Multisystem inflammatory syndrome in children (MIS-C) is a rare complication of SARS-CoV-2 infection that can result in serious illness in the paediatric population but our understanding of this syndrome is in its infancy. Translational studies in 2020 leveraging immune profiling have laid the foundation to enable further discovery in MIS-C.
Subject terms: Paediatric rheumatic diseases, SARS-CoV-2, Inflammation
Key advances
The immune response in multisystem inflammatory syndrome in children (MIS-C) seems to be distinct from that during acute SARS-CoV-2 infection8, but has both shared and distinct features compared with Kawasaki disease7,10.
The immune landscape shifts during the course of MIS-C, with the acute phase being characterized by activated innate immune cells and T cell and B cell lymphopenia, which normalize during recovery, and appropriate anti-viral antibody responses detected to SARS-CoV-2 (ref.6).
MIS-C and Kawasaki disease might share plasma protein profiles but differ in autoantibody targets9,10; whether they are distinct or represent a continuum of the same clinical syndrome remains to be determined.
In the early stages of the COVID-19 pandemic, healthy children were thought to have mild SARS-CoV-2 infections with favourable outcomes. In April 2020, reports began to emerge from COVID-19 epicenters describing clusters of children with features of Kawasaki disease and toxic shock syndrome1,2. This newly identified entity has many names and ultimately became known as multisystem inflammatory syndrome in children (MIS-C), as used by the WHO and CDC. As additional reports of MIS-C have surfaced, the clinical spectrum of this syndrome has broadened3–5, and studies have begun to unveil its immune landscape, which could help in our understanding of this condition6–10.
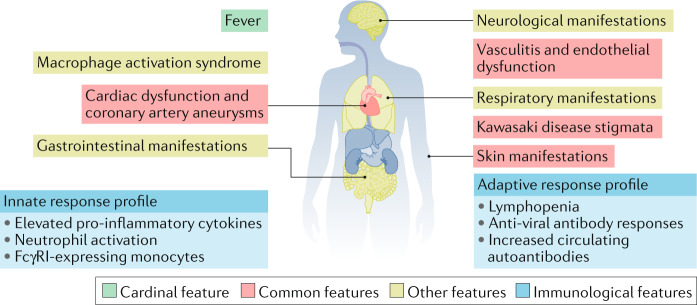
Emerging data show that MIS-C is characterized by the classic findings of inflammation, with fever as the cardinal feature, and multi-organ dysfunction that not only involves the skin, mucous membranes and heart but that also frequently affects the gastrointestinal, respiratory and neurologic systems (Fig. 1). However, the full clinical continuum of MIS-C is still being defined, and validated diagnostic criteria do not yet exist. As a result, researchers have employed varying case definitions of MIS-C so that patient populations are not necessarily comparable across studies5. This selection bias is important to consider because it affects our understanding of MIS-C.
Fig. 1. Emerging clinical and immunological features of MIS-C.
Multiple organs are affected in multisystem inflammatory syndrome in children (MIS-C). Most patients have evidence of prior SARS-CoV-2 exposure, and Kawasaki disease features and cardiac dysfunction are common. The immune response in MIS-C is distinct from that during the acute SARS-CoV-2 infection, and is associated with elevated pro-inflammatory cytokines, activated neutrophils and monocytes, cytopenias (thrombopenia and lymphopenia) and appropriate anti-viral antibody responses detected to SARS-CoV-2.
MIS-C is temporally linked to SARS-CoV-2, and occurs as a late manifestation of or response to the infection, with cases peaking 3–6 weeks after the highest rate of SARS-CoV-2 infection (as measured by PCR positivity) in a given location3,4. The majority of patients had neutralizing antibodies to SARS-CoV-2, with greater titres of IgG antibodies than IgM antibodies, further indicating a preceding SARS-CoV-2 infection2,3,6–10. Building on these findings, Diorio et al.8 evaluated the clinical and laboratory features of children with SARS-CoV-2 infections to clarify the differences between the early infectious phase of COVID-19 (severe COVID-19) and MIS-C. Compared with severe COVID-19, the PCR cycle thresholds for SARS-CoV-2 were higher for MIS-C, indicating a reduced viral burden and supporting the concept that MIS-C is a post-infectious process. Furthermore, this report identified demographics that differed between these two groups: patients with MIS-C were younger and less medically complex than patients with severe COVID-19. High levels of soluble C5b-9 (the membrane attack complex of the complement system) and evidence of microangiopathy on blood smears also suggested that endothelial dysfunction was central in the pathophysiology of both severe COVID-19 and MIS-C.
In a similar approach, Lee and colleagues evaluated the immunologic profile of MIS-C and identified the presence of T cell, B cell and natural killer cell cytopenias7. By comparing MIS-C to historic cohorts of Kawasaki disease (pre-pandemic Kawasaki disease), Lee et al. identified similarities and differences between these two childhood hyperinflammatory syndromes. Many patients with MIS-C had features of Kawasaki disease. However, the patients with MIS-C presented over a broader age range, had a greater degree of myocardial dysfunction, had more profound lymphopenia and thrombocytopenia, and more often showed signs of coagulopathy than the patients with pre-pandemic Kawasaki disease2,7,10. Whether MIS-C is distinct from Kawasaki disease or whether these two entities represent a continuum of the same clinical syndrome remains to be determined. Both reports by Diorio et al. and Lee et al. provide potentially useful diagnostic profiles of MIS-C; however, the results were derived from a small number of patients, and their generalizability awaits validation.
To gain further understanding of MIS-C, deeper immunophenotyping is required. Carter et al.6 undertook this approach by studying 25 patients with MIS-C from the acute phase of illness through to convalescence using high dimensional cytokine and flow cytometry panels. At disease onset, treatment-naive patients with MIS-C had high serum levels of multiple cytokines, and the acute phase was associated with activated neutrophils and monocytes that expressed high levels of FcγRI. Circulating levels of CD4+, CD8+ and γδT cells were decreased early in the course of MIS-C compared with age-matched healthy individuals, with the exception of CD4+CCR7+ T cells. Although patients with MIS-C are able to generate neutralizing antibodies to SARS-CoV-2, the patients had lower levels of total B cells, effector B cells and class switched memory B cells in the blood than healthy individuals. After resolution of MIS-C, these observed innate and adaptive immune system changes normalized, and the frequency of plasmablasts and regulatory T cells increased. This work by Carter and colleagues identified a shifting immune landscape over the course of illness in MIS-C and highlighted several immune cell populations that might be important in either promoting disease or mediating recovery in MIS-C.
Multi-dimensional immune profiling was also employed in two other important publications from 2020 — Gruber et al.9, and Consiglio et al.10 — that evaluated immune responses in MIS-C compared with pre-pandemic Kawasaki disease and/or acute COVID-19. In principal component analysis (PCA) of circulating immune proteins, patients with MIS-C clustered separately from adults and children with acute COVID-19 (refs9,10). Mass cytometry data from Gruber et al. showed a trend towards increased frequencies of circulating memory T cells in patients with acute COVID-19 compared with in patients with MIS-C, although most patients with MIS-C in this study were already being treated with immunomodulatory medications9. Comparisons of MIS-C with Kawasaki disease by Consiglio and colleagues yielded less conclusive findings. Patients with Kawasaki disease and patients with MIS-C clustered together in a PCA analysis of plasma proteins10. However, evaluation of immune cells by flow cytometry in MIS-C versus Kawasaki disease was limited owing to the small numbers of patients in the MIS-C group (n = 3).
Importantly, the work by Gruber et al.9 and Consiglio et al.10 has furthered our understanding of the humoral response in MIS-C. Both studies confirmed that patients with MIS-C generate appropriate antibody responses to SARS-CoV-2, as well as to other viruses. Compared with healthy individuals, patients with MIS-C had enrichment of both IgG and IgA autoantibodies directed towards peptides expressed in the endothelial, cardiac and gastrointestinal tissue as well as autoantibodies directed toward immune mediators9. Autoantibodies from both patients with Kawasaki disease and patients with MIS-C shared some targets, including proteins expressed by endothelial cells, whereas some autoantibodies were upregulated only in MIS-C or Kawasaki disease. Although these results are intriguing, the sample sizes were small, and it remains to be determined if these autoantibodies are primary mediators of disease in MIS-C or are generated secondarily as a result of tissue damage in the setting of infection.
Since MIS-C materialized as a complication of SARS-CoV-2 infections in children in early 2020, great strides have been made in characterizing the clinical presentation and immunophenotype of this syndrome, pointing to both innate and adaptive immunity together with vascular inflammation and endothelial dysfunction as important contributors to pathobiology. Yet, these studies represent only a beginning in our endeavour to understand MIS-C. To gain ground in this journey, future work will need to interrogate larger numbers of treatment-naive patients with MIS-C, along with appropriate febrile controls. To date, studies have focused on circulating immune perturbations; however, some cell populations of interest might have extravasated into affected tissues. Furthermore, the genetic susceptibilities that predispose patients to MIS-C are unknown, and the relationship between Kawasaki disease and MIS-C remains unresolved. The preliminary data generated by these translational research studies highlight the need for data sharing and cross-validation to bring disease understanding to a new level. Harmonizing case definitions and international collaborations will help accelerate the pace of advancement in MIS-C and make real change possible in the care and outcomes of this emerging condition.
Competing interests
The authors declare no competing interests.
References
- 1.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verdoni L, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dufort EM, et al. Multisystem inflammatory syndrome in shildren in New York State. New Engl. J. Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldstein LR, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. New Engl. J. Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrams JY, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J. Pediatr. 2020;226:45–54. doi: 10.1016/j.jpeds.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter MJ, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 7.Lee PY, et al. Distinct clinical and immunological features of SARS-COV-2-induced multisystem inflammatory syndrome in children. J. Clin. Invest. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diorio C, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J. Clin. Invest. 2020;130:5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruber CN, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:982–995. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Consiglio CR, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]