Abstract
Head and neck squamous cell carcinoma (HNSCC) is the sixth most prevalent malignancy worldwide, with high incidence and poor survival rates. Increased expression of microRNA-205-5p (miR-205-5p) may influence the outcomes of HNSCC, but the identities of miR-205-5p target genes and the potential signaling pathways related to HNSCC remain unclear. RT-qPCR was used to detect the expression levels of miR-205-5p in the plasma of patients with HNSCC. We also performed a meta-analysis using data from relevant literature, and the Gene Expression Omnibus (GEO) and the Cancer Genome Atlas (TCGA) databases to evaluate the expression level of miR-205-5p in HNSCC. Next, we predicted the potential miR-205-5p target genes in HNSCC. We also used Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) for enrichment analyses adapted to investigate the dynamics and possible mechanisms of miR-205-5p in HNSCC. Lastly, we predicted the potential miR-205-5p target genes by evaluating their expression level and using Spearman analysis. Expression of miR-205-5p was higher in HNSCC tissues compared to normal unafflicted tissue samples (P < 0.05), and the corresponding summary receiver operating characteristic (sROC) was 0.82.The pooled sensitivity, specificity, PLR, NLR, and DOR values were 0.78 (95% CI: 0.75-0.81), 0.67 (95% CI: 0.60-0.73), 2.34 (95% CI: 1.45-3.76), 0.34 (95% CI: 0.19-0.60), and 8.16 (95% CI: 4.01-16.64), respectively. Based on GO and KEGG analyses, we found that miR-205-5p was correlated with the progression of HNSCC through association with signaling pathways, including the drug metabolism-cytochrome P450 pathway. Analysis of the target genes revealed that flavin-containing monooxygenase isoform 2 (FMO2) and alcohol dehydrogenase 1B (ADH1B) may be important targets of miR-205-5p. In summary, miR-205-5p may have a significant role in the prognosis of HNSCC and may serve as a potential biomarker in HNSCC.
Keywords: microRNA-205-5p, head and neck squamous cell carcinoma, gene, molecular mechanism, signaling pathway
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most prevalent malignancy worldwide and occurs in areas of the upper digestive tract, including the nasal cavity, oral cavity, tongue, pharynx (nasal pharynx, oropharynx, hypopharynx), and larynx.1-3 The annual incidence of HNSCC is estimated to be approximately 50,000 in the USA, and over 500,000 worldwide.1 The causative factors that correlate with HNSCC include tobacco, alcohol, and human papilloma virus infection.4,5 Surgery, radiotherapy, and chemotherapy are the main treatment options for HNSCC patients.6 Despite the therapeutic advances over the recent decades, recurrence and metastasis remain the 2 primary reasons for the poor survival rates of patients diagnosed with HNSCC.7 Therefore, there is an urgent need to identify new biomarkers associated with HNSCC, and to examine the efficacy of new therapeutic strategies in HNSCC patients.8
MicroRNAs (miRNAs) are highly conserved noncoding RNAs of 19-24 nucleotides in length that can regulate gene expression by binding to the 3′ untranslated regions (3′UTR) of target messenger RNAs (mRNAs).9,10 Recently, miR-205-5p, located at 1q32.2, was found to be highly expressed in NPC patients.11 Further, studies have demonstrated that miR-205-5p is highly expressed in HNSCC cell lines and plays an important role in hepatocellular, lung, and breast cancers.12-16 However, the precise role and mechanism of action of miR-205-5p in signaling pathways associated with HNSCC remain unclear. The role of miR-205-5p as a potentially novel biomarker needs to be further examined to better understand its targets and molecular mechanisms, especially in relation to HNSCC.
Therefore, we sought to examine data obtained from current and relevant literature from the Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) databases. We performed a meta-analysis to assess the expression level of miR-205-5p in HNSCC patients. We also sought to examine and identify novel molecular mechanisms of miR-205-5p in HNSCC using the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) analyses. Our three major hypotheses were as follows: (1) Expression of miR-205-5p was significantly different in the HNSCC group compared to the control group; (2) miR-205-5p has predictive value in HNSCC, and (3) miR-205-5p plays a role in drug metabolism by inhibiting the specific target genes flavin-containing monooxygenase isoform 2 (FMO2), alcohol dehydrogenase 1B (ADH1B), and stimulating cytochrome P450 family 3 subfamily A member 5 (CYP3A5).
Materials and Methods
Reverse Transcription Quantitative Real-Time PCR
This study was approved by the ethics committee. Plasma samples were collected from 15 HNSCC patients and 12 healthy people from the Radiotherapy Department. Total RNA was extracted from the plasma using the miRNeasy Serum/Plasma Kit (Qiagen; Hilden, Germany). Reverse transcription quantitative real-time PCR (RT-qPCR) was performed on a CFX96 Real-time PCR Detection System (Bio-Rad) using SYBR-Green Master Mix (Takara, Tokyo, Japan). The expression level of the target genes was calculated using the 2-ΔΔCT method using U6 as an internal reference. The primers used for real-time PCR were as follows: U6 Forward 5′-CGGCGGTAGCTTATCAGACTGATG-3′ and Reverse 5′-CCAGTCGAGGGTCCGAGGTATT-3′; miR-205-5p Forward 5′- UCCUUCAUUCCACCGGAGUCUG-3′ and reverse mRQ 3′ primer (provided with the Mir-X miRNA qRT-PCR SYBR kit as antisense primer, Clontech). All experiments were repeated more than 3 times.
Literature Search and Data Acquisition from the GEO and TCGA Databases
We performed a comprehensive literature search using the China Biomedical Literature Database (CBM), China National Knowledge Infrastructure Database (CNKI), Technology Journal Database (VIP), Wanfang Database, China Science databases, PubMed, Web of Science, Science Direct, Wiley Online Library, EMBASE, and Cochrane Library databases. Simultaneously, expression data for miR-205-5p in HNSCC tissues and cell lines was obtained using the GEO database (https://www.ncbi.nlm.nih.gov/gds/) up to October 18, 2019.17 Our search strategy included the following terms and keywords: (MicroRNA-205-5p OR miRNA-205-5p OR miR-205-5p OR miRNA205-5p OR miR205-5p OR “miRNA 205-5p” OR “miR 205-5p” OR microRNA-205-5p OR miRNA-205-5p OR miR-205-5p) AND (“head and neck” OR “lip” OR “cheek” OR “nose” OR “nasal sinus” OR “nasal cavity” OR “paranasal sinuses” OR “oral cavity” OR “oral” OR “buccal” OR “tongue” OR “palatal” OR “tonsil” OR “tonsillar” OR “nasopharynx” OR “oropharynx” OR “oropharyngeal” OR “hypopharynx” OR “laryngopharynx” OR “larynx” OR “laryngopharyngeal” OR “laryngeal” OR “pharyngeal”) AND (HNSCC OR SCC OR “squamous cell carcinoma” OR “squamous cell cancer” OR “squamous cell tumor” OR “squamous cell neoplasm”).
Inclusion and Exclusion Criteria for the Studies and Data in the Search
The eligible results of the meta-analysis-based literature search from the TCGA and GEO databases were assessed independently by 2 researchers. An impartial reviewer resolved any disputes between the reviewers. We included studies that met the following criteria: (1) data that profiled miR-205-5p expression level; (2) data that compared miR-205-5p expression level between HNSCC and normal tissues; (3) data that were published in Chinese or English; and (4) data that provided adequate information to determine mean, standard deviation (SD), and 95% confidence interval (95% CI) values. Simultaneously, we excluded any studies that met the following criteria: (1) uncorrelated literature; (2) duplicate literature; (3) a lack of data that could be used for our statistical analyses. Further, we excluded studies that were mainly composed of (4) editorials, comments, reviews (excluding systematic reviews), conference proceedings, case reports, or letters. Key information extracted independently by each reviewer from eligible studies included: (1) the name of the first author; (2) the country in which the study was performed; (3) the date when the article or GSE was published; (4) the sample size in the study article or GSE; (5) the sampling methods; and (6) the analytical methods. Lastly, we contacted the respective authors for any study, as was necessary, when all information was not published in the study.
In addition, we extracted relevant data for the expression level of miR-205-5p in HNSCC and normal tissues, and for the available clinical parameters using the OncoLnc website (http://www.oncolnc.org/),18 a new portal of the TCGA database. The Statistical Package for Social Sciences Version 25.0 (SPSS 25.0) (Nanning, Guangxi Zhuang Autonomous Region, China) was used to examine and graph the relationship between the expression level of miR-205-5p and clinical parameters that were relevant to our study.
Possible Overlapping Target Genes of miR-205-5p in HNSCC
GEPIA (http://gepia.cancer-pku.cn) was used to search for differentially expressed genes (DEGs) in HNSCC samples and from TCGA database. Next, we selected DEGs that belonged to downregulated genes with at least a log2-fold change (FC) < −2. Furthermore, we utilized MiRWalk 3.0 to explore the potential miR-205-5p target genes with SUM ≥ 3.19 Ultimately, VENNY (https: //omictools. com/venny-tool) was used to identify overlapping genes.
Analysis of Signaling Pathways
GO20 terms of biological processes (BP), molecular function (MF), and cellular components (CC), and the KEGG21 pathway enrichment analyses were conducted using R (version 3.5.2) and DAVID 6.8 (https://david.ncifcrf.gov/summery.jsp), and visualized using Biological Networks Gene Oncology (BINGO) tool and Enrichment Map plug-in of Cytoscape. In addition, visual pathway analyses were performed using the BINGO tool and Cytoscape Enrichment Map plug-in. STRING (https://string-db.org/) was used to construct a network of protein-protein interactions (PPI) related to the crucial pathways. Moreover, we used the UALCOULD22 tool (http://ualcan.path.uab.edu/index.html) to identify the expression levels of the miR-205-5p target genes. We used LinkedOmics23 (http://www.linkedomics.org/), an online web portal within TCGA database, to perform Spearman’s correlation analysis to validate the correlation between the potential target genes, important pathways, and miR-205-5p.
Statistical Analyses
Data collected from the meta-analyses, TCGA (Nanning, Guangxi Zhuang Autonomous Region, China), and GEO databases were analyzed using STATA 15.0 software. MiR-205-5p expression level was evaluated using standard mean difference (SMD) values and 95% CI. We used a funnel plot with 95% CIs to determine the level of publication biases. We used GraphPad Prism 6 (Nanning, Guangxi Zhuang Autonomous Region, China) to generate scatter plots to show the level of expression miR-205-5p in selected samples. Moreover, we used the I2 index to assess the potential heterogeneity of the selected data. Data with values of I2 > 50% or values of P < 0.05 were included for analysis in a random-effects model, or alternatively, we used a fixed-effects model for analysis. All statistical analyses were performed using SPSS Version 25.0 Software. Values for the mean ± SD were used to show the measured data in graphical figures, and then t-tests were carried out to compare the values between groups. Nominal data rates are shown as percentages (%) and were compared using the X2 test. True positives, false positives, false negatives, and true negatives were graphically plotted to evaluate the predictive value of miR-205-5p. Meta-Disc Software was used to calculate and evaluate measures of specificity (SPE), sensitivity (SEN), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and summary receiver operating characteristic (sROC) curve. Furthermore, data for miR-205-5p expression level from TCGA and GEO databases were normalized to log2 due to potentially unreliable analyses of the original data. P < 0.05 was considered statistically significant.
Results
miR-205-5p Is Upregulated in Plasma of HNSCC Patients
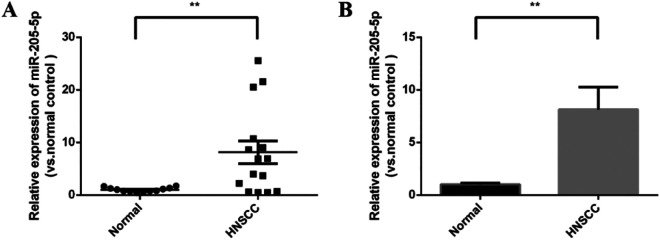
RT-qPCR results revealed that miR-205-5p was significantly upregulated in HNSCC plasma samples compared with normal plasma samples (p < 0.05, Figure 1).
Figure 1.
Levels of miR-205-5p in HNSCC and NC plasma samples. Compared with the control group (n = 12), miR-205-5p was significantly upregulated in HNSCC plasma (n = 15) (p < 0.01).
Literature Search and Data Extraction
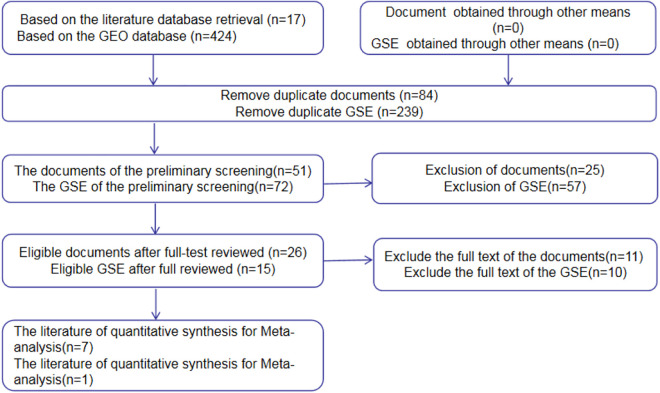
We first selected 17 papers from the CBM, CNKI, VIP, Wanfang, PubMed, Web of Science, Science Direct, Wiley Online Library, and EMBASE databases. Scanning of the summaries and full-texts of these papers (Figure 2) revealed that one study met all the inclusion criteria, and this was by Cao et al.24 (PMID: 22605671). We had expected to identify more articles that met the inclusion criteria. Data were then extracted from TCGA and GEO datasets, comprising GSE11163, GSE31277, GSE34496, GSE45238, GSE75630, GSE103931, and GSE107591 chips. The relevant information is summarized in Table 1. The data from TCGA, GEO, and the published article were all based on PCR analysis of tissue samples.
Figure 2.
Flow diagram for literature search and quality screening.
Table 1.
Features of Included Data.
| Researcher | Year | Country | Cancer/normal | Methods | Sample |
|---|---|---|---|---|---|
| GSE11163 | 2012 | USA | 16/5 | qPCR | Tissue |
| GSE32177 | 2018 | USA | 15/15 | qPCR | Tissue |
| GSE34496 | 2017 | USA | 44/25 | qPCR | Tissue |
| GSE45238 | 2019 | USA | 40/40 | qPCR | Tissue |
| GSE75630 | 2016 | USA | 28/18 | qPCR | Tissue |
| GSE103931 | 2018 | USA | 30/19 | qPCR | Tissue |
| GSE107591 | 2018 | USA | 24/23 | qPCR | Tissue |
| Cao et al | 2012 | China | 48/48 | qPCR | Tissue |
| TCGA | 2019 | USA | 523/44 | qPCR | Tissue |
MiR-205-5p Expression Level in Retrieved Data and Its Potential Predictive Role in HNSCC
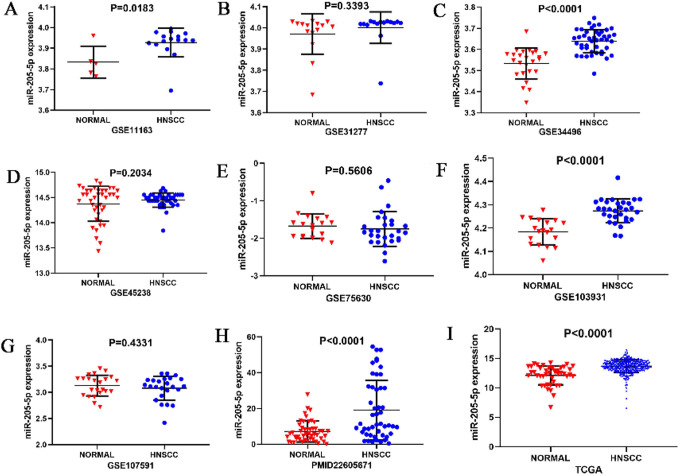
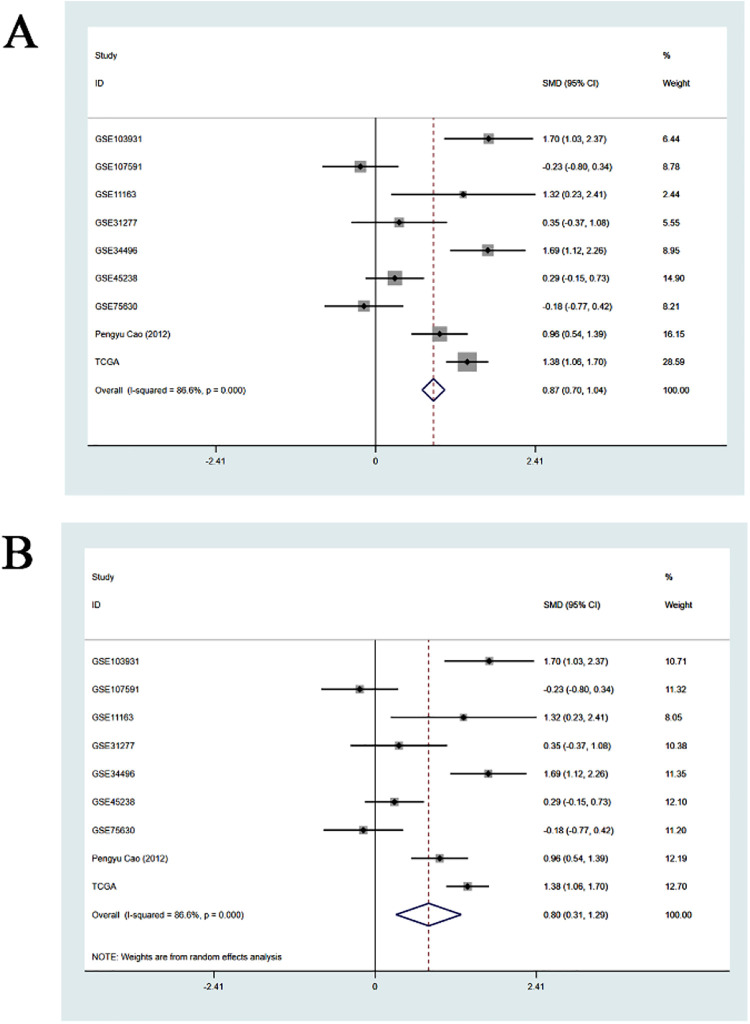
Scatter diagrams of the expression level of miR-205-5p in HNSCC and normal unafflicted tissues derived from TCGA and GEO databases are shown in Figure 3A-I. Five of the studies included revealed that miR-205-5p expression level was significantly higher in HNSCC tissues compared to normal unafflicted tissues (P < 0.05). In addition, Forest plots characterizing miR-205-5p expression level in HNSCC and normal unafflicted tissues from TCGA and GEO datasets using continuous variable meta-analysis are shown (Figure 4A-B). Results of the Forest plots also revealed overexpression of miR-205-5p in HNSCC tissues compared to normal tissues (P < 0.05). Because of the high heterogeneity (I2 = 86.6%, P = 0.000) in the selected studies (Figure 4A), the random-effects model with an overall resultant SMD of 0.87 (95% CI: 0.70. 1.04) was adopted as the best choice (Figure 4B). The results of the Funnel plot shown in Figure 5 indicate that the selected studies did not exhibit publication biases in the meta-analysis.
Figure 3.
Scatter diagrams for levels of expression of miR-205-5p in HNSCC patients. A. GSE11163; B.GSE31277; C.GSE34496; D.GSE45238; E.GSE75630; F.GSE103931; G.GSE107591; H.PMID22605671; I.TCGA.
Figure 4.
Comparisons of the levels of expression of miR-205-5p between HNSCC and normal tissues evaluated by forest plots based on data from published literature, and the TCGA and GEO database datasets. A. Fixed-effect model of Forest plots; B. Random-effect model of Forest plots.
Figure 5.
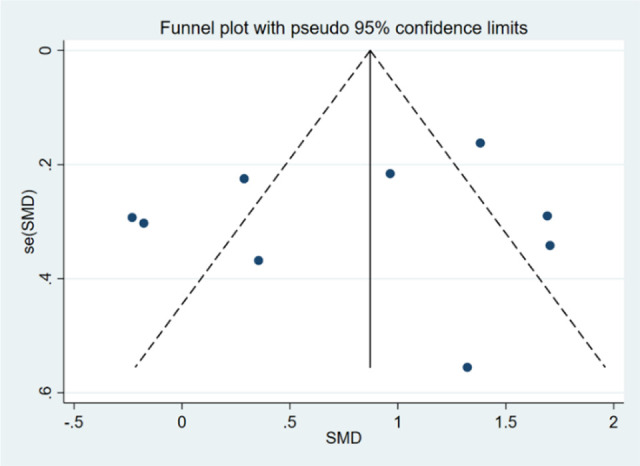
Bias analysis. Funnel plot with pseudo 95% confidence limits for the assessment of publication biases in meta-analysis.
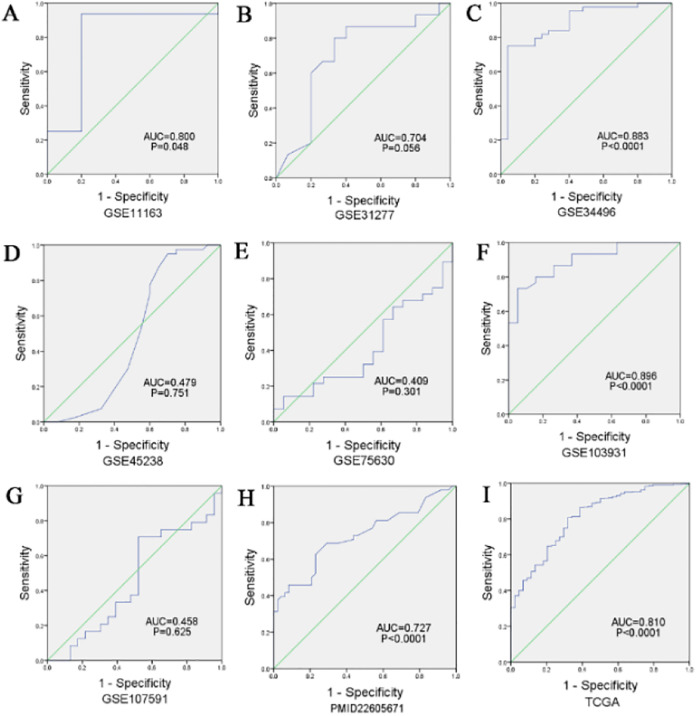
Based on the results of the analyses, the pooled sensitivity, specificity, PLR, NLR, and DOR values were 0.78 (95% CI: 0.75-0.81), 0.67 (95% CI: 0.60-0.73), 2.34 (95% CI: 1.45-3.76), 0.34 (95% CI: 0.19-0.60), and 8.16 (95% CI: 4.01-16.64), respectively. Next, we analyzed the potential predictive role of miR-205-5p expression level in HNSCC samples (Figure 6A-E). The ROC curves from each selected study are shown in Figure 7A-I. The diagnostic meta-analysis of AUC in the sROC curve was 0.82 (95% CI: 0.78-0.85; Figure 8).
Figure 6.
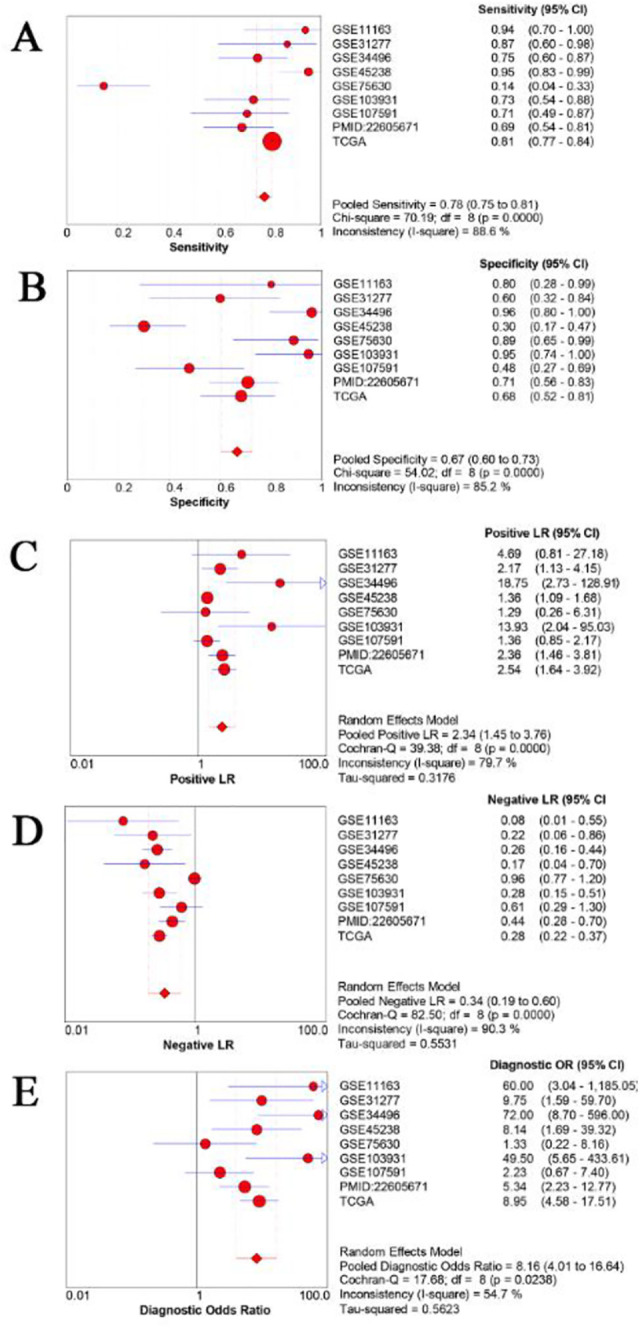
The performance of predictive role of miR-205-5p in HNSCC afflicted samples. A. Sensitivity; B. Specificity; C. Positive Likelihood Ratio (Positive LR); D. Negative Likelihood Ratio (Negative LR); E. Diagnostic Odds Ratio (DOR).
Figure 7.
Receiver operating characteristic (ROC) curves from each study indicating measures of diagnostic accuracy of miR-205-5p in HNSCC afflicted samples.
Figure 8.
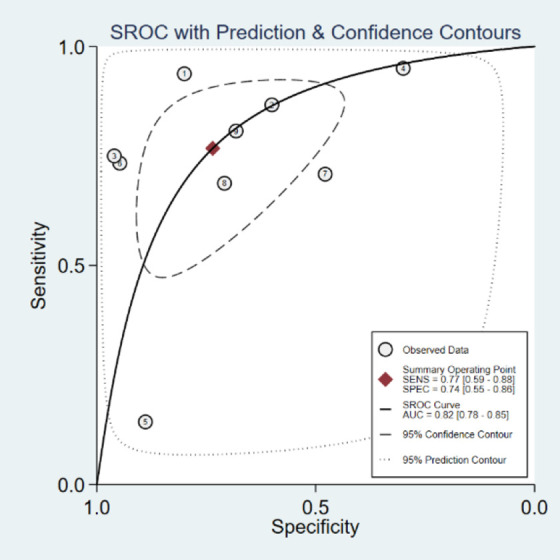
Summary receiver operating characteristic (sROC) curve for evaluation of diagnostic accuracy of miR-205-5p in HNSCC afflicted samples.
Role of miR-205-5p in HNSCC Progression
Based on the results from TCGA database, we identified 523 HNSCC tissue samples that were acceptable for analyses, and the results are shown in Table 2. The expression level of miR-205-5p was significantly correlated with unpaired tissue type and age, but not with sex, lympho-vascular invasion, pathologic grade, clinical grade, histological grade, and alcohol concentration.
Table 2.
The Correlation Between miR-205-5p Expression and Clinical Features From TCGA Data.
| Clinicopathological features | Terms | n | Mean ± SD | p-value |
|---|---|---|---|---|
| Unpaired tissue | Normal | 44 | 12.13 ± 1.61 | <0.0001 |
| HNSCC | 523 | 13.68 ± 0.05 | ||
| Gender | Male | 383 | 13.66 ± 1.13 | 0.657 |
| Female | 141 | 13.71 ± 0.89 | ||
| Age | <60 | 234 | 13.78 ± 1.02 | 0.039 |
| ≥60 | 290 | 13.59 ± 1.10 | ||
| Lymphovascular invasion | No | 232 | 13.69 ± 0.07 | 0.196 |
| Yes | 122 | 13.53 ± 1.21 | ||
| Pathologic grade | G1-G2 | 371 | 13.69 ± 0.05 | 0.691 |
| G3-G4 | 132 | 13.64 ± 1.34 | ||
| Clinical grade | Ⅰ-Ⅱ | 112 | 13.61 ± 1.08 | 0.463 |
| Ⅲ-Ⅳ | 412 | 13.70 ± 1.06 | ||
| T stage | T1-T2 | 188 | 13.66 ± 1.15 | 0.900 |
| T3-T4 | 324 | 13.67 ± 1.02 | ||
| N stage | N0 | 246 | 13.67 ± 0.98 | 0.859 |
| N1-N3 | 259 | 13.66 ± 1.15 | ||
| M stage | M0 | 498 | 13.67 ± 1.06 | 0.100 |
| M1 | 6 | 14.38 ± 0.46 | ||
| Alcohol | No | 164 | 13.69 ± 0.10 | 0.858 |
| Yes | 350 | 13.67 ± 0.10 |
Notes: SD, standard deviation; HNSCC, Head and neck squamous cell carcinoma; T stage, size or direct extent of the primary tumor; N stage, range of spread to regional lymph nodes; M stage, presence of distant metastasis.
Target Genes of miR-205-5p Were Assessed by Bioinformatic Analyses
The 7280 and 143 DEGs identified from the MiRWalk and GEPIA databases, respectively, were analyzed and found to include 57 overlapping target genes (Figure 9; Table 3). Enrichment analyses of GO and KEGG pathways were conducted to further study the mechanisms of miR-205-5p in HNSCC. For GO analyses, we used the BP annotations, including regulation of heart contraction, excretion, O-glycan processing, and CC terms of the extracellular exosome, Z disc, and extracellular space. We also used terms for MF, including iron ion binding, monooxygenase activity, and serine-type peptidase activity. These measures were significantly correlated with the identified target genes ( Figure 10A; Table 4). For KEGG pathway enrichment analyses, we found that the influence of miR-205-5p was important in the following crucial pathways: drug metabolism-cytochrome P450, retinol metabolism, and metabolism of xenobiotics by cytochrome P450 (Figure 10B; Table 5). Finally, we identified the networks of miR-205-5p target genes involved in the GO analyses and detailed them in Figure 11A-C. The PPI network analysis of these genes showed the involvement of the metabolism of xenobiotics by cytochrome P450 pathways (Figure 12). In brief, the bioinformatics analyses indicated that miR-205-5p affects specific genes that are important in the dynamics and mechanistics of HNSCC.
Figure 9.
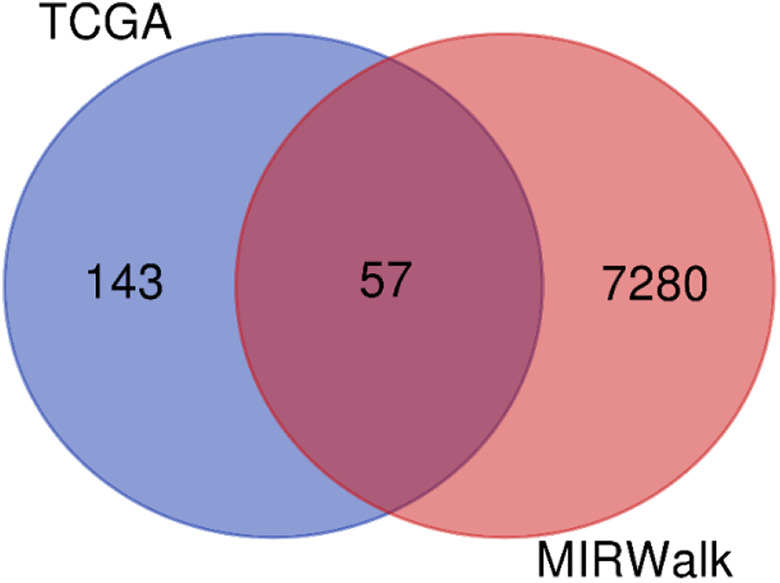
VENNY diagram showing the intersection of predicted miR-205-5p target genes.
Table 3.
The Promising Target Genes of miR-205 in HNSCC With TCGA and MiRWalk.
| Sources of genes | Genes |
|---|---|
| TCGA and MiRWalk | LDB3, CYP3A5, MUC20, SERPINB11, ALDH1L1, ALOX12 |
| UGT1A7, GABRP, FAM3D, GBP6, HOPX, PAX9, CXCR2, ATP13A4, NCCRP1, S100A1, MUC4, PPL | |
| SH3BGRL2, LTF, CRISP3, LYNX1, GPX3, KRT23, PADI1, UPK1A, ENDOU, GPD1, HLF, FMO2, KLK12, KRT78 | |
| PDK4, CGNL1, CYP4F22, AQP3, IL1RN, CA3, TNNI1 | |
| RORC, HSPB7, EYA2, SCIN, PIGR, ATP2A1, DES, TMPRSS2, ATP6V0A4, ADH1B, PPP1R3C | |
| PI16, MYOZ1, XIRP2, MYZAP, GCNT3, PPP1R1A, TGM1 |
Figure 10.
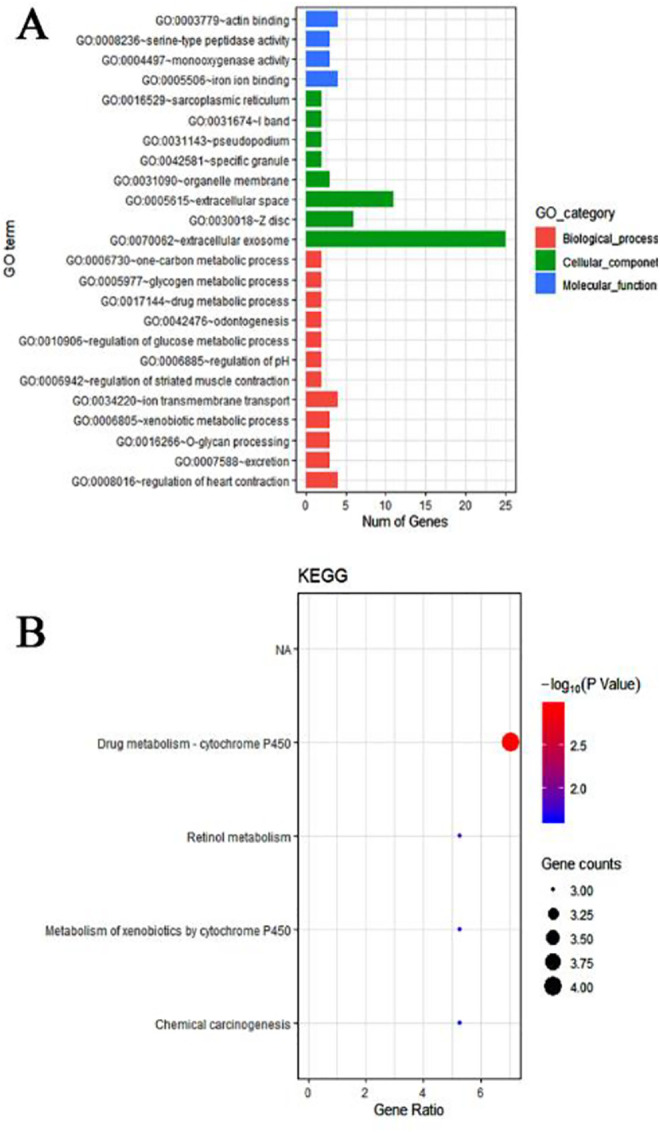
Enrichment analysis of GO and KEGG pathways of miR-205-5p target genes. A. Gene ontology (GO) of candidate genes; B. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis.
Table 4.
Predictive Genes of miR-205 by GO Analysis.
| GO ID | GO term | Counts(%) | Gene symbol | P-value |
|---|---|---|---|---|
| Biological process | ||||
| GO:0008016 | regulation of heart contraction | 4 | DES, HSPB7, HOPX, S100A1 | 1.18E-04 |
| GO:0007588 | excretion | 3 | UGT1A7, ATP6V0A4, AQP3 | 0.005844767 |
| GO:0016266 | O-glycan processing | 3 | GCNT3, MUC20, MUC4 | 0.014845857 |
| GO:0006805 | xenobiotic metabolic process | 3 | CYP3A5, FMO2, RORC | 0.024314658 |
| GO:0034220 | ion transmembrane transport | 4 | ATP2A1, ATP6V0A4, AQP3, ATP13A4 | 0.027210698 |
| GO:0006942 | regulation of striated muscle contraction | 2 | ATP2A1, TNNI1 | 0.027534149 |
| GO:0006885 | regulation of pH | 2 | PDK4, ATP6V0A4 | 0.048434249 |
| GO:0010906 | regulation of glucose metabolic process | 2 | PDK4, RORC | 0.065997490 |
| GO:0042476 | odontogenesis | 2 | PAX9, AQP3 | 0.080390330 |
| GO:0017144 | drug metabolic process | 2 | UGT1A7, FMO2 | 0.080390330 |
| GO:0005977 | glycogen metabolic process | 2 | PPP1R3C, PPP1R1A | 0.086086359 |
| GO:0006730 | one-carbon metabolic process | 2 | ALDH1L1, CA3 | 0.088921383 |
| Cellular component | ||||
| GO:0070062 | extracellular exosome | 25 | GPD1, TMPRSS2, CRISP3, GBP6, GCNT3, ALDH1L1, IL1RN, PIGR, PADI1, MUC4, DES, LYNX1, NCCRP1, UPK1A, PPL, SCIN, KRT78, TGM1, GPX3, KLK12, SH3BGRL2, LTF, ATP6V0A4, PI16, ALOX12 | 8.32E-07 |
| GO:0030018 | Z disc | 6 | DES, XIRP2, MYZAP, LDB3, MYOZ1, S100A1 | 3.07E-05 |
| GO:0005615 | extracellular space | 11 | CRISP3, PPP1R1A, GPX3, ENDOU, KRT78, IL1RN, KLK12, LTF, SERPINB11, PIGR, MUC4 | 0.007351259 |
| GO:0031090 | organelle membrane | 3 | CYP3A5, FMO2, CYP4F22 | 0.029370911 |
| GO:0042581 | specific granule | 2 | CRISP3, LTF | 0.03923172 |
| GO:0031143 | pseudopodium | 2 | LDB3, MYOZ1 | 0.050995984 |
| GO:0031674 | I band | 2 | ATP2A1, MYZAP | 0.068377576 |
| GO:0016529 | sarcoplasmic reticulum | 2 | ATP2A1, S100A1 | 0.099435479 |
| Molecular function | ||||
| GO:0005506 | iron ion binding | 4 | CYP3A5, CYP4F22, LTF, ALOX12 | 0.012284039 |
| GO:0004497 | monooxygenase activity | 3 | CYP3A5, FMO2, CYP4F22 | 0.014289818 |
| GO:0008236 | serine-type peptidase activity | 3 | TMPRSS2, ENDOU, KLK12 | 0.016715968 |
| GO:0003779 | actin binding | 4 | XIRP2, SCIN, MYOZ1, TNNI1 | 0.056676048 |
Table 5.
KEGG Pathway of Validated Target Genes of miR-205-5p.
| KEGG ID | KEGG term | Counts(%) | Gene symbol | P |
|---|---|---|---|---|
| hsa00982 | Drug metabolism—cytochrome P450 | 4 | UGT1A7, CYP3A5, FMO2, ADH1B | 0.001081795 |
| hsa00830 | Retinol metabolism | 3 | UGT1A7, CYP3A5, ADH1B | 0.015969869 |
| hsa00980 | Metabolism of xenobiotics by cytochrome P450 | 3 | UGT1A7, CYP3A5, ADH1B | 0.021008586 |
| hsa05204 | Chemical carcinogenesis | 3 | UGT1A7, CYP3A5, ADH1B | 0.024310918 |
Figure 11.
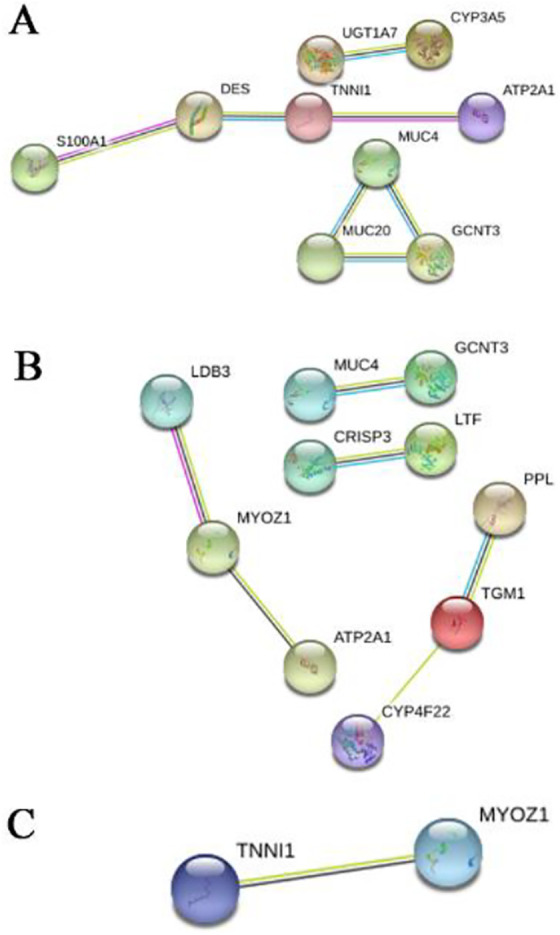
Gene Ontology (GO) analysis. A. biological process (BP); B. cellular componentry (CC), and C. molecular function (MF);
Figure 12.
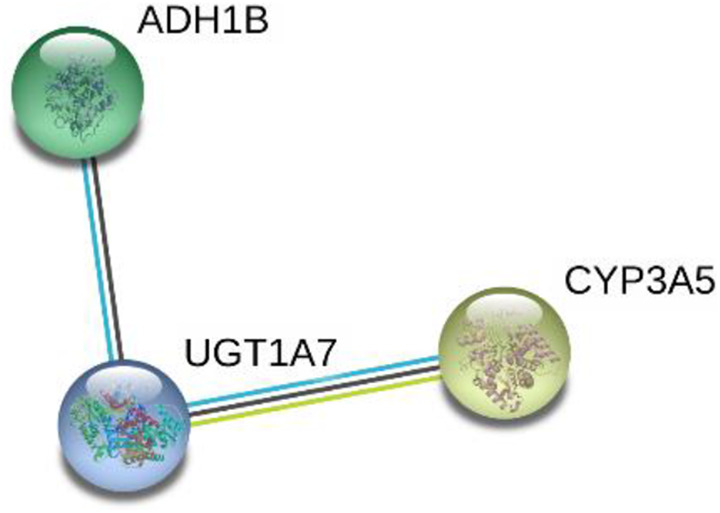
PPI network used to show bonds among target genes of miR-205-5p that are influence the dynamics the pathways of retinol metabolism, metabolism of xenobiotics by cytochrome P450, and chemical carcinogenesis. Nodes represent target genes of miR-205-5p and lines designate relationships between nodes.
Role of miR-205-5p Target Genes in the Drug Metabolism-Cytochrome P450 Pathway
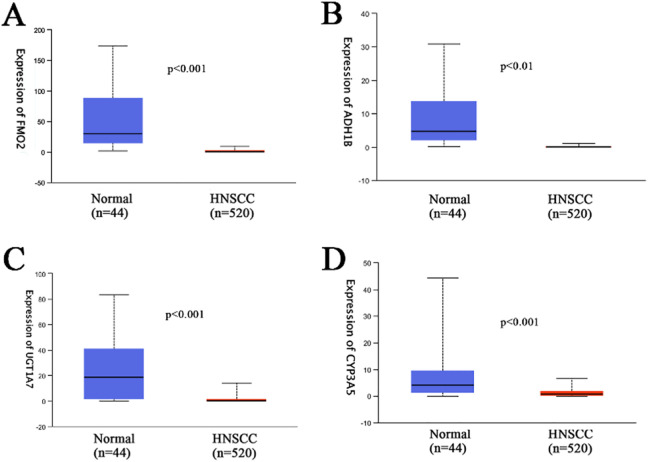
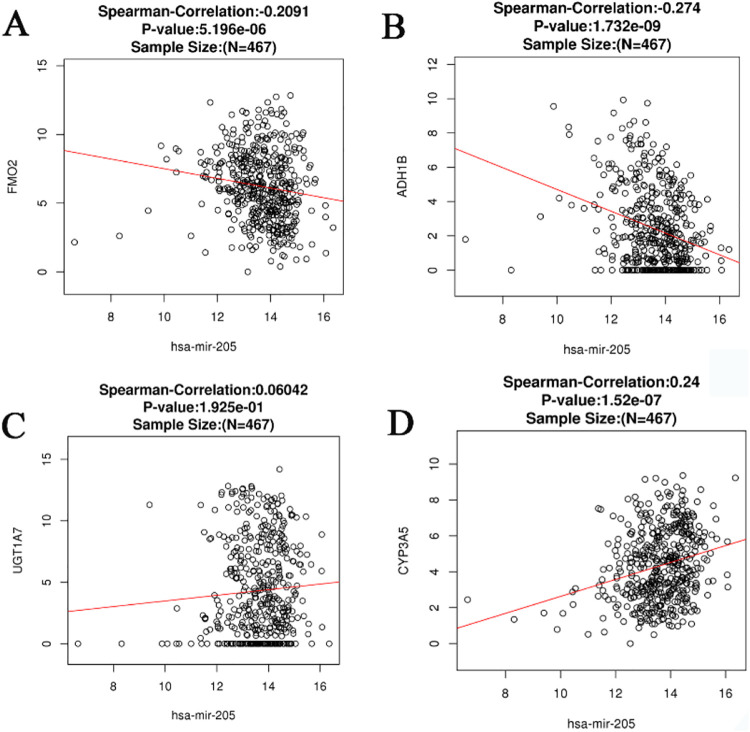
Screening of target genes was a critical measure and component of our study, and was carried out following the GO and KEGG analyses. All identified genes, including FMO2, ADH1B, UDP glucuronosyltransferase family 1 member A7 (UGT1A7), and CYP3A5 were found to be significantly downregulated in HNSCC samples relative to normal unafflicted tissues with respect to the drug metabolism-cytochrome P450 pathway (P < 0.05; Figure 13 A-D). Additionally, results from Spearman correlation analyses indicated that both FMO2 and ADH1B were negatively correlated with miR-205-5p expression level, whereas CYP3A5 indicated a positive correlation with miR-205-5p. In contrast, the examination of the relationship between the expression level of miR-205-5p and UGT1A7 did not indicate any statistically significant difference (Figure 14A-D).
Figure 13.
Levels of expression of 4 target genes of miR-205-5p and measures of correlation with the drug metabolism-cytochrome P450 pathway. A.FMO2; B.ADH1B; C.UGT1A7; D.CYP3A5.
Figure 14.
Spearman’s analysis of the correlation of the expression of miR-205-5p and the 4 target genes.
Discussion
Recent studies have indicated that miR-205-5p plays a key role in facilitating biological processes, including proliferation, migration, invasiveness, apoptosis, and senescence of neoplastic pancreatic cancer, cervical cancer, and endothelial progenitor cells (EPCs).25-29 Furthermore, recent research suggests that upregulation of miR-205-5p expression boosted vascular recanalization and stabilized angiogenesis in EPCs.28 Therefore, it has been postulated that miR-205-5p may be a novel diagnostic biomarker that can be used to predict and study cancer progression, and thus warrants further focused study. Tian et al reported that miR-205-5p induces apoptosis of laryngeal squamous cell carcinoma by targeting and influencing the Bcl-2 pathway, which suggests that miR-205-5p may correlate with the onset and progression of HNSCC.30 However, its functional mechanism remains largely undefined. Therefore, we attempted to examine and validate the possible molecular mechanisms of miR-205-5p in HNSCC using GO and KEGG analyses.
We carried out a meta-analysis using data from 768 HNSCC tissue samples and 237 unafflicted control samples that were derived from searching: 1) TCGA database, 2) 7 GSE datasets, and 3) a published article deemed eligible for our study. The results revealed high levels of miR-205-5p expression in HNSCC patients. Furthermore, we found that the measures for unpaired tissue type and age were the factors most closely associated with high levels of miR-205-5p expression by analyzing data from TCGA database. Our results suggest that miR-205-5p may be a novel biomarker for the prediction and assessment of cancer incidence. The diagnostic and prognostic roles of miR-205-5p were examined by Li and colleagues,31 and their results indicated that miR-205-5p could be used as a biomarker with relatively accurate results for the diagnosis of lung carcinoma. Lu and colleagues13 reported that miR-205-5p acts as a novel prognostic indicator for hepatocellular carcinoma. We sought to evaluate the predicted value of miR-205-5p in HNSCC. Therefore, we also sought to examine whether miR-205-5p represents a promising biomarker of tumorigenesis.
Recent accumulating evidence indicates that miR-205-5p plays an essential role in the occurrence of malignancies by regulating certain proteins and influencing signaling pathways.13,32 Gulei and colleagues33 demonstrated that epithelial to mesenchymal transition (EMT) was suppressed following the upregulation of miR-205-5p in colon carcinoma cells that as found to be associated with E-cadherin expression. Moreover, miRNA-205-5p was shown to be an optimal biomarker for the assessment of renal carcinoma cells via its relationship to the PI3K/Akt/mTOR signaling pathway.34 The pathways associated with miR-205-5p and their influence on HNSCC are being increasingly analyzed. For example, Zhang and colleagues35 reported that miR-205-5p functions to target PTEN via the PI3K/AKT signaling pathway during EMT in cisplatin-resistant NPC cells. The pathways identified in the current study, especially the drug metabolism-cytochrome P450 pathway, clearly warrant further investigation.
Cytochrome P450 enzymes constitute a system of metabolizing enzymes that govern as much as 80% of phase I metabolism of xenobiotics, and have been implicated in various types of cancers.36-38 Polymorphisms of some of the drug-metabolizing enzymes of the cytochrome P450 system have been reported to increase susceptibility to HNSCC.39 According to the results of our study, miR-205-5p target genes are strongly associated with the drug metabolism-cytochrome P450 pathway that is downregulated in HNSCC tissues. This result suggests that the identified target genes may influence the progression of HNSCC. Furthermore, the results suggest that both FMO2 and ADH1B were negatively correlated with miR-205-5p expression level, and that miR-205-5p may negatively regulate FMO2 and ADH1B.
FMO2 belongs to the FMO family of enzymes that are known to play a crucial role in drug metabolism.40 Phillips and colleagues showed that genetic variations in FMO2 affect the metabolism of drug substrates, which is consistent with findings in the pathway we studied.41 Owing to its high expression in lung tissues, FMO2 is characterized as a tumor suppressor in lung adenocarcinoma.42 However, to the best of our knowledge, there is limited research on the association between FMO2 and miR-205-5p. Thus, the results of our study are significant and may form the basis of further focused studies to examine the links between FMO2 and miR-205-5p in HNSCC patients. Our GO analyses revealed that FMO2 influences xenobiotic metabolism, drug metabolism, and organelle membranes. ADH1B is a class I member of ADH isoenzyme family that plays a key role in the transformation of ethanol to acetaldehyde.43 Polymorphisms of ADH1B affect the susceptibility to bladder carcinoma.44 Alcoholism is a major known risk factor for HNSCC4; thus, polymorphism of ADH1B may increase the exposure to acetaldehyde converted by ADH1B during alcohol metabolism.45 Similarly, Yan and colleagues demonstrated that ethanol metabolism might be regulated by ADH1B level.46 Our results suggest that miR-205-5p is likely to target FMO2 and ADH1B, thereby altering the metabolic processes in HNSCC patients. However, additional experiments and more molecular studies are necessary to further validate our findings.
Conclusions
Our study revealed that miR-205-5p is overexpressed in the HNSCC group compared to the normal control group, and has a predictive role in HNSCC. Furthermore, we identified several target genes that are highly associated with the drug metabolism-cytochrome P450 pathway, which is important because it influences the progression of HNSCC. Overall, our findings suggest that miR-205-5p as a potential biomarker for prognosis of HNSCC patients, and will help to promote further research on the oncogenic roles of miR-205-5p in HNSCC.
Abbreviations
- BiNGO
Biological Networks Gene Oncology tool
- BP
biological process
- CC
cellular component
- DAVID
Database for Annotation, Visualization and Integrated Discovery
- GO
Gene Ontology
- GEO
Gene Expression Omnibus
- HNSCC
Head and neck squamous cell carcinoma
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MF
molecular function
- miR
microRNA
- PPI
protein-protein interaction
- RT-qPCR
reverse transcription quantitative real-time PCR
- TCGA
The cancer genome atlas.
Footnotes
Author Notes: The study was approved by the Human Research Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. And written informed consent was obtained from all participants.
Author Contribution: Ziyan Zhou, MS and Chang Liu, MS authors have contributed equally to this work. MK designed the study and accessed the relevant information, and ZYZ and CL collected and analyzed the data. KL and MXL were involved in statistical analysis. BBL, ZRL, WC critically revised the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (No. 81760542), The Research Foundation of the Science and Technology Department of Guangxi Province, China (grant No. 2016GXNSFAA380252, 2018AB61001), the Research Foundation of the Health Department of Guangxi Province, China (No.S2018087), Guangxi Medical University Training Program for Distinguished Young Scholars (2017), Medical Excellence Award Funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University(2016).Guangxi Medical High-level Talents Training Program.The central government guide local science and technology development projects (ZY18057006).
ORCID iD: Ziyan Zhou, MS  https://orcid.org/0000-0002-2306-6307
https://orcid.org/0000-0002-2306-6307
Min Kang, MD  https://orcid.org/0000-0003-2287-8757
https://orcid.org/0000-0003-2287-8757
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2. Yang CX, Sedhom W, Song J, Lu SL. The role of MicroRNAs in recurrence and metastasis of head and neck squamous cell carcinoma. Cancers (Basel). 2019;11(3). doi:10.3390/cancers11030395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma P, Narwal A, Kamboj M. Detection of apoptosis in leukoplakia and oral squamous cell carcinoma using methyl green pyronin and hematoxylin and eosin. Iran J Pathol. 2020;15(3):189–196. doi:10.30699/ijp.2020.107263.2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marron M, Boffetta P, Zhang ZF, et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int J Epidemiol. 2010;39(1):182–196. doi:10.1093/ije/dyp291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castellsague X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):djv403 doi:10.1093/jnci/djv403 [DOI] [PubMed] [Google Scholar]
- 6. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386–396. doi:10.1016/j.mayocp.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 7. Tang X, He J, Li B, et al. Efficacy and safety of gefitinib in patients with advanced head and neck squamous cell carcinoma: a meta-analysis of randomized controlled trials. J Oncol. 2019;2019:6273438 doi:10.1155/2019/6273438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang S, Jin S, Liu MD, et al. Hsa-let-7e-5p inhibits the proliferation and metastasis of head and neck squamous cell carcinoma cells by targeting chemokine receptor 7. J Cancer. 2019;10(8):1941–1948. doi:10.7150/jca.29536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu J, Zhu H, Yang X, et al. MicroRNA-21 is a novel promising target in cancer radiation therapy. Tumour Biol. 2014;35(5):3975–3979. doi:10.1007/s13277-014-1623-8 [DOI] [PubMed] [Google Scholar]
- 10. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi:10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferdin J, Kunej T, Calin G. Non-coding RNAs: identification of cancer-associated microRNAs by gene profiling. Technol Cancer Res Treat. 2010;9(2):123–138. doi:10.1177/153303461000900202 [DOI] [PubMed] [Google Scholar]
- 12. Tran N, McLean T, Zhang X, et al. MicroRNA expression profiles in head and neck cancer cell lines. Biochem Biophys Res Commun. 2007;358(1):12–17. doi:10.1016/j.bbrc.2007.03.201 [DOI] [PubMed] [Google Scholar]
- 13. Lu J, Lin Y, Li F, et al. MiR-205 suppresses tumor growth, invasion, and epithelial-mesenchymal transition by targeting SEMA4C in hepatocellular carcinoma. FASEB J. 2018:fj201800113 R doi:10.1096/fj.201800113 R [DOI] [PubMed] [Google Scholar]
- 14. Park KS, Moon YW, Raffeld M, Lee DH, Wang Y, Giaccone G. High cripto-1 and low miR-205 expression levels as prognostic markers in early stage non-small cell lung cancer. Lung Cancer. 2018;116:38–45. doi:10.1016/j.lungcan.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang J, Wang X, Wen G, Ren Y. miRNA2055p functions as a tumor suppressor by negatively regulating VEGFA and PI3K/Akt/mTOR signaling in renal carcinoma cells. Oncol Rep. 2019;42(5):1677–1688. doi:10.3892/or.2019.7307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma X, Liang A-L, Liu Y-J. Research progress on the relationship between lung cancer drug-resistance and microRNAs. J Cancer. 2019;10(27):6865–6875. doi:10.7150/jca.31952. eCollection 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao L, Zhang LJ, Li SH, et al. Role of miR-452-5p in the tumorigenesis of prostate cancer: a study based on the Cancer Genome Atl(TCGA), Gene Expression Omnibus (GEO), and bioinformatics analysis. Pathol Res Pract. 2018;214(5):732–749. doi:10.1016/j.prp.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 18. Anaya J. OncoLnc: linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Computer Science. 2016;2:e67 doi:10.7287/peerj.preprints.1780v1 [Google Scholar]
- 19. Dweep H, Gretz N. miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nat Methods. 2015;12(8):697 doi:10.1038/nmeth.3485 [DOI] [PubMed] [Google Scholar]
- 20. Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40(null):D109–114. doi:10.1093/nar/gkr98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–29. doi:10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia (NewYork, NY). 2017;19(8):649–658. doi:10.1016/j.neo.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vasaikar SV, Straub P, Wang J, Zhang B. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018;46(null):D956–D963. doi:10.1093/nar/gkx1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cao P, Zhou L, Zhang J, et al. Comprehensive expression profiling of microRNAs in laryngeal squamous cell carcinoma. Head Neck. 2013;35(5):720–728. doi:10.1002/hed.23011 [DOI] [PubMed] [Google Scholar]
- 25. Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302(1):1–12. doi:10.1056/NEJMp058190 [DOI] [PubMed] [Google Scholar]
- 26. Qin R-F, Zhang J, Huo H-R, Yuan Z-J, Xue J-D. MiR-205 mediated APC regulation contributes to pancreatic cancer cell proliferation. World J Gastroenterol. 2019;25(28):3775–3786. doi:10.3748/wjg.v25.i28.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang F, Liu J, Xie B-B. Downregulation of microRNA-205 inhibits cell invasion and angiogenesis of cervical cancer through TSLC1-mediated Akt signaling pathway. J Cell Physiol. 2019;234(10):18626–18638. doi:10.1002/jcp.28501 [DOI] [PubMed] [Google Scholar]
- 28. Sun L-L, Xiao L, Du X-L, et al. MiR-205 promotes endothelial progenitor cell angiogenesis and deep vein thrombosis recanalization and resolution by targeting PTEN to regulate Akt/autophagy pathway and MMP2 expression. J Cell Mol Med. 2019;23(12):8493–8504. doi:10.1111/jcmm.14739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhuang L, Guo J, Yao Y, Li Z. miR-205 targets runt-related transcription factor 2 to inhibit human pancreatic cancer progression. Oncol Lett. 2019;17(1):843–848. doi:10.3892/ol.2018.9689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tian L, Zhang J, Ge J, et al. MicroRNA-205 suppresses proliferation and promotes apoptosis in laryngeal squamous cell carcinoma. Med Oncol. 2014;31(1):785 doi:10.1007/s12032-013-0785-3 [DOI] [PubMed] [Google Scholar]
- 31. Li J-H, Sun S-S, Li N, Lv P, Xie S-Y, Wang P-Y. MiR-205 as a promising biomarker in the diagnosis and prognosis of lung cancer. Oncotarget. 2017;8(54):91938–91949. doi:10.18632/oncotarget.20262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pang H, Yue X. MiR-205 serves as a prognostic factor and suppresses proliferation and invasion by targeting insulin-like growth factor receptor 1 in human cervical cancer. Tumour Biol. 2017;39(6):1010428317701308 doi:10.1177/1010428317701308 [DOI] [PubMed] [Google Scholar]
- 33. Gulei D, Magdo L, Jurj A, et al. The silent healer: miR-205-5p up-regulation inhibits epithelial to mesenchymal transition in colon cancer cells by indirectly up-regulating E-cadherin expression. Cell Death Dis. 2018;9(2):66 doi:10.1038/s41419-017-0102-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen W, Fu W, Deng Q, et al. Multiple metals exposure and chromosome damage: exploring the mediation effects of microRNAs and their potentials in lung carcinogenesis. Environ Int. 2019;122:291–300. doi:10.1016/j.envint.2018.11.020 [DOI] [PubMed] [Google Scholar]
- 35. Zhang P, Lu X, Shi Z, et al. miR-205-5p regulates epithelial-mesenchymal transition by targeting PTEN via PI3K/AKT signaling pathway in cisplatin-resistant nasopharyngeal carcinoma cells. Gene. 2019;710(undefined):103–113. doi:10.1016/j.gene.2019.05.058 [DOI] [PubMed] [Google Scholar]
- 36. Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103–141. doi:10.1016/j.pharmthera.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 37. Eichelbaum M, Ingelman-Sundberg M, Evans WE. Pharmacogenomics and individualized drug therapy. Ann Rev Med. 2006;57(undefined):119–137. doi:10.1146/annurev.med.56.082103.104724 [DOI] [PubMed] [Google Scholar]
- 38. Mittal B, Tulsyan S, Kumar S, Mittal RD, Agarwal G. Cytochrome P450 in cancer susceptibility and treatment. Adv Clin Chem. 2015;71(undefined):77–139. doi:10.1016/bs.acc.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 39. Maurya SS, Katiyar T, Dhawan A, et al. Gene-environment interactions in determining differences in genetic susceptibility to cancer in subsites of the head and neck. Environ Mol Mutagen. 2015;56(3):313–321. doi:10.1002/em.21920 [DOI] [PubMed] [Google Scholar]
- 40. Krueger SK, Williams DE. Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol Ther. 2005;106(3):357–387. doi:10.1016/j.pharmthera.2005.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Phillips IR, Shephard EA. Drug metabolism by flavin-containing monooxygenases of human and mouse. Expert Opin Drug Metab Toxicol. 2017;13(2):167–181. doi:10.1080/17425255.2017.1239718 [DOI] [PubMed] [Google Scholar]
- 42. Hsu Y-L, Hung J-Y, Lee Y-L, et al. Correction: identification of novel gene expression signature in lung adenocarcinoma by using next-generation sequencing data and bioinformatics analysis. Oncotarget. 2019;10(5):616 doi:10.18632/oncotarget.26609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ji YB, Lee SH, Kim KR, et al. Association between ADH1B and ADH1C polymorphisms and the risk of head and neck squamous cell carcinoma. Tumour Biol. 2015;36(6):4387–4396. doi:10.1007/s13277-015-3078-y [DOI] [PubMed] [Google Scholar]
- 44. Ruwali M, Dhawan A, Pant MC, Rahman Q, Khurana SM, Parmar D. Clinical management of head and neck cancer cases: role of pharmacogenetics of CYP2 and GSTs. Oncol Res Treat. 2016;39(4):221–226. doi:10.1159/000444608 [DOI] [PubMed] [Google Scholar]
- 45. Brennan P, Lewis S, Hashibe M, et al. Pooled analysis of alcohol dehydrogenase genotypes and head and neck cancer: a HuGE review. American journal of epidemiology. 2004;159(1):1–16. doi:10.1093/aje/kwh003 [DOI] [PubMed] [Google Scholar]
- 46. Yan Y, Yang J-Y, Mou Y-H, Wang L-H, Zhang H, Wu C-F. Possible metabolic pathways of ethanol responsible for oxidative DNA damage in human peripheral lymphocytes. Alcohol Clin Exp Res. 2011;35(1):1–9. doi:10.1111/j.1530-0277.2010.01316.x [DOI] [PubMed] [Google Scholar]