Abstract
Background
This study examined whether aspects of diet and nutrition risk explain variance in physical capacity and general health, after controlling for covariates, in Canadian adults with osteoarthritis (OA).
Methods
This was a cross-sectional study of baseline data from the Canadian Longitudinal Study on Aging (CLSA). Data from 1,404 participants with hand, hip, and/or knee OA were included. A series of regression analyses were conducted with independent variables of food intake (fiber and high calorie snack intake) and nutrition risk; and dependent variables of physical capacity and general health. Physical capacity was characterized through grip strength and a pooled index of four mobility tests. General health was characterized through an index of self-reported general health, mental health, and healthy aging.
Results
Higher fiber intake was related to greater mobility (p = .01). Food intake was not related to any other outcome. Nutrition risk was significantly associated with mobility (p < .001) and general health (p < .001); those with a high nutrition risk classification had poorer general health (p < .001, d = 0.65) than those at low nutrition risk. As well, those with moderate nutrition risk had poorer general health than those with low nutrition risk (p = .001, d = 0.31).
Conclusions
Nutrition risk screening for older adults with OA provides insight into behavioral characteristics associated with reduced mobility and poorer general health. Also, those consuming greater amounts of fiber demonstrated better mobility. Thus, this research suggests that quality of diet and nutritional behaviors can impact both physical and mental aspects of health in those with OA.
Keywords: Arthritis, Nutrition, Physical function, CLSA
Canadians are now living longer than previous generations (1). By 2024, we expect that 1 of 5 Canadians will be over age 65 and the majority will be overweight or obese (2,3). The cause of this obesity epidemic in developed nations is complex but reflects, at least in part, malnutrition; that is, a deficiency or excess of one or more essential nutrients that leads to functional changes in the body (4,5). While much aging research shows that frailty (including underweight, exhaustion, weakness, inactivity) (6), malnutrition and specifically protein undernutrition (7,8), and multimorbidity predict indices of functional decline and/or mortality among older adults (9,10), overnutrition and obesity are also associated with mortality and reduced quality of life among older community-dwelling adults (11). Compared to their age-matched counterparts, obese seniors are more likely to report impaired physical functioning (1) and more likely to live with multiple comorbidities, including but not limited to heart disease, diabetes, and chronic musculoskeletal conditions (12–16). Sarcopenic obesity, with etiologic factors of a poor quality diet and limited physical activity, places individuals at especially high risk for functional deficit (17,18). Unfortunately, obesity-related musculoskeletal conditions also present a barrier to exercise as a means of managing obesity (19).
One of the most prevalent obesity-related musculoskeletal conditions that reduces physical functioning among older adults is osteoarthritis (OA). OA is a chronic, degenerative joint condition that affects one in eight Canadians; this prevalence will rise dramatically in the coming decades (20). OA affects knees, hips and hands most often. OA reduces physical functioning through pain, muscle weakness, and psychological sequelae including anxiety and depression (21). A meta-analysis showed pooled odds ratio for OA incidence of 1.98 (95% confidence intervals [CI] 1.57–2.20; n = 22 studies) among overweight adults and 2.26 (95% CI 2.15–3.28; n = 22 studies) among obese adults (22). This obesity-related risk for OA is hypothesized to occur through multiple mechanisms: larger body mass places heavier loads on lower extremity joints (23), pro-inflammatory processes degrade joint tissues (24), and physical inactivity (21).
Given the link between obesity and OA among seniors, it is not surprising that interventions that reduce obesity also improve health outcomes in older adults with OA. An 18-month three-arm randomized controlled trial (RCT) of diet, exercise, and diet-and-exercise showed that, across all three interventions, at least 10% reduction in body mass improved physical function in 454 older overweight/obese adults with knee OA (25). While promising, two concerns exist. First, calorie restrictions implemented in similar trials are typically strict (eg, total consumption of 415–810 kcal/day in a formula-based diet) (26), which cannot be replicated with a regular diet. Second, these interventions do not address the nutrition behaviors that can contribute to obesity and/or OA.
Nutrition may be a key factor that exacerbates decrements in physical functioning among obese, older adults with OA. The intake of specific foods may influence OA risk. For example, higher dietary fiber intake relates with lower risk of knee pain among those with or at risk for OA (27). This finding may reflect a healthier lifestyle, higher socioeconomic status, and/or that fiber promotes a healthy body mass (28) and gut microbiome (29,30). Across adulthood, better diet quality (ie, greater consumption of fruits/vegetables, lower consumption of sugar, processed meats) was associated with better chair rise speed, standing balance time and timed-up-and-go speed (31). Nonetheless, nutrition reflects more than what is consumed. Nutrition risk (NUR) screening identifies characteristics, including behavioral, that are associated with malnutrition (32). Nutrition risk screening tools for community-living older adults examine risk factors such as appetite, physical challenges while eating such as swallowing, dietary intake of specific items, socialization and preparation of meals (33). Importantly, NUR predicts mortality (34) and quality of life (35) in vulnerable seniors—but NUR has not been explored in OA. If NUR exacerbates decrements in physical function and general health in OA, interventions should be refined to support healthy dietary behaviors (33). To-date, the impact of diet and NUR on physical capacity and health among seniors with OA is unknown.
The purpose of this study was to examine the extent to which aspects of diet and NUR explain the variance in physical capacity and general health after controlling for covariates in older adults with hand, hip or knee OA. It was hypothesized that consuming a greater intake of high calorie snacks and lower intake of high fiber cereals (ie, poorer diet quality), in addition to being at high NUR would be associated with poorer mobility, grip strength, and self-reported general health.
Methods
Canadian Longitudinal Study on Aging
This research was conducted using baseline data from the Canadian Longitudinal Study on Aging (CLSA). The CLSA is a national, longitudinal study collected across 11 cities, which examined qualitative and quantitative measures in adults aged 45–85 years (36). To be eligible for recruitment, participants needed to be able to respond in English or French and reside within 25–50 km from one of the 11 centers across Canada (University of Victoria, University of British Columbia, Simon Fraser University, University of Calgary, University of Manitoba, McMaster University, University of Ottawa, McGill University, Université de Sherbrooke, Dalhousie University and Memorial University of Newfoundland). CLSA study participants for the Comprehensive Database were recruited through provincial healthcare registration databases, random digit dialing and the Quebec Longitudinal Study on Nutrition and Aging (NuAge). Data for the current analysis was contained within the Comprehensive Database of the CLSA, which was collected from May 2012 to May 2015 through face-to-face interviews at home and at a data collection site. Ethics approval for analysis of this data set was obtained from the Hamilton Integrated Research Ethics Board.
Participants
Data from participants included in this analysis were extracted from the CLSA Comprehensive Dataset if they met the following inclusion criteria: between 45 and 85 years of age at baseline, and self-report that they had been diagnosed with one or more of hand, hip or knee OA by a physician. For the sample used in this analysis, the participants were excluded if they reported any of the following conditions: dementia or Alzheimer’s disease, Multiple Sclerosis, epilepsy, rheumatoid arthritis, stroke or a cerebrovascular accident, ministroke or transient ischemic attack, Parkinson’s disease, emphysema, chronic bronchitis, chronic obstructive pulmonary disease, or chronic changes in the lungs due to smoking, traumatic brain injury, clinical depression, respiratory issues following strenuous activity. Finally, only data from participants who completed all measurements of interest were included in the analyses; those with partial data sets were removed.
Independent Measures
Diet
Dietary variables were obtained by self-report using the Short Diet Questionnaire (NUT). The NUT asks participants to report the frequency of consumption for 36 different types of food. Participants reported a typical number of servings and the frequency of consumption (daily, weekly, monthly, or yearly). The NUT has demonstrated reasonable validity when compared with 24-hour diet recall (37). The current study specifically focused on question 1 of the NUT, which asked about high fiber cereal intake, and questions 25–28 which asked about high calorie snacks (ie, ice cream, salty snacks, cakes and pastries, chocolate bars), as indicators of better and worse diet quality. Within the CLSA database was a daily conversion for each question. For this study, the high calorie snacks were grouped as single independent variable (NUTHC), calculated to be the sum of the daily frequencies for each of the four high calorie snack categories. High fiber cereal was examined as a separate independent variable (NUTFBR).
Nutrition risk
Nutrition risk was assessed using the abbreviated version of the SCREEN II© tool (33). This tool includes 11 questions which ask participants about changes in their weight, appetite, specifics regarding their meal patterns (ie, whether they skip meals, meal preparation, eating alone), the frequency of consuming fruits and/or vegetables as well as drinking fluids, and physical challenges associated with eating (ie, choking, coughing). This tool produces valid and reliable data for detecting NUR among older adults, with a higher score indicating less risk (33). The CLSA database includes both a NUR score (NURSCR), as well as a NUR classification (NURCLS). Based on their score, participants were classified as either low risk (NURSCR ≥ 43), moderate risk (38 ≤ NURSCR < 43), or high risk (NURSCR < 38) (33).
Dependent Measures
Physical capacity
Measures of grip strength and mobility were used to characterize physical capacity. Grip strength was measured in the CLSA using a Tracker Freedom Wireless Grip Dynamometer. The average strength between three repeated trials was selected for analysis, rather than the peak, as it is reportedly more reliable (38). Rather than evaluate each measure of mobility individually, mobility was characterized as an index that included four measures: (a) 4-m walk, which measures the total time to walk a distance of 4 m, (b) one-leg standing balance, which is the best attained time for standing on one leg to a maximum of 60s, (c) chair rise, which is the average time to rise out of a chair five times, and (d) timed-up-and-go, which is the time to rise out of a chair, walk 3 m, and return to the chair to a seated position. A pooled index was created by first converting each of the four variables to standard normal variables using equation (1), and then calculating the sum of the four measures while also considering their directionality (equation 2). A higher mobility index score would indicate better mobility.
| (1) |
where
is the normalized value of a particular variable, x, for participant, i (i = 1: n number of participants)
and are the mean and standard deviation of that particular variable, x
| (2) |
where
is the normalized walk time
is the normalized chair rise time
is the normalized tug time
is the normalized standing balance time
General health
An index of self-reported general health was created using a collective score from three questions asked during the in-home questionnaire (equation 3). These questions asked participants to rate their (a) general health (b), mental health, and (c) healthy aging, on a scale from 1 (excellent) to 5 (poor). The index was created such that a higher score would indicate better general health.
| (3) |
Where,
is the 5-pt scale general health score
is the 5-pt scale mental health score
is the 5-pt scale healthy aging score
Covariates
Eleven covariates were included in these analyses. Variables selected were those that explain variance in physical capacity and general health in a population with OA. Covariates included age (39), sex (40), and body mass index (41). Scores on the Center for Epidemiological Studies Short Depression scale (CES-D-10) (42) was also included (43). This 10-item questionnaire elicits scores ranging from 0 to 30 with higher scores indicating more depressive symptoms. Socioeconomic variables including education level, income bracket, and social inequality were also included (43). Education level was classified into six categories with a score of 1 indicating no post-secondary degree certificate or diploma and six indicating a university degree or certificate above a bachelor’s degree. Five income brackets were listed, with a score of 1 reflecting a household income less than $20,000 and a score of 5 reflecting a household income at least $150,000. Social inequality was assessed through asking participants to rate their perceived level of social standing on 10-point scale, with a score of 1 representing lowest standing in the community to 10 representing highest standing in the community. Three comorbidities that coexist with OA and may explain variance in physical capacity and general health in OA were included as covariates: (a) heart disease (including congestive heart failure), (b) diabetes, borderline diabetes, or high blood sugar, and (c) kidney disease or failure (44,45). Lastly, OA type was also considered, with seven classifications including hand only, knee only, hip only, hand and knee, hand and hip, knee and hip, and all three forms.
Data and Statistical Analyses
A series of linear regression models were developed to express the relationship between independent variables and covariates with each of grip strength, mobility, and general health. First, a model solely considering all covariates (including OA type) was constructed. Then, a separate covariate model that did not include OA type was also considered. A likelihood-ratio test was performed to determine whether OA type was a significant contributor to the model. Pairwise comparisons were assessed between each OA type, using a Sidak correction for multiple comparisons. Next, separate models were developed for each independent variable: high calorie snack frequency (NUTHC), high fiber cereal frequency (NUTFBR), nutrition risk score (NURSCR), and nutrition risk classification (NURCLS); after accounting for the covariates, including type of OA. A likelihood-ratio test was performed to determine whether each of the models were statistically different from the covariate-only model. Effect sizes were calculated between NURCLS for each outcome measure. Furthermore, pairwise correlation coefficients with a Sidak correction were calculated between covariates and independent variables: NUTHC, NUTFBR, and NURSCR. As well, several model diagnostic tests were performed, and plots (standardized normal probability [P-P] plot, quantiles of variable against quantiles of normal distribution [Q-Q] plot, residual-versus-fitted plot, histogram of Studentized residuals) visually inspected to confirm normality, heteroskedasticity, and lack of co-linearity. All statistical analyses were conducted in Stata/IC 13.0 (StataCorp LP, College Station, TX).
Results
Participants
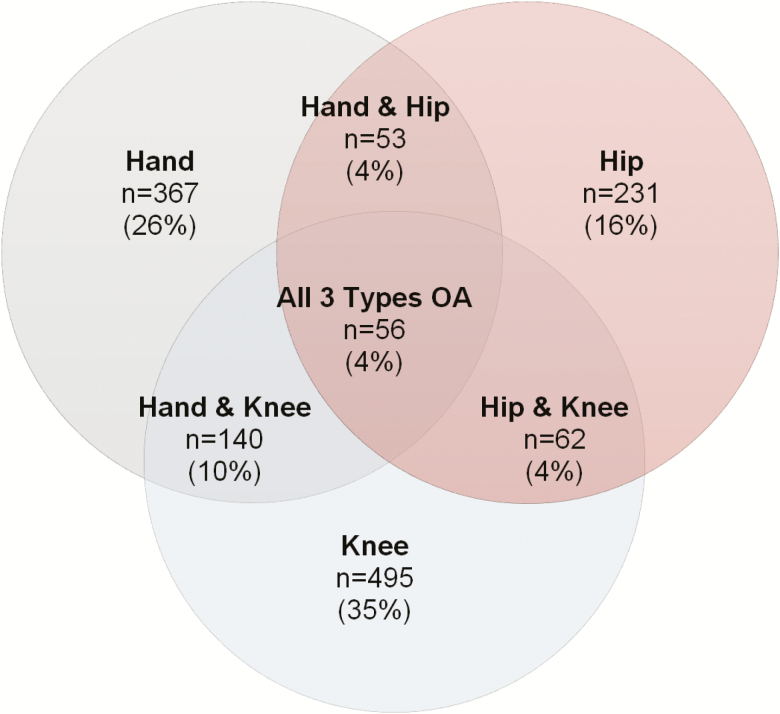
Data from 30,097 participants were received and screened for the inclusion and exclusion criteria (n = 4,129 from provincial health registries and n = 25,968 from random digit dialing and NuAge; overall response rate 0.1) (46). A total of 7,923 participants met the inclusion criteria for OA. Of these participants, 4,650 were removed based on the exclusion criteria. Of the remaining 3,723 participants, only 1,404 had completed data sets and were included in these analyses: 367 participants had hand OA, 495 had knee OA, 231 participants had hip OA, and 311 had multiple forms of OA (Table 1, Figure 1). Analyses revealed that for each of the measures (independent, dependent measures, and covariate), those with missing data were generally different than the participants included in the analysis. Specifically, those with missing data had significantly lower grip strength, mobility, and general health; older age; higher ratio of women to men; higher BMI; higher CES-D-10 scores; lower education, income and social inequality scores; and higher percentage of those having heart disease and diabetes.
Table 1.
Participant Characteristics (n = 1,404)
| Mean (range) | |
|---|---|
| Age | 66.1 (45, 85) y |
| Sex | n = 579 men; n = 825 women |
| BMI | 28.1 (18.1, 52.4) kg/m2 |
| Depressive symptoms (CES-D-10) score (/30) | 4.5 (0, 26) |
| Education level (1–6) | 4.2 (1, 6) |
| Income (1–5) | 3.3 (1, 5) |
| Social inequality (1–10) | 6.5 (1, 10) |
| Heart disease | Yes, n = 141; No, n = 1,263 |
| Diabetes | Yes, n = 234; No, n = 1,170 |
| Kidney disease | Yes, n = 36; No, n = 1,368 |
| NUTHC | 0.9 (0.0, 5.4) |
| NUTFBR | 0.6 (0.0, 11.0) |
| NURSCR | 40.4 (11.0, 48.0) |
| NURCLS | Low risk, n = 596 |
| Moderate risk, n = 440 | |
| High risk, n = 368 |
Note: BMI = Body mass index; CES-D = Center for Epidemiological Studies Short Depression scale
Figure 1.
Distribution of OA type across included participants from the CLSA data set. CLSA = Canadian Longitudinal Study on Aging.
Model Outcomes
Grip strength
The covariate model, particularly OA type, age, sex, depressive symptoms, and income explained a large amount of variance (63.67%, p < .0001) in average grip strength (Table 2). Older age, female sex, greater depressive symptoms, and lower income had a lower grip strength. As well, the covariate model that included OA type was significantly different from the model that did not (p = .0001). Specifically, after adjusting for multiple comparisons, knee OA alone had significantly higher grip strength than those with combined hand and hip OA (p = .022), and hand OA alone (p = .032). Also, hip OA alone had significantly higher grip strength than hand and hip OA (p = .003), and hand OA alone (p = .001). Neither dietary variables nor NUR were contributors to the model (p > .05; d < 0.13).
Table 2.
Regression Models Used to Predict Average Grip Strength
| Model | Explained Variance | Model Significance | Significant Predictors | Likelihood-Ratio Test (compared to Covariate) |
|---|---|---|---|---|
| Covariate model | R 2 = .6367 | F(16,1387) = 151.95 p < .0001 | OAtype (p = .0001) Age (p < .001; β = −0.25) Sex (p < .001; β= −0.73) BMI (p = .31; β = 0.02) CES-D-10 (p = .002; β = −0.05) EDU (p = .45; β = 0.01) INC (p = .002; β = 0.06) SEQ (p = .98; p < 0.001) HRT (p = .34; β = 0.02) DIA (p = .16; β = 0.02)KID (p = .97; β = 0.001) | |
| Covariates +NUTHC | R 2 = .6369 | F(17,1386) = 143.00 p < .0001 | NUTHC (p = .44; β = −0.01) | p = .44 |
| Covariates +NUTFBR | R 2 = .6368 | F(17,1386) = 142.97 p < .0001 | NUTFBR (p = .52; β = 0.01) | p = .52 |
| Covariates +NURSCR | R 2 = .6368 | F(17,1386) = 142.96 p < .0001 | NURSCR (p = .56; β = 0.01) | p = .55 |
| Covariates +NURCLS | R 2 = .6368 | F(18,1385) = 134.92 p < .0001 | NURCLS (p = .85) | p = .85 |
Note: Independent variables: frequency of high calorie snacks (NUTHC), frequency of high fiber cereal (NUTFBR), nutrition risk score (NURSCR), and nutrition risk classification (NURCLS, 2 = moderate risk, 3 = high-risk). Covariates: OA type, age, sex, body mass index (BMI), depressive symptoms (CES-D-10), education level (EDU), income (INC), social inequality (SEQ), heart disease (HRT), diabetes (DIA), and kidney disease (KID). Bolded values indicate significant findings.
Mobility index
The covariate model explained 36.99% of the variance (p < .0001) in the mobility index, with OA type, age, BMI, depressive symptoms, education, income, heart disease, and diabetes significantly contributing (p < .05) (Table 3). Older age, higher BMI, and greater depressive symptoms, lower education and income, and having heart disease and diabetes were associated with lower mobility index. As well, the covariate model that included OA type was significantly different from the model that did not (p < .0001). Specifically, after adjusting for multiple comparisons, those with combined hip and knee OA had a significantly lower mobility index than knee OA alone (p = .005), and combined hand and knee OA (p = .035). As well, hip OA had lower mobility than hand OA (p = .036). NUTHC did not contribute to the model, but both NUTFBR (p = .01) and NURSCR (p = .001), explained significantly more variance than the covariate model alone. Specifically, more frequent fiber intake and lower NUR were associated with greater mobility. Furthermore, though NURCLS was not statistically significant (p = .06), there was a moderate effect size between low and high NUR classification (d = 0.43), with higher NUR classification associated with a lower mobility index compared to a lower NUR (Table 3).
Table 3.
Regression Models Used to Predict Mobility Index
| Model | Explained Variance | Model Significance | Significant Predictors | Likelihood-Ratio Test (compared to covariate) |
|---|---|---|---|---|
| Covariate model | R 2 = .3699 | F(16,1387) = 50.89 p < .0001 | OAtype (p < .0001) Age (p < .001; β = −0.45) Sex (p = .43; β = −0.02) BMI (p < .001; β = −0.26)CES-D-10 (p < .001; β = −0.08)EDU (p = .003; β = 0.07)INC (p = .002; β = 0.08) SEQ (p = .13; β = 0.03) HRT (p = .05; β = 0.04)DIA (p = .003; β = 0.07)KID (p = .52; β = 0.01) | |
| Covariates +NUTHC | R 2 = .3700 | F(17,1386) = 47.88 p < .0001 | NUTHC (p = .74; β = −0.01) | p = .74 |
| Covariates +NUTFBR | R 2 = .3727 | F(17,1386) = 48.43 p < .0001 | NUT FBR (p = .01; β = 0.05) | p = .01 |
| Covariates +NURSCR | R 2 = .3751 | F(17,1386) = 48.94 p < .0001 | NUR SCR (p = .001; β = 0.08) | p = .001 |
| Covariates +NURCLS | R 2 = .3724 | F(18,1385) = 45.66 p < .0001 | NURCLS (p = .06) | p = .06 |
Note: Independent variables: frequency of high calorie snacks (NUTHC), frequency of high fiber cereal (NUTFBR), nutrition risk score (NURSCR), and nutrition risk classification (NURCLS, 2 = moderate risk, 3 = high-risk). Covariates: OA type, age, sex, body mass index (BMI), depressive symptoms (CES-D-10), education level (EDU), income (INC), social inequality (SEQ), heart disease (HRT), diabetes (DIA), and kidney disease (KID). Bolded values indicate significant findings.
General health index
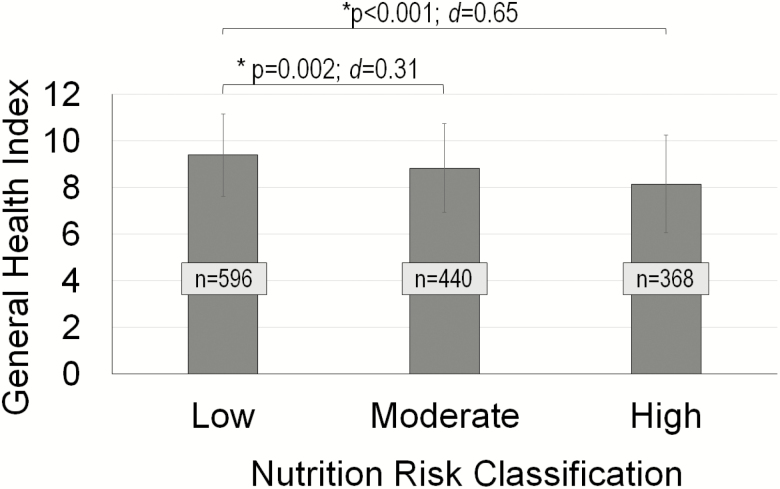
The covariate model explained 25.31% of the variance (p < .0001) in the general health index, with age, sex, BMI, depressive symptoms, education, social inequality, heart disease, and diabetes being significant (Table 4). Male sex, younger age, higher BMI and greater depressive symptoms, lower education, lower social inequality and having heart disease and diabetes were associated with a lower general health index. Neither dietary variables were associated with general health; however, both NUR score and classification (NURSCR, p < .001; NURCLS, p < .0001) were significant. Higher NUR (lower NURSCR) was associated with a lower general health index. Furthermore, those with high NUR classifications had a significantly lower general health index than low-risk classification, yielding a medium-large effect size (p < .001; d = 0.65). Additionally, there was a small-medium significant effect between low and moderate NUR classifications (p = .001; d = 0.31) with moderate risk associated with lower general health than low risk (Figure 2).
Table 4.
Regression Models Used to Predict General Health Index
| Model | Explained Variance | Model Significance | Significant Predictors | Likelihood-Ratio Test (compared to covariate) |
|---|---|---|---|---|
| Covariate model | R 2 = .2531 | F(16,1387) = 29.37 p < .0001 | OAtype (p = .89) Age (p = .001; β = 0.09)Sex (p = .006; β = 0.07)BMI (p < .001; β = −0.19)CES-D-10 (p < .001; β = −0.33)EDU (p = .004; β = 0.07) INC (p = .83; β = 0.01) SEQ (p < .001; β = 0.16)HRT (p < .001; β = 0.12)DIA (p = .003; β = 0.07)KID (p = .41; β = −0.02) | |
| Covariates +NUTHC | R 2 = .2538 | F(17,1386) = 27.73 p < .0001 | NUTHC (p = .24; β = −0.03) | p = .23 |
| Covariates +NUTFBR | R 2 = .2533 | F(17,1386) = 27.66 p < .0001 | NUTFBR (p = .47; β = 0.02) | p = .46 |
| Covariates +NURSCR | R 2 = .2674 | F(17,1386) = 29.76 p < .0001 | NUR SCR (p < .001; β = 0.13) | p < .0001 |
| Covariates +NURCLS | R 2 = .2666 | F(18,1385) = 27.97 p < .0001 | NUR CLS NUR CLS 2 (p = .001; β = −0.09)NURCLS3 (p < .001; β = −0.13) | p < .0001 |
Note: Independent variables: frequency of high calorie snacks (NUTHC), frequency of high fiber cereal (NUTFBR), nutrition risk score (NURSCR), and nutrition risk classification (NURCLS, 2 = moderate risk, 3 = high risk). Covariates: OA type, age, sex, body mass index (BMI), depressive symptoms (CES-D-10), education level (EDU), income (INC), social inequality (SEQ), heart disease (HRT), diabetes (DIA), and kidney disease (KID). Bolded values indicate significant findings.
Figure 2.
General health index (mean; error bars = standard deviation) by nutrition risk classification. Asterisk (*) indicates significant differences between classification groups (p < .05). p-Value, effect sizes (d), and sample size (n) are provided.
Independent variable and covariate relationships
Significant, albeit weak, correlations were demonstrated between NUR score and several of the included covariates. Specifically, a higher NURSCR (lower NUR) was associated with lower BMI (r = −.20, p < .0001), lower depressive symptoms (r = −.26, p < .0001), higher education (r = .14, p < .0001), income (r = .16, p < .0001), social inequality (r = .11, p < .0001), and lower presence of diabetes (r = .13, p < .0001). Age, sex, heart disease, and kidney disease were not related to NURSCR. Alternatively, a higher intake of high calorie snacks (NUTHC) only demonstrated relationships with older age (r = .08, p = .003) and male sex (r = −.06, p = .02), while a higher intake of high fiber cereal (NUTFBR) only demonstrated relationships with older age (r = .09, p = .0004) and lower BMI (r = −.10, p = .0003). It is important to note, that despite being significant, all of the relationships between independent variables and covariates were weak (r ≤ .26).
Discussion
Nutrition risk was significantly associated with mobility and general health, with higher risk related to poorer mobility and general health in persons with various types of OA. Frequency of intake of different dietary items, notably high calorie snacks and high fiber cereal, were not generally significant contributors to the models explaining most physical capacity and health outcomes. One exception was that greater intake of high fiber cereal was related to better mobility. Additionally, covariates which included OA type, age, sex, BMI, depressive symptoms (CES-D-10), socioeconomic variables (education, income, and social inequality), and certain comorbidities (heart disease, diabetes) together explained a large amount of variance in each of the dependent variables. While significant bivariate relationships were identified between several covariates with the dietary intake variables and NUR, these relationships were weak (r ≤ 0.26), and thus should be interpreted with caution.
Nutrition Risk Among Older Adults With OA
Among older adults with OA, NUR was significantly associated with lower mobility and general health. While this study is the first to explore NUR in OA, researchers have previously identified a relationship between NUR and both physical function and quality of life in older adults. An 18-month longitudinal study demonstrated that NUR predicted quality of life among frail community-dwelling seniors (p = .03) (35). Those at high NUR had approximately 2.2 fewer days per month with self-reported good physical health than those at low/moderate NUR. A 2-year longitudinal study in older adults further investigated the relationship between nutrition and functional capacity demonstrating that NUR at baseline was associated with functional decline of both activities of daily living (ADL) and instrumental ADL (IADL) (47). Specifically, for those at high NUR, 12.2% and 27.8% demonstrated a functional decline in ADL and IADL, respectively, compared to those at low NUR who had a decline of 1.9% and 8.2%, respectively. Recently, researchers have studied the predictive ability of NUR in explaining hospitalizations and mortality using multiple Canadian databases (Canadian Community Health Survey–Health Aging [CCHS–HA], Discharge Abstract Database and Canadian Mortality Database) (48). Among 9,878 participants over the age of 65, within a 25–36-month follow-up, the incidence of hospitalization and death was significantly higher among those at NUR, compared to those who were not. The NUR tool used in that study was the same as the current analysis.
The present analysis shows that NUR is an important concern for older adults with OA. We highlight potentially clinically meaningful differences between low and high NUR classifications for general health and potentially mobility, as demonstrated by medium-large effect sizes (high vs low NUR classification, general health d = .65; mobility d = .43). This suggests that nutrition problems in OA are not solely caused by what individuals eat, but also the eating behaviors that affect food consumption. The aforementioned longitudinal studies, in addition to the present research, highlight the utility of nutrition screening for identifying risk among this vulnerable population to subsequently develop effective prevention and rehabilitation strategies to improve physical function and health. To-date, the vast majority of nutrition interventions in OA have focused on calorie restriction to promote weight loss (25,49,50). The current findings suggest that overnutrition is only one element of nutrition challenges in OA. Behavioral characteristics associated with nutrition, including meal preparation, eating alone and physical challenges associated with eating, such as difficulty swallowing, should be explored as key factors contributing to malnutrition, physical capacity and general health in OA. Thus, this work provides insight into new avenues for conservative intervention to supplement exercise and dietary restriction in OA.
Dietary Intake and Physical Capacity and General Health in OA
The current analysis did not show overwhelming evidence for the relationship between high calorie snacks and high fiber cereal with physical capacity and general health outcomes. We found no evidence for a relationship between high calorie snack intake and physical capacity or general health; however, the current findings do suggest that there is the potential to enhance the impact of dietary interventions on mobility in OA through increasing fiber intake. Previously, researchers have studied specific diets or diet interventions and both clinical and performance outcomes. Messier and colleagues (25) conducted an 18-month RCT to examine the effects of a low calorie diet intervention, exercise intervention and a combined diet and exercise intervention on both biomechanical and clinical outcomes in older adults with clinical and radiographical knee OA. This study demonstrated that a combined intervention, that includes a calorie restricted diet, is important for health, pain, and physical function in OA. As fiber is in high amounts in nutrient dense (eg, fruits and vegetables, whole grains) foods that are also generally low in calories, the benefits of fiber may also be linked to consumption of these diet components. The association found in this study is supported by recent research that similarly demonstrated the importance of a high fiber diet for pain (27). Specifically, nutritional and clinical data obtained for those with or at risk of knee OA showed a significant association between a high fiber intake and lower risk of moderate and severe knee pain, with the odds ratio between highest and lowest quartiles of fiber intake being 0.57 (p = .0004) and 0.41 (p = .0006) for moderate and severe knee pain, respectively. This association has been presumed to exist due to the positive effect of fiber on reducing adiposity and inflammation, which are associated with pain in OA (27). It is also possible though, that high fiber intake reflects healthier living and socioeconomics; but without a full version of a food frequency questionnaire in the CLSA, we were unable to assess diet quality fully. High sugar and high fat diets may alter gut microbiota in ways that worsen systemic inflammation; while high fiber diets may prevent this intestinal dysbiosis (29,30). Nonetheless, while rehabilitation that includes exercise has shown considerable improvements in pain and function for knee OA, this research highlights the potential importance of also considering specific dietary intake, notably fiber, for its impact on mobility.
Effect of OA Type on Model Outcomes
Covariates were selected-based on literature supporting their impact on grip strength, mobility, and general health. Age, sex, and BMI were significant across the different outcome measures, as were depressive symptom scores and socioeconomic variables. Two comorbidities, heart disease and diabetes, were also shown to be related to poorer mobility and general health. As well, it is intuitive to expect that OA type would be a significant covariate, particularly in the mobility and grip strength models which more specifically address lower limb and upper limb challenges, respectively. In particular, OA type that included hand OA had lower grip strength than those that solely included knee or hip OA. For mobility, having multiple forms of lower limb OA was related to poorer mobility; combined hip and knee OA had significantly poorer mobility than either knee OA alone or combined hand and knee OA. As well, hip OA alone had poorer mobility than hand OA. Lastly, it was not surprising that OA type did not explain variance in general health. The challenges imposed by each OA type individually (hand, hip, or knee OA) or a combination of multiple forms could all negatively affect one’s self-reported general health. Thus, it is important to consider OA type as a variable that can influence the relationship between nutrition and physical capacity, particularly when outcome measures pose different physical challenges to different joints.
Limitations
This study has limitations. First, data from several potential participants were excluded from these analyses because of missing data from one or more variables (Supplementary Table 1). The differences identified between those with missing data and those included in the analyses preclude generalizing these results across the entire population with hip, knee, and hand OA. Nevertheless, when analyses were conducted with separate samples for each dependent variable (grip strength, n = 1,490; mobility, n = 1,552; general health, n = 1,648), rather than the common sample with a complete data set (n = 1,404), the results were identical.
The grip strength model (R2 = .6367) explained a greater amount of variance than both mobility (R2 = .3699) and general health (R2 = .2531) models. This finding suggests that other variables may account for the unexplained variance in the mobility and general health models. For example, comorbidities for which participants were excluded from the current analysis (ie, chronic obstructive pulmonary disease, dementia, cerebrovascular accident), physical fitness, objective measures of physical function or body composition likely further impact mobility and general health. In particular, multimorbidity is likely an important influence on mobility and general health. Multimorbidity is the co-occurrence of multiple diseases or conditions within an individual and is linked to poorer health outcomes (51), particularly with aging (52). Because the present analysis focused on establishing the association between OA disease and nutrition and therefore excluded individuals with other chronic health conditions, this analysis did not explore the role of multimorbidity. Beyond the focus on OA here, a broader perspective on the role of chronic disease burden mobility and general health, using tools designed to capture multimorbidity (53), should be the focus of future work. This analysis was also limited to exploring determinants of ADL (self-reported or objective measurement). Future work exploring ADL would be clinically useful. A comprehensive assessment of diet intake was unavailable for analysis of diet quality and future work should examine diet, preferably longitudinally, and its effect on functional outcomes in those with OA. Data used in these analyses were obtained from the CLSA Comprehensive Dataset. The researchers involved in conducting the analysis had no role in data collection or entry and thus would be unaware of methodological concerns or data entry errors.
In conclusion, NUR, as well as intake of fiber, are associated with mobility and general health among older adults with OA of the hand, hip, and knee. This research suggests that increasing fiber intake, potentially as a marker of diet quality, may be associated with better mobility in Canadian adults with hand, hip, and/or knee OA, while those with less NUR have better function and health. This study highlights the importance of considering both diet and NUR in future research investigating functional outcomes in older adults with OA and warrants development of nutrition interventions to mitigate impaired function and consequent frailty in those with OA.
Funding
J.C.-H. was supported through a Canadian Institutes of Health Research (CIHR) fellowship award. H.K. is the Schlegel-University of Waterloo Research Institute for Aging, Research Chair, Nutrition and Aging. Operating costs were supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant (353715 MRM). This research was made possible using the data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA). Funding for the Canadian Longitudinal Study on Aging (CLSA) is provided by the Government of Canada, through the Canadian Institutes of Health Research (CIHR) under grant reference: LSA 9447 and the Canada Foundation for Innovation. This research has been conducted using the CLSA Baseline Comprehensive Dataset 3.1, under Application Number 161014. The CLSA is led by Drs. Parminder Raina, Christina Wolfson, and Susan Kirkland.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Professor Paul Stratford and Anthony Gatti from the School of Rehabilitation Science at McMaster University for statistical support. The opinions expressed in this manuscript are the author’s own and do not reflect the views of the Canadian Longitudinal Study on Aging.
Conflict of Interest
None reported.
References
- 1. Rao DP, Patel P, Roberts KC, Thompson W. Obesity and healthy aging: social, functional and mental well-being among older Canadians. Health Promot Chronic Dis Prev Can. 2018;38:437–444. doi: 10.24095/hpcdp.38.12.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Statistics Canada. Canada’s Population Estimates: Age and Sex, July 1, 2018. Ottawa, Canada: Released at 8:30 am Eastern time in The Daily, Friday, January 25, 2019. [Google Scholar]
- 3. Public Health Agency of Canada. Obesity in Canadian Adults: It’s About More Than Just Weight. Ottawa, Canada: Government of Canada; 2017. [Google Scholar]
- 4. Cederholm T, Barazzoni R, Austin P, et al. ESPEN guideline: ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36:49–64. doi: 10.1016/j.clnu.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 5. Jelliffe DB. The assessment of the nutritional status of the community. Monogr Ser World Health Organ. 1966;53:1–271; ISBN: 9241400536. [PubMed] [Google Scholar]
- 6. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci. 2001;56:M146–157. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 7. Mendonça N, Granic A, Hill TR, et al. Protein intake and disability trajectories in very old adults: the Newcastle 85+ study. J Am Geriatr Soc. 2019;67:50–56. doi: 10.1111/jgs.15592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krok-Schoen JL, Archdeacon Price A, Luo M, Kelly OJ, Taylor CA. Low dietary protein intakes and associated dietary patterns and functional limitations in an aging population: a NHANES analysis. J Nutr Health Aging. 2019;23:338–347. doi: 10.1007/s12603-019-1174-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vermeiren S, Vella-Azzopardi R, Beckwée D, et al. ; Gerontopole Brussels Study Group Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17:1163.e1–1163.e17. doi: 10.1016/j.jamda.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 10. Wei MY, Kabeto MU, Galecki AT, Langa KM. Physical functioning decline and mortality in older adults with multimorbidity: joint modeling of longitudinal and survival data. J Gerontol A Biol Sci Med Sci. 2019;74:226–232. doi: 10.1093/gerona/gly038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bales CW, Buhr GT. Body mass trajectory, energy balance, and weight loss as determinants of health and mortality in older adults. Obes Facts. 2009;2:171–178. doi: 10.1159/000221008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Briggs AM, Cross MJ, Hoy DG, et al. Musculoskeletal health conditions represent a global threat to healthy aging: a report for the 2015 world health organization world report on ageing and health. Gerontologist. 2016;56(S2):S243–255. doi: 10.1093/geront/gnw002 [DOI] [PubMed] [Google Scholar]
- 13. Calders P, Van Ginckel A. Presence of comorbidities and prognosis of clinical symptoms in knee and/or hip osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2018;47:805–813. doi: 10.1016/j.semarthrit.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 14. Perruccio A, Badley E, Guan J. Burden of disease. In: Badley E, Glazier R, eds. Arthritis and Related Conditions in Ontario. Toronto, Ontario: Institute for Clinical Evaluative Sciences; 2004. [Google Scholar]
- 15. Salihu HM, Bonnema SM, Alio AP. Obesity: what is an elderly population growing into? Maturitas. 2009;63:7–12. doi: 10.1016/j.maturitas.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 16. Shlisky J, Bloom DE, Beaudreault AR, et al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv Nutr. 2017;8:17–26. doi: 10.3945/an.116.013474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barazzoni R, Bischoff SC, Boirie Y, et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr. 2018;37(6 Pt A):1787–1793. doi: 10.1016/j.clnu.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 18. Follis S, Cook A, Bea JW, et al. Association between sarcopenic obesity and falls in a multiethnic cohort of postmenopausal women. J Am Geriatr Soc. 2018;66:2314–2320. doi: 10.1111/jgs.15613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dobson F, Bennell KL, French SD, et al. Barriers and facilitators to exercise participation in people with hip and/or knee osteoarthritis: synthesis of the literature using behavior change theory. Am J Phys Med Rehabil. 2016;95:372–389. doi: 10.1097/PHM.0000000000000448 [DOI] [PubMed] [Google Scholar]
- 20. Bombardier C, Hawker G, Mosher D. The Impact of Arthritis in Canada: Today and Over the Next 30 Years. Toronto, Ontario: Arthritis Alliance of Canada; 2011. [Google Scholar]
- 21. Bennell KL, Wrigley TV, Hunt MA, Lim BW, Hinman RS. Update on the role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am. 2013;39:145–176. doi: 10.1016/j.rdc.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 22. Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:507–515. doi: 10.1016/j.joca.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 23. Runhaar J, Koes BW, Clockaerts S, Bierma-Zeinstra SM. A systematic review on changed biomechanics of lower extremities in obese individuals: a possible role in development of osteoarthritis. Obes Rev. 2011;12:1071–1082. doi: 10.1111/j.1467-789X.2011.00916.x [DOI] [PubMed] [Google Scholar]
- 24. Dumond H, Presle N, Terlain B, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118–3129. doi: 10.1002/art.11303 [DOI] [PubMed] [Google Scholar]
- 25. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. doi: 10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Christensen P, Frederiksen R, Bliddal H, et al. Comparison of three weight maintenance programs on cardiovascular risk, bone and vitamins in sedentary older adults. Obesity (Silver Spring). 2013;21:1982–1990. doi: 10.1002/oby.20413 [DOI] [PubMed] [Google Scholar]
- 27. Dai Z, Lu NA, Niu J, Felson DT, Zhang Y. Dietary fiber intake in relation to knee pain trajectory. Arthritis Care Res (Hoboken). 2017;69:1331–1339. doi: 10.1002/acr.23158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Felson DT, Lawrence RC, Hochberg MC, et al. Osteoarthritis: new insights. Part 2: treatment approaches. Ann Intern Med. 2000;133:726–737. doi: 10.7326/0003-4819-133-9-200011070-00015 [DOI] [PubMed] [Google Scholar]
- 29. Collins KH, Paul HA, Reimer RA, Seerattan RA, Hart DA, Herzog W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: studies in a rat model. Osteoarthritis Cartilage. 2015;23:1989–1998. doi: 10.1016/j.joca.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 30. Morris JL, Letson HL, Gillman R, et al. The CNS theory of osteoarthritis: opportunities beyond the joint. Semin Arthritis Rheum. 2019;49:331–336. doi: 10.1016/j.semarthrit.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 31. Robinson SM, Westbury LD, Cooper R, et al. Adult lifetime diet quality and physical performance in older age: findings from a British birth cohort. J Gerontol A Biol Sci Med Sci. 2018;73:1532–1537. doi: 10.1093/gerona/glx179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keller HH. Promoting food intake in older adults living in the community: a review. Appl Physiol Nutr Metab. 2007;32:991–1000. doi: 10.1139/H07-067 [DOI] [PubMed] [Google Scholar]
- 33. Keller HH, Goy R, Kane SL. Validity and reliability of SCREEN II (seniors in the community: risk evaluation for eating and nutrition, Version II). Eur J Clin Nutr. 2005;59:1149–1157. doi: 10.1038/sj.ejcn.1602225 [DOI] [PubMed] [Google Scholar]
- 34. Keller HH, Østbye T. Nutritional risk and time to death; predictive validity of SCREEN (seniors in the community risk evaluation for eating and nutrition). J Nutr Health Aging. 2003;7:274–279. [PubMed] [Google Scholar]
- 35. Keller HH, Østbye T, Goy R. Nutritional risk predicts quality of life in elderly community-living Canadians. J Gerontol A Biol Sci Med Sci. 2004;59:68–74. doi: 10.1093/gerona/59.1.m68 [DOI] [PubMed] [Google Scholar]
- 36. Raina PS, Wolfson C, Kirkland SA, et al. The Canadian longitudinal study on aging (CLSA). Can J Aging. 2009;28:221–229. doi: 10.1017/S0714980809990055 [DOI] [PubMed] [Google Scholar]
- 37. Shatenstein B, Payette H. Evaluation of the relative validity of the short diet questionnaire for assessing usual consumption frequencies of selected nutrients and foods. Nutrients. 2015;19:6362–6374. doi: 10.3390/nu7085282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9:222–226. doi: 10.1016/S0363-5023(84)80146-X [DOI] [PubMed] [Google Scholar]
- 39. Akhavan NS, Ormsbee L, Johnson SA, et al. Functionality in middle-aged and older overweight and obese individuals with knee osteoarthritis. Healthcare. 2018;6:74 (1–12). doi: 10.3390/healthcare6030074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kennedy D, Stratford PW, Pagura SM, Walsh M, Woodhouse LJ. Comparison of gender and group differences in self-report and physical performance measures in total hip and knee arthroplasty candidates. J Arthroplasty. 2002;17:70–77. doi: 10.1054/arth.2002.29324 [DOI] [PubMed] [Google Scholar]
- 41. Gill SV, Hicks GE, Zhang Y, Niu J, Apovian CM, White DK. The association of waist circumference with walking difficulty among adults with or at risk of knee osteoarthritis: the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2017;25:60–66. doi: 10.1016/j.joca.2016.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mohebbi M, Nguyen V, McNeil JJ, et al. Psychometric properties of a short form of the center for epidemiologic studies depression (CES-D-10) scale for screening depressive symptoms in healthy community dwelling older adults. Gen Hosp Psychiatry. 2018;51:118–125. doi: 10.1016/j.genhosppsych.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Forge BR, Sobal J, Krick JP. Relation of perceived health with psychosocial variables in elderly osteoarthritis patients. Psychol Rep. 1989;64:147–156. doi: 10.2466/pr0.1989.64.1.147 [DOI] [PubMed] [Google Scholar]
- 44. Ettinger WH, Davis MA, Neuhaus JM, Mallon KP. Long-term physical functioning in persons with knee osteoarthritis from NHANES. I: effects of comorbid medical conditions. J Clin Epidemiol. 1994;47:809–815. doi: 10.1016/0895-4356(94)90178-3 [DOI] [PubMed] [Google Scholar]
- 45. van Dijk GM, Veenhof C, Schellevis F, et al. Comorbidity, limitations in activities and pain in patients with osteoarthritis of the hip or knee. BMC Musculoskelet Disord. 2008;9:95. doi: 10.1186/1471-2474-9-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. CLSA CLS on A. CLSA Technical Document: Sampling and Computation of Response Rates and Sample Weights for the Tracking (Telephone Interview) Participants and Comprehensive Participants. 2017. https://www.clsa-elcv.ca/doc/1041 [Google Scholar]
- 47. Sugiura Y, Tanimoto Y, Imbe A, et al. Association between functional capacity decline and nutritional status based on the nutrition screening initiative checklist: a 2-year cohort study of Japanese community-dwelling elderly. PLoS One. 2016;28:1–10. doi: 10.1371/journal.pone.0166037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ramage-Morin PL, Gilmour H, Rotermann M. Nutritional risk, hospitalization and mortality among community-dwelling Canadians aged 65 or older. Health Rep. 2017;28:17–27. Catalogue no. 82-003-X. [PubMed] [Google Scholar]
- 49. Christensen P, Bliddal H, Riecke BF, Leeds AR, Astrup A, Christensen R. Comparison of a low-energy diet and a very low-energy diet in sedentary obese individuals: a pragmatic randomized controlled trial. Clin Obes. 2011;1:31–40. doi: 10.1111/j.1758-8111.2011.00006.x [DOI] [PubMed] [Google Scholar]
- 50. Riecke BF, Christensen R, Christensen P, et al. Comparing two low-energy diets for the treatment of knee osteoarthritis symptoms in obese patients: a pragmatic randomized clinical trial. Osteoarthritis Cartilage. 2010;18:746–754. doi: 10.1016/j.joca.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 51. Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu AL, Maltais D. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes. 2004;2:51. doi: 10.1186/1477-7525-2-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–2276. doi: 10.1001/archinte.162.20.2269 [DOI] [PubMed] [Google Scholar]
- 53. Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10:134–141. doi: 10.1370/afm.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.