequate images of the extrahepatic bile duct were able to be obtained in all studies. In patients with normal liver function, 95% of the ICG is captured by hepatocytes and excreted into the bile within 15 minutes after injection.28 For patients with impaired liver clearance, however, how long this process will take is uncertain. Therefore, exactly when ICG should be injected to obtain the best image of the bile ducts remains unknown. Another problem is the uncertainty about the dose of ICG injection. We used a dose of 2.5 mg in the present study. This dose is based on the current literature, but the dose that can provide the best bile duct image is unclear. Therefore, future studies should focus on the relationship between the optimal dose and timing of ICG administration and bile duct visualization. The present study had several limitations inherent to retrospective studies, and the number of patients was limited.
Conclusion
NIRF-C is a safe and effective approach in the performance of LC. It is easy to perform and does not require additional equipment or technicians. Hence, it is a highly safe approach that may help the surgeon to conduct LC more efficiently. In particular, NIRF-C is very helpful for patients with a BMI of <25 kg/m2. For patients with a BMI of >25 kg/m2, NIRF-C allows surgeons to repeatedly visualize the bile duct structure during LC.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060520979224 for Application of near-infrared fluorescent cholangiography using indocyanine green in laparoscopic cholecystectomy by Chusi Wang, Wenguang Peng, Jiarui Yang, Yuxuan Li, Jiawei Yang, Xueqiao Hu, Long Xia, Lei Zhang, Yuesi Zhong, Liang Qiao and Weidong Pan in Journal of International Medical Research
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was supported by the National Natural Science Foundation of China (82002587), Sun Yat-Sen University Clinical Research Program (YHJH201910), and China Postdoctoral Science Foundation (2020TQ0370).
ORCID iDs: Xueqiao Hu https://orcid.org/0000-0001-5638-4152
Liang Qiao https://orcid.org/0000-0002-4723-8935
References
- 1.Sable SA, Nagral S, Doctor N. Single-incision laparoscopic cholecystectomy is associated with a higher bile duct injury rate. Ann Surg 2015; 261: e79. [DOI] [PubMed] [Google Scholar]
- 2.Osayi SN, Wendling MR, Drosdeck JM, et al. Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc 2015; 29: 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spinoglio G, Priora F, Bianchi PP, et al. Real-time near-infrared (NIR) fluorescent cholangiography in single-site robotic cholecystectomy (SSRC): a single-institutional prospective study. Surg Endosc 2013; 27: 2156–2162. [DOI] [PubMed] [Google Scholar]
- 4.Stewart L. Iatrogenic biliary injuries: identification, classification, and management. Surg Clin North Am 2014; 94: 297–310. [DOI] [PubMed] [Google Scholar]
- 5.Khan OA, Balaji S, Branagan G, et al. Randomized clinical trial of routine on-table cholangiography during laparoscopic cholecystectomy. Br J Surg 2011; 98: 362–367. [DOI] [PubMed] [Google Scholar]
- 6.Buddingh KT, Nieuwenhuijs VB. The critical view of safety and routine intraoperative cholangiography complement each other as safety measures during cholecystectomy. J Gastrointest Surg 2011; 15: 1069–1070; author reply 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabone LE, Sarker S, Fisichella PM, et al. To ‘gram or not'? Indications for intraoperative cholangiogram. Surgery 2011; 150: 810–819. [DOI] [PubMed] [Google Scholar]
- 8.Landsman ML, Kwant G, Mook GA, et al. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J Appl Physiol 1976; 40: 575–583. [DOI] [PubMed] [Google Scholar]
- 9.Kokudo N, Ishizawa T. Clinical application of fluorescence imaging of liver cancer using indocyanine green. Liver Cancer 2012; 1: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wishart GC, Loh SW, Jones L, et al. A feasibility study (ICG-10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur J Surg Oncol 2012; 38: 651–656. [DOI] [PubMed] [Google Scholar]
- 11.Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg 2014; 98: 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardesty DA, Thind H, Zabramski JM, et al. Safety, efficacy, and cost of intraoperative indocyanine green angiography compared to intraoperative catheter angiography in cerebral aneurysm surgery. J Clin Neurosci 2014; 21: 1377–1382. [DOI] [PubMed] [Google Scholar]
- 13.Ruscito I, Gasparri ML, Braicu EI, et al. Sentinel node mapping in cervical and endometrial cancer: indocyanine green versus other conventional dyes—a meta-analysis. Ann Surg Oncol 2016; 23: 3749–3756. [DOI] [PubMed] [Google Scholar]
- 14.Ishizawa T, Bandai Y, Kokudo N. Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: an initial experience. Arch Surg 2009; 144: 381–382. [DOI] [PubMed] [Google Scholar]
- 15.Ishizawa T, Bandai Y, Ijichi M, et al. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg 2010; 97: 1369–1377. [DOI] [PubMed] [Google Scholar]
- 16.Prevot F, Rebibo L, Cosse C, et al. Effectiveness of intraoperative cholangiography using indocyanine green (versus contrast fluid) for the correct assessment of extrahepatic bile ducts during day-case laparoscopic cholecystectomy. J Gastrointest Surg 2014; 18: 1462–1468. [DOI] [PubMed] [Google Scholar]
- 17.Ishizawa T, Kaneko J, Inoue Y, et al. Application of fluorescent cholangiography to single-incision laparoscopic cholecystectomy. Surg Endosc 2011; 25: 2631–2636. [DOI] [PubMed] [Google Scholar]
- 18.Pesce A, Palmucci S, La Greca G, et al. Iatrogenic bile duct injury: impact and management challenges. Clin Exp Gastroenterol 2019; 12: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammad I, Stanford C, Morton MD, et al. Structurally modified indocyanine green dyes. Modification of the polyene linker. Dyes Pigm 2013; 99: 275–283. [Google Scholar]
- 20.Dip F, LoMenzo E, Sarotto L, et al. Randomized trial of near-infrared incisionless fluorescent cholangiography. Ann Surg 2019; 270: 992–999. [DOI] [PubMed] [Google Scholar]
- 21.Schwaitzberg SD, Scott DJ, Jones DB, et al. Threefold increased bile duct injury rate is associated with less surgeon experience in an insurance claims database: more rigorous training in biliary surgery may be needed. Surg Endosc 2014; 28: 3068–3073. [DOI] [PubMed] [Google Scholar]
- 22.Agnus V, Pesce A, Boni L, et al. Fluorescence-based cholangiography: preliminary results from the IHU-IRCAD-EAES EURO-FIGS registry. Surg Endosc 2020; 34: 3888–3896. [DOI] [PubMed] [Google Scholar]
- 23.Buchs NC, Hagen ME, Pugin F, et al. Intra-operative fluorescent cholangiography using indocyanin green during robotic single site cholecystectomy. Int J Med Robot 2012; 8: 436–440. [DOI] [PubMed] [Google Scholar]
- 24.Aoki T, Yasuda D, Shimizu Y, et al. Image-guided liver mapping using fluorescence navigation system with indocyanine green for anatomical hepatic resection. World J Surg 2008; 32: 1763–1767. [DOI] [PubMed] [Google Scholar]
- 25.Speich R, Saesseli B, Hoffmann U, et al. Anaphylactoid reactions after indocyanine-green administration. Ann Intern Med 1988; 109: 345–346. [DOI] [PubMed] [Google Scholar]
- 26.Lehrskov LL, Westen M, Larsen SS, et al. Fluorescence or X-ray cholangiography in elective laparoscopic cholecystectomy: a randomized clinical trial. Br J Surg 2020; 107: 655–661. [DOI] [PubMed] [Google Scholar]
- 27.Verbeek FP, Schaafsma BE, Tummers QR, et al. Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg Endosc 2014; 28: 1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaguchi Y, Ishizawa T, Miyata Y, et al. Portal uptake function in veno-occlusive regions evaluated by real-time fluorescent imaging using indocyanine green. J Hepatol 2013; 58: 247–253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060520979224 for Application of near-infrared fluorescent cholangiography using indocyanine green in laparoscopic cholecystectomy by Chusi Wang, Wenguang Peng, Jiarui Yang, Yuxuan Li, Jiawei Yang, Xueqiao Hu, Long Xia, Lei Zhang, Yuesi Zhong, Liang Qiao and Weidong Pan in Journal of International Medical Research