Abstract
Hypertensive disorders of pregnancy are one of the leading causes of poor pregnancy outcomes and are associated with increased rates of maternal mortality, preterm birth, small for gestational age newborns, stillbirth, and neonatal death. The overall and type-specific prevalence of hypertensive disorders of pregnancy and associated pregnancy outcomes are unknown in Sub-Saharan Africa. Therefore, this review aimed to identify the prevalence of hypertensive disorders of pregnancy and associated pregnancy outcomes in Sub-Saharan Africa. A systematic review and meta-analysis were conducted on observational facility-based studies irrespective of publication status, sample size, language, and follow-up duration from 19 countries between the years 2000 and 2018 in Sub-Saharan Africa. A review of studies using PubMed, EMBASE, African Index Medicus, and African Journals Online was completed with independent extraction of studies by review authors using the predefined inclusion criteria. Quality and risk of bias of individual studies were assessed using the Joanna Briggs Institute Checklist. Random effects model was used to estimate the pooled prevalence of hypertensive disorders of pregnancy and type-specific hypertensive disorders of pregnancy. A pooled adjusted odds ratio with 95% confidence interval for each study was calculated using comprehensive meta-analysis version 2 software to estimate the association of hypertensive disorders of pregnancy and its outcomes. The existence of heterogeneity was assessed using I2 and its corresponding P value. We assessed the presence of publication bias using the Egger’s test. Subgroup analysis was performed to assess the potential effect of variables, and a sensitivity analysis was conducted to assess any undue influence from studies. The analysis included 70 studies. The pooled prevalence of hypertensive disorders of pregnancy (all types combined), chronic hypertension, gestational hypertension, preeclampsia, and eclampsia were 8% (95% confidence interval = [5, 10]), 0.9% (95% confidence interval = [0.4, 1.8]), 4.1% (95% confidence interval = [2.4, 7]), 4.1% (95% confidence interval = [3.2, 5.1]), and 1.5% (95% confidence interval = [1, 2]), respectively. Compared with normotensive pregnant or postpartum women, women with hypertensive disorders of pregnancy were associated with increased risk of maternal mortality, odds ratio = 17 (95% confidence interval = [9.6, 28.8]); cesarean section, odds ratio = 3.1 (95% confidence interval = [1.7, 5.6]); perinatal mortality, odds ratio = 8.2 (95% confidence interval = [2.8, 24]); low birth weight, odds ratio = 3.2 (95% confidence interval = [2, 5]); and preterm delivery, odds ratio = 7.8 (95% confidence interval = [2.5, 25.3]) according to this analysis. The pooled prevalence of hypertensive disorders of pregnancy was high in Sub-Saharan Africa compared to those reported from other regions. Pregnant or postpartum women with hypertensive disorders of pregnancy have increased risk of maternal mortality, cesarean section, preterm delivery, perinatal mortality, and low birth weight newborn. Therefore, creating awareness of the risks of hypertensive disorders of pregnancy is essential. Pregnant women with hypertensive disorders need due attention to manage appropriately and more importantly to have favorable outcomes in this population.
Keywords: hypertensive disorders of pregnancy, pregnancy outcomes, prevalence, Sub-Saharan Africa
Background
Globally, over the past 25 years, the maternal mortality ratio (MMR) has decreased by almost 44%, from 385 maternal deaths per 100,000 live births in 1990 to an estimated 216 maternal deaths per 100,000 live births in 2015.1 Approximately 99% of the global maternal deaths in 2015 are from low- and middle-income countries (LMICs), with Sub-Saharan Africa (SSA) alone accounting for roughly 66%.2 In many regions of the world, maternal mortality has declined, but it has increased in 50 countries and 27 of which are in SSA.3 According to a recent report from the World Health Organization (WHO), in some African settings, hypertensive disorders of pregnancy (HDP) are the leading cause of maternal mortality.4 HDP are multisystem disorders, which include gestational hypertension, chronic hypertension, superimposed preeclampsia on chronic hypertension, and preeclampsia.5,6 They contribute to 14% and 16% maternal mortality worldwide and in SSA, respectively.7
In addition, HDP also contribute to adverse fetal outcomes.8 HDP increase the risk of preterm birth, stillbirth, small for gestational age, and neonatal death,9 and expose the mother to an emergency cesarean section, which increases the risk of low birth weight infants and neonatal death.10
Globally, 2.73% of women suffer from HDP while the incidence of chronic hypertension, preeclampsia, and eclampsia are 0.29%, 2.16% and 0.28%, respectively.11 However, in Africa, a relatively higher prevalence of HDP is reported, as it affects 1 in 10 women.12 Furthermore, there is concern that recent reports on the global burden of HDP underrepresent SSA populations, indicating the importance of further research addressing HDP among this population.13 The burden of hypertension has been increasing over the past few decades in SSA; however, a large percentage of the population with hypertension remains untreated, ineffectively treated, or even undiagnosed, contributing to the growing cardiovascular disorder problem in this region.14
Little is known about the prevalence of HDP in SSA despite widespread investigation in high-income countries.4 Hence, to the best of our awareness, we conducted the first systematic review and meta-analysis to estimate the overall and type-specific prevalence of HDP and associated pregnancy outcomes in SSA, to educate policymakers and guide strategies for early detection, prevention, and management of these disorders in the region. Therefore, the aim of this systematic review and meta-analysis was to determine the prevalence of HDP in SSA and investigate associated adverse pregnancy outcomes.
Methods
Eligibility criteria
Any observational facility-based study conducted to determine prevalence and/or adverse pregnancy outcomes of any type of HDP in SSA from 2000 to 2018 was included.
All observational studies (cohort and cross-sectional) were included regardless of publication status, sample size, language, or follow-up duration. The inclusion criteria comprised of the following: (1) facility-based studies; (2) sampling of a defined population or studies involving entire populations; (3) involving pregnant or postpartum participants; (4) reporting on the prevalence of chronic hypertension, superimposed preeclampsia, gestational hypertension, preeclampsia, and/or eclampsia and/or their pregnancy outcomes in a population of pregnant or postpartum women residing in SSA countries; and (5) defined HDP as blood pressure greater than 140/90 mm Hg15 or urine protein and elevated blood pressure above 140/90 mm Hg. Studies that did not meet the inclusion criteria were excluded. Longitudinal studies where prevalence of HDP or pregnancy outcomes were not determined were also excluded. In addition, case-control studies from pooled prevalence of HDP were also excluded from the analysis to minimize bias.
Information sources and search
A literature search was undertaken using the databases PUBMED and EMBASE. Studies were also retrieved by searches of African Index Medicus and the African Journals Online (AJOL) using all possible key subject headings. In addition to searching databases, supplementary approaches to identifying studies such as hand-searching of journals, checking reference lists, searching websites, and contacting authors were performed. MeSH and free text terms were used to increase sensitivity to potentially appropriate studies. When MeSH terms were not used (websites), search terms were recognized, and all possible substitutes and spellings were found and used in the search strategy.
The following search terms were used: prevalence, proportion, magnitude, epidemiology, hyperten$, “chronic hypertension,” hypertension, blood pressure, “high blood pressure,” preeclampsia, preeclampsia-eclampsia, pre-eclampsia-eclampsia, pre-eclampsia, eclampsia, preeclampsia superimposed, pre-eclampsia superimposed, superimposed preeclampsia, superimposed pre-eclampsia, pregnancy induced hyperten$, hypertension, pregnancy induced, hyperten$ disorders of pregnancy, hypertensive disorders in pregnancy, gestational hyperten$, gestational hypertension, hyperten$ gestational, pregnancy outcome, outcome of pregnancy, maternal mortality, maternal death, death of mother, perinatal mortality, perinatal death, stillbirth, small for gestational age, low birth weight, cesarean section, cesarean birth, prematurity, premature birth, preterm, preterm delivery, preterm labor, preterm birth, and Sub-Saharan Africa.
The search strategy for each database is available in Tables S1 and S2. The reference list of all relevant review papers was scrutinized to identify other potential data sources. The search, which was performed and overseen by two review authors (K.S.G. and N.A.), was restricted to studies in humans published between January 2000 and November 2018. Specific search terms, inclusion and exclusion criteria, searched databases, and eventual data needed to be collected and reported were considered during searching. The review authors collaborated, and consensus was needed throughout the process, with continued discussions on steps of searching during the review.
Study selection
Eligibility assessment of selected studies was performed independently by two authors (K.S.G. and N.A.) in two phases. First, titles and abstracts of retrieved literature were reviewed to check for eligibility for the second phase, which consists of full-text screening. During this phase, the authors further screened for eligibility to include in the study. Disagreements between the two reviewers were resolved by consensus and/or resolved through discussion with the third review author (B.M.).
Data collection process
First review author (K.S.G.) extracted the data from the eligible studies, which was then kept on a data extraction sheet and checked by the second review author (N.A.). Disagreements were resolved by the discussion between the two review authors, otherwise the third review author (B.M.) decided. The studies were checked for duplication based on name of authors, sample size, outcomes, and study location. Data on year of study, country of study, study design, type of HDP, sample size, blood pressure measurement, definition(s) used for HDP, and prevalence of HDP were extracted.
All citations identified by electronic databases and manual searches were organized, duplicates deleted, and each citation assigned a unique identification number. All extracted data were stored in a Microsoft Excel file format. PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines were used for all procedures and reporting.16
Data items
The participants were pregnant or postpartum women (population) with HDP (condition) in SSA (context). Pregnant or postpartum women (population) who are diagnosed with HDP (exposure) and normotensive pregnant or postpartum women (control) were seen for associated adverse pregnancy outcomes (outcome) in SSA.
The prevalence of HDP is the primary outcome of this study. The classification of HDP includes chronic hypertension, gestational hypertension, preeclampsia—de novo or superimposed on chronic hypertension and white coat hypertension.17–19 Hypertension in pregnancy is defined as a systolic blood pressure (SBP) of 140 mm Hg or more and/or diastolic blood pressure (DBP) of 90 mm Hg or more on two or more consecutive occasions during pregnancy.18 Gestational hypertension is an elevation of SBP greater than or equal to 140 mm Hg and DBP greater than or equal to 90 mm Hg without proteinuria in a previously normotensive non-proteinuria pregnant woman after 20 weeks of pregnancy.18,19
Preeclampsia is defined as women who develop both hypertension and proteinuria in pregnancy after 20 weeks of gestation.18 In this review, any forms of preeclampsia were included as preeclampsia (i.e. severe preeclampsia, mild preeclampsia, and superimposed preeclampsia). Eclampsia is the occurrence of convulsions or fit with SBP of >140 mm Hg and DBP of >90 mm Hg after 20 weeks of gestation, proteinuria of >2+, and signs and symptoms of severe preeclampsia.20,21 Chronic hypertension is defined as high blood pressure predating the pregnancy or recognized at less than 20 weeks of gestation.22
Preeclampsia superimposed on chronic hypertension refers to a sudden increase in blood pressure and new onset of proteinuria in women with hypertension and no proteinuria in early pregnancy (less than 20 weeks of gestation).23
The HDP definition was either reported by authors, in which case we depend on it; or we pooled data from eligible studies reporting on at least three of the four entities of HDP (i.e. chronic hypertension, gestational hypertension, preeclampsia/eclampsia, and superimposed preeclampsia/eclampsia) to acquire a rough approximation of the general prevalence of overall HDP in SSA, as limited studies reported an overall prevalence of HDP (four combined entities).12 Specific HDP in this review was each type of HDP-like chronic hypertension, gestational hypertension, preeclampsia, or eclampsia.
The secondary outcomes of this analysis include maternal mortality, cesarean section, perinatal mortality, preterm delivery, and low birth weight. Maternal mortality is defined as
the death of a pregnant woman or death within 42 days of termination of pregnancy, irrespective of site and the duration of the pregnancy, from any cause related to or intensified by the pregnancy or its management but not from unintentional or incidental causes.24
Cesarean section/birth is the delivery of a baby through incisions made in the mother’s abdomen and uterus.25 Perinatal mortality includes stillbirths (fetal loss after 28 weeks of gestation) and neonatal deaths (during first 7 days of life).26 Premature delivery is when birth occurs between 20 and 37 weeks of gestation.27 Low birth weight has been defined by the WHO as birth weight less than 2500 g28 with infants whose weight is less than the 10th percentile for gestational age classified as small for gestational age.29
Quality and risk of bias assessment
Quality and risk of bias of studies were assessed using the Joanna Briggs Institute assessment based on the Critical Appraisal Checklist for Studies Reporting Prevalence Data 2017. The study evaluated the quality of all included studies in accordance with the nine criteria. The criteria have a rating scale of 0, 1, and 2.30 Depending on the score achieved (from 0 to 18), studies were categorized as being high (>14), medium (11–14), or low (<11) quality.31 The review authors performed an assessment of the risk of bias independently and any studies which did not fulfill minimum criteria (low quality) were excluded from the meta-analysis.
Statistical analysis
The study used baseline data for cohort studies and the total number of pregnant women reported during a given period. Using studies which reported pregnancy outcomes, an unadjusted odds ratio (OR) with 95% confidence interval (CI) was calculated using comprehensive meta-analysis version 2 package. In addition to overall pooled prevalence for all forms of HDP, subgroup and sensitivity analysis was also performed. Considering the “synthesis of results,” the raw proportion was transformed into a suitable unit for meta-analysis using logit transformation. A random effects model was used to account for possible heterogeneity between studies, which defaults to the fixed effects model approach in the absence of heterogeneity to obtain an overall summary estimate of the prevalence across studies and pooled study-specific estimates.32 The P value of less than 0.05 was considered a significant association between HDP and pregnancy outcomes. Statistical heterogeneity was evaluated with the Cochran chi-square (χ2), and I2 statistic33 (low <25%, moderate 25%–50%, high >50%) was computed with its corresponding P value.
Risk of bias across studies
The study assessed the possibility of publication bias using Egger’s linear regression test as formal statistical test for publication bias.34 A P value of less than 0.10 on the Egger’s test was considered indicative of statistically significant publication bias.
Additional analyses
Subgroup analysis was conducted by specific types of HDP, design, and regions of SSA. Furthermore, the degree to which the main findings of a review were affected by changes in the methods or in the data used from individual studies was explored by the sensitivity analysis. The sensitivity analysis was also done considering diagnostic criteria, high prevalence, and wide CI.
Results
Study characteristics
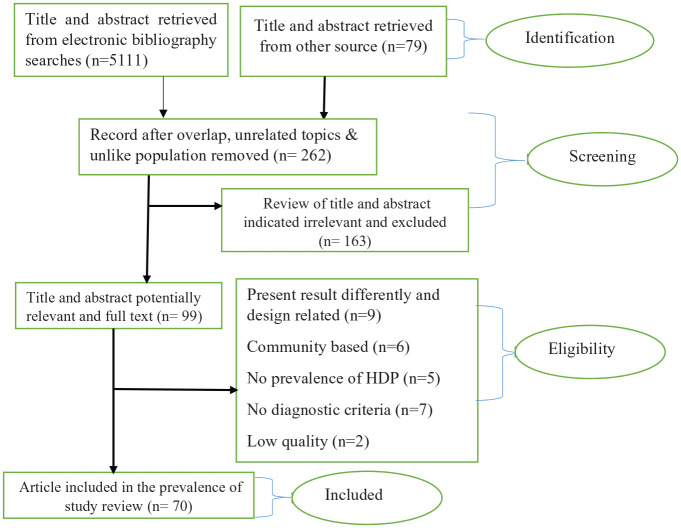
The literature search identified 1327 references through PubMed database and 3784 through EMBASE database (Figure 1). An additional 79 studies were found from African Index Medicus and AJOL. Following the removal of duplicates, 262 records were screened for relevance by reviewing the title and abstract. As a result, 99 full-text articles were assessed against the inclusion criteria. Consequently, 29 studies were excluded, including 9 studies which presented results differently, 6 community-based studies, 5 studies without prevalence and pregnancy outcomes of HDP, and 7 studies with no clear diagnostic criteria for HDP. Thus, 70 full-text studies remained for analysis (Table 1).
Figure 1.
Summary of literature search for prevalence of hypertensive disorders of pregnancy in Sub-Saharan Africa.
Table 1.
Summary characteristics of studies on hypertensive disorders of pregnancy in Sub-Saharan Africa..
| Type | Study design | Sample size | Diagnosis | Study period | Country | Prevalence |
|---|---|---|---|---|---|---|
| Chronic hypertension10 | Prospective cohort study | 789 | Elevated blood pressure at <20 weeks of gestation | Between July 2012 and March 2014 | Ghana | 2% |
| Chronic hypertension102 | Retrospective cohort study | 39,993 | BP 140/90 mm Hg or above recorded before 20 weeks of gestation | From July 2000 to December 2010 | Tanzania | 4.8% |
| Chronic hypertension50 | Cross-sectional study | 1856 | BP > 140/90 mm Hg before 20 weeks of gestation | Between 1 January and 28 February 2013 | Ghana | 1.2% |
| Chronic hypertension51 | Cross-sectional study | 2393 | Preexisting SBP of 140 mm Hg or greater and/or DBP of 90 mm Hg | From January 1997 to December 2002 | Nigeria | 1% |
| Chronic hypertension91 | Cohort study | 3491 | Preexisting BP of ⩾140/90 mm Hg observed before 20th week of gestation | From 15 May 2005 to 2007 | Nigeria | 0.3% |
| Chronic hypertension96 | Prospective cohort study | 3909 | SBP > 140 mm Hg and/or DBP > 90 mm Hg before 20 weeks of gestation | 2000 to 2008 | Tanzania | 1.2% |
| Chronic hypertension52 | Cross-sectional study | 5415 | SBP > 140 mm Hg and/or DBP > 90 mm Hg before 20 weeks of gestation | From 1 January 2010 to 1 December 2013 | Ethiopia | 0.1% |
| Chronic hypertension53 | Cross-sectional study | 25,792 | SBP > 140 mm Hg or DBP > 90 mm Hg before 20 weeks of gestation | 2008 to 2010 | Madagascar | 0.3% |
| Chronic hypertension39 | Longitudinal study | 216 | BP > 140/80 mm Hg before 20 weeks of gestation | March 2011 to December 2011 | Nigeria | 1.3% |
| Chronic hypertension97 | Prospective cohort study | 716 | SBP > 140 mm Hg or DBP > 90 mm Hg before 20 weeks of gestation | From July 2012 to March 2014 | Ghana | 2.2% |
| Chronic hypertension54 | Cross-sectional study | 1620 | SBP > 140 mm Hg or DBP > 90 mm Hg before 20 weeks of gestation | 1 October 2011 to 31 September 2012 | Togo | 1.2% |
| Chronic hypertension92 | Cohort study | 3247 | Patients with history or diagnosed hypertension before 20 weeks of amenorrhea | From 1 July 2012 to 31 March 2013 | Burkina Faso | 0.7% |
| Chronic hypertension9 | Prospective surveillance | 14,170 | SBP > 140 mm Hg or DBP > 90 mm Hg before 20 weeks of gestation | From August 2014 through July 2015 | Botswana | 4.8% |
| Eclampsia55 | Cross-sectional study | 42,963 | BP > 140/90 mm Hg plus convulsion | From 1 January 2009 to 30 December 2013 | Ethiopia | 0.7% |
| Eclampsia37 | Retrospective cohort study | 9086 | Grand mal seizure with features of preeclampsia and no history of a seizure disorder | From 1 January 2016 to 31 December 2016 | Zimbabwe | 0.3% |
| Eclampsia46 | Cross-sectional study | 13,682 | Generalized tonic-clonic convulsion during pregnancy excluding others cause of convulsion | From 1 January 1994 to 31 December 2003 | Nigeria | 0.9% |
| Eclampsia47 | Cross-sectional study | 2792 | Convulsions and preeclampsia | Between 1 April 2010 and 31 March 2011 | Nigeria | 2.2% |
| Eclampsia48 | Cross-sectional study | 4471 | Proteinuria at presentation and anticonvulsants used | Between 1 May 2005 and 30 April 2008 | Nigeria | 1.30% |
| Eclampsia49 | Cross-sectional study | 10,163 | When convulsions occur with syndrome of preeclampsia | Not identified | Nigeria | 1.2% |
| Eclampsia56 | Cross-sectional study | 306 | BP > 140/90 mm Hg and convulsion | From July to September 2015 | Namibia | 9.15% |
| Eclampsia57 | Cross-sectional study | 1863 | BP > 140/90 mm Hg and convulsion | From 1 April 2009 to 31 March 2010 | Ethiopia | 2% |
| Eclampsia58 | Cross-sectional study | 1981 | BP ⩾ 140/90 mm Hg plus proteinuria and convulsion | From 1 January 2010 to 31 December 2014 | Ethiopia | 5.4% |
| Eclampsia59 | Cross-sectional study | 2197 | ISSHP | Between 1 January 2002 and 31 December 2005 | Nigeria | 9.4% |
| Eclampsia60 | Cross-sectional study | 3500 | BP ⩾ 140/90 mm Hg plus convulsion | Between 1 May 2014 and 30 April 2015 | Ethiopia | 2.7% |
| Eclampsia61 | Cross-sectional study | 3952 | SBP ⩾ 140 mm Hg, DBP 90 mm Hg, proteinuria > 1+ and convulsions | Between 1 January 2005 to 31 December 2010 | Nigeria | 2.10% |
| Eclampsia62 | Cross-sectional study | 4316 | ISSHP | From 1 December 2015 to 31 July 2016 | Madagascar | 1.5% |
| Eclampsia90 | Follow-up study | 5860 | BP ⩾ 140/90 mm Hg and proteinuria and seizures | From April 2012 to March 2013 | South Africa | 0.5% |
| Eclampsia63 | Cross-sectional study | 5868 | ISSHP | Between 1 March 2000 and 28 February 2005 | Nigeria | 0.8% |
| Eclampsia64 | Cross-sectional study | 6598 | BP > 140/90 mm Hg plus convulsion | From 2008 to 2014 | Nigeria | 0.8% |
| Eclampsia65 | Cross-sectional study | 13,943 | SBP ⩾ 140 mm Hg, DBP 90 mm Hg, proteinuria > 1+ and convulsions | Between April 2008 and May 2009 | Nigeria | 5% |
| Eclampsia103 | Retrospective cohort study | 15,318 | Fit and >140/90 mm Hg at least 2+ proteinuria with or without edema and convulsion | From 1 January 1995 to 31 December 2004 | Nigeria | 4.3% |
| Eclampsia66 | Cross-sectional study | 17,169 | SBP ⩾ 140 mm Hg, DBP > 90 mm Hg and convulsion | From January 2008 to December 2016 | Nigeria | 0.84% |
| Eclampsia67 | Cross-sectional study | 17,592 | SBP > 140 mm Hg and/or DBP > 90 mm Hg and convulsion | Between 2003 and 2007 | Congo | 1.8% |
| Eclampsia68 | Cross-sectional study | 25,425 | BP > 140/90 mm Hg plus convulsion | During 18-month period | Zimbabwe | 0.6% |
| Eclampsia53 | Cross-sectional study | 25,792 | SBP > 140 mm Hg and/or DBP > 90 mm Hg and convulsion | 2008 to 2010 | Madagascar | 0.7% |
| Eclampsia40 | Cross-sectional study | 2500 | Convulsions not attributable to other conditions in a patient with preeclampsia | From 1 June 2012 to 31 June 2014 | Cameroon | 0.5% |
| Eclampsia69 | Cross-sectional study | 100 | BP > 140/90 mm Hg and 4+ urine protein and convulsion | Between 3 September and 12 October 2007 | Zanzibar | 2% |
| Eclampsia70 | Cross-sectional study | 320 | BP > 140/90 mm Hg and convulsion | From 8 July 2014 to 7 July 2015 | Ethiopia | 0.9% |
| Eclampsia71 | Cross-sectional study | 708 | Signs and symptoms of severe preeclampsia and convulsions or coma | From 1 July 2018 to 10 July 2018 | Ethiopia | 2.3% |
| Eclampsia72 | Cross-sectional study | 1100 | BP > 140/90 mm Hg and convulsion | From November 2004 to April 2005 | Cameroon | 1.6% |
| Eclampsia101 | Retrospective cohort study | 2094 | Standard criteria (Davey and MacGillivray, 1988; Huddle et al., 1993) | During 4-month period in 1997 | South Africa | 0.5% |
| Eclampsia73 | Cross-sectional study | 3398 | DBP ⩾ 90 mm Hg or SBP ⩾ 140 mm Hg and convulsion | Between July 2011 and December 2012 | Tanzania | 1.6% |
| Eclampsia91 | Cross-sectional study | 3491 | Generalized seizure occurred in a patient with HDP | From 15 May 2005 to 2007 | Nigeria | 2% |
| Eclampsia74 | Cross-sectional study | 3931 | SBP ⩾ 140 mm Hg, DBP 90 mm Hg, proteinuria > 1+ and convulsions | From 1 January 2003 to 31 December 2007 | Nigeria | 5.7% |
| Eclampsia75 | Cross-sectional study | 5562 | Convulsion > 24 weeks of pregnancy with BP > 140/90 mm Hg and proteinuria | From June 2009 to February 2010 | Tanzania | 1.37% |
| Eclampsia76 | Cross-sectional study | 6262 | BP > 140/90 mm Hg plus convulsion | From 1 January to 31 December 2009 | Nigeria | 0.91% |
| Eclampsia77 | Cross-sectional study | 8626 | SBP > 140 mm Hg or DBP > 90 mm Hg and convulsion | From September 2011 to August 2014 | Ethiopia | 0.6% |
| Eclamptia78 | Cross-sectional study | 35,741 | BP > 140/90 mm Hg plus convulsion | From October 1994 to September 1999 | Ethiopia | 0.7% |
| Eclamsia51 | Cross-sectional study | 2393 | SBP of 140 mm Hg or greater and/or DBP of 90 mm Hg or greater and convulsion | From January 1997 to December 2002 | Nigeria | 2.8% |
| HDP53 | Cross-sectional study | 25,792 | SBP > 140 mm Hg and/or DBP > 90 mm Hg | 2008 to 2010 | Madagascar | 5.11% |
| HDP55 | Cross-sectional study | 42,963 | SBP > 140 mm Hg or DBP > 90 mm Hg | 2009 to 2013 | Ethiopia | 8.1% |
| HDP35 | Cross-sectional study | 5248 | DBP 90 mm Hg or more recorded twice 4 to 6 h apart | From January 2003 to June 2003 | Malawi | 1.30% |
| HDP36 | Cross-sectional study | 6493 | DBP > 90 mm Hg, protein excretion of 300 mg and more | From 1 March 2000 to 28 February 2005 | Nigeria | 11.30% |
| HDP79 | Cross-sectional study | 160 | SBP > 140 mm Hg and/or DBP > 90 mm Hg | From May to June 2014 | Cameroon | 46% |
| HDP82 | Cross-sectional study | 289 | BP ⩾ 140/90 mm Hg | September 2012 | Zimbabwe | 21.5% |
| HDP70 | Cross-sectional study | 320 | BP > 140/90 mm Hg | From 8 July 2014 to 7 July 2015 | Ethiopia | 8.80% |
| HDP71 | Cross-sectional study | 708 | SBP ⩾ 140 mm Hg and/or DBP ⩾ 90 mm Hg | From 1 July 2018 to 10 July 2018 | Ethiopia | 9.9% |
| HDP50 | Cross-sectional study | 1856 | SBP > 140 mm Hg and/or DBP > 90 mm Hg | Between 1 January and 28 February 2013 | Ghana | 19.8% |
| HDP57 | Cross-sectional study | 1863 | SBP > 140 mm Hg or DBP > 90 mm Hg | From 1 April 2009 to 31 March 2010 | Ethiopia | 8.48% |
| HDP92 | Cohort study | 3247 | Based on European Society of Hypertension | From 1 July 2012 to 31 March 2013 | Burkina Faso | 9.60% |
| HDP95 | Longitudinal study | 3424 | SBP > 140 mm Hg or DBP > 90 mm Hg | For 1 year | Ethiopia | 5.3% |
| HDP91 | Cross-sectional study | 3491 | BP ⩾ 140 mm Hg (systolic)/90 mm Hg (diastolic) on at least two readings | From 15 May 2005 to 2007 | Nigeria | 6.2% |
| HDP52 | Cross-sectional study | 5415 | BP at least 140 mm Hg for systolic and/or 90 mm Hg for diastolic on at least two occasions | From 1 January 2010 to 1 December 2013 | Ethiopia | 2.4% |
| HDP90 | Cross-sectional study | 5860 | BP ⩾ 140/90 mm Hg | From April 2012 to March 2013 | South Africa | 12.5% |
| HDP9 | Prospective surveillance | 14,170 | At least one SBP > 140 mm Hg or DBP > 90 mm Hg | From August 2014 through July 2015 | Botswana | 22.20% |
| HDP77 | Cross-sectional study | 8626 | SBP > 140 mm Hg or DBP > 90 mm Hg | From September 2011 to August 2014 | Ethiopia | 3.90% |
| HDP10 | Prospective cohort study | 789 | SBP ⩾ 140 mm Hg and/or DBP ⩾ 90 mm Hg on two separate occasions | Between July 2012 and March 2014 | Ghana | 11.3% |
| HDP102 | Retrospective cohort study | 39,993 | SBP 140 mm Hg or more and DBP 90 mm Hg or above | From July 2000 to December 2010 | Tanzania | 4.8% |
| HDP38 | Retrospective cohort study | 249,771 | SBP of or above 140 mm Hg or DBP of or above 90 mm Hg | Between February 2006 and December 2012 | Zambia | 2.1% |
| HDP39 | Longitudinal study | 216 | BP > 140/80 mm Hg | From March to December 2011 | Nigeria | 17% |
| HDP5 | Retrospective cohort study | 30,750 | SBP > 140 mm Hg or DBP > 90 mm Hg | From 2008 to 2013 | Ethiopia | 3.6% |
| HDP69 | Cross-sectional study | 100 | Equal to or greater than 140/90 mm Hg blood pressure | Between 3 September and 12 October 2007 | Zanzibar | 2% |
| HDP97 | Prospective cohort study | 759 | SBP > 140 mm Hg or DBP > 90 mm Hg | From July 2012 to March 2014 | Ghana | 9.9% |
| HDP80 | Cross-sectional study | 910 | SBP at least 140 mm Hg or DBP at least 90 mm Hg | From August 2011 through May 2012 | Tanzania | 6.90% |
| HDP54 | Cross-sectional study | 1620 | BP ⩾ 140/90 mm Hg | From 1 October 2011 to 31 September 2012 | Togo | 12.3% |
| HDP101 | Retrospective cohort study | 2094 | Standard criteria (Davey and MacGillivray, 1988; Huddle et al., 1993) | During 4-month period in 1997 | South Africa | 18.2% |
| HDP51 | Cross-sectional study | 2393 | SBP of 140 mm Hg or greater and/or DBP of 90 mm Hg or greater on at least two readings | Between January 1997 and December 2002 | Nigeria | 5.30% |
| HDP100 | Prospective study | 3168 | SBP > 140 mm Hg and/or DBP > 90 mm Hg | From October 2011 to July 2012 | Sudan | 2.17% |
| HDP96 | Prospective cohort study | 3909 | BP 140 mm Hg SBP or more and DBP 90 mm Hg or above | Between 2000 and 2008 | Tanzania | 5.2% |
| HDP48 | Cross-sectional study | 4471 | SBP > 140 mm Hg or DBP > 90 mm Hg | Between 1 May 2005 and 30 April 2008 | Nigeria | 1% |
| HDP81 | Cross-sectional study | 7702 | BP > 140/90, proteinuria > 1+, with or without edema | From January 2009 to December 2012 | Ethiopia | 2.6% |
| GH93 | Cohort study | 180 | BP > 140/90 mm Hg measured on two separate occasions after 20 weeks of gestation | 2015 | Nigeria | 6.7% |
| GH82 | Cross-sectional study | 289 | BP ⩾ 140/90 mm Hg begins after 20 weeks of gestation, and resolves by the 6th week postpartum | From 2009 to 2011 | Zimbabwe | 19.4% |
| GH99 | Prospective longitudinal study | 416 | BP ⩾ 140/90 mm Hg on two occasions after 20 weeks of gestation | 2009 | Mauritius | 13.5% |
| GH71 | Cross-sectional study | 708 | De novo hypertension arising after mid-pregnancy without proteinuria | From 1 July 2018 to 10 July 2018 | Ethiopia | 9.9% |
| GH97 | Prospective cohort study | 759 | BP > 140/90 mm Hg after 20 weeks of gestation on two separate occasions | 2012 to 2014 | Ghana | 7.8% |
| GH98 | Prospective cohort study | 2630 | DBP of 90 mm Hg or higher or SBP of 140 mm Hg or higher | Between July 2006 and February 2009 | Nigeria | 28.9% |
| GH83 | Cross-sectional study | 16,985 | SBP > 140 mm Hg and/or DBP > 90 mm Hg | From July 2010 to June 2012 | Ethiopia | 0.5% |
| GH10 | Prospective cohort study | 789 | SBP ⩾ 140 mm Hg and/or DBP ⩾ 90 mm Hg on two separate occasions without proteinuria after 20 weeks of gestation | Between July 2012 and March 2014 | Ghana | 7.5% |
| GH53 | Cross-sectional study | 25,792 | SBP > 140 mm Hg and/or DBP > 90 mm Hg | 2008 to 2010 | Madagascar | 3.3% |
| GH39 | Longitudinal study | 216 | BP > 140/80 mm Hg | From March to December 2011 | Nigeria | 9.7% |
| GH69 | Cross-sectional study | 100 | BP > 140/90 mm Hg | Between 3 September and 12 October 2007 | Zanzibar | 9% |
| GH93 | Prospective study | 178 | BP higher than 140/90 mm Hg | Not specified | Nigeria | 6.7% |
| GH70 | Cross-sectional study | 320 | BP ⩾ 140/90 mm Hg | From 8 July 2014 to 7 July 2015 | Ethiopia | 1.3% |
| GH54 | Cross-sectional study | 1620 | SBP > 140 mm Hg and/or DBP > 90 mm Hg | From 1 October 2011 to 31 September 2012 | Togo | 4.1% |
| GH57 | Cross-sectional study | 1863 | DBP of 90 mm Hg or higher or SBP of 140 mm Hg or higher | From 1 April 2009 to 31 March 2010 | Ethiopia | 0.4% |
| GH51 | Cross-sectional study | 2393 | SBP of 140 mm Hg or greater and/or DBP of 90 mm Hg or greater on at least two readings | From January 1997 to December 2002 | Nigeria | 1.3% |
| GH92 | Cohort study | 3247 | SBP > 140 mm Hg and/or DBP > 90 mm Hg | From 1 July 2012 to 31 March 2013 | Burkina Faso | 0.8% |
| GH91 | Cohort study | 3491 | De novo hypertension did not show any features of preeclampsia | From 15 May 2005 to 2007 | Nigeria | 3.4% |
| GH96 | Prospective cohort study | 3909 | SBP > 140 mm Hg and/or DBP > 90 mm Hg | 2000 to 2008 | Tanzania | 0.5% |
| GH90 | Cross-sectional study | 5860 | BP ⩾ 140/90 mm Hg | From April 2012 to March 2013 | South Africa | 6.7% |
| Preeclampsia102 | Retrospective cohort study | 39,993 | BP 140/90 mm Hg or above with proteinuria | From July 2000 to December 2010 | Tanzania | 4.1% |
| Preeclampsia37 | Retrospective cohort study | 9086 | DBP > 110 mm Hg and proteinuria | From 1 January 2016 to 31 December 2016 | Zimbabwe | 1% |
| Preeclampsia40 | Cross-sectional study | 2500 | BP ⩾ 160/110 mm Hg and proteinuria | From 1 June 2012 to 31 June 2014 | Cameroon | 4.44% |
| Preeclampsia41 | Retrospective cohort study | 2337 | DBP ⩾ 110 mm Hg or SBP ⩾ 160 mm Hg or urine protein ⩾ 5 g/24 h | From 1 January 2005 to 31 December 2008 | Nigeria | 3.30% |
| Preeclampsia42 | Cross-sectional study | 11,585 | Standard criteria (Davey and MacGillivray, modified by the ISSHP) | 2002 to 2003 | South Africa | 11.5% |
| Preeclampsia84 | Cross-sectional study | 574 | SBP ⩾ 140 mm Hg and/or DBP ⩾ 90 mm Hg and proteinuria | From 1 July 2011 to 28 February 2012 | Ethiopia | 0.7% |
| Preeclampsia85 | Cross-sectional study | 422 | SBP ⩾ 140 mm Hg and/or DBP ⩾ 90 mm Hg and proteinuria | From 10 January to 9 February 2016 | Ethiopia | 18.25% |
| Preeclampsia71 | Cross-sectional study | 708 | SBP ⩾ 140 mm Hg and/or DBP ⩾ 90 mm Hg and proteinuria | From 1 July 2018 to 10 July 2018 | Ethiopia | 6.5% |
| Preeclampsia86 | Cross-sectional study | 1667 | ISSHP | From November 2005 to June 2006 | Mali | 7.8% |
| Preeclampsia50 | Cross-sectional study | 1856 | SBP of at least 140 mm Hg and/or a DBP of at least 90 mm Hg and proteinuria | Between 1 January and 28 February 2013 | Ghana | 7.5% |
| Preeclampsia58 | Cross-sectional study | 1981 | BP ⩾ 140/90 mm Hg plus proteinuria | From 1 January 2010 to 31 December 2014 | Ethiopia | 10.50% |
| Preeclampsia92 | Cohort study | 3247 | DBP of 90 mm Hg or higher, or SBP of 140 mm Hg or higher and proteinuria | From 1 July 2012 to 31 March 2013 | Burkina Faso | 1.9% |
| Preeclampsia73 | Cross-sectional study | 3398 | DBP ⩾ 90 mm Hg or SBP ⩾ 140 mm Hg with proteinuria | Between July 2011 and December 2012 | Tanzania | 0.8% |
| Preeclampsia96 | Prospective cohort study | 3909 | BP 140/90 mm Hg or above recorded after 20 weeks of gestation age combined with proteinuria | 2000 to 2008 | Tanzania | 3.5% |
| Preeclampsia62 | Cross-sectional study | 4316 | ISSHP | From 1 December 2015 to 31 July 2016 | Madagascar | 1.9% |
| Preeclampsia90 | Cohort study | 5860 | BP ⩾ 140/90 mm Hg and proteinuria after the 20th week of pregnancy | From April 2012 to March 2013 | South Africa | 1.4% |
| Preeclampsia81 | Cross-sectional study | 7702 | BP > 140/90 mm Hg proteinuria > 1+, with or without edema | From January 2009 to December 2012 | Ethiopia | 2.23% |
| Preeclampsia87 | Cross-sectional study | 8524 | BP > 140/90 mm Hg and proteinuria | From 2009 to 2011 | Nigeria | 1.20% |
| Preeclampsia94 | Longitudinal prospective analytical survey | 11,784 | BP 140/90 mm Hg or higher with proteinuria | From 1 January 1996 to 31 December 2003 | Ghana | 7.03% |
| Preeclampsia67 | Cross-sectional study | 17,592 | SBP > 140 mm Hg and/or DBP > 90 mm Hg and proteinuria | Between 2003 and 2007 | Congo | 8.5% |
| Preeclampsia10 | Prospective cohort study | 789 | SBP ⩾ 140 mm Hg and/or DBP ⩾ 90 mm Hg on two separate occasions with proteinuria after 20 weeks of gestation | Between July 2012 and March 2014 | Ghana | 1.8% |
| Preeclampsia53 | Cross-sectional study | 25,792 | SBP > 140 mm Hg and/or DBP > 90 mm Hg and proteinuria | 2008 to 2010 | Madagascar | 1.6% |
| Preeclampsia88 | Cross-sectional study | 33,832 | SBP > 140 mm Hg and/or DBP > 90 mm Hg and proteinuria | From April 1992 to March 1997 | South Africa | 7.1% |
| Preeclampsia55 | Cross-sectional study | 42,963 | BP ⩾ 140/90 mm Hg plus proteinuria | From 1 January 2009 to 30 December 2013 | Ethiopia | 3.5% |
| Preeclampsia39 | Longitudinal study | 216 | BP > 140/80 mm Hg and proteinuria | From March 2011 to December 2011 | Nigeria | 4.6% |
| Preeclampsia69 | Cross-sectional study | 100 | BP > 140/90 mm Hg and 4+ urine protein | Between 3 September and 12 October 2007 | Zanzibar | 9% |
| Preeclampsia93 | Cohort study | 180 | BP > 140/90 measured twice after 20 weeks of gestation plus proteinuria | 2015 | Nigeria | 7.3% |
| Preeclampsia70 | Cross-sectional study | 320 | SBP ⩾ 140 mm Hg, DBP > 90 mm Hg, proteinuria > 1+ | From 8 July 2014 to 7 July 2015 | Ethiopia | 6.6% |
| Preeclampsia99 | Prospective longitudinal study | 416 | SBP ⩾ 140 mm Hg and/or DBP ⩾ 90 mm Hg and albumin ⩾ 1+ | 2004 | Mauritius | 8.2% |
| Preeclampsia89 | Cross-sectional study | 490 | SBP ⩾ 140 mm Hg and/or DBP ⩾ 90 mm Hg and proteinuria | Between August and September 2013 | Ethiopia | 8.40% |
| Preeclampsia72 | Cross-sectional study | 1100 | BP > 140/90 mm Hg with proteinuria | From November 2004 through April 2005 | Cameroon | 7.7% |
| Preeclampsia57 | Cross-sectional study | 1863 | BP > 140/90 mm Hg with proteinuria | From 1 April 2009 to 31 March 2010 | Ethiopia | 5% |
| Preeclampsia77 | Cross-sectional study | 8626 | BP > 140/90 mm Hg and proteinuria | From November 2014 to March 2014 | Ethiopia | 2.1% |
BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; ISSHP: International Society for the Study of Hypertension in Pregnancy; HDP: hypertensive disorders of pregnancy; GH: gestational hypertension.
There were studies that used only DBP for diagnosis of HDP20,35–37 and other studies that used DBP cutoff of 80 mm Hg instead of 90 mm Hg.38,39 There were studies that included severe preeclampsia (DBP > 110 and proteinuria).37,40,41 Some studies used international organization diagnostic criteria (e.g. European Society of Hypertension and WHO standard),42–44 while eclampsia was also diagnosed based on the presence of hypertension/preeclampsia and convulsion in others.45–49 Since we retained the aforementioned studies, their effect on heterogeneity of meta-analysis was seen during subgroup and sensitivity analysis.
The characteristics of the cross-sectional,9,35,36,40,42,46–89 prospective, or retrospective cohort and longitudinal studies5,10,37–39,41,90–103 included in this review from 19 Sub-Saharan African countries are summarized in Table 1. The majority of studies were conducted in Nigeria, Ethiopia, and Tanzania, which are 21, 16, and 6, respectively. The majority of studies that included in the analysis were completed within 3 years of study period. The sample size of the studies ranged from 100 in Zanzibar69 to 249,771 in Zambia,38 with a total of 780,469 participants. Overall, 29,980 (3.8%) pregnant women from a total of 780,469 study participants were recorded as suffering from disorders during antepartum and/or intrapartum period.
Many studies consisted of retrospective record review with consecutive sampling of women at various stages of pregnancy till 6 weeks postpartum. The studies have reported overall HDP (Table 1), chronic hypertension, gestational hypertension, preeclampsia,41,72,84–86,94,104 and eclampsia.47,49,59,60,63,65,66,75,76,78,103,105–108
Prevalence of HDP
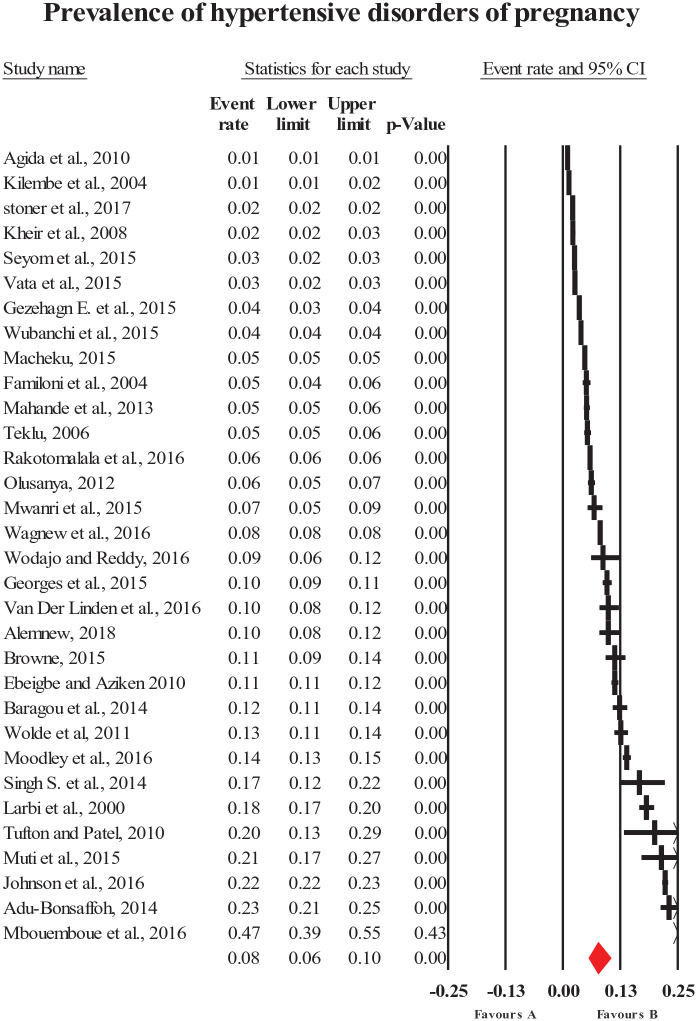
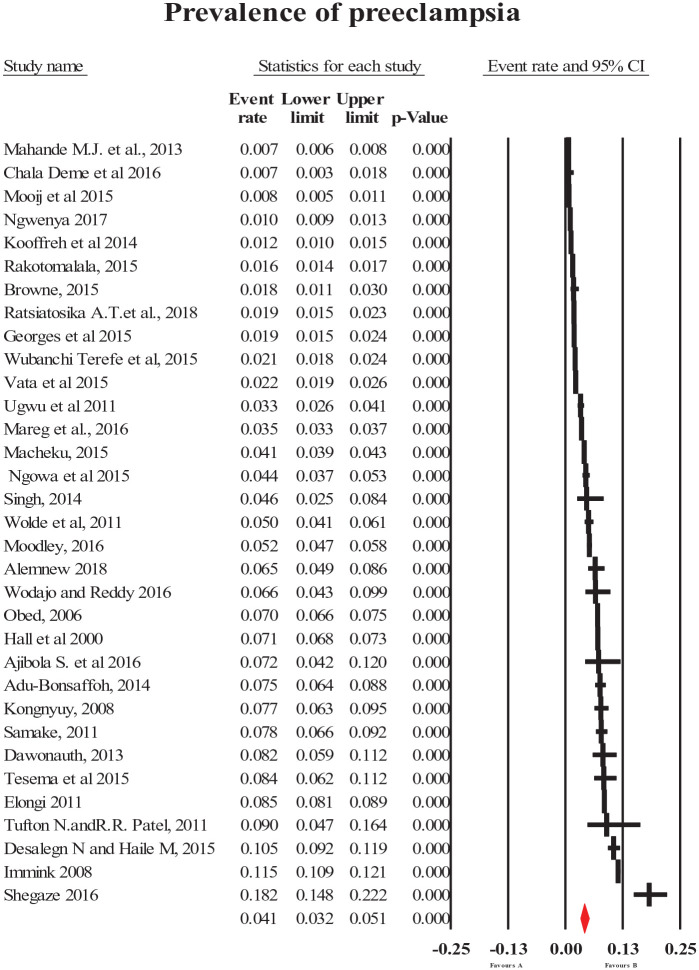
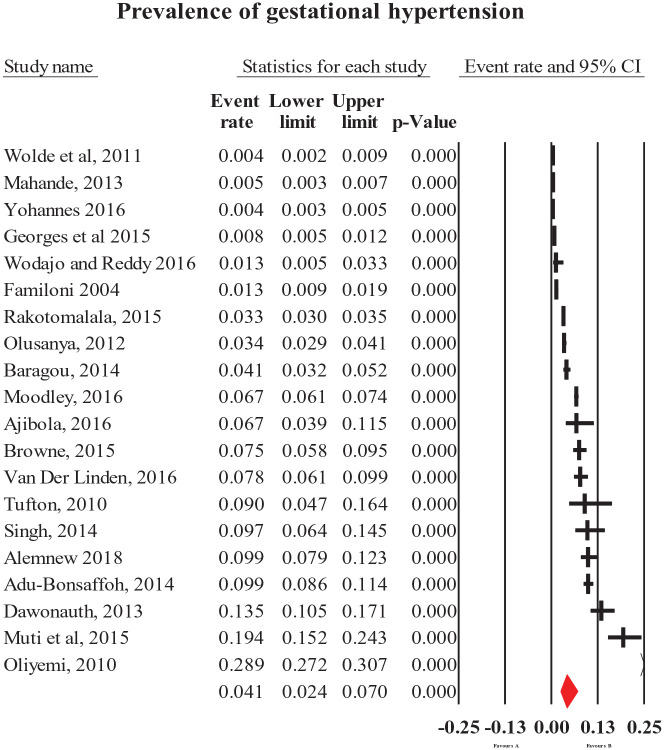
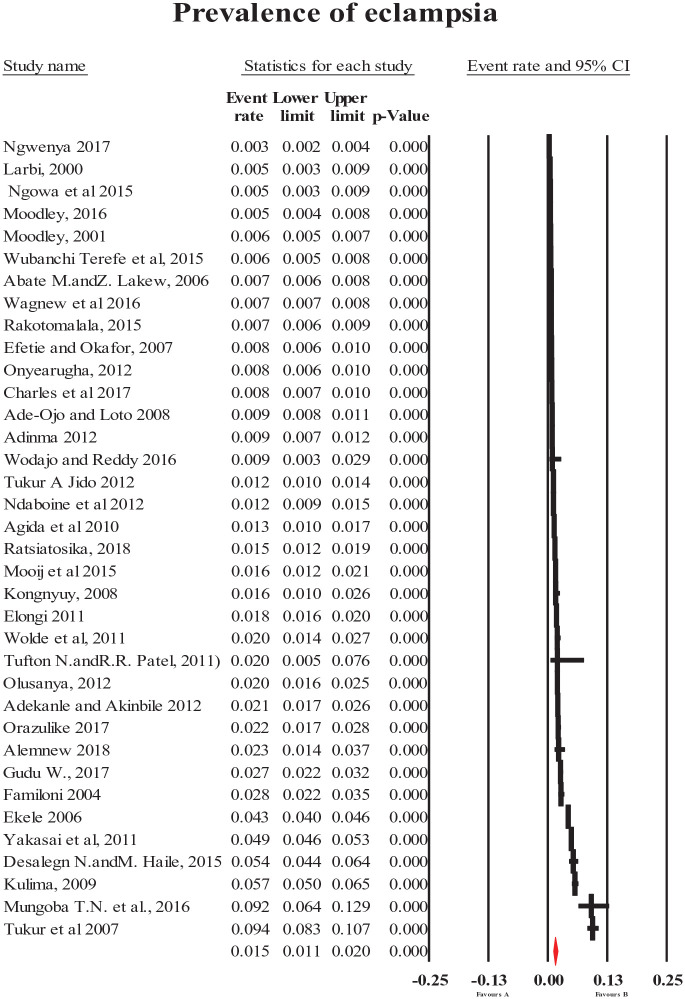
The pooled prevalence of HDP (all types combined) was 8% (95% CI = [6, 10]; Figure 2). The most common forms of specific HDP were preeclampsia and gestational hypertension with a prevalence of 4.1% (95% CI = [3.2, 5.1]; Figure 3) and 4.1% (95% CI = [2.4, 7]; Figure 4), respectively. The less common types of specific HDP were chronic hypertension (0.9%; 95% CI = [0.4, 1.8]; Figure 5) and eclampsia (1.5%; 95% CI = [1.1, 2]; Figure 6). Although there was substantial heterogeneity (Table 2), no publication bias was found for overall and type-specific HDP.
Figure 2.
Meta-analysis of prevalence of overall hypertensive disorder of pregnancy.
Figure 3.
Meta-analysis of prevalence of preeclampsia.
Figure 4.
Meta-analysis of prevalence of gestational hypertension.
Figure 5.
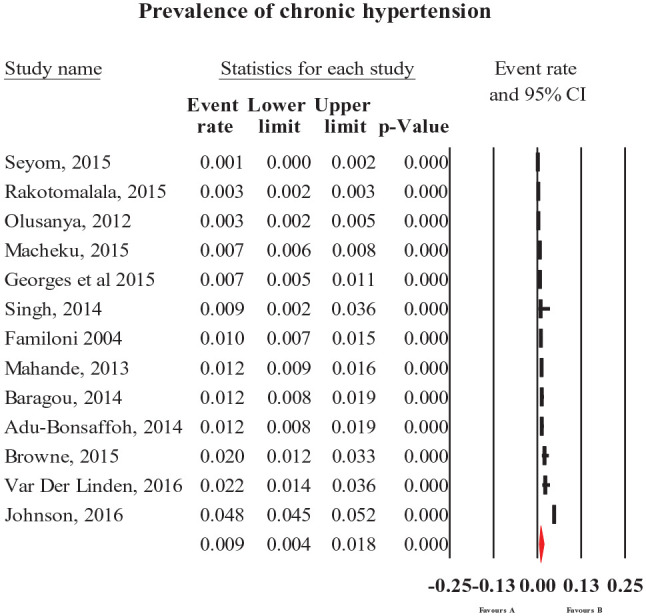
Meta-analysis of prevalence of chronic hypertension.
Figure 6.
Meta-analysis of prevalence of eclampsia.
Table 2.
Summary statistics of the prevalence of hypertensive disorders of pregnancy in Sub-Saharan Africa.
| Prevalence [95% confidence interval] | Number of studies | Number of participants | I2 | P value of heterogeneity | P value of Egger’s test | |
|---|---|---|---|---|---|---|
| Overall HDP | 8 [5, 10] | 32 | 478,570 | 99.8 | <0.0001 | 0.27 |
| Chronic hypertension | 0.9 [0.4, 1.8] | 13 | 103,607 | 99.1 | <0.0001 | 0.10 |
| Gestational hypertension | 4.1 [2.4, 7] | 20 | 73,421 | 99.4 | <0.0001 | 0.33 |
| Preeclampsia | 4.1 [3.2, 5.1] | 33 | 271,630 | 99.2 | <0.0001 | 0.11 |
| Eclampsia | 1.5 [1, 2] | 36 | 311,063 | 99.0 | <0.001 | 0.035 |
I2: heterogeneity; HDP: hypertensive disorders of pregnancy.
Subgroup analysis
Subgroup analysis of HDP by design and region revealed substantial heterogeneity across the studies in terms of HDP prevalence (I2 > 83%, P < 0.0001). There was a tendency for study-specific prevalence to vary by study design and region, with the cross-sectional and western SSA studies reporting significantly higher prevalence (P < 0.0001).
Subgroup analysis of HDP prevalence by SSA region showed a significantly higher pooled prevalence in west (2%; 95% CI = [1.3, 2.9] for eclampsia) and central (6.7%; 95% CI = [3.1, 13.8] for preeclampsia) SSA. The subgroup analysis by study design also revealed high pooled prevalence in cross-sectional (3.1%, 95% CI = [2.9, 3.3] for chronic hypertension; 4.7%, 95% CI = [3.5, 6.1] for preeclampsia; and 1.6%, 95% CI = [1.2, 2.3] for eclampsia) and cohort (5.7%, 95% CI = [2.8, 11.2] for gestational hypertension) study designs. The lowest prevalence estimates were observed in eastern (0.5%, 95% CI = [0.1, 2.3] for chronic hypertension; 1.5%, 95% CI = [0.6, 3.8] for gestational hypertension; and 3.6%, 95% CI = [2.5, 5.3] for preeclampsia) and southern (1%, 95% CI = [0.5, 1.7] for eclampsia) SSA (Table 3).
Table 3.
Subgroup analysis of prevalence of hypertensive disorders of pregnancy by study design and regions in Sub-Saharan Africa.
| Subgroup | Number of studies | Prevalence [95% CI] | Test of heterogeneity (I2) | P value |
|---|---|---|---|---|
| CH by design | ||||
| Cohort study | 6 | 0.8 [0.7, 0.9] | 89.1 | <0.0001 |
| Cross-sectional study | 7 | 3.1 [2.9, 3.3] | 99.2 | <0.0001 |
| CH by region of SSA | ||||
| East SSA | 3 | 0.5 [0.1, 2.3] | 94.1 | <0.0001 |
| South SSA | 2 | 1.2 [0.2, 7.5] | 99.8 | <0.0001 |
| West SSA | 7 | 1 [0.4, 2.9] | 83.7 | <0.0001 |
| Eclampsia by design | ||||
| Cohort study | 4 | 0.8 [0.3–2] | 99.1 | <0.0001 |
| Cross-sectional study | 32 | 1.6 [1.2–2.3] | 98.9 | <0.0001 |
| Eclampsia by region | ||||
| Central SSA | 3 | 1.2 [0.5–3] | 89.9 | <0.0001 |
| East SSA | 10 | 1.5 [0.9–2.4] | 981 | <0.0001 |
| South SSA | 8 | 1 [0.5–1.7] | 97.0 | <0.0001 |
| West SSA | 15 | 2 [1.3–2.9] | 99.0 | <0.0001 |
| GH by design | ||||
| Cohort study | 9 | 5.7 [2.8–11.2] | 99.3 | <0.0001 |
| Cross-sectional study | 11 | 3.2 [1.7–6.1] | 98.8 | <0.0001 |
| GH by region | ||||
| East SSA | 6 | 1.5 [0.6–3.8] | 98.8 | <0.0001 |
| South SSA | 4 | 8.9 [3.0–23.6] | 99.1 | <0.0001 |
| West SSA | 9 | 5.6 [2.7–11.5] | 99.3 | <0.0001 |
| Preeclampsia by design | ||||
| Cohort study | 10 | 2.9 [1.9–4.5] | 99.0 | <0.0001 |
| Cross-sectional study | 23 | 4.7 [3.5–6.1] | 99.3 | <0.0001 |
| Preeclampsia by region | ||||
| Central SSA | 3 | 6.7 [3.1–14] | 95.7 | <0.0001 |
| East SSA | 13 | 3.6 [2.5–5.3] | 98.8 | <0.0001 |
| South SSA | 8 | 4.2 [2.6–6.7] | 99.6 | <0.0001 |
| West SSA | 9 | 3.9 [2.4–6.1] | 98.2 | <0.0001 |
CI: confidence interval; CH: chronic hypertension; SSA: Sub-Saharan Africa; GH: gestational hypertension.
The studies in the analysis included a mix of cross-sectional, prospective, or retrospective cohort and longitudinal studies; prospective and longitudinal studies reported on the prevalence of various HDP outcomes. The analyses were accomplished by consecutively omitting one study at a time to endorse the results. Sensitivity analysis was completed using diagnostic criteria, sample size, and width of CI, although change in heterogeneity was insignificant.
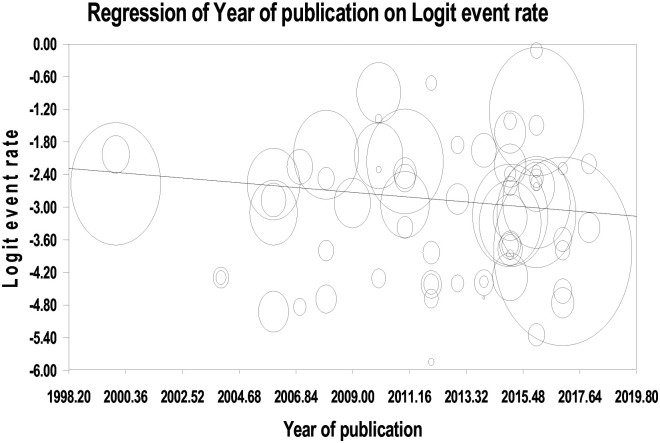
The meta-regression indicates that the number of publications on HDP is increasing which shows that hypertensive disorders are currently receiving attention (Figure 7). This is aligned with the current shift of attention to non-communicable diseases such as hypertension and cardiovascular disease specifically in the LMICs.
Figure 7.
Meta-regression of studies on hypertensive disorders of pregnancy by year of publication in SSA.
Pregnancy outcomes of HDP
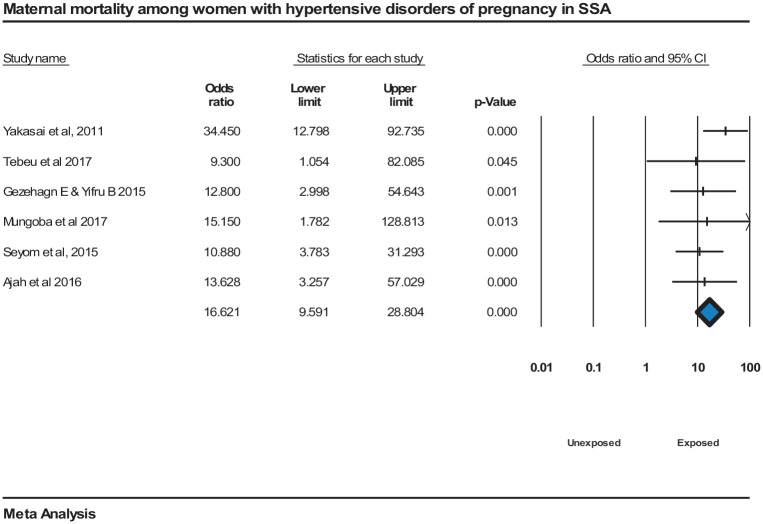
The meta-analysis shows that maternal mortality was significantly associated with HDP, OR = 17 (95% CI = [9.6, 28.8]) with homogeneity among the studies’ findings, I2 = 0.000, P = <0.000152,56,65,109–111 (Figure 8).
Figure 8.
Forest plot displaying maternal mortality associated with hypertensive disorders of pregnancy in SSA.
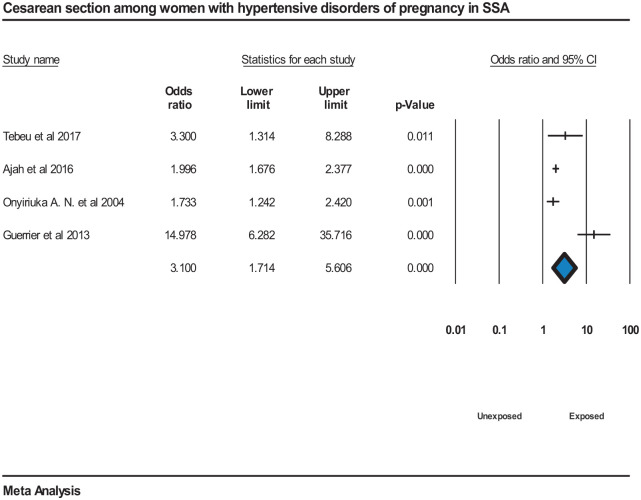
The meta-analysis also showed pregnant women with hypertensive disorders were more likely to have cesarean section compared to normotensive pregnant women, OR = 3.1 (95% CI = [1.7, 5.6]). However, there was high heterogeneity among the studies, I2 = 86.45%, P = <0.000144,109,110,112 (Figure 9).
Figure 9.
Forest plot displaying cesarean section among women with hypertensive disorders of pregnancy in SSA.
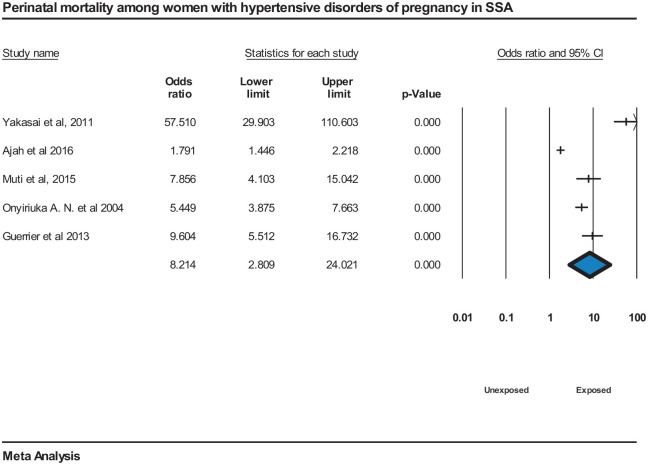
Perinatal mortality was significantly associated with HDP, OR = 8.2 (95% CI = [2.8, 24]), although the meta-analysis revealed large heterogeneity, I2 = 96.9%, P = <0.000144,65,82,109,112 (Figure 10).
Figure 10.
Forest plot displaying perinatal mortality among women with hypertensive disorders of pregnancy in SSA.
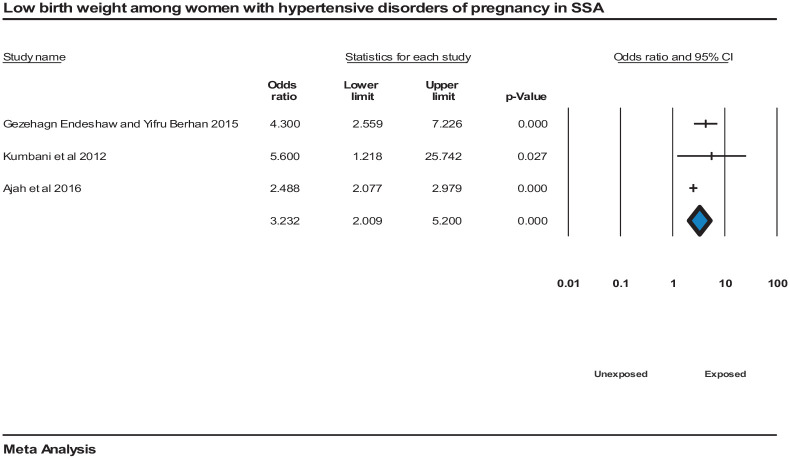
The pooled risk of having a low birth weight newborn was high among women who have HDP compared to normotensive pregnant women, OR = 3.2 (95% CI = [2, 5]). However, the meta-analysis revealed high heterogeneity among the studies, I2 = 57.7%, P = 0.00019,109,111 (Figure 11).
Figure 11.
Forest plot displaying low birth weight newborn among women with hypertensive disorders of pregnancy in SSA.
HDP have significant pooled association with preterm delivery, OR = 7.8 (95% CI = [2.5, 25.3]), though the meta-analysis of studies had high heterogeneity, I2 = 84.34%, P = 0.001.52,113,114
Publication bias
There was no evidence of publication bias across studies reporting on the prevalence of HDP, chronic hypertension, gestational hypertension, and preeclampsia with the Egger’s test for bias giving a P value of 0.27, 0.10, 0.33, and 0.11, respectively. However, there was some indication of publication bias across studies reporting on eclampsia prevalence, with the Egger’s test for bias giving a P value of 0.037 (Table 2).
Duval and Tweedie’s trim and fill analysis using the random effect model analysis was performed for overall HDP, chronic hypertension, gestational hypertension, preeclampsia, and eclampsia. The adjusted values were zero to the right or left of the mean for overall HDP, gestational hypertension, and preeclampsia, and 2 to the left of the mean and 4 to the right of the mean for chronic hypertension and eclampsia, respectively, although insignificant.
Discussion
This meta-analysis confirms that HDP are associated with adverse pregnancy outcomes. Maternal and fetal complications increase with HDP; however, appropriate maternal and perinatal care can prevent adverse outcomes such as eclampsia, maternal and perinatal death.
The overall prevalence of HDP was 8% in SSA which is in line with global estimates (5.2%–8.2%),13 slightly lower than a review from Africa (10%),12 and higher than the pooled prevalence of HDP in Ethiopia (6.1%).115 This may be due to sample size differences, inclusion criteria, and vast geographical differences. The finding is also higher than the global secondary analysis of the WHO’s multicountry survey prevalence of 2.7%.11 The difference might be due to variability in maternal risk factors, socio-demographic factors, and the difference in prenatal care service utilization. Most studies were facility-based from the record review, which may result in inflated numbers. Due to poor health-seeking behavior, women typically present late and with advanced disease states in SSA that contributed to high maternal and perinatal morbidity and mortality.7,12,116 Furthermore, there may be delayed and lack of intervention due to poor infrastructure. As a result, most studies had a relatively high prevalence of eclampsia that affected overall pooled analysis.
The pooled prevalence of preeclampsia was 4.1% in SSA, and this finding is in line with the prevalence of preeclampsia in Africa (5.3%),12 the United States (3%–5%),117,118 and global estimate (1.8%–4.4%).13 The pooled prevalence of eclampsia was 1.5% according to this review, which is similar to a review performed in Africa (1.47%)12 and global estimates (0.2%–9.2%),13 but slightly higher than a survey performed in China (0.9%) and WHO multicountry survey prevalence of 0.3%.11 The difference may be due to racial differences since this study focused on Sub-Saharan African countries.12,119–121 Furthermore, the variation may be attributed to differences in ethnic background, age distribution, socioeconomic status, parity, and study methodology. The pregnant women in SSA may have presented immediately with convulsions and right away diagnosed with eclampsia and no diagnosis ever of high blood pressure or anything. Estimates also varied substantially according to the SSA region, with a significantly higher prevalence of HDP among women in central and western SSA compared to other parts of SSA.
There was high heterogeneity between studies which showed an insignificant decrease when subgroup analysis was performed. However, the meta-analysis did not identify any other underlying causes of heterogeneity, suggesting that either populations with HDP are varied or the determination of HDP and outcomes may not be consistent. Meanwhile, it might be due to differences in geographical locations, diagnostic and measurement methods of disorders, socioeconomic status, and sample size. Hence, it also highlights the necessity to standardize HDP definition in SSA.12
The meta-analysis has shown that maternal mortality has a significant association with HDP. Mothers with hypertensive disorders were more exposed to maternal mortality and morbidities as compared to those who were not affected.51 Moreover, according to the studies in 29 countries from Africa, Asia, Latin America, and the Middle East, the risk of death was nearly four times higher for women with preeclampsia than non-preeclamptic women, and for those with eclampsia, the risk increased exponentially.11
Women with HDP are more likely to have a cesarean section compared to normotensive pregnant women and significant pooled association indicates increased perinatal mortality. Similarly, the studies have shown the risk of fetal and neonatal deaths, as well as preterm birth and admission to a Neonatal Intensive Care Unit were increased in both preeclampsia and eclampsia.11
The pooled risk of preterm delivery and low birth weight newborn was high among women with HDP compared to normotensive pregnant women. Similarly, the studies have shown the more significant the severity of hypertension, the increased risk of complications associated with the pregnancy, and the greater the possibility of pregnancy termination.122,123 HDP are associated with prematurity (which may be iatrogenic) and low birth weight even when corrected for gestation,124 which is in line with the analysis of this study.
The pooled ORs of HDP and cesarean section, perinatal mortality, low birth weight, and preterm delivery have shown high heterogeneity. The geographic differences, ethnicity, socioeconomic status, adjustment for confounding, sampling error (within and between), and design aspects may contribute.
These findings have important policy implications for the treatment of HDP and pregnancy outcomes in SSA. The strong influence of HDP on maternal mortality, cesarean section, perinatal mortality, low birth weight, and preterm birth suggests that safe motherhood programs need to explore applicable methods of increasing maternal service utilization among women of reproductive age, pregnant mothers, and mothers diagnosed with HDP in SSA. The review has revealed that preeclampsia and eclampsia prevalence is high. Therefore, attention should be given to improving women’s health-seeking behavior, preeclampsia and eclampsia management protocol adherence. This calls for a rigorous evaluation of the effectiveness of existing antenatal and intranatal care programs in which prevention and management of HDP will be undertaken in SSA to identify gaps in policy and program implementation. Targeted interventions to strengthen the infrastructure of antenatal and intranatal care both for women and newborns should be considered.
Duval and Tweedie’s trim and fill analysis using the random effect analysis showed adjusted values of 2 to the left and 4 to the right of the mean for chronic hypertension and eclampsia, respectively, although non-significant. Publication bias may be due to difficulty in accessing gray literature in SSA and less publication of non-significant results. In the analysis, checking study quality, sensitivity analysis, and acknowledging potential sources of bias were completed.
“Strengths and weaknesses” of study
Studies were carefully selected according to a rigorous search strategy to enable unbiased inclusion of retrospective or prospective studied cohorts and cross-sectional studies. These studies were included if they calculated prevalence/proportion or mentioned pregnancy outcomes.
Despite the selection of relevant and appropriately performed studies, there was a range of reported incidences of HDP. There was difficulty in selecting studies of (any type of) HDP and compiling the relevant data, particularly because HDP include various types of disorders and individual studies did not always assess every type. As a result, the overall prevalence of HDP may be liable to bias. There may also be bias due to differences in the selection of women studied, difficulties in measuring HDP, and true differences within the population of women. In addition, the authors failed to define the control group in the studies.
Most papers did not report relevant baseline demographics defining the studied population and this was not considered in the meta-analysis, which limited the assessment of confounders. Coexisting factors including maternal age and ethnicity, recognized to be associated with both HDP and adverse pregnancy outcome, may contribute to confounding, but their relative effects are unknown. Few studies in the meta-analysis reported control data, so a direct comparison of outcomes between women with HDP and normotensive women was not possible. We found substantial heterogeneity which may be partly explained by characteristics of the studies shown by subgroup analysis. The included studies were facility-based that may have a slightly elevated proportion of HDP compared to population-based study; hence, the findings may not represent true prevalence in the community. Also, a significant proportion of studies that included data with HDP and pregnancy outcomes were retrospective studies.
Conclusion
The overall pooled prevalence of HDP was high compared to those reported from other regions. The meta-analysis indicates that HDP are associated with maternal mortality and cesarean section as well as adverse pregnancy outcomes such as preterm delivery, low birth weight, and perinatal mortality.
The meta-analysis supports the importance of increased antenatal surveillance for women with HDP to enable early identification of complications. Women should receive counseling to optimize their health during pregnancy and to inform them of the increased maternal and fetal risks associated with their hypertension. Strategies to predict those at greatest risk of HDP, determine optimal drug treatments, and reduce adverse pregnancy outcomes are needed.
Supplemental Material
Supplemental material, sj-pdf-1-whe-10.1177_1745506520973105 for Prevalence of hypertensive disorders of pregnancy and pregnancy outcomes in Sub-Saharan Africa: A systematic review and meta-analysis by Kasiye Shiferaw Gemechu, Nega Assefa and Bizatu Mengistie in Women’s Health
Acknowledgments
We would like to acknowledge all the authors of the studies included in this review. We also thank Abdulbasit Musa for unreserved support, and Ogbudu Emmanuel for language edition. Finally, we appreciate Tara Wilfong and Rosanna Glazik for their constructive comments and language edition.
Footnotes
Author contributions: All authors involved in the design, selection of articles, data extraction, statistical analysis, and manuscript writing. All authors read and approved the final draft of the manuscript.
Availability of data and materials: All data pertaining to this study are contained and presented in this document.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kasiye Shiferaw Gemechu  https://orcid.org/0000-0001-6087-1682
https://orcid.org/0000-0001-6087-1682
Nega Assefa  https://orcid.org/0000-0003-0341-2329
https://orcid.org/0000-0003-0341-2329
Supplemental material: Supplemental material for this article is available online.
References
- 1. Bongaarts J. WHO, UNICEF, UNFPA, World Bank Group, and United Nations Population Division trends in maternal mortality: 1990 to 2015 Geneva: World Health Organization, 2015. Popul Dev Rev 2016; 42: 726–726. [Google Scholar]
- 2. World Health Organization (WHO). Trends in maternal mortality: 1990 to 2015: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: WHO, 2015. [Google Scholar]
- 3. Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 980–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fokom-Domgue J, Noubiap JJ. Diagnosis of hypertensive disorders of pregnancy in Sub-Saharan Africa: a poorly assessed but increasingly important issue. J Clin Hypertens 2015; 17(1): 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Endeshaw G, Berhan Y. Perinatal outcome in women with hypertensive disorders of pregnancy: a retrospective cohort study. Int Sch Res Notices 2015; 2015: 208043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Von Dadelszen P, Magee LA. Preventing deaths due to the hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2016; 36: 83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014; 2(6): e323–e333. [DOI] [PubMed] [Google Scholar]
- 8. Nakimuli A, Nakubulwa S, Kakaire O, et al. The burden of maternal morbidity and mortality attributable to hypertensive disorders in pregnancy: a prospective cohort study from Uganda. BMC Pregnancy Childbirth 2016; 16: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson KM, Zash R, Haviland MJ, et al. Hypertensive disease in pregnancy in Botswana: prevalence and impact on perinatal outcomes. Pregnancy Hypertens 2016; 6: 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Browne JL, Vissers KM, Antwi E, et al. Perinatal outcomes after hypertensive disorders in pregnancy in a low resource setting. Trop Med Int Health 2015; 20(12): 1778–1786. [DOI] [PubMed] [Google Scholar]
- 11. Abalos E, Cuesta C, Carroli G, et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG 2014; 121(Suppl. 1): 14–24. [DOI] [PubMed] [Google Scholar]
- 12. Noubiap JJ, Bigna JJ, Nyaga UF, et al. The burden of hypertensive disorders of pregnancy in Africa: a systematic review and meta-analysis. J Clin Hypertens 2019; 21(4): 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res 2017; 40: 213–220. [DOI] [PubMed] [Google Scholar]
- 14. Ataklte F, Erqou S, Kaptoge S, et al. Burden of undiagnosed hypertension in Sub-Saharan Africa. Hypertension 2015; 65: 291–298. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization (WHO). A global brief on hypertension: silent killer, global public health crisis: World Health Day 2013. Geneva: WHO, 2013. [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tranquilli A, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens 2014; 4(2): 97–104. [DOI] [PubMed] [Google Scholar]
- 18. Brown MA, Lindheimer MD, De Swiet M, et al. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Abingdon: Taylor & Francis, 2001. [DOI] [PubMed] [Google Scholar]
- 19. American College of Obstetricians Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on hypertension in pregnancy. Obstet Gynecol 2013; 122(5): 1122–1131. [DOI] [PubMed] [Google Scholar]
- 20. Gaym A, Bailey P, Pearson L, et al. Disease burden due to pre-eclampsia/eclampsia and the Ethiopian health system’s response. Int J Gynaecol Obstet 2011; 115(1): 112–116. [DOI] [PubMed] [Google Scholar]
- 21. Watanabe K, Matsubara K, Nakamoto O, et al. Outline of the new definition and classification of “Hypertensive Disorders of Pregnancy (HDP)”; a revised JSSHP statement of 2005. Hypertens Res Pregnancy 2018; 6: 33–37. [Google Scholar]
- 22. Brown MA, Magee LA, Kenny L, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 2018; 13: 291–310. [DOI] [PubMed] [Google Scholar]
- 23. Vigil-De Gracia P, Montufar-Rueda C, Ruiz J. Expectant management of severe preeclampsia and preeclampsia superimposed on chronic hypertension between 24 and 34 weeks’ gestation. Eur J Obstet Gynecol Reprod Biol 2003; 107: 24–27. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization (WHO). International statistical classification of diseases and related health problems. Geneva: WHO, 2011, p. 201. [Google Scholar]
- 25. American College of Obstetricians and Gynecologists. Cesarean birth, 2018, https://www.acog.org/womens-health/faqs/cesarean-birth
- 26. Berhan Y, Berhan A. Perinatal mortality trends in Ethiopia. Ethiop J Health Sci 2014; 24(Suppl.): 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American College of Obstetricians and Gynecologists. Preterm (premature) labor and birth, 2016. [Google Scholar]
- 28. UNICEF and WHO. Low birthweight: country, regional and global estimates. New York: United Nations Children’s Fund, 2004. [Google Scholar]
- 29. Stavis RL. Small-for-Gestational-Age (SGA) infant: (dysmaturity; intrauterine growth restriction), https://www.merckmanuals.com/professional/pediatrics/perinatal-problems/small-for-gestational-age-sga-infant#v1087269 (2018, accessed 15 November 2018).
- 30. Joanna Briggs Institute. The Joanna Briggs Institute critical appraisal tools for use in JBI systematic reviews checklist for systematic reviews and research syntheses, 2017, https://joannabriggs.org/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Systematic_Reviews2017_0.pdf
- 31. Yu Z, Han S, Zhu J, et al. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS ONE 2013; 8: e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 33. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kilembe FD. Hypertensive disorders of pregnancy: prevalence, maternal complications and perinatal outcomes at Lilongwe Central Hospital, Malawi. Oslo: University of Oslo, 2004. [Google Scholar]
- 36. Ebeigbe PN, Aziken ME. Early onset pregnancy-induced hypertension/eclampsia in Benin City, Nigeria. Niger J Clin Pract 2010; 13(4): 388–393. [PubMed] [Google Scholar]
- 37. Ngwenya S. Severe preeclampsia and eclampsia: incidence, complications, and perinatal outcomes at a low-resource setting, Mpilo Central Hospital, Bulawayo, Zimbabwe. Int J Womens Health 2017; 9: 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stoner MC, Vwalika B, Smid MC, et al. A retrospective study of HIV, antiretroviral therapy, and pregnancy-associated hypertension among women in Lusaka, Zambia. Int J Gynaecol Obstet 2016; 134(3): 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh S, Ahmed EB, Egondu SC, et al. Hypertensive disorders in pregnancy among pregnant women in a Nigerian Teaching Hospital. Niger Med J 2014; 55(5): 384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ngowa JDK, Kasia JM, Alim J, et al. Maternal and perinatal complications of severe preeclampsia in three referral hospitals in Yaoundé, Cameroon. Open J Obstet Gynecol 2015; 5: 723–730. [Google Scholar]
- 41. Ugwu EO, Dim CC, Okonkwo CD, et al. Maternal and perinatal outcome of severe pre-eclampsia in Enugu, Nigeria after introduction of Magnesium sulfate. Niger J Clin Pract 2011; 14(4): 418–421. [DOI] [PubMed] [Google Scholar]
- 42. Immink A, Scherjon S, Wolterbeek R, et al. Seasonal influence on the admittance of pre-eclampsia patients in Tygerberg Hospital. Acta Obstet Gynecol Scand 2008; 87(1): 36–42. [DOI] [PubMed] [Google Scholar]
- 43. Georges MRC, Yameogo AR, Mandi DG, et al. Hypertension in pregnancy at the teaching hospital of Yalgado Ouédraogo, Burkina Faso. J Hypertens 2015; 4: 199. [Google Scholar]
- 44. Guerrier G, Oluyide B, Keramarou M, et al. Factors associated with severe preeclampsia and eclampsia in Jahun, Nigeria. Int J Womens Health 2013; 5: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buambo-Bamanga SF, Makoumbou P, Ngbalé R, et al. Eclampsia in the university teaching hospital in Brazzaville, Congo. Clin Mother Child Health 2009; 6: 1129–1133. [Google Scholar]
- 46. Ade-Ojo IP, Loto OM. Outcome of eclampsia at the Obafemi Awolowo University Teaching Hospital complex, ILE-IFE. Niger J Clin Pract 2008; 11(3): 279–284. [PubMed] [Google Scholar]
- 47. Oputa V, Orazulike N, Alegbeleye J, et al. Eclampsia at the University of Port Harcourt Teaching Hospital Port Harcourt, Nigeria. IOSR J Dental Med Sci 2017; 16: 77–81. [Google Scholar]
- 48. Agida ET, Adeka BI, Jibril KA. Pregnancy outcome in eclamptics at the University of Abuja Teaching Hospital, Gwagwalada, Abuja: a 3 year review. Niger J Clin Pract 2010; 13(4): 394–398. [PubMed] [Google Scholar]
- 49. Jido TA. Ecalmpsia: maternal and fetal outcome. Afr Health Sci 2012; 12(2): 148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adu-Bonsaffoh K, Obed SA, Seffah JD. Maternal outcomes of hypertensive disorders in pregnancy at Korle Bu Teaching Hospital, Ghana. Int J Gynaecol Obstet 2014; 127(3): 238–242. [DOI] [PubMed] [Google Scholar]
- 51. Familoni OB, Adefuye PO, Olunuga TO. Pattern and factors affecting the outcome of pregnancy in hypertensive patients. J Natl Med Assoc 2004; 96(12): 1626–1631. [PMC free article] [PubMed] [Google Scholar]
- 52. Seyom E, Abera M, Tesfaye M, et al. Maternal and fetal outcome of pregnancy related hypertension in Mettu Karl Referral Hospital, Ethiopia. J Ovarian Res 2015; 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rakotomalala Z, Randriambololona D, Andriampanarivo H, et al. Facteurs de mauvais pronostic en cas de pré-éclampsie à Madagascar. Méd Sante Trop 2016; 26: 78–82. [DOI] [PubMed] [Google Scholar]
- 54. Baragou S, Goeh-Akue E, Pio M, et al. [Hypertension and pregnancy in Lome (Sub-Saharan Africa): epidemiology, diagnosis and risk factors]. Ann Cardiol Angeiol 2014; 63: 145–150. [DOI] [PubMed] [Google Scholar]
- 55. Wagnew M, Dessalegn M, Worku A, et al. Trends of preeclampsia/eclampsia and maternal and neonatal outcomes among women delivering in addis ababa selected government hospitals, Ethiopia: a retrospective cross-sectional study. Pan Afr Med J 2016; 25(Suppl. 2): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mungoba TN, Mitonga KH, David SA, et al. Factors associated with adverse pregnancy outcomes among women who delivered at intermediate hospital oshakati, Namibia. Int J Med 2016; 5: 6939. [Google Scholar]
- 57. Wolde Z, Segni H, Woldie M. Hypertensive disorders of pregnancy in Jimma University specialized hospital. Ethiop J Health Sci 2011; 21(3): 147–154. [PMC free article] [PubMed] [Google Scholar]
- 58. Desalegn N, Haile M. Causes of admission and out comes among preeclampsia and eclampsia mothers admitted to Jimma University specialized hospital intensive care unit. Clin Med Res 2015; 4: 154–159. [Google Scholar]
- 59. Tukur J, Umar B, Rabi’u A. Pattern of eclampsia in a tertiary health facility situated in a semi-rural town in Northern Nigeria. Ann Afr Med 2007; 6(4): 164–167. [DOI] [PubMed] [Google Scholar]
- 60. Gudu W. Prodromal symptoms, health care seeking in response to symptoms and associated factors in eclamptic patients. BMC Pregnancy Childbirth 2017; 17: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Adekanle DA, Akinbile TO. Eclampsia and pregnancy outcome at Lautech Teaching Hospital, Osogbo, SouthWest, Nigeria. Clinics Mother Child Health 2012; 9: 1–4. [Google Scholar]
- 62. Ratsiatosika AT, Razafimanantsoa E, Andriantoky VB, et al. Incidence and natural history of preeclampsia/eclampsia at the university maternity of Antananarivo, Madagascar: high prevalence of the early-onset condition. J Matern Fetal Neonatal Med 2019; 32: 3266–3271. [DOI] [PubMed] [Google Scholar]
- 63. Efetie E, Okafor UV. Maternal outcome in eclamptic patients in Abuja, Nigeria: a 5 year review. Niger J Clin Pract 2007; 10(4): 309–313. [PubMed] [Google Scholar]
- 64. Esike COU, Chukwuemeka UI, Anozie OB, et al. Eclampsia in rural Nigeria: the unmitigating catastrophe. Ann Afr Med 2017; 16(4): 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yakasai I, Gaya SA. Maternal and fetal outcome in patients with eclampsia at Murtala Muhammad specialist Hospital Kano, Nigeria. Ann Afr Med 2011; 10(4): 305–309. [DOI] [PubMed] [Google Scholar]
- 66. Charles A, Victor P, Jonathan K, et al. Eclampsia and pregnancy outcome at Jos University Teaching Hospital, Jos, Plateau State, Nigeria. J Gynaecol Obst 2017; 5: 46–49. [Google Scholar]
- 67. Elongi J-P, Tandu B, Spitz B, et al. Influence de la variation saisonnière sur la prévalence de la pré-éclampsie à Kinshasa. Gynécol Obstét Fertil 2011; 39: 132–135. [DOI] [PubMed] [Google Scholar]
- 68. Majoko F, Mujaji C. Maternal outcome in eclampsia at Harare Maternity Hospital. Cent Afr J Med 2001; 47(5): 123–128. [DOI] [PubMed] [Google Scholar]
- 69. Tufton N, Patel RR. Prevalence of hypertensive disorders in a prenatal clinic in Zanzibar. Int J Gynaecol Obstet 2011; 112: 69–70. [DOI] [PubMed] [Google Scholar]
- 70. Shambel W, Surender R. Hypertensive disorders of pregnancy and associated factors among admitted pregnant cases in Dessie town referral hospital, North East Ethiopia. Med Res Chronicles 2016; 3: 297–306. [Google Scholar]
- 71. Alemnew Bayensagn S, Lemmi T, Assefa N. Prevalence and associated factors of pregnancy induced hypertension disorder among women delivered in Gelemso General Hospital, Oromia Regional State, Eastern Ethiopia. Harar, Ethiopia: Haramaya University, 2018. [Google Scholar]
- 72. Kongnyuy EJ, Nana PN, Fomulu N, et al. Adverse perinatal outcomes of adolescent pregnancies in Cameroon. Matern Child Health J 2008; 12(2): 149–154. [DOI] [PubMed] [Google Scholar]
- 73. Mooij R, Lugumila J, Mwashambwa MY, et al. Characteristics and outcomes of patients with eclampsia and severe pre-eclampsia in a rural hospital in Western Tanzania: a retrospective medical record study. BMC Pregnancy Childbirth 2015; 15: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kullima A, Kawuwa MB, Audu B, et al. A 5-year review of maternal mortality associated with eclampsia in a tertiary institution in northern Nigeria. Ann Afr Med 2009; 8: 81–84. [DOI] [PubMed] [Google Scholar]
- 75. Ndaboine EM, Kihunrwa A, Rumanyika R, et al. Maternal and perinatal outcomes among eclamptic patients admitted to Bugando Medical Centre, Mwanza, Tanzania. Afr J Reprod Health 2012; 16(1): 35–41. [PubMed] [Google Scholar]
- 76. Adinma ED. Pattern of clinical presentation of eclampsia at Nnamdi Azikiwe University Teaching Hospital, Nnewi, Southeastern Nigeria. Niger J Med 2012; 21(3): 313–316. [PubMed] [Google Scholar]
- 77. Terefe W, Getachew Y, Hiruye A, et al. Patterns of hypertensive disorders of pregnancy and associated factors at Debre Berhan Referral Hospital, North Shoa, Amhara Region. Ethiop Med J 2015; 2(Suppl.): 57–65. [PubMed] [Google Scholar]
- 78. Abate M, Lakew Z. Eclampsia a 5 years retrospective review of 216 cases managed in two teaching hospitals in Addis Ababa. Ethiop Med J 2006; 44(1): 27–31. [PubMed] [Google Scholar]
- 79. Pancha Mbouemboue O. A study on factors related to hypertensive disorders in pregnancy in Ngaoundere (Adamawa Region, Cameroon). Clin Med Res 2016; 5: 6–12. [Google Scholar]
- 80. Mwanri AW, Kinabo JL, Ramaiya K, et al. High blood pressure and associated risk factors among women attending antenatal clinics in Tanzania. J Hypertens 2015; 33: 940–947. [DOI] [PubMed] [Google Scholar]
- 81. Vata PK, Chauhan NM, Nallathambi A, et al. Assessment of prevalence of preeclampsia from Dilla region of Ethiopia. BMC Res Notes 2015; 8: 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Muti M, Tshimanga M, Notion GT, et al. Prevalence of pregnancy induced hypertension and pregnancy outcomes among women seeking maternity services in Harare, Zimbabwe. BMC Cardiovasc Disord 2015; 15: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tesfay Y, Berhe S, Aregay A. Risk factors of pregnancy related hypertension among women attending maternal health care service in selected public hospitals of Tigray, Ethiopia. Int J Dev Res 2016; 6: 8904–8911. [Google Scholar]
- 84. Deme C, Edao B, Jaya G, et al. Prevalence of hypertension, anemia, asymptomatic urinary tract infection, syphilis, HIV and hepatitis B virus infection among pregnant women attending an antenatal clinic at a rural hospital in Southern Ethiopia. Southeast Asian J Trop Med Public Health 2016; 47(5): 1032–1039. [PubMed] [Google Scholar]
- 85. Mulugeta S, Yohannes M, Wubeshet E, et al. Magnitude and associated factors of preeclampsia among pregnant women who attend Antenatal Care Service in Public Health Institutions in Arba Minch Town, Southern Ethiopia, 2016. Gynecol Obstet 2016; 6: 12. [Google Scholar]
- 86. Samaké BM, Traoré M, Goita L, et al. [Epidemiogic and clinical profile of severe pre-eclampsia at the university hospital of Gabriel Touré]. Mali Med 2011; 26(4): 5–7. [PubMed] [Google Scholar]
- 87. Kooffreh ME, Ekott M, Ekpoudom DO. The prevalence of pre-eclampsia among pregnant women in the University of Calabar Teaching Hospital, Calabar. Saudi J Health Sci 2014; 3: 133–136. [Google Scholar]
- 88. Hall D, Odendaal H, Steyn D, et al. Expectant management of early onset, severe pre-eclampsia: maternal outcome. BJOG 2000; 107(10): 1252–1257. [DOI] [PubMed] [Google Scholar]
- 89. Tessema GA, Tekeste A, Ayele TA. Preeclampsia and associated factors among pregnant women attending antenatal care in Dessie referral hospital, Northeast Ethiopia: a hospital-based study. BMC Pregnancy and Childbirth 2015; 15: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moodley J, Onyangunga O, Maharaj NR. Hypertensive disorders in primigravid black South African women: a one-year descriptive analysis. Hypertens Pregnancy 2016; 35(4): 529–535. [DOI] [PubMed] [Google Scholar]
- 91. Olusanya BO, Solanke OA. Perinatal outcomes associated with maternal hypertensive disorders of pregnancy in a developing country. Hypertens Pregnancy 2012; 31(1): 120–130. [DOI] [PubMed] [Google Scholar]
- 92. Georges MA, Germain YR, Camille MD, et al. Hypertension in pregnancy at the teaching hospital of Yalgado Ouedraogo, Burkina Faso. J Hypertens 2015; 4: 2167. [Google Scholar]
- 93. Ajibola S, Adeyemo T, Afolabi B, et al. Utility of a single mid-trimester measurement of plasminogen activator Type 1 and fibronectin to predict preeclampsia in pregnancy. Niger Med J 2016; 57(4): 213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Obed S, Patience A. Birth weight and ponderal index in pre-eclampsia: a comparative study. Ghana Med J 2006; 40(1): 8–13. [PMC free article] [PubMed] [Google Scholar]
- 95. Teklu S, Gaym A. Prevalence and clinical correlates of the hypertensive disorders of pregnancy at Tikur Anbessa Hospital, Addis Ababa, Ethiopia. Ethiop Med J 2006; 44(1): 17–26. [PubMed] [Google Scholar]
- 96. Mahande MJ, Daltveit AK, Mmbaga BT, et al. Recurrence of preeclampsia in northern Tanzania: a registry-based cohort study. PLoS ONE 2013; 8(11): e79116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Van Der Linden EL, Browne JL, Vissers KM, et al. Maternal body mass index and adverse pregnancy outcomes: a Ghanaian cohort study. Obesity 2016; 24(1): 215–222. [DOI] [PubMed] [Google Scholar]
- 98. Olayemi O, Strobino D, Aimakhu C, et al. Influence of duration of sexual cohabitation on the risk of hypertension in nulliparous parturients in Ibadan: a cohort study. Aust N Z J Obstet Gynaecol 2010; 50(1): 40–44. [DOI] [PubMed] [Google Scholar]
- 99. Dawonauth L, Rademacher L, L’Omelette AD, et al. Urinary inositol phosphoglycan-P type: near patient test to detect preeclampsia prior to clinical onset of the disease. A study on 416 pregnant Mauritian women. J Reprod Immunol 2014; 101–102: 148–152. [DOI] [PubMed] [Google Scholar]
- 100. Kheir AEM, Ali RA, Kononna AAM. Neonatal outcome in hypertensive disorders of pregnancy in a tertiary neonatal unit in Sudan. J Med Med Res 2014; 2: 59–65. [Google Scholar]
- 101. Larbi RKT, Buchmann EJ, Matshidze PR. Pregnancy outcomes in urban black South African women aged 35 years and older. J Obstet Gynaecol 2000; 20(3): 259–262. [DOI] [PubMed] [Google Scholar]
- 102. Macheku GS, Philemon RN, Oneko O, et al. Frequency, risk factors and feto-maternal outcomes of abruptio placentae in Northern Tanzania: a registry-based retrospective cohort study. BMC Pregnancy Childbirth 2015; 15: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ekele B, Bello S, Adamu AN. Clusters of eclampsia in a Nigerian teaching hospital. Int J Gynaecol Obstet 2007; 96(1): 62–66. [DOI] [PubMed] [Google Scholar]
- 104. Hall D, Odendaal H, Kirsten G, et al. Expectant management of early onset, severe pre-eclampsia: perinatal outcome. BJOG 2000; 107(10): 1258–1264. [DOI] [PubMed] [Google Scholar]
- 105. Adekanle D, Akinbile T. Eclampsia and pregnancy outcome at LAUTECH teaching hospital, Osogbo, SouthWest, Nigeria. Clin Mother Child Health 2012; 9(1): 1–3. [Google Scholar]
- 106. Ade-Ojo I, Loto OM. Outcome of eclampsia at the Obafemi Awolowo University Teaching Hospital Complex, Ile-Ife. Niger J Clin Pract 2008; 11(3): 279–284. [PubMed] [Google Scholar]
- 107. Kidanto HL, Wangwe P, Kilewo CD, et al. Improved quality of management of eclampsia patients through criteria based audit at Muhimbili National Hospital, Dar es Salaam, Tanzania. Bridging the quality gap. BMC Pregnancy Childbirth 2012; 12: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mungoba TN, Mitonga HK, David SA, et al. Factors associated with adverse pregnancy outcomes among women who delivered at Intermediate Hospital Oshakati. Int J Med 2017; 5: 5–9. [Google Scholar]
- 109. Ajah LO, Ozonu NC, Ezeonu PO, et al. The feto-maternal outcome of preeclampsia with severe features and eclampsia in Abakaliki, South-East Nigeria. J Clin Diagn Res 2016; 10: QC18–QC21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tebeu PM, Halle G, Ngowa JDK, et al. Outcome of pregnancy in pre-eclampsia and eclampsia at the regional hospital Maroua-Cameroon. Int J Reprod Med Gynecol 2017; 3: 034–039. [Google Scholar]
- 111. Berhan Y, Endeshaw G. Maternal mortality predictors in women with hypertensive disorders of pregnancy: a retrospective cohort study. Ethiop J Health Sci 2015; 25: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Onyiriuka AN, Okolo AA. Perinatal outcome in patients with pre-eclampsia in Benin City, Nigeria. Trop J Obstet Gynaecol 2004; 21: 148–152. [Google Scholar]
- 113. Bramham K, Parnell B, Nelson-Piercy C, et al. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ 2014; 348: g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kiondo P, Tumwesigye NM, Wandabwa J, et al. Adverse neonatal outcomes in women with pre-eclampsia in Mulago Hospital, Kampala, Uganda: a cross-sectional study. Pan Afr Med J 2014; 17(Suppl. 1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Berhe AK, Kassa GM, Fekadu GA, et al. Prevalence of hypertensive disorders of pregnancy in Ethiopia: a systemic review and meta-analysis. BMC Pregnancy Childbirth 2018; 18: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Allanson ER, Muller M, Pattinson RC. Causes of perinatal mortality and associated maternal complications in a South African province: challenges in predicting poor outcomes. BMC Pregnancy Childbirth 2015; 15: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ 2013; 347: f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mol BW, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. The Lancet 2016; 387: 999–1011. [DOI] [PubMed] [Google Scholar]
- 119. Bragulat E, De La Sierra A, Antonio MT, et al. Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension 2001; 37(2 Pt 2): 444–448. [DOI] [PubMed] [Google Scholar]
- 120. Miranda ML, Swamy GK, Edwards S, et al. Disparities in maternal hypertension and pregnancy outcomes: evidence from North Carolina, 1994–2003. Public Health Rep 2010; 125(4): 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ghosh G, Grewal J, Männistö T, et al. Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethn Dis 2014; 24: 283–289. [PMC free article] [PubMed] [Google Scholar]
- 122. Saleh MM, Selinger M. Evaluation of the role of day assessment unit in the management of pregnancy induced hypertension. J Obstet Gynaecol 2009; 25: 651–655. [DOI] [PubMed] [Google Scholar]
- 123. Henry CS, Biedermann SA, Campbell MF, et al. Spectrum of hypertensive emergencies in pregnancy. Crit Care Clin 2004; 20(4): 697–712, ix. [DOI] [PubMed] [Google Scholar]
- 124. Thoulass JC, Robertson L, Denadai L, et al. Hypertensive disorders of pregnancy and adult offspring cardiometabolic outcomes: a systematic review of the literature and meta-analysis. J Epidemiol Community Health 2015; 70: 414–422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-whe-10.1177_1745506520973105 for Prevalence of hypertensive disorders of pregnancy and pregnancy outcomes in Sub-Saharan Africa: A systematic review and meta-analysis by Kasiye Shiferaw Gemechu, Nega Assefa and Bizatu Mengistie in Women’s Health