Abstract
Alkyl chlorides are bench-stable chemical feed-stocks that remain among the most underutilized electrophile classes in transition metal catalysis. Overcoming intrinsic limitations of C(sp3)–Cl bond activation, we report the development of a novel organosilane reagent that can participate in chlorine atom abstraction under mild photocatalytic conditions. In particular, we describe the application of this mechanism to a dual nickel/photoredox catalytic protocol that enables the first cross-electrophile coupling of unactivated alkyl chlorides and aryl chlorides. Employing these low toxicity, abundant, and commercially available organochloride building blocks, this methodology allows access to a broad array of highly functionalized C(sp2)–C(sp3) coupled adducts, including numerous drug analogs.
Graphical Abstract
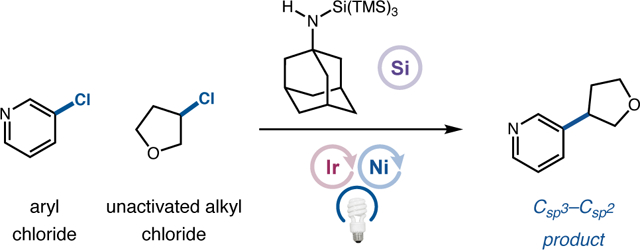
Nickel-catalyzed cross-electrophile coupling has become a well-accepted and powerful strategy for the rapid assembly of C(sp3)-rich drug-like molecules, permitting convergent access to novel chemical space while introducing desirable physicochemical and pharmacokinetic properties.1 Seminal studies by Weix, Gong, Reisman, and others have established the viability and synthetic utility of this approach, wherein a metal reductant such as Zn or Mn obviates the requirement for prefunctionalized, and in many cases air-sensitive, organometallic reagents.2,3 In 2016, our laboratory disclosed an alternative strategy for the cross-electrophile coupling of aryl bromides and alkyl bromides via the use of silane-mediated bromine atom abstraction in combination with dual nickel/photoredox catalysis.4,5 Under these robust and mild conditions, a broad collection of C(sp2)–C(sp3) coupled products can be prepared in high efficiency, and this methodology has witnessed widespread application throughout the pharmaceutical sector, driven primarily by its degree of success with drug-like substrates.6 Following these initial reports, a number of cross-electrophile protocols have leveraged silane-mediated halogen atom abstraction7 in a series of novel transformations that include alkyl-alkyl coupling, trifluoromethylation, alkyl fluorination, as well as alkene hydrosulfamoylation.8,9
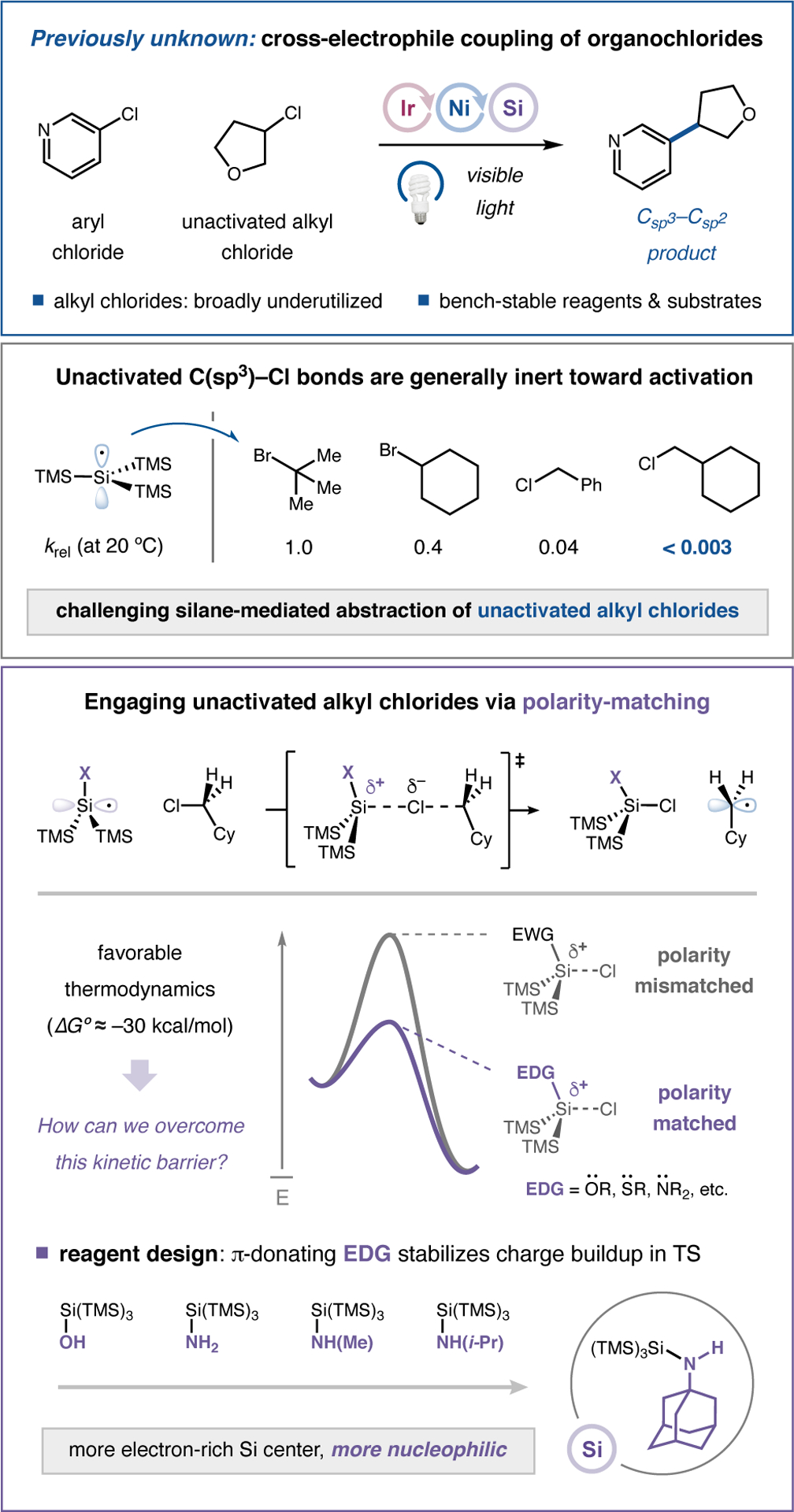
Given the impact and widespread application of cross-electrophile coupling technologies, it is remarkable to consider that simple alkyl chlorides remain effectively unknown as viable reaction partners,10 with the vast majority of systems utilizing C(sp3)–bromides,11 iodides,12 and sulfonates.13 In comparison, the use of organochlorides offers a host of chemical, safety, and economic advantages that include: (i) abundant and diverse structural representation across both commercial and natural sources;14 (ii) reduced toxicity (e.g., as carcinogens) in comparison to most available electrophiles;15 (iii) chemical stability, with respect to handling, and tolerance in multistep sequences;16 and (iv) low sourcing and production costs on scale.17 In practice, however, the benefits arising from the intrinsic chemical stability of alkyl chlorides have prohibited their implementation in nickel-catalyzed cross-electrophile couplings.18 Within the realm of metal reductant-mediated nickel catalysis, strong C(sp3)–Cl bonds prevent the necessary oxidative addition steps, while the accompanying reduction potentials preclude outer-sphere electron-transfer.19 Moreover, within photoredox pathways, the low polarizability of the C–Cl bond kinetically retards chlorine atom transfer in the silyl abstraction event (a step that would otherwise be highly exergonic).7c For example, an aliphatic bromide will typically undergo halogen atom abstraction by supersilyl radical with a rate that is several orders of magnitude faster than the corresponding alkyl chloride (Figure 1). To overcome these limitations, we recently sought to employ polarity matching as a design element for the development of new silane reagents in an effort to significantly lower the kinetic barrier to chlorine atom transfer.20 Herein, we report the successful implementation of these ideals and present the first examples of nickel cross-electrophile coupling using abundant, less toxic, and inexpensive alkyl chlorides.
Figure 1.
Cross-electrophile coupling of organic chlorides
Design plan.
Given the inherent kinetic challenges associated with radical-mediated C(sp3)–Cl activation, we questioned whether we could induce an increased polarity-matching effect between an unactivated C–Cl bond and the silyl abstraction reagent via judicious selection of substituents that would impose increased electron density on an open-shell silicon species. Recognizing that π-donors are well-established to increase the nucleophilic character of adjacent spin-centers, we hypothesized that the incorporation of a heteroatom (i.e., nitrogen)21 into the silane reagent might significantly improve its polarity complementarity with C–Cl bonds and thereby dramatically lower the barrier to chlorine atom abstraction. Moreover, we envisioned that a bulky N-alkyl substituent could significantly improve the electron-releasing capacity of the nitrogen donor via induction while simultaneously conferring hydrolytic stability to the labile Si–N bond.22 To this end, we disclose the discovery and development of novel organosilicon reagents that fulfill these design criteria, and we highlight the value of silane 3, a 1-ada-mantylamine-substituted supersilyl agent that is bench-stable, inexpensive, and broadly useful for photocatalytic alkyl chloride activation (Table 1).
Table 1.
Control Reactions of Optimized Conditionsa
 | |||
|---|---|---|---|
| entry | conditions | silane | Yieldb |
| 1 | as above | 3 | 73% |
| 2 | as above | TMS3SiNH(tBu) | 72% |
| 3 | as above | TMS3SiNH(iPr) | 45% |
| 4 | as above | TMS3SiNH(nBu) | 30% |
| 5 | as above | TMS3SiOH | 0% (100%)c |
| 6 | as above | TMS3SiH | 0% (77%)c |
| 7 | DMA as solvent | 3 | 60% |
| 8 | 4,4′-dtbbpy as ligand | 3 | 20% |
| 9 | no photocatalyst | 3 | 0% |
| 10 | no nickel catalyst | 3 | 0% |
| 11 | no silane | none | 0% |
| 12 | no light | 3 | 0% |
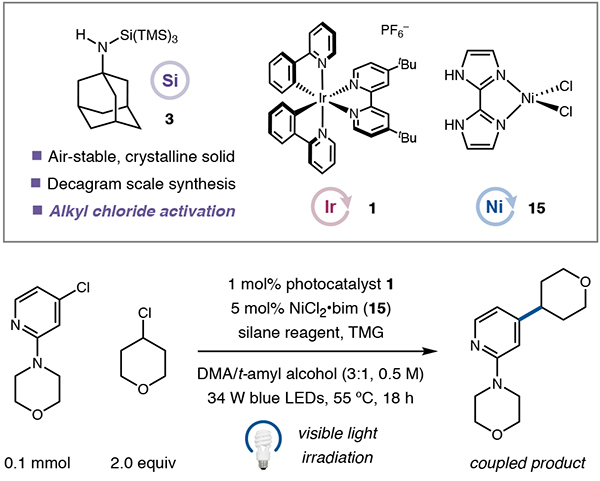
Performed with silane reagent (1.2 equiv), TMG (3.0 equiv), aryl chloride (0.1 mmol), and alkyl chloride (2.0 equiv) in DMA/t-amyl alcohol (3:1, 0.5 M) without fans.
Yields determined by 1H NMR using mesitylene as internal standard. See SI for experimental details.
Recovery of alkyl chloride in parenthesis. bim, 2,2′-biimidazole; TMG, 1,1,3,3-tetramethylguanidine. DMA, N,N-dimethyla-cetamide.
A proposed mechanism for this new cross-chloride coupling is described in Figure 2.23 Upon irradiation with visible light, the photocatalyst [Ir(ppy)2(dtbbpy)](PF6) (1) is known to access the long-lived triplet excited state 2.24 Central to our reaction design, we envisioned that this mildly-oxidizing species (E1/2red [*IrIII/IrII] = +0.76 V vs saturated calomel electrode (SCE) in DMA/t-amyl alcohol; see Supporting Information (SI)) should engage a suitable silane reagent (3) in single-electron transfer (SET) to furnish the reduced IrII complex 4 and N-centered radical 5. Subsequent radical aza-Brook rearrangement25 would unveil the electron-rich α-amino silicon-centered radical 6, which is poised to readily abstract a chlorine atom from an aliphatic chloride 7 to furnish the corresponding alkyl radical 8. At the same time, low-valent Ni0 catalyst 9 is expected to undergo oxidative addition into aryl chloride 10 to afford NiII-aryl intermediate 11. Oxidative radical capture of the open-shell alkyl species 8 would deliver NiIII-(alkyl)(aryl) complex 12, which upon reductive elimination should release the desired C(sp2)–C(sp3) product 13. Finally, single-electron reduction of the resulting NiI intermediate 14 by the IrII species 4 (E1/2red [IrIII/IrII] = −1.38 V vs SCE in DMA/t-amyl alcohol) closes both catalytic cycles, simultaneously regenerating the ground-state photocatalyst 1 and the Ni0 catalyst 9.
Figure 2.

Design plan for cross-electrophile coupling
Optimization and reaction scope.
Following an extensive survey of various supersilane derivatives, catalysts, and solvents, we determined that under optimal conditions (photocatalyst 1 (1 mol%), NiCl2•bim26 15 (5 mol%), and TMG as base), the 1-adamantyl aminosilane reagent 3 facilitates a cross-electrophile coupling mechanism that provides the desired product in excellent yield (Table 1, entry 1, 73% yield). The highly crystalline aminosilane reagent 3 can now be purchased (Milli-poreSigma, #915319) or be easily prepared in a single step from commercial materials on a decagram scale (see SI), and all other reagents and catalysts are commercially available. Importantly, reagent 3 was found to have a relatively low oxidation potential (Epa = +0.86 V vs SCE in DMA/t-amyl alcohol), which permits activation by excited photocatalyst 2 via SET under mild conditions, consistent with excited-state potentials and Stern-Volmer quenching experiments (see Figure S8). Subsequent rearrangement pathways27 were interrogated through a series of computational studies using density functional theory, which established the feasibility of our proposed aza-Brook rearrangement (see Figure S9 and accompanying discussion). The t-butylamine-derived supersilane performs comparably (entry 2), but due to operational difficulties in handling this waxy solid, the crystalline 1-ada-mantylamine derivative (see Figure S2 for X-ray structure) was selected as the reagent of choice. Consistent with our design hypothesis, the introduction of less electron-rich amines resulted in substantially diminished reaction efficiencies (entries 3 and 4), while silane reagents previously used in photoredox cross-electrophile coupling (i.e., supersilanol and supersilane) were ineffective at alkyl chloride activation under all conditions employed (entries 5 and 6). Decreased yields were also observed when DMA was used without a co-solvent (entry 7) or when NiCl2•dtbbpy was used in lieu of 15 (entry 8). Control experiments established that the iridium photocatalyst, light, aminosilane reagent, and nickel catalyst were all necessary for product formation (entries 9–12).
With these optimized conditions in hand, we directed our studies toward exploring the scope of this organochloride cross-electrophile coupling. As summarized in Table 2, we were delighted to find that our silyl-radical activation approach served as a broadly applicable platform for coupling a wide array of alkyl chlorides and aryl chlorides. With respect to the alkyl chloride coupling partner, a variety of five-, six-, and seven-membered cyclic systems performed well (16–20, 66–77% yield). Secondary acyclic alkyl chlorides, as well as hindered bridged bicyclic and neopentyl substrates, were also found to be competent electrophiles (21–23, 66–77% yield). While halogen atom abstraction from primary alkyl chlorides was anticipated to be kinetically challenging based on literature precedent,7c we were pleased to find that a number of functionalized primary substrates could be successfully engaged in our coupling methodology. In particular, alkyl chloride partners containing cyclic and acyclic ethers can be employed to access the desired C(sp2)–C(sp3) adducts in good yield (24 and 25, 72% and 57% yield, respectively). Gratifyingly, electrophilic moieties, such as esters, nitriles, and ketones, were also well-tolerated under our standard protocol (26–28, 58–62% yield). Moreover, alkyl fragments incorporating protected functional groups were successfully introduced, including primary alcohols, aldehydes, and vicinal diols (29–31, 61–74% yield). Notably, aliphatic substrates containing nitrogen heteroarenes can also be coupled with useful efficiencies (e.g., 32, 64% yield).
Table 2.
Scope of Silane-Mediated Cross-Electrophile Coupling of Unactivated Alkyl Chlorides and Aryl Chloridesa

|
All yields are isolated. Photocatalyst 1 (1 mol%), NiCl2•bim (5 mol%), aminosilane reagent 3 (1.2 equiv), TMG (3.0 equiv), aryl chloride (0.5 mmol), and alkyl chloride (1.0 mmol) were irradiated by blue LEDs in DMA/t-amyl alcohol (3:1, 0.5 M) without fans, equilibrating at 50–55 °C.
4,4′,5,5′-tetramethyl-2,2′-biimidazole as ligand.
3 mol% Ni catalyst and 0.6 mol% photocatalyst.
dr > 20:1.
10 mol% nickel and 2 mol% photocatalyst.
2,2′-bibenzimidazole as ligand.
BTMG (3.0 equiv) as base.
2.5 equiv 3.
DMA (0.5 M) as solvent.
DMA/t-amyl alcohol (3:1, 1.0 M) as solvent.
[Ir(dF(H)ppy)2(dtbbpy)](PF6) as photocatalyst.
DMA/t-amyl alcohol (1:2, 0.3 M) as solvent.
[Ir(dF(Me)ppy)2(dtbbpy)](PF6) as photocatalyst.
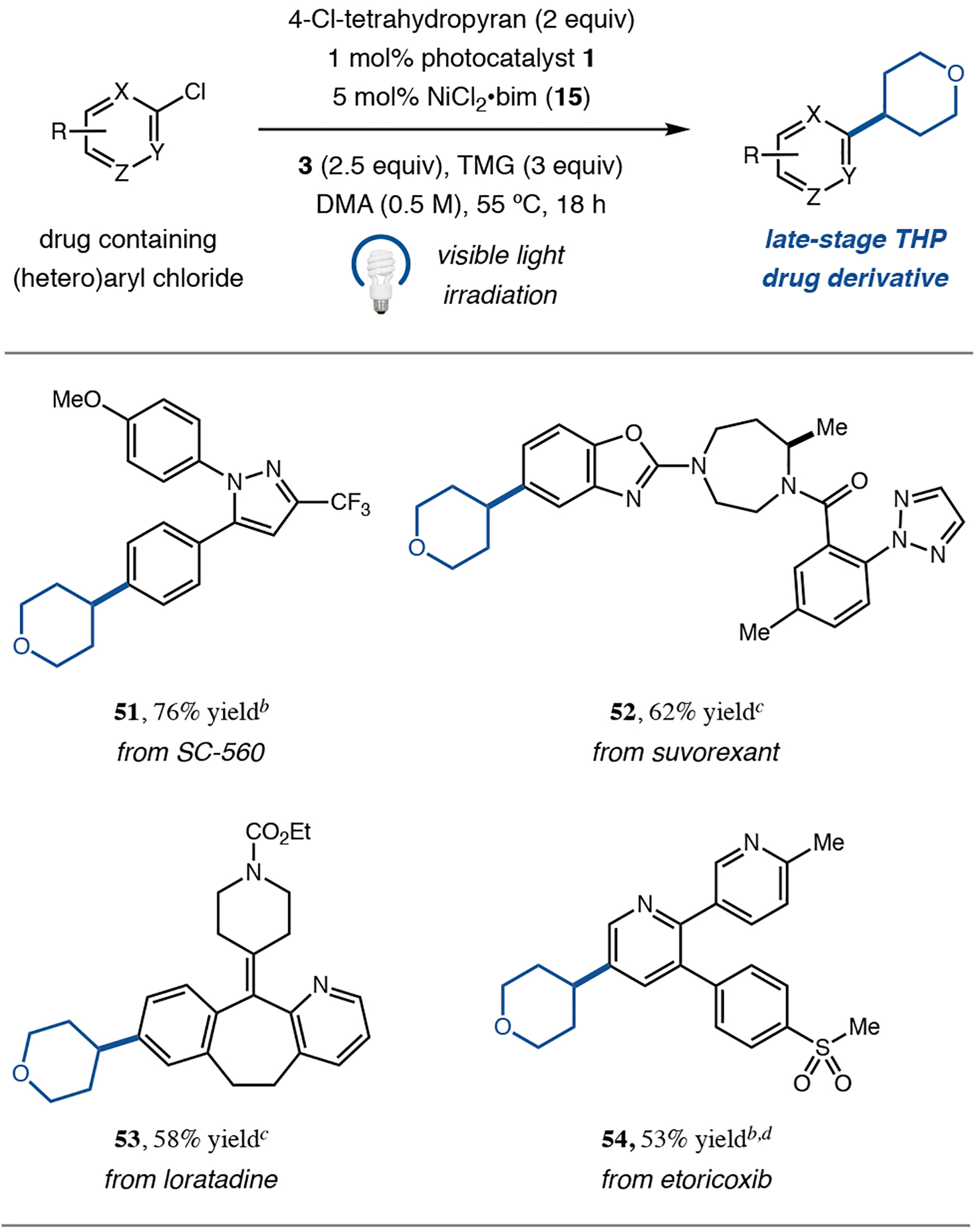
Next, we turned our attention to the scope of the aryl chloride coupling partner. Our investigations revealed that both electron-rich and electron-deficient chlorobenzene derivatives could be employed to provide the corresponding adducts in good yield (33–36, 65–75% yield). Given the abundance of heteroarene substructures in pharmaceutical agents,28 we were delighted to find that 2-, 3-, and 4-chloropyridines, as well as extended aromatic systems such as quinoline, could be readily alkylated with good efficiency (37–40, 61–72% yield). Pyrimidines with diverse substitution patterns were also competent aryl electrophiles, enabling access to diazine products (41 and 42, 57% and 62% yield, respectively). In addition, we were pleased to find that nitrogen-abundant heteroaryl fragments, such as azaindole, pyrrolopyrimidine, and azaindazole, were combined with the parent alkyl chloride scaffold without difficulty (43–45, 62–73% yield). Five-membered heterocycles such as pyrazole could also be coupled with good yield using this new protocol (46, 67% yield). Perhaps most notable, a number of heteroaryl chlorides could be readily employed for which the corresponding aryl bromides are not commercially available (designated by ★), illustrating the immediate utility of this approach in preparing value-added products from synthetically accessible precursors (47–50, 60–68% yield). Finally, in an effort to demonstrate the applicability of our method to the late-stage elaboration of drug-like molecules, we tested several known medicinal agents and drug candidates containing aryl chlorides in this new transformation. As shown in Table 3, we were delighted to find that the desired C(sp2)–C(sp3) adducts could be formed in good yield (51–54, 53–76% yield), illustrating the compatibility of our reaction with medicinally relevant functional groups such as triazoles, amides, sulfones, and carbamates. These results further support the generic utility of our method for application in medicinal chemistry settings.29
Table 3.
Application to Late-Stage Functionalizationa
|
Isolated yields for reactions performed on 0.5 mmol scale.
In DMA/t-amyl alcohol (3:1, 0.5 M).
With 2,2′-bi-1H-benzimidazole as ligand.
With 3 equiv TMG and 1.25 equiv 3.
In summary, we have developed the first general cross-electrophile coupling of unactivated alkyl chlorides and aryl chlorides via the merger of nickel and photoredox catalysis. Our reaction conditions enable the formation of a broad range of C(sp2)–C(sp3) coupled products from widely abundant and bench-stable organic chlorides, including several drug derivatives. In particular, our approach has employed a novel 1-adamantyl aminosilane 3 that exploits polarity-matching effects to achieve the kinetically challenging halogen atom abstraction from unactivated alkyl chlorides. Mechanistic studies exploring the activation of the reagent and subsequent chlorine atom abstraction are ongoing and will be reported in due course.
Supplementary Material
ACKNOWLEDGEMENT
No competing financial interests have been declared.
The authors are grateful for financial support provided by the National Institute of General Medical Sciences (NIGMS), the NIH (under award no. R35GM134897-01), the Princeton Catalysis Initiative, and kind gifts from Merck, Janssen, BMS, Genentech, Celgene and Pfizer. H.A.S acknowledges Princeton University for a first-year fellowship and acknowledges Princeton University, E. Taylor, and the Taylor family for an Edward C. Taylor Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIGMS. The authors thank P. Jeffrey for assistance with X-ray structure determination.
Footnotes
Supporting Information.
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and spectral data (PDF)
REFERENCES
- (1).For general reviews:; (a) Knappke CE; Grupe S; Gartner D; Corpet M; Gosmini C; Jacobi von Wangelin A Reductive Cross-Coupling Reactions between Two Electrophiles. Chem. Eur. J 2014, 20, 6828–6842. [DOI] [PubMed] [Google Scholar]; (b) Weix DJ Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res 2015, 48, 1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Richmond E; Moran J Recent Advances in Nickel Catalysis Enabled by Stoichiometric Metallic Reducing Agents. Synthesis 2018, 50, 499–513. [Google Scholar]
- (2).(a) Everson DA; Shrestha R; Weix DJ Nickel-Catalyzed Reductive Cross-Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc 2010, 132, 920–92. [DOI] [PubMed] [Google Scholar]; (b) Yu X; Yang T; Wang S; Xu H; Gong H Nickel-Catalyzed Reductive Cross-Coupling of Unactivated Alkyl Halides. Org. Lett 2011, 13, 2138–2141. [DOI] [PubMed] [Google Scholar]; (c) Fujihara T; Nogi K; Xu T; Terao J; Tsuji Y Nickel-Catalyzed Carboxylation of Aryl and Vinyl Chlorides Employing Carbon Dioxide. J. Am. Chem. Soc 2012, 134, 9106–9109. [DOI] [PubMed] [Google Scholar]; (d) León T; Correa A; Martin R Ni-Catalyzed Direct Carboxylation of Benzyl Halides with CO2. J. Am. Chem. Soc 2013, 135, 1221–1224. [DOI] [PubMed] [Google Scholar]; (e) Cherney AH; Reisman SE Nickel-Catalyzed Asymmetric Reductive Cross-Coupling Between Vinyl and Benzyl Electrophiles. J. Am. Chem. Soc 2014, 136, 14365–14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).For earlier examples of cross-electrophile coupling with transition metal catalysts other than nickel:; (a) Jacobi von Wangelin A; Czaplik W; Mayer M Direct Cobalt-Catalyzed Cross-Coupling Between Aryl and Alkyl Halides. Synlett 2009, 2009, 2931–2934. [Google Scholar]; (b) Amatore M; Gosmini C Direct Method for Carbon-Carbon Bond Formation: The Functional Group Tolerant Cobalt-Catalyzed Alkylation of Aryl Halides. Chem. Eur. J 2010, 16, 5848–5852. [DOI] [PubMed] [Google Scholar]; (c) Krasovskiy A; Duplais C; Lipshutz BH Zn-Mediated, Pd-Catalyzed Cross-Couplings in Water at Room Temperature Without Prior Formation of Organozinc Reagents. J. Am. Chem. Soc 2009, 131, 15592–15593. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Bhonde VR; O’Neill BT; Buchwald SL An Improved System for the Aqueous Lipshutz-Negishi Cross-Coupling of Alkyl Halides with Aryl Electrophiles. Angew. Chem., Int. Ed 2016, 55, 1849–1853. [DOI] [PubMed] [Google Scholar]
- (4).For general reviews on metallaphotoredox catalysis:; (a) Twilton J; Le C; Zhang P; Shaw MH; Evans RW; MacMillan DWC The merger of transition metal and photocatalysis. Nat. Rev. Chem 2017, 1, 0052. [Google Scholar]; (b) Skubi KL; Blum TR; Yoon TP Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev 2016, 116, 10035–10074. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Levin MD; Kim S; Toste FD Photoredox Catalysis Unlocks Single-Electron Elementary Steps in Transition Metal Catalyzed Cross-Coupling. ACS Cent. Sci 2016, 2, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Zhang P; Le CC; MacMillan DW Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling. J. Am. Chem. Soc 2016, 138, 8084–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) Zhang R; Li G; Wismer M; Vachal P; Colletti SL; Shi ZC Profiling and Application of Photoredox C(sp3)–C(sp2) Cross-Coupling in Medicinal Chemistry. ACS Med. Chem. Lett 2018, 9, 773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pomberger A; Mo Y; Nandiwale KY; Schultz VL; Duvadie R; Robinson RI; Altinoglu EI; Jensen KF A Continuous Stirred-Tank Reactor (CSTR) Cascade for Handling Solid-Containing Photochemical Reactions. Org. Process Res. Dev 2019, 23, 2699–2706. [Google Scholar]; (c) Dombrowski AW; Gesmundo NJ; Aguirre AL; Sarris KA; Young JM; Bodgan AR; Martin MC; Gedeon S; Wang Y Expanding the Medicinal Chemist Toolbox: Comparing Seven C(sp2)–C(sp3) Cross-Coupling Methods by Library Synthesis. ACS Med. Chem. Lett 2020, 11, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Chatgilialoglu C; Ingold KU; Scaiano JC Absolute rate constants for the reaction of triethylsilyl radicals with ring-substituted benzyl chlorides. J. Org. Chem 1987, 52, 938–940. [Google Scholar]; (b) Jiang X-K; Ding WF-X; Zhang Y-H The nucleophilic silyl radical: dual-parameter correlation analysis of the relative rates of bromine-atom abstraction reactions as measured by a rigorous methodology. Tetrahedron 1997, 53, 8479–8490. [Google Scholar]; (c) Chatgilialoglu C; Griller D; Lesage M Rate constants for the reactions of tris(trimethylsilyl)silyl radicals with organic halides. J. Org. Chem 1989, 54, 2492–2494. [Google Scholar]; For a recent review, see; Chatgilialoglu C; Ferreri C; Landais Y; Timokhin VI Thirty Years of (TMS)3SiH: A Milestone in Radical-Based Synthetic Chemistry. Chem. Rev 2018, 118, 6516–6572. [DOI] [PubMed] [Google Scholar]
- (8).For abstraction from alkyl bromides:; (a) Bacauanu V; Cardinal S; Yamauchi M; Kondo M; Fernandez DF; Remy R; MacMillan DWC Metallaphotoredox Difluoromethylation of Aryl Bromides. Angew. Chem. Int., Ed 2018, 57, 12543–12548. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) ElMarrouni A; Ritts CB; Balsells J Silyl-mediated photoredox-catalyzed Giese reaction: addition of non-activated alkyl bromides. Chem. Sci 2018, 9, 6639–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Smith RT; Zhang X; Rincon JA; Agejas J; Mateos C; Barberis M; Garcia-Cerrada S; de Frutos O; MacMillan DWC Metallaphotoredox-Catalyzed Cross-Electrophile Csp3-Csp3 Coupling of Aliphatic Bromides. J. Am. Chem. Soc 2018, 140, 17433–17438. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kornfilt DJP; MacMillan DWC Copper-Catalyzed Trifluoromethylation of Alkyl Bromides. J. Am. Chem. Soc 2019, 141, 6853–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Lovett GH; Chen S; Xue X-S; Houk KN; MacMillan DWC Open-Shell Fluorination of Alkyl Bromides: Unexpected Selectivity in a Silyl Radical-Mediated Chain Process. J. Am. Chem. Soc 2019, 141, 20031–20036. [DOI] [PubMed] [Google Scholar]; (f) Hell SM; Meyer CF; Laudadio G; Misale A; Willis MC; Noël T; Trabanco AA; Gouverneur V Silyl Radical-Mediated Activation of Sulfamoyl Chlorides Enables Direct Access to Aliphatic Sulfonamides from Alkenes. J. Am. Chem. Soc 2020, 142, 720–725. [DOI] [PubMed] [Google Scholar]
- (9).For abstraction from aryl bromides and activated alkyl chlorides:; (a) Le C; Chen TQ; Liang T; Zhang P; MacMillan DWC A radical approach to the copper oxidative addition problem: Trifluoromethylation of bromoarenes. Science 2018, 360, 1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen TQ; MacMillan DWC A Metallaphotoredox Strategy for the Cross-Electrophile Coupling of α-Chloro Carbonyls with Aryl Halides. Angew. Chem., Int. Ed 2019, 58, 14584–14588. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wiles RJ; Phelan JP; Molander GA Metal-free defluorinative arylation of trifluoromethyl alkenes via photoredox catalysis. Chem. Commun 2019, 55, 7599–7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).For selected examples of traditional nucleophile-electrophile couplings with alkyl chlorides:; (a) Lu Z; Fu GC Alkyl-Alkyl Suzuki Cross-Coupling of Unactivated Secondary Alkyl Chlorides. Angew. Chem., Int. Ed 2010, 49, 6676–6678. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lu Z; Wilsily A; Fu GC Stereoconvergent Amine-Directed Alkyl-Alkyl Suzuki Reactions of Unactivated Secondary Alkyl Chlorides. J. Am. Chem. Soc 2011, 133, 8154–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Atack TC; Cook SP Manganese-Catalyzed Borylation of Unactivated Alkyl Chlorides. J. Am. Chem. Soc 2016, 138, 6139–6142. [DOI] [PubMed] [Google Scholar]
- (11).(a) Everson DA; Jones BA; Weix DJ Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides. J. Am. Chem. Soc 2012, 134, 6146–6159. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molander GA; Traister KM; O’Neill BT Reductive Cross-Coupling of Nonaromatic, Heterocyclic Bromides with Aryl and Heteroaryl Bromides. J. Org. Chem 2014, 79, 5771–5780. [DOI] [PubMed] [Google Scholar]; (c) Wang X; Wang S; Xue W; Gong H Nickel-Catalyzed Reductive Coupling of Aryl Bromides with Tertiary Alkyl Halides. J. Am. Chem. Soc 2015, 137, 11562–11565. [DOI] [PubMed] [Google Scholar]; (d) Knauber T; Chandrasekaran R; Tucker JW; Chen JM; Reese M; Rankic DA; Sach N; Helal C Ru/Ni Dual Catalytic Desulfinative Photoredox Csp2-Csp3 Cross-Coupling of Alkyl Sulfinate Salts and Aryl Halides. Org. Lett 2017, 19, 6566–6569. [DOI] [PubMed] [Google Scholar]
- (12).(a) Yu X; Yang T; Wang S; Xu H; Gong H Nickel-Catalyzed Reductive Cross-Coupling of Unactivated Alkyl Halides. Org. Lett 2011, 13, 2138–2141. [DOI] [PubMed] [Google Scholar]; (b) Huihui KM; Caputo JA; Melchor Z; Olivares AM; Spiewak AM; Johnson KA; DiBenedetto TA; Kim S; Ackerman LK; Weix DJ Decarboxylative Cross-Electrophile Coupling of N-Hydroxyphthalimide esters with aryl iodides. J. Am. Chem. Soc 2016, 138, 5016–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Liang Z; Xue W; Lin K; Gong H Nickel-Catalyzed Reductive Methylation of Alkyl Halides and Acid Chlorides with Methyl p-Tosylate. Org. Lett 2014, 16, 5620–5623. [DOI] [PubMed] [Google Scholar]; (b) Molander GA; Traister KM; O’Neill BT Engaging Nonaromatic, Heterocyclic Tosylates in Reductive Cross-Coupling with Aryl and Heteroaryl Bromides. J. Org. Chem 2015, 80, 2907–2911. [DOI] [PubMed] [Google Scholar]; (c) Duan J; Du Y-F; Pang X; Shu X-Z Ni-catalyzed cross-electrophile coupling between vinyl/aryl and alkyl sulfonates: synthesis of cycloalkenes and modification of peptides. Chem. Sci 2019, 10, 8706–8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) Ertl P; Schuhmann T A Systematic Cheminformatics Analysis of Functional Groups Occurring in Natural Products. J. Nat. Prod 2019, 82, 1258–1263. [DOI] [PubMed] [Google Scholar]; (b) Gribble GW Naturally Occurring Organohalogen Compounds. Acc. Chem. Res 1998, 31, 141–152. [Google Scholar]
- (15).(a) Sobol Z; Engel ME; Rubitski E; Ku WW; Aubrecht J; Schiestl RH Genotoxicity profiles of common alkyl halide esters with alkylating activity. Mutat. Res 2007, 633, 80–84. [DOI] [PubMed] [Google Scholar]; (b) Schiffman SS; Rother KI Sucralose, a synthetic organochlorine sweetener: overview of biological issues. J. Toxicol. Enviorn. Health B Crit. Rev 2013, 16, 399–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).For examples in total synthesis:; (a) Shibuya GM; Kanady JS; Vanderwal CD Stereoselective Dichlorination of Allylic Alcohol Derivatives to Access Key Stereochemical Arrays of the Chlorosul-folipids. J. Am. Chem. Soc 2008, 130, 12514–12518. [DOI] [PubMed] [Google Scholar]; (b) Snyder SA; Tang Z-Y; Gupta R Total Synthesis of (–)-Napyradiomycin A1 via Asymmetric Chlorination of an Isolated Olefin. J. Am. Chem. Soc 2009, 131, 5744–5745. [DOI] [PubMed] [Google Scholar]; (c) Nilewski C; Geisser RW; Carreira EM Total synthesis of a chlorosulpholipid cytotoxin associated with seafood poisoning. Nature 2009, 457, 573–576. [DOI] [PubMed] [Google Scholar]; (d) Bucher C; Deans RM; Burns NZ Highly Selective Synthesis of Halomon, Plocamenone, and Isoplocamenone. J. Am. Chem. Soc 2015, 137, 12784–12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).(a) Frisch AC; Beller M Catalysts for Cross-Coupling Reactions with Non-activated Alkyl Halides. Angew. Chem. Int. Ed 2005, 44, 674–688. [DOI] [PubMed] [Google Scholar]; (b) Grushin VV; Alper H Transformations of Chloroarenes, Catalyzed by Transition-Metal Complexes. Chem. Rev 1994, 94, 1047–1062. [Google Scholar]
- (18).For unactivated alkyl chlorides in net reductive C–C bond-forming reactions:; (a) Prinsell MR; Everson D A; Weix, D. J. Nickel-Catalyzed, Sodium iodide-promoted reductive dimerization of alkyl halides, alkyl pseudohalides, and allylic acetates. Chem. Commun 2010, 46, 5743–5745. [DOI] [PubMed] [Google Scholar]; (b) Tollefson EJ; Erickson LW; Jarvo ER Stereospecific Intramolecular Reductive Cross-Electrophile Coupling Reactions for Cyclopropane Synthesis. J. Am. Chem. Soc 2015, 137, 9760–9763. [DOI] [PubMed] [Google Scholar]; (c) Bojresson M; Moragas T; Martin R Ni-Catalyzed Carboxylation of Unactivated Alkyl Chlorides with CO2. J. Am. Chem. Soc 2016, 138, 7504–7507. [DOI] [PubMed] [Google Scholar]; (d) Wu X; Hao W; Ye K; Jiang B; Pombar G; Song Z; Lin S Ti-Catalyzed Radical Alkylation of Secondary and Tertiary Alkyl Chlorides Using Michael Acceptors. J. Am. Chem. Soc 2018, 140, 14836–14843. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Claros M; Ungeheuer F; Franco F; Martin-Diaconescu V; Casitas A; Fillol-Lloret J Reductive cyclization of unactivated alkyl chlorides with tethered alkenes under visible-light photoredox catalysis. Angew. Chem. Int. Ed 2019, 58, 4869–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Giedyk M; Narobe R; Weiss S; Touraud D; Kunz W; König B Photocatalytic activation of alkyl chlorides by assembly promoted single electron transfer in microheterogeneous solutions. Nat. Catal 2020, 3, 40–47. [Google Scholar]
- (19).(a) Blanksby SJ and Ellison GB Bond Dissociation Energies of Organic Molecules. Acc. Chem. Res 2003, 36, 255–263. [DOI] [PubMed] [Google Scholar]; (b) Roth HG; Romero NA; Nicewicz DA Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett 2016, 27, 714–723. [Google Scholar]
- (20).Silyl radicals are nucleophilic reaction partners; see reference 7b. For a general review on radical polarity:; Roberts BP Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev 1999, 28, 25–35. [Google Scholar]
- (21).(a) De Vleeschouwer F; Van Speybroeck V; Waroquier M; Geerlings P; De Proft F Electrophilicity and Nucleophilicity Index for Radicals. Org. Lett 2007, 9, 2721–2724. [DOI] [PubMed] [Google Scholar]; (b) Hu J; Wang J; Nguyen TH; Zheng N The chemistry of amine radical cations produced by visible light photoredox catalysis. Beilstein J. Org. Chem 2013, 9, 1977–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nakajima K; Miyake Y; Nishibayashi Y Synthetic Utilization of α-Aminoalkyl Radicals and Related Species in Visible Light Photoredox Catalysis. Acc. Chem. Res 2016, 49, 1946. [DOI] [PubMed] [Google Scholar]
- (22).Steric protection towards hydrolysis is achieved by increasing the bulk of silicon substituents:; Wutz PGM Greene’s Protective Groups in Organic Synthesis, 5th ed; John Wiley: Hoboken, NJ, 2014; pp 1086–1087. [Google Scholar]
- (23).For recent mechanistic studies on cross-electrophile coupling:; (a) Biswas S; Weix DJ Mechanism and Selectivity in Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc 2013, 135, 16192–16197. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shu W; Garcia-Dominguez A; Quiros MT; Mondal R; Cardenas DJ; Nevado C Ni-Catalyzed Reductive Dicarbofunctionalization of Nonactivated Alkenes: Scope and Mechanistic Insights. J. Am. Chem. Soc 2019, 141, 13812–13821. [DOI] [PubMed] [Google Scholar]; (c) Lin Q; Diao T Mechanism of Ni-Catalyzed Reductive 1,2-Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc 2019, 141, 17937–17948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).(a) Slinker JD; Gorodetsky AA; Lowry MS; Wang J; Parker S; Rohl R; Bernhard S; Malliaras GG Efficient Yellow Electroluminescence from a Single Layer of a Cyclometalated Iridium Complex. J. Am. Chem. Soc 2004, 126, 2763–2767. [DOI] [PubMed] [Google Scholar]; (b) Lowry MS; Goldsmith JI; Slinker JD; Rohl R; Pascal RA; Malliaras GG; Bernhard S Single-Layer Electroluminescent Devices and Photoinduced Hydrogen Production from an Ionic Iridium(III) Complex. Chem. Mater 2005, 17, 5712–5719. [Google Scholar]
- (25).For the related radical aza-Brook rearrangement with silyl migration from carbon to nitrogen, see:; (a) Harris JM; MacInnes I; Walton JC; Maillard B 1,2-Migration of the trimethylsilyl group in free radicals. J. Organomet. Chem 1991, 3, C25–C28. [Google Scholar]; (b) Harris JM; Walton JC; Maillard B; Grelier S; Picard J-P Hydrogen abstraction from silylamines; an investigation of the 1,2-migration of the trimethylsilyl group in aminyl radicals. J. Chem. Soc., Perkin Trans. 2 1993, 11, 2119–2123. [Google Scholar]; (c) Schiesser CH; Styles ML On the radical Brook and related reactions: an ab initio study of some (1,2)-silyl, germyl and stannyl translocations. J. Chem. Soc., Perkin Trans 2 1997, 2355–2340. [Google Scholar]
- (26).This ligand is known to undergo deprotonation under basic conditions, giving a more electron-rich nickel complex. For synthesis and characterization of related 2,2′-biimidazole-ligated nickel complexes:; (a) Mighell AD; Reimann CW; Mauer FA The crystal and molecular structure of diaquobis-(2,2′-biimidazole)nickel(II) dinitrate. Acta Cryst 1969, B25, 60–66. [Google Scholar]; (b) Abushamleh AS; Goodwin HA Coordination of 2,2′-biimidazole with iron, cobalt, nickel and copper. Aust. J. Chem 1979, 32, 513–518. [Google Scholar]
- (27).Other pathways are also envisioned to feasibly reveal a silyl radical analogous to 6. For example, organosilane amine radical cations undergo well-precedented rearrangement chemistry with concomitant desilylation to unveil open-shell carbon intermediates:; (a) Miyake Y; Ashida Y; Nakajima K; Nishibayashi Y Visible-light-mediated addition of α-aminoalkyl radicals generated from α-silylamines to α,β-unsatured carbonyl compounds. Chem. Commun 48, 2012, 6966–6968. [DOI] [PubMed] [Google Scholar]; (b) Espelt LR; McPherson IS; Wiensch EM; Yoon TP Enantioselective Conjugate Additions of α-Amino Radicals via Cooperative Photoredox and Lewis Acid Catalysis. J. Am. Chem. Soc 2015, 137, 2454–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bootwicha T; Feilner JM; Myers EL; Aggarwal VK Iterative assembly line synthesis of polypropionates with full stereocontrol. Nat. Chem 2017, 9, 896–902. [DOI] [PubMed] [Google Scholar]; For a general review, see:; Cho DW; Yoon UC; Mariano PS Studies Leading to the Development of a Single-Electron Transfer (SET) Photochemical Strategy for Syntheses of Macrocyclic Polyethers, Polythioethers, and Polyamides. Acc. Chem. Res 2011, 44, 204–215. [DOI] [PubMed] [Google Scholar]
- (28).Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]
- (29).For additional examples and limitations of alkyl and aryl chloride substrates, see Supplementary Information, Figure S7.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


