Abstract
Patient: Male, 61-year-old
Final Diagnosis: Phlegmasia cerulea dolens
Symptoms: Bilateral leg swelling • breathlessness • cough • fever
Medication: —
Clinical Procedure: —
Specialty: Cardiology • Infectious Diseases • Medicine, General and Internal
Objective:
Rare disease
Background:
Coronavirus disease 2019 (COVID-19) is a novel infectious disease with an evolving understanding of its clinical manifestations, complications, and therapeutic implications. Thromboembolic disease and coagulopathy are common and have been seen in COVID-19 patients. Phlegmasia cerulea dolens had been reported in previous cases associated with malignancy which is a known cause of a procoagulable state. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may also induce a procoagulable state and be associated with PCD.
Case Report:
A 61-year-old man presented with a painful, swollen limb and gangrene, findings consistent with a diagnosis of PCD due to venous thrombosis. The patient tested positive for SARS-CoV-2 infection after a nasopharyngeal swab sample using the XPRSARS-COV2-10 reverse transcription polymerase chain reaction kit.
He had bilateral leg swelling with a gangrenous left fourth digit in the presence of a palpable peripheral pulse. His venous duplex showed bilateral acute deep venous thrombosis, whereas his arterial Doppler scan was normal and his skin biopsy was negative for vasculitis. One of our screening blood tests was suggestive of an antiphospholipid-like syndrome. These clinical and radiologic findings were consistent with PCD. This patient was promptly anticoagulated; other supportive treatments were also initiated. He had a significant resolution of his pedal swelling with the associated revitalization of his previously gangrenous toe.
Conclusions:
This case report shows the importance of testing for SARS-CoV-2 infection in patients who present with unusual thrombotic symptoms and signs and highlights the potential severity of these thrombotic complications.
MeSH Keywords: COVID-19, Gangrene, Venous Thrombosis
Background
A new type of viral pneumonia appeared in Wuhan, Hubei Province, China in December 2019 [1]. This viral pneumonia was named “2019 novel coronavirus” by the World Health Organization (WHO) on January 12, 2020 [2]. Coronaviruses are single-stranded positive-sense ribonucleic acid viruses with the ability to undergo mutation. Coronaviruses are known as etiologic agents for respiratory infection [3]. The coronavirus disease 2019 (COVID-19) is an illness of novel dimensions in terms of infectivity, morbidity, and mortality but without specific treatment [1]. The WHO declared the COVID-19 outbreak a pandemic on March 11, 2020 [4].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2)-induced COVID 19 has been shown to affect people with preexisting comorbidities and cause serious disease [5,6]. Venous thrombosis and thromboembolic disease have been reported as highly prevalent and a major poor prognostic factor in COVID-19 patients [7]. In a single-center cohort study by Middeldorp et al. [8] of the 75 patients hospitalized in the intensive care unit (ICU) for COVID-19, venous thromboembolism was observed in 47% of them despite being on prophylactic antithrombotic drugs. In a systematic review article by Fontana et al. [9] the risk of venous thromboembolism ranged from 4.4% to 8.2% in all patients hospitalized for COVID-19, whereas the risk was significantly higher, up to 53.8%, for COVID-19 patients admitted into the ICU. Deep vein thrombosis (DVT) contributes 10–15% of hospital mortality in patients without COVID-19. The combined poor prognostic effect of DVT and COVID-19 cannot be overemphasized [10]. Early and rapid diagnosis of SARSCoV-2 infection using the WHO guidelines for testing with reverse transcription polymerase chain reaction (RT-PCR) is needed to establish a diagnosis and to commence anticoagulation to mitigate venous thromboembolism, which is a known complication. Some of the uncommon manifestations of venous thrombosis include phlegmasia alba dolens, phlegmasia ceru-lean dolens (PCD), and venous gangrene [10]. Warkentin [11] first described the diagnosis of PCD as evidence of limb necrosis in the presence of palpable or Doppler-identifiable arterial pulses, which suggests microvascular thrombosis. A similar diagnostic guideline was used by Maiti et al. [12]. PCD can be divided into 3 stages on the basis of severity: (I) noncomplicated PCD, (II) impending venous gangrene, and (III) venous gangrene [13]. Our index case had stage III.
A case of venous gangrene associated with SARS-CoV-2 infection in a pediatric age group had been reported by Visveswaran et al. [14] and a similar case was also reported by Morales et al. [15]. This report is a case of a 61-year-old man who presented with a painful, swollen limb and gangrene consistent with a diagnosis of PCD stage III and who tested positive for SARS-CoV-2 infection.
Case Report
A 61-year-old man with a premorbid history of hypertension was admitted after a 2-week history of fever, chills, nonproductive cough, shortness of breath, and bilateral pedal swelling. Shortness of breath was present both at rest and on exertion. At rest there was associated wheezing; however, he had no orthopnea, paroxysmal nocturnal dyspnea, or chest pain. He noticed painful bilateral leg swelling about a week before presentation, with associated dark discoloration of his left fourth toe that progressed during the illness.
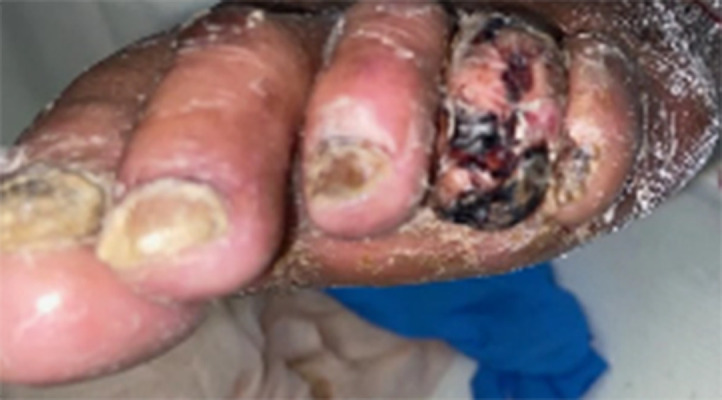
His initial vital signs showed a temperature of 38.1°C, blood pressure 135/82 mmHg, heart rate (HR) 82 beats per minute, respiration rate 24/min, and oxygen saturation of 90% on room air. The pulmonary exam revealed rhonchi in both upper lung zones and rales bibasally. The abdomen was soft, nontender, and had no palpable organomegaly. The cardiac examination noted a regular HR and rhythm with first and second heart sounds. The musculoskeletal exam found bilateral calf tenderness with tense bilateral pitting pedal edema; pedal pulses were present bilaterally, but there was a bluish discoloration of his left fourth digit (Figure 1).
Figure 1.
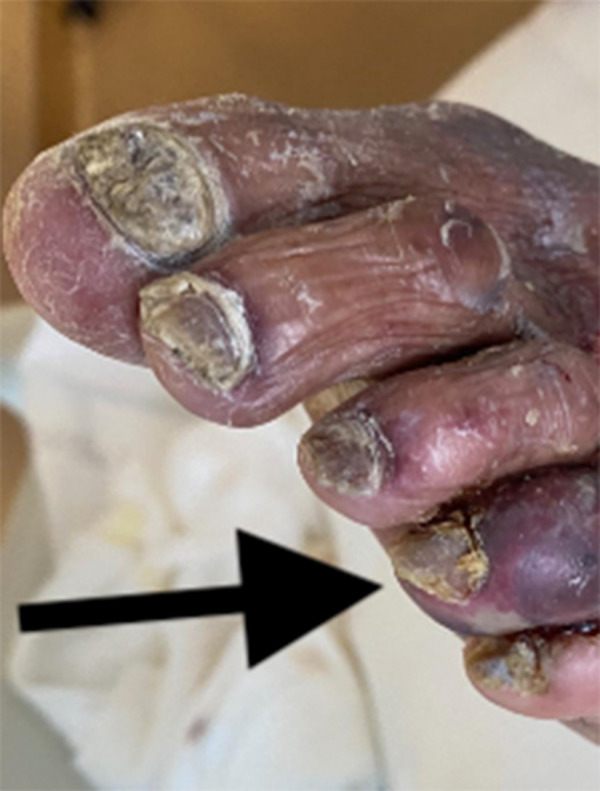
Dark arrow pointing at the gangrenous left fourth toe of the index case before commencing heparin.
Our patient’s SARS-CoV-2 infection was diagnosed from a nasopharyngeal swab sample using the XPRSARS-COV2-10 RT-PCR kit (Cepheid, 904 Caribbean Drive, Sunnyvale, CA, USA) for use with GeneXpert detection system (Cepheid), which has received U.S. Food and Drug Administration emergency use authorization, as recommended by current testing guidelines from the WHO on use of laboratory methods for SARS diagnosis 2020.URL: https://www.who.int/csr/sars/labmethods/en/. His initial blood test was positive for antiphospholipid-like syndrome, with high concentrations of inflammatory markers (Table 1).
Table 1.
Blood results.
| Markers of coagulopathy | Result |
|---|---|
| D-Dimer | 3,612 ng/dL (0–230 ng/dl) high |
| Cardiolipin antibodies | Positive |
| Cardiolipin immunoglobulin (Ig)G | 60.5 GPL (0.0–12.5) GPL high |
| Cardiolipin IgM | 44.5 MPL (0.0–12.5 MPL) high |
| Cardiolipin IgA | 8.8 APL (0.0–12.5 APL) normal |
| Beta-2 glycoprotein | Positive |
| Beta-2 glycoprotein IgM | 61.9 SMU (≤20.0 SMU) high |
| Beta-2 glycoprotein IgG and IgA | 5 SGU and 5.9 SAU respectively (normal) |
| Homocysteine | 6.8 μmol/l (≤15.0 μmol/L) normal |
| Factor V Leiden mutation | Negative |
| Prothrombin mutation analysis | Negative for prothrombin G20210A mutation |
| International normalized ratio | 1.1 |
| Activated partial thromboplastin time | 31.6 (25.1–36.5) normal |
| Fibrinogen | 283 (200–393 mg/dL) normal |
| Markers of infection/inflammation | Result |
|---|---|
| Procalcitonin | 0.28 ng/mL (0.02–0.08 ng/mL) high |
| C-reactive protein | 6.11 mg/dL (0.00–0.40 mg/dL) high |
| Ferritin | 444 ng/mL (30–400 ng/mL) high |
| Blood culture | Negative |
| Blood biochemistry | Result |
|---|---|
| Creatinine | 1.4 mg/dL (0.7–1.2mg/dl) high |
| Blood urea nitrogen | 35 mg/dL (6.0–20.0 mg/dl) high |
| Lactic acid | 3.1 mmol/L (0.5–2.0 mmol/L) high |
| Probrain natriuretic peptide | 1,716 pg/mL (1.0–125.0 pg/mL) high |
| Arterial blood gas | pH 7.30 low |
| Lactase dehydrogenase | 758 U/L (135–225 U/L) high |
| Albumin | 3.5 mg/dL (3.5–5.2 mg/dL) normal |
| Total protein | 8.0 g/dL (6.6–8.7 g/dL) normal |
| Alkaline phosphatase | 352 U/L (40–129 U/L) high |
| Aspartate transaminase | 74 U/L (0–40 U/L) high |
| Alanine transaminase | 61 U/L (0–41 U/L) high |
| Total bilirubin | 1.3 mg/dL (0.0–1.2 mg/dL) high |
| Blood count | Result |
|---|---|
| White blood cell count | 19,530 cell/mm3 (4.30–11.00 cell/mm3) |
| Hemoglobin | 11.1 g/dL (14.0–18.0 g/dL) |
| Platelet | 148×103/μL (150–450×103/μL) |
Electrocardiography (EKG) showed normal sinus rhythm. A chest X-ray on admission showed bibasilar pulmonary infiltrates compatible with pneumonia.
Venous Doppler ultrasound of the bilateral lower extremities revealed thrombosis. There was evidence of acute DVT in the left lower extremity (Figures 2, 3); the popliteal vein, gastrocnemius vein, and soleal veins showed no compression and no color Doppler flow. The right lower extremity revealed evidence of acute DVT in the femoral, popliteal, peroneal, and soleal veins as there was neither compression nor color Doppler flow.
Figure 2.
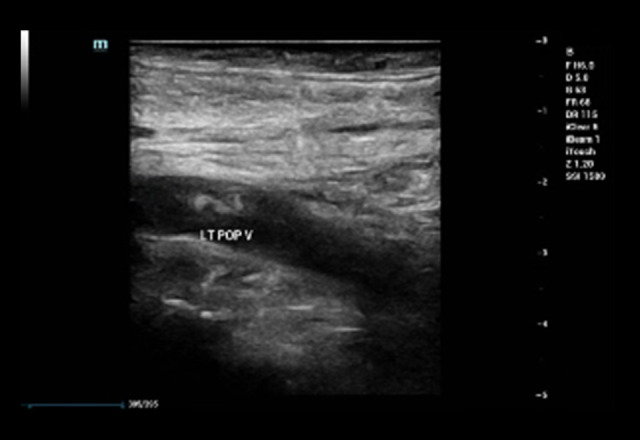
Longitudinal venous duplex during acute phase showing thrombus in the left popliteal vein.
Figure 3.
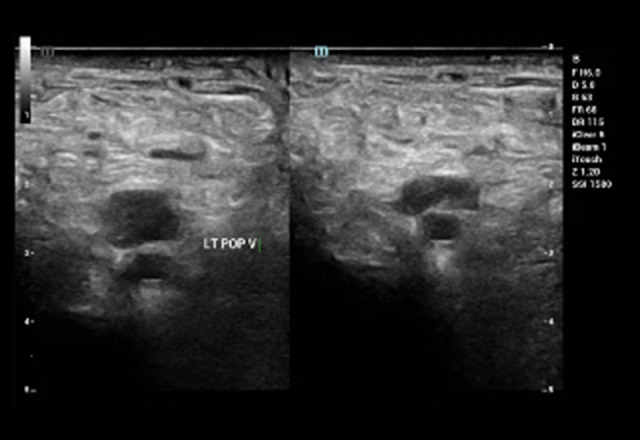
Sagittal venous duplex during acute phase showing thrombus in the left popliteal vein.
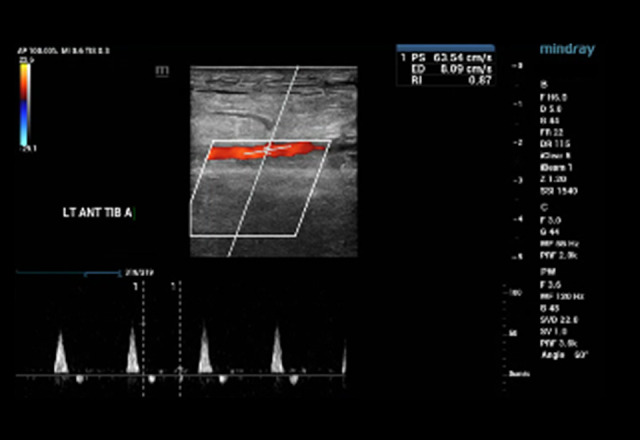
An arterial duplex scan of bilateral lower extremity shows patent common femoral, femoral, popliteal, posterior tibial, and anterior tibial arteries with no evidence of plaque/stenosis and with triphasic waveforms (Figures 4, 5). A biopsy of the left foot epidermis and dermal skin showed ulcers associated with stasis changes and hemorrhages but no evidence of vasculitis. Periodic acid Schiff stain was negative for fungal infection.
Figure 4.
Arterial Doppler ultrasound and spectral finding of the left posterior tibial artery was normal.
Figure 5.
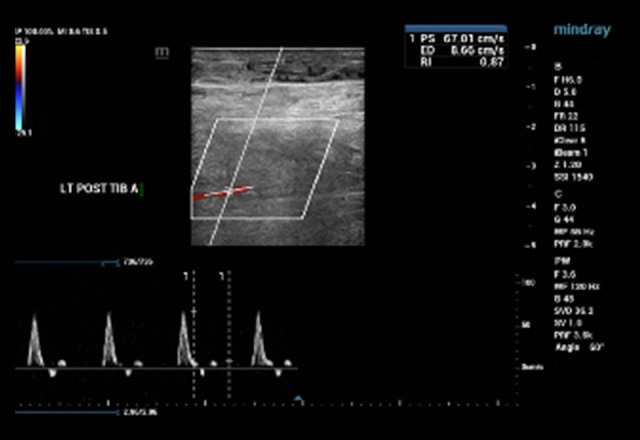
Arterial Doppler ultrasound with the color flow of the left anterior tibial artery was normal.
Diagnosis and treatment
The patient was admitted for pneumonia secondary to coronavirus infection and bilateral lower-extremity DVT complicated with venous gangrene of the left fourth toe. Vasculitis was ruled out in this patient by a negative skin biopsy, so peripheral arterial disease also was less likely, as he had a normal arterial duplex of the lower-limb arteries.
The patient was managed with a nonrebreather oxygen mask (fraction of inspired oxygen [FiO2] 40%), antivirus, antibiotics, heparin, and symptomatic and supportive treatment. Additionally, he was placed on bilateral Unna boots by the podiatry unit and the same management was agreed on by the vascular surgeons while awaiting demarcation, but there was no demarcation as the gangrenous toe and pedal edema improved remarkably after 1 week on heparin and the improvement was progressive and sustained all through his admission (Figure 6). However, the patient’s hypoxemic respiratory failure worsened and his oxygen requirement had to be increased from 8 L/min to 15 L/min (FiO2 of 100%) to maintain an oxygen saturation of 88%. He could no longer speak in full sentences without getting short of breath and his kidney function deteriorated as he had an elevation of creatinine and blood urea nitrogen. On hospital day 12, because of his multi-organ dysfunction and overall progressive decline, a chest radiograph was repeated. Chest X-rays displayed the progression of bilateral interstitial infiltrating shadows with new effusions.
Figure 6.
Improved vitalization of the left fourth toe of the index case after commencing heparin.
Chest computed tomographic angiography was subsequently performed and showed no evidence of pulmonary emboli, but revealed bilateral pleural effusions, large on the right and moderate on the left. Also present were ground-glass opacities in both upper lobes and the superior segments of both lower lobes. He was subsequently managed in the ICU for worsening hypoxemic respiratory failure and had a chest tube placed in the right pleural space, which drained hemorrhagic pleural effusions. After 30 days in the ICU, he had a repeat COVID-19 test, which was negative; the gangrenous toe became revitalized and his respiratory symptoms resolved. He was subsequently transferred to the rehabilitation unit for physical therapy. He was discharged 60 days after being admitted, having made significant clinical improvement.
Discussion
COVID-19 is a new disease caused by SARS-CoV-2 [16]. The viral particles move to the pulmonary terminal structure, causing an early alveolar exudation and lymphocytic infiltration in the pulmonary interstitium. The most common clinical manifestations are fever, cough, and shortness of breath [17]. In this patient who presented to our hospital with these symptoms, SARS-CoV-2 infection was confirmed by real-time RT-PCR.
A chest X-ray showed bilateral infiltrates. He also had painful bilateral pedal edema with a gangrenous left fourth toe in the presence of palpable peripheral pulses, normal arterial flow bilaterally, bilateral DVT, negative skin biopsy for vasculitis, positive antiphospholipid-like syndrome, elevated D-dimer, and other inflammatory markers. We believe that PCD stage III likely was triggered by SARS-CoV-2-induced procoagulable state. A similar case in a pediatric patient was published by Visveswaran et al. [14]. Venous gangrene as seen in our index case is an uncommon form of DVT that presents with pain and discoloration of the affected limb in the presence of radiologic evidence of DVT and the absence of an arterial occlusion [11].
The traditional Virchow’s triad of stasis, hypercoagulability, and endothelial dysfunction holds in COVID-19 patients; perhaps each component of the aforementioned triad is exacerbated by the SARS-CoV-2 infection [18].
Acute respiratory infections caused by various pathogens, including influenza virus, respiratory syncytial virus, and bacteria, are well-recognized triggers for thromboembolic events [19]. High concentrations of C-reactive protein (CRP) have been seen in patients with a first episode of venous thrombosis, suggesting a link with systemic inflammation; likewise, our patient had elevated CRP levels [20].
Patients with SARS-CoV-2 infection may be dehydrated because of fever, may have other secondary infections, and may be on prolonged bed rest as seen in our index case; these are all risk factors for DVT. SARS-CoV-2 infection is known to cause an antiphospholipid-like syndrome (hypercoagulability), which was also seen in our index case, ultimately putting our patient at high risk for thrombosis and venous gangrene, which he developed [21].
Given the high inflammatory burden of SARS-CoV-2 infection, the multiplier effect of a significant systemic inflammatory response and localized vascular inflammation as a result of endothelial dysfunction at the venous level in patients with background cardiovascular disease such as obesity with prolonged hospitalization (stasis) due to SARS-CoV-2 infection may likely trigger venous thromboembolism, as seen in our index case [22].
Increased amounts of circulating tissue factor, which sits at the apex of the coagulation cascade, is more prevalent in COVID-19 patients, and this may be the most important trigger for venous thrombosis and venous gangrene, as in this case [23–25]. Morales et al. [15] reported a case of PCD in a patient with SARS-CoV-2 infection and premorbid history of provoked DVT, whereas our index case had no history of DVT but presented de novo with bilateral DVT and PCD. Investigational therapies such as heparin for treating SARS-CoV-2 infection may have combined anticoagulation and antiviral properties [25]. Heparin was used in our index case in an attempt to mitigate the SARS-CoV-2 infection-induced venous gangrene both as a possible antiviral, anti-inflammatory, and an anticoagulant [26]. We also might have taken advantage of the possible anti-infective and anti-inflammatory properties of the medications used, which include: cefepime, azithromycin, and hydroxychloroquine, which was then the acceptable mode of care in an attempt to mitigate the background infection and inflammation that precipitated venous gangrene in our index case.
Conclusions
Cardiovascular abnormalities have been described in COVID-19 patients from previous studies, and venous thromboembolism is a major culprit in the excess mortality seen in these patients [5,27]. Compared with patients with cardiovascular disease without SARS-CoV-2 infection, those with a combined burden are likely to have higher mortality.
This paper looked at the complex interactions between background cardiovascular risk factors, SARS-CoV-2 infection, which is known to trigger a procoagulability mechanism, and the therapeutic implications. It is our opinion that the evaluation of the risk of DVT, early testing for SARS-CoV-2 infection in those with unusual thrombotic symptoms, and treatment of such thrombotic complications are of paramount importance in abating the morbidity and mortality in COVID-19 patients, as the pool of evidence from various studies shows that early initiation of anticoagulation with heparin in patients with SARS-CoV-2 infection, especially those with cardiovascular risk, can improve outcome [26,28–30].
Acknowledgments
We thank our Program Director, Dr. Shobhana Chaudhari, for giving us an enabling environment for clinical practice and research.
References:
- 1.Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709–10. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 3.Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660–94. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–10. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kollias A, Kyriakoulis KG, Dimakakos E, et al. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br J Haematol. 2020;189:846–47. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontana P, Casini A, Robert-Ebadi H, et al. Venous thromboembolism in COVID-19: Systematic review of reported risks and current guidelines. Swiss Med Wkly. 2020;150:w20301. doi: 10.4414/smw.2020.20301. [DOI] [PubMed] [Google Scholar]
- 10.Bhardwaj R, Kandoria A, Sharma R, et al. A case of venous gangrene, treated successfully with thrombolytic therapy and skin grafting. J Assoc Phys India. 2008;56:640–42. [PubMed] [Google Scholar]
- 11.Warkentin TE. Venous limb gangrene during warfarin treatment of cancer-associated deep venous thrombosis. Ann Int Med. 2001;135(8 Part 1):589–93. doi: 10.7326/0003-4819-135-8_part_1-200110160-00009. [DOI] [PubMed] [Google Scholar]
- 12.Maiti A, Das A, Smith DT. Phlegmasia cerulea dolens. Postgrad Med J. 2016;92(1093):690. doi: 10.1136/postgradmedj-2016-134185. [DOI] [PubMed] [Google Scholar]
- 13.Chinsakchai K, ten Duis K, Moll FL, et al. Trends in management of phlegmasia cerulea dolens. Vasc Endovasc Surg. 2011;45(1):5–14. doi: 10.1177/1538574410388309. [DOI] [PubMed] [Google Scholar]
- 14.Visveswaran GK, Morparia K, Narang S, et al. SARS-CoV-2 infection and thrombosis: Phlegmasia cerulea dolens presenting with venous gangrene in a child. J Pediatr. 2020;226:281–84. doi: 10.1016/j.jpeds.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales MH, Leigh CL, Simon EL. COVID-19 infection with extensive thrombosis: A case of phlegmasia cerulea dolens. Am J Emerg Med. 2020;38:1978–980. doi: 10.1016/j.ajem.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–18. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zu ZY, Jiang MD, Xu PP, et al. Coronavirus disease 2019 (COVID-19): A perspective from China. Radiology. 2020;296(2):E15–25. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virchow R. Gesammelte abhandlungen zur wissenschaftlichen medicin: Grote. Berlin: Meidinger; 1862. [Google Scholar]
- 19.Cowan LT, Lutsey PL, Pankow JS, et al. Inpatient and outpatient infection as a trigger of cardiovascular disease: The ARIC Study. J Am Heart Assoc. 2018;7(22):e009683. doi: 10.1161/JAHA.118.009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamphuisen PW, Eikenboom JC, Vos HL, et al. Increased levels of factor VIII and fibrinogen in patients with venous thrombosis are not caused by acute phase reactions. Thromb Haemost. 1999;81(5):680–83. [PubMed] [Google Scholar]
- 21.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobato NS, Filgueira FP, Akamine EH, et al. Mechanisms of endothelial dys-function in obesity-associated hypertension. Braz J Med Biol Res. 2012;45(5):392–400. doi: 10.1590/S0100-879X2012007500058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hron G, Kollars M, Weber H, et al. Tissue factor-positive microparticles: Cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost. 2007;97(1):119–23. [PubMed] [Google Scholar]
- 24.Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3(8):1590–96. doi: 10.1111/j.1538-7836.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- 25.Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020;18(5):1020–22. doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costanzo L, Palumbo FP, Ardita G, et al. Coagulopathy, thromboembolic complications, and the use of heparin in COVID-19 pneumonia. J Vasc Surg Venous Lymphat Disord. 2020;8(5):711–16. doi: 10.1016/j.jvsv.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T, Chen R, Liu C, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362–63. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hippensteel JA, LaRiviere WB, Colbert JF, et al. Heparin as a therapy for COVID-19: Current evidence and future possibilities. Am J Physiol Lung Cell Mol Physiol. 2020;319(2):L211–17. doi: 10.1152/ajplung.00199.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miesbach W, Makris M. COVID-19: Coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020;26:1076029620938149. doi: 10.1177/1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–99. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]


